A Scoping Review of Neurotoxic and Behavioral Outcomes Following Polychlorinated Biphenyl (PCB) Exposure in Post-Weaned Rodents
Abstract
1. Introduction
2. Methods
2.1. Selection of Articles
2.1.1. Search Strategy
2.1.2. Title and Abstract Screening
2.1.3. Automated Screening Using ChatGPT
2.1.4. Analysis of Screened Articles
2.2. Method Screening
2.2.1. Manual Screening of Methods Sections
2.2.2. Automated Methods Screening Using ChatGPT
2.2.3. Analysis of Methods Screening Results
2.3. Full-Text Assessment
2.4. Limitations
2.5. Statistical Analysis
3. Results and Discussion
3.1. Article Selection
3.2. Inter-Reviewer Reliability Assessment
3.3. Neurotoxic Outcomes
3.3.1. Histological/Morphological Changes
3.3.2. Calcium Modulation
3.3.3. Oxidative Stress and DNA Modifications
3.3.4. Neurotransmitters and Receptors
Biosynthesis of Neurotransmitters
Dopamine and Metabolites
Dopamine Receptors and Transporters
Serotonin and Norepinephrine
Glutamate
3.3.5. Apoptosis
3.3.6. Additional Drivers of PCB-Induced Neurotoxicity
Enzymes
Receptors
Iron Metabolism
Amino Acids
Gene Expression
Signaling Pathways
Cytokine/Chemokine
DNA Methylation
3.4. Behavioral Outcomes
3.4.1. Locomotor and Exploration
3.4.2. Anxiety-like Behavior
3.4.3. Learning and Memory
3.4.4. Motor Coordination and Balance
3.4.5. Social and Sociosexual Behavior
3.4.6. Operant Conditioning and Behavioral Flexibility
3.4.7. Reproductive and Maternal Behavior
3.4.8. Cognitive Flexibility and Decision-Making
3.4.9. Impulsivity and Timing
4. Conclusions
4.1. Neurotoxic Outcomes
4.2. Behavioral Outcomes
4.3. Limitations
| Behavioral Test | Behavioral Domain | Key Brain Regions Implicated | Principal Molecular Pathways/ Mechanisms | Proposed Mechanistic Interpretation (Cross-Scale Link) | References |
|---|---|---|---|---|---|
| OFT | Locomotor and Exploration; Anxiety-Like Behavior | Striatum, Prefrontal Cortex, Amygdala | Dopaminergic signaling disruption; serotonergic/GABAergic imbalance; Ca2+ dysregulation | Altered dopaminergic tone and excitatory–inhibitory imbalance led to hyperactivity or increased thigmotaxis indicative of anxiety-like responses. | [258,259,260] |
| EPM | Anxiety-Like Behavior | Amygdala, Hippocampus | Serotonergic and GABAergic disruption; oxidative stress | Neurochemical imbalance enhances amygdala excitability, increasing anxiety and reducing open-arm exploration. | [187,261,262] |
| Light–Dark Box | Anxiety-Like Behavior | Amygdala, Prefrontal Cortex | Serotonergic and GABAergic imbalance; neuroinflammation | Heightened inflammatory and neurotransmitter dysregulation promotes avoidance of the light zone and risk-averse exploration. | [263,264,265] |
| MWM | Learning and Memory | Hippocampus, Cortex | Oxidative stress; Ca2+ homeostasis disruption; BDNF–TrkB axis impairment; thyroid hormone signaling | Oxidative injury and reduced neurotrophic support impair hippocampal plasticity and spatial learning. | [266,267,268] |
| 8-Arm Radial Maze | Learning and Memory | Hippocampus, Prefrontal Cortex | Oxidative stress; mitochondrial dysfunction | Energy deficits and oxidative damage reduce accuracy and increase latency in memory retrieval. | [198,269,270] |
| CFA | Learning and Memory | Insular Cortex, Amygdala | Synaptic plasticity gene dysregulation; oxidative stress | Impaired synaptic encoding of aversive cues due to oxidative stress and reduced immediate-early gene activation. | [271,272,273] |
| Rotarod | Motor Coordination and Balance | Cerebellum, Motor Cortex, Striatum | Dopaminergic disruption; mitochondrial dysfunction; apoptosis | Motor impairments arise from cerebellar mitochondrial toxicity and dopaminergic neuron loss. | [274,275,276] |
| Three-Chamber Social Interaction | Social and Sociosexual Behavior | Prefrontal Cortex, Hippocampus, Amygdala | BDNF–TrkB axis disruption; neuroinflammation | Reduced neurotrophic signaling and cytokine-mediated synaptic pruning impair social approach behavior. | [217,277,278] |
| Social Novelty Test | Social and Sociosexual Behavior | Hippocampus, Prefrontal Cortex | BDNF–TrkB impairment; oxidative stress | Diminished hippocampal plasticity and oxidative injury impair discrimination between familiar and novel animals. | [279,280,281] |
| USV | Social and Sociosexual Behavior | Amygdala, Periaqueductal Gray | Synaptic plasticity gene dysregulation; neuroinflammation | Altered synaptic signaling and neuroimmune activation disrupt communication and social bonding cues. | [218,282,283] |
| Operant Conditioning | Operant Conditioning and Behavioral Flexibility | Striatum, Prefrontal Cortex | Dopaminergic disruption; oxidative stress | Impaired reward processing and task acquisition due to frontostriatal dysfunction. | [284,285,286] |
| Maternal Care | Reproductive and Maternal Behavior | Hypothalamus, Prefrontal Cortex | Thyroid hormone disruption; dopaminergic signaling | Endocrine disruption and altered reward circuits reduce pup-directed behaviors. | [232,287,288] |
| Set-Shifting/Reversal Learning | Cognitive Flexibility and Decision-Making | Prefrontal Cortex | Neuroinflammation; synaptic gene dysregulation | Prefrontal cytokine activation and reduced synaptic remodeling impair task- switching and adaptability. | [289,290,291] |
4.4. Future Directions and Translational Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HIAA | 5-hydroxyindoleacetic acid |
| AChE | Acetylcholinesterase |
| AhR | Aryl hydrocarbon receptor |
| ANOVA | Analysis of variance |
| Ars | Androgen receptors |
| AF-6 | Afadin |
| Avpr1a | Vasopressin receptor 1a |
| Bad | BCL2-associated death promoter |
| Bax | BCL2-associated X protein |
| Bcl2 | B-cell leukemia/lymphoma 2 |
| BDNF | Brain-derived neurotrophic factor |
| Bid | BH3 interacting-domain death agonist |
| BNST | Bed nucleus of the stria terminalis |
| CAR | Constitutive androstane receptor |
| CAT | Catalase |
| CASP | Caspase |
| CBL | Cerebellar |
| CFA | Conditioned flavor aversion |
| cGMP | Cyclic guanosine monophosphate |
| Cldn5 | Claudin-5 |
| CNS | Central nervous system |
| COMT | Catechol-O-methyltransferase |
| CREB | Cyclic AMP-response element binding protein |
| CRF | Continuous reinforcement |
| Cu/Zn SOD | Cu/Zn superoxide dismutase |
| CYPs | Cytochrome P450 monooxygenases |
| DA | Dopamine |
| DAT | Dopamine transporter |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| DRL | Differential reinforcement of low rates of responding |
| DRL1 | DRL training at the 1 s interval |
| DRL10 | DRL training at the 10 s interval |
| DRL15 | DRL training at the 15 s interval |
| DRL5 | DRL training at the 5 s interval |
| EAC1 | Excitatory amino acid carrier 1 |
| EPM | Elevated Plus Maze |
| ERK | Extracellular signal-regulated kinase |
| ERs | Estrogen receptors |
| EXT | Extinction |
| Fas | Tumor necrosis factor receptor superfamily member 6 |
| FasL | Fas ligand |
| FI | Fixed interval |
| GLAST | Glutamate aspartate transporter |
| GLT-1 | Glutamate transporter 1 |
| GPV | Glial plasmalemmal vesicle |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSTs | Glutathione-S-transferases |
| HO-1 | Heme oxygenase-1 |
| HVA | Homovanillic acid |
| i.p. | Intraperitoneal |
| Jam3 | Junction adhesion molecule-3 |
| LTME | Long-term memory error |
| MAP | Microtubule-associated protein |
| MeA | Medial amygdala |
| Mn SOD | Manganese SOD |
| MR | Muscarinic receptor |
| MWM | Morris water maze |
| NAPDH-d | NADPH-diaphorase |
| NE | Norepinephrine |
| Nefl | Neurofilament light chain |
| NF-kB | Nuclear factor kappa B |
| NMDA | N-methyl-D-aspartate |
| NOS | Nitric oxide synthase |
| NTKRB | Neurotrophin tyrosine kinase receptor B |
| Ocln | Occludin |
| OFT | Open Field Test |
| OH-PCBs | Hydroxylated metabolites |
| Oprm1 | mu opioid receptor |
| PCBs | Polychlorinated biphenyls |
| PKA | Protein Kinase A |
| PKC | Protein Kinase C |
| PPARs | Peroxisome proliferator-activated receptors |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| PVN | Paraventricular nucleus |
| PXR | Pregnane X receptor |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RyRs | Ryanodine receptors |
| SAM | School Air Mixture |
| SNpc | Substantia nigra pars compacta |
| SON | Supraoptic nucleus |
| STME | Short-term memory errors |
| STR | Striatal |
| SULTS | Sulfotransferases |
| TBARS | Thiobarbituric acid reactive substances |
| TH | Tyrosine hydroxylase |
| TH− | TH-negative |
| TH+ | Tyrosine hydroxylase-positive |
| TME | Total memory error |
| TNFa | Tumor necrosis factor alpha |
| UGTs | UDP-glucuronosyltransferases |
| U.S. | United States |
| USVs | Ultrasonic vocalizations |
| VMH | Ventromedial hypothalamus |
| VTA | Ventral tegmental area |
| ZO | Zona Occludens |
Appendix A
| PCB Source | Dose (mg/kg/day) a | Exposure Route | Vehicle | Exposure Period | Age | N b | Sex | Brain Region | Analysis | Result c | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1254 d | 62.1 ± 17.7 μg/m3 | Inh e | LA f | 28 days | Adult (239 ± 8g) | 8 | F g | Whole Brain | Histology H&E h | - Gross histopathological changes in HC i - Features of necrosis or apoptosis | [24] |
| A1254 A1221 | 45.5 ± 5.9 μg/m3 | Inh | LA | 6 d/w j for 13 weeks | Adult (241 ± 9g) | S k: 8 E l: 12 | F | Whole Brain | Histology H&E | - Histopathological changes | [25] |
| A1254 | 62.1 ± 17.7 μg/m3 | Inh | LA | 28 days | Adult (239 ± 8g) | 8 | F | Whole Brain | OS m Markers | ↓ ROS n/RNS o | [24] |
| A1254 A1221 | 45.5 ± 5.9 μg/m3 | Inh | LA | 6 d/w for 13 weeks | Adult (241 ± 9g) | S: 8 E: 12 | F | Whole Brain | OS Markers | - ROS/RNS | [25] |
| A1221 | 0 or 1 | IP p | 4% DMSO q | PND24 r, 26, 28 | PND24 | 9–12 | M s/F | BNST t MeA u PVN v VMH w | DNA Methylation | - DNA methylation | [163] |
| A1254 | 0 or 25 | PO x | CO y on VW z | 3 days, or 1, 2, or 8 weeks | 12 weeks | 3–4 | M | Striatal Dialysate | DA aa Concentrations | ↑ DA after 3 days ↓ DA after 1, 2, and 8 weeks | [132] |
| A1254 | 0 or 25 | PO | CO on VW | 3 days, or 1, 2, or 8 weeks | 12 weeks | 3–6 | M | ST bb | DA Concentrations | - DA levels | [132] |
| A1254 A1221 | 45.5 ± 5.9 μg/m3 | Inh | LA | 6 d/w for 13 weeks | Adult (241 ± 9g) | S: 8 E: 12 | F | ST | DA and DOPAC cc Concentrations | - DA and DOPAC levels | [25] |
| PCB 180 | 0, 19.4, 64.3, 194, 643, 2000, 6430 or 13000 | PO | CO | 28 days | 6 weeks | 5 | M/F | CE dd | DA and Nicotinic Receptors | - Specific binding to DRD1 ee/DRD5 receptors - High or low affinity sites on nicotinic receptor | [119] |
| PCB 180 | 0, 19.4, 64.3, 194, 643, 2000, 6430 or 13,000 | PO | CO | 28 days | 6 weeks | 5 | M/F | CE | Brain AA ff | ↓ GSH gg in males - γ-Amino butyric acid, aspartate, glutamate, serine, glycine, taurine, glutamine, alanine, | [119] |
| A1254 A1221 | 45.5 ± 5.9 μg/m3 | Inh | LA | 6 d/w for 13 weeks | Adult (241 ± 9g) | S: 8 E: 12 | F | Whole Brain | Cytokine and Chemokine | - Cytokine or chemokine concentrations | [25] |
| A1221 | 0 or 1 | IP | 4% DMSO | PND24, 26, 28 | P24 | 9–12 | M/F | PoA hh | Gene Expression | ↓ Expression of Ar ii and Oprm1 jj in males | [163] |
| A1221 | 0 or 1 | IP | 4% DMSO | PND24, 26, 28 | P24 | 9–12 | M/F | PFC kk | Gene Expression | ↑ Expression of Oprm1 in males | [163] |
| A1221 | 0 or 1 | IP | 4% DMSO | PND24, 26, 28 | P24 | 9–12 | M/F | Lateral septum | Gene Expression | ↓ Expression of Avpr1a ll in males | [163] |
| A1221 | 0 or 1 | IP | 4% DMSO | P24, 26, 28 | P24 | 9–12 | M/F | PFC | Gene × behavior interactions | ↑ Correlation of no-hormone stimulus time and Oprm1 | [163] |
| A1221 | 0 or 1 | IP | 4% DMSO | P24, 26, 28 | P24 | 9–12 | M/F | BNST MeA PVN VMH | Gene Expression | - Gene expression | [163] |
| A1254 A1221 | 45.5 ± 5.9 μg/m3 | Inh | LA | 6 d/w for 13 weeks | Adult (241 ± 9g) | S: 8 E: 12 | F | Brain | RNAseq and Pathway Analysis | ↑ 274 genes ↓ 58 genes Pathways altered NS, CD, VF, + IR | [25] |
| A1254 | 0 or 30 | PO | CO in Cheeto | 14 days | 3–5 months | 3–6 | M | SON mm | Hyperosmotic Challenge and NOS Activity | - NAPDH-d density | [151] |
| PCB 153 | 0 or 20 | PO | CO | GD10-GD16 nn | 15 weeks | 5 | F | CC CE HC ST | Muscarinic Receptor | ↑ MR oo density in CC ↓ MR density in CE - MR density in HC or ST | [164] |
| PCB Source | Dose (mg/kg/day) | Exposure Route | Vehicle | Exposure Period | Age | N a | Sex | Brain Region | Analysis | Result b | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1254 c | 0 or 2 | IP d | CO e | 30 days | Adult (180–200 g) | 6 | M f | CC g CE h | Histology Methylene Blue | ↑ Degenerated neurons | [106] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 6 | M | CE | Histology H&E i | ↑ Shrinkage and degeneration of Purkinje neurons | [105] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 4 | M | CC | Histology H&E/Luxol Fast Blue | ↑ Pyknotic CB j, NC k, IBA l ↓ Pyramidal, Betz, stellate cells, myelin density | [107] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 m | 6 | M | CC | Histology H&E | ↑ Neuronal shrinkage and degeneration ↑ Pyknotic nuclei with PPNS n | [104] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC o | Histology Cresyl Fast Violet | ↑ Disruption in CA4 p | [110] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | Histology H&E | ↑ Degeneration of pyramidal cells ↑ Neuronal shrinkage of HC layer | [109] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | Histology H&E | ↑ Degeneration of pyramidal cells ↑ Neuronal shrinkage of HC layer | [108] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CE CC HC | Histology H&E | ↑ Pyknotic nuclei with PPNS in CC ↑ Degeneration of pyramidal cells in HC ↑ Purkinje cells in CE with shrinkage, degeneration, and moderate atrophy | [103] |
| A1254 | 0 or 10 | PO q | RO r | 14 days | Young Adult (200–250 g) | DND s | M | HC | Histology Toluidine Blue | ↓ Number of neurons | [111] |
| A1254 | 0 or 10 | PO | RO | 14 days | Young Adult (200–250 g) | DND | M | FBC t HC | Transmission Electron Microscopy | ↑ Damaged neuron nuclei in FBC and HC ↑ RER u damage in HC | [111] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CC | Cacna1d v Expression | ↑ mRNA expression of Cacna1d | [104] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | Cacna1d Expression | ↓ mRNA expression of Cacna1d | [109] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 6 | M | CC | Protein Expression | ↑ NMDA w receptor expression ↓ PKAxα levels ↑ Calpain expression ↑ NcaCP y level | [113] |
| A1254 | 0 or 10 | PO | RO | 14 days | Young Adult (200–250 g) | DND | M | HC | Ca2+ Efflux | ↓ NMDA Ca2+ release | [111] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult | 6 | M | CE | OS z Markers | ↑ LP aa, HP bb, and PC cc | [105] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CE HC CC | mRNA Expression | ↓ Expression of Cu/Zn SOD dd ↓ Expression of GPx-4 ee | [103] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CC | OS Markers | ↑ LP, HP, and PC | [104] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | OS Markers | ↑ LP, HP, HR ff, and TBARS gg | [110] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | OS Markers | ↑ LP, HP, and PC | [116] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | OS Markers | ↑ LP, HP, and PC | [108] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CE HC CC | OS Markers | ↑ LP and HR ↓ Glutathione | [118] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CE HC CC | OS Markers | ↑ LP, HP, and HR ↓ Glutathione | [117] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | Antioxidant Enzymes | ↓ SOD, CAT hh, GPx, GST ii, and GR jj | [110] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CE HC CC | Antioxidant Enzyme Activity | ↓ Total SOD, Cu/Zn and Mn SOD ↓ Activity of GPx | [103] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | DNA Fragmentation | Streak of fragmentation | [108] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CC | ATPase Activity | ↓ Na/K, Ca, and Mg ATPase activity | [104] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 6 | M | CE | ATPase Activity | ↓ Na/K, Ca, and Mg ATPase activity | [105] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | ATPase Activity | ↓ Na/K, Ca, and Mg ATPase activity | [109] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CE CC HC | ATPase Activity | ↓ Na/K, Ca, and Mg ATPase activity | [117] |
| A1254 A1260 | 0, 500 or 1000 | PO | CO | 1 day | Adult (250–300 g) | 36 | M | Caudate LOT kk | Dopamine Metabolites | ↓ DA and DOPAC in caudate ↓ DOPAC/DA ratio in LOT - DA, DOPAC in LOT - HVA ll, HVA/DA ratio | [134] |
| A1254 | 0, 500 or 1000 | PO | AOC mm | 30 days | Adult (350–375 g) | 9 | M | ST nn | Dopamine Metabolites | ↓ DA, HVA, and DOPAC/DA, HVA/DA ratios | [133] |
| A1254 | 0, 500 or 1000 | PO | AOC | 30 days | Adult (350–375 g) | 9 | M | LOT | Dopamine Metabolites | ↓ DOPAC, HVA, and DOPAC/DA, HVA/DA ratios - DA levels | [133] |
| A1254 A1260 | 0, 500 or 1000 | PO | CO | 1 day | Adult (250–300 g) | 36 | M | FC oo | Neurotransmitter Concentrations | ↓ 5-HT pp ↑ 5-HIAA qq, 5-HIAA/5-HT ratio | [139] |
| A1254 A1260 | 0, 500 or 1000 | PO | CO | 1 day | Adult (250–300 g) | 36 | M | HC | Neurotransmitter Concentrations | ↓ 5-HT ↑ 5-HIAA/5-HT ratio levels - 5-HIAA levels | [139] |
| A1254 A1260 | 0, 500 or 1000 | PO | CO | 1 day | Adult (250–300 g) | 36 | M | LOT | Neurotransmitter Concentrations | ↑ 5-HT and 5-HIAA - 5-HIAA/5HT ratio | [139] |
| A1254 A1260 | 0, 500 or 1000 | PO | CO | 1 day | Adult (250–300 g) | 36 | M | BS rr HT ss | Neurotransmitter Concentrations | ↑ 5-HIAA/5HT ratio - 5-HT and 5-HIAA | [139] |
| A1254 | 0, 500 or 1000 | PO | AOC | 30 days | Adult (350–375 g) | 9 | M | ST LOT | Neurotransmitter Concentration/Activity | - NE tt or 5-HT | [133] |
| A1254 A1260 | 0, 500 or 1000 | PO | CO | 1 day | Adult (250–300 g) | 36 | M | DFC uu HC | NE Concentration | ↓ NE | [138] |
| A1254 A1260 | 0, 500 or 1000 | PO | CO | 1 day | Adult (250–300 g) | 36 | M | MBH vv BS | NE Concentration | - NE | [138] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 6 | M | CE | DR ww Expression | ↑ DRD1 xx and DRD5 yy mRNA and protein ↓ DRD2 zz, DRD3 aaa and DRD4 bbb mRNA and protein | [105] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CC | DR Expression | ↑ DRD1 and DRD5 mRNA ↓ DRD2, DRD3, and DRD4 mRNA | [104] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | DR Expression | ↑ DRD1 and DRD5 mRNA and protein ↓ DRD2, DRD3 and DRD4 mRNA and protein | [109] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 6 | M | CE | TH ccc Expression | ↓ TH protein - TH mRNA | [105] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | TH expression | - TH mRNA | [109] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 6 | M | CC CE | Apoptotic Gene Expression | ↑ NF-kB ddd expression | [106] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 4 | M | CC | Bcl2 eee Signaling Protein | ↑ Bid fff, Bad ggg, Bax hhh, CASP3 iii, CASP9 jjj ↓ Bcl2 | [107] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | Bcl2 Signaling mRNA | ↑ Bid, Bad, Bax, CASP3, CASP9 ↓ Bcl2 | [108] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 6 | M | CC CE | Bcl2 Signaling mRNA | ↑ Bad ↓ Bcl2 | [106] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | Apoptotic Signaling Expression | ↑ TNFa kkk, NF-kB, Fas lll, and FasL mmm mRNA and protein | [108] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 6 | M | CC CE | Apoptotic Gene Expression | ↑ FasL and CASP8 nnn | [106] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | Caspase Expression and Activity | ↑ CASP8, 9, and 3 mRNA and protein ↑ CASP3 activity | [108] |
| A1254 | 0 or 10 | PO | RO | 14 days | Young Adult (200–250 g) | DND | M | HC micro-dialysate | cGMP and Amino Acid Levels | ↓ NDMA-evoked cGMP and Glu ↓ Basal Glu, Arg, and Gln - Basal cGMP, Tau, Asp | [111] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CC | Creatine Kinase Activity | ↓ Creatine kinase activity | [104] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CE CC HC | Creatine Kinase Activity | ↓ Creatine kinase activity | [118] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 6 | M | CE | Functional Enzyme Activity | ↓ Creatine kinase A and AChE ooo | [105] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CC | AChE Activity | ↓ Mean AChE activity | [104] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | CE CC HC | AChE Activity | ↓ Specific activity of AChE | [117] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 6 | M | CC | Neurotrophin Signaling Expression | ↓ Expression of GLAST ppp, BDNF qqq, NTRKB rrr, MAP1 sss, ERK1 ttt, ERK2 uuu, CREB vvv | [113] |
| A1254 | 0 or 10 | PO | RO | 14 days | Young Adult (200–250 g) | DND | M | FB CE | Glutamate Transporter Expression | ↓ GLT-1 www mRNA and protein in FB - GLT-1 in CE - GLAST in CE and FB | [141] |
| A1254 | 0 or 10 | PO | RO | 14 days | Young Adult (200–250 g) | DND | M | Whole Brain SS xxx and GS yyy | [3H] Glutamate Transport | ↑ Glutamate uptake in SS ↓ Glutamate uptake in GS ↓ Accumulation of amino acids ↑ KCl-stimulated release of glutamate - Rate of [3H] glutamate release | [141] |
| A1254 | 0 or 10 | PO | RO | 14 days | Young Adult (200–250 g) | DND | M | FB CE | Glutamate Transporter Expression | ↑ EAAC1 zzz protein in FB ↓ EAAC1 mRNA in FB ↓ EAAC1 mRNA and protein in CE | [141] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | mRNA Expression | ↓ Estrogen α and β ↓ Occludin, claudin, ZO1 aaaa and 2, AF6 bbbb and JAM cccc | [116] |
| A1254 | 0 or 2 | IP | CO | 30 days | PND90 | 6 | M | HC | Protein Expression | ↓ Ras dddd + Raf eeee protein levels | [116] |
| A1254 | 0 or 2 | IP | CO | 30 days | Adult (180–200 g) | 6 | M | CC CE | mRNA and Protein Expression | ↓ Nefl ffff mRNA and protein | [106] |
| PCB Source | Dose (mg/kg/day) a | Exposure Route | Vehicle | Exposure Period | Age | N b | Sex | Brain Region | Analysis | Result c | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1254 d | 0, 10 or 30 | PO e | CO f | 5 d/w g, 4 weeks | PND60 h | 26, 25, 35 | M i | FC j, CE k, ST l, MS m, and MT n | Ca2+ Buffering and PKC o | ↓ Ca2+ uptake in microsomes ↓ Ca2+ uptake in mitochondria of CE ↓ Total PKC activity in CE ↑ Membrane-bound PKC activity in CE | [114] |
| A1254 | 0, 10 or 30 | PO | CO | 5 d/w, 4 weeks | PND60 | 26, 25, 35 | M | FC ST | Neurotransmitter Concentrations | - 5-HT p, NE q, DA r, HVA s ↑ DOPAC t and 5-HIAA u in ST ↑ NE, DA, DOPAC, HVA, and 5-HIAA in ST vs. FC | [114] |
| A1254 | 0, 10 or 30 | PO | CO | 5 d/w, 4 weeks | PND60 | 26, 25, 35 | M | ST | Tyrosine Hydroxylase | - TH v immunoreactivity - TH specific activity | [114] |
| PCB Source | Dose (mg/kg/day) | Exposure Route | Vehicle | Exposure Period | Age | N a | Sex | Brain Region | Analysis | Result b | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1254 c | 0, 6, 12 or 25 | PO d | SO e on VW f | 4 weeks | 9 weeks | 20 | M f | ST g, CE h | OS g Markers | ↑ MDA h, 4-HNE i, PO j ↑ Accumulation in ST than CE | [120] |
| A1254 | 0, 6, 12 or 25 | PO | SO on VW | 4 weeks | 9 weeks | 20 | M | ST, CE | OS Protein Expression | ↑ HO-1 k, MnSOD l, CuZnSOD protein expression | [120] |
| A1254 | 0, 6, 12 or 25 | PO | SO on VW | 4 weeks | 9 weeks | 20 | M | ST | DA m and Metabolites | ↑ DA levels - DOPAC n, HVA o, HVA/DA ratio | [120] |
| A1254 | 0, 6, 12 or 25 | PO | SO on VW | 4 weeks | 9 weeks | 20 | M | ST | TH p and DAT q Expression | ↓ TH and DAT | [120] |
| A1254 | 0, 6, 12 or 25 | PO | SO on VW | 4 weeks | 9 weeks | 20 | M | ST CE | Iron levels | ↑ Iron and Iron+ cells in ST ↓ Iron levels CE - Iron staining CE | [120] |
| A1254 | 0, 6, 12 or 25 | PO | SO on VW | 4 weeks | 9 weeks | 20 | M | ST | Iron Regulatory Protein Expression | ↑ Ferritin levels at 7 days ↓ Ferritin levels at 14 days ↑ TfR1 r after 28 days | [120] |
| A1254 | 0, 6, 12 or 25 | PO | SO on VW | 4 weeks | 9 weeks | 20 | M | SNPC s VTA t | Neuronal cell number | ↑ Cell death in TH+ and TH− cells | [120] |
| PCB Source | Dose (mg/kg/day) a | Exposure Route | Vehicle | Exposure Period | Age | N b | Sex | Behavioral Category | Analysis | Result c | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCB 180 | 0, 19.4, 64.3, 194, 643, 2000, 6430 or 13,000 μg/kg/day | PO d | CO e | 28 days | 6 weeks | 5 | M f F g | Locomotor and Exploration | Open Field Test | ↑ Time and distance moved in inner zone in F ↓ Habituation across days 2–5 for time and distance moved in the inner zone ↑/↓ Quadratic relationship between dose and total distance moved - Habituation of total activity - Observed in M | [119] |
| A1221 h | 0 or 1 | IP i | 4% DMSO j | PND24 k, 26, 28 | PND24 | VM l: 12 EM m: 10 VF n: 10 EF o: 12 | M F | Anxiety | Juvenile Elevated Plus Maze | - Time spent on arms and number of entries into open/closed arms | [192] |
| A1221 | 0 or 1 | IP | 4% DMSO | PND24, 26, 28 | PND24 | VM: 12 EM: 10 VF: 10 EF: 12 | M F | Anxiety | Adult Elevated Plus Maze | - Time spent on arms or number of entries into open/closed arms in adults exposed to juvenile PCB | [192] |
| A1254 A1221 | 45.5 ± 5.9 μg/m3 | Inh p | LA q | 6 d/w r for 13 weeks | Adult (241 ± 9 g) | S s: 8 E t: 12 | F | Anxiety | Elevated Plus Maze | ↑ Duration and number of entries in the closed arm | [25] |
| A1221 | 0 or 1 | IP | 4% DMSO | PND24, 26, 28 | PND24 | VM: 12 EM: 10 VF: 10 EF: 12 | F M | Anxiety | Juvenile Light/Dark Box | - Time spent on the light side | [192] |
| A1221 | 0 or 1 | IP | 4% DMSO | PND24, 26, 28 | PND24 | VM: 12 EM: 10 VF: 10 EF: 12 | F M | Anxiety | Adult Light/Dark Box | - Time spent on the light side | [192] |
| A1221 | 0 or 1 | IP | 4% DMSO | PND24, 26, 28 | PND24 | VM: 12 EM: 10 VF: 10 EF: 12 | F M | Social and Sociosexual Behavior | Juvenile Sociability | - Time near stimulus - Choice of familiar or novel animal ↑ Latency to stimulus in F | [192] |
| A1221 | 0 or 1 | IP | 4% DMSO | PND24, 26, 28 | PND24 | VM: 12 EM: 10 VF: 10 EF: 12 | F M | Social and Sociosexual Behavior | Adult Socio-sexual USV u | - Number of USVs produced | [192] |
| A1221 | 0 or 1 | IP | 4% DMSO | PND24, 26, 28 | PND24 | VM: 12 EM: 10 VF: 10 EF: 12 | F M | Social and Sociosexual Behavior | Adult Socio-sexual Choice | ↑ Time in hormone stimulus zone ↑ Time with stimuli | [192] |
| A1248 | 0 or 1 mL oil containing 0.5 μg/g PCB | PO | COR v | 30 days | PND37 | 14 | M | Operant Conditioning and Behavioral Flexibility | Operant Training Fixed Interval Presses | ↑ Lever presses | [230] |
| A1248 | 0 or 1 mL oil containing 0.5 μg/g PCB | PO | COR | 30 days | PND37 | 14 | M | Operant Conditioning and Behavioral Flexibility | Operant Training Response Bursts | - Response bursts | [230] |
| A1248 | 0 or 1 mL oil containing 0.5 μg/g PCB | PO | COR | 30 days | PND37 | 14 | M | Operant Conditioning and Behavioral Flexibility | Operant Training Reinforcements Delivered | - Mean number of reinforcements delivered | [230] |
| A1248 | 0 or 1 mL oil containing 0.5 μg/g PCB | PO | COR | 30 days | PND37 | 14 | M | Operant Conditioning and Behavioral Flexibility | Operant Training Extinction Presses | - Mean number of lever presses | [230] |
| A1248 | 0 or 1 mL oil containing 0.5 μg/g PCB | PO | COR | 30 days | PND37 | 14 | M | Operant Conditioning and Behavioral Flexibility | Operant Training Transition Presses | ↑ Lever presses in the first session than the second | [230] |
| A1248 | 0 or 562 ng/m3 | Inh | LA | 30 days | PND37 | S: 23 E: 24 | M F | Operant Conditioning and Behavioral Flexibility | Operant Training | ↑ Bar pressing rates, differences over time, hyperactivity at 90 and 120 s, and number of response bursts ↑ Number of responses in males | [231] |
| A1254 | 62.1 ± 17.7 μg/m3 | Inh | LA | 28 days | Adult (239 ±8 g) | 8 | F | Learning and Memory | Morris Water Maze | - Escape latency ↓ Platform crossings | [24] |
| A1254 A1221 | 45.5 ± 5.9 μg/m3 | Inh | LA | 6 d/w for 13 weeks | Adult (241 ± 9 g) | S: 8 E: 12 | F | Learning and Memory | Morris Water Maze | - Escape latency, travel distance, or time in target quadrant ↓ Platform crossings | [25] |
| A1221 | 0 or 1 | IP | 4% DMSO | PND24, 26, 28 | PND24 | VM: 12 EM: 10 VF: 10 EF: 12 | F M | Social and Sociosexual Behavior | Affiliative USV | - Number of USVs produced | [192] |
| A1221 | 0 or 1 | IP | 4% DMSO | PND24, 26, 28 | PND24 | VM: 12 EM: 10 VF: 10 EF: 12 | F M | Social and Sociosexual Behavior | Affiliative Behavior | ↑ Latency to hop in F - Number of hops - Nape contacts | [192] |
| PCB Source | Dose (mg/kg/day) a | Exposure Route | Vehicle | Exposure Period | Age | N b | Sex | Brain Region | Analysis | Result c | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1254 d | 62.1 ± 17.7 μg/m3 | Inh e | LA f | 28 days | Adult (239 ± 8g) | 8 | F g | Whole Brain | Histology H&E h | - Gross histopathological changes in HC i - Features of necrosis or apoptosis | [24] |
| A1254 A1221 | 45.5 ± 5.9 μg/m3 | Inh | LA | 6 d/w j for 13 weeks | Adult (241 ± 9 g) | S k: 8 E l: 12 | F | Whole Brain | Histology H&E | - Histopathological changes | [25] |
| A1254 m | 0, 10 or 30 | PO n | CO o | 5 d/w p for 4 weeks | PND60 q | 26, 25, 35 | M r | Locomotor and Exploration | Motor Activity | ↓ Horizontal activity in 30 mg/kg/day -Vertical activity | [114] |
| A1254 | 0, 100, 300 or 1000 | PO | CO | 1 day | PND67 | 9 | M | Locomotor and Exploration | Motor Activity | ↓ Horizontal activity in 1000 mg/kg/day ↓ Vertical activity in 300 and 1000 mg/kg/day | [184] |
| A1254 | 0, 10, 30 or 100 | PO | CO | 5 d/w for 4 weeks s | PND67 | 9 | M | Locomotor and Exploration | Motor Activity | ↓ Horizontal activity ↓ Vertical activity | [184] |
| A1254 | 0, 1, 3 or 10 | PO | CO | 5 d/w for 6 weeks | PND67 | 9 | M | Locomotor and Exploration | Motor Activity | - Horizontal and vertical activity | [184] |
| A1221 A1254 | 0, 2.5 or 5 | IP t | SO u | 1 week | PND60 | 12 | F v | Reproductive and Maternal Behavior | Lordosis and Pacing Test | - Approach latency, mount return latency, intromission return latency, postejaculatory refractory period, and lordosis quotient | [238] |
| PCB 77 | 0 or 2 | SQ w | CO | GD6x-18 | Adult | 6 | F | Reproductive and Maternal Behavior | Maternal Auto-grooming | - Time spent auto-grooming | [239] |
| PCB 77 | 0 or 2 | SQ | CO | GD6-18 | Adult | 6 | F | Reproductive and Maternal Behavior | Licking and Grooming Pups | ↑ Time spent licking and grooming pups | [239] |
| PCB 77 | 0 or 2 | SQ | CO | GD6-18 | Adult | 6 | F | Reproductive and Maternal Behavior | Nursing Bouts | ↑ Number of nursing bouts | [239] |
| PCB 77 | 0 or 2 | SQ | CO | GD6-18 | Adult | 6 | F | Reproductive and Maternal Behavior | Nursing | - Time nursing | [239] |
| PCB 77 | 0 or 2 | SQ | CO | GD6-18 | Adult | 6 | F | Reproductive and Maternal Behavior | Time on Nest | ↑ Time spent on nest | [239] |
| PCB 77 | 0 or 2 | SQ | CO | GD6-18 | Adult | 6 | F | Reproductive and Maternal Behavior | High Crouch Nursing Posture | ↓ Time in high crouch nursing posture | [239] |
| FRM y,z | 0, 3 or 6 | PO | CO | PND27-50 | PND27 | 14, 13, 14 | F M | Cognitive Flexibility and Decision-Making | Set Shifting—Omissions and Response Latencies | ↑ Average trial omissions in M - Position discrimination and position reversal omissions in M - Set shifting omissions | [248] |
| FRM | 0, 3 or 6 | PO | CO | PND27-50 | PND27 | 14, 13, 14 | F M | Cognitive Flexibility and Decision-Making | Set Shifting—Visual Cue Discrimination | ↑ Errors to criterion in M - Errors to criterion in F | [248] |
| FRM | 0, 3 or 6 | PO | CO | PND27-50 | PND27 | 14, 13, 14 | F M | Cognitive Flexibility and Decision-Making | Set Shifting—Shift to Position | - Number of errors | [248] |
| FRM | 0, 3 or 6 | PO | CO | PND27-50 | PND27 | 14, 13, 14 | F M | Cognitive Flexibility and Decision-Making | Set Shifting—Response Reversal | ↓ Number of errors in M - Number of errors in F | [248] |
| FRM | 0, 3 or 6 | PO | CO | PND27-50 | PND27 | 14, 13, 14 | F M | Cognitive Flexibility and Decision-Making | Set Shifting—Error Analyss | ↓ Number of pervasive errors in M | [248] |
| FRM | 0, 3 or 6 | PO | CO | PND27-50 | PND27 | 14, 13, 14 | F M | Impulsivity and Timing | DRL aa—Training | - Total presses or efficiency ratio | [248] |
| FRM | 0, 3 or 6 | PO | CO | PND27-50 | PND27 | 14, 13, 14 | F M | Impulsivity and Timing | DRL - DRL15 training | - Total presses or efficiency ratio | [248] |
| FRM | 0, 3 or 6 | PO | CO | PND27-50 | PND27 | 14, 13, 14 | F M | Impulsivity and Timing | DRL—Extinction | - Main effect of exposure or exposure × session interaction | [248] |
| A1254 | 0, 100 or 300 | PO | CO | 1 day | PND76 | 6 | M | Learning and Memory | Flavor Aversion | ↓ Saccharin intake - Fluid intake | [184] |
| A1254 | 0, 10 or 30 | PO | CO | 1 day | PND76 | 6 | M | Learning and Memory | Flavor Aversion | ↓ Saccharin intake - Fluid intake | [184] |
| A1254 | 0, 15 or 25 | PO | CO | 1 day | PND76 | 6 | M | Learning and Memory | Flavor Aversion | ↓ Saccharin intake ↓ Fluid intake | [184] |
| A1254 | 0, 7.5 or 15 | PO | CO | 14 days | PND76 | 6 | M | Learning and Memory | Flavor Aversion | ↓ Saccharin intake ↓ Fluid intake 15mg/kg | [184] |
| A1254 | 0, 3.5 or 7.5 | PO | CO | 14 days | PND76 | 6 | M | Learning and Memory | Flavor Aversion | - Saccharin intake | [184] |
| PCB Source | Dose (mg/kg/day) a | Exposure Route | Vehicle | Exposure Period | Age | N b | Sex | Brain Region | Analysis | Result c | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C57Bl/6 Mice | |||||||||||
| A1254 d | 0, 6, 12 or 25 | PO e | VWC f SO g | 4 weeks | 9 weeks | 20 | M h | Locomotor and Exploration | Locomotor Activity | ↑ Vertical, horizontal, and ambulatory activity | [120] |
| Swiss Albino Mice | |||||||||||
| Mixture i (PCB 28, 52, 101, 138, 153, 180) | 0, 1, 10, or 100 ng/kg/day | PO | RO j | GD k 0-21 | 11 weeks | 10 | F l | Reproductive and Maternal Behavior | Next Building Activity | - Nest quality | [240] |
| Mixture (PCB 28, 52, 101, 138, 153, 180) | 0, 1, 10, or 100 ng/kg/day | PO | RO | GD 0-21 | 11 weeks | 10 | F | Reproductive and Maternal Behavior | Pup Retrieving Test | - Latency period to retrieve pup and carry back to nest | [240] |
References
- Learn About Polychlorinated Biphenyls. Available online: www.epa.gov/pcbs/learn-about-polychlorinated-biphenyls (accessed on 16 March 2025).
- IARC. Polychlorinated Biphenyls and Polybrominated Biphenyls; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2016. [Google Scholar]
- Erickson, M.D.; Kaley, R.G. Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res. Int. 2011, 18, 135–151. [Google Scholar] [CrossRef]
- MacIntosh, D.L.; Minegishi, T.; Fragala, M.A.; Allen, J.G.; Coghlan, K.M.; Stewart, J.H.; McCarthy, J.F. Mitigation of building-related polychlorinated biphenyls in indoor air of a school. Environ. Health 2012, 11, 24. [Google Scholar] [CrossRef]
- Thomas, K.; Xue, J.; Williams, R.; Jones, P.; Whitaker, D. Polychlorinated Biphenyls (PCBs) in School Buildings: Sources, Environmental Levels, and Exposures; United States Environmental Protection Agency (U.S. EPA): Washington, DC, USA, 2012. [Google Scholar]
- Herrick, R.F.; McClean, M.D.; Meeker, J.D.; Baxter, L.K.; Weymouth, G.A. An unrecognized source of PCB contamination in schools and other buildings. Environ. Health Perspect. 2004, 112, 1051–1053. [Google Scholar] [CrossRef]
- Herrick, R.F.; Meeker, J.D.; Altshul, L. Serum PCB levels and congener profiles among teachers in PCB-containing schools: A pilot study. Environ. Health 2011, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Herrick, R.F.; Stewart, J.H.; Allen, J.G. Review of PCBs in US schools: A brief history, an estimate of the number of impacted schools, and an approach for evaluating indoor air samples. Environ. Sci. Pollut. Res. Int. 2016, 23, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Bannavti, M.K.; Jahnke, J.C.; Marek, R.F.; Just, C.L.; Hornbuckle, K.C. Room-to-room variability of airborne polychlorinated biphenyls in schools and the application of air sampling for targeted source evaluation. Environ. Sci. Technol. 2021, 55, 9460–9468, Correction in Environ. Sci. Technol. 2021, 55, 10892. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.B.X.; Marek, R.F.; Hornbuckle, K.C. Polyurethane foam emission samplers to identify sources of airborne polychlorinated biphenyls from glass-block windows and other room surfaces in a Vermont school. Environ. Sci. Technol. 2023, 57, 14310–14318. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Andersen, Z.J.; Frederiksen, M.; Specht, I.O.; Hougaard, K.S.; Ebbehøj, N.; Bailey, J.; Giwercman, A.; Steenland, K.; Longnecker, M.P.; et al. Health effects of PCBs in residences and schools (HESPERUS): PCB—Health cohort profile. Sci. Rep. 2016, 6, 24571. [Google Scholar] [CrossRef]
- Borja, J.; Taleon, D.M.; Auresenia, J.; Gallardo, S. Polychlorinated biphenyls and their biodegradation. Process Biochem. 2005, 40, 1999–2013. [Google Scholar] [CrossRef]
- Hu, D.; Hornbuckle, K.C. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 2010, 44, 2822–2827. [Google Scholar] [CrossRef]
- Liu, X.; Mullin, M.R.; Egeghy, P.; Woodward, K.A.; Compton, K.C.; Nickel, B.; Aguilar, M.; Folk Iv, E. Inadvertently generated PCBs in consumer products: Concentrations, fate and transport, and preliminary exposure assessment. Environ. Sci. Technol. 2022, 56, 12228–12236. [Google Scholar] [CrossRef]
- Mastin, J.; Harner, T.; Schuster, J.K.; South, L. A review of PCB-11 and other unintentionally produced PCB congeners in outdoor air. Atmos. Pollut. Res. 2022, 13, 101364. [Google Scholar] [CrossRef]
- Louis, C.; Covaci, A.; Crocker, D.E.; Debier, C. Lipophilicity of PCBs and fatty acids determines their mobilisation from blubber of weaned northern elephant seal pups. Sci. Total Environ. 2016, 541, 599–602. [Google Scholar] [CrossRef]
- Harrad, S. Beyond the Stockholm Convention: An Introduction to Current Issues and Future Challenges in POPs Research. In Persistent Organic Pollutants; John Wiley and Sons: Hoboken, NJ, USA, 2009; pp. 1–4. [Google Scholar]
- Hornbuckle, K.C.; Carlson, D.L.; Swackhamer, D.L.; Baker, J.E.; Eisenreich, S.J. Polychlorinated Biphenyls in the Great Lakes. In Persistent Organic Pollutants in the Great Lakes; Hites, R.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 13–70. [Google Scholar]
- Boon, J.P.; Oostingh, I.; van der Meer, J.; Hillebrand, M.T.J. A model for the bioaccumulation of chlorobiphenyl congeners in marine mammals. Eur. J. Pharmacol. 1994, 270, 237–251. [Google Scholar] [CrossRef]
- Borlakoglu, J.T.; Haegele, K.D. Comparative aspects on the bioaccumulation, metabolism and toxicity with PCBs. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 1991, 100, 327–338. [Google Scholar] [CrossRef]
- Weitekamp, C.A.; Phillips, L.J.; Carlson, L.M.; DeLuca, N.M.; Cohen Hubal, E.A.; Lehmann, G.M. A state-of-the-science review of polychlorinated biphenyl exposures at background levels: Relative contributions of exposure routes. Sci. Total Environ. 2021, 776, 145912. [Google Scholar] [CrossRef] [PubMed]
- Bullert, A.J.; Li, X.; Gautam, B.; Wang, H.; Adamcakova-Dodd, A.; Wang, K.; Thorne, P.S.; Lehmler, H.-J. Distribution of 2,2′,5,5′-tetrachlorobiphenyl (PCB52) metabolites in adolescent rats after acute nose-only inhalation exposure. Environ. Sci. Technol. 2024, 58, 6105–6116. [Google Scholar] [CrossRef]
- Helm-Kwasny, B.K.; Bullert, A.; Wang, H.; Chimenti, M.S.; Adamcakova-Dodd, A.; Jing, X.; Li, X.; Meyerholz, D.K.; Thorne, P.S.; Lehmler, H.-J.; et al. Upregulation of fatty acid synthesis genes in the livers of adolescent female rats caused by inhalation exposure to PCB52 (2,2′,5,5′-tetrachlorobiphenyl). Environ. Toxicol. Pharmacol. 2024, 110, 104520. [Google Scholar] [CrossRef]
- Wang, H.; Adamcakova-Dodd, A.; Flor, S.; Gosse, L.; Klenov, V.E.; Stolwijk, J.M.; Lehmler, H.J.; Hornbuckle, K.C.; Ludewig, G.; Robertson, L.W.; et al. Comprehensive subchronic inhalation toxicity assessment of an indoor school air mixture of PCBs. Environ. Sci. Technol. 2020, 54, 15976–15985. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Adamcakova-Dodd, A.; Lehmler, H.J.; Hornbuckle, K.C.; Thorne, P.S. Toxicity assessment of 91-day repeated inhalation exposure to an indoor school air mixture of PCBs. Environ. Sci. Technol. 2022, 56, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, K.; Uwimana, E.; Adamcakova-Dodd, A.; Thorne, P.S.; Lehmler, H.-J.; Robertson, L.W. Disposition of phenolic and sulfated metabolites after inhalation exposure to 4-chlorobiphenyl (PCB3) in female rats. Chem. Res. Toxicol. 2014, 27, 1411–1420. [Google Scholar] [CrossRef]
- Hu, X.; Adamcakova-Dodd, A.; Lehmler, H.-J.; Gibson-Corley, K.; Thorne, P.S. Toxicity evaluation of exposure to an atmospheric mixture of polychlorinated biphenyls by nose-only and whole-body inhalation regimens. Environ. Sci. Technol. 2015, 49, 11875–11883. [Google Scholar] [CrossRef]
- Dhakal, K.; Adamcakova-Dodd, A.; Lehmler, H.-J.; Thorne, P.S.; Robertson, L.W. Sulfate conjugates are urinary markers of inhalation exposure to 4-chlorobiphenyl (PCB3). Chem. Res. Toxicol. 2013, 26, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Adamcakova-Dodd, A.; Lehmler, H.-J.; Hu, D.; Kania-Korwel, I.; Hornbuckle, K.C.; Thorne, P.S. Time course of congener uptake and elimination in rats after short-term inhalation exposure to an airborne polychlorinated biphenyl (PCB) mixture. Environ. Sci. Technol. 2010, 44, 6893–6900. [Google Scholar] [CrossRef]
- Hu, X.; Adamcakova-Dodd, A.; Lehmler, H.-J.; Hu, D.; Hornbuckle, K.; Thorne, P.S. Subchronic inhalation exposure study of an airborne polychlorinated biphenyl mixture resembling the Chicago ambient air congener profile. Environ. Sci. Technol. 2012, 46, 9653–9662. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lehmler, H.-J.; Adamcakova-Dodd, A.; Thorne, P.S. Elimination of inhaled 3,3′-dichlorobiphenyl and the formation of the 4-hydroxylated metabolite. Environ. Sci. Technol. 2013, 47, 4743–4751. [Google Scholar] [CrossRef]
- Hu, X.; Adamcakova-Dodd, A.; Thorne, P.S. The fate of inhaled 14C-labeled PCB11 and its metabolites in vivo. Environ. Int. 2014, 63, 92–100. [Google Scholar] [CrossRef]
- Ampleman, M.D.; Martinez, A.; DeWall, J.; Rawn, D.F.K.; Hornbuckle, K.C.; Thorne, P.S. Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ. Sci. Technol. 2015, 49, 1156–1164. [Google Scholar] [CrossRef]
- Lehmann, G.M.; Christensen, K.; Maddaloni, M.; Phillips, L.J. Evaluating health risks from inhaled polychlorinated biphenyls: Research needs for addressing uncertainty. Environ. Health Perspect. 2015, 123, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Hammel, S.C.; Andersen, H.V.; Knudsen, L.E.; Frederiksen, M. Inhalation and dermal absorption as dominant pathways of PCB exposure for residents of contaminated apartment buildings. Int. J. Hyg. Environ. Health 2023, 247, 114056. [Google Scholar] [CrossRef]
- Othman, N.; Ismail, Z.; Selamat, M.I.; Sheikh Abdul Kadir, S.H.; Shibraumalisi, N.A. A review of polychlorinated biphenyls (PCBs) pollution in the air: Where and how much are we exposed to? Int. J. Environ. Res. Public Health 2022, 19, 13923. [Google Scholar] [CrossRef]
- Heiger-Bernays, W.J.; Tomsho, K.S.; Basra, K.; Petropoulos, Z.E.; Crawford, K.; Martinez, A.; Hornbuckle, K.C.; Scammell, M.K. Human health risks due to airborne polychlorinated biphenyls are highest in New Bedford Harbor communities living closest to the harbor. Sci. Total Environ. 2020, 710, 135576. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hefti, M.M.; Marek, R.F.; Hornbuckle, K.C.; Wang, K.; Lehmler, H.J. Assessment of polychlorinated biphenyls and their hydroxylated metabolites in postmortem human brain samples: Age and brain region differences. Environ. Sci. Technol. 2022, 56, 9515–9526. [Google Scholar] [CrossRef]
- Sasaki, N.; Jones, L.E.; Morse, G.S.; Carpenter, D.O. Mixture effects of polychlorinated biphenyls (PCBs) and three organochlorine pesticides on cognitive function in Mohawk Adults at Akwesasne. Int. J. Environ. Res. Public Health 2023, 20, 1148. [Google Scholar] [CrossRef]
- Robertson, L.W.; Ludewig, G. Polychlorinated biphenyl (PCB) carcinogenicity with special emphasis on airborne PCBs. Gefahrst Reinhalt Luft 2011, 71, 25–32. [Google Scholar]
- Silberhorn, E.M.; Glauert, H.P.; Robertson, L.W. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit. Rev. Toxicol. 1990, 20, 440–496. [Google Scholar] [CrossRef]
- Barrett, J.R. Diminished protection? Early childhood PCB exposure and reduced immune response to vaccinations. Environ. Health Perspect. 2010, 118, A445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heilmann, C.; Grandjean, P.; Weihe, P.; Nielsen, F.; Budtz-Jørgensen, E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006, 3, e311. [Google Scholar] [CrossRef]
- Meeker, J.D.; Hauser, R. Exposure to polychlorinated biphenyls (PCBs) and male reproduction. Syst. Biol. Reprod. Med. 2010, 56, 122–131. [Google Scholar] [CrossRef]
- Wu, C.; Du, X.; Liu, H.; Chen, X.; Ge, K.; Meng, R.; Zhang, Z.; Zhang, H. Advances in polychlorinated biphenyls-induced female reproductive toxicity. Sci. Total Environ. 2024, 918, 170543. [Google Scholar] [CrossRef] [PubMed]
- Govarts, E.; Nieuwenhuijsen, M.; Schoeters, G.; Ballester, F.; Bloemen, K.; de Boer, M.; Chevrier, C.; Eggesbø, M.; Guxens, M.; Krämer, U.; et al. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): A meta-analysis within 12 European birth cohorts. Environ. Health Perspect. 2012, 120, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, B.; Stahlmann, R. Developmental toxicity of polychlorinated biphenyls (PCBs): A systematic review of experimental data. Arch. Toxicol. 2004, 78, 252–268, Correction in Arch. Toxicol. 2004, 78, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Pessah, I.N.; Lein, P.J.; Seegal, R.F.; Sagiv, S.K. Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol. 2019, 138, 363–387. [Google Scholar] [CrossRef] [PubMed]
- Schantz, S.L. Developmental neurotoxicity of PCBs in humans: What do we know and where do we go from here? Neurotoxicol. Teratol. 1996, 18, 217–227. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, I.K.; Jin, S.H.; Steffes, M.; Jacobs, D.R., Jr. Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: Results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care 2007, 30, 622–628. [Google Scholar] [CrossRef]
- Buha Djordjevic, A.; Antonijevic, E.; Curcic, M.; Milovanovic, V.; Antonijevic, B. Endocrine-disrupting mechanisms of polychlorinated biphenyls. Curr. Opin. Toxicol. 2020, 19, 42–49, Correction in Curr. Opin. Toxicol. 2022, 32, 100375. [Google Scholar] [CrossRef]
- Everett, C.J.; Mainous, A.G., 3rd; Frithsen, I.L.; Player, M.S.; Matheson, E.M. Association of polychlorinated biphenyls with hypertension in the 1999–2002 National Health and Nutrition Examination Survey. Environ. Res. 2008, 108, 94–97. [Google Scholar] [CrossRef]
- Grimm, F.A.; Klaren, W.D.; Li, X.; Lehmler, H.J.; Karmakar, M.; Robertson, L.W.; Chiu, W.A.; Rusyn, I. Cardiovascular effects of polychlorinated biphenyls and their major metabolites. Environ. Health Perspect. 2020, 128, 77008. [Google Scholar] [CrossRef]
- Kilburn, K.H.; Warsaw, R.H.; Shields, M.G. Neurobehavioral dysfunction in firemen exposed to polycholorinated biphenyls (PCBs): Possible improvement after detoxification. Arch. Environ. Health 1989, 44, 345–350. [Google Scholar] [CrossRef]
- Learn About Dioxin. Available online: www.epa.gov/dioxin/learn-about-dioxin (accessed on 11 September 2025).
- Zhang, W.; Sargis, R.M.; Volden, P.A.; Carmean, C.M.; Sun, X.J.; Brady, M.J. PCB 126 and other dioxin-like PCBs specifically suppress hepatic PEPCK expression via the aryl hydrocarbon receptor. PLoS ONE 2012, 7, e37103. [Google Scholar] [CrossRef]
- Dioxins and Dioxin-Like Substances. Available online: www.who.int/teams/environment-climate-change-and-health/chemical-safety-and-health/health-impacts/chemicals/dioxins (accessed on 11 September 2025).
- Langeveld, W.T.; Meijer, M.; Westerink, R.H. Differential effects of 20 non-dioxin-like PCBs on basal and depolarization-evoked intracellular calcium levels in PC12 cells. Toxicol. Sci. 2012, 126, 487–496. [Google Scholar] [CrossRef]
- Bjermo, H.; Darnerud, P.O.; Lignell, S.; Pearson, M.; Rantakokko, P.; Nälsén, C.; Enghardt Barbieri, H.; Kiviranta, H.; Lindroos, A.K.; Glynn, A. Fish intake and breastfeeding time are associated with serum concentrations of organochlorines in a Swedish population. Environ. Int. 2013, 51, 88–96. [Google Scholar] [CrossRef]
- Gährs, M.; Roos, R.; Andersson, P.L.; Schrenk, D. Role of the nuclear xenobiotic receptors CAR and PXR in induction of cytochromes P450 by non-dioxinlike polychlorinated biphenyls in cultured rat hepatocytes. Toxicol. Appl. Pharmacol. 2013, 272, 77–85. [Google Scholar] [CrossRef]
- Pessah, I.N.; Cherednichenko, G.; Lein, P.J. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol. Ther. 2010, 125, 260–285. [Google Scholar] [CrossRef]
- Yaghoobi, B.; Miller, G.W.; Holland, E.B.; Li, X.; Harvey, D.; Li, S.; Lehmler, H.J.; Pessah, I.N.; Lein, P.J. Ryanodine receptor-active non-dioxin-like polychlorinated biphenyls cause neurobehavioral deficits in larval zebrafish. Front. Toxicol. 2022, 4, 947795. [Google Scholar] [CrossRef]
- Takeuchi, S.; Shiraishi, F.; Kitamura, S.; Kuroki, H.; Jin, K.; Kojima, H. Characterization of steroid hormone receptor activities in 100 hydroxylated polychlorinated biphenyls, including congeners identified in humans. Toxicology 2011, 289, 112–121. [Google Scholar] [CrossRef]
- Amir, S.; Shah, S.T.A.; Mamoulakis, C.; Docea, A.O.; Kalantzi, O.I.; Zachariou, A.; Calina, D.; Carvalho, F.; Sofikitis, N.; Makrigiannakis, A.; et al. Endocrine disruptors acting on estrogen and androgen pathways cause reproductive disorders through multiple mechanisms: A review. Int. J. Environ. Res. Public Health 2021, 18, 1464. [Google Scholar] [CrossRef]
- Shan, Q.; Li, H.; Chen, N.; Qu, F.; Guo, J. Understanding the multiple effects of PCBs on lipid metabolism. Diabetes Metab. Syndr. Obes. 2020, 13, 3691–3702. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Khweek, A.A.; Beg, M.A. Peroxisome proliferator-activated receptors as potential targets for carcinogenic activity of polychlorinated biphenyls: A computational perspective. Anticancer Res. 2016, 36, 6117. [Google Scholar] [CrossRef]
- Grimm, F.A.; Hu, D.; Kania-Korwel, I.; Lehmler, H.J.; Ludewig, G.; Hornbuckle, K.C.; Duffel, M.W.; Bergman, Å.; Robertson, L.W. Metabolism and metabolites of polychlorinated biphenyls. Crit. Rev. Toxicol. 2015, 45, 245–272. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Li, X.; Flor, S.; Ruiz, P.; Kruve, A.; Ludewig, G.; Lehmler, H.-J. Metabolism of 3-chlorobiphenyl (PCB 2) in a human-relevant cell line: Evidence of dechlorinated metabolites. Environ. Sci. Technol. 2022, 56, 12460–12472. [Google Scholar] [CrossRef]
- Ernhart, C.B. A critical review of low-level prenatal lead exposure in the human: 1. Effects on the fetus and newborn. Reprod. Toxicol. 1992, 6, 9–19. [Google Scholar] [CrossRef]
- Matthews, H.B.; Kato, S. The metabolism and disposition of halogenated aromatics. Ann. N.Y. Acad. Sci. 1979, 320, 131–137. [Google Scholar] [CrossRef]
- Mise, S.; Haga, Y.; Itoh, T.; Kato, A.; Fukuda, I.; Goto, E.; Yamamoto, K.; Yabu, M.; Matsumura, C.; Nakano, T.; et al. From the cover: Structural determinants of the position of 2,3′,4,4′,5-pentachlorobiphenyl (CB118) hydroxylation by mammalian cytochrome P450 monooxygenases. Toxicol. Sci. 2016, 152, 340–348. [Google Scholar] [CrossRef]
- Yabu, M.; Haga, Y.; Itoh, T.; Goto, E.; Suzuki, M.; Yamazaki, K.; Mise, S.; Yamamoto, K.; Matsumura, C.; Nakano, T.; et al. Hydroxylation and dechlorination of 3,3′,4,4′-tetrachlorobiphenyl (CB77) by rat and human CYP1A1s and critical roles of amino acids composing their substrate-binding cavity. Sci. Total Environ. 2022, 837, 155848. [Google Scholar] [CrossRef]
- Warner, N.A.; Martin, J.W.; Wong, C.S. Chiral polychlorinated biphenyls are biotransformed enantioselectively by mammalian cytochrome P-450 isozymes to form hydroxylated metabolites. Environ. Sci. Technol. 2009, 43, 114–121. [Google Scholar] [CrossRef]
- Lu, Z.; Kania-Korwel, I.; Lehmler, H.-J.; Wong, C.S. Stereoselective formation of mono- and dihydroxylated polychlorinated biphenyls by rat cytochrome P450 2B1. Environ. Sci. Technol. 2013, 47, 12184–12192. [Google Scholar] [CrossRef]
- Kania-Korwel, I.; Lehmler, H.J. Chiral polychlorinated biphenyls: Absorption, metabolism and excretion--a review. Environ. Sci. Pollut. Res. Int. 2016, 23, 2042–2057. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Interaction Profile for: Persistent Chemicals Found in Breast Milk (Chlorinated Dibenzo-p-Dioxins, Hexachlorobenzene, p,p′-DDE, Methylmercury, and Polychlorinated Biphenyls); Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2004. [Google Scholar]
- Safe, S.H. Polychlorinated biphenyls (PCBs): Environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit. Rev. Toxicol. 1994, 24, 87–149. [Google Scholar] [CrossRef]
- Song, Y.; Wagner, B.A.; Lehmler, H.-J.; Buettner, G.R. Semiquinone radicals from oxygenated polychlorinated biphenyls: Electron paramagnetic resonance studies. Chem. Res. Toxicol. 2008, 21, 1359–1367. [Google Scholar] [CrossRef]
- Amaro, A.R.; Oakley, G.G.; Bauer, U.; Spielmann, H.P.; Robertson, L.W. Metabolic activation of PCBs to quinones: Reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chem. Res. Toxicol. 1996, 9, 623–629. [Google Scholar] [CrossRef]
- Tehrani, R.; Van Aken, B. Hydroxylated polychlorinated biphenyls in the environment: Sources, fate, and toxicities. Environ. Sci. Pollut. Res. Int. 2014, 21, 6334–6345. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Song, E.; Song, Y. A critical review of polychlorinated biphenyls metabolism, metabolites, and their correlation with oxidative stress. Chem. Res. Toxicol. 2020, 33, 2022–2042. [Google Scholar] [CrossRef]
- Tampal, N.; Lehmler, H.-J.; Espandiari, P.; Malmberg, T.; Robertson, L.W. Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs). Chem. Res. Toxicol. 2002, 15, 1259–1266. [Google Scholar] [CrossRef]
- Ekuase, E.J.; Liu, Y.; Lehmler, H.-J.; Robertson, L.W.; Duffel, M.W. Structure–activity relationships for hydroxylated polychlorinated biphenyls as inhibitors of the sulfation of dehydroepiandrosterone catalyzed by human hydroxysteroid sulfotransferase SULT2A1. Chem. Res. Toxicol. 2011, 24, 1720–1728. [Google Scholar] [CrossRef]
- Song, Y.; Wagner, B.A.; Witmer, J.R.; Lehmler, H.-J.; Buettner, G.R. Nonenzymatic displacement of chlorine and formation of free radicals upon the reaction of glutathione with PCB quinones. Proc. Natl. Acad. Sci. USA 2009, 106, 9725–9730. [Google Scholar] [CrossRef]
- Rezek, J.; Macek, T.; Mackova, M.; Triska, J.; Ruzickova, K. Hydroxy-PCBs, methoxy-PCBs and hydroxy-methoxy-PCBs: Metabolites of polychlorinated biphenyls formed in vitro by tobacco cells. Environ. Sci. Technol. 2008, 42, 5746–5751. [Google Scholar] [CrossRef]
- Mio, T.; Sumino, K. Mechanism of biosynthesis of methylsulfones from PCBs and related compounds. Environ. Health Perspect. 1985, 59, 129–135. [Google Scholar] [CrossRef][Green Version]
- Klocke, C.; Sethi, S.; Lein, P.J. The developmental neurotoxicity of legacy vs contemporary polychlorinated biphenyls (PCBs): Similarities and differences. Environ. Sci. Pollut. Res. Int. 2020, 27, 8885–8896. [Google Scholar] [CrossRef]
- Klocke, C.; Lein, P.J. Evidence implicating non-dioxin-like congeners as the key mediators of polychlorinated biphenyl (PCB) developmental neurotoxicity. Int. J. Mol. Sci. 2020, 21, 1013. [Google Scholar] [CrossRef]
- Bell, M.R. Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. Curr. Opin. Pharmacol. 2014, 19, 134–144. [Google Scholar] [CrossRef]
- Torres-Rojas, C.; Jones, B.C. Sex differences in neurotoxicogenetics. Front. Genet. 2018, 9, 196. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243, Correction in J. Med. Libr. Assoc. 2017, 105, 111. [Google Scholar] [CrossRef]
- Alshami, A.; Elsayed, M.; Ali, E.; Eltoukhy, A.E.E.; Zayed, T. Harnessing the power of ChatGPT for automating systematic review process: Methodology, case study, limitations, and future directions. Systems 2023, 11, 351. [Google Scholar] [CrossRef]
- Pollock, D.; Peters, M.D.J.; Khalil, H.; McInerney, P.; Alexander, L.; Tricco, A.C.; Evans, C.; de Moraes, É.; Godfrey, C.M.; Pieper, D.; et al. Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI Evid. Synth. 2023, 21, 520–532. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Nee, J.C.; Landis, J.R. Large sample variance of kappa in the case of different sets of raters. Psychol. Bull. 1979, 86, 974–977. [Google Scholar] [CrossRef]
- Beattie, M.K.; Gerstenberger, S.; Hoffman, R.; Dellinger, J.A. Rodent neurotoxicity bioassays for screening contaminated Great Lakes fish. Environ. Toxicol. Chem. 1996, 15, 313–318. [Google Scholar] [CrossRef]
- Hertzler, D.R. Neurotoxic behavioral effects of Lake Ontario salmon diets in rats. Neurotoxicol. Teratol. 1990, 12, 139–143. [Google Scholar] [CrossRef]
- Stewart, P.; Pagano, J.; Sargent, D.; Darvill, T.; Lonky, E.; Reihman, J. Effects of Great Lakes fish consumption on brain PCB pattern, concentration, and progressive-ratio performance. Environ. Res. 2000, 82, 18–32. [Google Scholar] [CrossRef]
- Daly, H.B. Laboratory rat experiments show consumption of Lake Ontario salmon causes behavioral changes: Support for wildlife and human research results. J. Great Lakes Res. 1993, 19, 784–788. [Google Scholar] [CrossRef]
- Flanigan, K.A.S.; Czuba, M.I.; Riesgo, V.R.; Rúa, M.A.; Stevenson, L.M.; Willing, J. Developmental exposure to corn grown on Lake Erie dredged material: A preliminary analysis. Front. Behav. Neurosci. 2023, 17, 987239. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Venkataraman, P.; Selvakumar, K.; Krishnamoorthy, G.; Muthusami, S.; Rameshkumar, R.; Prakash, S.; Arunakaran, J. Effect of melatonin on PCB (Aroclor 1254) induced neuronal damage and changes in Cu/Zn superoxide dismutase and glutathione peroxidase-4 mRNA expression in cerebral cortex, cerebellum and hippocampus of adult rats. Neurosci. Res. 2010, 66, 189–197. [Google Scholar] [CrossRef]
- Pratheepa Kumari, R.; Selvakumar, K.; Bavithra, S.; Zumaana, R.; Krishnamoorthy, G.; Arunakaran, J. Role of quercetin on PCBs (Aroclor-1254) induced impairment of dopaminergic receptor mRNA expression in cerebral cortex of adult male rats. Neurochem. Res. 2011, 36, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Bavithra, S.; Selvakumar, K.; Pratheepa Kumari, R.; Krishnamoorthy, G.; Venkataraman, P.; Arunakaran, J. Polychlorinated biphenyl (PCBs)-induced oxidative stress plays a critical role on cerebellar dopaminergic receptor expression: Ameliorative role of quercetin. Neurotox. Res. 2012, 21, 149–159. [Google Scholar] [CrossRef]
- Bavithra, S.; Selvakumar, K.; Krishnamoorthy, G.; Venkataraman, P.; Arunakaran, J. Melatonin attenuates polychlorinated biphenyls induced apoptosis in the neuronal cells of cerebral cortex and cerebellum of adult male rats-In vivo. Environ. Toxicol. Pharmacol. 2013, 36, 152–163. [Google Scholar] [CrossRef]
- Bavithra, S.; Selvakumar, K.; Sundareswaran, L.; Arunakaran, J. Neuroprotective effect of melatonin against PCBs induced behavioural, molecular and histological changes in cerebral cortex of adult male Wistar rats. Neurochem. Res. 2017, 42, 428–438. [Google Scholar] [CrossRef]
- Selvakumar, K.; Bavithra, S.; Suganthi, M.; Benson, C.S.; Elumalai, P.; Arunkumar, R.; Krishnamoorthy, G.; Venkataraman, P.; Arunakaran, J. Protective role of quercetin on PCBs-induced oxidative stress and apoptosis in hippocampus of adult rats. Neurochem. Res. 2012, 37, 708–721. [Google Scholar] [CrossRef]
- Selvakumar, K.; Bavithra, S.; Krishnamoorthy, G.; Ganesh, A.; Venkataraman, P.; Arunakaran, J. Impact of quercetin on PCBs (Aroclor-1254)-induced impairment of dopaminergic receptors expression in hippocampus of adult male Wistar rats. Biomed. Prev. Nutr. 2013, 3, 42–52. [Google Scholar] [CrossRef]
- Selvakumar, K.; Bavithra, S.; Ganesh, L.; Krishnamoorthy, G.; Venkataraman, P.; Arunakaran, J. Polychlorinated biphenyls induced oxidative stress mediated neurodegeneration in hippocampus and behavioral changes of adult rats: Anxiolytic-like effects of quercetin. Toxicol. Lett. 2013, 222, 45–54. [Google Scholar] [CrossRef]
- Hilgier, W.; Łazarewicz, J.W.; Strużynska, L.; Frontczak-Baniewicz, M.; Albrecht, J. Repeated exposure of adult rats to Aroclor 1254 induces neuronal injury and impairs the neurochemical manifestations of the NMDA receptor-mediated intracellular signaling in the hippocampus. Neurotoxicology 2012, 33, 16–22. [Google Scholar] [CrossRef]
- Zündorf, G.; Reiser, G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal. 2011, 14, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Bavithra, S.; Sugantha Priya, E.; Selvakumar, K.; Krishnamoorthy, G.; Arunakaran, J. Effect of melatonin on glutamate: BDNF signaling in the cerebral cortex of polychlorinated biphenyls (PCBs)—Exposed adult male rats. Neurochem. Res. 2015, 40, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Kodavanti, P.R.; Derr-Yellin, E.C.; Mundy, W.R.; Shafer, T.J.; Herr, D.W.; Barone, S.; Choksi, N.Y.; MacPhail, R.C.; Tilson, H.A. Repeated exposure of adult rats to Aroclor 1254 causes brain region-specific changes in intracellular Ca2+ buffering and protein kinase C activity in the absence of changes in tyrosine hydroxylase. Toxicol. Appl. Pharmacol. 1998, 153, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Selvakumar, K.; Bavithra, S.; Krishnamoorthy, G.; Arunakaran, J. Impact of quercetin on tight junctional proteins and BDNF signaling molecules in hippocampus of PCBs-exposed rats. Interdiscip. Toxicol. 2019, 11, 294–305. [Google Scholar] [CrossRef]
- Venkataraman, P.; Krishnamoorthy, G.; Vengatesh, G.; Srinivasan, N.; Aruldhas, M.M.; Arunakaran, J. Protective role of melatonin on PCB (Aroclor 1254) induced oxidative stress and changes in acetylcholine esterase and membrane bound ATPases in cerebellum, cerebral cortex and hippocampus of adult rat brain. Int. J. Dev. Neurosci. 2008, 26, 585–591. [Google Scholar] [CrossRef]
- Venkataraman, P.; Krishnamoorthy, G.; Selvakumar, K.; Arunakaran, J. Oxidative stress alters creatine kinase system in serum and brain regions of polychlorinated biphenyl (Aroclor 1254)-exposed rats: Protective role of melatonin. Basic Clin. Pharmacol. Toxicol. 2009, 105, 92–97. [Google Scholar] [CrossRef]
- Viluksela, M.; Heikkinen, P.; Van Der Ven, L.T.M.; Rendel, F.; Roos, R.; Esteban, J.; Korkalainen, M.; Lensu, S.; Miettinen, H.M.; Savolainen, K.; et al. Toxicological profile of ultrapure 2,2′,3,4,4′,5,5′- heptachlorbiphenyl (PCB 180) in adult rats. PLoS ONE 2014, 9, e104639. [Google Scholar] [CrossRef]
- Lee, D.W.; Notter, S.A.; Thiruchelvam, M.; Dever, D.P.; Fitzpatrick, R.; Kostyniak, P.J.; Cory-Slechta, D.A.; Opanashuk, L.A. Subchronic polychlorinated biphenyl (Aroclor 1254) exposure produces oxidative damage and neuronal death of ventral midbrain dopaminergic systems. Toxicol. Sci. 2012, 125, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters-key factors in neurological and neurodegenerative disorders of the central nervous system. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Chakravarthy, H.; Sharma, S.; Shree, S.; Bhat, A.R.; Pradeepkiran, J.A.; Devanathan, V. Neurotransmitter systems in the etiology of major neurological disorders: Emerging insights and therapeutic implications. Ageing Res. Rev. 2023, 89, 101994. [Google Scholar] [CrossRef]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- Speranza, L.; Miniaci, M.C.; Volpicelli, F. The role of dopamine in neurological, psychiatric, and metabolic disorders and cancer: A complex web of interactions. Biomedicines 2025, 13, 492. [Google Scholar] [CrossRef] [PubMed]
- Money, K.M.; Stanwood, G.D. Developmental origins of brain disorders: Roles for dopamine. Front. Cell. Neurosci. 2013, 7, 260. [Google Scholar] [CrossRef] [PubMed]
- Enayah, S.H.; Vanle, B.C.; Fuortes, L.J.; Doorn, J.A.; Ludewig, G. PCB95 and PCB153 change dopamine levels and turn-over in PC12 cells. Toxicology 2018, 394, 93–101. [Google Scholar] [CrossRef]
- Dreiem, A.; Okoniewski, R.J.; Brosch, K.O.; Miller, V.M.; Seegal, R.F. Polychlorinated biphenyls and polybrominated diphenyl ethers alter striatal dopamine neurochemistry in synaptosomes from developing rats in an additive manner. Toxicol. Sci. 2010, 118, 150–159. [Google Scholar] [CrossRef]
- Mariussen, E.; Mørch Andersen, J.; Fonnum, F. The effect of polychlorinated biphenyls on the uptake of dopamine and other neurotransmitters into rat brain synaptic vesicles. Toxicol. Appl. Pharmacol. 1999, 161, 274–282. [Google Scholar] [CrossRef]
- Chishti, M.A.; Fisher, J.P.; Seegal, R.F. Aroclors 1254 and 1260 reduce dopamine concentrations in rat striatal slices. Neurotoxicology 1996, 17, 653–660. [Google Scholar]
- Seegal, R.F.; Bush, B.; Shain, W. Lightly chlorinated ortho-substituted PCB congeners decrease dopamine in nonhuman primate brain and in tissue culture. Toxicol. Appl. Pharmacol. 1990, 106, 136–144. [Google Scholar] [CrossRef]
- Mariussen, E.; Fonnum, F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology 2001, 159, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Seegal, R.F.; Okoniewski, R.J.; Brosch, K.O.; Bemis, J.C. Polychlorinated biphenyls alter extraneuronal but not tissue dopamine concentrations in adult rat striatum: An in vivo microdialysis study. Environ. Health Perspect. 2002, 110, 1113–1117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seegal, R.F.; Bush, B.; Brosch, K.O. Sub-chronic exposure of the adult rat to Aroclor 1254 yields regionally-specific changes in central dopaminergic function. Neurotoxicology 1991, 12, 55–65. [Google Scholar] [PubMed][Green Version]
- Seegal, R.F.; Brosch, K.O.; Bush, B. Polychlorinated biphenyls produce regional alteration of dopamine metabolism in rat brain. Toxicol. Lett. 1986, 30, 197–202. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Lin, W.-P.; Huang, L.-P.; Zhao, B.; Zhang, C.-C.; Yin, D.-M. Dopamine D2 receptor regulates cortical synaptic pruning in rodents. Nat. Commun. 2021, 12, 6444. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef]
- Rosin, D.L.; Martin, B.R. Neurochemical and behavioral effects of polychlorinated biphenyls in mice. Neurotoxicology 1981, 2, 749–764. [Google Scholar]
- Seegal, R.F.; Bush, B.; Brosch, K.O. Polychlorinated biphenyls induced regional changes in brain norepinephrine concentrations in adult rats. Neurotoxicology 1985, 6, 13–23. [Google Scholar]
- Seegal, R.F.; Brosch, K.O.; Bush, B. Regional alterations in serotonin metabolism induced by oral exposure of rats to polychlorinated biphenyls. Neurotoxicology 1986, 7, 155–165. [Google Scholar]
- Mattson, M.P. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann. N.Y. Acad. Sci. 2008, 1144, 97–112. [Google Scholar] [CrossRef]
- Strużyńska, L.; Sulkowski, G.; Dąbrowska-Bouta, B. Aroclor 1254 selectively inhibits expression of glial GLT-1 glutamate transporter in the forebrain of chronically exposed adult rat. Toxicology 2012, 300, 12–18. [Google Scholar] [CrossRef]
- Hollville, E.; Romero, S.E.; Deshmukh, M. Apoptotic cell death regulation in neurons. FEBS J. 2019, 286, 3276–3298. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Chang, H.Y.; Sang, T.K. Neuronal cell death mechanisms in major neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Rahimi, N.; Dimri, M. G Protein Coupled Receptors; StatPearls: St. Petersburg, FL, USA, 2023; NBK518966. [Google Scholar]
- Salvador, G.A.; Uranga, R.M.; Giusto, N.M. Iron and mechanisms of neurotoxicity. Int. J. Alzheimers Dis. 2010, 2011, 720658. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, T.; Budd, S.L.; Lipton, S.A. Excitatory amino acid neurotoxicity. Adv. Exp. Med. Biol. 2002, 513, 3–40. [Google Scholar] [CrossRef]
- Garthwaite, J. Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci. 2008, 27, 2783–2802. [Google Scholar] [CrossRef]
- Benarroch, E.E. Na+, K+ -ATPase. Neurology 2011, 76, 287–293. [Google Scholar] [CrossRef]
- Andres, R.H.; Ducray, A.D.; Schlattner, U.; Wallimann, T.; Widmer, H.R. Functions and effects of creatine in the central nervous system. Brain Res. Bull. 2008, 76, 329–343. [Google Scholar] [CrossRef]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef]
- Coburn, C.G.; Watson-Siriboe, A.; Hou, B.; Cheetham, C.; Gillard, E.R.; Lin, L.; León-Olea, M.; Sánchez-Islas, E.; Mucio-Ramírez, S.; Currás-Collazo, M.C. Permanently compromised NADPH-diaphorase activity within the osmotically activated supraoptic nucleus after in utero but not adult exposure to Aroclor 1254. Neurotoxicology 2015, 47, 37–46. [Google Scholar] [CrossRef]
- Zhang, X.; Lee, W.; Bian, J.S. Recent advances in the study of Na+/K+ -ATPase in neurodegenerative diseases. Cells 2022, 11, 4075. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Dong, W.; Lin, X.; Bian, J. Na+/K+-ATPase: Ion pump, signal transducer, or cytoprotective protein, and novel biological functions. Neural Regen. Res. 2024, 19, 2684–2697. [Google Scholar] [CrossRef]
- Mata, A.M.; Sepulveda, M.R. Plasma membrane Ca-ATPases in the nervous system during development and ageing. World J. Biol. Chem. 2010, 1, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Nozadze, E.; Arutinova, N.; Tsakadze, L.; Shioshvili, L.; Leladze, M.; Dzneladze, S.; Chkadua, G. Molecular mechanism of Mg-ATPase activity. J. Membr. Biol. 2015, 248, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, W.; Wallimann, T. Functional aspects of creatine kinase in brain. Dev. Neurosci. 1993, 15, 249–260. [Google Scholar] [CrossRef]
- Adhihetty, P.J.; Beal, M.F. Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases. Neuromolecular Med. 2008, 10, 275–290. [Google Scholar] [CrossRef]
- Zimmerman, G.; Soreq, H. Termination and beyond: Acetylcholinesterase as a modulator of synaptic transmission. Cell Tissue Res. 2006, 326, 655–669. [Google Scholar] [CrossRef]
- Knorr, D.Y.; Demirbas, D.; Heinrich, R. Multifaceted promotion of apoptosis by acetylcholinesterase. Front. Cell Death 2023, 2, 1169966. [Google Scholar] [CrossRef]
- Yohn, S.E.; Harvey, P.D.; Brannan, S.K.; Horan, W.P. The potential of muscarinic M1 and M4 receptor activators for the treatment of cognitive impairment associated with schizophrenia. Front. Psychiatry 2024, 15, 1421554. [Google Scholar] [CrossRef]
- Reeves, K.C.; Shah, N.; Muñoz, B.; Atwood, B.K. Opioid receptor-mediated regulation of neurotransmission in the brain. Front. Mol. Neurosci. 2022, 15, 919773. [Google Scholar] [CrossRef]
- Hwang, W.J.; Lee, T.Y.; Kim, N.S.; Kwon, J.S. The role of estrogen receptors and their signaling across psychiatric disorders. Int. J. Mol. Sci. 2021, 22, 373. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.R.; Hart, B.G.; Gore, A.C. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 2. Sex-specific neuromolecular effects in the brain. Mol. Cell Endocrinol. 2016, 420, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Coccini, T.; Randine, G.; Castoldi, A.F.; Grandjean, P.; Ostendorp, G.; Heinzow, B.; Manzo, L. Effects of developmental co-exposure to methylmercury and 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) on cholinergic muscarinic receptors in rat brain. Neurotoxicology 2006, 27, 468–477. [Google Scholar] [CrossRef]
- Gao, Q.; Yiyang, Z.; Yu, C.; Wei, H.; Wenwen, J.; Chunting, Z.; Hao, Y.; Jianshun, L.; Zhenlang, L.; Lin, W. Role of iron in brain development, aging, and neurodegenerative diseases. Ann. Med. 2025, 57, 2472871. [Google Scholar] [CrossRef]
- Levi, S.; Ripamonti, M.; Moro, A.S.; Cozzi, A. Iron imbalance in neurodegeneration. Mol. Psychiatry 2024, 29, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Zwingmann, C.; Leibfritz, D. Glial–Neuronal Shuttle Systems. In Handbook of Neurochemistry and Molecular Neurobiology: Brain Energetics; Integration of Molecular and Cellular Processes; Lajtha, A., Gibson, G.E., Dienel, G.A., Eds.; Springer: Boston, MA, USA, 2007; pp. 197–238. [Google Scholar]
- Meriney, S.D.; Fanselow, E.E. Amino Acid Neurotransmitters. In Synaptic Transmission; Meriney, S.D., Fanselow, E.E., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 399–419. [Google Scholar]
- Albrecht, J.; Sidoryk-Węgrzynowicz, M.; Zielińska, M.; Aschner, M. Roles of glutamine in neurotransmission. Neuron Glia Biol. 2010, 6, 263–276. [Google Scholar] [CrossRef]
- Cairns, N.J.; Lee, V.M.; Trojanowski, J.Q. The cytoskeleton in neurodegenerative diseases. J. Pathol. 2004, 204, 438–449. [Google Scholar] [CrossRef]
- Zhao, F.; Li, B.; Yang, W.; Ge, T.; Cui, R. Brain-immune interaction mechanisms: Implications for cognitive dysfunction in psychiatric disorders. Cell Prolif. 2022, 55, e13295. [Google Scholar] [CrossRef]
- Hudson, N.; Campbell, M. Tight Junctions of the Neurovascular Unit. Front. Mol. Neurosci. 2021, 14, 752781. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.T.; Pierre, V.J.; Ploski, J.E.; Queen, K.; Schafe, G.E. The NO-cGMP-PKG signaling pathway regulates synaptic plasticity and fear memory consolidation in the lateral amygdala via activation of ERK/MAP kinase. Learn. Mem. 2008, 15, 792–805. [Google Scholar] [CrossRef]
- Albert-Gascó, H.; Ros-Bernal, F.; Castillo-Gómez, E.; Olucha-Bordonau, F.E. MAP/ERK signaling in developing cognitive and emotional function and Its effect on pathological and neurodegenerative processes. Int. J. Mol. Sci. 2020, 21, 4471. [Google Scholar] [CrossRef] [PubMed]
- Ziebell, J.M.; Morganti-Kossmann, M.C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 2010, 7, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; MacLean, A.G.; Philipp, M.T. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediat. Inflamm. 2013, 2013, 480739. [Google Scholar] [CrossRef]
- Mallick, R.; Duttaroy, A.K. Epigenetic modification impacting brain functions: Effects of physical activity, micronutrients, caffeine, toxins, and addictive substances. Neurochem. Int. 2023, 171, 105627. [Google Scholar] [CrossRef]
- Rasmi, Y.; Shokati, A.; Hassan, A.; Aziz, S.G.; Bastani, S.; Jalali, L.; Moradi, F.; Alipour, S. The role of DNA methylation in progression of neurological disorders and neurodegenerative diseases as well as the prospect of using DNA methylation inhibitors as therapeutic agents for such disorders. IBRO Neurosci. Rep. 2023, 14, 28–37. [Google Scholar] [CrossRef]
- Lossi, L.; Castagna, C.; Merighi, A. An An overview of the epigenetic modifications in the brain under normal and pathological conditions. Int. J. Mol. Sci. 2024, 25, 3881. [Google Scholar] [CrossRef]
- Pisula, W. Exploratory Behavior. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T.K., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 2558–2566. [Google Scholar] [CrossRef]
- Võikar, V.; Stanford, S.C. The Open Field Test. In Psychiatric Vulnerability, Mood, and Anxiety Disorders: Tests and Models in Mice and Rats; Harro, J., Ed.; Springer: New York, NY, USA, 2023; pp. 9–29. [Google Scholar] [CrossRef]
- Leussis, M.P.; Bolivar, V.J. Habituation in rodents: A review of behavior, neurobiology, and genetics. Neurosci. Biobehav. Rev. 2006, 30, 1045–1064. [Google Scholar] [CrossRef]
- Graham, D.L.; Meyer, J.S.; Stanwood, G.D. Behavioral Phenotyping in Developmental Neurotoxicology—Simple Approaches Using Unconditioned Behaviors in Rodents. In Handbook of Developmental Neurotoxicology, 2nd ed.; Slikker, W., Paule, M.G., Wang, C., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 287–308. [Google Scholar]
- Nishida, N.; Farmer, J.D.; Kodavanti, P.R.; Tilson, H.A.; MacPhail, R.C. Effects of acute and repeated exposures to Aroclor 1254 in adult rats: Motor activity and flavor aversion conditioning. Fundam. Appl. Toxicol. 1997, 40, 68–74. [Google Scholar] [CrossRef][Green Version]
- Rodgers, R.J.; Dalvi, A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997, 21, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Himanshu; Dharmila; Sarkar, D.; Nutan. A review of behavioral tests to evaluate different types of anxiety and anti-anxiety effects. Clin. Psychopharmacol. Neurosci. 2020, 18, 341–351. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated plus maze for mice. J. Vis. Exp. 2008, 22, 1088. [Google Scholar] [CrossRef]
- La-Vu, M.; Tobias, B.C.; Schuette, P.J.; Adhikari, A. To approach or avoid: An introductory overview of the study of anxiety using rodent assays. Front. Behav. Neurosci. 2020, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.; Goodwin, F.K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 1980, 13, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Campos-Cardoso, R.; Godoy, L.D.; Lazarini-Lopes, W.; Novaes, L.S.; dos Santos, N.B.; Perfetti, J.G.; Garcia-Cairasco, N.; Munhoz, C.D.; Padovan, C.M. Exploring the light/dark box test: Protocols and implications for neuroscience research. J. Neurosci. Methods 2023, 384, 109748. [Google Scholar] [CrossRef]
- Bell, M.R.; Thompson, L.M.; Rodriguez, K.; Gore, A.C. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 1. Sexually dimorphic effects on social and anxiety-like behaviors. Horm. Behav. 2016, 78, 168–177. [Google Scholar] [CrossRef]
- Lang, B.; Kahnau, P.; Hohlbaum, K.; Mieske, P.; Andresen, N.P.; Boon, M.N.; Thöne-Reineke, C.; Lewejohann, L.; Diederich, K. Challenges and advanced concepts for the assessment of learning and memory function in mice. Front. Behav. Neurosci. 2023, 17, 1230082. [Google Scholar] [CrossRef]
- Sridhar, S.; Khamaj, A.; Asthana, M.K. Cognitive neuroscience perspective on memory: Overview and summary. Front. Hum. Neurosci. 2023, 17, 1217093. [Google Scholar] [CrossRef]
- Morris, R.G.M. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981, 12, 239–260. [Google Scholar] [CrossRef]
- Olton, D.S.; Samuelson, R.J. Remembrance of places passed: Spatial memory in rats. J. Exp. Psychol. Anim. Behav. Process. 1976, 2, 97–116. [Google Scholar] [CrossRef]
- Garcia, J.; Kimeldorf, D.J.; Koelling, R.A. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science 1955, 122, 157–158. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Assessing spatial learning and memory in rodents. ILAR J. 2014, 55, 310–332. [Google Scholar] [CrossRef]
- Dudchenko, P.A. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004, 28, 699–709. [Google Scholar] [CrossRef]
- Bermúdez-Rattoni, F. Molecular mechanisms of taste-recognition memory. Nat. Rev. Neurosci. 2004, 5, 209–217. [Google Scholar] [CrossRef]
- Matsumoto, K.; Tanaka, K. The role of the medial prefrontal cortex in achieving goals. Curr. Opin. Neurobiol. 2004, 14, 178–185. [Google Scholar] [CrossRef]
- Squire, L.R. Memory systems of the brain: A brief history and current perspective. Neurobiol. Learn. Mem. 2004, 82, 171–177. [Google Scholar] [CrossRef]
- Olton, D.S.; Paras, B.C. Spatial memory and hippocampal function. Neuropsychologia 1979, 17, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-García, J.M.; da Guarda, S.N.F.; Gerwig, M.; Habas, C.; Hagura, N.; Ivry, R.B.; Mariën, P.; et al. Consensus paper: Roles of the cerebellum in motor control—The diversity of ideas on cerebellar involvement in movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef]
- Tilson, H.A. Behavioral indices of neurotoxicity: What can be measured? Neurotoxicol. Teratol. 1987, 9, 427–443. [Google Scholar] [CrossRef]
- Dunham, N.W.; Miya, T.S. A note on a simple apparatus for detecting neurological deficit in rats and mice. J. Am. Pharm. Assoc. 1957, 46, 208–209. [Google Scholar] [CrossRef]
- Jones, B.J.; Roberts, D.J. The quantitative measurement of motor in coordination in naive mice using an accelerating rotarod. J. Pharm. Pharmacol. 1968, 20, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.J.; Morton, J.; Dunnett, S.B. Motor coordination and balance in rodents. Curr. Protoc. Neurosci. 2001, 15, 8.12.1–8.12.14. [Google Scholar] [CrossRef]
- Mann, A.; Chesselet, M.-F. Techniques for Motor Assessment in Rodents. In Movement Disorders, 2nd ed.; LeDoux, M.S., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 139–157. [Google Scholar]
- Ölveczky, B.P. Motoring ahead with rodents. Curr. Opin. Neurobiol. 2011, 21, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Meredith, G.E.; Kang, U.J. Behavioral models of Parkinson’s disease in rodents: A new look at an old problem. Mov. Disord. 2006, 21, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Plowman, E.K.; Kleim, J.A. Behavioral and neurophysiological correlates of striatal dopamine depletion: A rodent model of Parkinson’s disease. J. Commun. Disord. 2011, 44, 549–556. [Google Scholar] [CrossRef][Green Version]
- Willott, J.F. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar][Green Version]
- Moy, S.S.; Nadler, J.J.; Perez, A.; Barbaro, R.P.; Johns, J.M.; Magnuson, T.R.; Piven, J.; Crawley, J.N. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004, 3, 287–302. [Google Scholar] [CrossRef]
- Rein, B.; Ma, K.; Yan, Z. A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat. Protoc. 2020, 15, 3464–3477. [Google Scholar] [CrossRef]
- Cum, M.; Santiago Pérez, J.A.; Wangia, E.; Lopez, N.; Wright, E.S.; Iwata, R.L.; Li, A.; Chambers, A.R.; Padilla-Coreano, N. A systematic review and meta-analysis of how social memory is studied. Sci. Rep. 2024, 14, 2221. [Google Scholar] [CrossRef]
- Premoli, M.; Memo, M.; Bonini, S.A. Ultrasonic vocalizations in mice: Relevance for ethologic and neurodevelopmental disorders studies. Neural Regen. Res. 2021, 16, 1158–1167. [Google Scholar] [CrossRef]
- Mercado, E.; Zhuo, J. Do rodents smell with sound? Neurosci. Biobehav. Rev. 2024, 167, 105908. [Google Scholar] [CrossRef]
- Carlsson, M.A.; Swedberg, M.D.B. A behavioural operant discrimination model for assessment and pharmacological manipulation of visual function in rats. Brain Res. 2010, 1321, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Dayan, P.; Balleine, B.W. Reward, motivation, and reinforcement learning. Neuron 2002, 36, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Staddon, J.E.; Cerutti, D.T. Operant conditioning. Annu. Rev. Psychol. 2003, 54, 115–144. [Google Scholar] [CrossRef]
- Rossi, M.A.; Yin, H.H. Methods for studying habitual behavior in mice. Curr. Protoc. Neurosci. 2012, 8, 8.29.1–8.29.9. [Google Scholar] [CrossRef]
- Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998, 80, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Shiflett, M.W.; Balleine, B.W. Molecular substrates of action control in cortico-striatal circuits. Prog. Neurobiol. 2011, 95, 1–13. [Google Scholar] [CrossRef]
- Nist, A.N.; Shahan, T.A. The extinction burst: Impact of reinforcement time and level of analysis on measured prevalence. J. Exp. Anal. Behav. 2021, 116, 131–148. [Google Scholar] [CrossRef]
- Bari, A.; Robbins, T.W. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol. 2013, 108, 44–79. [Google Scholar] [CrossRef]
- Dalley, J.W.; Everitt, B.J.; Robbins, T.W. Impulsivity, compulsivity, and top-down cognitive control. Neuron 2011, 69, 680–694. [Google Scholar] [CrossRef]
- Burton, C.L.; Longaretti, A.; Zlatanovic, A.; Gomes, G.M.; Tonini, R. Striatal insights: A cellular and molecular perspective on repetitive behaviors in pathology. Front. Cell. Neurosci. 2024, 18, 1386715. [Google Scholar] [CrossRef]
- Berger, D.F.; Lombardo, J.P.; Jeffers, P.M.; Hunt, A.E.; Bush, B.; Casey, A.; Quimby, F. Hyperactivity and impulsiveness in rats fed diets supplemented with either Aroclor 1248 or PCB-contaminated St. Lawrence river fish. Behav. Brain Res. 2001, 126, 1–11. [Google Scholar] [CrossRef]
- Lombardo, J.P.; Berger, D.F.; Hunt, A.; Carpenter, D.O. Inhalation of polychlorinated biphenyls (PCB) produces hyperactivity in rats. J. Toxicol. Environ. Health A 2015, 78, 1142–1153. [Google Scholar] [CrossRef]
- Bridges, R.S. Neuroendocrine regulation of maternal behavior. Front. Neuroendocrinol. 2015, 36, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Erskine, M.S. Solicitation behavior in the estrous female rat: A review. Horm. Behav. 1989, 23, 473–502. [Google Scholar] [CrossRef]
- Fleming, A.S.; Rosenblatt, J.S. Maternal behavior in the virgin and lactating rat. J. Comp. Physiol. Psychol. 1974, 86, 957–972. [Google Scholar] [CrossRef]
- Gammie, S.C. Current models and future directions for understanding the neural circuitries of maternal behaviors in rodents. Behav. Cogn. Neurosci. Rev. 2005, 4, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Curley, J.P.; Champagne, F.A. Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Front. Neuroendocrinol. 2016, 40, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Huang, L.; Corona, A.; Pagliaro, A.H.; Shea, S.D. A dopaminergic reward prediction error signal shapes maternal behavior in mice. Neuron 2023, 111, 557–570. [Google Scholar] [CrossRef]
- Chung, Y.W.; Nunez, A.A.; Clemens, L.G. Effects of neonatal polychlorinated biphenyl exposure on female sexual behavior. Physiol. Behav. 2001, 74, 363–370. [Google Scholar] [CrossRef]
- Cummings, J.A.; Nunez, A.A.; Clemens, L.G. A cross-fostering analysis of the effects of PCB 77 on the maternal behavior of rats. Physiol. Behav. 2005, 85, 83–91. [Google Scholar] [CrossRef]
- Elnar, A.A.; Diesel, B.; Desor, F.; Feidt, C.; Bouayed, J.; Kiemer, A.K.; Soulimani, R. Neurodevelopmental and behavioral toxicity via lactational exposure to the sum of six indicator non-dioxin-like-polychlorinated biphenyls (∑6 NDL-PCBs) in mice. Toxicology 2012, 299, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Dalley, J.W.; Cardinal, R.N.; Robbins, T.W. Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neurosci. Biobehav. Rev. 2004, 28, 771–784. [Google Scholar] [CrossRef]
- Floresco, S.B.; Block, A.E.; Tse, M.T.L. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav. Brain Res. 2008, 190, 85–96. [Google Scholar] [CrossRef]
- Brady, A.M.; Floresco, S.B. Operant procedures for assessing behavioral flexibility in rats. J. Vis. Exp. 2015, 96, e52387. [Google Scholar] [CrossRef]
- Li, X.; Suh, Y.P.; Cui, J.Y.; Lehmler, H.-J. Dataset for Characterization of the Fox River Mixture (FRM); Westra, B., Ed.; University of Iowa: Iowa City, IA, USA, 2023. [Google Scholar] [CrossRef]
- Kostyniak, P.J.; Hansen, L.G.; Widholm, J.J.; Fitzpatrick, R.D.; Olson, J.R.; Helferich, J.L.; Kim, K.H.; Sable, H.J.K.; Seegal, R.F.; Pessah, I.N.; et al. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol. Sci. 2005, 88, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.L.; Li, X.; Lehmler, H.J.; Phillips, B.; Shen, D.; Cui, J.Y. Gut microbiota modulates interactions between polychlorinated biphenyls and bile acid homeostasis. Toxicol. Sci. 2018, 166, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.J.; Suh, Y.P.; Dursun, I.; Li, X.; da Costa Souza, F.; Grodzki, A.C.; Cui, J.Y.; Lehmler, H.-J.; Lein, P.J. Developmental exposure to the Fox River PCB mixture modulates behavior in juvenile mice. Neurotoxicology 2024, 103, 146–161. [Google Scholar] [CrossRef]
- Monaikul, S.; Eubig, P.; Floresco, S.; Schantz, S. Strategy set-shifting and response inhibition in adult rats exposed to an environmental polychlorinated biphenyl mixture during adolescence. Neurotoxicol. Teratol. 2017, 63, 14–23. [Google Scholar] [CrossRef]
- Ferster, C.B.; Skinner, B.F. Schedules of Reinforcement; Appleton-Century-Crofts: New York, NY, USA, 1957. [Google Scholar]
- Liao, R.-M.; Pattij, T. Neural basis of operant behaviors maintained on the differential-reinforcement-of-low-rate (DRL) schedule in rodents. Brain Res. Bull. 2022, 185, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tilson, H.A.; Davis, G.J.; McLachlan, J.A.; Lucier, G.W. The effects of polychlorinated biphenyls given prenatally on the neurobehavioral development of mice. Environ. Res. 1979, 18, 466–474. [Google Scholar] [CrossRef]
- Beery, A.K. Inclusion of females does not increase variability in rodent research studies. Curr. Opin. Behav. Sci. 2018, 23, 143–149. [Google Scholar] [CrossRef]
- Hånell, A.; Marklund, N. Structured evaluation of rodent behavioral tests used in drug discovery research. Front. Behav. Neurosci. 2014, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Peng, T.; Xu, M.; Lin, S.; Hu, B.; Chu, T.; Liu, B.; Xu, Y.; Ding, W.; Li, L.; et al. Spatial multi-omics: Deciphering technological landscape of integration of multi-omics and its applications. J. Hematol. Oncol. 2024, 17, 72. [Google Scholar] [CrossRef]
- Shi, C.; Cheng, L.; Yu, Y.; Chen, S.; Dai, Y.; Yang, J.; Zhang, H.; Chen, J.; Geng, N. Multi-omics integration analysis: Tools and applications in environmental toxicology. Environ. Pollut. 2024, 360, 124675. [Google Scholar] [CrossRef] [PubMed]
- Alam El Din, D.-M.; Moenkemoeller, L.; Loeffler, A.; Habibollahi, F.; Schenkman, J.; Mitra, A.; van der Molen, T.; Ding, L.; Laird, J.; Schenke, M.; et al. Human neural organoid microphysiological systems show the building blocks necessary for basic learning and memory. Commun. Biol. 2025, 8, 1237. [Google Scholar] [CrossRef]
- Lee, C.-J.; Nam, Y.; Rim, Y.A.; Ju, J.H. Advanced animal replacement testing strategies using stem cell and organoids. Int. J. Stem Cells 2025, 18, 107–125. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Diaz-delCastillo, M.; Kong, L.; Daniels, N.; MacIntosh-Smith, W.; Abdallah, A.; Domanski, D.; Sofrenovic, D.; Yeung, T.P.S.; Valiente, D.; et al. A systematic review and meta-analysis of thigmotactic behaviour in the open field test in rodent models associated with persistent pain. PLoS ONE 2023, 18, e0290382. [Google Scholar] [CrossRef]
- Spielewoy, C.; Roubert, C.; Hamon, M.; Nosten-Bertrand, M.; Betancur, C.; Giros, B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav. Pharmacol. 2000, 11, 279–290. [Google Scholar] [CrossRef]
- Bertan, F.; Wischhof, L.; Sosulina, L.; Mittag, M.; Dalügge, D.; Fornarelli, A.; Gardoni, F.; Marcello, E.; Di Luca, M.; Fuhrmann, M.; et al. Loss of ryanodine receptor 2 impairs neuronal activity-dependent remodeling of dendritic spines and triggers compensatory neuronal hyperexcitability. Cell Death Differ. 2020, 27, 3354–3373, Correction in Cell Death Differ. 2021, 28, 1134. [Google Scholar] [CrossRef] [PubMed]
- Hogg, S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol. Biochem. Behav. 1996, 54, 21–30. [Google Scholar] [CrossRef]
- León-Rodríguez, A.; Fernández-Arjona, M.d.M.; Grondona, J.M.; Pedraza, C.; López-Ávalos, M.D. Anxiety-like behavior and microglial activation in the amygdala after acute neuroinflammation induced by microbial neuraminidase. Sci. Rep. 2022, 12, 11581. [Google Scholar] [CrossRef]
- Comai, S.; De Gregorio, D.; Posa, L.; Ochoa-Sanchez, R.; Bedini, A.; Gobbi, G. Dysfunction of serotonergic activity and emotional responses across the light-dark cycle in mice lacking melatonin MT2 receptors. J. Pineal. Res. 2020, 69, e12653. [Google Scholar] [CrossRef]
- Miller, S.M.; Piasecki, C.C.; Lonstein, J.S. Use of the light-dark box to compare the anxiety-related behavior of virgin and postpartum female rats. Pharmacol. Biochem. Behav. 2011, 100, 130–137. [Google Scholar] [CrossRef]
- Antunes, G.F.; Gouveia, F.V.; Kuroki, M.A.; Oliveira Martins, D.; Pagano, R.L.; Pinheiro Campos, A.C.; Martinez, R.C.R. Neuroinflammation in the prefrontal-amygdala-hippocampus network is associated with maladaptive avoidance behaviour. Heliyon 2024, 10, e30427. [Google Scholar] [CrossRef]
- Patki, G.; Solanki, N.; Atrooz, F.; Allam, F.; Salim, S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013, 1539, 73–86. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L.; Zhu, L.; Chen, D.; Cai, K.; Liu, Z.; Chen, A. Moderate exercise combined with enriched environment enhances learning and memory through BDNF/TrkB signaling pathway in rats. Int. J. Environ. Res. Public Health 2021, 18, 8283. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.Z.; Hassan, Z.; Che Has, A.T. Morris water maze: A versatile and pertinent tool for assessing spatial learning and memory. Exp. Anim. 2022, 71, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Olesen, M.A.; Torres, A.K.; Jara, C.; Murphy, M.P.; Tapia-Rojas, C. Premature synaptic mitochondrial dysfunction in the hippocampus during aging contributes to memory loss. Redox Biol. 2020, 34, 101558. [Google Scholar] [CrossRef] [PubMed]
- Comyn, T.; Preat, T.; Pavlowsky, A.; Plaçais, P.-Y. Mitochondrial plasticity: An emergent concept in neuronal plasticity and memory. Neurobiol. Dis. 2024, 203, 106740. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ueji, K. Brain mechanisms of flavor learning. Front. Syst. Neurosci. 2011, 5, 76. [Google Scholar] [CrossRef]
- Yiannakas, A.; Rosenblum, K. The insula and taste learning. Front. Mol. Neurosci. 2017, 10, 335. [Google Scholar] [CrossRef]
- Kargl, D.; Kaczanowska, J.; Ulonska, S.; Groessl, F.; Piszczek, L.; Lazovic, J.; Buehler, K.; Haubensak, W. The amygdala instructs insular feedback for affective learning. eLife 2020, 9, e60336. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 2018, 285, 3657–3668. [Google Scholar] [CrossRef]
- Colebrooke, R.E.; Humby, T.; Lynch, P.J.; McGowan, D.P.; Xia, J.; Emson, P.C. Age-related decline in striatal dopamine content and motor performance occurs in the absence of nigral cell loss in a genetic mouse model of Parkinson’s disease. Eur. J. Neurosci. 2006, 24, 2622–2630. [Google Scholar] [CrossRef]
- Shiotsuki, H.; Yoshimi, K.; Shimo, Y.; Funayama, M.; Takamatsu, Y.; Ikeda, K.; Takahashi, R.; Kitazawa, S.; Hattori, N. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods 2010, 189, 180–185. [Google Scholar] [CrossRef]
- Ni, R.J.; Yuan, W.J.; Wang, Y.Y.; Yang, X.; Wei, J.X.; Zhao, L.S.; Wang, Q.; Tang, X.D.; Ma, X.H. Microglia-mediated inflammation and synaptic pruning contribute to sleep deprivation-induced mania in a sex-specific manner. Transl. Psychiatry 2025, 15, 285. [Google Scholar] [CrossRef]
- Krasner, H.; Ong, C.V.; Hewitt, P.; Vida, T.A. From stress to synapse: The neuronal atrophy pathway to mood dysregulation. Int. J. Mol. Sci. 2025, 26, 3219. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.C.; Selvakumar, B.; Weil, Z.M.; Snyder, S.H.; Nelson, R.J. Neuronal nitric oxide synthase and NADPH oxidase interact to affect cognitive, affective, and social behaviors in mice. Behav. Brain Res. 2013, 256, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-X.; Su, Y.-A.; Wang, Q.; Zheng, J.-Y.; Zhang, C.-C.; Wang, T.; Liu, X.; Ma, Y.-N.; Li, X.-X.; Zhang, X.-Q.; et al. The causal involvement of the BDNF-TrkB pathway in dentate gyrus in early-life stress-induced cognitive deficits in male mice. Transl. Psychiatry 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, L.; Huang, A.J.Y.; Boehringer, R.; Robert, V.; Wintzer, M.E.; Polygalov, D.; Weitemier, A.Z.; Tao, Y.; Gu, M.; et al. A hypothalamic novelty signal modulates hippocampal memory. Nature 2020, 586, 270–274. [Google Scholar] [CrossRef]
- Sharif, A.; Matsumoto, J.; Choijiljav, C.; Badarch, A.; Setogawa, T.; Nishijo, H.; Nishimaru, H. Characterization of ultrasonic vocalization-modulated neurons in rat motor cortex based on their activity modulation and axonal projection to the periaqueductal gray. eNeuro 2024, 11, ENEURO.0452-23.2024. [Google Scholar] [CrossRef]
- Michael, V.; Goffinet, J.; Pearson, J.; Wang, F.; Tschida, K.; Mooney, R. Circuit and synaptic organization of forebrain-to-midbrain pathways that promote and suppress vocalization. eLife 2020, 9, e63493. [Google Scholar] [CrossRef]
- Arnsten, A.F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Johnson, A.W.; Jaaro-Peled, H.; Shahani, N.; Sedlak, T.W.; Zoubovsky, S.; Burruss, D.; Emiliani, F.; Sawa, A.; Gallagher, M. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc. Natl. Acad. Sci. USA 2013, 110, 12462–12467. [Google Scholar] [CrossRef]
- Ríos-Bonilla, K.M.; Aga, D.S.; Lee, J.; König, M.; Qin, W.; Cristobal, J.R.; Atilla-Gokcumen, G.E.; Escher, B.I. Neurotoxic effects of mixtures of perfluoroalkyl substances (PFAS) at environmental and human blood concentrations. Environ. Sci. Technol. 2024, 58, 16774–16784. [Google Scholar] [CrossRef]
- Meaney, M.J.; Diorio, J.; Francis, D.; Weaver, S.; Yau, J.; Chapman, K.; Seckl, J.R. Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: The effects of thyroid hormones and serotonin. J. Neurosci. 2000, 20, 3926–3935. [Google Scholar] [CrossRef] [PubMed]
- Day, K.R.; Shea, S.D. Dopamine dynamics underlying maternal motivation and reward. Neurosci. Res. 2025, 218, 104928. [Google Scholar] [CrossRef]
- Bissonette, G.B.; Powell, E.M. Reversal learning and attentional set-shifting in mice. Neuropharmacology 2012, 62, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Zipp, F.; Bittner, S.; Schafer, D.P. Cytokines as emerging regulators of central nervous system synapses. Immunity 2023, 56, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Liss, A.; Siddiqi, M.T.; Marsland, P.; Varodayan, F.P. Neuroimmune regulation of the prefrontal cortex tetrapartite synapse. Neuropharmacology 2025, 269, 110335. [Google Scholar] [CrossRef] [PubMed]
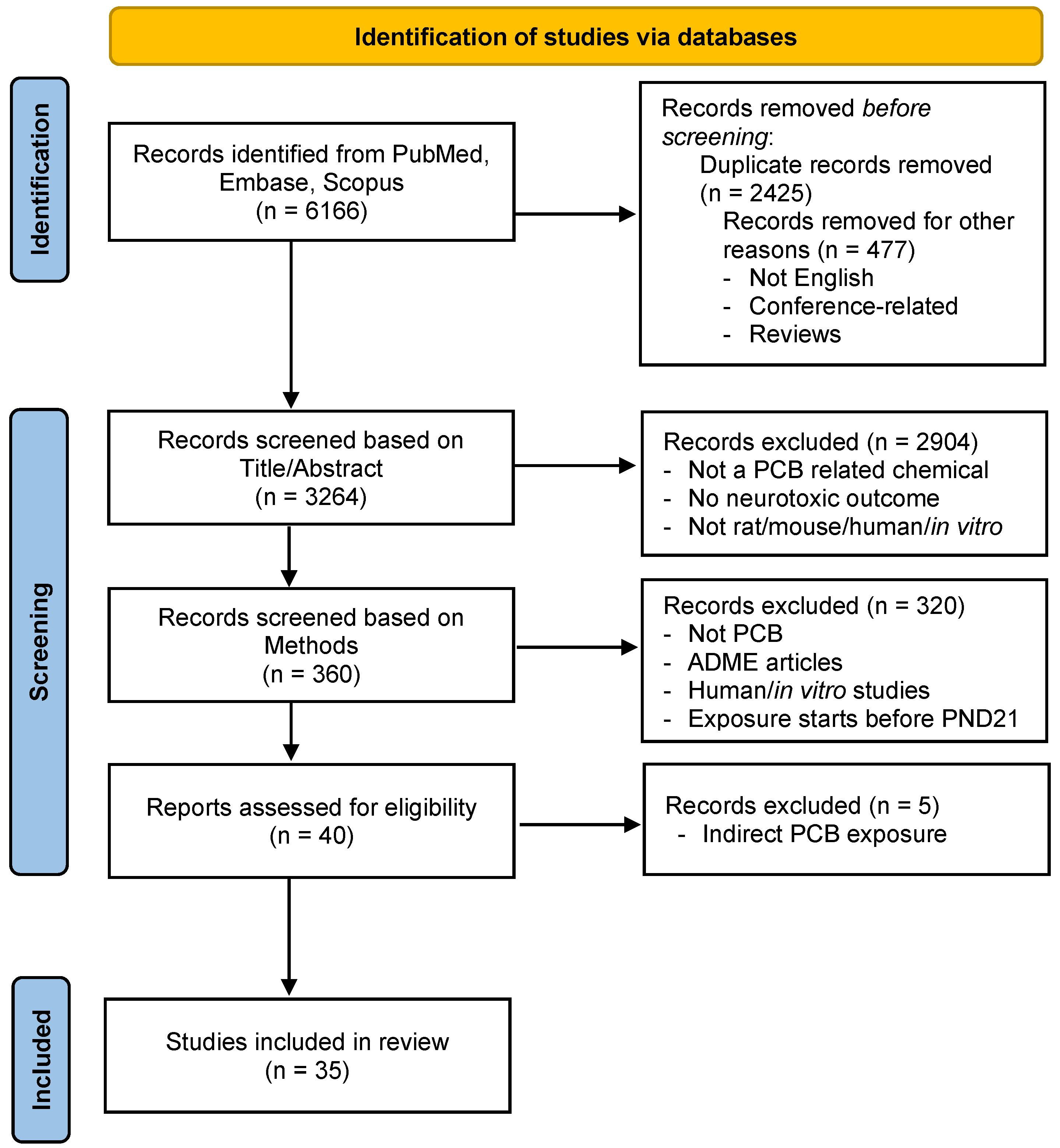
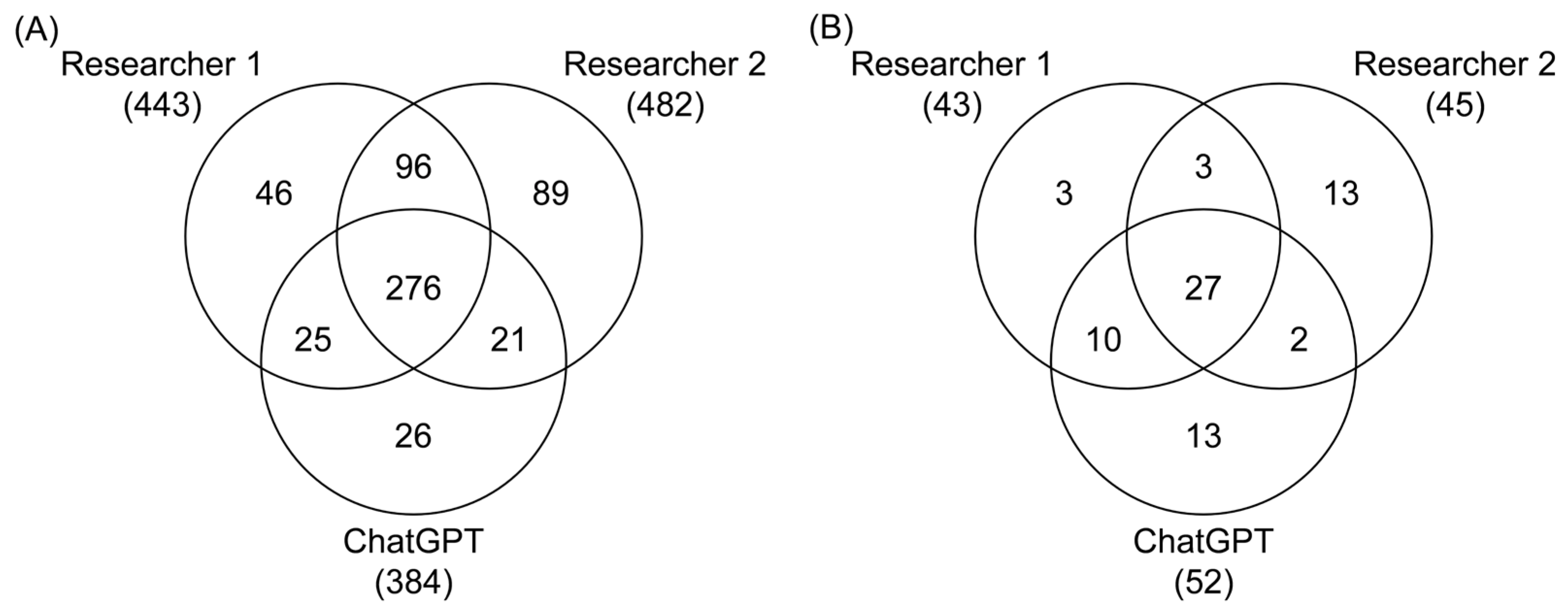
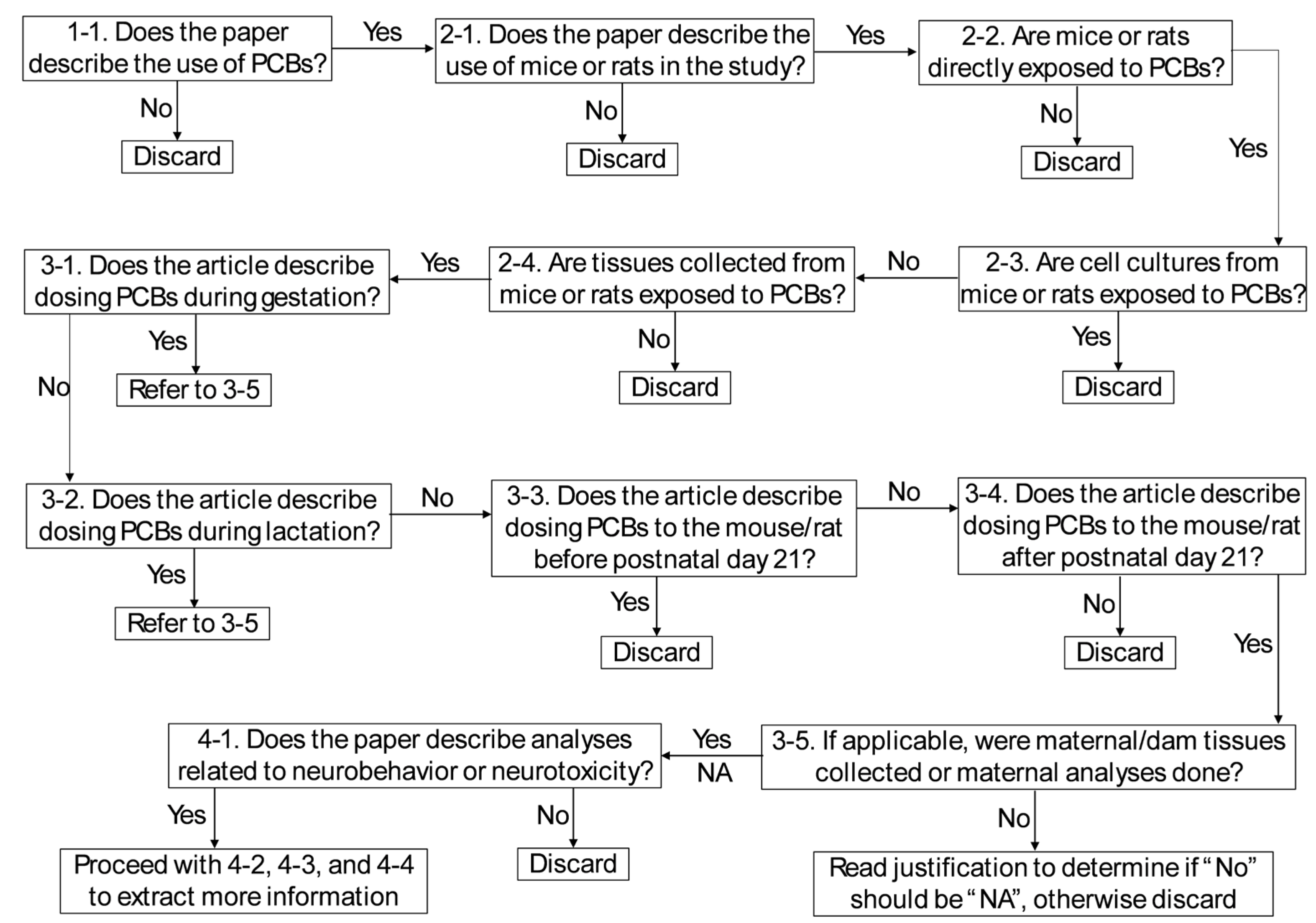
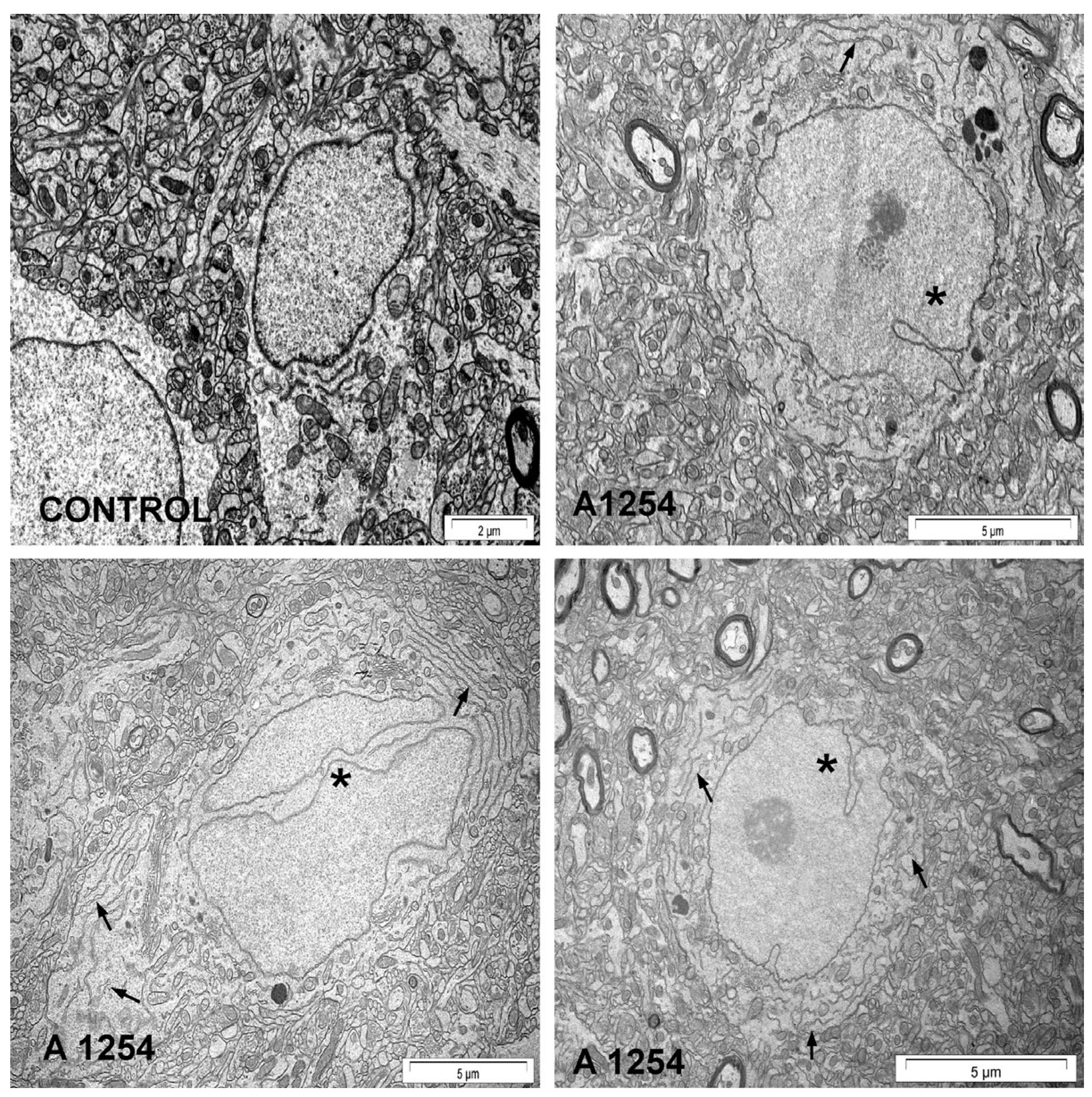

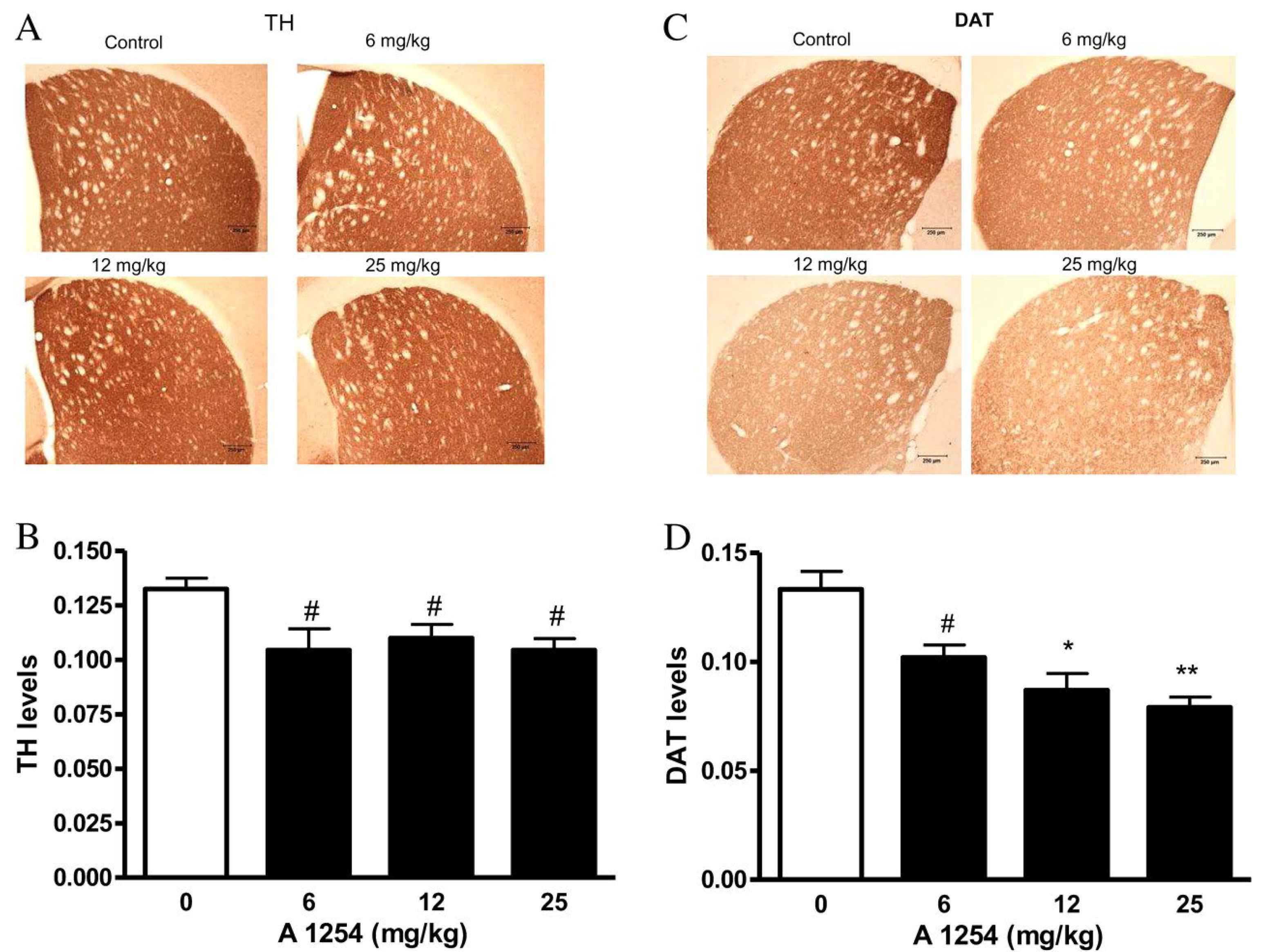
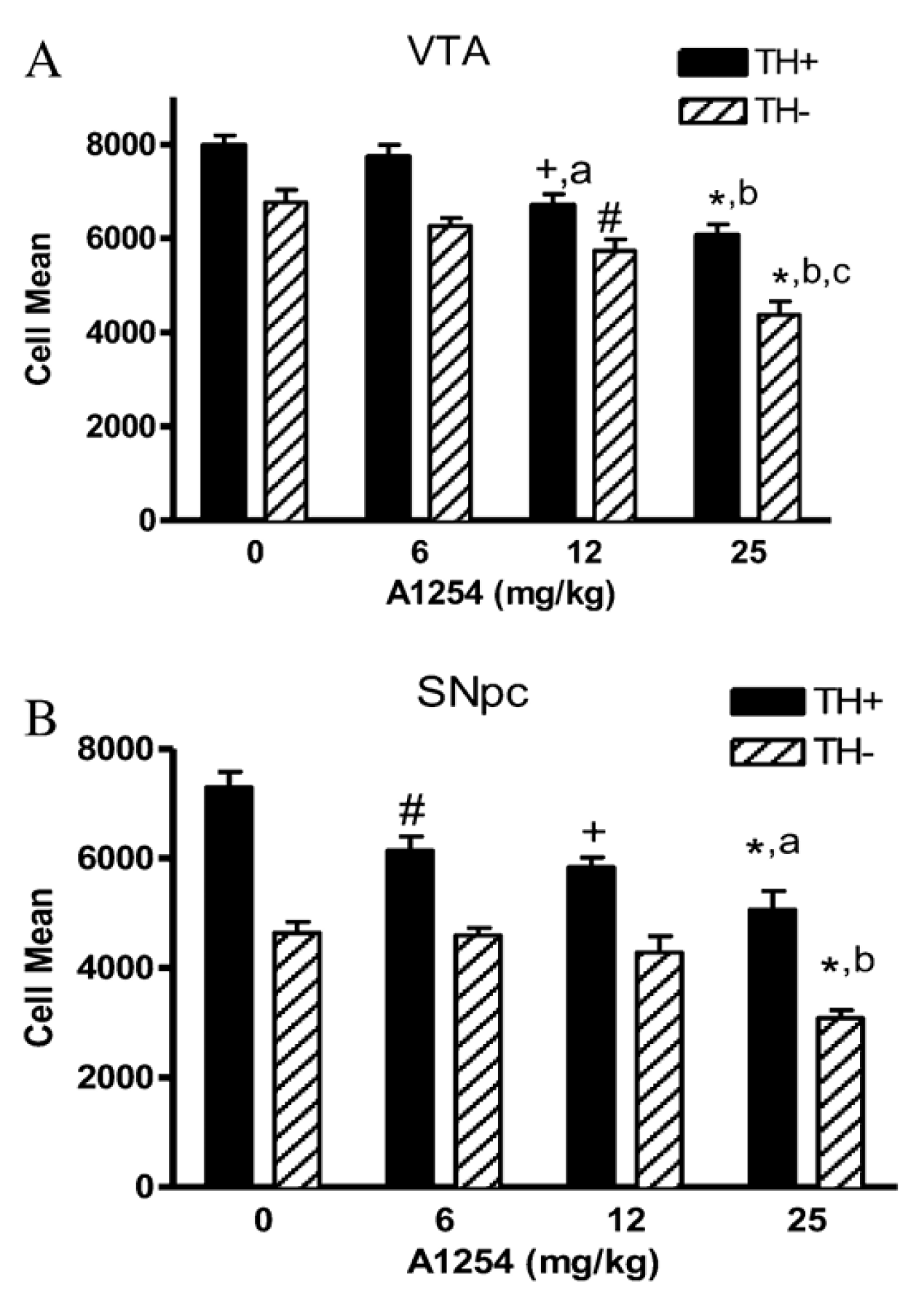
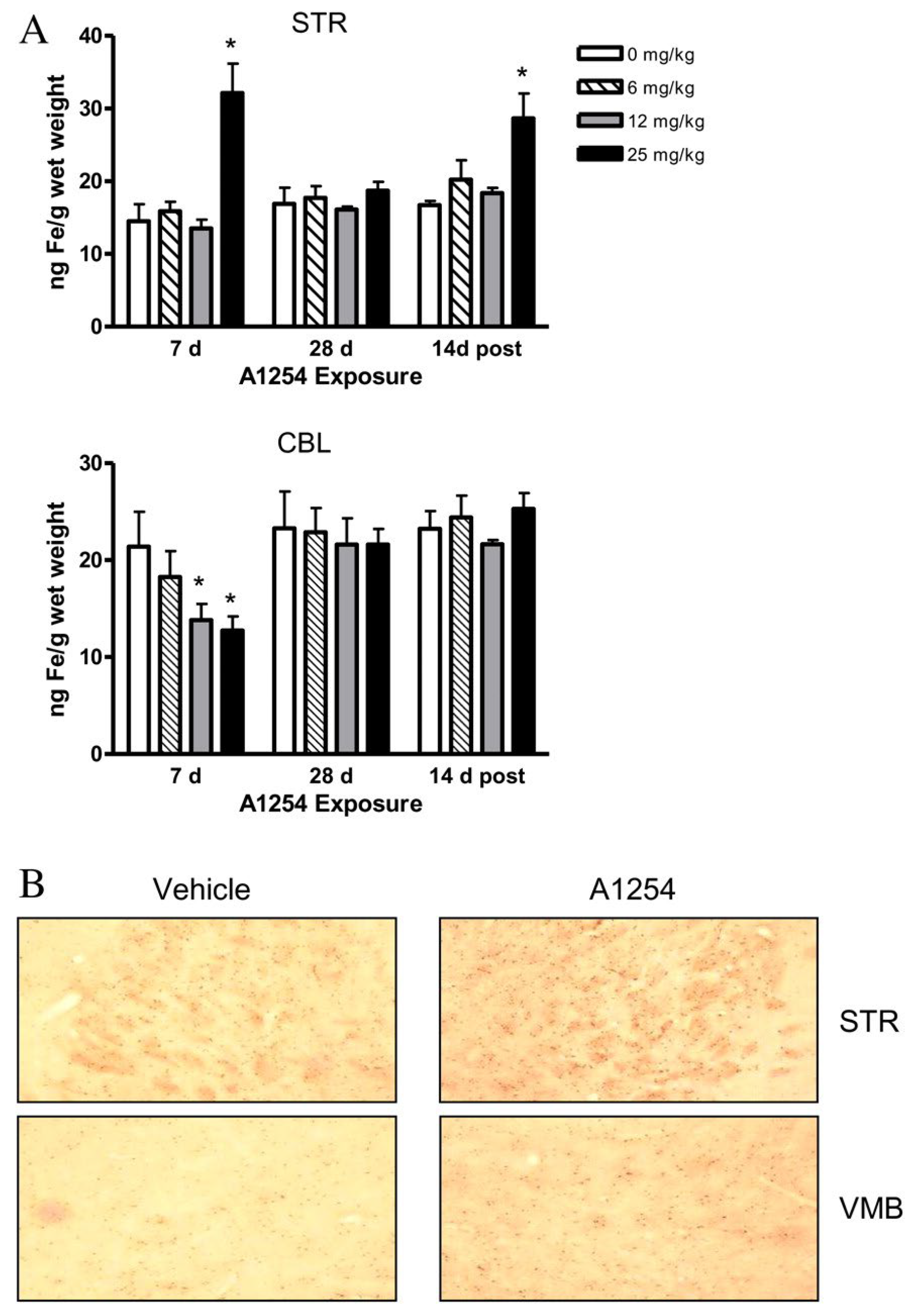
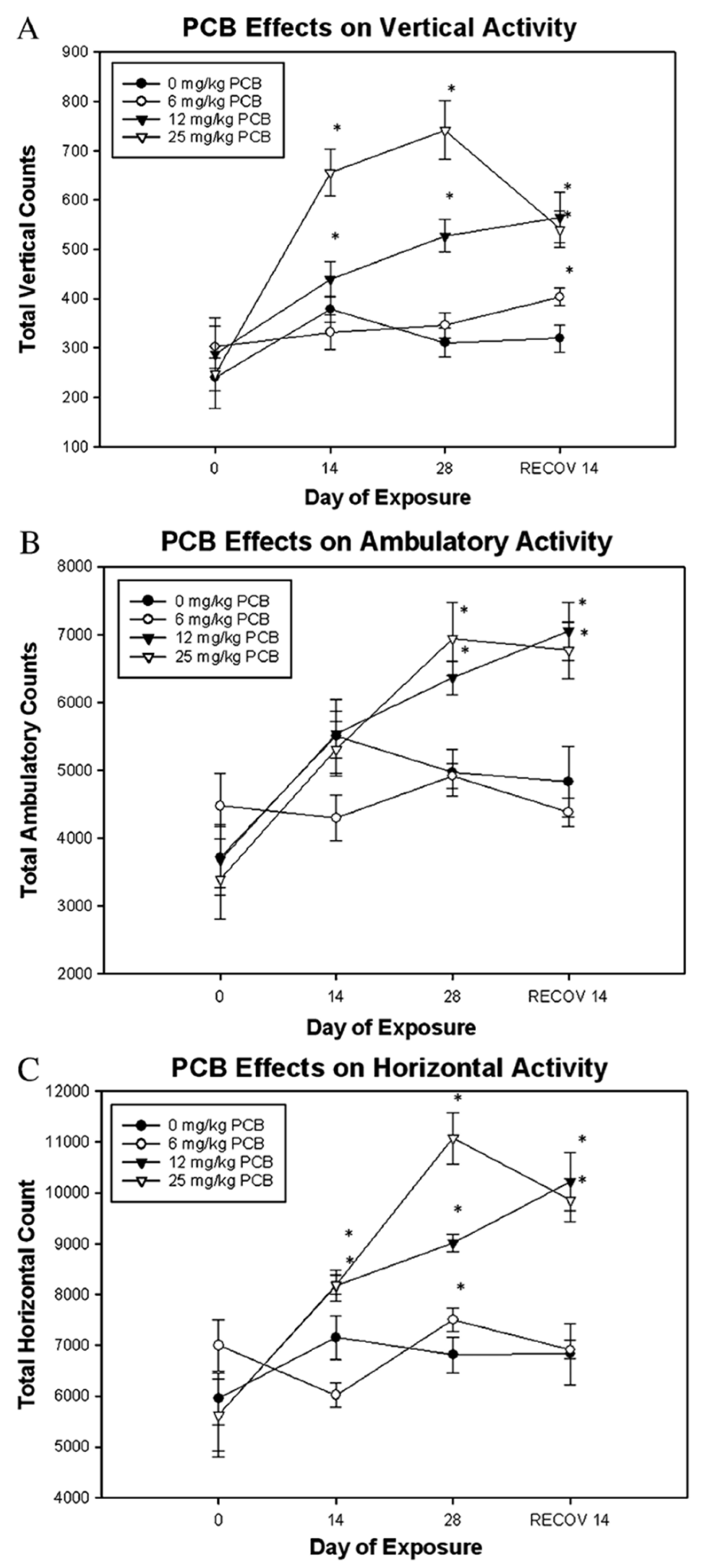
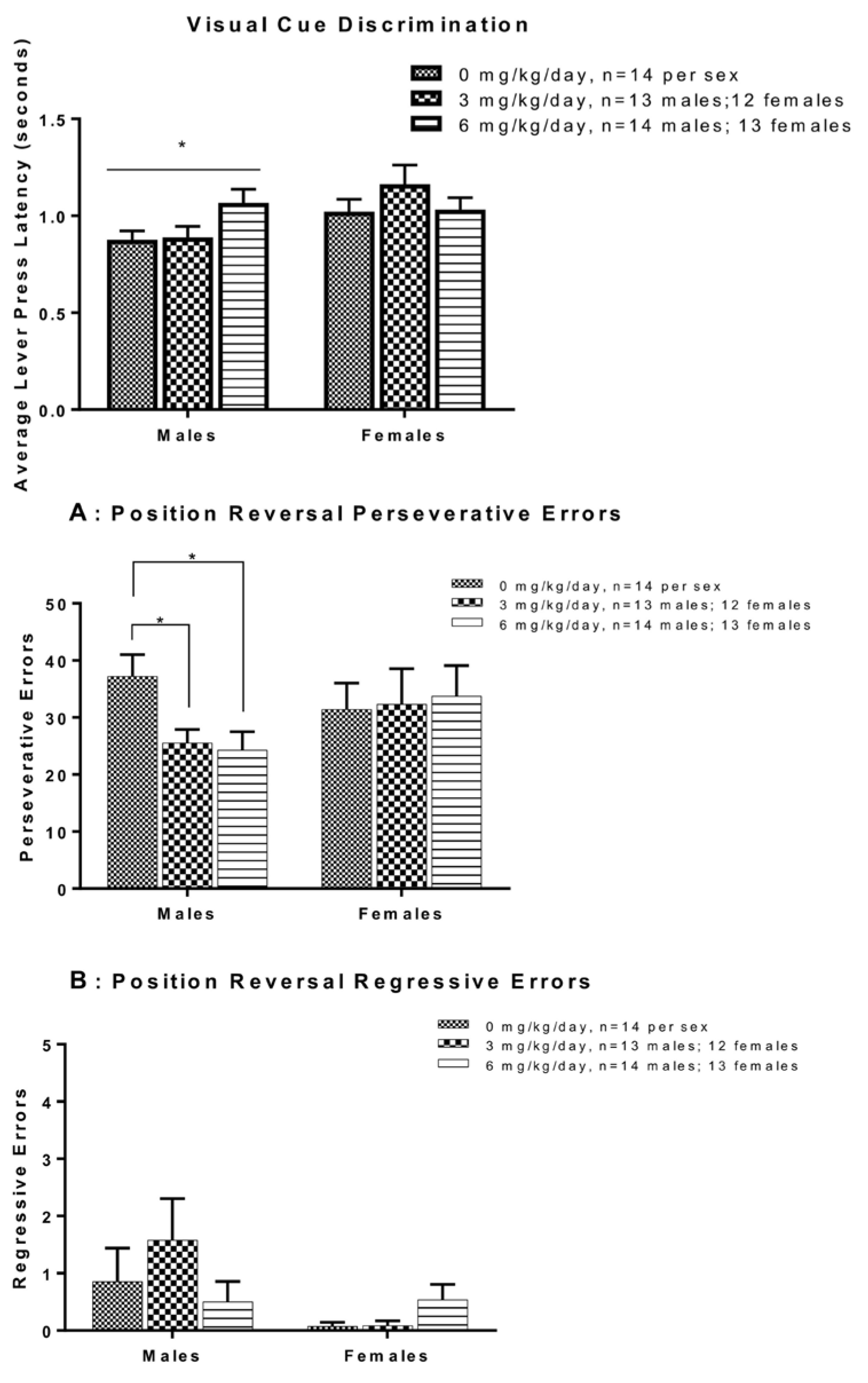
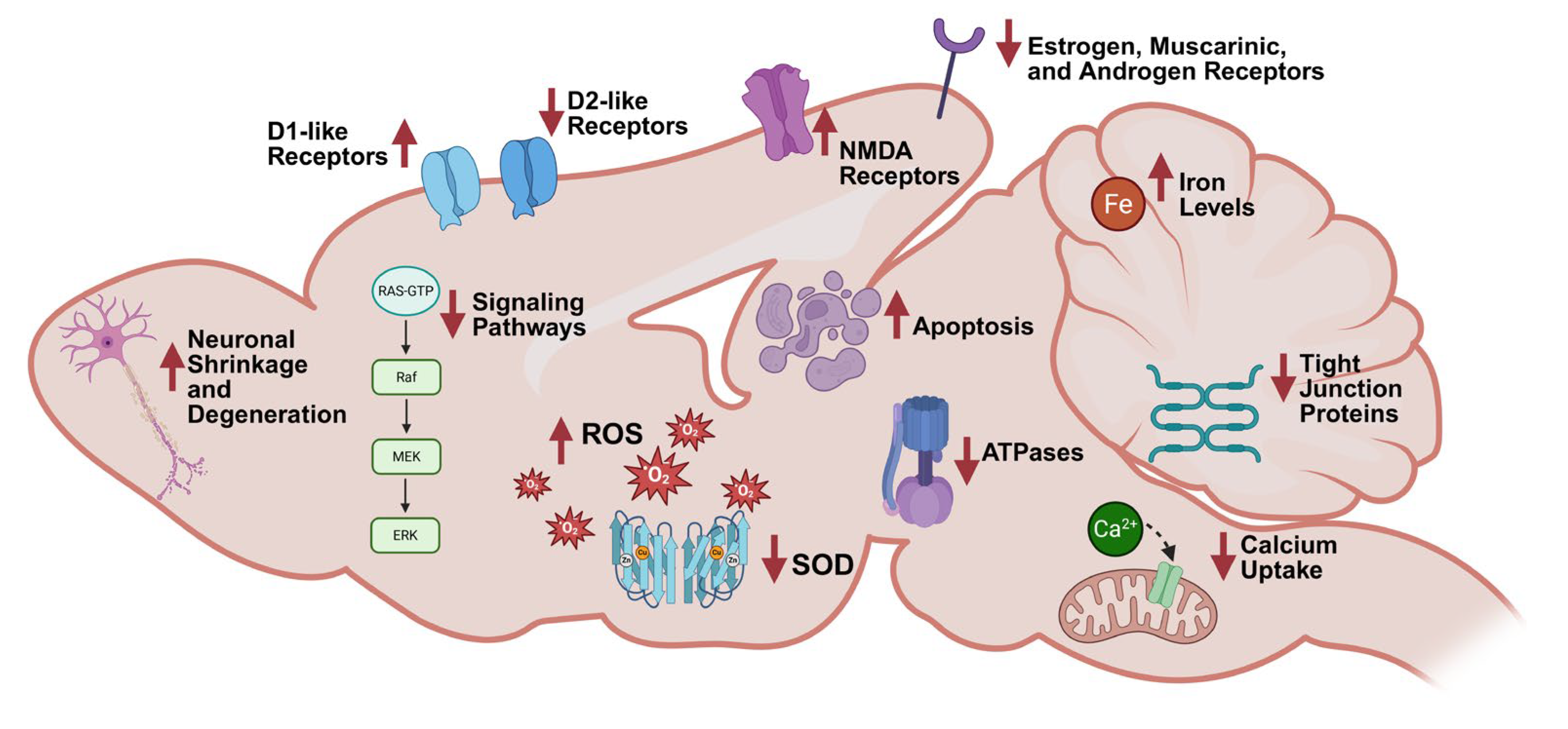
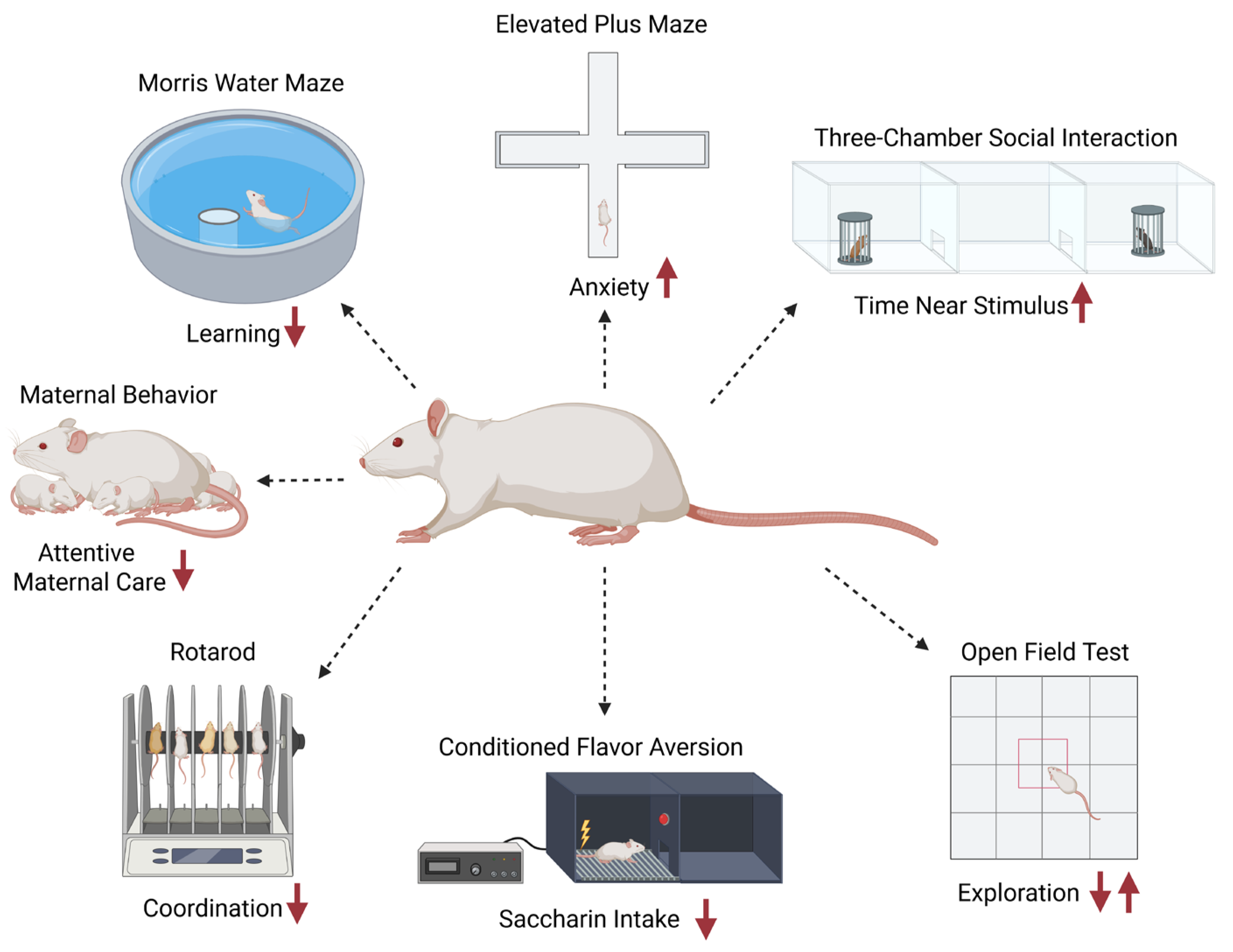
| ID | Question |
|---|---|
| 1 | What are polychlorinated biphenyls? |
| 2 | How are people exposed to PCBs? |
| 3 | How do PCBs impact health? |
| 4 | How are PCBs neurotoxic? |
| 5 | How do PCBs impact behavior? |
| 6 | How do PCBs impact neurobehavior? |
| 7 | How are PCBs studied in mice and rats? |
| Author/Title/Year | Mouse/Rat/Human/In Vitro Related | PCB or Related Chemicals | Neurotoxic Outcome Listed | Not Related | Explanation |
|---|---|---|---|---|---|
| Nilsson, C. B. et al. (2000). “2,3,7,8-tetrachlorodibenzo-p-dioxin increases serum and kidney retinoic acid levels and kidney retinol esterification in the rat.” | X | X | Involves rats and TCDD, a related chemical to PCBs. | ||
| Nilsson, E. et al. (2012). “Environmentally induced epigenetic transgenerational inheritance of ovarian disease.” | X | X | Involves rats and various environmental chemicals, including dioxins. | ||
| Ning, S. and X. Xiaobai (1997). “Reductive metabolism of 4-nitrobiphenyl by rat liver fraction.” | X | Involves rats but not related to PCBs or neurotoxic outcomes. | |||
| Ninou, I. et al. (2018). “Pharmacologic targeting of the ATX/LPA axis attenuates bleomycin-induced pulmonary fibrosis.” | X | Not related to PCBs or neurotoxic outcomes. | |||
| Nishida, N. et al. (1997). “Effects of acute and repeated exposures to Aroclor 1254 in adult rats: motor activity and flavor aversion conditioning.” | X | X | X | Involves rats, Aroclor 1254 (a type of PCB), and studies neurotoxic outcomes. |
| ID | Question |
|---|---|
| 1. PCBs | |
| 1-1 | Does the paper describe the use of PCBs? Must only be PCB congeners, not related substances. If the answer is no, do not proceed any further with the assessment. |
| 2. Mouse/Rat | |
| 2-1 | Does the paper describe the use of mice or rats in the study? |
| 2-2 | Are mice or rats directly exposed to PCBs? |
| 2-3 | Are cell cultures from mice or rats exposed to PCBs? |
| 2-4 | Are tissues collected from mice or rats exposed to PCBs? |
| 3. Exposure Timeframe | |
| 3-1 | Does the article describe dosing PCBs during gestation? |
| 3-2 | Does the article describe dosing PCBs during lactation? |
| 3-3 | Does the article describe dosing PCBs to the mouse/rat before postnatal day 21? |
| 3-4 | Does the article mention dosing after weaning/post-natal day 21? |
| 3-5 | If applicable, were maternal/dam tissues collected or maternal analyses done? |
| 4. Neuro-Outcomes | |
| 4-1 | Does the paper describe analyses related to neurobehavior or neurotoxicity? |
| 4-2 | Does the paper describe behavioral analysis? |
| 4-3 | Does the paper describe gene analysis? |
| 4-4 | Does the paper describe receptor changes? |
| Author/Title/Year | Sub-Part | Yes/No | Explanation |
|---|---|---|---|
| Coburn, C. G. et al., 2015. Permanently compromised NADPH-diaphorase activity within the osmotically activated supraoptic nucleus after in utero but not adult exposure to Aroclor 1254. Neurotoxicology, 47, 37–46. | 1-1 | Yes | The paper describes the use of Aroclor 1254, a commercial PCB mixture. |
| 2-1 | Yes | The study uses male Sprague-Dawley rats. | |
| 2-2 | Yes | Rats were exposed to Aroclor 1254 through cheese puff snacks infused with the PCB mixture. | |
| 2-3 | No | The study does not describe the use of cell cultures from mice or rats exposed to PCBs. | |
| 2-4 | Yes | Brain tissues were collected from rats exposed to Aroclor 1254 for histochemical analysis. | |
| 3-1 | Yes | In utero exposure was accomplished by feeding pregnant dams cheese puff snacks infused with Aroclor 1254 for 10 days during gestation (GD 10–19). | |
| 3-2 | No | The article does not describe dosing PCBs during lactation. | |
| 3-3 | Yes | Pups were exposed to PCBs in utero and allowed to suckle until weaning at PND 22. | |
| 3-4 | Yes | Adult rats were exposed to PCBs at 3–5 months old, and some were allowed to age until 14–16 months before sacrifice. | |
| 3-5 | No | The study does not mention collecting maternal/dam tissues or conducting maternal analyses. | |
| 4-1 | Yes | The paper describes the assessment of NADPH-diaphorase activity, which is related to neurotoxicity. | |
| 4-2 | No | The paper does not describe behavioral analysis. | |
| 4-3 | No | The paper does not describe gene analysis. | |
| 4-4 | No | The paper does not describe receptor changes. |
| Comparison | A | B | C | D | Agreement | Kappa | SE | 95% CI | Interpretation |
|---|---|---|---|---|---|---|---|---|---|
| Reviewer 1 & Reviewer 2 | 372 | 71 | 110 | 2711 | 94.45% | 0.772 | 0.02 | 0.740–0.804 | Substantial Agreement |
| Reviewer 1 & ChatGPT | 301 | 142 | 47 | 2774 | 94.21% | 0.729 | 0.02 | 0.692–0.765 | Substantial Agreement |
| Reviewer 2 & ChatGPT | 297 | 51 | 185 | 2731 | 92.77% | 0.675 | 0.02 | 0.637–0.714 | Substantial Agreement |
| Comparison | A | B | C | D | Agreement | Kappa | SE | 95% CI | Interpretation |
|---|---|---|---|---|---|---|---|---|---|
| Reviewer 1 & Reviewer 2 | 30 | 13 | 15 | 302 | 92.22% | 0.638 | 0.06 | 0.514–0.761 | Moderate- Substantial Agreement |
| Reviewer 1 & ChatGPT | 37 | 6 | 15 | 302 | 94.17% | 0.746 | 0.05 | 0.642–0.849 | Moderate- Almost Perfect Agreement |
| Reviewer 2 & ChatGPT | 29 | 23 | 16 | 292 | 89.17% | 0.536 | 0.07 | 0.407–0.664 | Fair-Moderate Agreement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breese, N.M.; Heim, S.G.; Samuelson, R.J.; Lehmler, H.-J. A Scoping Review of Neurotoxic and Behavioral Outcomes Following Polychlorinated Biphenyl (PCB) Exposure in Post-Weaned Rodents. Int. J. Mol. Sci. 2025, 26, 10829. https://doi.org/10.3390/ijms262210829
Breese NM, Heim SG, Samuelson RJ, Lehmler H-J. A Scoping Review of Neurotoxic and Behavioral Outcomes Following Polychlorinated Biphenyl (PCB) Exposure in Post-Weaned Rodents. International Journal of Molecular Sciences. 2025; 26(22):10829. https://doi.org/10.3390/ijms262210829
Chicago/Turabian StyleBreese, Nicole M., Sophia G. Heim, Riley J. Samuelson, and Hans-Joachim Lehmler. 2025. "A Scoping Review of Neurotoxic and Behavioral Outcomes Following Polychlorinated Biphenyl (PCB) Exposure in Post-Weaned Rodents" International Journal of Molecular Sciences 26, no. 22: 10829. https://doi.org/10.3390/ijms262210829
APA StyleBreese, N. M., Heim, S. G., Samuelson, R. J., & Lehmler, H.-J. (2025). A Scoping Review of Neurotoxic and Behavioral Outcomes Following Polychlorinated Biphenyl (PCB) Exposure in Post-Weaned Rodents. International Journal of Molecular Sciences, 26(22), 10829. https://doi.org/10.3390/ijms262210829








