Synergistic Effects of Curcumin and Antibiotics Against Drug-Sensitive and Multidrug-Resistant Mycobacterium tuberculosis
Abstract
1. Introduction
2. Results
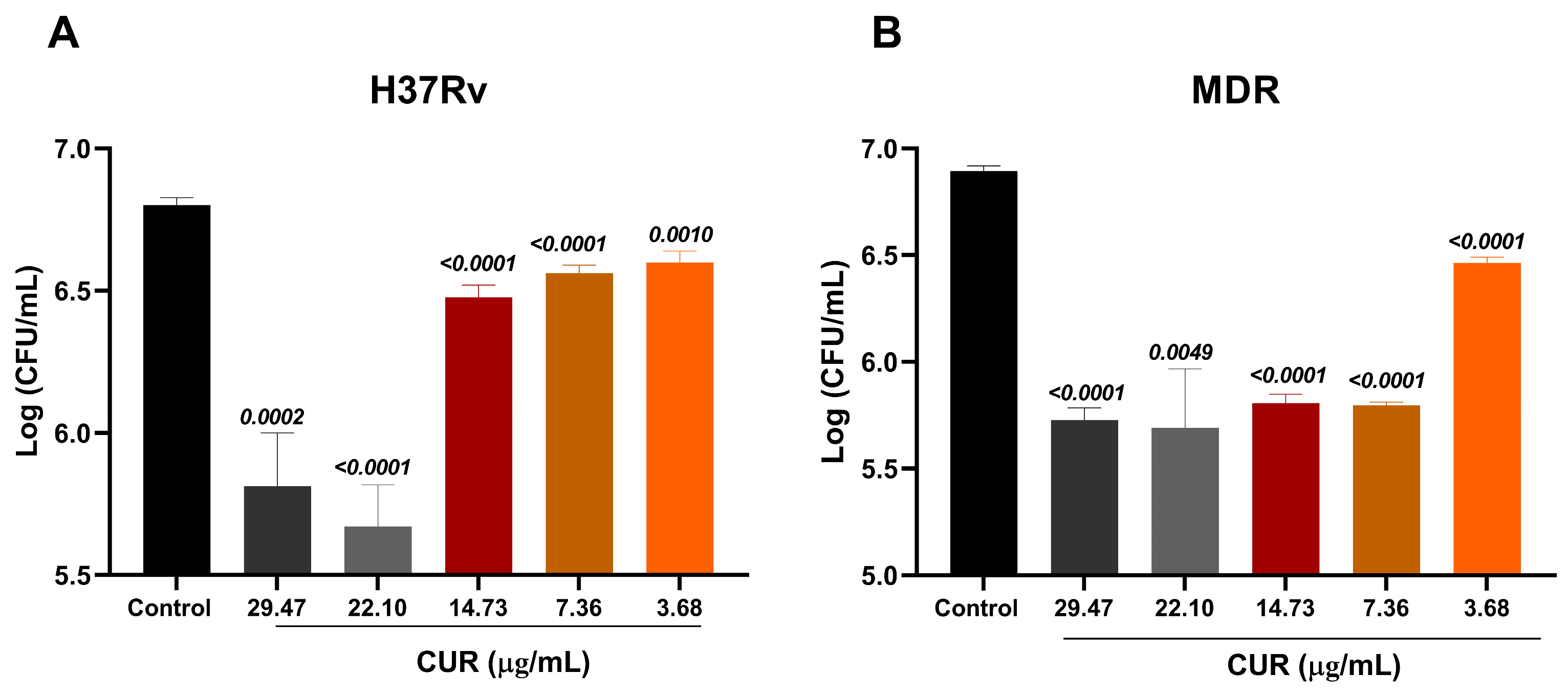
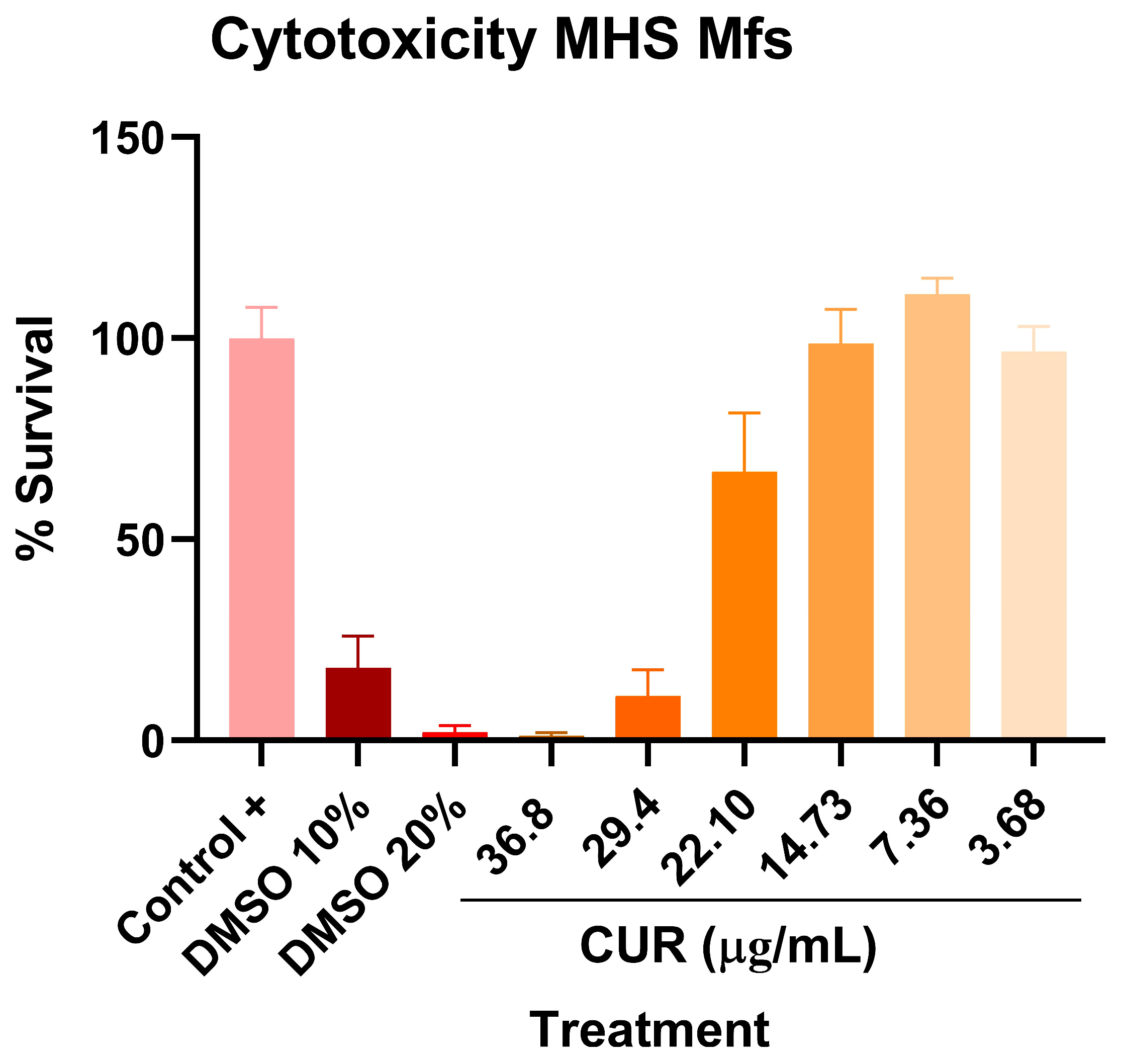
2.1. Antimicrobial Activity of Curcumin on Mtb
2.2. Effect of Curcumin in Combination with First and Second-Line ABX Against Mtb
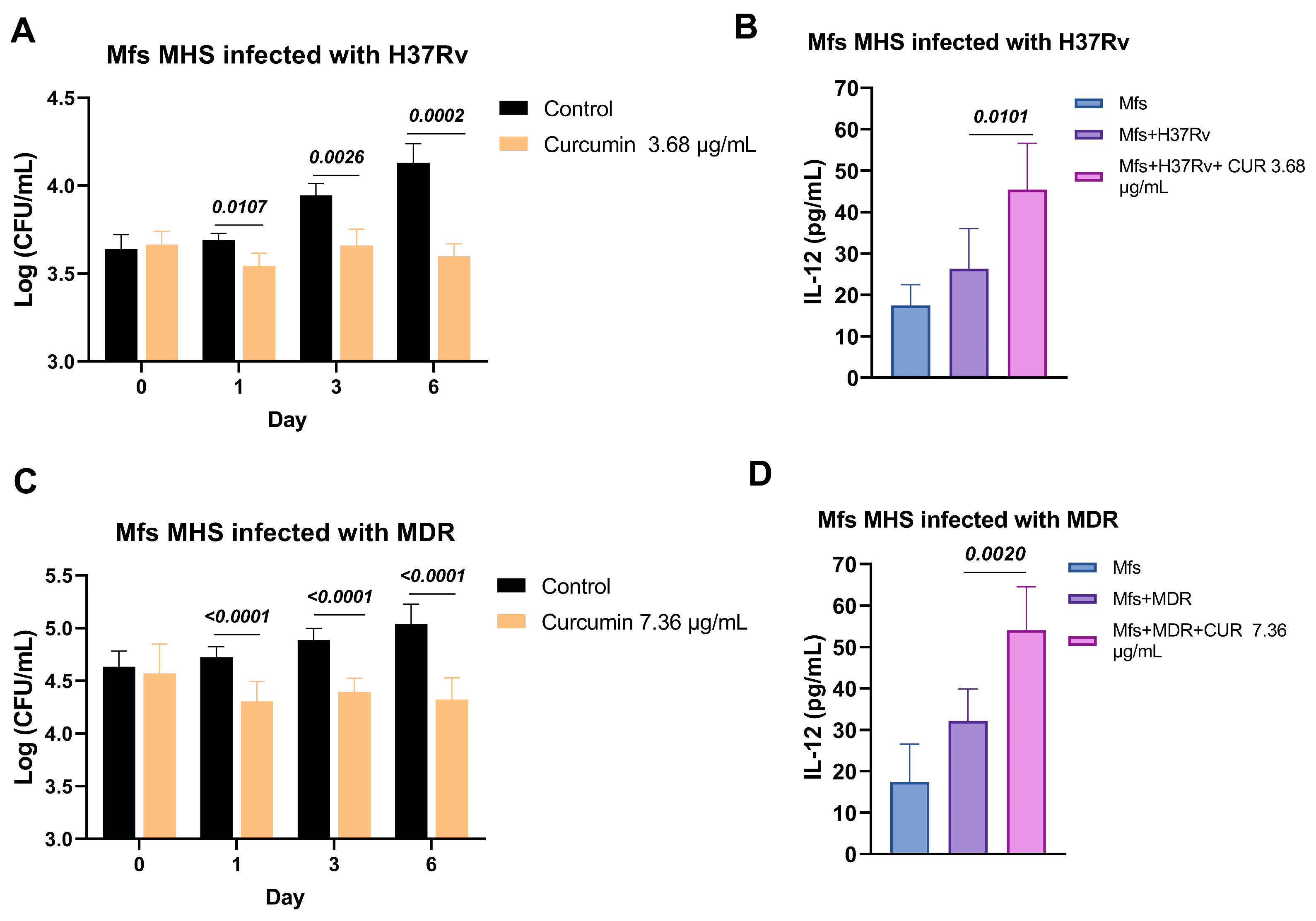
2.3. Effect of Curcumin in Macrophages Infected with Mtb
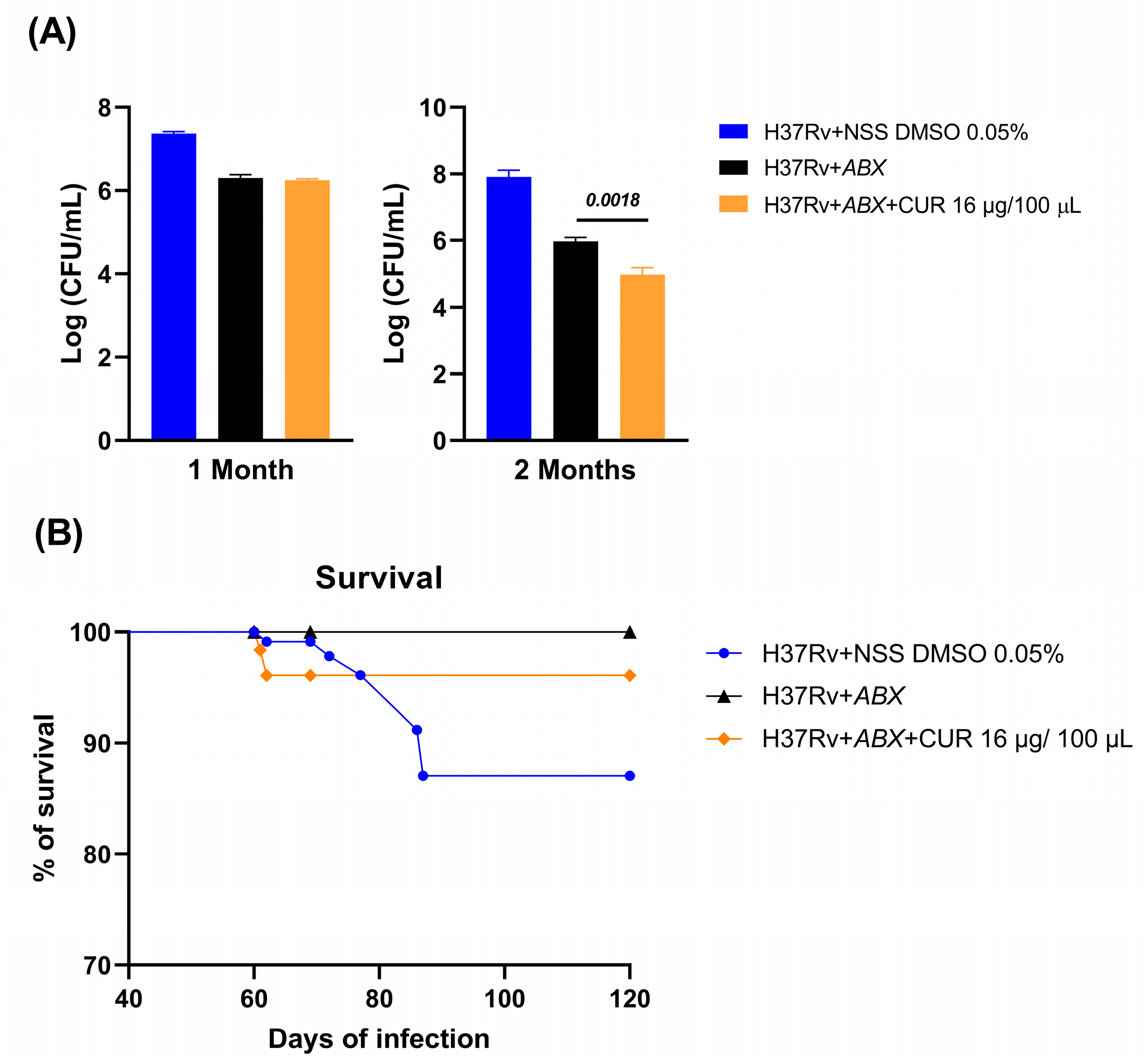
2.4. Effect of Curcumin Administration in Conjunction with ABX on Advanced Pulmonary TB
2.5. Effect of Curcumin Administration in Combination with ABX on the Sickness Behavior of Mice with Advanced TB
3. Discussion
4. Materials and Methods
4.1. Experimental Strategy
4.2. Preparation of Curcumin
4.3. Determination of the Antimicrobial Activity of Curcumin on Mtb In Vitro
4.4. Determination of the Combined Antimicrobial Activity of Curcumin with First and Second-Line ABX
4.5. Evaluation of the Effect of Curcumin on Macrophage Infection and Bacillary Load Assay
4.6. Use of Animals
4.7. The Murine Model of Pulmonary TB
4.8. Treatment with Curcumin and First-Line ABX
4.9. CFU Methodology for Determining Pulmonary Bacillary Loads
4.10. Preparation of Lung Tissue for Morphometric Analysis
4.11. Behavioral Studies
4.12. Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis of TNF Expression
4.13. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABX | Antibiotics |
| AMK | Amikacin |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| BDNF | Brain-Derived Neurotrophic Factor |
| BSL-3 | Biosafety Level 3 |
| CFU | Colony Forming Unit |
| COX-2 | Cyclooxygenase-2 |
| DMSO | Dimethyl sulfoxide |
| FBS | Fetal Bovine Serum |
| FICI | Fractional Inhibitory Concentration Index |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| H&E | Hematoxylin and Eosin |
| IL-12 | Interleukin-12 |
| INCMNSZ | Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán |
| INH | Isoniazid |
| MDR | Multidrug-resistant |
| MIC | Minimum Inhibitory Concentration |
| MOI | Multiplicity of Infection |
| MOX | Moxifloxacin |
| Mtb | Mycobacterium tuberculosis |
| NFκB | Nuclear Factor kappa B |
| NSS | Normal Saline Solution |
| OADC | Oleic, Albumin, Dextrose, and Catalase |
| OD | Optical Density |
| PBS | Phosphate-Buffered Saline |
| PGE2 | Prostaglandin E2 |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| PYZ | Pyrazinamide |
| RAi | Red de Apoyo a la Investigación |
| RIF | Rifampicin |
| RPMI | Roswell Park Memorial Institute medium |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SD | Standard Deviation |
| SDS | Sodium Dodecyl Sulfate |
| SEM | Standard Error of the Mean |
| TB | Tuberculosis |
| TNF | Tumor Necrosis Factor |
| XDR | Extensively drug-resistant |
References
- Pandit, R.; Singh, P.K.; Kumar, V. Natural Remedies against Multi-Drug Resistant Mycobacterium tuberculosis. J. Tuberc. Res. 2015, 3, 171–183. [Google Scholar] [CrossRef]
- Tung, B.T.; Nham, D.T.; Hai, N.T.; Thu, D.K. Curcuma longa, the Polyphenolic Curcumin Compound and Pharmacological Effects on Liver; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128144671. [Google Scholar]
- Iweala, E.J.; Uche, M.E.; Dike, E.D.; Etumnu, L.R.; Dokunmu, T.M.; Oluwapelumi, A.E.; Okoro, B.C.; Dania, O.E.; Adebayo, A.H.; Ugbogu, E.A. Curcuma longa (Turmeric): Ethnomedicinal uses, phytochemistry, pharmacological activities and toxicity profiles—A review. Pharmacol. Res. Mod. Chin. Med. 2023, 6, 100222. [Google Scholar] [CrossRef]
- Sudarshan, K.; Yarlagadda, S.; Sengupta, S. Recent Advances in the Synthesis of Diarylheptanoids. Chem. Asian J. 2024, 19, e202400380. [Google Scholar] [CrossRef]
- Bhandary, S.; Shetty, M.S.; Sharma, D.; Tanna, D.A.; Jain, M. The Medicinal Chemistry of Curcuma longa: A Narrative Review. Bangladesh J. Med. Sci. 2023, 22, 67–71. [Google Scholar] [CrossRef]
- Vaithiyalingam, M.; Sumathi, D.L.; Sabarathinam, S. Isolation and In silico Study of Curcumin from Curcuma longa and Its Anti-Diabetic Activity. Appl. Biochem. Biotechnol. 2023, 195, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef] [PubMed]
- Rahlwes, K.C.; Dias, B.R.S.; Campos, P.C.; Alvarez-Arguedas, S.; Shiloh, M.U. Pathogenicity and virulence of Mycobacterium tuberculosis. Virulence 2023, 14, 2150449. [Google Scholar] [CrossRef] [PubMed]
- Migliori, G.B.; Ong, C.W.M.; Petrone, L.; D’Ambrosio, L.; Centis, R.; Goletti, D. The definition of tuberculosis infection based on the spectrum of tuberculosis disease. Breathe 2021, 17, 210079. [Google Scholar] [CrossRef]
- World Health Organization. 2024 Global Tuberculosis (TB) Report; World Health Organization: Geneva, Switzerland, 2024; ISBN 9789240101531. [Google Scholar]
- Peloquin, C.A.; Davies, G.R. The Treatment of Tuberculosis. Clin. Pharmacol. Ther. 2021, 110, 1455–1466. [Google Scholar] [CrossRef]
- Heidary, M.; Shirani, M.; Moradi, M.; Goudarzi, M.; Pouriran, R.; Rezaeian, T.; Khoshnood, S. Tuberculosis challenges: Resistance, co-infection, diagnosis, and treatment. Eur. J. Microbiol. Immunol. 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Belete, T.M. Recent Progress in the Development of Novel Mycobacterium Cell Wall Inhibitor to Combat Drug-Resistant Tuberculosis. Microbiol. Insights 2022, 15, 11786361221099878. [Google Scholar] [CrossRef]
- Dartois, V.A.; Rubin, E.J. Anti-tuberculosis treatment strategies and drug development: Challenges and priorities. Nat. Rev. Microbiol. 2022, 20, 685–701. [Google Scholar] [CrossRef]
- Cox, H.S.; Furin, J.J.; Mitnick, C.D.; Daniels, C.; Cox, V.; Goemaere, E. The need to accelerate access to new drugs for multidrug-resistant tuberculosis. Bull. World Health Organ. 2015, 93, 491–497. [Google Scholar] [CrossRef]
- Brigden, G.; Nyang’wa, B.T.; du Cros, P.; Varaine, F.; Hughes, J.; Rich, M.; Horsburgh, C.R., Jr.; Mitnick, C.D.; Nuermberger, E.; Mcilleron, H.; et al. Principles for designing future regimens for multidrug-resistant tuberculosis. Bull. World Health Organ. 2014, 92, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Oberley-Deegan, R.E.; Bai, A.; Ovrutsky, A.R.; Kinney, W.H.; Weaver, M.; Zhang, G.; Honda, J.R.; Chan, E.D. Curcumin enhances human macrophage control of Mycobacterium tuberculosis infection. Respirology 2016, 21, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, W.W.; Cheng, X.; Xiang, H.R.; He, B.; Zhang, Q.Z.; Peng, W.X. Curcumin attenuates isoniazid-induced hepatotoxicity by upregulating the SIRT1/PGC-1α/NRF1 pathway. J. Appl. Toxicol. 2022, 42, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Lara-Espinosa, J.V.; Arce-Aceves, M.F.; López-Torres, M.O.; Lozano-Ordaz, V.; Mata-Espinosa, D.; Barrios-Payán, J.; Silva-Islas, C.A.; Maldonado, P.D.; Marquina-Castillo, B.; Hernández-Pando, R. Effect of Curcumin in Experimental Pulmonary Tuberculosis: Antimycobacterial Activity in the Lungs and Anti-Inflammatory Effect in the Brain. Int. J. Mol. Sci. 2022, 23, 1964. [Google Scholar] [CrossRef]
- Zumla, A.; Rao, M.; Parida, S.K.; Keshavjee, S.; Cassell, G.; Wallis, R.; Axelsson-Robertsson, R.; Doherty, M.; Andersson, J.; Maeurer, M. Inflammation and tuberculosis: Host-directed therapies. J. Intern. Med. 2015, 277, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Tiberi, S.; du Plessis, N.; Walzl, G.; Vjecha, M.J.; Rao, M.; Ntoumi, F.; Mfinanga, S.; Kapata, N.; Mwaba, P.; McHugh, T.D.; et al. Tuberculosis: Progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect. Dis. 2018, 18, e183–e198, Erratum in Lancet Infect. Dis. 2018, 18, 598. https://doi.org/10.1016/S1473-3099(18)30283-4. [Google Scholar] [CrossRef]
- Marini, E.; Di Giulio, M.; Magi, G.; Di Lodovico, S.; Cimarelli, M.E.; Brenciani, A.; Nostro, A.; Cellini, L.; Facinelli, B. Curcumin, an antibiotic resistance breaker against a multiresistant clinical isolate of Mycobacterium abscessus. Phytother. Res. 2018, 32, 488–495. [Google Scholar] [CrossRef]
- Tousif, S.; Singh, D.K.; Mukherjee, S.; Ahmad, S.; Arya, R.; Nanda, R.; Ranganathan, A.; Bhattacharyya, M.; Van Kaer, L.; Kar, S.K.; et al. Nanoparticle-formulated curcumin prevents posttherapeutic disease reactivation and reinfection with Mycobacterium tuberculosis following isoniazid therapy. Front. Immunol. 2017, 8, 739. [Google Scholar] [CrossRef]
- Gupta, P.K. Inhibition of Intracellular Survival of Multi Drug Resistant Clinical Isolates of Mycobacterium tuberculosis in Macrophages by Curcumin. Open Antimicrob. Agents J. 2013, 4, 1–5. [Google Scholar] [CrossRef]
- Ahmad, S.; Bhattacharya, D.; Kar, S.; Ranganathan, A.; Van Kaer, L.; Das, G. Curcumin nanoparticles enhance mycobacterium bovis BCG vaccine efficacy by modulating host immune responses. Infect. Immun. 2019, 87, e00291-19. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12 and its role in the generation of TH1 cells. Immunol. Today 1993, 14, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gately, M.K.; Wang, E.; Gong, J.; Wolf, S.F.; Lu, S.; Modlin, R.L.; Barnes, P.F. Interleukin 12 at the site of disease in tuberculosis. J. Clin. Investig. 1994, 93, 1733–1739. [Google Scholar] [CrossRef]
- Réus, G.Z.; Fries, G.R.; Stertz, L.; Badawy, M.; Passos, I.C.; Barichello, T.; Kapczinski, F.; Quevedo, J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015, 300, 141–154. [Google Scholar] [CrossRef]
- Kaufmann, F.N.; Gazal, M.; Bastos, C.R.; Kaster, M.P.; Ghisleni, G. Curcumin in depressive disorders: An overview of potential mechanisms, preclinical and clinical findings. Eur. J. Pharmacol. 2016, 784, 192–198. [Google Scholar] [CrossRef]
- Tiwari, V.; Chopra, K. Attenuation of oxidative stress, neuroinflammation, and apoptosis by curcumin prevents cognitive deficits in rats postnatally exposed to ethanol. Psychopharmacology 2012, 224, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Liu, W.; Liu, L.; Xiao, C.; Wang, Y.; Jiao, J.S. Curcumin protects neuron against cerebral ischemia-induced inflammation through improving PPAR-gamma function. Evid.-Based Complement. Altern. Med. 2013, 2013, 470975. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Xu, Y.; Lian, Y.; Xie, N.; Wu, T.; Zhang, H.; Sun, L.; Zhang, R.; Wang, Z. Curcumin improves amyloid β-peptide (1–42) induced spatial memory deficits through BDNF-ERK signaling pathway. PLoS ONE 2015, 10, e0131525. [Google Scholar] [CrossRef]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef]
- Haider, S.; Naqvi, F.; Batool, Z.; Tabassum, S.; Sadir, S.; Liaquat, L.; Naqvi, F.; Zuberi, N.A.; Shakeel, H.; Perveen, T. Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats. Brain Res. Bull. 2015, 115, 1–8. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M.; Ajarem, J.S.; Ahmad, M. Protective Effect of Curcumin on Anxiety, Learning Behavior, Neuromuscular Activities, Brain Neurotransmitters and Oxidative Stress Enzymes in Cadmium Intoxicated Mice. J. Behav. Brain Sci. 2013, 3, 74–84. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Curcumin for depression: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Huang, H.; Liu, Z. The efficacy and acceptability of curcumin for the treatment of depression or depressive symptoms: A systematic review and meta-analysis. J. Affect. Disord. 2021, 282, 242–251, Erratum in J. Affect. Disord. 2021, 289, 179. https://doi.org/10.1016/j.jad.2021.03.070. [Google Scholar] [CrossRef]
- Molina-Salinas, G.M.; Ramos-Guerra, M.C.; Vargas-Villarreal, J.; Mata-Cárdenas, B.D.; Becerril-Montes, P.; Said-Fernández, S. Bactericidal activity of organic extracts from Flourensia cernua DC against strains of Mycobacterium tuberculosis. Arch. Med. Res. 2006, 37, 45–49. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Mata-Espinosa, D.; Lara-Espinosa, J.V.; Barrios-Payán, J.; Hernández-Pando, R. The Use of Viral Vectors for Gene Therapy and Vaccination in Tuberculosis. Pharmaceuticals 2023, 16, 1475. [Google Scholar] [CrossRef]
- Rodríguez-Flores, E.M.; Mata-Espinosa, D.; Barrios-Payan, J.; Marquina-Castillo, B.; Castañón-Arreola, M.; Hernández-Pando, R. A significant therapeutic effect of silymarin administered alone, or in combination with chemotherapy, in experimental pulmonary tuberculosis caused by drug-sensitive or drug-resistant strains: In vitro and in vivo studies. PLoS ONE 2019, 14, e0217457. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Espinosa, O.; Mata-Espinosa, D.; Francisco-Cruz, A.; López-Torres, M.O.; Hernández-Bazán, S.; Barrios-Payán, J.; Marquina-Castillo, B.; Carretero, M.; del Río, M.; Hernández-Pando, R. Immunotherapeutic effect of adenovirus encoding antimicrobial peptides in experimental pulmonary tuberculosis. J. Leukoc. Biol. 2021, 110, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Lara-Espinosa, J.V.; Santana-Martínez, R.A.; Maldonado, P.D.; Zetter, M.; Becerril-Villanueva, E.; Pérez-Sánchez, G.; Pavón, L.; Mata-Espinosa, D.; Barrios-Payán, J.; López-Torres, M.O.; et al. Experimental pulmonary tuberculosis in the absence of detectable brain infection induces neuroinflammation and behavioural abnormalities in male balb/c mice. Int. J. Mol. Sci. 2020, 21, 9483. [Google Scholar] [CrossRef]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Neave, N.; Aggleton, J.P. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp. Brain Res. 1997, 113, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
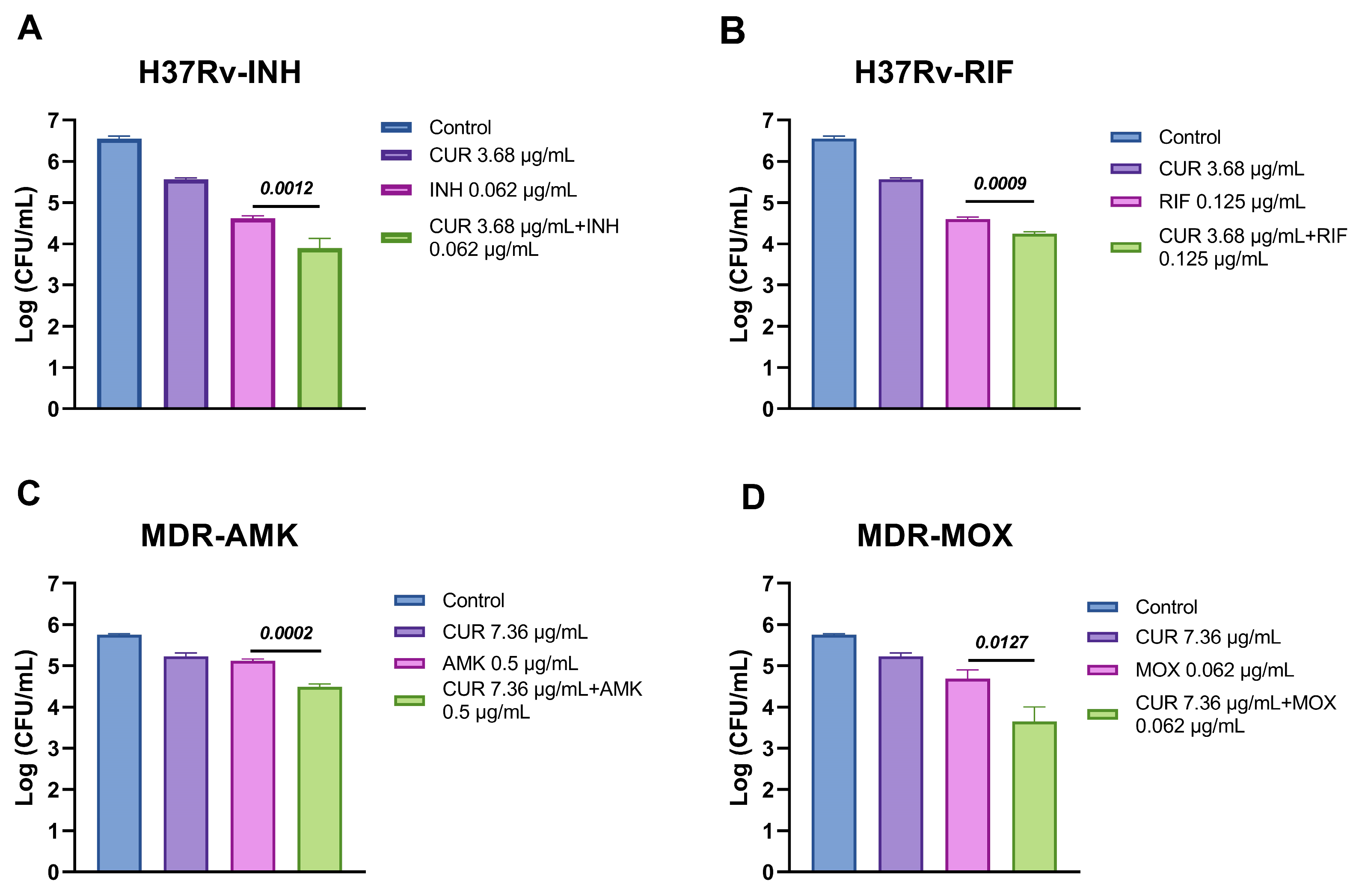



| Mtb | Compound | MIC | CUR + ABX | FICI | Result |
|---|---|---|---|---|---|
| H37Rv | CUR | 3.68 μg/mL | 1.84 μg/mL | 1 | Additive |
| INH | 0.0625 μg/mL | 0.03125 μg/mL | |||
| CUR | 3.68 μg/mL | 0.92095 μg/mL | 0.75 | Partial synergy | |
| RIF | 0.125 μg/mL | 0.0625 μg/mL | |||
| MDR | CUR | 7.36 μg/mL | 1.84 μg/mL | 0.75 | Partial synergy |
| AMK | 0.5 μg/mL | 0.25 μg/mL | |||
| CUR | 7.36 μg/mL | 1.84 μg/mL | 0.75 | Partial synergy | |
| MOX | 0.0625 μg/mL | 0.03125 μg/mL |
| Group | Number of Mice |
|---|---|
| H37Rv + NSS | 24 (12 per time of euthanasia) |
| H37Rv + ABX | 24 (12 per time of euthanasia) |
| H37Rv + ABX+ Curcumin | 24 (12 per time of euthanasia) |
| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | 5′-CATTGTGGAAGGGCTATGA-3′ | 5′-GGAAGGCCATGCCAGTGAGC-3′ |
| TNF | 5′-GCCGAGAAAGGCTGCTTG-3′ | 5′-TGTGGCTTCGACCTCTACCTC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Espinosa, J.V.; Barrios-Payán, J.; Lozano-Ordaz, V.; Mata-Espinosa, D.; Becerril-Villanueva, E.; Ponce-Regalado, M.D.; Hernández-Pando, R. Synergistic Effects of Curcumin and Antibiotics Against Drug-Sensitive and Multidrug-Resistant Mycobacterium tuberculosis. Int. J. Mol. Sci. 2025, 26, 10414. https://doi.org/10.3390/ijms262110414
Lara-Espinosa JV, Barrios-Payán J, Lozano-Ordaz V, Mata-Espinosa D, Becerril-Villanueva E, Ponce-Regalado MD, Hernández-Pando R. Synergistic Effects of Curcumin and Antibiotics Against Drug-Sensitive and Multidrug-Resistant Mycobacterium tuberculosis. International Journal of Molecular Sciences. 2025; 26(21):10414. https://doi.org/10.3390/ijms262110414
Chicago/Turabian StyleLara-Espinosa, Jacqueline V., Jorge Barrios-Payán, Vasti Lozano-Ordaz, Dulce Mata-Espinosa, Enrique Becerril-Villanueva, María Dolores Ponce-Regalado, and Rogelio Hernández-Pando. 2025. "Synergistic Effects of Curcumin and Antibiotics Against Drug-Sensitive and Multidrug-Resistant Mycobacterium tuberculosis" International Journal of Molecular Sciences 26, no. 21: 10414. https://doi.org/10.3390/ijms262110414
APA StyleLara-Espinosa, J. V., Barrios-Payán, J., Lozano-Ordaz, V., Mata-Espinosa, D., Becerril-Villanueva, E., Ponce-Regalado, M. D., & Hernández-Pando, R. (2025). Synergistic Effects of Curcumin and Antibiotics Against Drug-Sensitive and Multidrug-Resistant Mycobacterium tuberculosis. International Journal of Molecular Sciences, 26(21), 10414. https://doi.org/10.3390/ijms262110414








