Human Body Malodor and Deodorants: The Present and the Future
Abstract
1. Malodors in the Body
1.1. Definition
1.2. Causes of Malodor Formation
1.2.1. Gene Expression
1.2.2. Diet Consumption
1.2.3. Age
1.2.4. Gender
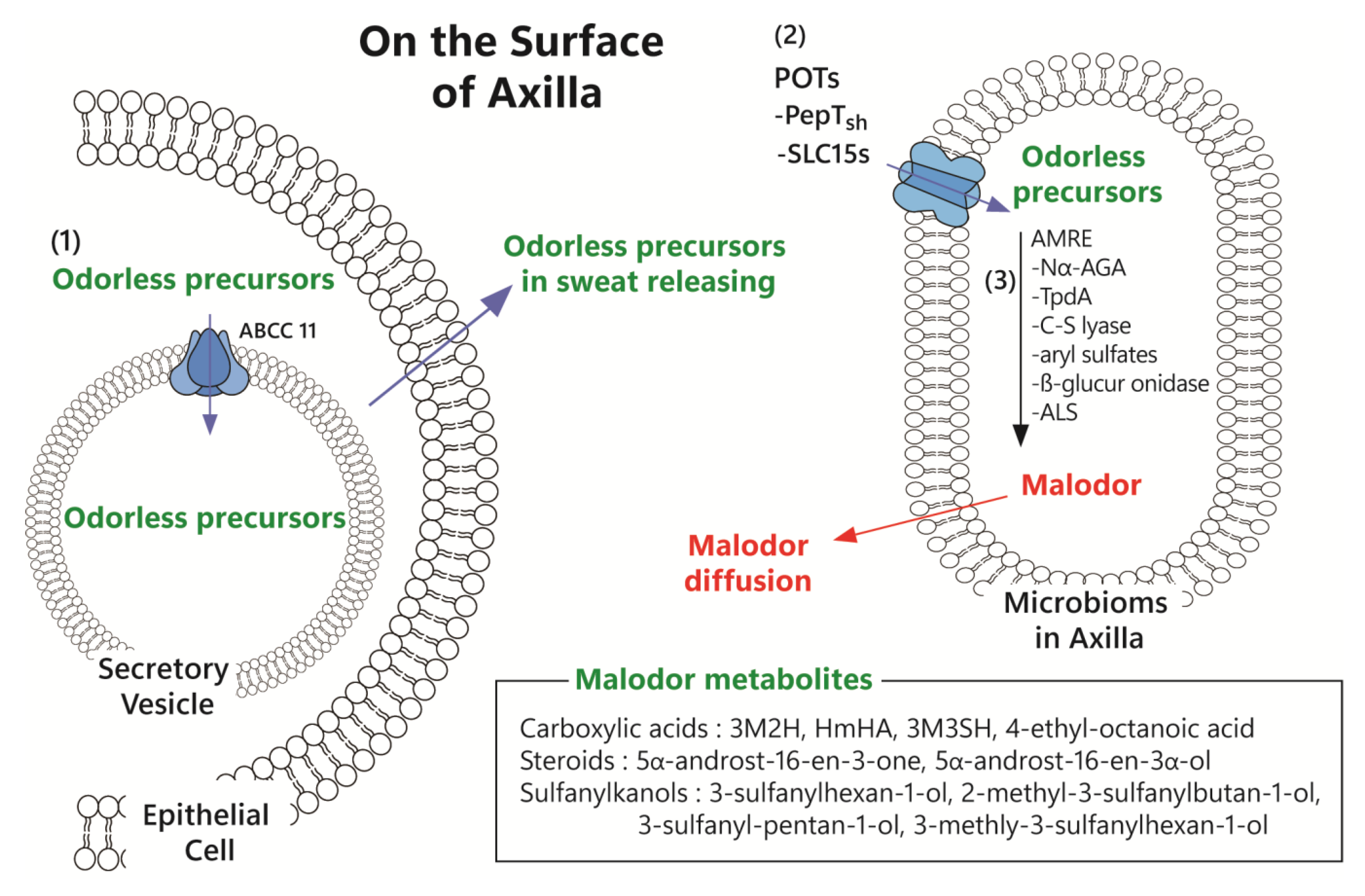
1.3. Mechanism of Malodor Formation in the Axilla
1.3.1. Sweating and Release by Secretory Vesicles
1.3.2. Transport of Odorless Precursors via Active Transport in the Microbiome
1.3.3. Fermentation of Precursors via Converting Enzyme and Malodor Diffusion
2. Deodorants
2.1. Definition
2.2. Ingredient Classification Based on the Functions
2.2.1. Anti-Sweating Effect
2.2.2. Antiproliferative Effect on Malodor-Forming Bacteria
2.2.3. Masking/Neutralizing Effect Against Malodor
2.2.4. Deodorizing Effect of Natural Products
3. Intervention Strategy for Next-Generation Deodorants
3.1. ABCC11 Pump Controller
3.2. Bacterial Active Pump Controller
3.3. Bacterial Converting Enzyme Modifier
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3M2H | (E)-3-methyl-2-hexenoic acid |
| HMHA | 4-ethyl-octanoic acid and 3-hydroxy-3-methyl-hexanoic acid |
| C. striatum | Corynebacterium striatum |
| NαAGA | Nα-acyl-glutamine aminoacylase |
| AMRE | Axillary-malodor-releasing enzyme |
| S. hominis | Staphyloccus hominis |
| C-S lyase | cystathione-β-lyase |
| 3M3SH | 3-methyl-3-sufanylhexan-l-ol |
| ABC | ATP-binding cassette |
| TpdA | Thiol precursor dipeptidase A |
| M | Muscarinic |
| EU | European Union |
| D4 | cyclotetrasiloxane |
| D5 | cyclopentasiloxane, decamethylcyclopentasiloxane |
| D6 | cyclohexasiloxane |
| D7 | cyclohepatiloxane |
| FDA | US Food and Drug Administration |
| HCO | Hydrogenated castor oil |
| Triclosan | 2,4,4′-trichloro-2′-hydroxydiphenyl ether |
| PQ-16 | copolymers of 1-vinyl-2-pyrolidone and 1-vinyl-3-methylimidazolium chloride |
| linalool | 3,7-dimethyl-1,6-octadien-3-ol |
| dl-citronellol | 3,7-dimethyl-6-octen-1-ol |
| citral | acyclic monoterpene aldehyde |
| benzyl salicylate | 2-hydroxybenzoic acid |
| hexyl cinnamal | 2-hexyl-3-phenyl-2-propenal |
| OECD | The Organisation for Economic Cooperation and Development |
| geraniol | (2E)-3,7-dimethylocta-2,6-dien-1-ol |
| limonene | 1-methyl-4-(1-methyl phenyl) cyclohexene |
| farnesol | 3,7,11-trimethyl-2,6,10-dodecatrien-1-ol |
| florhydral | Isopropylphenylbutanal |
| linalyl acetate | 3,7-dimethylocta-1,6-dien-3-yl acetate |
| 2-isopropyl-5-methylphenol | Isomeric phenolic monoterpenes thymol |
| carvacrol | 2-methyl-5-(propan-2-yl)phenol |
| MK-571 | 5-(3-(2-(7-chloroquinolin-2-yl)ethenyl)phenyl0-8-dimethylcarbamyl-4,6-dithiaoctanoic acid sodium salt hydrate |
| 5-FU | 5-fluorouracil |
| FdUMP | 5-fluoro-2′-deoxyuridine 5′-monophosphate |
| Ara-C | Cytosine arabinoside |
| MTA | Pemetrexed |
| MTX | Methotrexate |
| PMEA | 9′-(2′-phosphonyl-methoxyethyl)adenine |
| POT | Proton-coupled oligopeptide transporter |
| PepTsh | Peptide transporter Staphyloccus hominis |
| SLC | Solute carrier |
| PepT | Peptide transporter |
| PhT | Peptide/histidine transpoter |
| S. epidermidis | Staphylococcus epidermidis |
| S. aureus | Staphylococcus aureus |
| ALS | Acetolactate synthase |
References
- Teerasumran, P.; Velliou, E.; Bai, S.; Cai, Q. Deodorants and antiperspirants: New trends in their active agents and testing methods. Int. J. Cosme. Sci. 2023, 45, 426–443. [Google Scholar] [CrossRef]
- Low, P.A. Chapter 51. Sweating. In Primer on the Autonomic Nervous System, 3rd ed.; Biaggioni, I., Brwoning, K., Fink, G., Jordan, J., Low, P.A., Paton, J.F.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 249–251. [Google Scholar]
- Gartner, L.P.; Hiatt, J.L. Chapter11. Integument. In Color Atlas and Text of Histology, 6th ed.; Lippincott Williams & Wilkins a Wolters Kluwer Business: Philadelphia, PA, USA, 2014; pp. 254–274. [Google Scholar]
- Semkova, K.; Gergovska, M.; Kazandjieve, J.; Tsankov, N. Hyperhidrosis, bromhidrosis, and chromhidrosis: Fold (intertriginous) dermatoses. Clin. Dermatol. 2015, 33, 483–491. [Google Scholar] [CrossRef]
- Labors, J.N.; Preti, G.; Hoelzle, E.; Leyden, J.; Kligman, A. Steroid analysis of human apocrine secretion. Steroids 1979, 34, 249–258. [Google Scholar] [CrossRef]
- Callewaert, C.; Kerckhof, F.-M.; Granitsiotis, M.S.; Gele, M.V.; de Wiele, T.V.; Boon, N.; Boon, N. Characterization of Staphylococcus and Corynebacterium clusters in the human axillary region. PLoS ONE 2013, 8, e70538. [Google Scholar] [CrossRef] [PubMed]
- Burnett, G.R.; Stephen, A.S.; Pizzey, R.L.; Bradshaw, D.J. In vitro effects of novel toothpaste actives on components of oral malodor. Int. Dent. J. 2011, 61, 67–73. [Google Scholar] [CrossRef]
- James, A.G.; Cox, D.; Worrall, K. Microbiological and biochemical origins of human foot malodor. Flavour Fragr. J. 2013, 28, 231–237. [Google Scholar] [CrossRef]
- Claus, R.; Alsing, W. Occurrence of 5alpha-androst-16en-3-one, a boar pheromone, in man and its relationship to testosterone. J. Endocrinol. 1976, 68, 483–484. [Google Scholar] [CrossRef]
- Brooksbank, B.W.; Brown, R.; Gustafsson, J.A. The detection of 5alpha-androst-16-en-3alpha-ol in human male axillary sweat. Experientia 1974, 30, 864–865. [Google Scholar] [CrossRef] [PubMed]
- Gautschi, M.; Natsch, A.; Schroder, F. Biochemistry of human axilla malodor and chemistry of deodorant ingredients. Chimia 2007, 61, 27–32. [Google Scholar] [CrossRef]
- Zeng, X.N.; Leyden, J.J.; Lawley, H.J.; Sawano, K.; Nohara, I.; Preti, G. Analysis of characteristic odors from human male axillae. J. Chem. Ecol. 1991, 17, 1469–1492. [Google Scholar] [CrossRef]
- Natsch, A.; Gfeller, H.; Gygax, P.; Schmid, J.; Acuna, G. A specific bacterial aminoacylase cleaves odorant precursors secreted in the human axilla. J. Biol. Chem. 2003, 278, 5718–5727. [Google Scholar] [CrossRef]
- Natsch, A.; Schmid, J.; Flachsmann, F. Identification of odoriferous sulfanylalkanols in human axilla secretions and their formation through cleavage of cysteine precursors by a C-S lyase isolated from axilla bacteria. Chem. Biodivers. 2004, 1, 1058–1072. [Google Scholar] [CrossRef]
- Bawdon, D.; Cox, D.S.; Ashford, D.; James, A.G.; Thomas, G.H. Identification of axillary Staphylococcus sp. Involved in the production of the malodorous thioalcohol 3-methyl-3-sufanylhexan-1-ol. FEMS Microbiol. Lett. 2015, 362, fnv111. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Rzhetskey, A.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef]
- Tammur, J.; Prades, C.; Arnould, I.; Rzhetsky, A.; Hutchinson, A.; Adachi, M.; Schuetz, J.D.; Swoboda, K.J.; Ptacek, L.J.; Rosier, M.; et al. Two new genes from the human ATP-binding cassette transporter superfamily, ABCC11 and ABCC12, tandemly duplicated on chromosome 16q12. Gene 2001, 273, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Harker, M.; Carvell, A.-M.; Marti, V.P.J.; Riazanskaia, S.; Kelso, H.; Tayor, D.; Grimshaw, S.; Arnold, D.S.; Zillmer, R.; Shaw, J.; et al. Functional characterization of a SNP in the ABCC11 allele-Effects on axillary skin metabolism, odour generation and association behaviours. J. Dermatol. Sci. 2014, 73, 23–30. [Google Scholar] [CrossRef]
- Yoshiura, K.-i.; Kinoshita, A.; Ishida, T.; Ninokata, A.; Ishikawa, T.; Kaname, T.; Bannai, M.; Tokunaga, K.; Sonoda, S.; Komaki, R.; et al. A SNP in the ABCC11 gene is the determinant of human earwax type. Nat. Genet. 2006, 38, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Havlicek, J.; Lenochova, P. The effect of meat consumption on body odor attractiveness. Chem. Senses 2006, 31, 747–752. [Google Scholar] [CrossRef]
- Zuniga, A.; Stevenson, R.J.; Mahmut, M.K.; Stephen, I.D. Diet quality and the attractiveness of male body odor. Evol. Hum. Behav. 2017, 38, 136–143. [Google Scholar] [CrossRef]
- Fialova, J.; Roberts, S.C.; Havlicek, J. Consumption of garlic positively affects hedonic perception of axillary body odour. Appetite 2016, 97, 5–8. [Google Scholar] [CrossRef]
- Fialova, J.; Hoffmann, R.; Roberts, S.C.; Havlicek, J. The effect of complete caloric intake restriction on human body odour quality. Physiol. Behav. 2019, 210, 112554. [Google Scholar] [CrossRef] [PubMed]
- Mitro, S.; Gordon, A.R.; Olsson, M.J.; Lundstrom, J.N. The smell of age: Perception and discrimination of body odors of different ages. PLoS ONE 2012, 7, e38110. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Chen, W.C.; Thornton, M.J.; Qin, K.; Rosenfield, R. Sexual hormones in human skin. Horm. Metab. Res. 2007, 39, 85–95. [Google Scholar] [CrossRef]
- Gallagher, M.; Wysocki, C.J.; Leyden, J.J.; Spielman, A.I.; Sun, X.; Preti, G. Analyses of volatile organic compounds form human skin. Br. J. Dermatol. 2008, 159, 780–791. [Google Scholar] [CrossRef]
- Ishino, K.; Wakita, C.; Shbata, T.; Toyokuni, S.; Machida, S.; Matsuda, S.; Matsuda, T.; Uchida, K. Lipid peroxidation generates body odor component trans-2-nonenal covalently bound to protein in vivo. J. Biol. Chem. 2010, 285, 15302–15313. [Google Scholar] [CrossRef]
- Haze, S.; Gozu, Y.; Nakamura, S.; Kohno, Y.; Sawano, K.; Ohta, H.; Yamazaki, K. Nonenal newly found in huyman body odor tends to increase with aging. J. Investig. Dermatol. 2001, 116, 520–524. [Google Scholar] [CrossRef]
- Hold, B.; Schliedt, M. The importance of human odour in non-verbal communication. Z. Tierpsychol. 1977, 43, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Russell, M. Human olfactory communication. Nature 1976, 260, 520–522. [Google Scholar] [CrossRef]
- Lindqvist, A. Preference and gender associations of perfumes applied on human skin. J. Sens. Stud. 2013, 27, 490–497. [Google Scholar] [CrossRef]
- Muitic, S.; Moellers, E.M.; Wiesmann, M.; Freiherr, J. Chemosensory communication of gender information: Masculinity bias in body odor perception and femininity bias introduced by chemosignals during social perception. Front. Psychol. 2016, 6, 1980. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, R.; Gangestad, S.W. The scent of symmetry: A human sex pheromone that signals fitness? Evol. Hum. Behav. 1999, 20, 175–201. [Google Scholar] [CrossRef]
- Jenskinson, D.M.; Montgomery, I.; Elder, H.Y. Studies on the nature of the peripheral sudomotor control mechanism. J. Anat. 1978, 125, 625–639. [Google Scholar]
- Amano, T.; Fujiii, N.; Kenny, G.P.; Inoue, Y.; Kondo, N. Does α1-adrenergic receptor blockade modulate sweating during incremental exercise in young endurance-trained men? Eur. J. Appl. Bhysiol. 2020, 120, 1123–1129. [Google Scholar] [CrossRef]
- Lindsay, S.L.; Holmes, S.; Corbett, A.D.; Harker, M.; Bovell, D.L. Innervation and receptor profiles of the human apocrine (epitrichial) sweat gland: Routes for intervention in bromhidrosis. Brit. J. Dermatol. 2008, 159, 653–660. [Google Scholar] [CrossRef]
- Shang, D.; Zhang, X.; Sun, M.; Wei, Y.; Wen, Y. Strong association of the SNP rs17822931 with wet earwax and bromhidrosis in a Chinese family. J. Genet. 2013, 92, 289–291. [Google Scholar] [CrossRef]
- Nakano, M.; Miwa, N.; Hirano, A.; Yoshiura, K.; Niikawa, N. A strong association of axillary osmidrosis with the wet earwax type determined by genotyping of the ABCC11 gene. BMC Genet. 2009, 10, 42. [Google Scholar] [CrossRef]
- Natsch, A.; Emter, R. The specific biochemistry of human axilla odour formation viewed in an evolutionary context. Phil. Trans. R. Soc. B 2020, 375, 20190269. [Google Scholar] [CrossRef]
- Martin, A.; Saathoff, M.; Kuhn, F.; Max, H.; Terstegen, L.; Natsch, A. A functional ABCC11 allele is essential in the biochemical formation of human axillary odor. J. Invest. Dermatol. 2010, 130, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.; Bergmann, S.; Schmidt-Rose, T.; Max, H.; Martin, A.; Enthaler, B.; Terstegen, L.; Schweiger, D.; Kalbacher, H.; Wenck, H.; et al. Glutathion-conjugated sulfanylalkanols are substrates for ABCC11 and γ-glutamyl transferase 1: A potential new pathway for the formation of odorant precursors in the apocrine sweat gland. Exp. Dermatol. 2014, 23, 247–252. [Google Scholar] [CrossRef]
- Herman, R.; Kinniment-williams, B.; Rudden, M.; James, A.G.; Wilkinson, A.J.; Murphy, B.; Thomas, G.H. Identification of a Staphylococcal depeptidase involved in the production of human body odor. J. Biol. Chem. 2024, 300, 107928. [Google Scholar] [CrossRef] [PubMed]
- Rudden, M.; Herman, R.; Rose, M.; Bawdon, D.; Cox, D.S.; Dodson, E.; Holden, M.T.G.; Wilkinson, A.J.; James, A.G.; Thomas, G.H. The molecular basis of thioalcohol production in human body odour. Sci. Rep. 2020, 10, 12500. [Google Scholar] [CrossRef]
- Troccaz, M.; Borchard, G.; Vuilleumier, C.; Raviot-Derrien, S.; Niclass, Y.; Beccucci, S.; Starkenmann, C. Gender-specific differences between the concentrations of nonvolatile (R/(S)-3-methyl-3-sulfanylhexan-1-ol and (R/(S)-3-hydroxy-3-methyl-hexanoic acid odor precursors in axillary secretions. Chem. Senses 2009, 34, 203–210. [Google Scholar] [CrossRef]
- Owsienko, D.; Goppelt, L.; Hierl, K.; Schafer, L.; Croy, I.; Loos, H.M. Body odor samples from infants and post-pubertal children differ in their volatile profiles. Commun. Chem. 2024, 7, 53. [Google Scholar] [CrossRef]
- Callewaert, C.; Hutapea, P.; de Wiele, T.V.; Boon, N. Deodorants and antiperspirants affect the axillary bacterial community. Arch. Dermatol. Res. 2014, 306, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Minhas, S.G.; Bawdon, D.; Herman, R.; Rudden, M.; Stone, A.P.; James, A.G.; Thomas, G.H.; Newstead, S. Structural basis of malodour precursor transport in the human axilla. eLife 2018, 7, e34995. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.; Gower, D.B. Axillary 5 alpha-androst-16-en-3-one, cholesterol and squalene in men; preliminary evidence for 5 alpha-androst-16-en-3-one being a product of bacterial action. J. Steroid Biochem. 1982, 17, 517–522. [Google Scholar] [CrossRef]
- Troccaz, M.; Starkenmann, C.; Niclass, Y.; van de Waal, M.; Clark, A.J. 3-Methyl-3-sulfanylhexan-1-ol as a major descriptor for axilla-sweat odour profile. Chem. Biodivers. 2004, 1, 1022–1035. [Google Scholar] [CrossRef]
- Kalinowska-Lis, U. Overview of active ingredients used in deodorants and antiperspirants available on EU market. Appl. Sci. 2025, 15, 5068. [Google Scholar] [CrossRef]
- Stillians, A.W. The control of localized hyperhidrosis. JAMA. 1916, LXVII, 2015–2016. [Google Scholar] [CrossRef]
- Johnson, W., Jr.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of cyclomethicone, cyclotetrasiloxane, cyclopentasiloxane, cyclohexailoxane, and cycloheptasiloxane. Int. J. Toxicol. 2011, 30, 149S–227S. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, B.P.; Price, B.B.; van Egmond, R.A. Accounting for intended use application in characterizing the contributions of cycolopentasiloxane (D5) to aquatic loadings following personal care product use: Antiperspirants, skin care products and hair care products. Chemosphere 2013, 93, 735–740. [Google Scholar] [CrossRef]
- 1 Final report on the safety assessment of stearyl alcohol, oleyl alcohol, and octyl dodecanol. J. Am. Coll. Toxicol. 1985, 4, 1–29. [CrossRef]
- Atsmon, D. Oil Crops of the World; Robbelen, G., Downey, R.K., Ashri, A., Eds.; McGraw-Hill: New York, NY, USA, 1989; pp. 483–488. [Google Scholar]
- Abraham, T.W.; Hofer, R. 10.03 Lipid-based polymer building blocks and polymers. Polym. Sci. A Compr. Ref. 2012, 10, 15–58. [Google Scholar] [CrossRef]
- de Oliveira, E.C.V.; Salvador, D.S.; Holsback, V.; Shultz, J.D.; Michniak-Kohn, B.B.; Leonardi, G.R. Deodorants and antiperspirants: Identification of new strategies and perspectives to prevent and control malodor and sweat of the body. Int. J. Dermatol. 2021, 60, 613–619. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics—A critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Traupe, B.; Folster, H.; Max, H.; Schulz, J. Effective axillary malodour reduction by polyquaternium-16-containing deodorants. Int. J. Cosmet. Sci. 2017, 39, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ermenlieva, N.; Georgieva, E.; Milev, M. Antibacterial and antifungal activity of antiperspirant cosmetic products. J. IMAB 2020, 26, 3374–3377. [Google Scholar] [CrossRef]
- FEMA. FEMA Database: Linalool (FEMA No. 2635); Flavor and Extract Manufacturers’ Association: Washington, DC, USA, 1997; p. 53. Available online: https://www.femaflavor.org/flavor-library/linalool (accessed on 1 September 2025).
- Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Rosol, T.J.; Harman, C.; Taylor, S.V. GRAS 29 Flavoring substances. Food Technol. 2020, 74, 44–65. Available online: http://www.femaflavor.org/sites/default/files/2020-03/GRAS%2029.pdf (accessed on 1 September 2025).
- Smith, R.L.; Waddell, W.J.; Cohen, S.M.; Feron, V.J.; Marnett, L.J.; Portoghese, P.S.; Rietjens, I.; Adams, T.B.; Lucas Gavin, C.; McGowen, M.M.; et al. GRAS favoring substances 24. Food Technol. 2009, 63, 46–105. Available online: http://www.ift.org/~/media/Food%20Technology/pdf/2009/06/0609feat_GRAS24text.pdf (accessed on 2 September 2025).
- Lapczynski, A.; McGuinty, D.; Jones, L.; Bhatai, S.; Letizia, C.S.; Api, A.M. Fragrance material review on benzyl salicylate. Food Chem. Toxicol. 2007, 45, S362–S380. [Google Scholar] [CrossRef]
- Basketter, D.; White, I.R.; McFadden, J.P.; Kimber, I. Hexyl cinnamal: Consideration of skin-sensitizing properties and suitability as a positive control. Cutan. Ocul. Toxicol. 2014, 34, 227–231. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Ravichandran, C.; Badguja, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef]
- Lapczynski, A.; Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on farnesol. Food Chem. Toxicol. 2008, 46, S149–S156. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Bhatia, S.; Bruze, M.; Calow, P.; Dagli, M.L.; Dekant, W.; Fryer, A.D.; Kromidas, L.; Cava, S.L.; et al. RIFM fragrance ingredient safety assessment, isopropylphenylbutanal, CAS registry number 125109-85-5. Food Chem. Toxicol. 2016, 97, S230–S236. [Google Scholar] [CrossRef]
- Letizia, C.S.; Cocchiara, J.; Lalko, J.; Api, A.M. Fragrance material review on linalyl acetate. Food Chem. Toxicol. 2003, 41, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Henmi, A.; Shoji, M.; Nomura, M.; Inoue, T. Fatty acid composition and applications of Eriobotrya japonica seed oil. J. Oleo Sci. 2019, 7, 599–606. [Google Scholar] [CrossRef]
- Kim, M.-J.; Tagele, S.B.; Jo, H.; Kim, M.-C.; Jung, Y.; Park, Y.-J.; So, J.-H.; Kim, H.J.; Kim, H.J.; Lee, D.-G.; et al. Effect of a bioconverted product of Lotus corniculatus seed on the axillary microbiome and body odor. Sci. Rep. 2021, 11, 10138. [Google Scholar] [CrossRef]
- Shahtalebi, M.A.; Ghanadian, M.; Farzan, A.; Shiri, N.; Shokri, D.; Fatemi, S.A. Deodorant effects of a sage extract stick: Antibacterial activity and sensory evaluation of axillary deodorancy. J. Res. Med. Sci. 2013, 18, 833–839. [Google Scholar] [PubMed]
- Huang, P.; Wang, Z.; Shi, Y.; Zhang, R.; Feng, X.; Kan, J. Deodorizing effects of rosemary extract on silver carp (Hypophthalmichthys molitrix) and determination of its deodorizing components. J. Food Sci. 2022, 87, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.; Truong, F.; Adal, A.M.; Sarker, L.S.; Mahmoud, S.S. Lavandula essential oils: A current review of applications in medicinal, food, and cosmetic industries of lavender. Nat. Prod. Commun. 2018, 13, 1403–1417. [Google Scholar] [CrossRef]
- Yousef, J.; Hasanat, A.A.; Othman, S.; Alrahma, S.; Alloubani, A. Effects of lavender oil on odor elimination, ostomy adjustment and quality of life in patients with permanent colostomy: A randomized controlled trial. Euro. J. Oncol. Nurs. 2024, 68, 102471. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and thyme essential oil—New insights into selected therapeutic applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Quatrale, R.P.; Waldman, A.H.; Rogers, J.G.; Felger, C.B. The mechanism of antiperspirant action by aluminum salts. I. The effect of cellophane tape stripping on aluminum salt-inhibited eccrine sweat glands. J. Soc. Cosmet. Chem. 1981, 32, 67–73. [Google Scholar]
- Karsai, S.; Weiβ, C.; Lutgerath, C.; Ott, I.; Faulhaber, J. Comparison of novel aluminium lactate versus aluminium chloride-based antiperspirant in excessive axillary perspiration: First prospective cohort study. Dermatol. Ther. 2021, 34, e15020. [Google Scholar] [CrossRef]
- Gorgogietas, V.A.; Tsialtas, I.; Sotirious, N.; Laschou, V.C.; Karra, A.G.; Leonidas, D.D.; Chrousos, G.P.; Protopapa, E.; Psarra, A.-M.G. Potential interference of aluminum chlorohydrate with estrogen receptor signaling in breast cancer cells. J. Mol. Biochem. 2018, 7, 1–13. [Google Scholar]
- Mirick, D.K.; Davis, S.; Thomas, D.B. Antiperspirant use and the risk of breast cancer. J. Natl. Cancer Inst. 2002, 94, 1578–1580. [Google Scholar] [CrossRef]
- Taghipour, K.; Tatnall, F.; Orton, D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermat. 2008, 58, 168–169. [Google Scholar] [CrossRef]
- Jungermann, E.; Taber, D. A new broad spectrum antibacterial soap, I: General properties. J. Am. Oil Chem. 1971, 48, 318–325. [Google Scholar] [CrossRef]
- Rogos, J.; Zak, O.; Solf, R.; Visher, W.A.; Weirich, E.G. Antimicrobial spectrum of triclosan, a broad spectrum antimicrobial agent for topical application. II: Comparison with other antibacterial agents. Dermatologica 1979, 158, 72–79. [Google Scholar] [CrossRef]
- Bhargava, H.N.; Leonard, P.A. Triclosan: Applications and safety. Am. J. Inf. Control 1996, 24, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation and human health effects. J. Toxicol. Environ. Health B Crit. Ver. 2017, 20, 447–469. [Google Scholar] [CrossRef] [PubMed]
- McGee, T.; Rankin, K.M.; Baydar, A. The design principles of axilla deodorant fragrances. Ann. N. Y. Acad. Sci. 1998, 855, 841–846. [Google Scholar] [CrossRef]
- Bedoukian, P.Z. Perfumery and Flavoring Synthetics, 3rd. ed.; Wheaton, I.L., Ed.; Allured Publishing Corp: Carol Stream, IL, USA, 1986; pp. 267–282. [Google Scholar]
- Santos, P.L.; Matos, J.P.S.C.F.; Picot, L.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Junior, L.J. Citronellol, a monoterpene alcohol with promising pharmacological activities—A systematic review. Food Chem. Toxicol. 2019, 123, 459–469. [Google Scholar] [CrossRef]
- Shahina, Z.; Dahms, T.E.S. A comparative review of eugenol and citral anticandidal mechanisms: Partners in crimes against fungi. Molecules 2024, 29, 5536. [Google Scholar] [CrossRef]
- Burdock, G.A. Encyclopedia of Food and Colours Additives; CRC Presses: Boca Raton, FL, USA, 1995; Volume 1. [Google Scholar]
- Sikdar, D.C.; Menon, R.; Duseja, K.; Kumar, P.; Swami, P. Extraction of citrus oil from orange (Citrus sinensis) peels by steam distillation and its characterization. Int. J. Tech. Res. Appl. 2016, 4, 341–346. [Google Scholar]
- National Library of Medicine. Florhydral. PubChem 2025, 63, e00506-19. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/86209 (accessed on 1 September 2025).
- Zhang, Y.; Long, Y.; Yu, S.; Yang, M.; Guan, Y.; Zhang, D.; Wan, J.; Liu, S.; Shi, A.; Li, N.; et al. Natural volatile oils derived from herbal medicines: A promising therapy way for treating depressive disorder. Pharmacol. Res. 2021, 164, 105376. [Google Scholar] [CrossRef]
- Tonzetich, J.; Richter, V.J. Evaluation of volatile odoriferous components of saliva. Arch. Oral Biol. 1964, 9, 39–45. [Google Scholar] [CrossRef]
- Zeng, Q.C.; Wu, A.Z.; Pika, J. The effect of green tea extract on the removal of sulfur-containing oral malodor volatiles in vitro and its potential application in chewing gum. J. Breath Res. 2010, 4, 036005. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Hayashi, S.; Craker, L.E. Culture, history and applications of medicinal and aromatic plants in Japan. Aromat. Med. Plants 2017, 95–110. [Google Scholar] [CrossRef]
- Dalmarco, J.B.; Dalmarco, E.M.; Koelzer, J.; Pizzolatti, M.G.; Frode, T.S. Isolation and identification bioactive compounds responsible for the anti-bacterial efficacy of Lotus corniculatus var. Sao Gabriel. Int. J. Green Pharm. 2010, 4, 108–114. [Google Scholar] [CrossRef]
- Yerlikaya, S.; Baloglu, M.C.; Diuzheva, A.; Jeko, J.; Cziaky, Z.; Zengin, G. Investigation of chemical profile, biological properties of Lotus corniculatus L. extracts and their apoptotic-autophagic effects on breast cancer cells. J. Pharm. Biomed. Anal. 2019, 174, 286–299. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 13, 433–440. [Google Scholar] [CrossRef]
- Heinrich, M.; Kufer, J.; Leonti, M.; Pardo-de-Santayana, M. Ethnobotany and ethnopharmacology-interdisciplinary links with the historical sciences. J. Ethnopharamcol. 2006, 107, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, G.; Alirezalu, A.; Ghosta, Y.; Jarrahi, A.; Safavi, S.; Abbas-Mohammadi, M.; Barba, F.J.; Munekata, P.E.S.; Dominguez, R.; Lorenzo, J.M. Composition, antifungal, phytotoxic, insecticidal activities of Thymus kotschyanus essential oil. Molecules 2020, 25, 1152. [Google Scholar] [CrossRef]
- Prokop-Prigge, K.A.; Thaler, E.; Wysocki, C.J.; Preti, G. Identification of volatile organic compounds in human cerumen. J. Chromatogr. B 2014, 953–954, 48–52. [Google Scholar] [CrossRef]
- Saito, H.; Toyoda, Y.; Hirata, H.; Ota-Kontani, A.; Tsuchiya, Y.; Takada, T.; Suzuki, H. Soy isoflavone genistein inhibits an axillary osmidrosis risk factor ABCC11: In vitro screening and fractional approach for ABCC11-inhibitory activities in plant extracts and dietary flavonoids. Nutrients 2020, 12, 2452. [Google Scholar] [CrossRef]
- Tun-Yhong, W.; Chinpaisal, C.; Pamonsinlapatham, P.; Kaewkitichai, S. Tenofovir disoproxil fumarate is a new substrate of ATP-binding cassette subfamily C member 11. Antimicrob. Agents Chemother. 2017, 61, e01725–16, Correction in Antimicrob. Agents Chemother. 2019, 63, e00506–19. [Google Scholar] [CrossRef]
- Miura, K.; Yoshiura, K.-i.; Miura, S.; Shimada, T.; Yamasaki, K.; Yoshida, A.; Nakayama, D.; Shibata, Y.; Niikawa, N.; Masuzaki, H. A strong association between human earwax-type and apocrine colostrum secretion form the mammary gland. Hum. Genet. 2007, 121, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, Y.; Ishikawa, T. Pharmacogenomics of human ABC transporter ABCC11 (MRP8): Potential risk of breast cancer and chemotherapy failure. Anti-Cancer Agents Med. Chem. 2010, 10, 617–624. [Google Scholar] [CrossRef]
- Parker, J.L.; Li, C.; Brinth, A.; Wang, Z.; Vogeley, L.; Solcan, N.; Ledderboge-Vucinic, G.; Swanson, J.M.J.; Caffrey, M.; Voth, G.A.; et al. Proton movement and coupling in the POT family of peptide transporters. Proc. Natl. Acad. Sci. USA 2017, 114, 13182–13187. [Google Scholar] [CrossRef]
- Smith, D.E.; Clemencon, B.; Hediger, M.A. Proton-coupled oligopeptide transporter family SLC15: Physiological, pharmacological and pathological implications. Mol. Asp. Med. 2013, 34, 323–336. [Google Scholar] [CrossRef]
- Emter, R.; Natsch, A. The sequential action of a dipeptidase and a β-lyase in required for the release of the human body odorant 3-methyl-3-sulfanylhexan-1-ol from a secreted Cys-Gly-(S) conjugate by Corynebacteria. J. Biol. Chem. 2008, 283, 20645–60652. [Google Scholar] [CrossRef]
- Natsch, A.; Gfeller, H.; Gygax, P.; Schmid, J. Isolation of a bacterial enzyme releasing axillary malodor and its use as a screening target for novel deodorant formulations. Int. J. Cosmet. Sci. 2005, 27, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Natsch, A. What makes us smell: The biochemistry of body odour and the design of new deodorant ingredients. Chimia 2015, 69, 414–420. [Google Scholar] [CrossRef]
- Schwartz, M.; Poirier, N.; Moreno, J.; Proskura, A.; Lelievre, M.; Heydel, J.-M.; Neiers, F. Microbial β C-S lyases: Enzymes with multifaceted roles in flavor generation. Int. J. Mol. Sci. 2024, 25, 6412. [Google Scholar] [CrossRef]
- Fidan, O.; Karipcin, A.D.; Kose, A.H.; Anaz, A.; Demirsoy, B.N.; Arslansoy, N.; Sun, L.; Mujwar, S. Doscovery of a C-S lyase inhibitor for the prevention of human body malodor formation: Tannic acid inhibits the thioalcohol production in Staphylococcus hominis. Int. Microbiol. 2025, 28, 411–422. [Google Scholar] [CrossRef]
- Charig, A.; Froebe, C.; Simone, A.; Eigen, E. Inhibitor of odor-producing axillary bacterial exoenymes. J. Soc. Cosmet. Chem. 1991, 42, 133–145. [Google Scholar]
- Froebe, C.; Simone, A.; Charig, A.; Eigen, E. Axillary malodor production: A new mechanism. J. Soc. Cosmet. Chem. 1990, 41, 173–185. [Google Scholar]
- Eigen, E.; Froebe, C. Deodorant Compositions Comprising Inhibitors of Odor-Producing Axillary Bacterial Exoenzymes. U.S. Patent 5,676,937, 14 October 1997. [Google Scholar]
- Kumar, M.; Myagmardoloonjin, B.; Keshari, S.; Negari, I.P.; Huang, C.-M. 5-methyl furfural reduces the production of malodors by inhibiting sodium L-lactate fermentation of Staphylococcus epidermidis: Implication for deodorants targeting the fermenting skin microbiome. Microorganisms 2019, 7, 239. [Google Scholar] [CrossRef]
- Hara, T.; Matsui, H.; Shimizu, H. Suppression of microbial metabolic pathways inhibits the generation of the human body odor component diacetyl by Staphyloccuss spp. PLoS ONE 2014, 9, e111833. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Lourith, N. Body malodours and their topical treatment agents. Int. J. Cosmet. Sci. 2011, 33, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Fortune Business Insights. Deodorant Market Size, Share & Industry Analysis. By Product Type (Spray, Roll-On, Stick, and Others), by Packing Material (Metal, Plastic, and Others), by End-User (Mean and Women), and Regional Forecast, 2025–2032. 2025. Available online: https://www.fortunebusinessinsights.com/deodorant-market-102687 (accessed on 1 September 2025).
- SanMiguel, A.J.; Meisel, J.S.; Horwinski, J.; Zheng, Q.; Grice, E. Topical antimicrobial treatments can elicit shifts to resident skin bacterial communities and reduce colonization by Staphylococcus aureus competitors. Antimicrob. Agents Chemother. 2017, 61, e0077-17. [Google Scholar] [CrossRef] [PubMed]

| Precursor | Metabolite | Classification | Key Bacteria | Enzyme | Odor | Reference |
|---|---|---|---|---|---|---|
| Nα-3M2H-glutamine conjugate | 3M2H | Carboxylic acid | Corynebacterium striatum Ax20 | Nα-acyl-glutamine amonacylase (N-AGA) | Cheesy/sweaty | [13,14] |
| Nα-HMHA-glutamine conjugate | HMHA | Corynebacterium spp. | Nα-acyl-glutamine amonacylase (N-AGA) | Acidic, pungent | [14,44] | |
| Nα-4-ethyloctanoyl-glutamine conjugate | 4-ethyl-octanoic acid | Corynebacterium spp. | Nα-acyl-glutamine amonacylase (N-AGA) | Goaty | [14,45] | |
| Glutathione-pathway dipeptide | Cyc-Gly-(S)-3M3SH | Staphylococcus hominis | C-S lyase PatB PepTsh peptidases | - | [42,46,47] | |
| Not known as amino-acid conjugate | 5α-androst-16-en-3α-ol (androstenol) | steroid | - | - | Musky | [48] |
| Not known as amino-acid conjugate | 5α-androst-16-en-3-one (androstenone) | - | - | Urine/musky | [48] | |
| (S)-3M3SH | Cys-Gly-(S)-3M3SH | sulfanylalknol | Staphylococcus spp. | PatB-like C-S lysase | Sulfury, very pungent | [42,44,49] |
| Cysteine/dipeptide conjugate | 3-sulfanylhexan-1-ol (3SH) | Staphylococcus spp. | PatB-like C-S lysase | Grapefruit | [14,49] | |
| Cysteine/dipeptide conjugate | 2-methyl-3-sulfanylbutan-1-ol | Staphylococcus spp. | PatB-like C-S lysase | Onion | [14] | |
| Cycteine/dipeptide conjugate | 3-sulfanyl-pentan-1-ol | Staphylococcus spp. | PatB-like C-S lysase | Sulfury | [14] |
| Function | Ingredient | References |
|---|---|---|
| Anti-sweating | Aluminum-based compounds (aluminum chlorohydrate, aluminium sesquichlorohydrate, aluminium bromide, aluminium zirconium tetrachlorohydroate, aluminium lactate, and potassium alum) | [51] |
| Cyclomethiocone (mixture of cyclotetrasiloxane (D4), cyclopentasiloxane (D5), cyclohexasiloxane (D6), and cyclohepatiloxane (D7)) | [52,53] | |
| Stearyl alcohol | [54] | |
| Castor oil/hydrogenated caster oil (HCO) | [55,56] | |
|
Anti-proliferation
of malodor-forming bacteria | Triclosan (2,4,4′-trichloro-2′-hydroxydiphenyl ether) | [57] |
| Essential oil | [57,58] | |
| polymeric quaternary ammonium compound (PQ-16, copolymers of 1-vinyl-2-pyrolidone and 1-vinyl-3-methylimidazolium chloride) | [59] | |
| aluminum chlorohydrate | [60] | |
|
Masking/neutralizing effects
against malodor (fragrance) | Linalool (3,7-dimethyl-1,6-octadien-3-ol) | [61] |
| dl-citronellol (3,7-dimethyl-6-octen-1-ol) | [62] | |
| Citral (acyclic monoterpene aldehyde) | [63] | |
| Benzyl salicylate (2-hydroxybenzoic acid) | [64] | |
| Hexyl cinnamal (2-hexyl-3-phenyl-2-propenal) | [65] | |
| Geraniol ((2E)-3,7-dimethylocta-2,6-dien-1-ol) | [66] | |
| Limonene (1ne-methyl-4-(1-methyl phenyl)) | [67] | |
| Farnesol (3,7,11-trimethyl-2,6,10-dodecatrien-1-ol) | [68] | |
| Florhydral (isopropylphenylbutanal) | [69] | |
| Linalyl acetate (bergamol, 3,7-dimethylocta-1,6-dien-3-yl acetate) | [70] | |
|
Deodorizing effect
by natural products | Eribotrya japonca | [71] |
| Lotus corniculatus | [72] | |
| Salvia officinalis (sage) | [73] | |
| Rosmarinus officinalis L. (rosemary) | [74] | |
| Lavandular angustifolia Mill. (lavender) | [75,76] | |
| Thyme herbs (Thymus vulgaris L. and Thymus zygis L.) | [77] |
| No. | Items | Chemical Structure | No. | Items | Chemical Structure |
|---|---|---|---|---|---|
| 1 | Amylcinnamal | 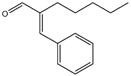 | 14 | Farnesol | 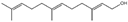 |
| 2 | Amylcinnamyl alcohol | 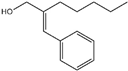 | 15 | Geraniol | 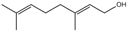 |
| 3 | Anicyl alcohol (4-methoxybenzyl alcohol) |  | 16 | Hexyl cinnamicaldehyde (alpha-Hexylcinnamaldehyde) |  |
| 4 | Benzyl alcohol |  | 17 | Hydroxyl-citronellal | 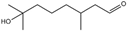 |
| 5 | Benzyl benzoate | 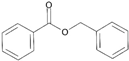 | 18 | Hydroxy-methylpentyl-cyclohexenecarboxaldehyde | 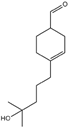 |
| 6 | Benzyl cinnamate | 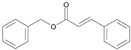 | 19 | Isoeugenol | 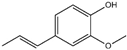 |
| 7 | Benzyl salicylate | 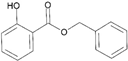 | 20 | D-limonene | 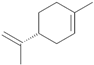 |
| 8 | Cinnamyl alcohol | 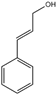 | 21 | Linalool | 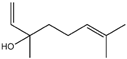 |
| 9 | Cinnamal (Cinnamaldehyde) | 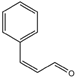 | 22 | Methyl heptin carbonate |  |
| 10 | Citral |  | 23 | 3-methyl-4-(2,6,6-tri-methyl-2-cyclohexen-1-yl)-3-buten-2-one (α-Cetone) | 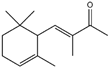 |
| 11 | β-Citronellol | 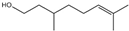 | 24 | Oak moss and treemoss extract | - |
| 12 | Coumarin | 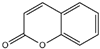 | 25 | Treemoss extract | - |
| 13 | eugenol | 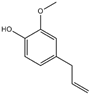 | 26 | 2-(4-tert-butylbenzyl) propionaldehyde (Lilial) | 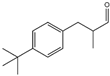 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, H.T.; Choi, H.-S.; Cho, S.-S.; Park, D.-H. Human Body Malodor and Deodorants: The Present and the Future. Int. J. Mol. Sci. 2025, 26, 10415. https://doi.org/10.3390/ijms262110415
Son HT, Choi H-S, Cho S-S, Park D-H. Human Body Malodor and Deodorants: The Present and the Future. International Journal of Molecular Sciences. 2025; 26(21):10415. https://doi.org/10.3390/ijms262110415
Chicago/Turabian StyleSon, Hyun Tae, Hyo-Seung Choi, Seung-Sik Cho, and Dae-Hun Park. 2025. "Human Body Malodor and Deodorants: The Present and the Future" International Journal of Molecular Sciences 26, no. 21: 10415. https://doi.org/10.3390/ijms262110415
APA StyleSon, H. T., Choi, H.-S., Cho, S.-S., & Park, D.-H. (2025). Human Body Malodor and Deodorants: The Present and the Future. International Journal of Molecular Sciences, 26(21), 10415. https://doi.org/10.3390/ijms262110415










