Sialylation Inhibition Impairs Migration and Promotes Adhesion of GBM Cells
Abstract
1. Introduction
2. Results
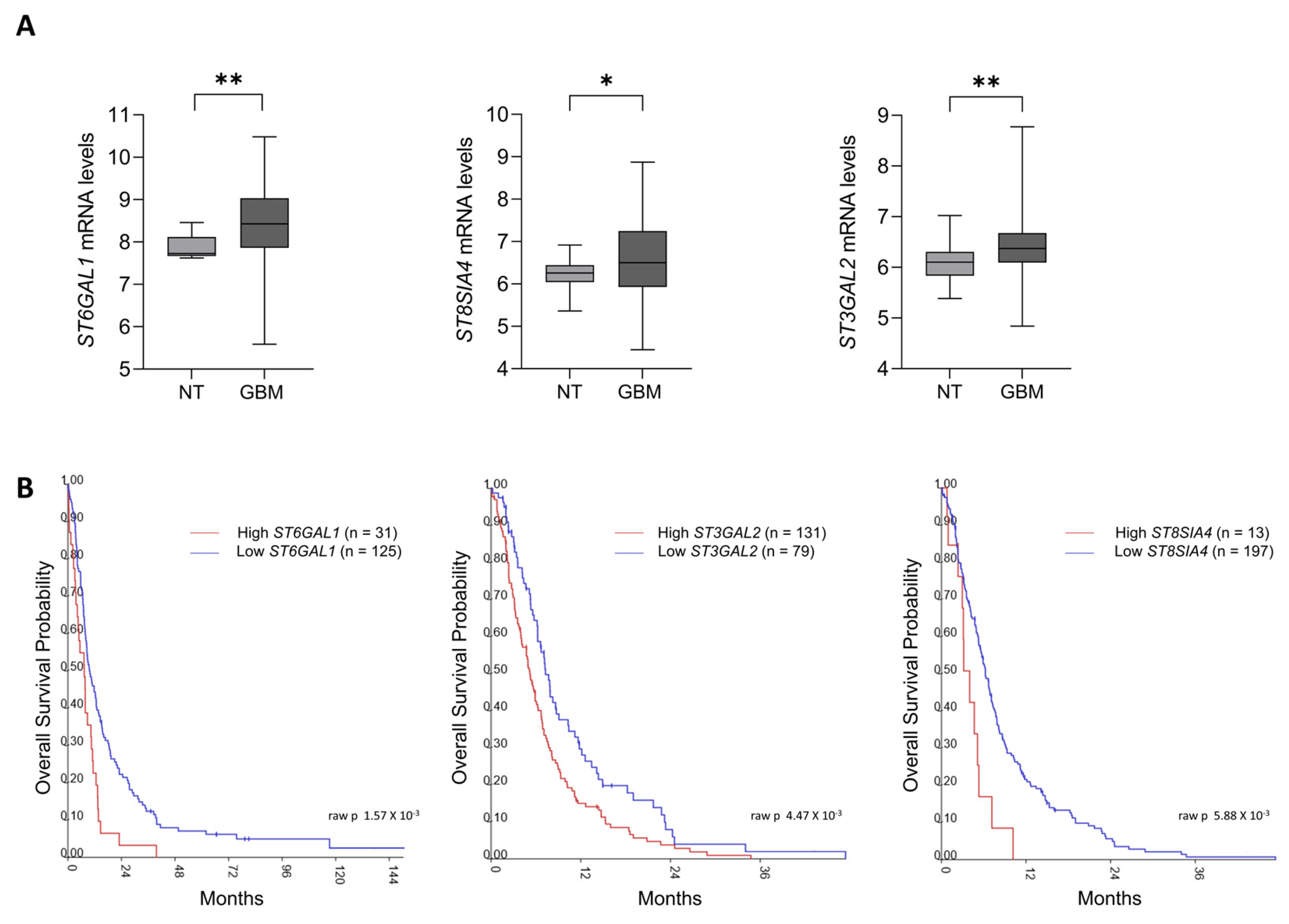
2.1. Sialyltransferases Up-Regulation Correlates with Poor Survival in GBM Patients
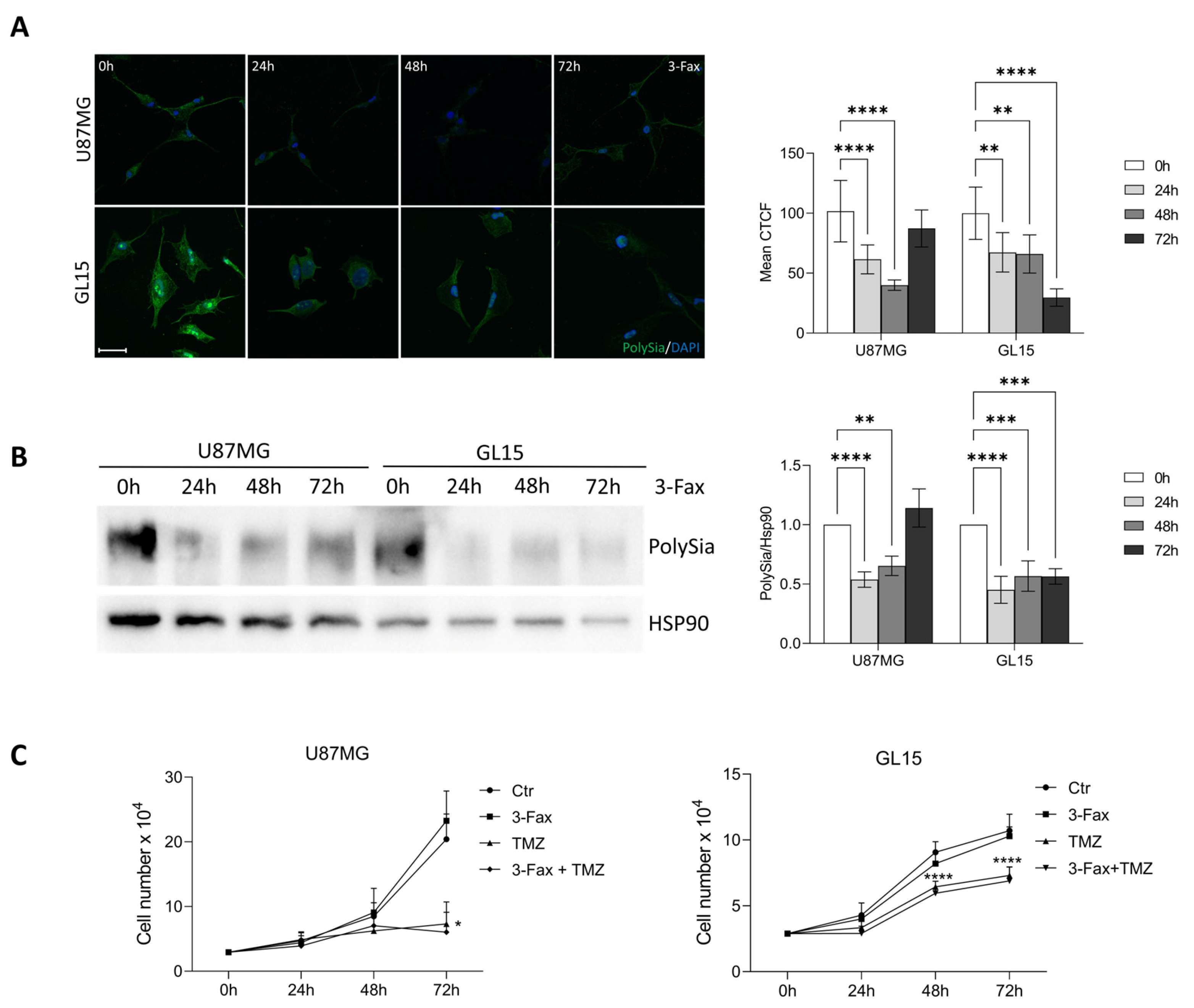
2.2. GBM Cell Lines Express Differential Levels of Sialyltransferases and Sias
2.3. Sialylation Inhibition Doesn’t Affect GBM Cell Proliferation and TMZ Sensitivity
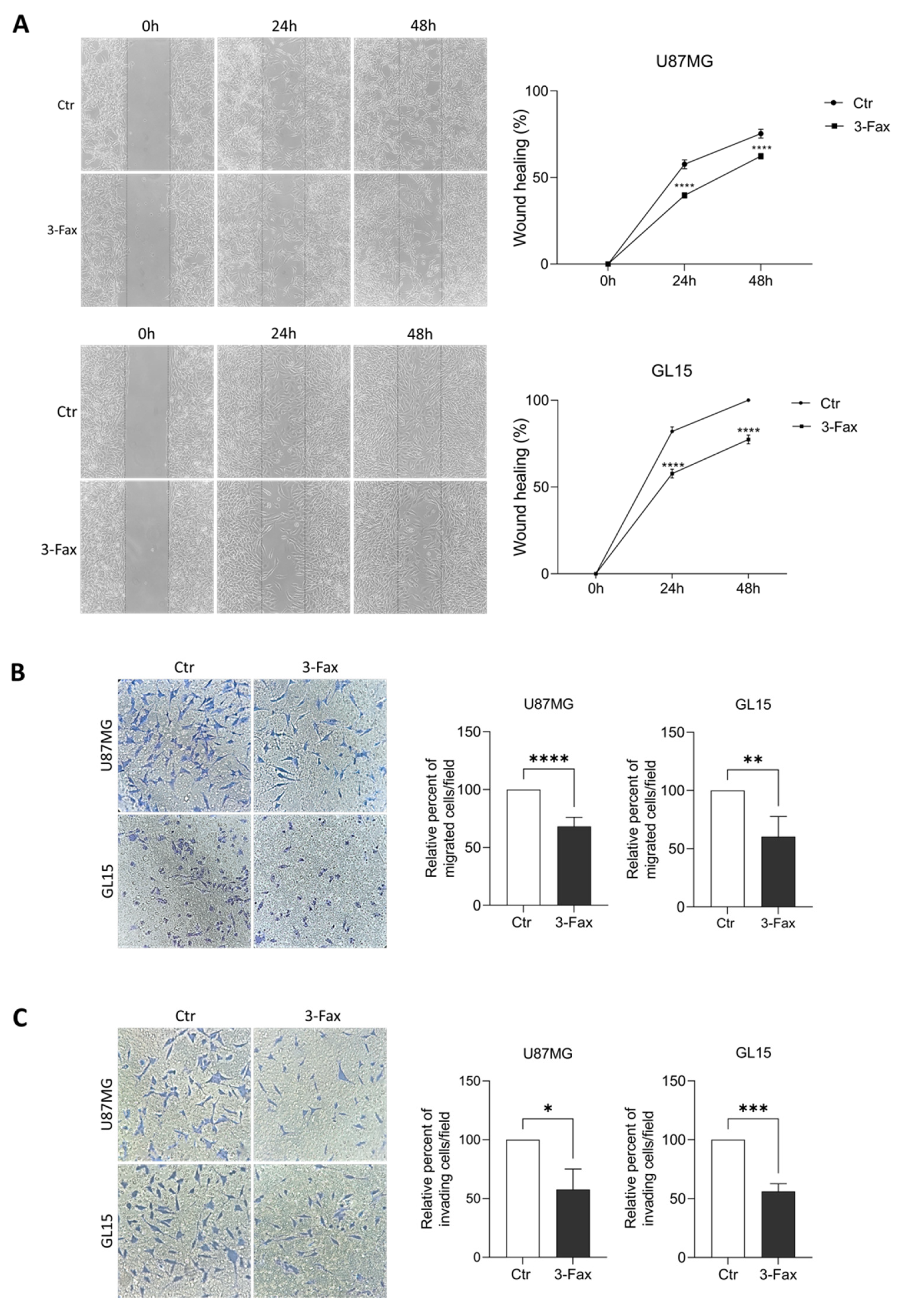
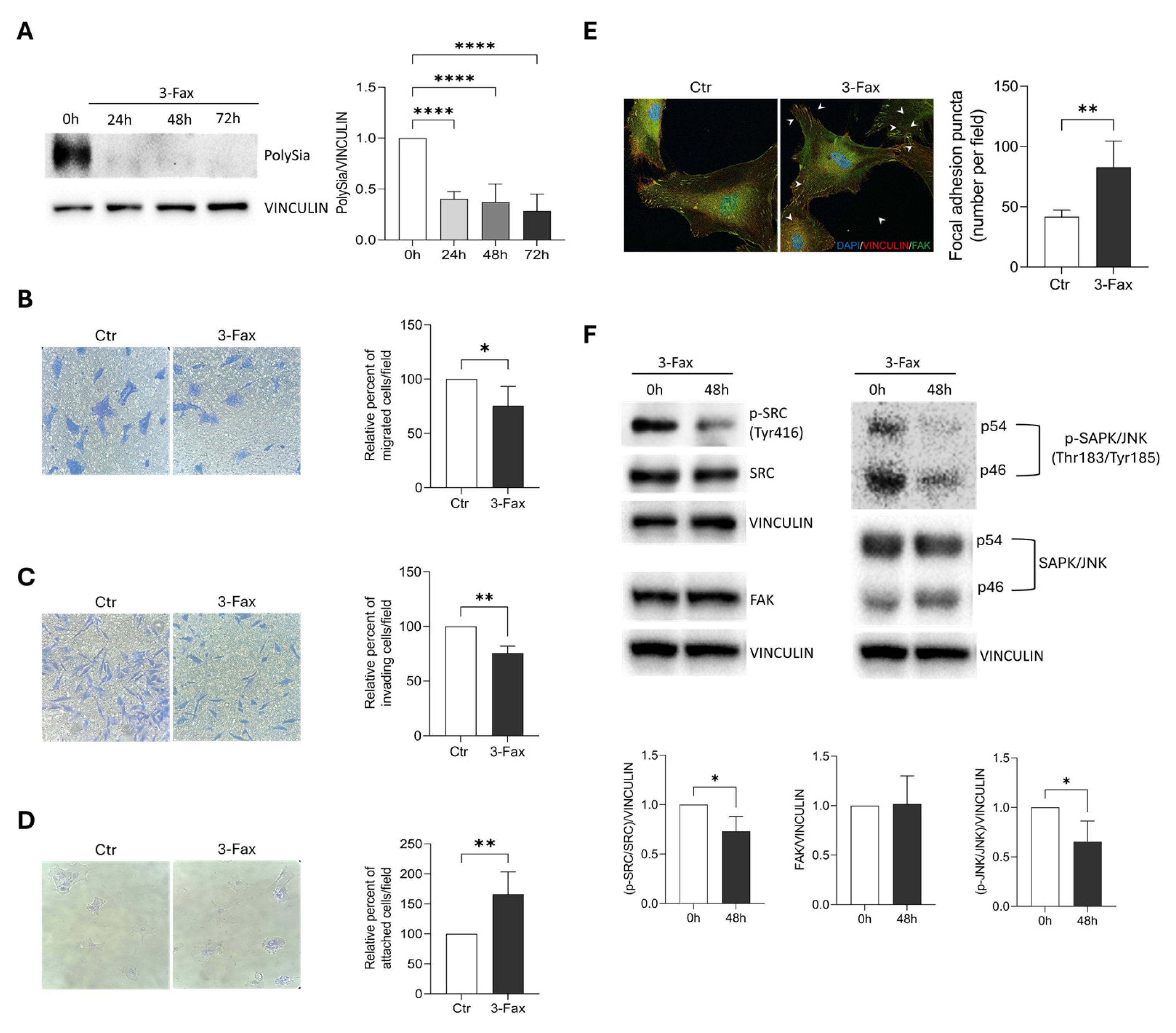
2.4. Sialylation Inhibition Affects Migration and Invasion Ability of GBM Cells
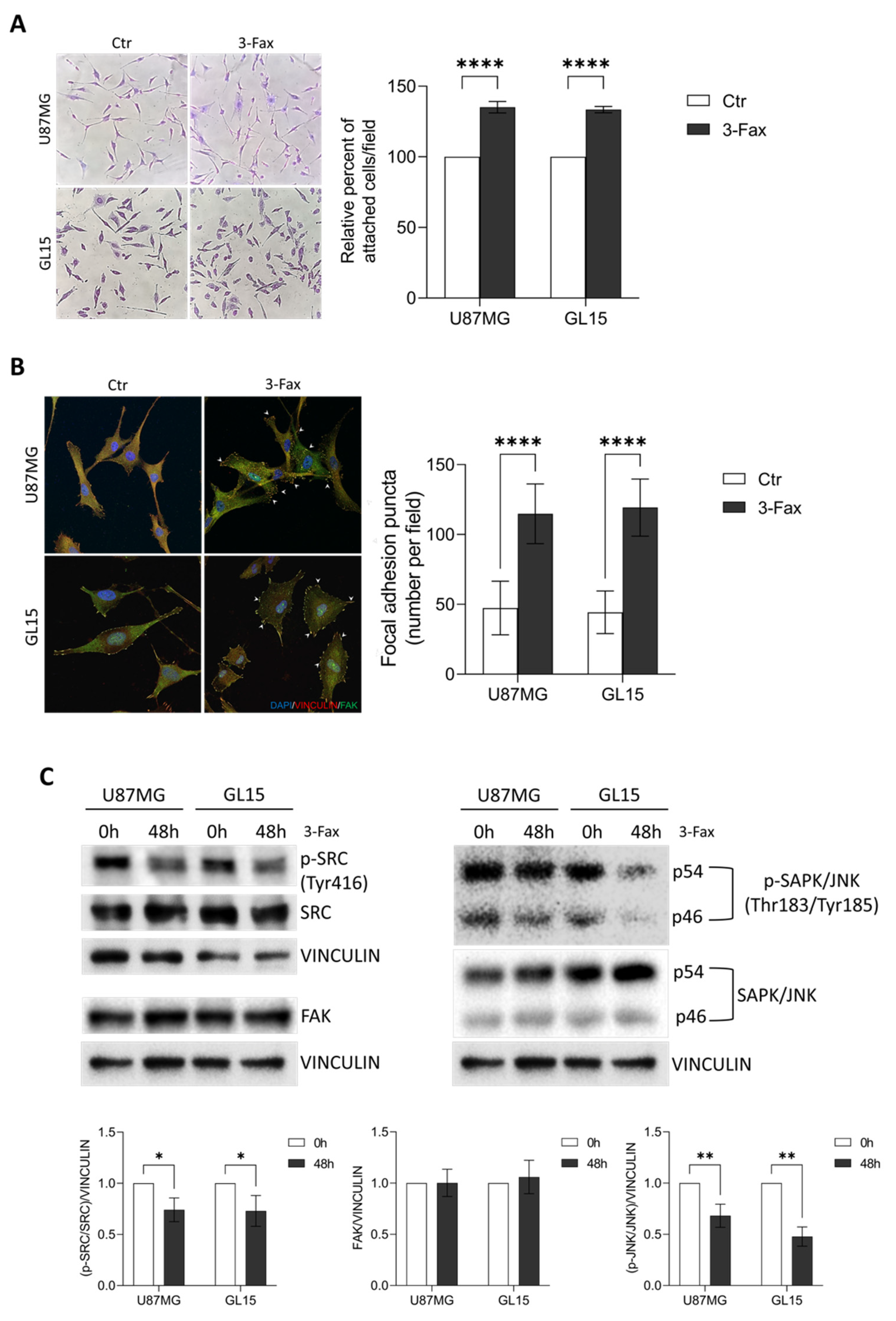
2.5. Sialylation Inhibition Enhances Cell-Matrix Adhesion in GBM Cells
2.6. Sialylation Inhibition Affects Migration and Invasion Properties of Primary GBM Cells
3. Discussion
4. Materials and Methods
4.1. Analysis of Publicly Available Patients’s Transcriptomic Data
4.2. RNA Isolation and Quantitative RT-PCR
4.3. Cell Culture and Treatments
4.4. Lectin Cytochemistry
4.5. Immunocytochemistry
4.6. Proliferation Assay
4.7. Wound Healing Assay
4.8. Transwell Migration and Invasion Assay
4.9. Cell Adhesion Assay
4.10. Western Blotting and Antibodies
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, M.; Suzuki, M.; Nakayama, J.; Suzuki, A.; Angata, K.; Chen, S.; Sakai, K.; Hagihara, K.; Yamaguchi, Y.; Fukuda, M. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology 2005, 15, 887–894. [Google Scholar] [CrossRef]
- Putthisen, S.; Silsirivanit, A.; Panawan, O.; Niibori-Nambu, A.; Nishiyama-Ikeda, Y.; Ma-In, P.; Luang, S.; Ohta, K.; Muisuk, K.; Wongkham, S.; et al. Targeting alpha2,3-sialylated glycan in glioma stem-like cells by Maackia amurensis lectin-II: A promising strategy for glioma treatment. Exp. Cell Res. 2022, 410, 112949. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.; Scibetta, S.; Pepe, G.; Mangino, G.; Capocci, L.; Moons, S.J.; Boltje, T.J.; Fazi, F.; Petrozza, V.; Di Pardo, A.; et al. Polysialic Acid Sustains the Hypoxia-Induced Migration and Undifferentiated State of Human Glioblastoma Cells. Int. J. Mol. Sci. 2022, 23, 9563. [Google Scholar] [CrossRef] [PubMed]
- Schildhauer, P.; Selke, P.; Scheller, C.; Strauss, C.; Horstkorte, R.; Leisz, S.; Scheer, M. Glycation Leads to Increased Invasion of Glioblastoma Cells. Cells 2023, 12, 1219. [Google Scholar] [CrossRef]
- Smith, H.L.; Wadhwani, N.; Horbinski, C. Major Features of the 2021 WHO Classification of CNS Tumors. Neurotherapeutics 2022, 19, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Djuzenova, C.S.; Fiedler, V.; Memmel, S.; Katzer, A.; Hartmann, S.; Krohne, G.; Zimmermann, H.; Scholz, C.J.; Polat, B.; Flentje, M.; et al. Actin cytoskeleton organization, cell surface modification and invasion rate of 5 glioblastoma cell lines differing in PTEN and p53 status. Exp. Cell Res. 2015, 330, 346–357. [Google Scholar] [CrossRef]
- GC, S.; Tuy, K.; Rickenbacker, L.; Jones, R.; Chakraborty, A.; Miller, C.R.; Beierle, E.A.; Hanumanthu, V.S.; Tran, A.N.; Mobley, J.A.; et al. α2,6 Sialylation mediated by ST6GAL1 promotes glioblastoma growth. JCI Insight 2022, 7, e158799. [Google Scholar] [CrossRef]
- Schildhauer, P.; Selke, P.; Staege, M.S.; Harder, A.; Scheller, C.; Strauss, C.; Horstkorte, R.; Scheer, M.; Leisz, S. Glycation Interferes with the Expression of Sialyltransferases and Leads to Increased Polysialylation in Glioblastoma Cells. Cells 2023, 12, 2758. [Google Scholar] [CrossRef]
- Avci, N.G.; Ebrahimzadeh-Pustchi, S.; Akay, Y.M.; Esquenazi, Y.; Tandon, N.; Zhu, J.J.; Akay, M. NF-κB inhibitor with Temozolomide results in significant apoptosis in glioblastoma via the NF-κB (p65) and actin cytoskeleton regulatory pathways. Sci. Rep. 2020, 10, 13352. [Google Scholar] [CrossRef]
- Mathew, E.N.; Berry, B.C.; Yang, H.W.; Carroll, R.S.; Johnson, M.D. Delivering Therapeutics to Glioblastoma: Overcoming Biological Constraints. Int. J. Mol. Sci. 2022, 23, 1711. [Google Scholar] [CrossRef]
- Silsirivanit, A. Glycan and Glycosylation as a Target for Treatment of Glioblastoma. In Glioblastoma-Current Evidence; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Munkley, J. Aberrant Sialylation in Cancer: Therapeutic Opportunities. Cancers 2022, 14, 4248. [Google Scholar] [CrossRef]
- Gnanapragassam, V.S.; Bork, K.; Galuska, C.E.; Galuska, S.P.; Glanz, D.; Nagasundaram, M.; Bache, M.; Vordermark, D.; Kohla, G.; Kannicht, C.; et al. Sialic Acid Metabolic Engineering: A Potential Strategy for the Neuroblastoma Therapy. PLoS ONE 2014, 9, e105403. [Google Scholar] [CrossRef]
- Whited, J.; Zhang, X.; Nie, H.; Wang, D.; Li, Y.; Sun, X.L. Recent Chemical Biology Approaches for Profiling Cell Surface Sialylation Status. ACS Chem. Biol. 2018, 13, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009, 19, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Schnaar, R.L.; Schauer, R. Sialic Acids and Other Nonulosonic Acids. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017; pp. 179–195. [Google Scholar]
- Varki, A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 2007, 446, 1023–1029. [Google Scholar] [CrossRef]
- Schauer, R. Sialic acids as link to Japanese scientists. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2016, 92, 109–120. [Google Scholar] [CrossRef]
- Marini, M.; Ambrosini, S.; Sarchielli, E.; Thyrion, G.D.Z.; Bonaccini, L.; Vannelli, G.B.; Sgambati, E. Expression of sialic acids in human adult skeletal muscle tissue. Acta Histochem. 2014, 116, 926–935. [Google Scholar] [CrossRef]
- Marini, M.; Sarchielli, E.; Zappoli Thyrion, G.D.; Ambrosini, S.; Sgambati, E. Sialic acid expression in human fetal skeletal muscle during limb early myogenesis. Histol. Histopathol. 2017, 32, 1207–1221. [Google Scholar] [CrossRef]
- Li, F.; Ding, J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell 2019, 10, 550–565. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.H.; Stanley, P.; Bertozzi, C.R.; Hart, G.W.; Etzler, M.E. (Eds.) Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009; ISBN 9780879697709. [Google Scholar]
- Schauer, R.; Kamerling, J.P. Exploration of the Sialic Acid World. Adv. Carbohydr. Chem. Biochem. 2018, 75, 1–213. [Google Scholar] [CrossRef]
- Manetti, M.; Marini, M.; Perna, A.; Tani, A.; Sgambati, E. Sialylation status and its relationship with morphofunctional changes in human adult testis during sexually mature life and aging: A narrative review. Acta Histochem. 2024, 126, 152143. [Google Scholar] [CrossRef] [PubMed]
- Putthisen, S.; Panawan, O.; Luang, S.; Araki, N.; Wongkham, S.; Silsirivanit, A. Suppression of sialylation increases sensitivity of glioblastoma cells to cisplatin and 5-fluorouracil. In Proceedings of the 7th International Conference on Biochemistry and Molecular Biology, Online, 6–7 July 2021. [Google Scholar]
- Schultz, M.J.; Swindall, A.F.; Bellis, S.L. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 2012, 31, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Büll, C.; den Brok, M.H.; Adema, G.J. Sweet escape: Sialic acids in tumor immune evasion. Biochim. Biophys. Acta Rev. Cancer 2014, 1846, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Büll, C.; Stoel, M.A.; Den Brok, M.H.; Adema, G.J. Sialic acids sweeten a tumor’s life. Cancer Res. 2014, 74, 3199–3204. [Google Scholar] [CrossRef]
- Inagaki, Y.; Gao, J.; Song, P.; Kokudo, N.; Nakata, M.; Tang, W. Clinicopathological utility of sialoglycoconjugates in diagnosing and treating colorectal cancer. World J. Gastroenterol. 2014, 20, 6123–6132. [Google Scholar] [CrossRef]
- Seifert, A.; Glanz, D.; Glaubitz, N.; Horstkorte, R.; Bork, K. Polysialylation of the neural cell adhesion molecule: Interfering with polysialylation and migration in neuroblastoma cells. Arch. Biochem. Biophys. 2012, 524, 56–63. [Google Scholar] [CrossRef]
- Ghosh, S. Sialic acids: Biomarkers in endocrinal cancers. Glycoconj. J. 2015, 32, 79–85. [Google Scholar] [CrossRef]
- Pearce, O.M.T.; Läubli, H. Sialic acids in cancer biology and immunity. Glycobiology 2015, 26, 111–128. [Google Scholar] [CrossRef]
- Vajaria, B.N.; Patel, K.R.; Begum, R.; Patel, P.S. Sialylation: An Avenue to Target Cancer Cells. Pathol. Oncol. Res. 2016, 22, 443–447. [Google Scholar] [CrossRef]
- Lübbers, J.; Rodríguez, E.; van Kooyk, Y. Modulation of Immune Tolerance via Siglec-Sialic Acid Interactions. Front. Immunol. 2018, 9, 2807. [Google Scholar] [CrossRef]
- Rodrigues, E.; Macauley, M.S. Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities. Cancers 2018, 10, 207. [Google Scholar] [CrossRef]
- Marini, M.; Tani, A.; Manetti, M.; Sgambati, E. Characterization and distribution of sialic acids in human testicular seminoma. Acta Histochem. 2020, 122, 151532. [Google Scholar] [CrossRef]
- Sgambati, E.; Tani, A.; Leri, M.; Delfino, G.; Zecchi-Orlandini, S.; Bucciantini, M.; Nosi, D. Correlation between Sialylation Status and Cell Susceptibility to Amyloid Toxicity. Cells 2022, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Beyer, S.; Kimani, M.; Zhang, Y.; Verhassel, A.; Sternbæk, L.; Wang, T.; Persson, J.L.; Härkönen, P.; Johansson, E.; Caraballo, R.; et al. Fluorescent Molecularly Imprinted Polymer Layers against Sialic Acid on Silica-Coated Polystyrene Cores-Assessment of the Binding Behavior to Cancer Cells. Cancers 2022, 14, 1875. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.J.L.P.; Fu, C.W.; Li, W.S. Sialyltransferase Inhibitors for the Treatment of Cancer Metastasis: Current Challenges and Future Perspectives. Molecules 2021, 26, 5673. [Google Scholar] [CrossRef]
- Dawson, G.; Moskal, J.R.; Dawson, S.A. Transfection of 2,6 and 2,3-Sialyltransferase genes and GlcNAc-transferase genes into human glioma cell line U-373 MG affects glycoconjugate expression and enhances cell death. J. Neurochem. 2004, 89, 1436–1444. [Google Scholar] [CrossRef]
- Fioretto, B.S.; Rosa, I.; Tani, A.; Andreucci, E.; Romano, E.; Sgambati, E.; Manetti, M. Blockade of Sialylation with Decrease in Polysialic Acid Levels Counteracts Transforming Growth Factor β1-Induced Skin Fibroblast-to-Myofibroblast Transition. Cells 2024, 13, 1067. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Lobo, A.; Li, P.F.; Zhang, Y.F. Sialylated glycoproteins and sialyltransferases in digestive cancers: Mechanisms, diagnostic biomarkers, and therapeutic targets. Crit. Rev. Oncol. Hematol. 2024, 197, 104330. [Google Scholar] [CrossRef]
- Macauley, M.S.; Arlian, B.M.; Rillahan, C.D.; Pang, P.C.; Bortell, N.; Marcondes, M.C.G.; Haslam, S.M.; Dell, A.; Paulson, J.C. Systemic blockade of sialylation in mice with a global inhibitor of sialyltransferases. J. Biol. Chem. 2014, 289, 35149–35158. [Google Scholar] [CrossRef]
- Rillahan, C.D.; Antonopoulos, A.; Lefort, C.T.; Sonon, R.; Azadi, P.; Ley, K.; Dell, A.; Haslam, S.M.; Paulson, J.C. Global metabolic inhibitors of sialyl-and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 2012, 8, 661–668. [Google Scholar] [CrossRef]
- Koster, J.; Volckmann, R.; Zwijnenburg, D.; Molenaar, P.; Versteeg, R. Abstract 2490: R2: Genomics Analysis and Visualization Platform. Cancer Res. 2019, 79, 2490. [Google Scholar] [CrossRef]
- Gravendeel, L.A.M.; Kouwenhoven, M.C.M.; Gevaert, O.; de Rooi, J.J.; Stubbs, A.P.; Duijm, J.E.; Daemen, A.; Bleeker, F.E.; Bralten, L.B.C.; Kloosterhof, N.K.; et al. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res. 2009, 69, 9065–9072. [Google Scholar] [CrossRef]
- Gusev, Y.; Bhuvaneshwar, K.; Song, L.; Zenklusen, J.C.; Fine, H.; Madhavan, S. The REMBRANDT study, a large collection of genomic data from brain cancer patients. Sci. Data 2018, 5, 180158. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, A.J.; Ruff, E.M.; Martindale, C.; Lovegrove, N.; Short, S.C. Cytotoxic effects of temozolomide and radiation are additive- and schedule-dependent. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Van Slambrouck, S.; Grijelmo, C.; De Wever, O.; Bruyneel, E.; Emami, S.; Gespach, C.; Steelant, W.F.A. Activation of the FAK-Src molecular scaffolds and P130Cas-JNK signaling cascades by alpha1-integrins during colon cancer cell invasion. Int. J. Oncol. 2007, 31, 1501–1508. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.Y.; Tang, Y.A.; Huang, S.M.; Juan, H.F.; Wu, L.W.; Sun, Y.C.; Wang, S.C.; Wu, K.W.; Balraj, G.; Chang, T.T.; et al. A novel sialyltransferase inhibitor suppresses FAK/paxillin signaling and cancer angiogenesis and metastasis pathways. Cancer Res. 2011, 71, 473–483. [Google Scholar] [CrossRef]
- Uddin, M.S.; Al Mamun, A.; Alghamdi, B.S.; Tewari, D.; Jeandet, P.; Sarwar, M.S.; Ashraf, G.M. Epigenetics of glioblastoma multiforme: From molecular mechanisms to therapeutic approaches. Semin. Cancer Biol. 2022, 83, 100–120. [Google Scholar] [CrossRef]
- Chong, Y.K.; Sandanaraj, E.; Koh, L.W.H.; Thangaveloo, M.; Tan, M.S.Y.; Koh, G.R.H.; Toh, T.B.; Lim, G.G.Y.; Holbrook, J.D.; Kon, O.L.; et al. ST3GAL1-Associated Transcriptomic Program in Glioblastoma Tumor Growth, Invasion, and Prognosis. J. Natl. Cancer Inst. 2016, 108, djv326. [Google Scholar] [CrossRef]
- Scibetta, S.; Pepe, G.; Iuliano, M.; Iaiza, A.; Palazzo, E.; Quadri, M.; Boltje, T.J.; Fazi, F.; Petrozza, V.; Di Bartolomeo, S.; et al. Polysialylation of Glioblastoma Cells Is Regulated by Autophagy Under Nutrient Deprivation. Int. J. Mol. Sci. 2025, 26, 7625. [Google Scholar] [CrossRef]
- Büll, C.; Boltje, T.J.; Wassink, M.; de Graaf, A.M.A.; van Delft, F.L.; den Brok, M.H.; Adema, G.J. Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Mol. Cancer Ther. 2013, 12, 1935–1946. [Google Scholar] [CrossRef]
- Natoni, A.; Farrell, M.L.; Harris, S.; Falank, C.; Kirkham-McCarthy, L.; Macauley, M.S.; Reagan, M.R.; O’Dwyer, M. Sialyltransferase inhibition leads to inhibition of tumor cell interactions with E-selectin, VCAM1, and MADCAM1, and improves survival in a human multiple myeloma mouse model. Haematologica 2020, 105, 457–467. [Google Scholar] [CrossRef]
- Hou, S.; Hang, Q.; Isaji, T.; Lu, J.; Fukuda, T.; Gu, J. Importance of membrane-proximal N-glycosylation on integrin β1 in its activation and complex formation. FASEB J. 2016, 30, 4120–4131. [Google Scholar] [CrossRef]
- Seales, E.C.; Jurado, G.A.; Brunson, B.A.; Wakefield, J.K.; Frost, A.R.; Bellis, S.L. Hypersialylation of β1 Integrins, Observed in Colon Adenocarcinoma, May Contribute to Cancer Progression by Up-Regulating Cell Motility. Cancer Res. 2005, 65, 4645–4652. [Google Scholar] [CrossRef]
- Holdbrooks, A.T.; Britain, C.M.; Bellis, S.L. ST6Gal-I sialyltransferase promotes tumor necrosis factor (TNF)-mediated cancer cell survival via sialylation of the TNF receptor 1 (TNFR1) death receptor. J. Biol. Chem. 2018, 293, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Swindall, A.F.; Bellis, S.L. Sialylation of the Fas death receptor by St6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J. Biol. Chem. 2011, 286, 22982–22990. [Google Scholar] [CrossRef] [PubMed]
- Britain, C.M.; Holdbrooks, A.T.; Anderson, J.C.; Willey, C.D.; Bellis, S.L. Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. J. Ovarian Res. 2018, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Britain, C.M.; Bhalerao, N.; Silva, A.D.; Chakraborty, A.; Buchsbaum, D.J.; Crowley, M.R.; Crossman, D.K.; Edwards, Y.J.K.; Bellis, S.L. Glycosyltransferase ST6Gal-I promotes the epithelial to mesenchymal transition in pancreatic cancer cells. J. Biol. Chem. 2021, 296, 100034. [Google Scholar] [CrossRef]
- Ankenbauer, K.E.; Rao, T.C.; Mattheyses, A.L.; Bellis, S.L. Sialylation of EGFR by ST6GAL1 induces receptor activation and modulates trafficking dynamics. J. Biol. Chem. 2023, 299, 105217. [Google Scholar] [CrossRef]
- Rao, T.C.; Beggs, R.R.; Ankenbauer, K.E.; Hwang, J.; Pui-Yan Ma, V.; Salaita, K.; Bellis, S.L.; Mattheyses, A.L. ST6Gal-I-mediated sialylation of the epidermal growth factor receptor modulates cell mechanics and enhances invasion. J. Biol. Chem. 2022, 298, 101726. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, M.; Linhai, Y.; Song, Y.; Gui, X.; Tan, G.; Li, J.; Liu, Y.; Deng, Z.; Chen, X.; et al. Increasing HER2 α2,6 sialylation facilitates gastric cancer progression and resistance via the Akt and ERK pathways. Oncol. Rep. 2018, 40, 2997–3005. [Google Scholar] [CrossRef]
- Ray, P.; Tan, Y.S.; Somnay, V.; Mehta, R.; Sitto, M.; Ahsan, A.; Nyati, S.; Naughton, J.P.; Bridges, A.; Zhao, L.; et al. Differential protein stability of EGFR mutants determines responsiveness to tyrosine kinase inhibitors. Oncotarget 2016, 7, 68597–68613. [Google Scholar] [CrossRef] [PubMed]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance1. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Colardo, M.; Segatto, M.; Di Bartolomeo, S. Targeting RTK-PI3K-MTOR Axis in Gliomas: An Update. Int. J. Mol. Sci. 2021, 22, 4899. [Google Scholar] [CrossRef] [PubMed]
- Colella, B.; Colardo, M.; Iannone, G.; Contadini, C.; Saiz-Ladera, C.; Fuoco, C.; Barilà, D.; Velasco, G.; Segatto, M.; Di Bartolomeo, S. mTOR Inhibition Leads to Src-Mediated EGFR Internalisation and Degradation in Glioma Cells. Cancers 2020, 12, 2266. [Google Scholar] [CrossRef]
- Burridge, K. Focal adhesions: A personal perspective on a half century of progress. FEBS J. 2017, 284, 3355–3361. [Google Scholar] [CrossRef]
- Horton, E.R.; Byron, A.; Askari, J.A.; Ng, D.H.J.; Millon-Frémillon, A.; Robertson, J.; Koper, E.J.; Paul, N.R.; Warwood, S.; Knight, D.; et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat. Cell Biol. 2015, 17, 1577–1587. [Google Scholar] [CrossRef]
- Schiller, H.B.; Friedel, C.C.; Boulegue, C.; Fässler, R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 2011, 12, 259–266. [Google Scholar] [CrossRef]
- Zaidel-Bar, R.; Itzkovitz, S.; Ma’ayan, A.; Iyengar, R.; Geiger, B. Functional atlas of the integrin adhesome. Nat. Cell Biol. 2007, 9, 858–867. [Google Scholar] [CrossRef]
- Devi, S.S.; Yadav, R.; Arya, R. Altered Actin Dynamics in Cell Migration of GNE Mutant Cells. Front. Cell Dev. Biol. 2021, 9, 603742. [Google Scholar] [CrossRef]
- Pepe, G.; Capocci, L.; Marracino, F.; Realini, N.; Lenzi, P.; Martinello, K.; Bovier, T.F.; Bichell, T.J.; Scarselli, P.; Di Cicco, C.; et al. Treatment with THI, an inhibitor of sphingosine-1-phosphate lyase, modulates glycosphingolipid metabolism and results therapeutically effective in experimental models of Huntington’s disease. Mol. Ther. 2023, 31, 282–299. [Google Scholar] [CrossRef]
- Catalano, M.; D’Alessandro, G.; Lepore, F.; Corazzari, M.; Caldarola, S.; Valacca, C.; Faienza, F.; Esposito, V.; Limatola, C.; Cecconi, F.; et al. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Mol. Oncol. 2015, 9, 1612–1625. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargano, D.; Calvitto, M.; Niro, A.; Pepe, G.; Martella, N.; Tani, A.; Rosa, P.; Maglione, V.; Musci, G.; Cutone, A.; et al. Sialylation Inhibition Impairs Migration and Promotes Adhesion of GBM Cells. Int. J. Mol. Sci. 2025, 26, 10708. https://doi.org/10.3390/ijms262110708
Gargano D, Calvitto M, Niro A, Pepe G, Martella N, Tani A, Rosa P, Maglione V, Musci G, Cutone A, et al. Sialylation Inhibition Impairs Migration and Promotes Adhesion of GBM Cells. International Journal of Molecular Sciences. 2025; 26(21):10708. https://doi.org/10.3390/ijms262110708
Chicago/Turabian StyleGargano, Deborah, Mariangela Calvitto, Antonella Niro, Giuseppe Pepe, Noemi Martella, Alessia Tani, Paolo Rosa, Vittorio Maglione, Giovanni Musci, Antimo Cutone, and et al. 2025. "Sialylation Inhibition Impairs Migration and Promotes Adhesion of GBM Cells" International Journal of Molecular Sciences 26, no. 21: 10708. https://doi.org/10.3390/ijms262110708
APA StyleGargano, D., Calvitto, M., Niro, A., Pepe, G., Martella, N., Tani, A., Rosa, P., Maglione, V., Musci, G., Cutone, A., Di Bartolomeo, S., & Sgambati, E. (2025). Sialylation Inhibition Impairs Migration and Promotes Adhesion of GBM Cells. International Journal of Molecular Sciences, 26(21), 10708. https://doi.org/10.3390/ijms262110708









