Editorial Board Members’ Collection Series: “Neuroinflammation”
Conflicts of Interest
References
- Dumurgier, J.; Tzourio, C. Epidemiology of Neurological Diseases in Older Adults. Rev. Neurol. 2020, 176, 642–648. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Calderone, A.; Latella, D.; Cardile, D.; Gangemi, A.; Corallo, F.; Rifici, C.; Quartarone, A.; Calabrò, R.S. The Role of Neuroinflammation in Shaping Neuroplasticity and Recovery Outcomes Following Traumatic Brain Injury: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 11708. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xie, J.; Li, Z.; He, X.; Wei, P.; Sander, J.W.; Zhao, G. The Role of Neuroinflammation and Network Anomalies in Drug-Resistant Epilepsy. Neurosci. Bull. 2025, 41, 881–905. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, S.; Stegnjaić, G.; Stanisavljević, S.; Lazarević, M.; Pilipović, I.; Sakač, M.; Miljković, Đ. Anti-Neuroinflammatory Effects of a Novel Bile Acid Derivative. Int. J. Mol. Sci. 2024, 25, 7136. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Zhu, G.; Zeng, Q.; Gao, R.; Qiu, J.; Su, W.; Wang, R. Human C15orf39 Inhibits Inflammatory Response via PRMT2 in Human Microglial HMC3 Cell Line. Int. J. Mol. Sci. 2024, 25, 6025. [Google Scholar] [CrossRef] [PubMed]
- Andraka, J.M.; Sharma, N.; Marchalant, Y. New Insights on the Effects of Krill Oil Supplementation, a High-Fat Diet, and Aging on Hippocampal-Dependent Memory, Neuroinflammation, Synaptic Density, and Neurogenesis. Int. J. Mol. Sci. 2024, 25, 11554. [Google Scholar] [CrossRef]
- Villamar, M.F.; Santos Portilla, A.; Fregni, F.; Zafonte, R. Noninvasive Brain Stimulation to Modulate Neuroplasticity in Traumatic Brain Injury. Neuromodulation Technol. Neural Interface 2012, 15, 326–338. [Google Scholar] [CrossRef]
- McNerney, M.W.; Gurkoff, G.G.; Beard, C.; Berryhill, M.E. The Rehabilitation Potential of Neurostimulation for Mild Traumatic Brain Injury in Animal and Human Studies. Brain Sci. 2023, 13, 1402. [Google Scholar] [CrossRef]
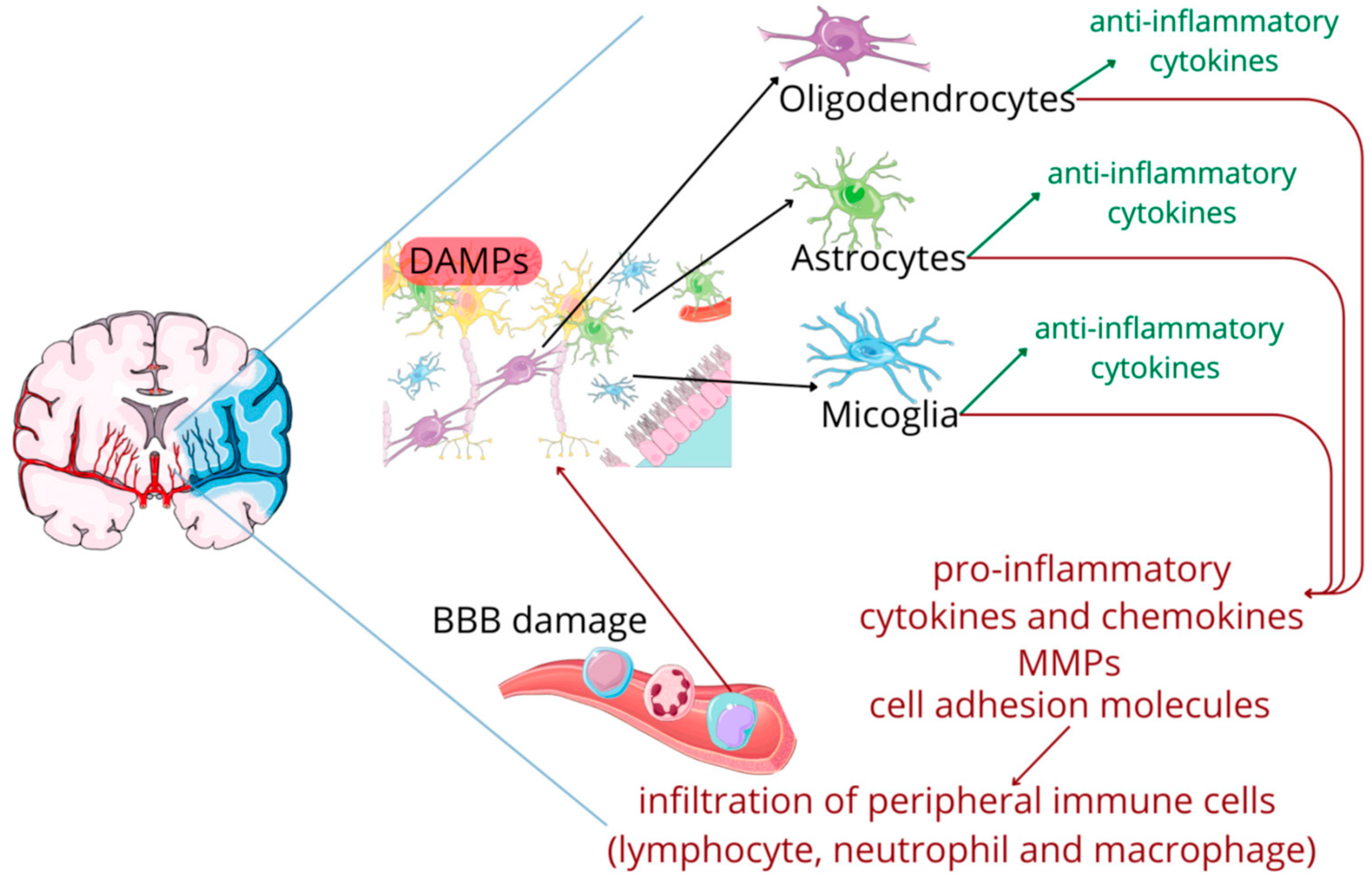
- Donat, C.K.; Scott, G.; Gentleman, S.M.; Sastre, M. Microglial Activation in Traumatic Brain Injury. Front. Aging Neurosci. 2017, 9, 208. [Google Scholar] [CrossRef]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-Derived Microparticles Mediate Neuroinflammation after Traumatic Brain Injury. J. Neuroinflamm. 2017, 14, 47. [Google Scholar] [CrossRef]
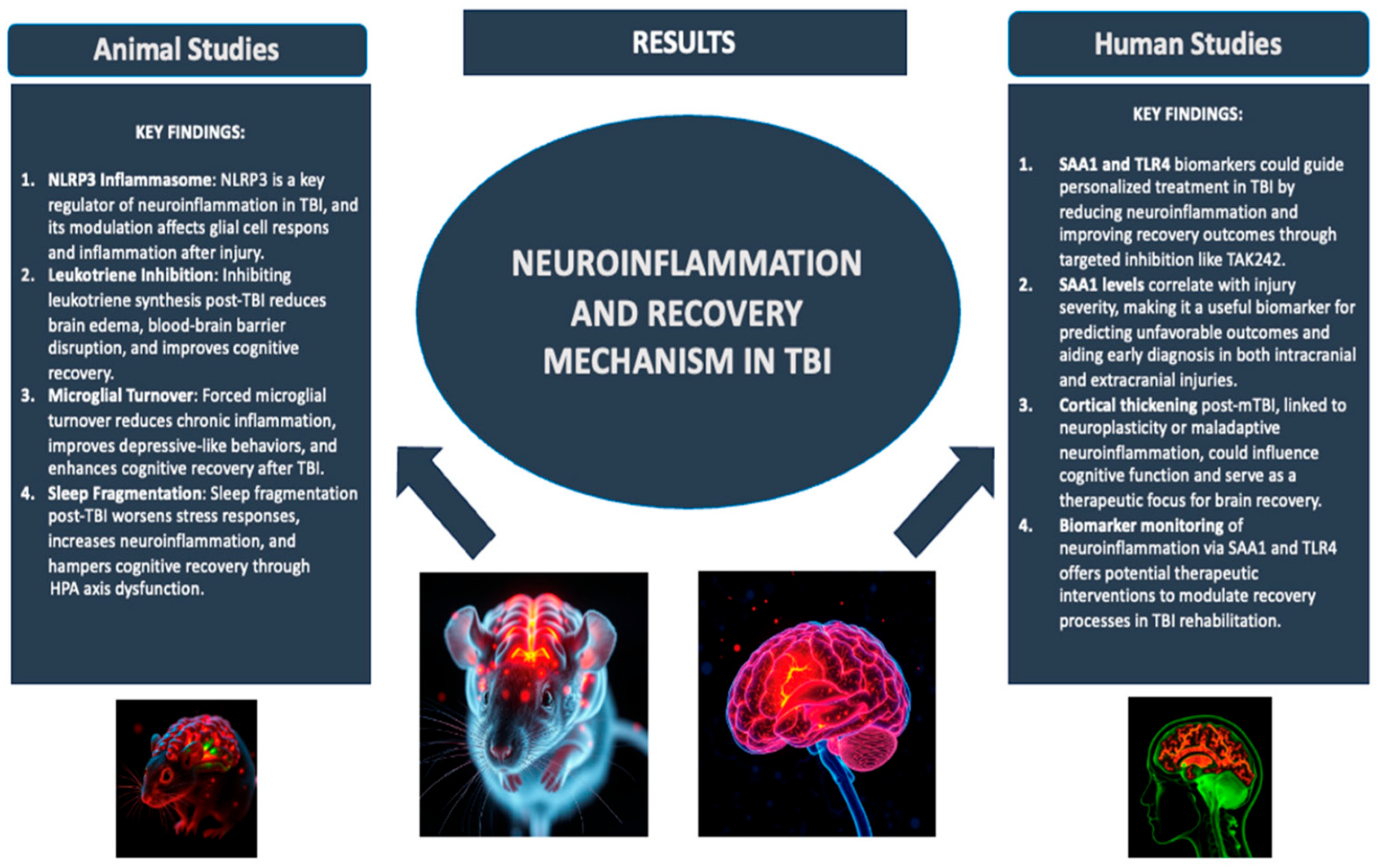
- Bray, C.E.; Witcher, K.G.; Adekunle-Adegbite, D.; Ouvina, M.; Witzel, M.; Hans, E.; Tapp, Z.M.; Packer, J.; Goodman, E.; Zhao, F.; et al. Chronic Cortical Inflammation, Cognitive Impairment, and Immune Reactivity Associated with Diffuse Brain Injury Are Ameliorated by Forced Turnover of Microglia. J. Neurosci. 2022, 42, 4215–4228. [Google Scholar] [CrossRef]
- Corser-Jensen, C.E.; Goodell, D.J.; Freund, R.K.; Serbedzija, P.; Murphy, R.C.; Farias, S.E.; Dell’Acqua, M.L.; Frey, L.C.; Serkova, N.; Heidenreich, K.A. Blocking Leukotriene Synthesis Attenuates the Pathophysiology of Traumatic Brain Injury and Associated Cognitive Deficits. Exp. Neurol. 2014, 256, 7–16. [Google Scholar] [CrossRef]
- Witcher, K.G.; Bray, C.E.; Chunchai, T.; Zhao, F.; O’Neil, S.M.; Gordillo, A.J.; Campbell, W.A.; McKim, D.B.; Liu, X.; Dziabis, J.E.; et al. Traumatic Brain Injury Causes Chronic Cortical Inflammation and Neuronal Dysfunction Mediated by Microglia. J. Neurosci. 2021, 41, 1597–1616. [Google Scholar] [CrossRef]
- Tapp, Z.M.; Cornelius, S.; Oberster, A.; Kumar, J.E.; Atluri, R.; Witcher, K.G.; Oliver, B.; Bray, C.; Velasquez, J.; Zhao, F.; et al. Sleep Fragmentation Engages Stress-Responsive Circuitry, Enhances Inflammation and Compromises Hippocampal Function Following Traumatic Brain Injury. Exp. Neurol. 2022, 353, 114058. [Google Scholar] [CrossRef] [PubMed]
- Farré-Alins, V.; Palomino-Antolín, A.; Narros-Fernández, P.; Lopez-Rodriguez, A.B.; Decouty-Perez, C.; Muñoz-Montero, A.; Zamorano-Fernández, J.; Mansilla-Fernández, B.; Giner-García, J.; García-Feijoo, P.; et al. Serum Amyloid A1/Toll-Like Receptor-4 Axis, an Important Link between Inflammation and Outcome of TBI Patients. Biomedicines 2021, 9, 599. [Google Scholar] [CrossRef]
- Carabias, C.S.; Castaño-León, A.M.; Blanca Navarro, B.; Panero, I.; Eiriz, C.; Gómez, P.A.; Egea, J.; Lagares, A. Serum Amyloid A1 as a Potential Intracranial and Extracranial Clinical Severity Biomarker in Traumatic Brain Injury. J. Intensive Care Med. 2020, 35, 1180–1195. [Google Scholar] [CrossRef]
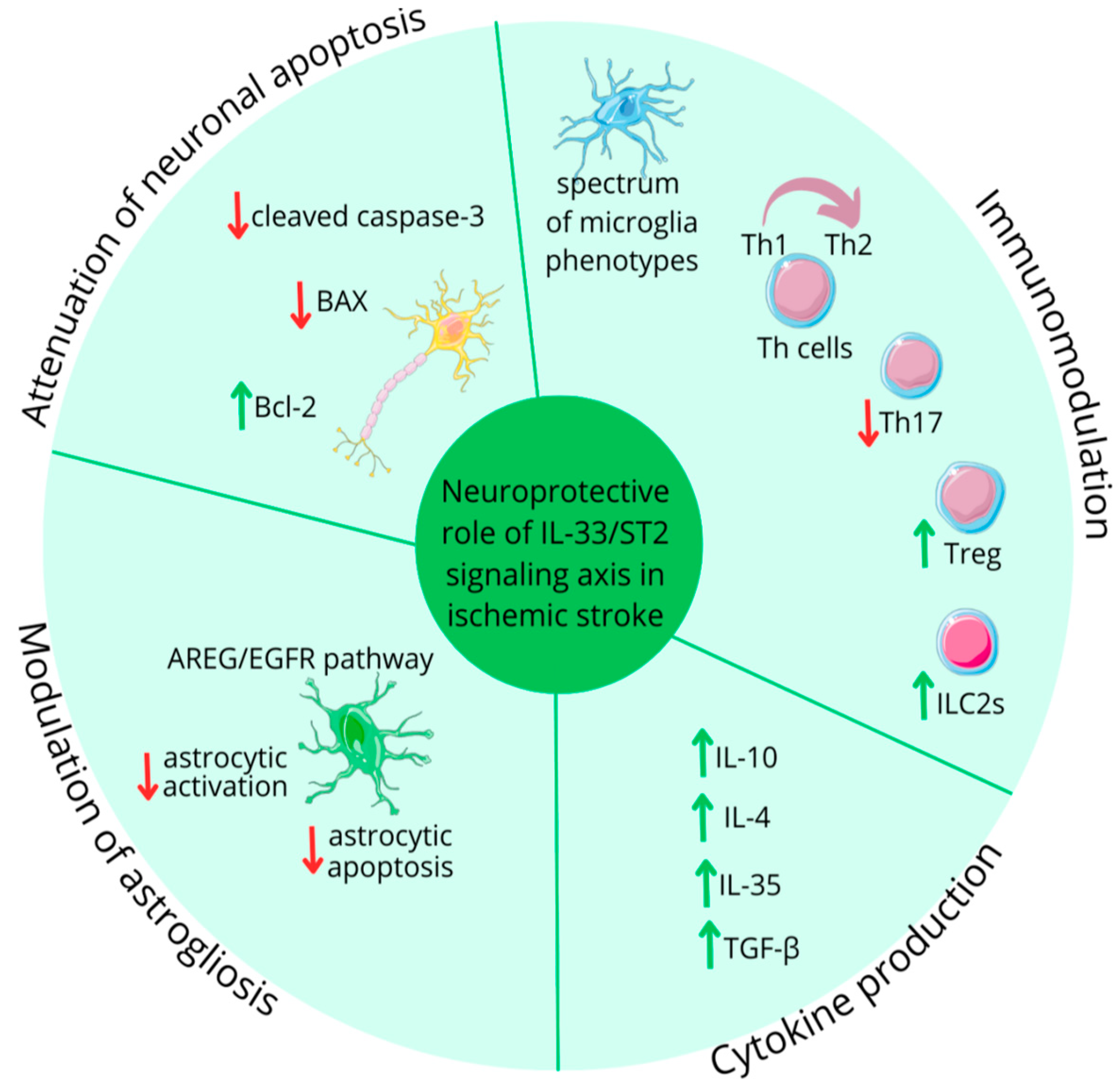
- Matys, P.; Mirończuk, A.; Starosz, A.; Grubczak, K.; Kochanowicz, J.; Kułakowska, A.; Kapica-Topczewska, K. Expanding Role of Interleukin-1 Family Cytokines in Acute Ischemic Stroke. Int. J. Mol. Sci. 2024, 25, 10515. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cai, L.; Wu, Y.; Jiang, M.; Zhang, Y.; Ren, W.; Song, Y.; Li, L.; Lei, Z.; Wu, Y.; et al. Emerging Functions and Therapeutic Targets of IL--38 in Central Nervous System Diseases. CNS Neurosci. Ther. 2024, 30, e14550. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Mastrangelo, F.; D’Ovidio, C.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Frydas, I.; Kritas, S.K.; Trimarchi, M.; Carinci, F.; et al. Activation of Mast Cells by Neuropeptides: The Role of Pro-Inflammatory and Anti-Inflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 4811. [Google Scholar] [CrossRef]
- Ito, M.; Komai, K.; Mise-Omata, S.; Iizuka-Koga, M.; Noguchi, Y.; Kondo, T.; Sakai, R.; Matsuo, K.; Nakayama, T.; Yoshie, O.; et al. Brain Regulatory T Cells Suppress Astrogliosis and Potentiate Neurological Recovery. Nature 2019, 565, 246–250. [Google Scholar] [CrossRef]
- Pusceddu, I.; Dieplinger, B.; Mueller, T. ST2 and the ST2/IL-33 Signalling Pathway–Biochemistry and Pathophysiology in Animal Models and Humans. Clin. Chim. Acta 2019, 495, 493–500. [Google Scholar] [CrossRef]
- Ma, L.; Su, H.; Wang, Y.; Zhou, Y.; Kang, Z.; Xu, Y.; Gao, J. Interleukin-1β (IL-1β) C-511T Polymorphism Is Associated with Susceptibility to Coronary Artery Disease in Type 2 Diabetic Patients. Eur. J. Inflamm. 2020, 18, 205873922091804. [Google Scholar] [CrossRef]
- Riccitelli, G.C.; Gironi, R.; Melli, G.; Kaelin-Lang, A. The Effect of Repetitive Transcranial Magnetic Stimulation Treatment on Plasma BDNF Concentration and Executive Functions in Parkinson’s Disease: A Theoretical Translational Medicine Approach. Int. J. Mol. Sci. 2025, 26, 1205. [Google Scholar] [CrossRef] [PubMed]
- Hyman, C.; Hofer, M.; Barde, Y.-A.; Juhasz, M.; Yancopoulos, G.D.; Squinto, S.P.; Lindsay, R.M. BDNF Is a Neurotrophic Factor for Dopaminergic Neurons of the Substantia Nigra. Nature 1991, 350, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Xu, B. BDNF Signaling and Survival of Striatal Neurons. Front. Cell. Neurosci. 2014, 8, 254. [Google Scholar] [CrossRef]
- Choung, J.S.; Kim, J.M.; Ko, M.-H.; Cho, D.S.; Kim, M. Therapeutic Efficacy of Repetitive Transcranial Magnetic Stimulation in an Animal Model of Alzheimer’s Disease. Sci. Rep. 2021, 11, 437, Erratum in Sci. Rep. 2021, 11, 7350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apolloni, S.; Koppula, S. Editorial Board Members’ Collection Series: “Neuroinflammation”. Int. J. Mol. Sci. 2025, 26, 10473. https://doi.org/10.3390/ijms262110473
Apolloni S, Koppula S. Editorial Board Members’ Collection Series: “Neuroinflammation”. International Journal of Molecular Sciences. 2025; 26(21):10473. https://doi.org/10.3390/ijms262110473
Chicago/Turabian StyleApolloni, Savina, and Sushruta Koppula. 2025. "Editorial Board Members’ Collection Series: “Neuroinflammation”" International Journal of Molecular Sciences 26, no. 21: 10473. https://doi.org/10.3390/ijms262110473
APA StyleApolloni, S., & Koppula, S. (2025). Editorial Board Members’ Collection Series: “Neuroinflammation”. International Journal of Molecular Sciences, 26(21), 10473. https://doi.org/10.3390/ijms262110473





