3.1. Identification of CPK Family Genes C. quinoa
Calcium-dependent protein kinases (CPKs) constitute one of the main families of kinases in plants, playing crucial roles in physiological processes and responses to multiple types of stress [
17,
30]. In this study, 16
CqCPK genes were identified. The conserved grouping of proteins observed in
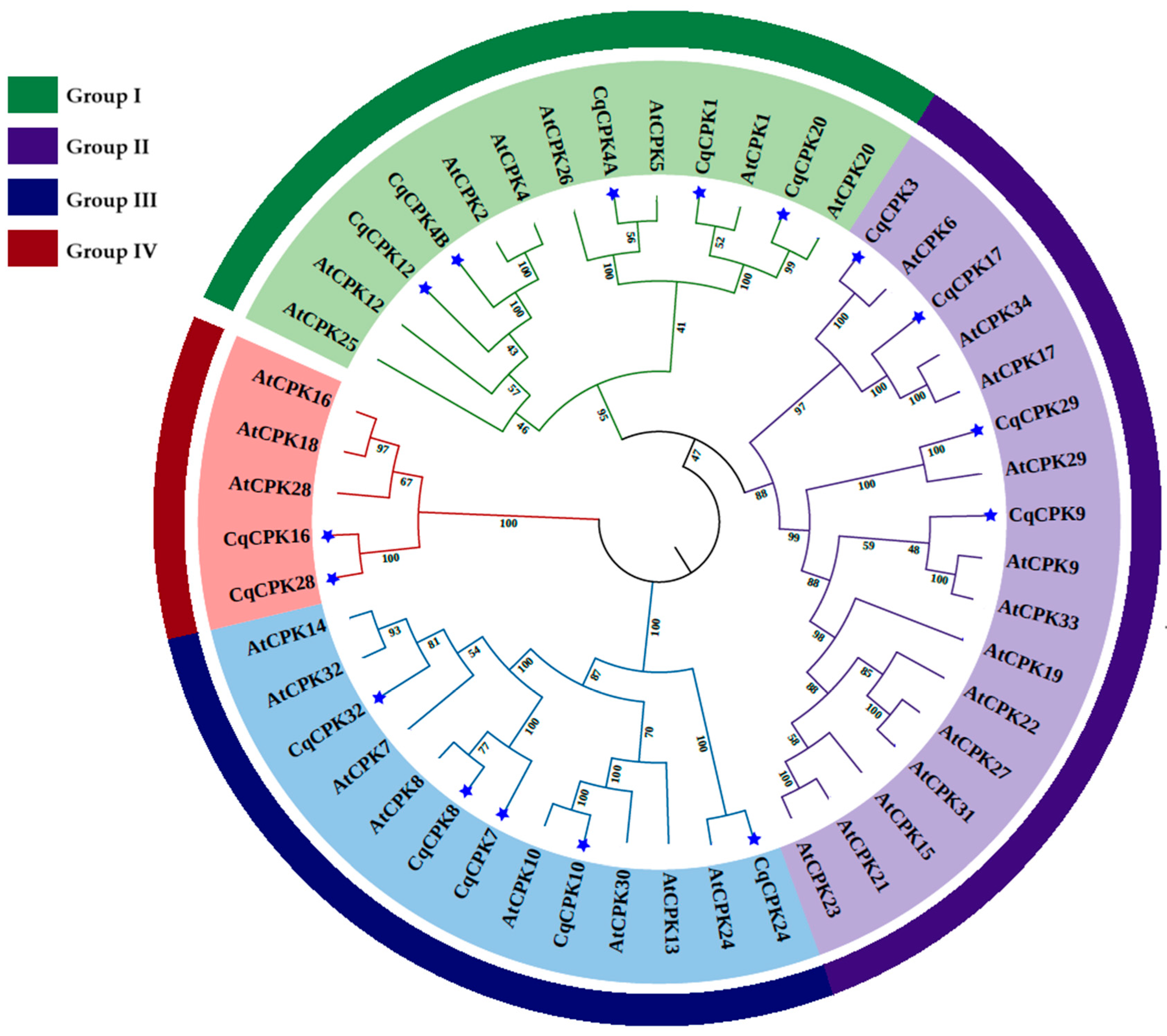
Figure 3 suggests that the subgroups maintain close evolutionary relationships and possibly share similar biological functions. Phylogenetic analysis grouped these genes into four subgroups (I, II, III, and IV) (
Figure 1A), consistent with CPK classifications in other species, such as
Arabidopsis thaliana,
Oryza sativa,
A. comosus, and
Gossypium hirsutum [
30,
32,
33,
34]. All
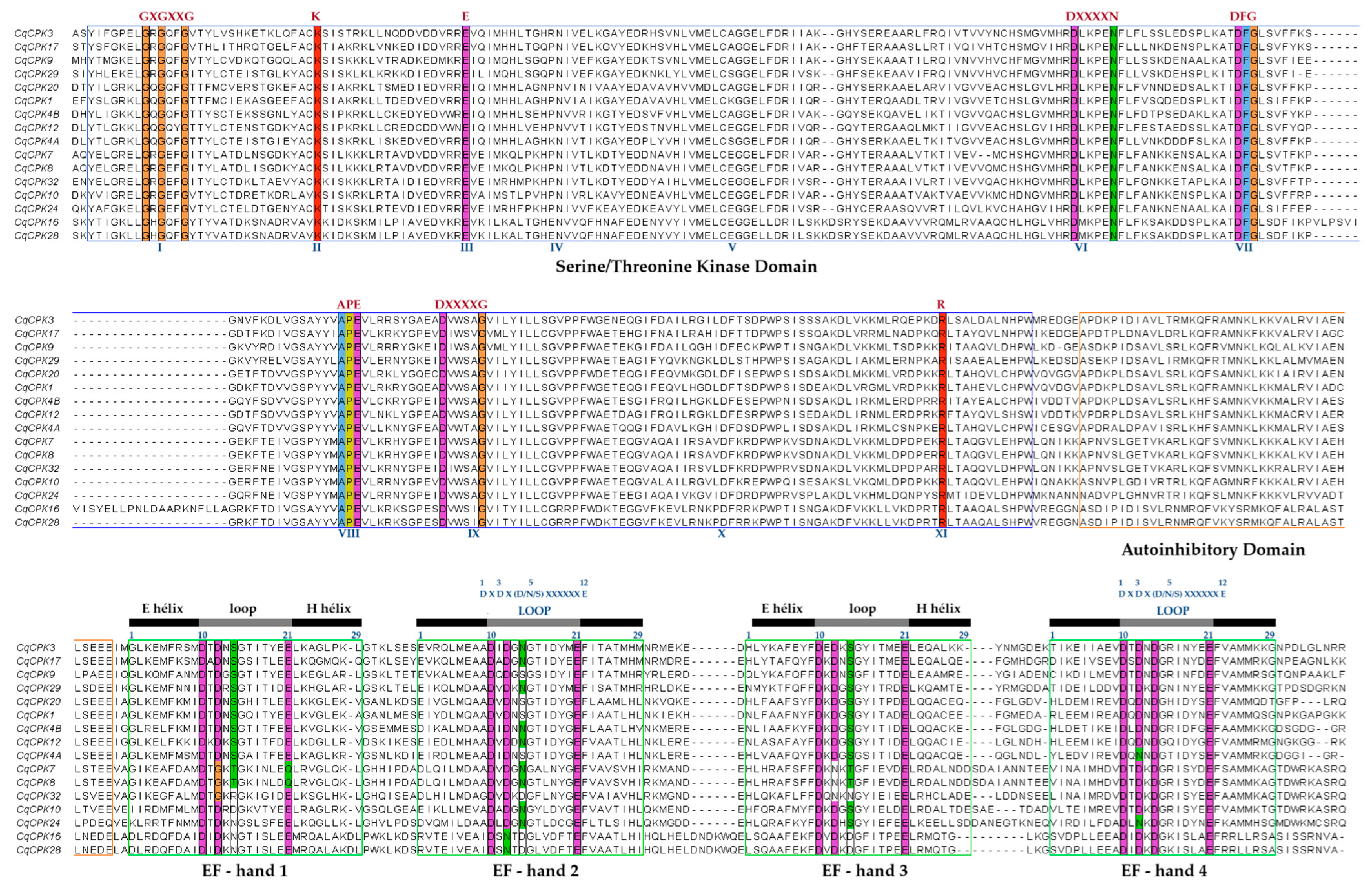
CqCPKs in quinoa had characteristic structural domains (
Figure 1B), such as a variable N-terminal domain, an autoinhibitory domain, a phosphorylation kinase domain, and four EF-hand motifs arranged in two pairs [
35]. Which optimises their calcium-binding capacity and structural stability in their active form. This structural conservation has been observed in other studies in species such as
O. sativa,
Fragaria × ananassa, and
A. comosus [
30,
33,
36].
Analysis of conserved MEME motifs (
Figure 1C) revealed that CqCPKs contain nine main motifs that show high conservation in organisation and number in all CqCPKs. Similar motif organisation was found in research on
Glycyrrhiza uralensis [
37] and
Chorchorus capsularis [
38]. Sequence alignment analysis (
Figure 2) identified highly conserved residues within the functional domains, supporting their evolutionary and functional importance. In agreement with previous studies by Hanks [
14] and Takahashi & Ito, it was observed that serine/threonine kinase contains eleven typically conserved subdomains. The amino-terminal end is essential for ATP binding, containing the conserved sequence GXGXXG in subdomain I, which is a glycine-rich loop whose function is phosphate binding, while lysine (K) in subdomain II and glutamic acid (E) in subdomain III participate in the stabilisation and orientation of ATP. At the carboxy-terminal end, the conserved sequence DXXXXN was identified in subdomain VIb, the catalytic ATP-binding domain, while the DFG sequence in subdomain VII plays a structural role in the coordination of magnesium ions. In addition, conserved APE motifs have been detected in subdomain VIII, DXXXXG in subdomain IX, and a highly conserved arginine (R) in subdomain XI. These results are consistent with the reports of Nolen et al. [
39]. Furthermore, according to Wang et al. [
40], the conservation of these residues suggests strong evolutionary pressure to maintain the functional and structural integrity of the kinase domain.
EF-hand motifs, whose main function is to coordinate calcium ion (Ca
+2) binding, have a helix-loop-helix conformation (approximately 29 aa), with the loop (DXDX S/N/D XXXXXE) typically consisting of 12 aa located at positions 10 to 21, as previously described by Liese & Romeis [
11]. his region provides flexibility for calcium ion binding, thanks to the presence of glutamic acid (E), a negatively charged amino acid, at position 12 of the loop. This is a bidentate ligand that provides two oxygen atoms, necessary for complete calcium stabilisation, a characteristic consistently reported in various EF-hand proteins by Kundu et al. [
18], McCormack et al. [
41], and Grabarek [
42]. The conservation of the EF-hand motif is also observed in species such as
G. uralensis [
37],
Zea mays [
40], and
C. capsularis [
38]. In addition, variations were identified in the sequences of the EF-hand motif of
CqCPK7 and
CqCPK8, where glutamic acid (E) at position 12 of the loop was replaced by glutamine (Q). This change suggests a possible alteration in calcium ion affinity, which could result in decreased sensitivity or a partial loss of calcium uptake and a consequent conformational change in the entire CPK protein. This finding is similar to that reported in
A. thaliana by Liese & Romeis [
11], where EF-hand 1 of
AtCPK23 is degenerate due to the substitution of glutamic acid (E) for glutamine (Q), which has been associated with a decrease in affinity for calcium ions and, therefore, with a reduced functional capacity in calcium uptake and response to calcium signals.
Structurally,
CqCPK genes show variation in the number of exons (
Figure 1D). Consistent patterns were observed within each group: members of group I had seven exons, while those in group II had eight, with the exception of
CqCPK3, which has nine. In group III, seven and eight exons were observed, while the two members of group IV retained 12 exons, suggesting that this latter group has been under greater evolutionary pressure to maintain its structure. Studies in
A. thaliana [
32],
A. comosus [
30],
Vittis spp. [
43],
G. hirsutum [
34] show similar patterns. The reduction in the number of exons in certain CqCPK genes could be explained by genomic deletion events, structural rearrangements, or selective pressures that favour faster or more efficient transcription under adverse conditions such as salinity. This hypothesis is supported by recent studies by Hernández-Urrieta et al. [
44], that show how environmental stress modulates gene structure to favour adaptive responses. In particular, it has been reported that salinity and other factors induce changes in alternative splicing, affecting the inclusion or exclusion of exons and generating more functional isoforms under adverse conditions. Comparative studies on MADS-box genes, such as those by Yu et al. [
45], have shown that structural changes in exon-intron organisation are frequent and may be linked to functional specialisation, suggesting an evolutionary background that explains the structural variability present in
CqCPK genes.
The comparative analysis between
C. quinoa and
A. thaliana (
Figure 3) showed that
CPK genes in both species are grouped into four subgroups (I–IV), a pattern consistent with that reported in other model species such as
Vittis spp. [
19,
43]
O. sativa [
33] and
A. comosus [
30], suggesting strong evolutionary pressure to maintain similar functions within each subfamily in different species. The high similarity in the
CPK genes of
A. thaliana and
C. quinoa indicates that they are closely related to each other, suggesting that they may retain similar or complementary functions, as they maintain greater conservation in key functional regions, supporting their possible involvement in specific responses, their subcellular localisation, or their participation in specific signalling pathways, as reported by Li, et al. [
46] where phylogenetically related
CPK genes can be located in the same subcellular compartments or participate in common responses to biotic or abiotic stimuli.
The physicochemical characterisation (
Table 1) reveals sizes ranging from 470 to amino acids and estimated molecular weights between 52,599 and 66,797 kDa. These values are within the range reported for CPK proteins in other species such as
Triticum aestivum [
47] and
G. hirsutum [
34], suggesting general structural conservation between species. The isoelectric point (pI) ranges from 5.02 to 9.15, similar to that observed in
A. comosus [
30] and
G. uralensis [
37]. The aliphatic index, which is related to the thermal stability of proteins, ranged from 76.85 to 92.49, suggesting high stability, in agreement with that reported in wheat by Liu et al. [
47].
Most CqCPK proteins, except
CqCPK3,
CqCPK4B,
CqCPK12,
CqCPK20,
CqCPK28, and
CqCPK29, undergo post-translational modifications, specifically N-myristoylation and S-palmitoylation sites, according to Cheng et al. [
32]. Post-translational modifications are essential for their subcellular localisation and function. In
O. sativa [
48],
OsCPK19 has been shown to possess both types of modifications, which determine its association with the cell membrane. In accordance with this model, in the present study, the prediction of myristoylation and palmitoylation sites showed that four isoforms (
CqCPK9,
CqCPK10,
CqCPK16, and
CqCPK24) simultaneously contain myristoylation and palmitoylation sites, while six (
CqCPK1,
CqCPK4A,
CqCPK7,
CqCPK8,
CqCPK17,
CqCPK32) have only palmitoylation sites. This pattern is consistent with what has been reported in species such as
Fragaria [
36] and
Vitis spp. [
43]. However, the prediction of subcellular localisation suggests that CPKs in quinoa, despite undergoing these modifications, are not restricted solely to the plasma membrane. On the contrary, they can be located in different cellular compartments, including the nucleus, mitochondria, and cytoplasm, with the latter being the most frequent. This pattern is consistent with reports in
G. hirsutum [
34]
Z. mays [
40] and
Vitis spp. [
43], where some CPKs with post-translational modifications were detected in compartments other than the membrane. Furthermore, in
T. aestivum [
49], it has been observed that
TaCPK3 and
TaCPK15 are associated with the plasma membrane even in the absence of post-translational modifications. This indicates that, although post-translational modifications may favour anchoring to the membrane, this association is complex and may be influenced by other factors that allow them to perform diverse and specialised functions within the cell.
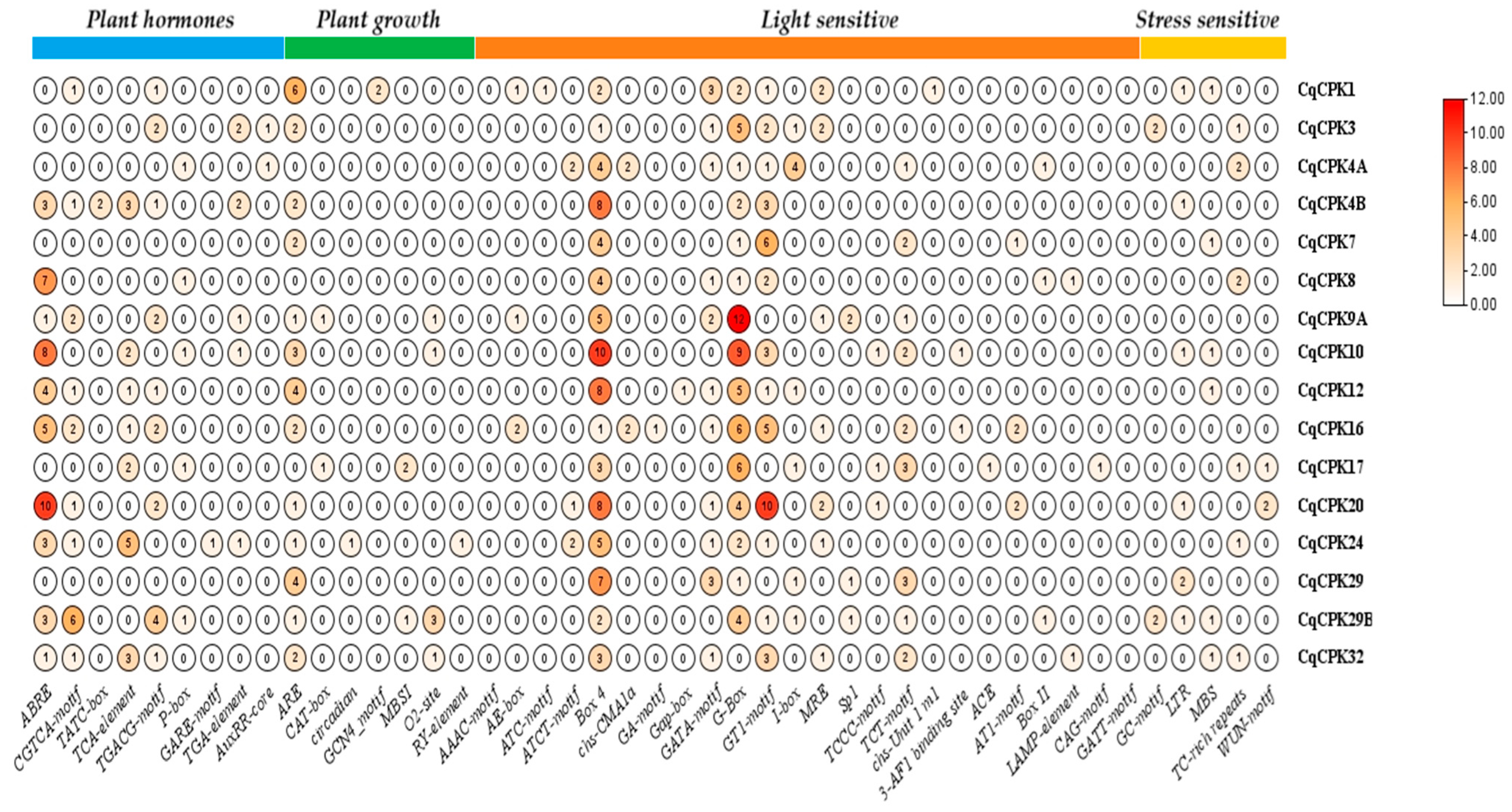
Analysis of the promoter regions (
Figure 4) of
CqCPK genes reveals a diversity of
cis-regulatory elements associated with hormonal signalling pathways, environmental stimuli and developmental processes, which could suggest the possible functions and regulatory mechanisms of the genes, giving them the ability to adapt to adverse conditions. The presence of elements related to phytohormone pathways (ABA, GA, MeJA, SA, and AUX), as well as motifs sensitive to abiotic stress (MBS, ARE, TC-rich repeats, and LTR), indicates that these genes could be integrated into adaptive response networks against adverse conditions such as salinity, drought, or low temperatures. In particular,
CqCPK12,
CqCPK17,
CqCPK20 and
CqCPK32 showed a high density of motifs associated with regulation by ABA, MeJA and typical stress response elements such as ARE, MBS and TC-rich repeats. This combination suggests that these genes could be integrated into rapid and efficient signalling pathways against oxidative damage and osmotic imbalance induced by salt stress, reinforcing their functional role as sensors and modulators of the adaptive response. Similar findings have been reported in species such as
G. uralensis [
37],
G. hirsutum [
34],
C. capsularis [
38],
Setaria italica [
50], and
Carya illinoinensis [
29]. These studies have confirmed that these elements are common in genes involved in responses to abiotic stress, such as salinity, drought and cold.
Together, the CqCPK genes, especially CqCPK12, CqCPK17, CqCPK20, and CqCPK32, show structural and regulatory characteristics that predict their involvement in the response to abiotic stress. Their structural conservation and the presence of cis-elements associated with hormonal and environmental signals reinforce their potential role as key modulators in the adaptation of C. quinoa to adverse conditions.
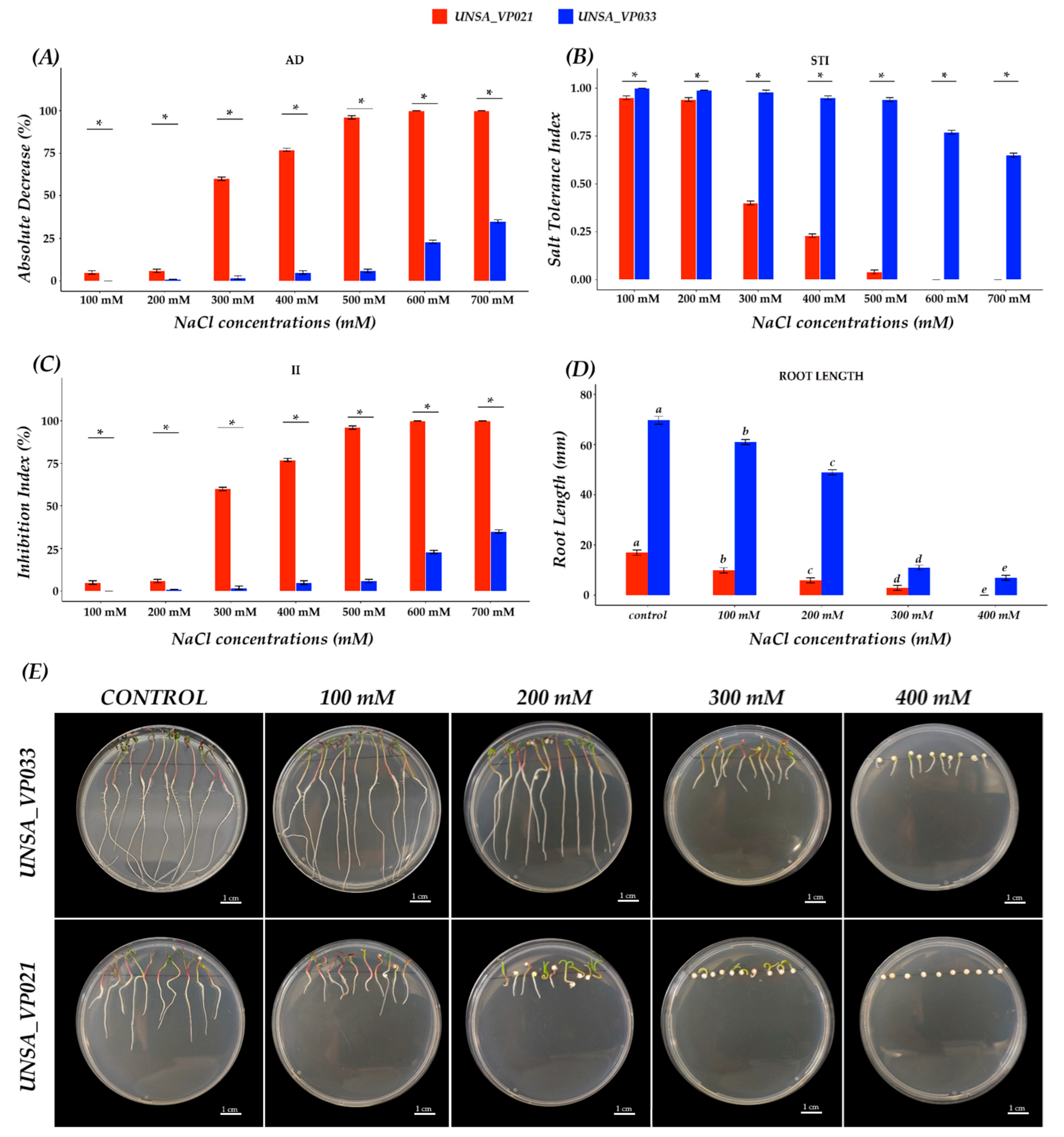
3.2. Evaluation of Salt Tolerance in a Halotolerant and a Halosensitive Accession of C. quinoa
C. quinoa is recognised for its salinity tolerance. Studies conducted by Al-Naggar et al. and Prajapat et al. [
50,
51] have shown that this capacity varies significantly between genotypes and stages of development, reflecting a differential genotypic and phenological response to salt stress. In this study, accessions UNSA_VP021 and UNSA_VP033 showed contrasting responses to salt stress during germination.
The absolute decrease (DA) rate in germination (
Figure 5A) showed a negative impact of the saline treatment compared to the control. The accessions showed a differential response to salinity stress, particularly above 500 mM, with accession UNSA_VP021 showing a reduction of 96 to 100%, being the most affected, indicating high sensitivity to saline stress. Meanwhile, accession UNSA_VP033 showed a germination DA of only 36%, suggesting greater tolerance. These results confirm that a higher DA is associated with greater susceptibility to salinity, as reported by Ravelombola et al. [
52]. Similarly, the inhibition index (II), widely used as a parameter to assess the impact of stress on plants, followed the same pattern, with UNSA_VP021 showing a very high II (above 90%), reinforcing its profile in line with that reported by Qureshi et al. [
53]. In contrast, the salt tolerance index (STI) reflects a plant’s ability to maintain its yield under saline conditions [
54], highlighting that higher STI values indicate a greater probability of salt tolerance. In this study, significant differences were observed between accessions, with UNSA_VP033 presenting the highest STI (above 0.64). These results suggest that accession UNSA_VP021 is highly sensitive to salinity, while UNSA_VP033 exhibits marked tolerance during the germination stage.
In addition, a direct relationship was observed between tolerance indices and root length under saline conditions, with accession UNSA_VP033 maintaining a greater root length compared to UNSA_VP021, whose root development was severely affected by the increase in salinity. Although both accessions showed a significant reduction in root length as the NaCl concentration increased, UNSA_VP033 managed to sustain relatively superior root growth. These results are consistent with studies conducted on
A. thaliana [
55] and
Glycine max [
56], where salinity has been shown to reduce root growth, especially in sensitive genotypes.
Taken together, the results of the DA, II, and STI analyses and the root length evaluation clearly demonstrate that UNSA_VP021 is a sensitive accession, while UNSA_VP033 shows a tolerant response to salt stress, as described in the study by Alvarez-Vasquez et al. [
57].
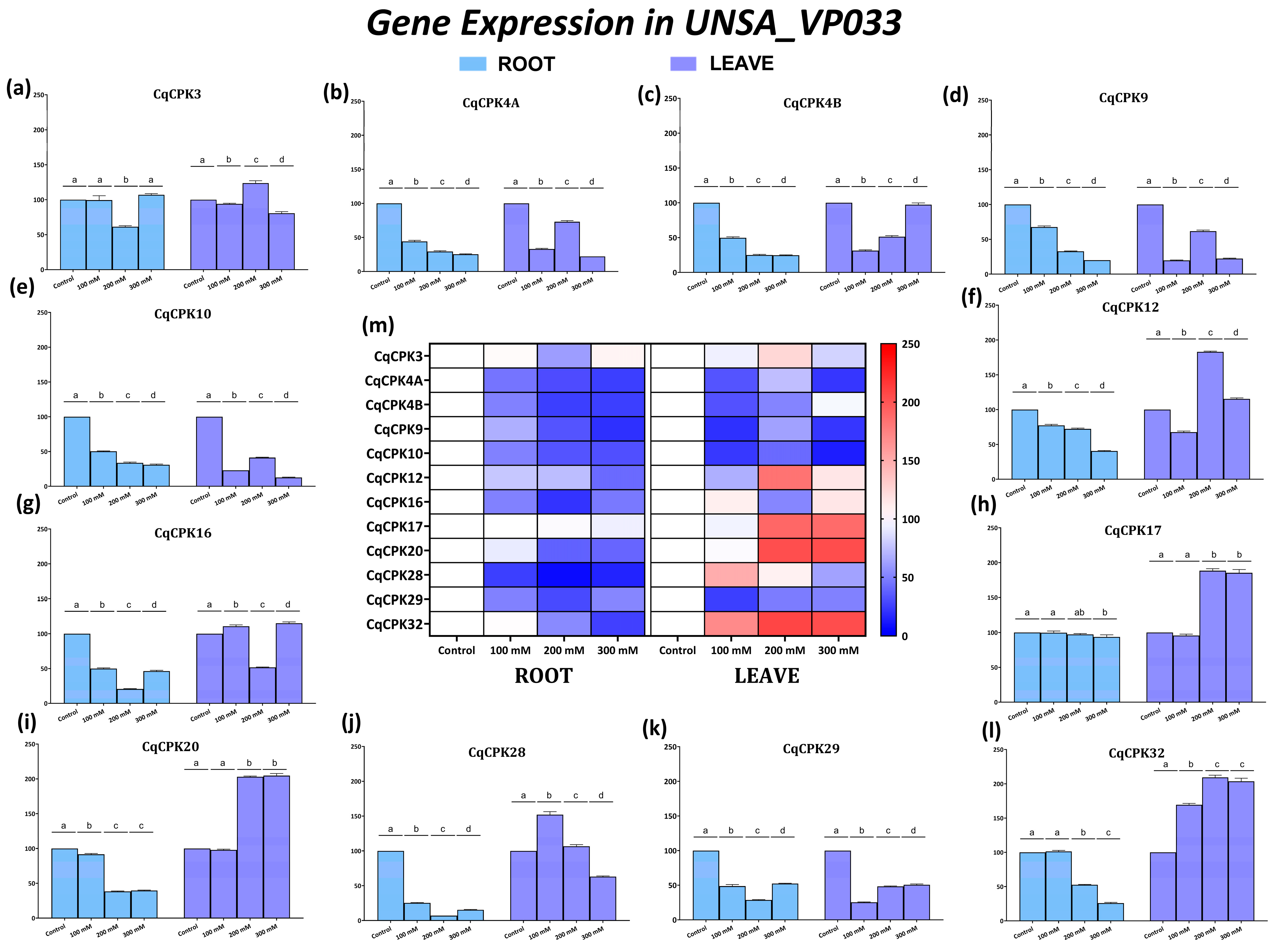
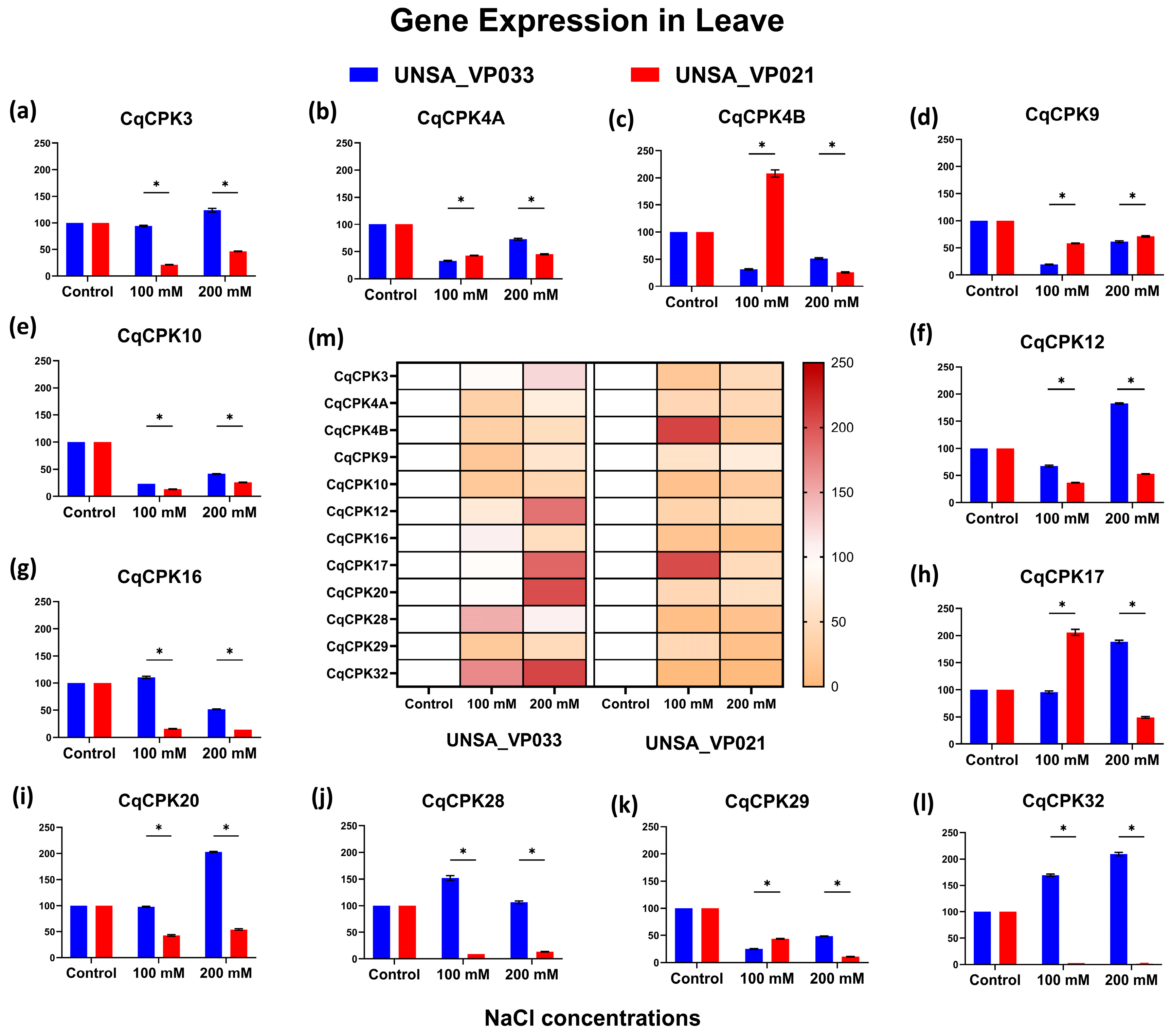
3.3. Analysis of Gene Expression in a Tolerant Accession and a Sensitive Accession in C. quinoa
The gene expression results for
CqCPK genes reveal a differential response to salt stress in
C. quinoa, with a clear contrast between accessions UNSA_VP033 (tolerant) and UNSA_VP021 (sensitive). In leaves (
Figure 6), UNSA_VP033 showed significant overexpression of
CqCPK12,
CqCPK17,
CqCPK20, and
CqCPK32 at 200 mM, a pattern that was maintained even at 300 mM (see
Figure A1). This sustained activation suggests the involvement of these genes in the modulation of calcium-dependent mechanisms, possibly related to stomatal regulation, osmoprotectant production and redox balance (ROS) control. In contrast, in UNSA_VP021, all genes were found to be underexpressed at 200 mM, which would indicate a limited capacity for transcriptional activation in response to stress. The higher expression of
CqCPK in the aerial part of UNSA_VP033 coincides with reports in other species such as
A. thaliana [
21,
55],
O. sativa [
23,
33],
G. hirsutum [
24],
A. comosus [
30], where
CPK genes play key roles in signalling and adaptive response to salt stress.
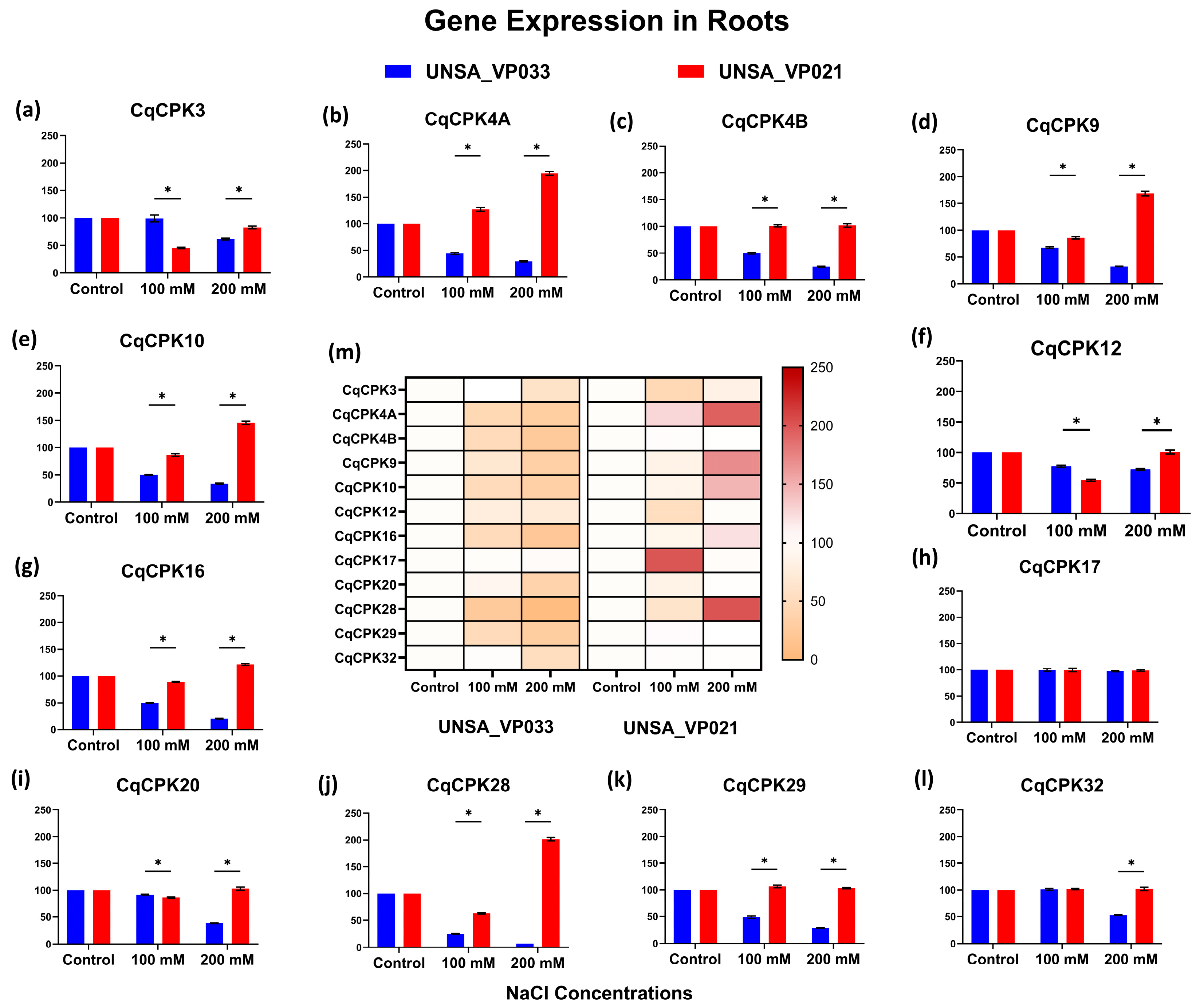
In roots (
Figure 7), expression at 200 mM was opposite; in the tolerant accession, all genes evaluated were underexpressed, except for
CqCPK17, which remained stable. In contrast, in UNSA_VP021, expression was more variable. These results suggest that UNSA_VP033 focuses its adaptive response on the aerial part, probably thanks to more efficient ionic homeostasis, which includes Na
+ exclusion in the root and regulated transport to the upper tissues. In contrast, the sensitive accession activates signalling pathways in the root, possibly as a strategy to limit Na
+ absorption from the early stages of stress. This behaviour is supported by previous studies by Hariadi et al., Panuccio et al., and Claros et al. [
58,
59,
60], which highlight the involvement of the root in ion exclusion and osmotic regulation under saline stress.
The variability in the expression of
CqCPK genes between tissues and genotypes could represent a functional compensation strategy, where different organs assume specific roles to maintain homeostasis under stress. As pointed out by Kong et al. [
61] and Zhang et al. [
30]
CPK gene expression can be highly tissue-specific, as in the case of
ZmCPK37 (root) and
ZmCPK22 (leaves) in maize, or
AcoCPK16 in pineapple leaves and flowers. Taken together, the differential expression of
CqCPKs in roots and leaves suggests that
C. quinoa accessions adopt divergent adaptive strategies in response to salinity. UNSA_VP033 appears to maintain a more efficient response from the aerial part, while UNSA_VP021 attempts to compensate through responses at the root level. Therefore, these results support the hypothesis that
CPK genes are critical components in the signalling pathways that modulate the response to salt stress.