Anti-Metastatic Effects of Plukenetia volubilis (Sacha Inchi) Husk Extract via EGFR and EMT Pathways and Other Antitumor Effects in Colon Cancer
Abstract
1. Introduction
2. Results
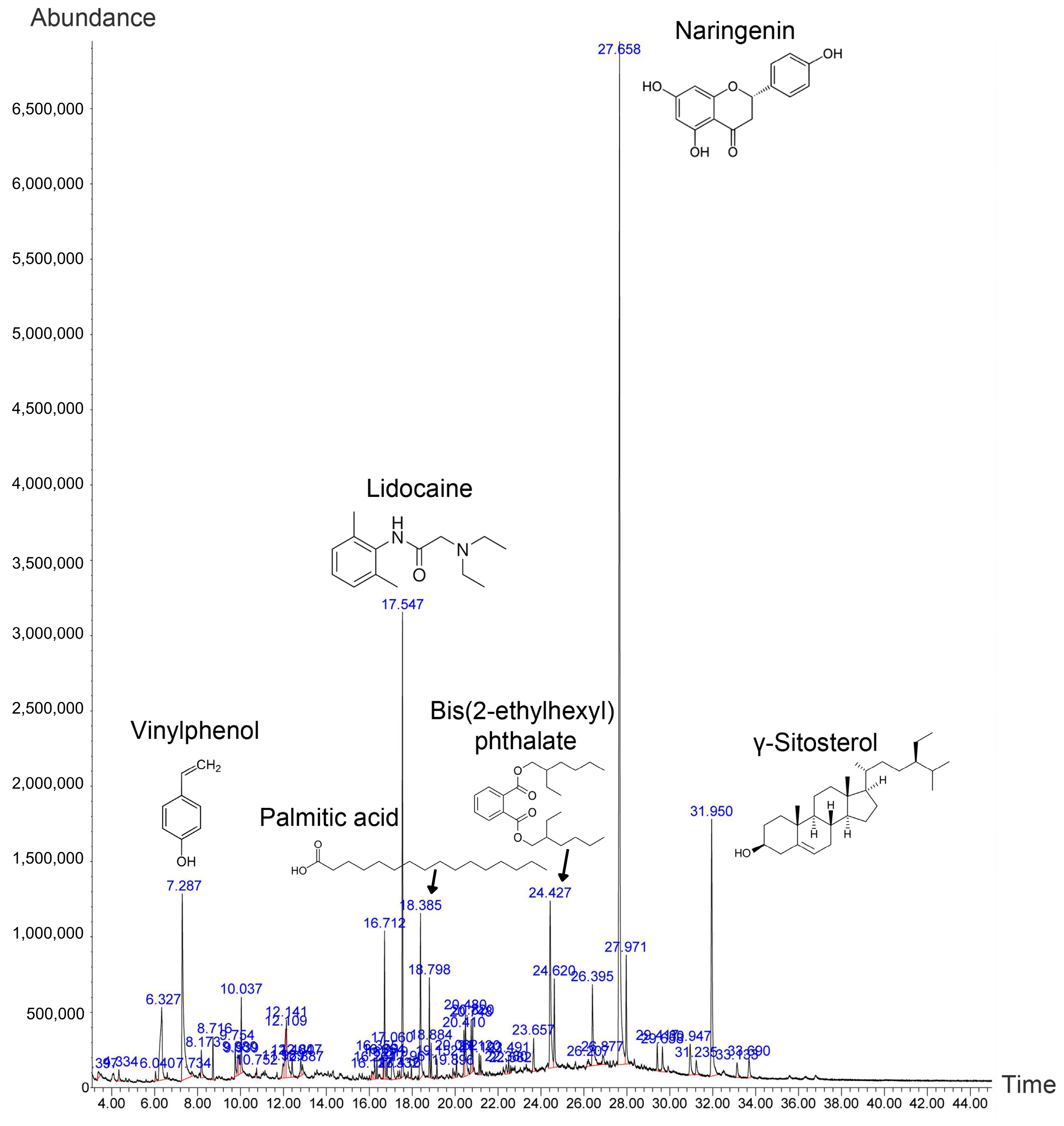
2.1. Phytochemical Screening of the Husk Extract Using Mass Spectrometry
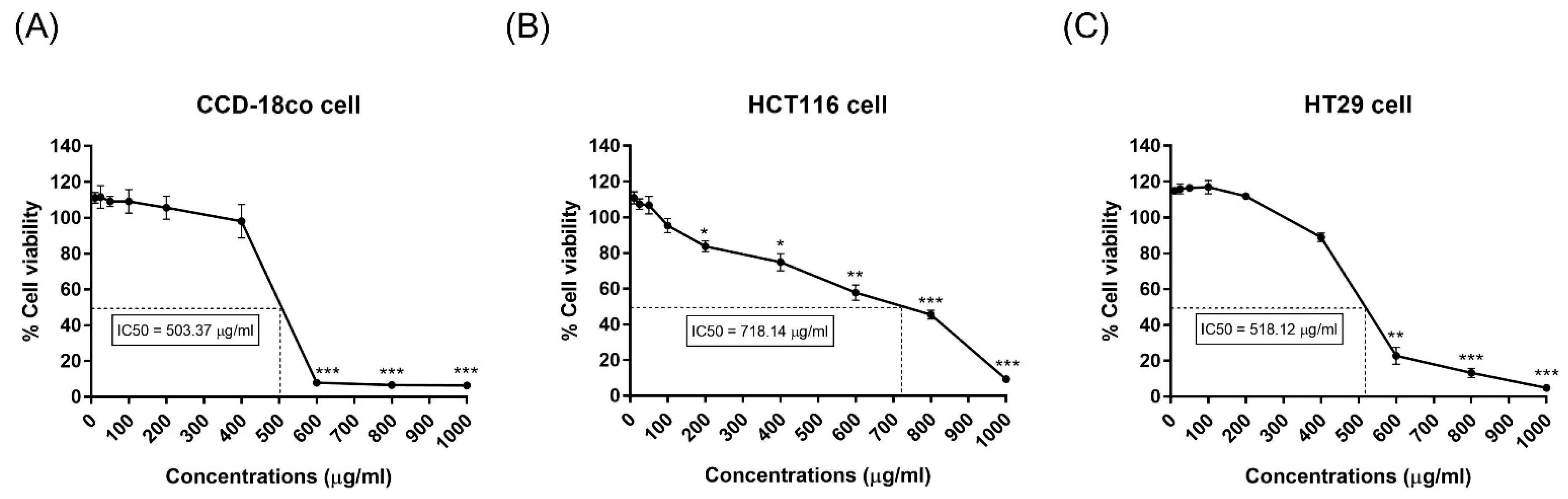
2.2. Cytotoxicity of the Husk Extract Against Cell Lines
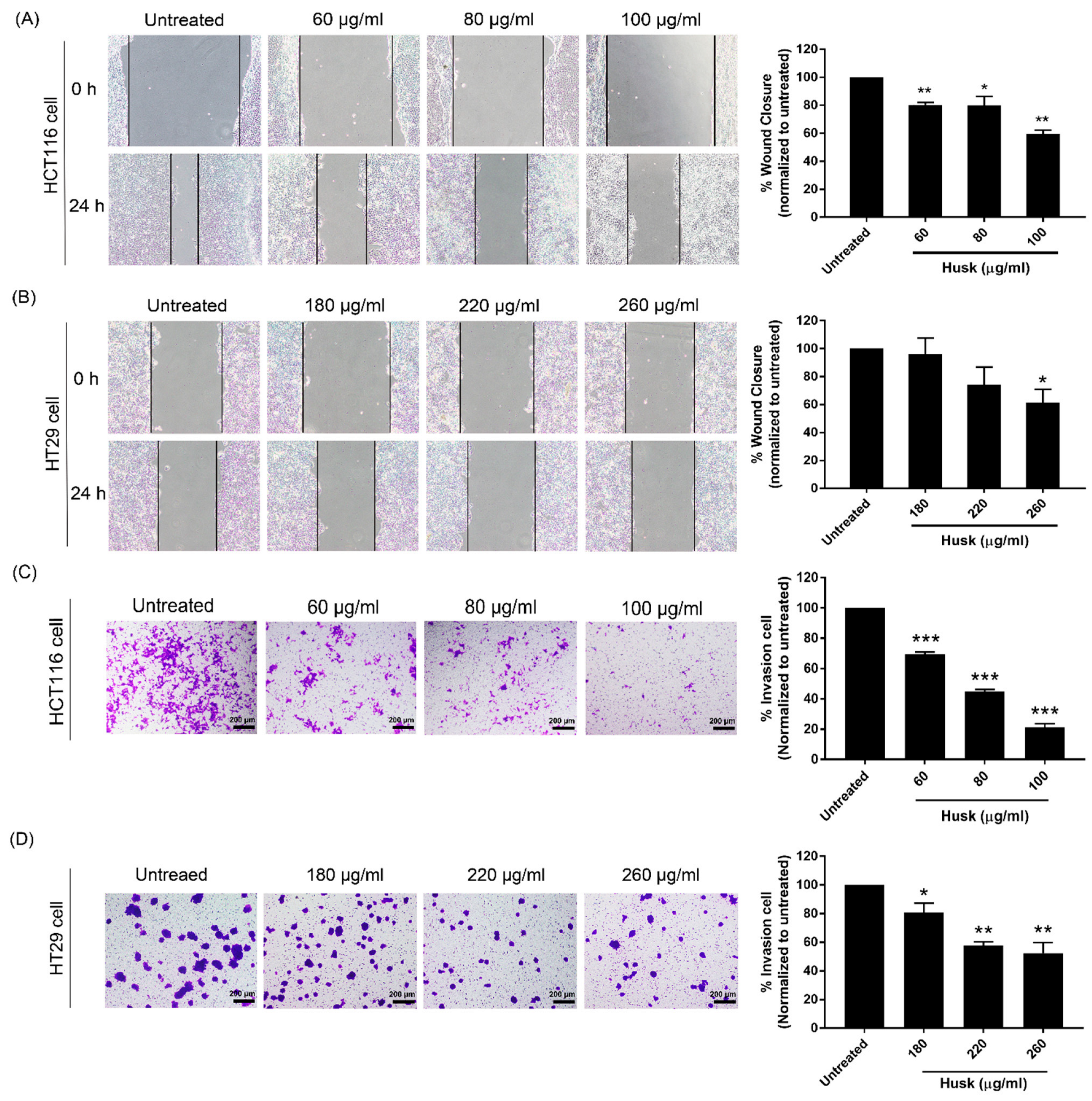
2.3. The Husk Extract Inhibits the Migration and Invasion of Colon Cancer Cells
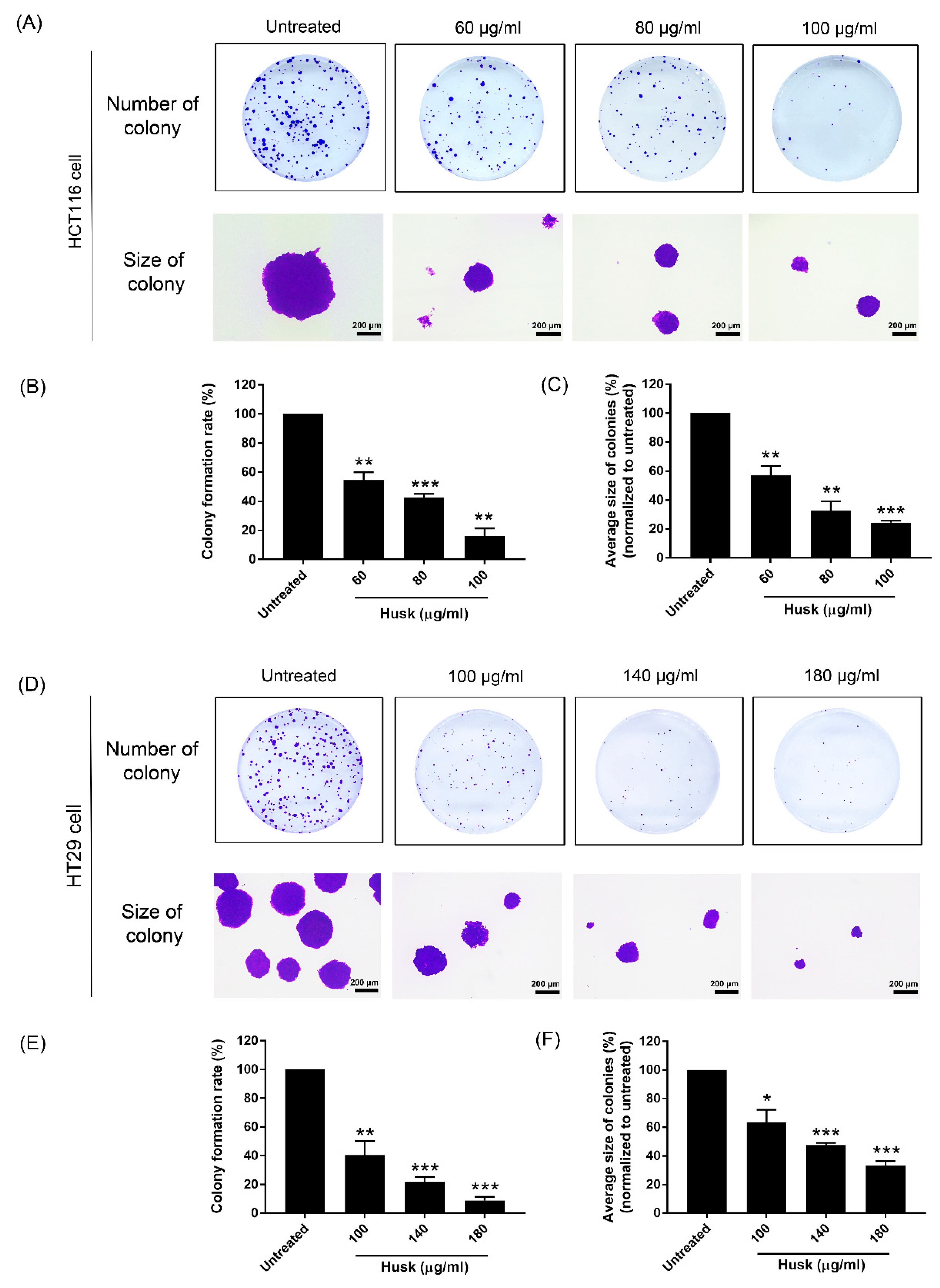
2.4. Effect of the Husk Extract on the Colony Formation of Colon Cancer Cell Growth
2.5. Effect of Husk Extract Treatment on the Gene Expression of Colon Cancer Cells
2.6. Effect of Husk Extract Treatment on Colorectal Cancer Cell Proteomes
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Extraction
4.2. Phytochemical Evaluation
4.2.1. Determination of Total Phenols by Folin–Ciocalteu Reagent Method
4.2.2. Determination of Total Flavonoid Content by Aluminum Chloride Colorimetric Method
4.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
4.4. Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
4.5. Cell Cultures
4.6. MTT Assay
4.7. Cell Migration by Wound Scratch Assay
4.8. Cell Invasion by Transwell Assay
4.9. Colony Formation
4.10. Transcriptome
4.11. Sample Preparation and Proteomic Analysis by Quadrupole Time-of-Flight (QTOF) Mass Spectrometry
4.12. Protein Identification and Functional Annotation
4.13. Western Blot Analysis
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CES | Collision energy spread |
| DEGs | Differentially expressed genes |
| DMSO | Dimethyl Sulfoxide |
| DP | Decluttering potential |
| DTT | Dithiothreitol |
| EMT | Epithelial–Mesenchymal Transition |
| FA | Formic acid |
| GC-MS | Gas chromatography–mass spectrometry |
| GO | Gene Ontology |
| IAA | Alkylating reagent |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| MET | Mesenchymal–Epithelial Transition |
| MMP | Matrix metalloproteinases |
| NIST | National Institute of Standards and Technology |
| PBS | Phosphate-buffered saline |
| PBST | Phosphate-buffered saline and 20% Tween |
| RNAseq | RNA sequencing |
| PMSF | Phenyl-Methyl-Sulfonyl-Fluoride |
| QTOF | Quadrupole Time-of-Flight |
| SRA | Sequence Read Archive |
| SD | Standard deviation |
| SDS-PAGE | Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis |
| TFC | Total flavonoid content |
| TPC | Total phenolic content |
| TIMPs | Tissue inhibitors of metalloproteinases |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Cancer statistics: A comparison between world health organization (WHO) and global burden of disease (GBD). Eur. J. Public Health 2020, 30, 1026–1027. [Google Scholar] [CrossRef]
- Spano, D.; Heck, C.; De Antonellis, P.; Christofori, G.; Zollo, M. Molecular networks that regulate cancer metastasis. Semin. Cancer Biol. 2012, 22, 234–249. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X. Characteristics and significance of the pre-metastatic niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Okegawa, T.; Pong, R.-C.; Li, Y.; Hsieh, J.-T. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim. Pol. 2004, 51, 445–457. [Google Scholar] [CrossRef]
- Pepper, M.S.; Montesano, R.; Mandriota, S.J.; Orci, L.; Vassalli, J.-D. Angiogenesis: A paradigm for balanced extracellular proteolysis during cell migration and morphogenesis. Enzym. Protein 1996, 49, 138–162. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S.; Mirza, H.; Conner, C.E.; Lorenz, A.F.; Drews, M.H.; Bahou, W.F.; Jesty, J. Vascular endothelial groth factor induces tissue factor and matrix metalloproteinase production in endothelial cells: Conversion of prothrombin to thrombin results in progelatininase a activation and cell proliferation. Int. J. Cancer 1998, 75, 780–786. [Google Scholar] [CrossRef]
- Zhao, X.; Xiu, J.; Yang, H.; Han, W.; Jin, Y. Network pharmacology and bioinformatics study of six medicinal food homologous plants against colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 930. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alkafaas, S.S.; Saad, A.M.; Mohammed, D.M.; Korma, S.A.; Salem, H.M.; Abd El-Mageed, T.A.; Elsalahaty, M.I.; Elkafas, S.S.; Mosa, W.F. Medicinal plants: Nutritional, immunological and therapeutic role in treating cancer-related malnutrition: A comprehensive review. Cancer Cell Int. 2025, 25, 266. [Google Scholar] [CrossRef]
- Kangra, K.; Kakkar, S.; Mittal, V.; Kumar, V.; Aggarwal, N.; Chopra, H.; Malik, T.; Garg, V. Incredible use of plant-derived bioactives as anticancer agents. RSC Adv. 2025, 15, 1721–1746. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An emerging anticancer flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, X.; Zhuang, J.; Hao, H. The Anticancer Perspective of Tangeretin: A Small Review. Molecules 2025, 30, 300. [Google Scholar] [CrossRef]
- Dincheva, I.; Badjakov, I.; Galunska, B. New Insights in the Research on Bioactive Compounds from Plant Origins with Nutraceutical and Pharmaceutical Potential II. Plants 2025, 14, 500. [Google Scholar] [CrossRef]
- Preedy, V.R.; Watson, R.R. Nuts and Seeds in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Keawkim, K.; Lorjaroenphon, Y.; Vangnai, K.; Jom, K.N. Metabolite–flavor profile, phenolic content, and antioxidant activity changes in sacha inchi (Plukenetia volubilis L.) seeds during germination. Foods 2021, 10, 2476. [Google Scholar] [CrossRef]
- Goyal, A.; Tanwar, B.; Sihag, M.K.; Sharma, V. Sacha inchi (Plukenetia volubilis L.): An emerging source of nutrients, omega-3 fatty acid and phytochemicals. Food Chem. 2022, 373, 131459. [Google Scholar] [CrossRef]
- Qiu, Z.; Cheng, Y.; Liu, H.; Li, T.; Jiang, Y.; Lu, Y.; Jiang, D.; Zhang, X.; Wang, X.; Kang, Z. Screening colorectal cancer associated autoantigens through multi-omics analysis and diagnostic performance evaluation of corresponding autoantibodies. BMC Cancer 2025, 25, 713. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Hu, Y.; Dai, Z.; Lou, Q.; Xu, W.; Chen, L. Precision medicine research progress based on colorectal cancer organoids. Discov. Oncol. 2025, 16, 1181. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Yang, L.; Yin, F.-T.; Sun, Y.; Li, X.-H.; Li, J.; Wang, X.-J. Multi-omics approaches in colorectal cancer screening and diagnosis, recent updates and future perspectives. Cancers 2022, 14, 5545. [Google Scholar] [CrossRef]
- Basu, B.; De Sampaio, P.C.; Mohammed, H.; Fogarasi, M.; Corrie, P.; Watkins, N.; Smethurst, P.; English, W.; Ouwehand, W.; Murphy, G. Inhibition of MT1-MMP activity using functional antibody fragments selected against its hemopexin domain. Int. J. Biochem. Cell Biol. 2012, 44, 393–403. [Google Scholar] [CrossRef]
- Chirinos, R.; Necochea, O.; Pedreschi, R.; Campos, D. Sacha inchi (Plukenetia volubilis L.) shell: An alternative source of phenolic compounds and antioxidants. Int. J. Food Sci. Technol. 2016, 51, 986–993. [Google Scholar] [CrossRef]
- Sainakham, M.; Mungmai, L. In vitro anti-oxidative activity and tyrosinase inhibition of Inca peanut (Plukenetia volubilis L.) shell extracts from different preparation methods. Thai J. Sci. Technol. 2020, 9, 407–417. [Google Scholar]
- Sanchez-Reinoso, Z.; Mora-Adames, W.I.; Fuenmayor, C.A.; Darghan-Contreras, A.E.; Gardana, C.; Gutiérrez, L.-F. Microwave-assisted extraction of phenolic compounds from Sacha Inchi shell: Optimization, physicochemical properties and evaluation of their antioxidant activity. Chem. Eng. Process. Process Intensif. 2020, 153, 107922. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Ma, J.; Smith-Warner, S.A.; Lee, J.E.; Giovannucci, E. Vitamin B6 and colorectal cancer: Current evidence and future directions. World J. Gastroenterol. WJG 2013, 19, 1005. [Google Scholar] [CrossRef]
- Prasongsub, W.; Pimsan, N.; Buranapattarachote, C.; Punturee, K. Anti-HMG-CoA reductase and antioxidant activities of Sacha inchi (Plukenetia volubilis L.) nutshell extract. J. Assoc. Med. Sci. 2021, 54, 18–26. [Google Scholar]
- Kittibunchakul, S.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Temviriyanukul, P.; Suttisansanee, U. Evaluation of sacha inchi (Plukenetia volubilis L.) by-products as valuable and sustainable sources of health benefits. Horticulturae 2022, 8, 344. [Google Scholar] [CrossRef]
- Boateng, J.; Verghese, M.; Walker, L.; Ogutu, S. Effect of processing on antioxidant contents in selected dry beans (Phaseolus spp. L.). LWT-Food Sci. Technol. 2008, 41, 1541–1547. [Google Scholar] [CrossRef]
- Rana, J.N.; Gul, K.; Mumtaz, S. Isorhamnetin: Reviewing Recent Developments in Anticancer Mechanisms and Nanoformulation-Driven Delivery. Int. J. Mol. Sci. 2025, 26, 7381. [Google Scholar] [CrossRef]
- Cheng, H.; Jiang, X.; Zhang, Q.; Ma, J.; Cheng, R.; Yong, H.; Shi, H.; Zhou, X.; Ge, L.; Gao, G. Naringin inhibits colorectal cancer cell growth by repressing the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2020, 19, 3798–3804. [Google Scholar] [CrossRef]
- Chaitanya, N.S.; Devi, A.; Sahu, S.; Alugoju, P. Molecular mechanisms of action of Trehalose in cancer: A comprehensive review. Life Sci. 2021, 269, 118968. [Google Scholar] [CrossRef]
- Hirakawa, N.; Okauchi, R.; Miura, Y.; Yagasaki, K. Anti-invasive activity of niacin and trigonelline against cancer cells. Biosci. Biotechnol. Biochem. 2005, 69, 653–658. [Google Scholar] [CrossRef]
- Xu, W.; Liu, J.; Li, C.; Wu, H.-Z.; Liu, Y.-W. Kaempferol-7-O-β-d-glucoside (KG) isolated from Smilax china L. rhizome induces G2/M phase arrest and apoptosis on HeLa cells in a p53-independent manner. Cancer Lett. 2008, 264, 229–240. [Google Scholar] [CrossRef]
- Zhu, D.; Li, B.; Wang, C.; Jiang, P.; Tang, F.; Li, Y. Echinocystic acid induces the apoptosis, and inhibits the migration and invasion of non-small cell lung cancer cells. Med. Oncol. 2023, 40, 182. [Google Scholar] [CrossRef]
- Kikuyama, F.; Suzuki, S.; Jibiki, A.; Yokoyama, Y.; Kawazoe, H.; Kitanaka, S.; Nakamura, T. Ingenol mebutate inhibits the growth of pancreatic cancer cells in vitro via STING with an efficacy comparable to that of clinically used anticancer agents. J. Nat. Med. 2023, 77, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, G.; Sun, C.; Li, H.; Fu, Y.; Xu, W. Apoptosis effects of dihydrokaempferol isolated from Bauhinia championii on synoviocytes. Evid.-Based Complement. Altern. Med. 2018, 2018, 9806160. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Chelakkot, V.S.; Licursi, M.; Rutihinda, S.G.; Som, J.; Derwish, L.; King, J.J.; Pongnopparat, T.; Mearow, K.; Larijani, M. Enhancement of cancer-specific protoporphyrin IX fluorescence by targeting oncogenic Ras/MEK pathway. Theranostics 2018, 8, 2134. [Google Scholar] [CrossRef]
- Qu, X.; Yang, L.; Shi, Q.; Wang, X.; Wang, D.; Wu, G. Lidocaine inhibits proliferation and induces apoptosis in colorectal cancer cells by upregulating mir-520a-3p and targeting EGFR. Pathol. Res. Pract. 2018, 214, 1974–1979. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Hong, Z.; Li, Y.; Deng, X.; Chen, M.; Pan, J.; Chen, Z.; Zhang, X.; Wang, C.; Qiu, C. Comprehensive analysis of triphenyl phosphate: An environmental explanation of colorectal cancer progression. Ecotoxicol. Environ. Saf. 2022, 241, 113778, Correction in Ecotoxicol. Environ. Saf. 2022, 242, 113911. [Google Scholar] [CrossRef]
- Cai, Z.; Lim, D.; Liu, G.; Ding, W.; Wang, Z.; Tian, Z.; Peng, J.; Dong, C.; Zhang, F.; Feng, Z. 2-hexyl-4-pentynoic acid (HPTA), a novel radiosensitizer to breast cancer cells through increasing the instability of DNA repair proteins. Res. Sq. 2020, 4, 204–213. [Google Scholar]
- Rana, N.; Huang, S.; Patel, P.; Samuni, U.; Sabatino, D. Synthesis, characterization and anti-cancer activity of a peptide nucleolipid bioconjugate. Bioorganic Med. Chem. Lett. 2016, 26, 3567–3571. [Google Scholar] [CrossRef]
- Gupta, P.; Srivastava, S.K. Antitumor activity of phenethyl isothiocyanate in HER2-positive breast cancer models. BMC Med. 2012, 10, 80. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, P.; Du, W.; Xu, R.; Yan, G.; Deng, Y.; Li, X.; Chen, Y. Metabolically stable diphenylamine derivatives suppress androgen receptor and BET protein in prostate cancer. Biochem. Pharmacol. 2020, 177, 113946. [Google Scholar] [CrossRef]
- Hwang, C.J.; Choi, J.Y.; Park, M.H.; Song, M.J.; Oh, K.W.; Son, D.J.; Lee, S.H.; Han, S.B.; Hong, J.T. Inhibitory effect of carnosol on phthalic anhydride-induced atopic dermatitis via inhibition of STAT3. Biomol. Ther. 2017, 25, 535. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.-X. Synergistic enhancement of the antitumor activity of 5-fluorouracil by bornyl acetate in SGC-7901 human gastric cancer cells and the determination of the underlying mechanism of action. Gastric Cancer 2016, 4, 5. [Google Scholar]
- He, Z.; Lin, J.; Peng, D.; Zeng, J.; Pan, X.; Zheng, R.; Li, P.; Du, B. Peptide fractions from Sacha inchi induced apoptosis in HepG2 cells via P53 activation and a mitochondria-mediated pathway. J. Sci. Food Agric. 2023, 103, 7621–7630. [Google Scholar] [CrossRef]
- Chang, H.-L.; Chang, Y.-M.; Lai, S.-C.; Chen, K.-M.; Wang, K.-C.; Chiu, T.-T.; Chang, F.-H.; Hsu, L.-S. Naringenin inhibits migration of lung cancer cells via the inhibition of matrix metalloproteinases 2 and -9. Exp. Ther. Med. 2017, 13, 739–744. [Google Scholar] [CrossRef]
- Sarojamma, V.; Vadde, R. Naringenin in the prevention of colon cancer: An updated review. Onco Ther. 2022, 9, 25–41. [Google Scholar] [CrossRef]
- Alhalmi, A.; Amin, S.; Ralli, T.; Ali, K.S.; Kohli, K. Therapeutic role of naringin in cancer: Molecular pathways, synergy with other agents, and nanocarrier innovations. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 3595–3615. [Google Scholar] [CrossRef] [PubMed]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.-L. Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef]
- Kim, M.; Baek, M.; Kim, D.J. Protein tyrosine signaling and its potential therapeutic implications in carcinogenesis. Curr. Pharm. Des. 2017, 23, 4226–4246. [Google Scholar] [CrossRef] [PubMed]
- Tabana, Y.; Dahham, S.; Shah, A.; Majid, A. Major signaling pathways of colorectal carcinogenesis. Recent Adv. Colon Cancer 2016, 1, 1–2. [Google Scholar]
- Jeong, W.-J.; Ro, E.J.; Choi, K.-Y. Interaction between Wnt/β-catenin and RAS-ERK pathways and an anti-cancer strategy via degradations of β-catenin and RAS by targeting the Wnt/β-catenin pathway. NPJ Precis. Oncol. 2018, 2, 5. [Google Scholar] [CrossRef]
- Saletti, P.; Molinari, F.; De Dosso, S.; Frattini, M. EGFR signaling in colorectal cancer: A clinical perspective. Gastrointest. Cancer Targets Ther. 2015, 5, 21–38. [Google Scholar]
- Hutchinson, R.A.; Adams, R.A.; McArt, D.G.; Salto-Tellez, M.; Jasani, B.; Hamilton, P.W. Epidermal growth factor receptor immunohistochemistry: New opportunities in metastatic colorectal cancer. J. Transl. Med. 2015, 13, 217. [Google Scholar] [CrossRef]
- Koveitypour, Z.; Panahi, F.; Vakilian, M.; Peymani, M.; Seyed Forootan, F.; Nasr Esfahani, M.H.; Ghaedi, K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019, 9, 97. [Google Scholar] [CrossRef]
- Stefani, C.; Miricescu, D.; Stanescu-Spinu, I.-I.; Nica, R.I.; Greabu, M.; Totan, A.R.; Jinga, M. Growth factors, PI3K/AKT/mTOR and MAPK signaling pathways in colorectal cancer pathogenesis: Where are we now? Int. J. Mol. Sci. 2021, 22, 10260. [Google Scholar] [CrossRef]
- Qu, X.; Hamidi, H.; Johnson, R.M.; Sokol, E.S.; Lin, E.; Eng, C.; Kim, T.W.; Bendell, J.; Sivakumar, S.; Kaplan, B. Ligand-activated EGFR/MAPK signaling but not PI3K, are key resistance mechanisms to EGFR-therapy in colorectal cancer. Nat. Commun. 2025, 16, 4332. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Yoshida, S.; Inoue, T.; Sekido, Y.; Hata, T.; Hamabe, A.; Ogino, T.; Miyoshi, N.; Uemura, M.; Yamamoto, H. The role of KRAS mutations in colorectal cancer: Biological insights, clinical implications, and future therapeutic perspectives. Cancers 2025, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Tat, T.; Jurj, A.; Selicean, C.; Pasca, S.; Ionescu, D. Antiproliferative effects of propofol and lidocaine on the colon adenocarcinoma microenvironment. J. Buon. 2019, 24, 106–115. [Google Scholar]
- Bundscherer, A.C.; Malsy, M.; Bitzinger, D.I.; Wiese, C.H.; Gruber, M.A.; Graf, B.M. Effects of lidocaine on HT-29 and SW480 colon cancer cells in vitro. Anticancer Res. 2017, 37, 1941–1945. [Google Scholar] [PubMed]
- Yoon, S.-O.; Park, S.-J.; Yun, C.-H.; Chung, A.-S. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. BMB Rep. 2003, 36, 128–137. [Google Scholar] [CrossRef]
- Zhou, H.; Huang, S. Role of mTOR signaling in tumor cell motility, invasion and metastasis. Adv. Cancer Drug Targets 2016, 3, 207–244. [Google Scholar]
- Bauvois, B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2012, 1825, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lustosa, S.A.S.; de Souza Viana, L.; Affonso Jr, R.J.; Silva, S.R.M.; Denadai, M.V.A.; de Toledo, S.R.C.; Oliveira, I.D.; Matos, D. Expression Profiling Using a cDNA Array and Immunohistochemistry for the Extracellular Matrix Genes FN-1, ITGA-3, ITGB-5, MMP-2, and MMP-9 in Colorectal Carcinoma Progression and Dissemination. Sci. World J. 2014, 2014, 102541. [Google Scholar] [CrossRef]
- Jolly, M.K.; Ware, K.E.; Gilja, S.; Somarelli, J.A.; Levine, H. EMT and MET: Necessary or permissive for metastasis? Mol. Oncol. 2017, 11, 755–769. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Nieto, M.A. Epithelial plasticity: A common theme in embryonic and cancer cells. Science 2013, 342, 1234850. [Google Scholar] [CrossRef]
- Hahn, S.; Jackstadt, R.; Siemens, H.; Hünten, S.; Hermeking, H. SNAIL and miR-34a feed-forward regulation of ZNF281/ZBP99 promotes epithelial–mesenchymal transition. EMBO J. 2013, 32, 3079–3095. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, N.; Han, Q.; Lu, W.; Wang, L.; Yang, D.; Zheng, M.; Zhang, Z.; Liu, H.; Lee, T.H. Pin1 inhibition reverses the acquired resistance of human hepatocellular carcinoma cells to Regorafenib via the Gli1/Snail/E-cadherin pathway. Cancer Lett. 2019, 444, 82–93, Erratum in Cancer Lett. 2024, 604, 217257. [Google Scholar] [CrossRef]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarker s and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, H.; Xia, W.; Li, M.; Niu, Y.; Fu, X. Investigation of the effects of glabridin on the proliferation, apoptosis, and migration of the human colon cancer cell lines SW480 and SW620 and its mechanism based on reverse virtual screening and proteomics. Oxidative Med. Cell. Longev. 2023, 2023, 1117431. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A. Targeting the RAS upstream and downstream signaling pathway for cancer treatment. Eur. J. Pharmacol. 2024, 979, 176727. [Google Scholar] [CrossRef] [PubMed]
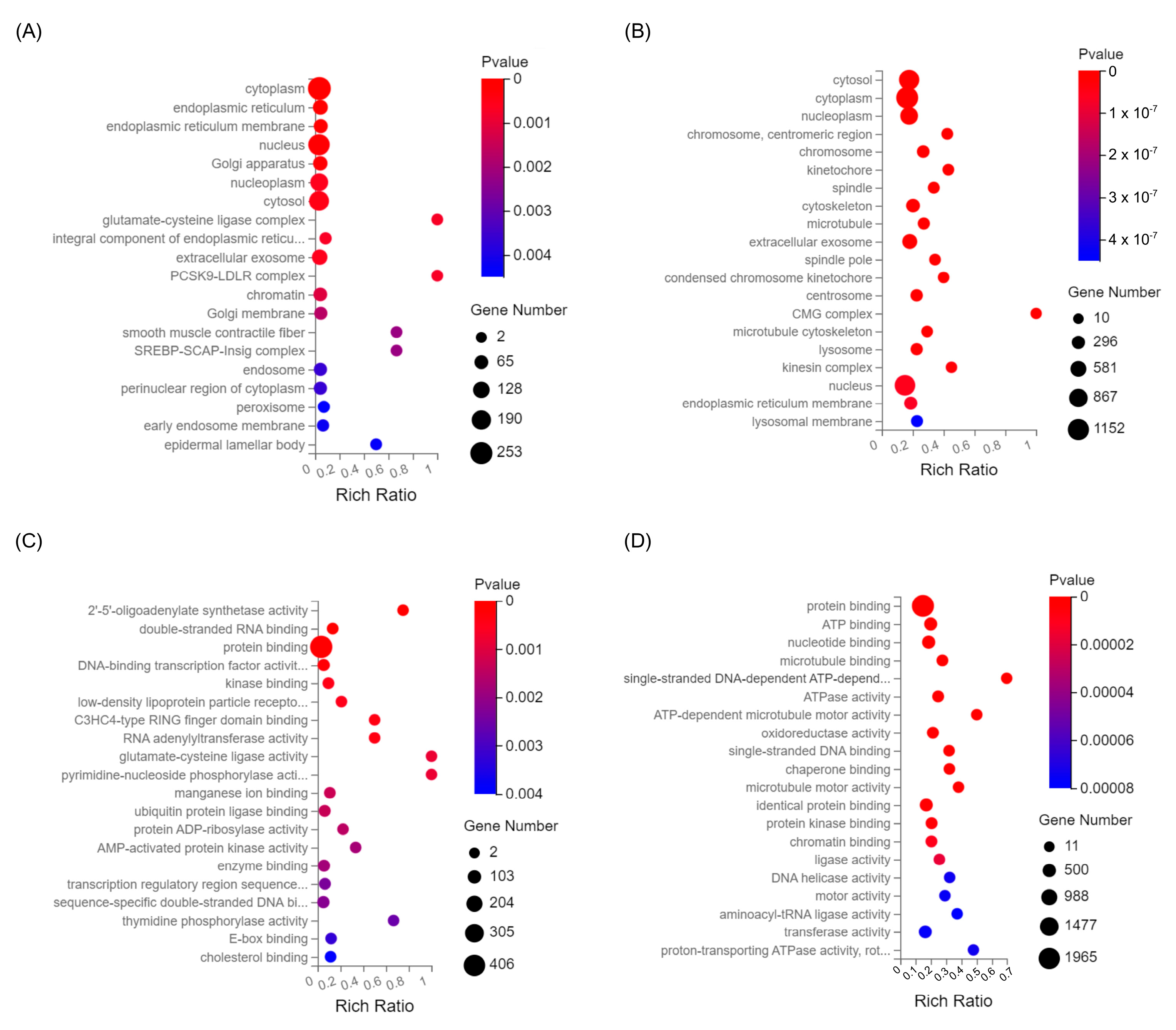

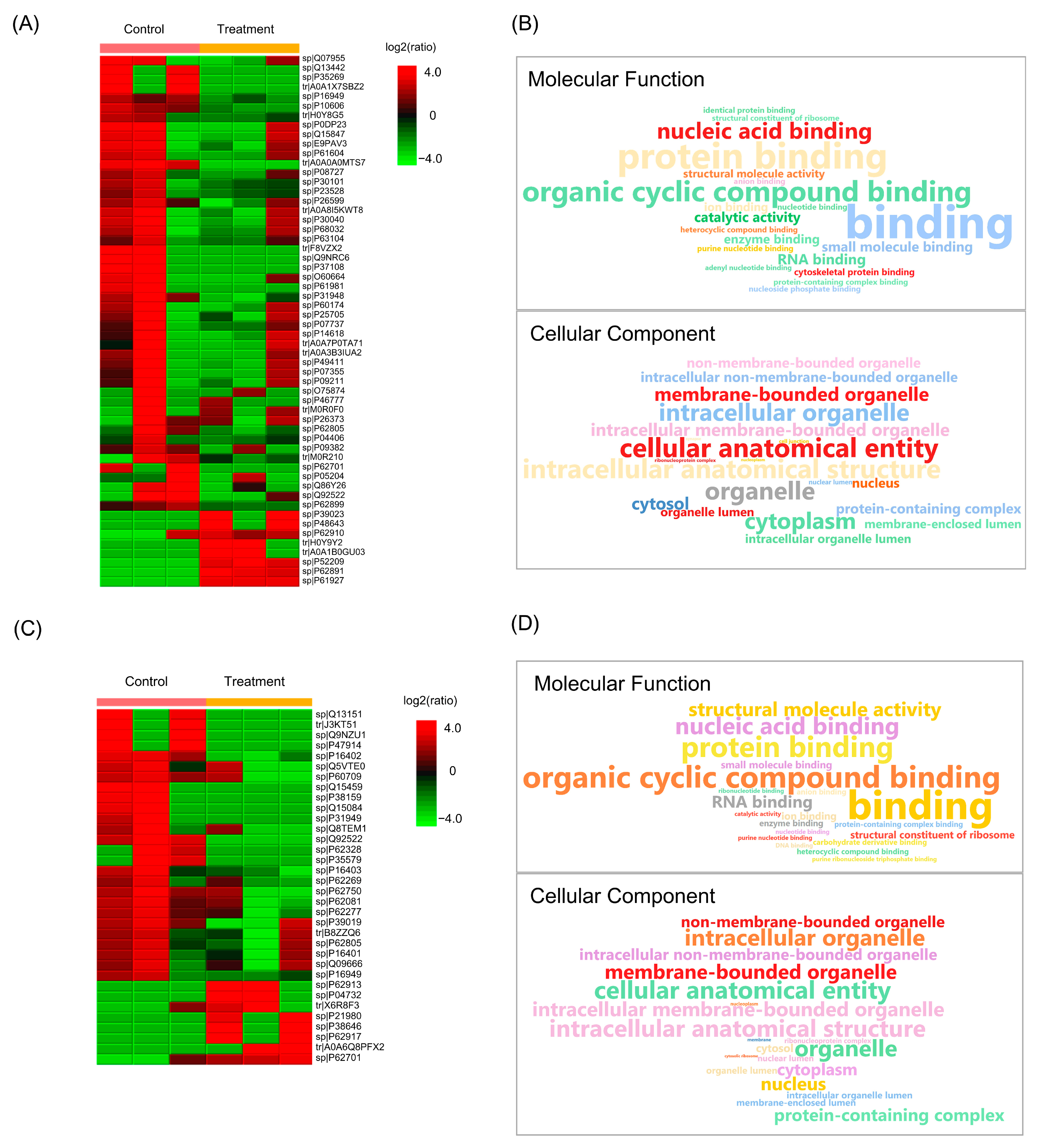
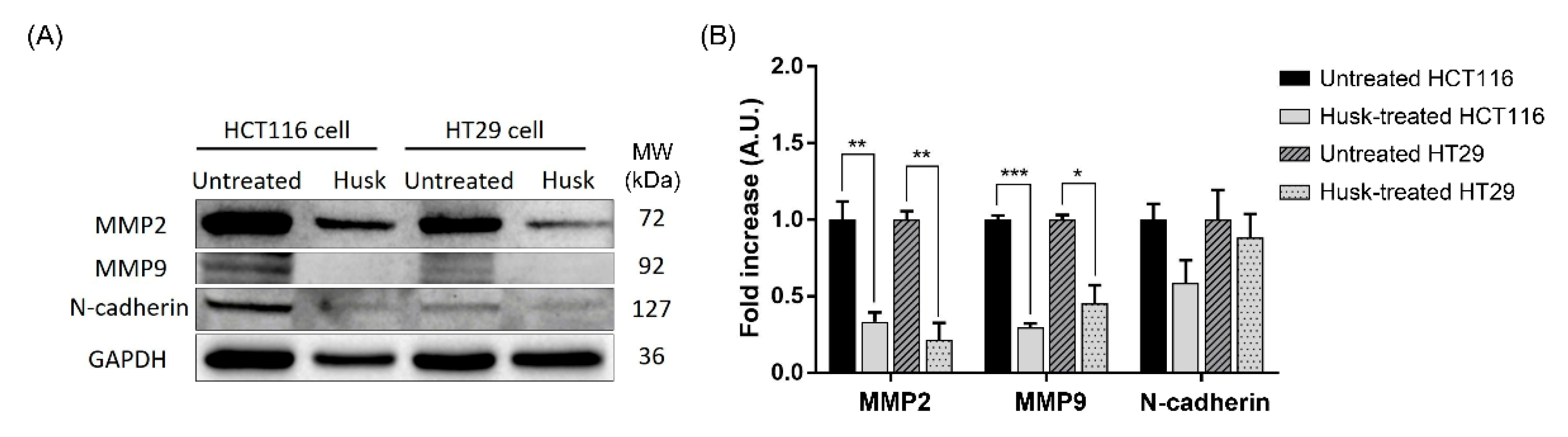

| Cell Line (Treatment Concentration) | Total DEGs | Significantly Upregulated | Significantly Downregulated |
|---|---|---|---|
| HCT116 (100 µg/mL extract) | 15,589 | 7632 | 7957 |
| HT29 (250 µg/mL extract) | 15,566 | 7726 | 7840 |
| Compound | Class | Activity | Ref. |
|---|---|---|---|
| Alpha, beta-trehalose | Disaccharide | Targeting cell progression, angiogenesis, and metastasis pathways at molecular level and targeting EGFR, PI3K, Akt, VEGF, and MMP9 proteins inside the cell. | [30] |
| Vitamin B6 | Pyridine | Reducing inflammation, cell proliferation, oxidative stress, and anti-colorectal cancer. | [24] |
| Trigonelline | Pyridine | Inhibiting the invasion of hepatoma cells (AH109A). | [31] |
| Kaempferol 7-O-glucoside | Flavonoid glycoside | Anti-tumor effect through cell cycle arrest and apoptotic induction in cervix carcinoma (HeLa cells). | [32] |
| Echinocystic acid 3-glucoside | Triterpenoid glycoside | Induces the apoptosis and inhibits the migration and invasion of non-small-cell lung cancer cells. | [33] |
| Ingenol | Diterpene ester | Inhibits the growth of pancreatic cancer cells in vitro via STING | [34] |
| Dihydrokaempferol | Flavonoid | Suppression of apoptotic activity in rheumatoid arthritis | [35] |
| Protoporphyrin IX | Porphyrin | Inhibition of oncogenic Ras/MEK significantly in vitro and in vivo (colon and breast cancer) | [36] |
| Naringenin | Flavonoid | Inhibits colorectal cancer cell growth by repressing the PI3K/AKT/mTOR signaling pathway | [29] |
| Luteolin | Flavonoid | Induction of apoptosis and inhibition of cell proliferation, metastasis, and angiogenesis. | [37] |
| Triphenyl phosphate | phenyl phosphate | Induces cell proliferation and migration ability in colorectal cancer. | [38] |
| Mono-2-ethylhexyl phthalate | phenolic | Progression of CRC through AKT-β-catenin signaling. | [39] |
| 2-Hexyl-4-pentynoic acid | Carboxylic acid | A novel radiosensitizer to breast cancer cells through increasing the instability of DNA repair proteins. | [40] |
| Palmitamide | Fatty acid | Anticancer activities on lung cancer cell line (NCI-60). | [41] |
| Phenyl isothiocyanate | Phenolic | Inducing apoptosis in breast cancer (HER2)-expressing tumor cells in vitro and in vivo. | [42] |
| Diphenylamine | Phenylamine | Suppresses androgen receptor and protein in prostate cancer. | [43] |
| Phthalic anhydride | Phenyl anhydride | Inhibits pro-inflammatory mediators through suppression of STAT3 activation in mice. | [44] |
| Bomyl acetate | Ester | Anticancer activity in human gastric cancer (SGC-7901) cells by inducing apoptosis, DNA fragmentation, and G2/M cell cycle arrest. | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osotprasit, S.; Suwansa-Ard, S.; Cummins, S.F.; Wang, T.; Samrit, T.; Chaiwichien, A.; Smith, S.J.; Changklungmoa, N.; Kueakhai, P. Anti-Metastatic Effects of Plukenetia volubilis (Sacha Inchi) Husk Extract via EGFR and EMT Pathways and Other Antitumor Effects in Colon Cancer. Int. J. Mol. Sci. 2025, 26, 10514. https://doi.org/10.3390/ijms262110514
Osotprasit S, Suwansa-Ard S, Cummins SF, Wang T, Samrit T, Chaiwichien A, Smith SJ, Changklungmoa N, Kueakhai P. Anti-Metastatic Effects of Plukenetia volubilis (Sacha Inchi) Husk Extract via EGFR and EMT Pathways and Other Antitumor Effects in Colon Cancer. International Journal of Molecular Sciences. 2025; 26(21):10514. https://doi.org/10.3390/ijms262110514
Chicago/Turabian StyleOsotprasit, Supawadee, Saowaros Suwansa-Ard, Scott F. Cummins, Tianfang Wang, Tepparit Samrit, Athit Chaiwichien, Stuart J. Smith, Narin Changklungmoa, and Pornanan Kueakhai. 2025. "Anti-Metastatic Effects of Plukenetia volubilis (Sacha Inchi) Husk Extract via EGFR and EMT Pathways and Other Antitumor Effects in Colon Cancer" International Journal of Molecular Sciences 26, no. 21: 10514. https://doi.org/10.3390/ijms262110514
APA StyleOsotprasit, S., Suwansa-Ard, S., Cummins, S. F., Wang, T., Samrit, T., Chaiwichien, A., Smith, S. J., Changklungmoa, N., & Kueakhai, P. (2025). Anti-Metastatic Effects of Plukenetia volubilis (Sacha Inchi) Husk Extract via EGFR and EMT Pathways and Other Antitumor Effects in Colon Cancer. International Journal of Molecular Sciences, 26(21), 10514. https://doi.org/10.3390/ijms262110514






