Differential Binding of ΔFN3 Proteins of Bifidobacterium longum GT15 and Bifidobacterium bifidum 791 to Cytokines Determined by Surface Plasmon Resonance and De Novo Molecular Modeling
Abstract
1. Introduction
2. Results
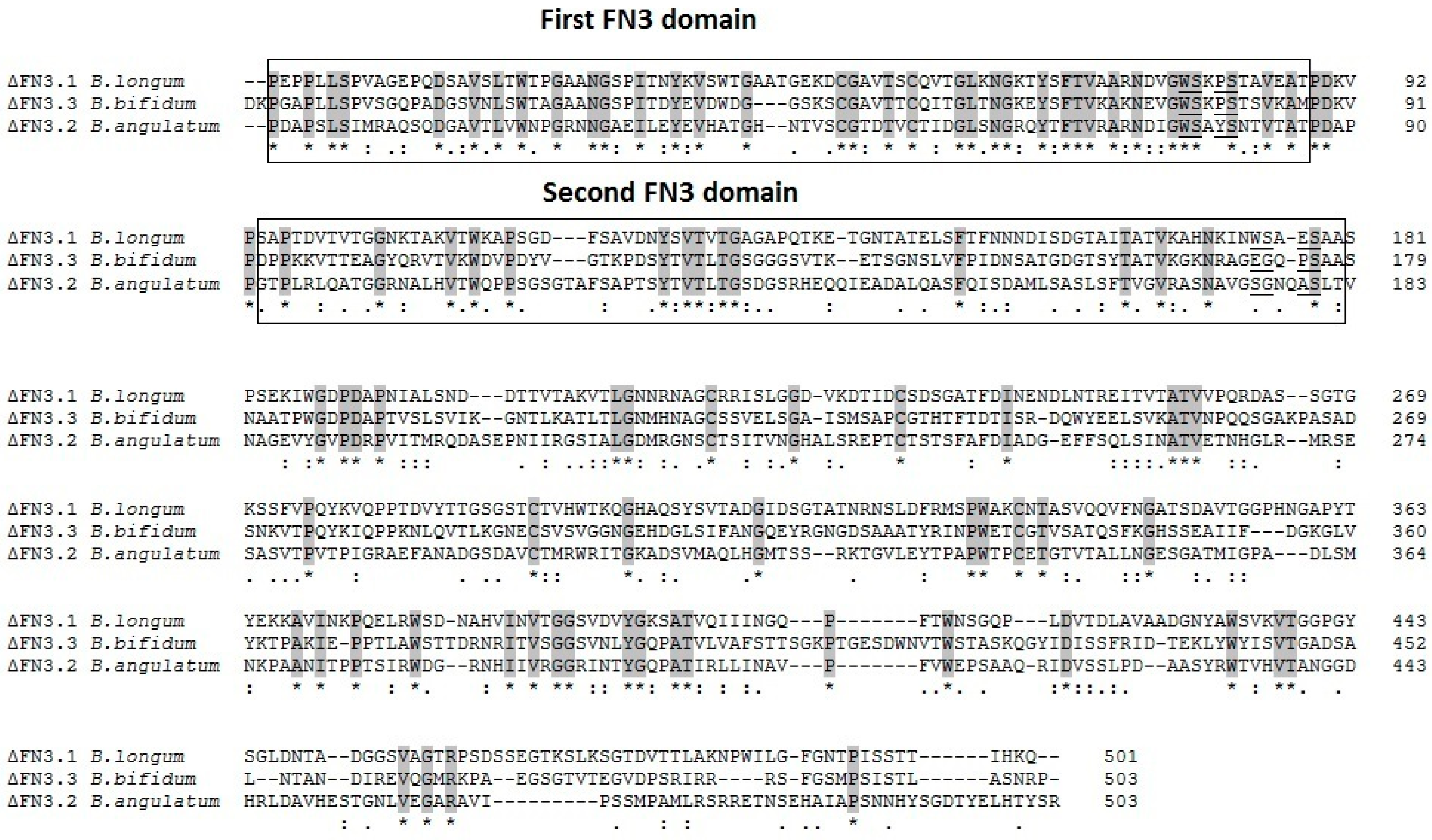
2.1. Analysis of Amino Acid Sequences of Bifidobacterial ΔFN3 Fragments
2.2. Cloning and Expression of Genes Encoding ΔFN3.2 B. angulatum and ΔFN3.3 B. bifidum Isolation and Purification of Recombinant Proteins
2.3. Interactions of ΔFN3.1 B. longum GT15 and ΔFN3.3 B. bifidum 791 with TNF-α, IL-6, IL-8, and IL-10
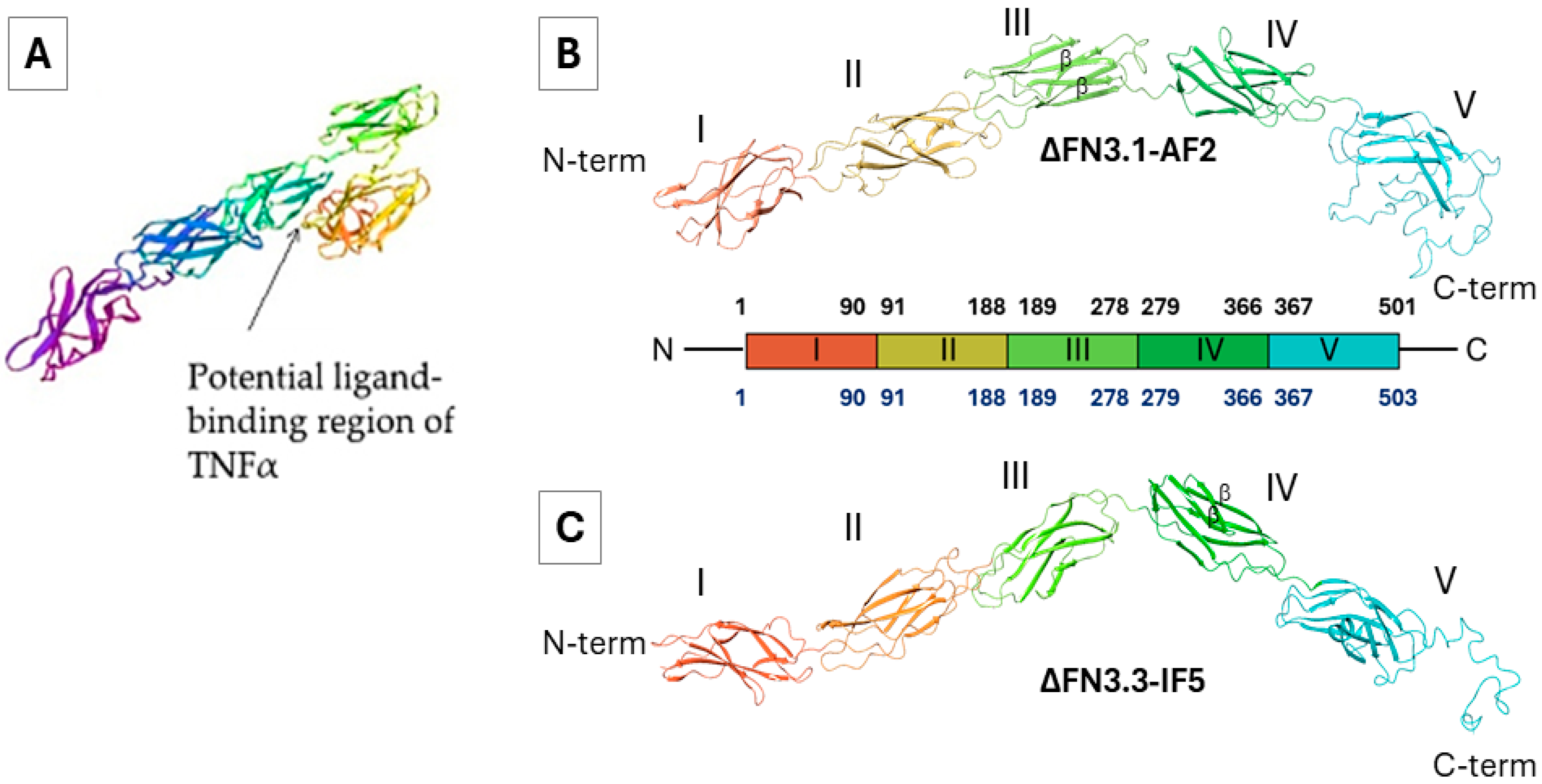
2.4. Prediction of Tertiary Structures
2.5. Molecular Docking
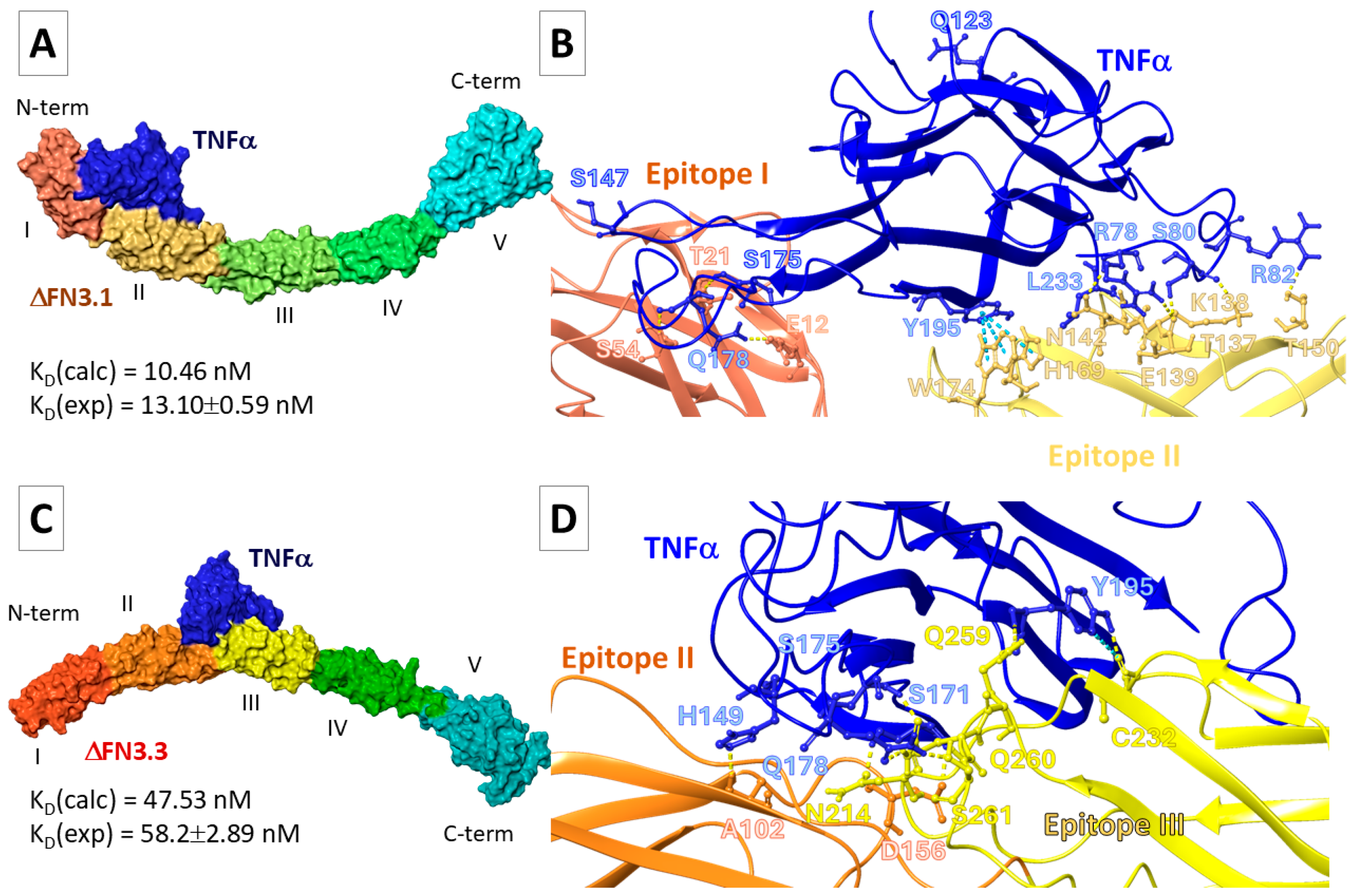
2.5.1. FN3-TNF-α Interaction
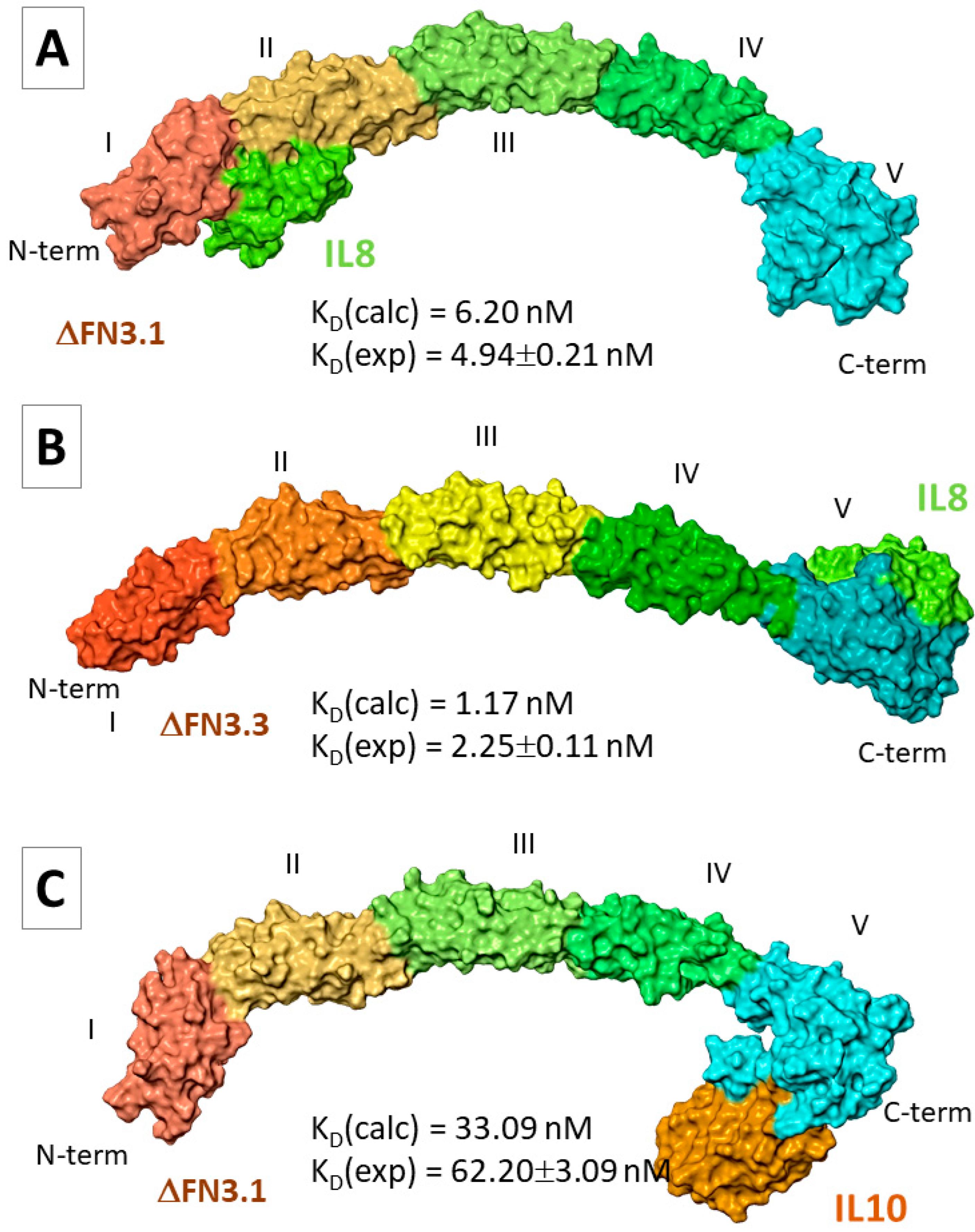
2.5.2. FN3–Interleukin Interactions
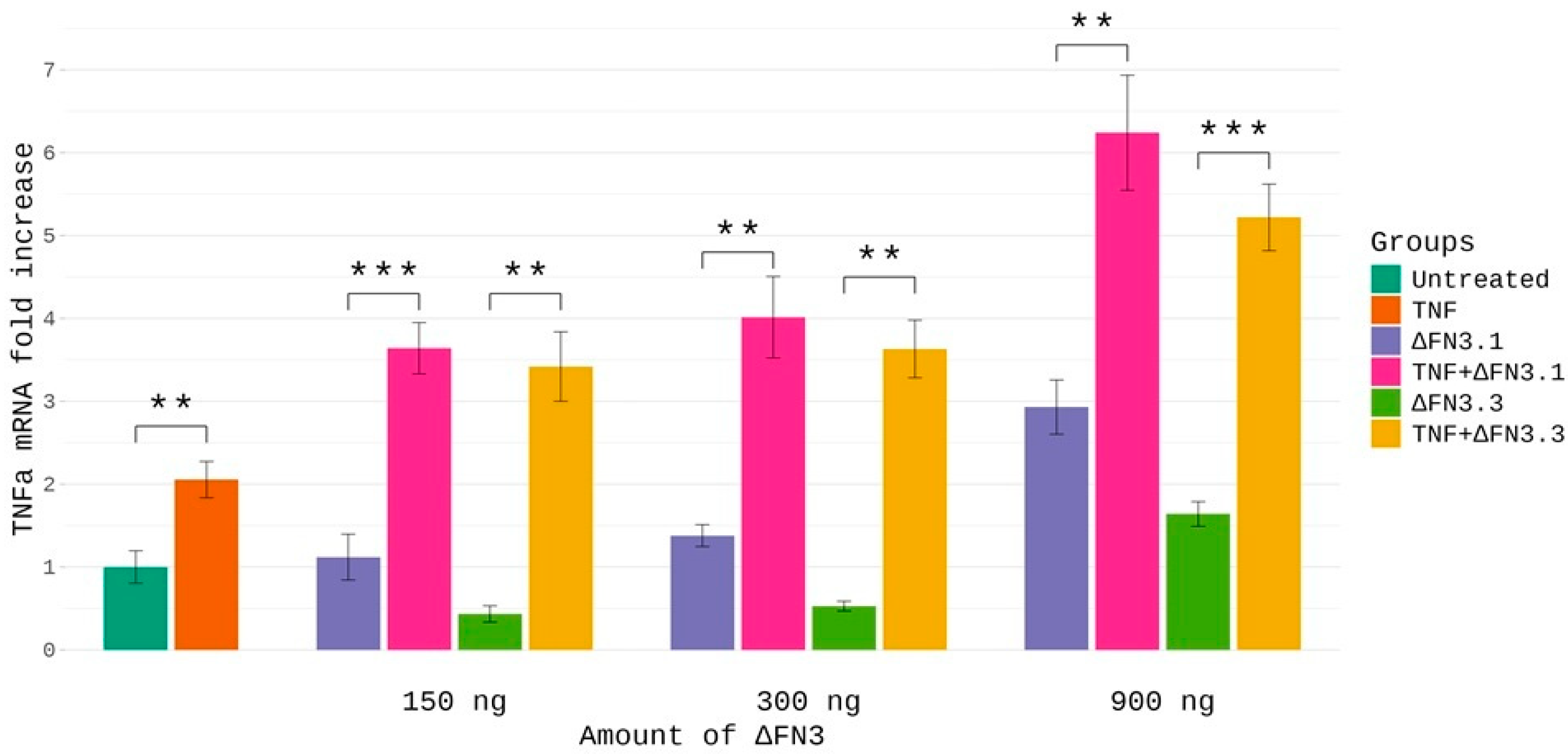
2.6. Effects of ΔFN3 and TNF-α on Cytokine mRNA Abundance in THP-1 Cells
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmid Vectors, Culture Media, and Conditions
4.2. DNA Manipulations
4.3. Expression in E. coli and Purification of Recombinant ΔFN3 Proteins
4.4. Interaction of ΔFN3 Proteins with TNF-α, IL-8, IL-6, and IL-10
4.5. Molecular Modeling Studies
4.5.1. Prediction of Tertiary Structure of ∆FN3 Proteins
4.5.2. Protein–Protein Docking
4.5.3. MD Simulations
4.5.4. Estimation of Binding Energy and KD
4.6. Detection of Cytokine mRNA by Reverse Transcription-Polymerase Chain Reaction
4.7. Bioinformatic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C-score | Confidence Score |
| DARS | Decoys As the Reference State |
| GMQS | Global Model Quality Score |
| MD simulations | Molecular dynamic simulations |
| pLDDT | Predicted Local Distance Difference Test |
References
- Sharma, A.; Sharma, G.; Im, S.H. Gut microbiota in regulatory T cell generation and function: Mechanisms and health implications. Gut Microbes 2025, 17, 2516702. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Collado, M.C.; Wopereis, H.; Salminen, S.; Knol, J.; Roeselers, G. The Bifidogenic Effect Revisited-Ecology and Health Perspectives of Bifidobacterial Colonization in Early Life. Microorganisms 2020, 8, 1855. [Google Scholar] [CrossRef] [PubMed]
- Rabe, H.; Lundell, A.C.; Sjöberg, F.; Ljung, A.; Strömbeck, A.; Gio-Batta, M.; Maglio, C.; Nordström, I.; Andersson, K.; Nookaew, I.; et al. Neonatal gut colonization by Bifidobacterium is associated with higher childhood cytokine responses. Gut Microbes 2020, 12, 1847628. [Google Scholar] [CrossRef]
- Ennis, D.; Shmorak, S.; Jantscher-Krenn, E.; Yassour, M. Longitudinal quantification of Bifidobacterium longum subsp. infantis reveals late colonization in the infant gut independent of maternal milk HMO composition. Nat. Commun. 2024, 15, 894. [Google Scholar] [CrossRef]
- Shang, J.; Wan, F.; Zhao, L.; Meng, X.; Li, B. Potential Immunomodulatory Activity of a Selected Strain Bifidobacterium bifidum H3-R2 as Evidenced in vitro and in Immunosuppressed Mice. Front. Microbiol. 2020, 11, 2089. [Google Scholar] [CrossRef]
- Vinkler, M.; Fiddaman, S.R.; Těšický, M.; O’Connor, E.A.; Savage, A.E.; Lenz, T.L.; Smith, A.L.; Kaufman, J.; Bolnick, D.I.; Davies, C.S. Understanding the evolution of immune genes in jawed vertebrates. J. Evol. Biol. 2023, 36, 847–873. [Google Scholar] [CrossRef]
- Alessandri, G.; van Sinderen, D.; Ventura, M. The genus bifidobacterium: From genomics to functionality of an important component of the mammalian gut microbiota running title: Bifidobacterial adaptation to and interaction with the host. Comput. Struct. Biotechnol. J. 2021, 19, 1472–1487. [Google Scholar] [CrossRef]
- Liwen, Z.; Yu, W.; Liang, M.; Kaihong, X.; Baojin, C. A low abundance of Bifidobacterium but not Lactobacillius in the feces of Chinese children with wheezing diseases. Medicine 2018, 97, e12745. [Google Scholar] [CrossRef]
- Choi, Y.J.; Shin, S.H.; Shin, H.S. Immunomodulatory Effects of Bifidobacterium spp. and Use of Bifidobacterium breve and Bifidobacterium longum on Acute Diarrhea in Children. J. Microbiol. Biotechnol. 2022, 9, 1186–1194. [Google Scholar] [CrossRef]
- Lim, H.J.; Shin, H.S. Antimicrobial and Immunomodulatory Effects of Bifidobacterium Strains: A Review. J. Microbiol. Biotechnol. 2020, 30, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Nezametdinova, V.Z.; Mavletova, D.A.; Alekseeva, M.G.; Chekalina, M.S.; Zakharevich, N.V.; Danilenko, V.N. Species-specific serine-threonine protein kinase Pkb2 of Bifidobacterium longum subsp. longum: Genetic environment and substrate specificity. Anaerobe 2018, 51, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Dyachkova, M.S.; Chekalin, E.V.; Danilenko, V.N. Positive Selection in Bifidobacterium Genes Drives Species-Specific Host-Bacteria Communication. Front. Microbiol. 2019, 10, 2374. [Google Scholar] [CrossRef]
- Nezametdinova, V.Z.; Zakharevich, N.V.; Alekseeva, M.G.; Averina, O.V.; Mavletova, D.A.; Danilenko, V.N. Identification and characterization of the serine/threonine protein kinases in Bifidobacterium. Arch. Microbiol. 2014, 196, 125–136. [Google Scholar] [CrossRef]
- Zakharevich, N.V.; Averina, O.V.; Klimina, K.M.; Kudryavtseva, A.V.; Kasianov, A.S.; Makeev, V.J.; Danilenko, V.N. Complete Genome Sequence of Bifidobacterium longum GT15: Identification and Characterization of Unique and Global Regulatory Genes. Microb. Ecol. 2015, 70, 819–834. [Google Scholar] [CrossRef]
- Nezametdinova, V.Z.; Yunes, R.A.; Dukhinova, M.S.; Alekseeva, M.G.; Danilenko, V.N. The Role of the PFNA Operon of Bifidobacteria in the Recognition of Host’s Immune Signals: Prospects for the Use of the FN3 Protein in the Treatment of COVID-19. Int. J. Mol. Sci. 2021, 22, 9219. [Google Scholar] [CrossRef] [PubMed]
- Westermann, C.; Gleinser, M.; Corr, S.C.; Riedel, C.U. A Critical Evaluation of Bifidobacterial Adhesion to the Host Tissue. Front. Microbiol. 2016, 7, 1220. [Google Scholar] [CrossRef] [PubMed]
- Dyakov, I.N.; Mavletova, D.A.; Chernyshova, I.N.; Snegireva, N.A.; Gavrilova, M.V.; Bushkova, K.K.; Dyachkova, M.S.; Alekseeva, M.G.; Danilenko, V.N. FN3 protein fragment containing two type III fibronectin domains from B. longum GT15 binds to human tumor necrosis factor alpha in vitro. Anaerobe 2020, 65, 102247. [Google Scholar] [CrossRef]
- Henderson, B.; Nair, S.; Pallas, J.; Williams, M.A. Fibronectin: A multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol. Rev. 2011, 35, 147–200. [Google Scholar] [CrossRef]
- Speziale, P.; Arciola, C.R.; Pietrocola, G. Fibronectin and Its Role in Human Infective Diseases. Cells 2019, 8, 1516. [Google Scholar] [CrossRef]
- Malagrinò, F.; Pennacchietti, V.; Santorelli, D.; Pagano, L.; Nardella, C.; Diop, A.; Toto, A.; Gianni, S. On the Effects of Disordered Tails, Supertertiary Structure and Quinary Interactions on the Folding and Function of Protein Domains. Biomolecules 2022, 12, 209. [Google Scholar] [CrossRef]
- Valk, V.; van der Kaaij, R.M.; Dijkhuizen, L. The evolutionary origin and possible functional roles of FNIII domains in two Microbacterium aurum B8. A granular starch degrading enzymes, and in other carbohydrate acting enzymes. Amylase 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Sun, H.; Guo, Z.; Hong, H.; Zhang, Z.; Zhang, Y.; Wang, Y.; Le, S.; Chen, H. Free Energy Landscape of Type III Fibronectin Domain with Identified Intermediate State and Hierarchical Symmetry. Phys. Rev. Lett. 2023, 131, 218402. [Google Scholar] [CrossRef]
- Koide, A.; Koide, S. Use of Phage Display and Other Molecular Display Methods for the Development of Monobodies. Cold Spring Harb. Protoc. 2024, 5, 107982. [Google Scholar] [CrossRef]
- Zhu, N.; Smallwood, P.M.; Rattner, A.; Chang, T.H.; Williams, J.; Wang, Y.; Nathans, J. Utility of protein-protein binding surfaces composed of anti-parallel alpha-helices and beta-sheets selected by phage display. J. Biol. Chem. 2024, 300, 107283. [Google Scholar] [CrossRef] [PubMed]
- Siupka, P.; Hamming, O.T.; Kang, L.; Gad, H.H.; Hartmann, R. A conserved sugar bridge connected to the WSXWS motif has an important role for transport of IL-21R to the plasma membrane. Genes Immun. 2015, 6, 405–413. [Google Scholar] [CrossRef]
- Singh, S.; Chaturvedi, N.; Rai, G. De novo modeling and structural characterization of IL9 IL9 receptor complex: A potential drug target for hematopoietic stem cell therapy. Netw. Model. Anal. Health Inform. Bioinform. 2020, 9, 29. [Google Scholar] [CrossRef]
- Singh, S.; Rai, G. Structural Insights into the IL12:IL12 Receptor Complex Assembly by Molecular Modeling, Docking, and Molecular Dynamics Simulation. Comb. Chem. High Throughput Screen. 2022, 25, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.G.; Kragelund, B.B. Who climbs the tryptophan ladder? On the structure and function of the WSXWS motif in cytokine receptors and thrombospondin repeats. Cytokine Growth Factor Rev. 2014, 25, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Szabó, R.; Láng, O.; Láng, J.; Illyés, E.; Kőhidai, L.; Hudecz, F. Effect of SXWS/WSXWS peptides on chemotaxis and adhesion of the macrophage-like cell line J774. J. Mol. Recognit. 2015, 28, 253–260. [Google Scholar] [CrossRef]
- Zakharevich, N.V.; Nezametdinova, V.Z.; Averina, O.V.; Chekalina, M.S.; Alekseeva, M.G.; Danilenko, V.N. Complete Genome Sequence of Bifidobacterium angulatum GT102: Potential Genes and Systems of Communication with Host. Russ. J. Genet. 2019, 55, 847–864. [Google Scholar] [CrossRef]
- Alekseeva, M.G.; Dyakov, I.N.; Bushkova, K.K.; Mavletova, D.A.; Yunes, R.A.; Chernyshova, I.N.; Masalitin, I.A.; Koshenko, T.A.; Nezametdinova, V.Z.; Danilenko, V.N. Study of the binding of ΔFN3.1 fragments of the Bifidobacterium longum GT15 with TNFα and prevalence of domain-containing proteins in groups of bacteria of the human gut microbiota. Microbiome Res. Rep. 2023, 2, 10. [Google Scholar] [CrossRef]
- Veselovsky, V.A.; Dyachkova, M.S.; Menyaylo, E.A.; Polyaeva, P.S.; Olekhnovich, E.I.; Shitikov, E.A.; Bespiatykh, D.A.; Semashko, T.A.; Kasianov, A.S.; Ilina, E.N.; et al. Gene Networks Underlying the Resistance of Bifidobacterium longum to Inflammatory Factors. Front. Immunol. 2020, 11, 595877. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a 3-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods 2021, 1, 100014. [Google Scholar] [CrossRef]
- Zhang, C.; Freddolino, L.; Zhang, Y. COFACTOR: Improved protein function prediction by combining structure, sequence and protein–protein interaction information. Nucleic Acids Res. 2017, 45, 291–299. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, 174–181. [Google Scholar] [CrossRef] [PubMed]
- McGuffin, L.J.; Edmunds, N.S.; Genc, A.G.; Alharbi, S.M.A.; Salehe, B.R.; Adiyaman, R. Prediction of protein structures, functions and interactions using the IntFOLD7, MultiFOLD and ModFOLDdock servers. Nucleic Acids Res. 2023, 51, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Mustaev, E.; Khamitov, E.M. Predicting the PARP1 Tertiary Structure by Molecular Modeling Methods. J. Struct. Chem. 2025, 66, 898–910. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Liang, S.; Dai, J.; Hou, S.; Su, L.; Zhang, D.; Guo, H.; Hu, S.; Wang, H.; Rao, Z.; Guo, Y.; et al. Structural Basis for Treating Tumor Necrosis Factor α (TNFα)-associated Diseases with the Therapeutic Antibody Infliximab. J. Biol. Chem. 2013, 288, 13799–13807. [Google Scholar] [CrossRef]
- Carrington, B.; Myers, W.K.; Horanyi, P.; Calmianol, M.; Lawson, A.D.G. Natural Conformational Sampling of Human TNFα Visualized by Double Electron-Electron Resonance. Biophys. J. 2017, 113, 371–380. [Google Scholar] [CrossRef]
- Somers, W.; Stahl, M.; Seehra, J.S. Å crystal structure of interleukin 6: Implications for a novel mode of receptor dimerization and signaling. EMBO J. 1997, 16, 989–997. [Google Scholar] [CrossRef]
- Yoon, S.I.; Logsdon, N.J.; Sheikh, F.; Donnelly, R.P.; Walter, M.R. Conformational Changes Mediate Interleukin-10 Receptor 2 (IL-10R2) Binding to IL-10 and Assembly of the Signaling Complex. Protein Struct. Fold. 2006, 281, 35088–35096. [Google Scholar] [CrossRef] [PubMed]
- Daëron, M. The immune system as a system of relations. Front. Immunol. 2022, 13, 984678. [Google Scholar] [CrossRef]
- Wang, R.; Lan, C.; Benlagha, K.; Olsen, N.; Camara, S.; Miller, H.; Kubo, M.; Heegaard, S.; Lee, P.; Yang, L.; et al. The interaction of innate immune and adaptive immune system. MedComm 2024, 5, e714. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, R.N.; Camacho, J.V.; Peñata, E.Z.; Lemus, Y.B.; López-Fernández, C.; Escorcia, L.G.; Fernández-Ponce, C.; Cobos, M.R.; Moreno, J.F.; Fiorillo-Moreno, O. Multiscale information processing in the immune system. Front. Immunol. 2025, 16, 1563992. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From clinical significance to quantification. Adv. Sci. 2021, 15, 2004433. [Google Scholar] [CrossRef]
- Mendes, V.; Galvão, I.; Vieira, A.T. Mechanisms by Which the Gut Microbiota Influences Cytokine Production and Modulates Host Inflammatory Responses. J. Interferon Cytokine Res. 2019, 39, 393–409. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Saredy, J.; Yuan, Z.; Yang, X.; Wang, H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020, 37, 101759. [Google Scholar] [CrossRef]
- Shukla, A.; Tangney, M. Bacterial exopolysaccharides and live biotherapeutics: Orchestrating immune modulation and therapeutic potential in the gut microbiome era. Biomed. Pharmacother. 2025, 191, 118510. [Google Scholar] [CrossRef]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.P.; Fragiadakis, G.K.; Sonnenburg, J.L. Pursuing human-relevant gut microbiota-immune interactions. Immunity 2019, 51, 225–239. [Google Scholar] [CrossRef]
- Gao, B.; Gupta, R.S. Phylogenetic Framework and Molecular Signatures for the Main Clades of the Phylum Actinobacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 66–112. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef]
- Duranti, S.; Longhi, G.; Ventura, M.; van Sinderen, D.; Turroni, F. Exploring the ecology of Bifidobacteria and their genetic adaptation to the mammalian gut. Microorganisms 2020, 9, 8. [Google Scholar] [CrossRef]
- Olajide, T.S.; Ijomone, O.M. Targeting gut microbiota as a therapeutic approach for neurodegenerative diseases. Neuroprotection 2025, 3, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Mopuru, R.; Chaturvedi, S.; Burkholder, B.M. Relapsing Thrombotic Thrombocytopenic Purpura (TTP) in a Patient Treated with Infliximab for Chronic Uveitis. Ocul. Immunol. Inflamm. 2022, 30, 241–243. [Google Scholar] [CrossRef]
- El-Tahan, R.R.; Ghoneim, A.M.; El-Mashad, N. TNF-α gene polymorphisms and expression. Springerplus 2016, 5, 1508. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.; Hazeldine, J.; Moiemen, N.; Harrison, P. IL-8 Induces Neutrophil Extracellular Trap Formation in Severe Thermal Injury. Int. J. Mol. Sci. 2024, 25, 7216. [Google Scholar] [CrossRef]
- Copaescu, A.; Smibert, O.; Gibson, A.; Phillips, E.J.; Trubiano, J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J. Allergy Clin. Immunol. 2020, 146, 518–534. [Google Scholar] [CrossRef]
- Inoue, H.; Nojima, H.; Okayama, H. High efficiency transformation of Escherichia coli with plasmids. Gene 1990, 96, 23–28. [Google Scholar] [CrossRef]
- Mierendorf, R.; Yeager, K.; Novy, R. Innovations. Newsl. Novagen Inc. 1994, 1, 1–3. [Google Scholar]
- Maniatis, T.; Fritsch, E.F.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor: New York, USA, 1984. [Google Scholar]
- Mariani, V.; Biasini, M.; Barbato, A.; Schwede, T. lDDT: A local superposition-free score for comparing protein structures and models using distance difference tests. Bioinformatics 2013, 29, 2722–2728. [Google Scholar] [CrossRef]
- Zheng, W.; Wuyun, Q.; Li, Y.; Liu, Q.; Zhou, X.; Peng, C.; Zhu, Y.; Freddolino, L.; Zhang, Y. Deep-learning-based single-domain and multidomain protein structure prediction with D-I-TASSER. Nat. Biotechnol. 2025, 23, 1–26. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, W.; Li, Y.; Pearce, R.; Zhang, C.; Bell, E.W.; Zhang, G.; Zhang, Y. I-TASSER-MTD: A deep-learning-based platform for multi-domain protein structure and function prediction. Nat. Protoc. 2022, 17, 2326–2353. [Google Scholar] [CrossRef]
- McGuffin, L.J.; Shuid, A.N.; Kempster, R.; Maghrabi, A.H.A.; Nealon, J.O.; Salehe, B.R.; Atkins, J.D.; Roche, D.B. Accurate template-based modeling in CASP12 using the IntFOLD4-TS, ModFOLD6, and ReFOLD methods. Proteins 2018, 86, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Brenke, R.; Comeau, S.R.; Vajda, S. PIPER: An FFT-Based Protein Docking Program with Pairwise Potentials. PROTEINS: Struct. Funct. Bioinform. 2006, 65, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein-protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the ACM/IEEE SC2006 Conference on High Performance Networking and Computing, Tampa, FL, USA, 11–17 November 2006. [Google Scholar]
- Frey, B.J.; Dueck, D. Clustering by Passing Messages Between Data Points. Science 2007, 315, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Haimes, J.; Kelley, M. Demonstration of a ΔΔCq Calculation Method to Compute Relative Gene Expression from qPCR Data; A Horizon Discovery Group Company: Lafayette, CO, USA, 2015; pp. 1–4. [Google Scholar]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. Available online: https://www.biomedcentral.com/1471-2105/13/134 (accessed on 24 April 2025). [CrossRef] [PubMed]
| TNFα | Ka (1/Ms) * | Kd (1/s) | KD (nM) |
|---|---|---|---|
| ΔFN3.1 | 3.43 × 10−5 | 451 × 10−5 | 13.1 ± 0.6 |
| ΔFN3.3 | 13.1 × 10−5 | 76.1 × 10−5 | 58.2 ± 2.9 |
| Albumin | Below the level of detection | ||
| ΔFN3.1 | Ka (1/Ms) | Ka (1/Ms) | Ka (1/Ms) |
|---|---|---|---|
| pH 7.4 | |||
| IL-6 | Below the level of detection | ||
| IL-8 | 330 × 10−5 | 1.6 × 10−5 | 4.9 ± 0.2 |
| IL-10 | 22.5 × 10−5 | 140 × 10−5 | 62.2 ± 3.1 |
| pH 8.0 | |||
| IL-6 | Below the level of detection | ||
| IL-8 | 397 × 10−5 | 1.6 × 10−5 | 4.0 ± 0.2 |
| IL-10 | Below the level of detection | ||
| ΔFN3.1 | Ka (1/Ms) | Ka (1/Ms) | Ka (1/Ms) |
|---|---|---|---|
| pH 7.4 | |||
| IL-6 | Below the level of detection | ||
| IL-8 | 878 × 10−5 | 2.0 × 10−5 | 2.3 ± 0.1 |
| IL-10 | Below the level of detection | ||
| pH 8.0 | |||
| IL-6 | Below the level of detection | ||
| IL-8 | 879 × 10−5 | 1.1 × 10−5 | 1.2 ± 0.04 |
| IL-10 | Below the level of detection | ||
| Protein | Amino Acid Residues | Location |
|---|---|---|
| ΔFN3.1 | Trp78, Ser79, Pro81, Ser82 | Cytokine receptor motif (FN3- domain I) |
| Trp174, Ser175, Glu177, Ser178 | Cytokine receptor motif (FN3- domain II) | |
| Ala43, Ala51, Thr111, Pro417, Ala424 | [31] | |
| ΔFN3.3 | Trp77, Ser78, Pro80, Ser81 | Cytokine receptor motif (FN3- domain I) |
| Glu172, Gly173, Pro175, Ser176 | Cytokine receptor motif (FN3- domain II) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alekseeva, M.G.; Borisevich, S.S.; Yusupova, A.R.; Reznikova, D.A.; Mavletova, D.A.; Nesterov, A.A.; Ilyina, M.G.; Akimova, N.I.; Shtil, A.A.; Danilenko, V.N. Differential Binding of ΔFN3 Proteins of Bifidobacterium longum GT15 and Bifidobacterium bifidum 791 to Cytokines Determined by Surface Plasmon Resonance and De Novo Molecular Modeling. Int. J. Mol. Sci. 2025, 26, 10560. https://doi.org/10.3390/ijms262110560
Alekseeva MG, Borisevich SS, Yusupova AR, Reznikova DA, Mavletova DA, Nesterov AA, Ilyina MG, Akimova NI, Shtil AA, Danilenko VN. Differential Binding of ΔFN3 Proteins of Bifidobacterium longum GT15 and Bifidobacterium bifidum 791 to Cytokines Determined by Surface Plasmon Resonance and De Novo Molecular Modeling. International Journal of Molecular Sciences. 2025; 26(21):10560. https://doi.org/10.3390/ijms262110560
Chicago/Turabian StyleAlekseeva, Maria G., Sophia S. Borisevich, Alfia R. Yusupova, Diana A. Reznikova, Dilara A. Mavletova, Andrey A. Nesterov, Margarita G. Ilyina, Natalia I. Akimova, Alexander A. Shtil, and Valery N. Danilenko. 2025. "Differential Binding of ΔFN3 Proteins of Bifidobacterium longum GT15 and Bifidobacterium bifidum 791 to Cytokines Determined by Surface Plasmon Resonance and De Novo Molecular Modeling" International Journal of Molecular Sciences 26, no. 21: 10560. https://doi.org/10.3390/ijms262110560
APA StyleAlekseeva, M. G., Borisevich, S. S., Yusupova, A. R., Reznikova, D. A., Mavletova, D. A., Nesterov, A. A., Ilyina, M. G., Akimova, N. I., Shtil, A. A., & Danilenko, V. N. (2025). Differential Binding of ΔFN3 Proteins of Bifidobacterium longum GT15 and Bifidobacterium bifidum 791 to Cytokines Determined by Surface Plasmon Resonance and De Novo Molecular Modeling. International Journal of Molecular Sciences, 26(21), 10560. https://doi.org/10.3390/ijms262110560








