Novel Spinel Li–Cr Nano-Ferrites: Structure, Morphology, and Electrical/Dielectric Properties
Abstract
1. Introduction
2. Results
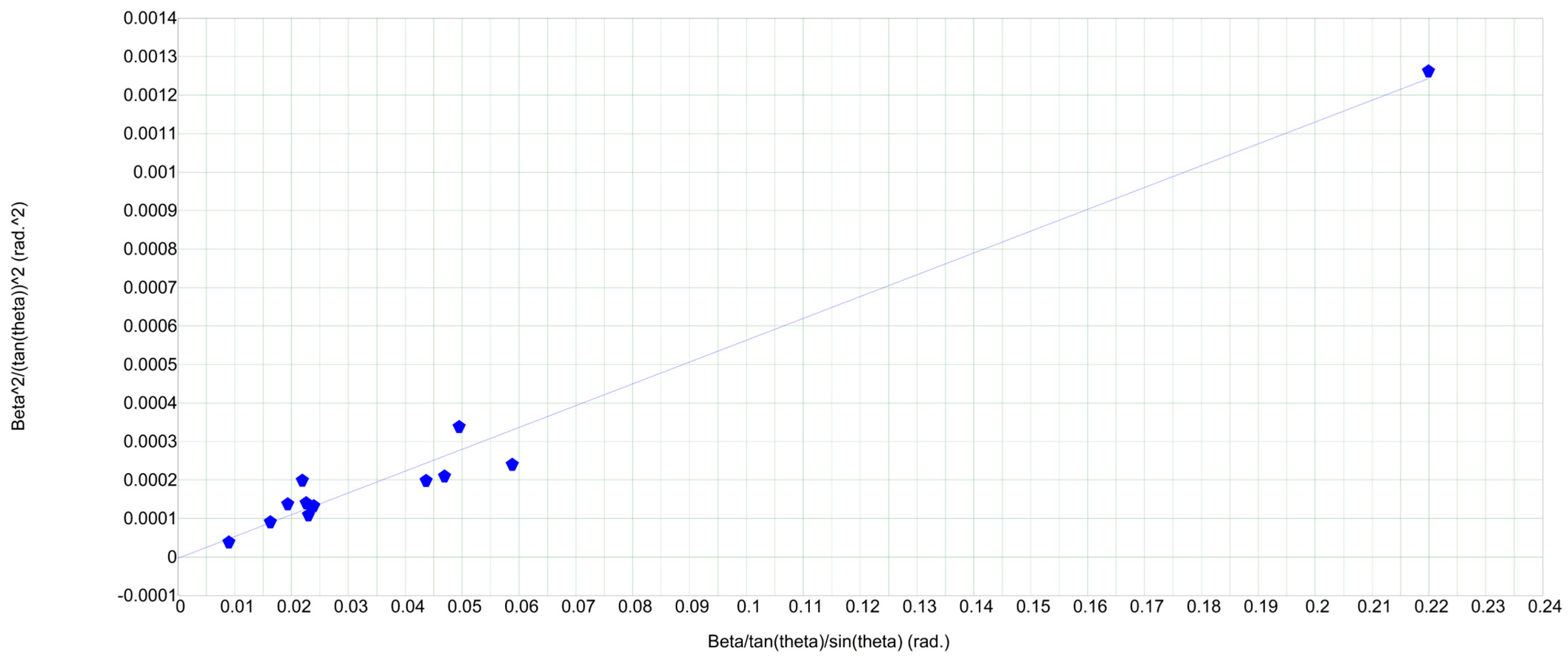
2.1. X-Ray Diffraction (XRD) Analysis
2.2. Raman Spectroscopy
2.3. Scanning Electron Microscope
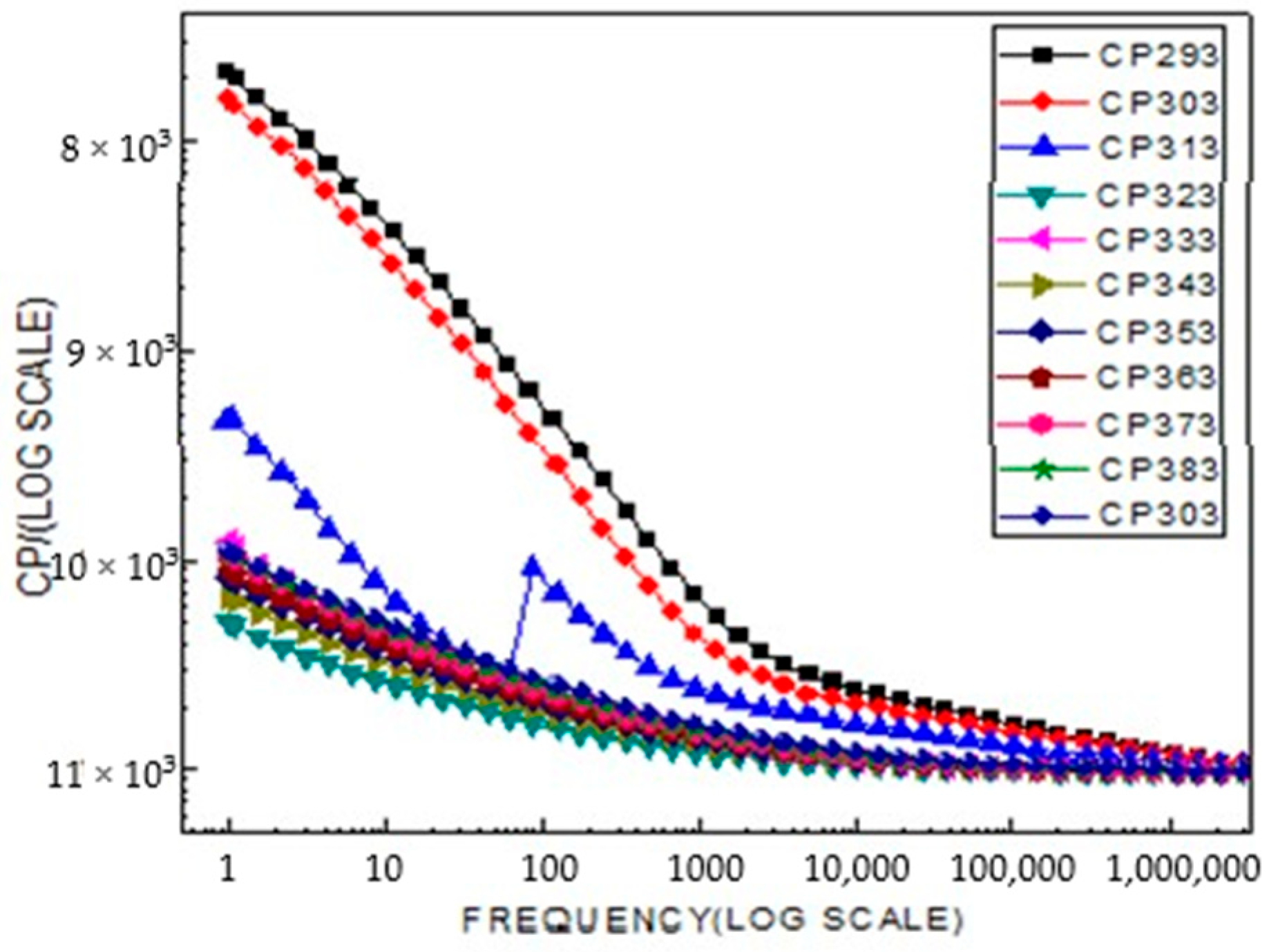
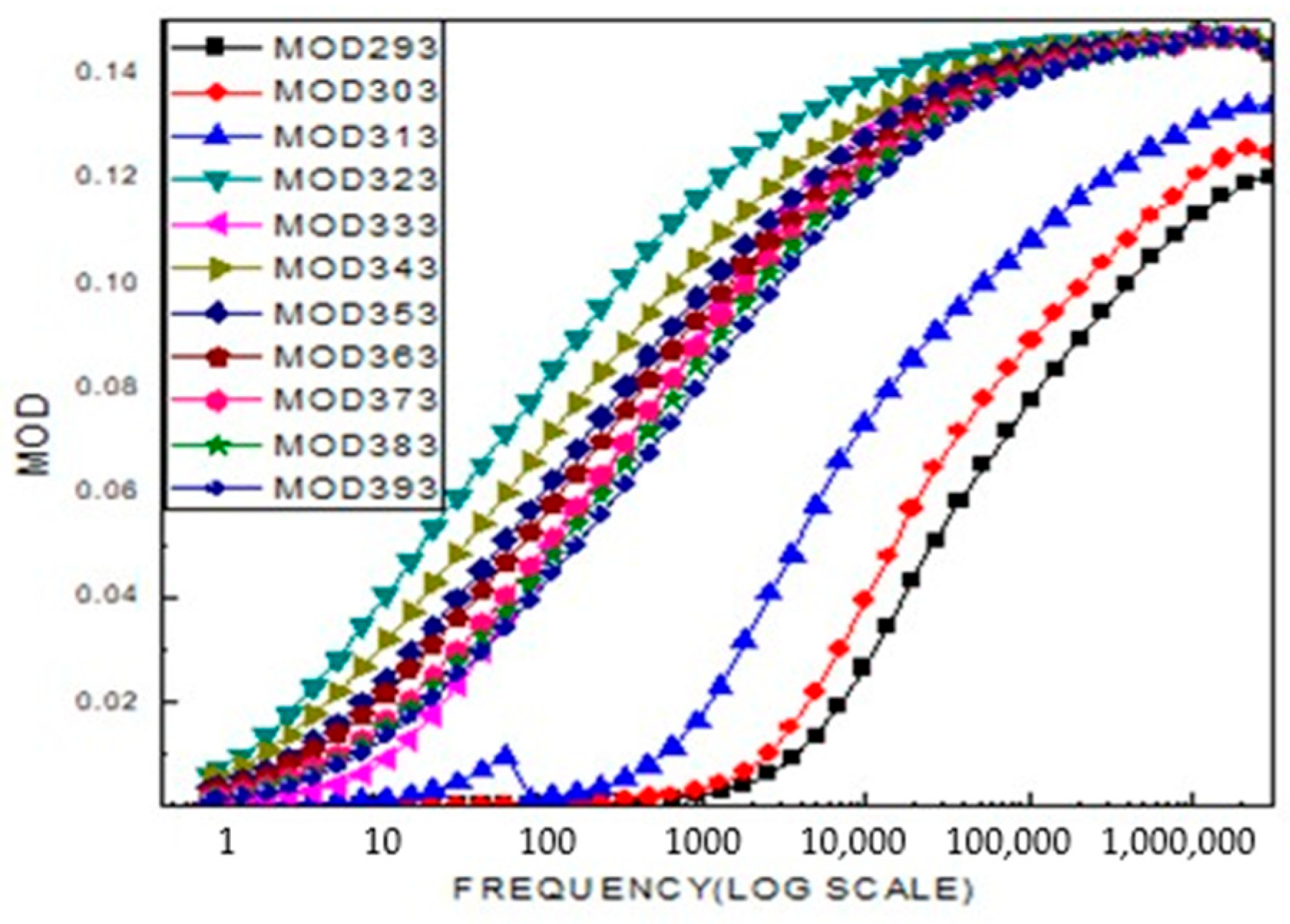
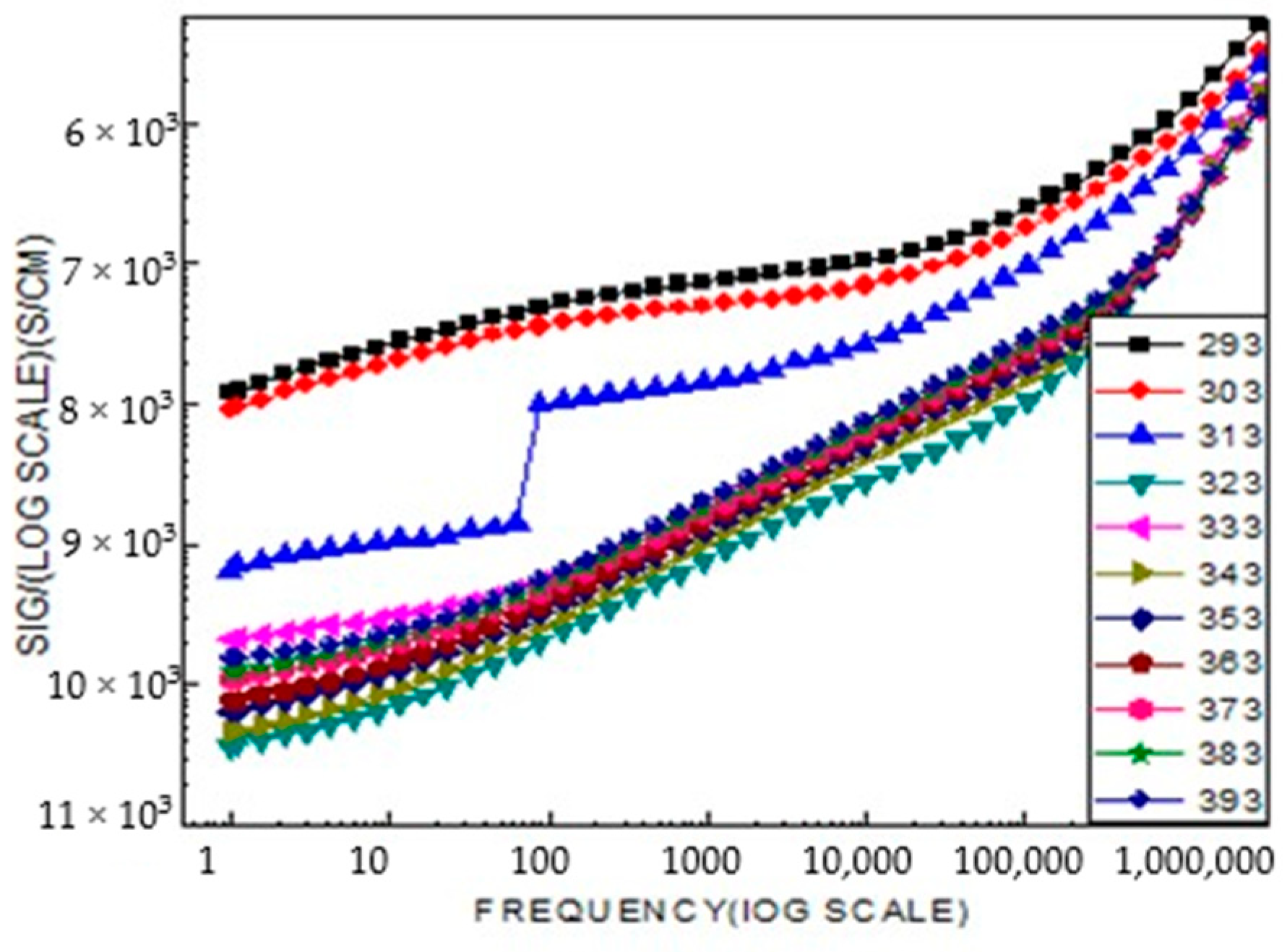
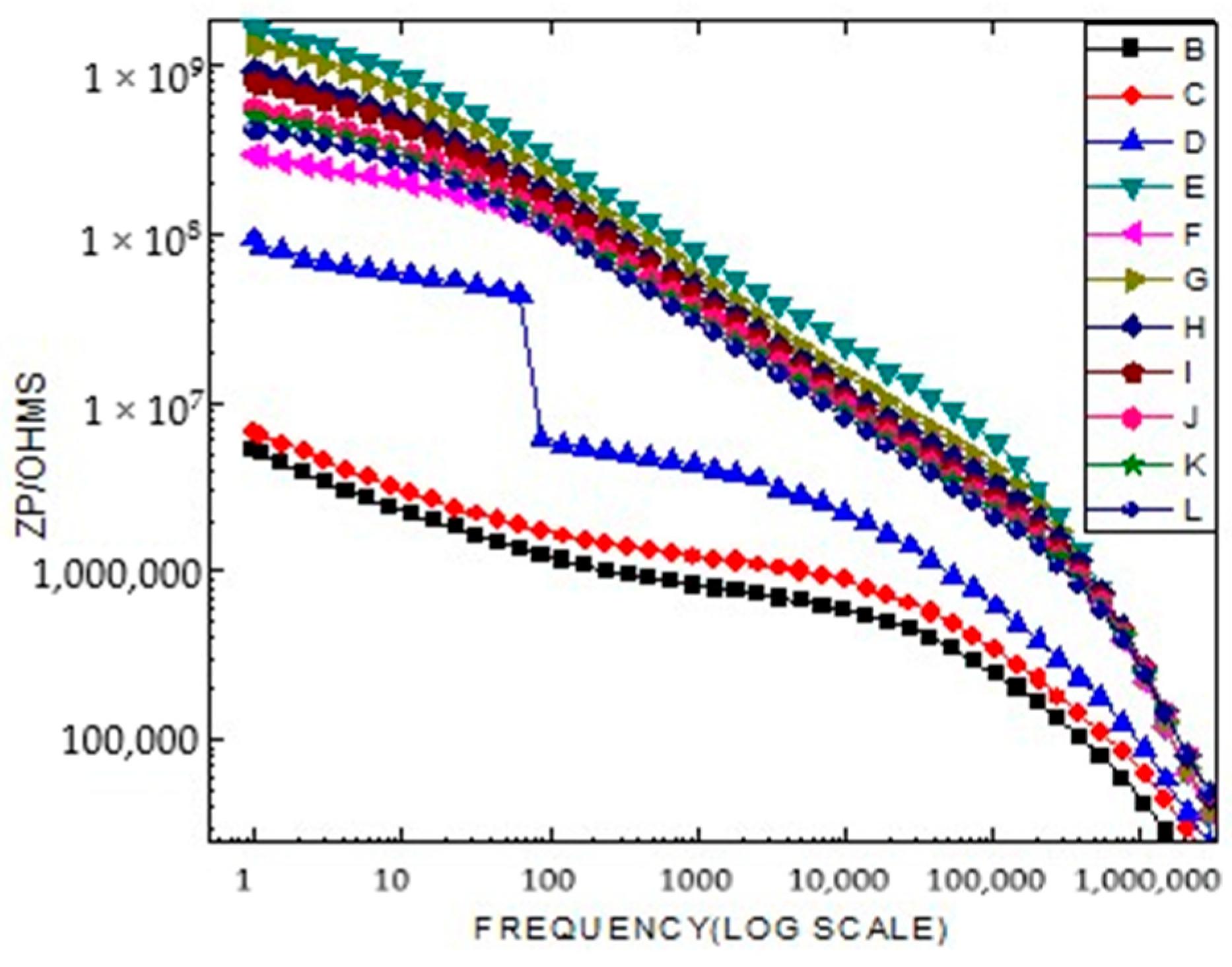
2.4. Broad Band Spectrometer Analysis
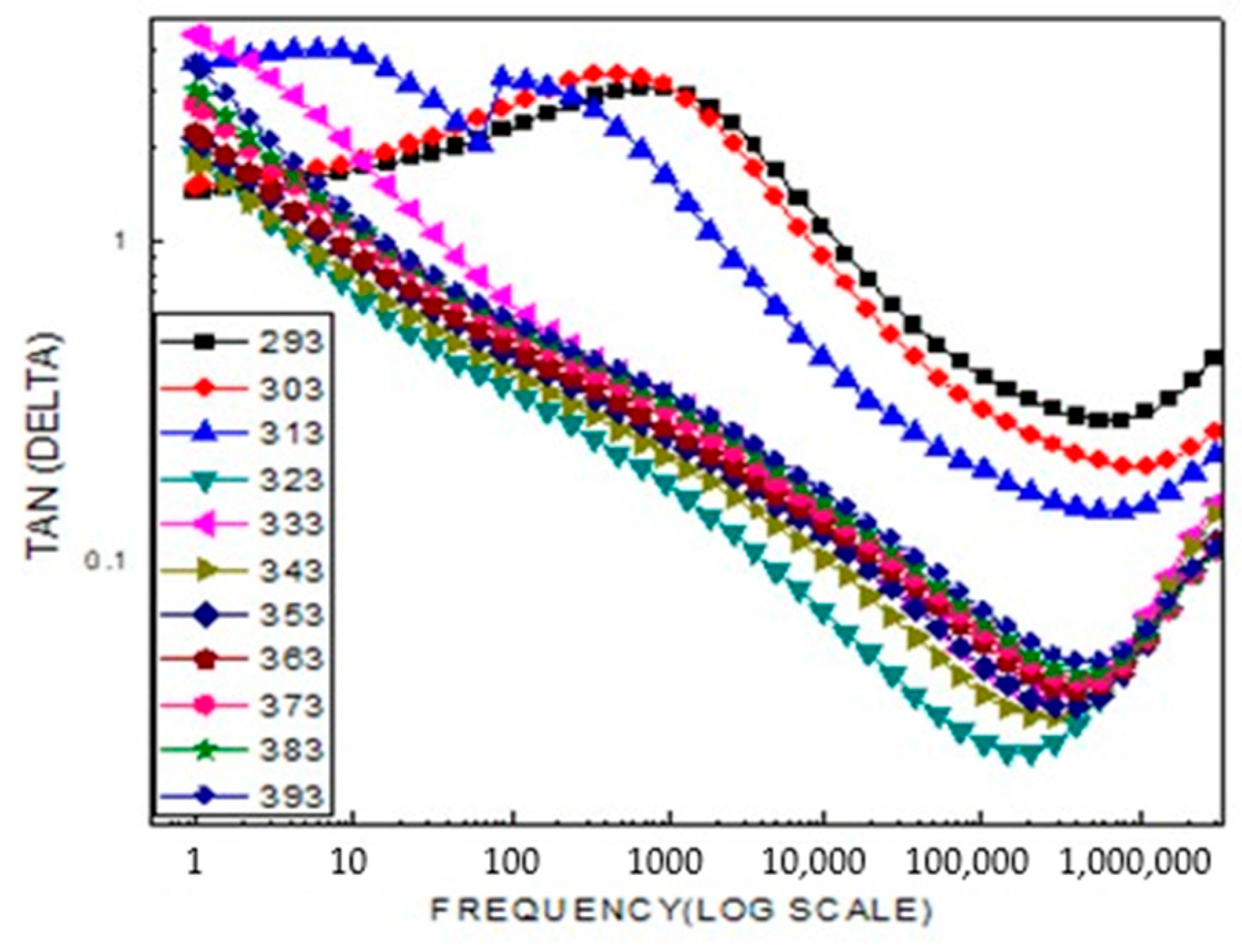
2.5. Electrophysical Research
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mataev, M.М.; Madiyarova, A.M.; Patrin, G.S.; Abdraimova, М.R.; Nurbekova, M.A.; Tursunova, Z.I. Synthesis and physico–chemical characteristics of complex ferrite CrNaFe2O5. Chem. J. Kaz. 2024, 1, 109–118. [Google Scholar] [CrossRef]
- Mataev, M.M.; Madiyarova, A.M.; Patrin, G.S.; Abdraimova, M.R.; Nurbekova, M.A.; Durmenbayeva, Z.D. Synthesis of New Complex Ferrite Li0.5MnFe1.5O4: Chemical–Physical and Electrophysical Research. Materials 2024, 17, 3754. [Google Scholar] [CrossRef]
- Saad, A.A.; Khan, W.; Dhiman, P.; Naqvi, A.H.; Singh, M. Structural, optical and magnetic properties of perovskite (La1−x Sr x )(Fe1−x Nix )O3, (x = 0.0, 0.1 & 0.2) nanoparticles. Electron. Mater. Lett. 2013, 9, 77–81. [Google Scholar]
- Benali, A.; Azizi, S.; Bejar, M.; Dhahri, E.; Graça, M.F.P. Structural, electrical and ethanol sensing properties of double-doping LaFeO3 perovskite oxides. Ceram. Int. 2014, 40, 14367–14373. [Google Scholar] [CrossRef]
- Ming, Q.; Nersesyan, M.D.; Wagner, A.; Ritchie, J.; Richardson, J.T.; Luss, D.; Jacobson, A.J.; Yang, Y.L. Combustion synthesis and characterization of Sr and Ga doped LaFeO3. Solid State Ion. 1999, 122, 113–121. [Google Scholar] [CrossRef]
- Zhaisanbaeva, M.E.; Patrin, G.S.; Seitbekova, K.S.; Nurbekova, M.A.; Mataev, M.M. Synthesis and Structural Studies of Perovskite-Ytterbium Manganate and Spinel-Cobalt Chromite Nanomaterials. Eng. Sci. 2025, 34, 1469. [Google Scholar] [CrossRef]
- Khetre, S.M.; Jadhav, H.V.; Jagadale, P.N.; Kulalb, S.R.; Bamane, S.R. Studies on electrical and dielectric properties of LaFeO3. Adv. Appl. Sci. Res. 2011, 2, 503–511. [Google Scholar]
- Sora, I.N.; Fontana, F.; Passalacqua, R.; Ampelli, C.; Perathoner, S.; Centi, G.; Parrino, F.; Palmisano, L. Photoelectrochemical properties of doped lanthanum orthoferrites. Electrochim. Acta 2013, 109, 710–715. [Google Scholar] [CrossRef]
- Varandili, S.B.; Babaei, A.; Ataie, A.; Khodadadi, A.A.; Kazerooni, H. Nano-structured Pd doped LaFe(Co)O3 perovskite; synthesis, characterization and catalytic behavior. Mater. Chem. Phys. 2018, 205, 228–239. [Google Scholar] [CrossRef]
- Mataev, M.M.; Abdraimova, M.R.; Tursinova, Z.I.; Kezdikbaeva, A.T. Thermodynamic Properties of Complex, Ferrite BiCa3Fe5O12. Orient. J. Chem. 2017, 33, 3200–3203. [Google Scholar] [CrossRef]
- Fujii, T.; Matsusue, I.; Takada, J. Superparamagnetic Behaviour and Induced Ferrimagnetism of LaFeO3 Nanoparticles. Adv. Asp. Spectrosc. 2012, 373–390. [Google Scholar] [CrossRef]
- Haye, E.; Capon, F.; Barrat, S.; Boulet, P.; Andre, E.; Carteret, C.; Bruyere, S. Properties of rare-earth orthoferrites perovskite driven by steric hindrance. J. Alloys Compd. 2016, 657, 631–638. [Google Scholar] [CrossRef]
- Deraz, N.M.; Shaban, S. Optimization of catalytic, surface and magnetic properties of nanocrystalline manganese ferrite. J. Anal. Appl. Pyrolysis. 2009, 86, 173–179. [Google Scholar] [CrossRef]
- Deraz, N.M.; El-Aiash, M.K.; Ali, S.A. Novel preparation and physicochemical characterization of a nanocrystalline cobalt ferrite system. Adsorpt. Sci. Technol. 2009, 27, 797–810. [Google Scholar] [CrossRef]
- Deraz, N.M.; Alarifi, A.; Shaban, S.A. Removal of sulfur from commercial kerosene using nanocrystalline NiFe2O4 based sorbents. J. Saudi Chem. Soc. 2010, 14, 357–362. [Google Scholar] [CrossRef]
- Köseoğlu, Y.; Baykal, A.; Gözüak, F.; Kavas, H. Structural and magnetic properties of CoxZn1−xFe2O4 nanocrystals synthesized by microwave method. Polyhedron 2009, 28, 2887–2892. [Google Scholar] [CrossRef]
- Mataev, M.M.; Myrzakhmetova, N.O.; Kuanysheva, Z.K.; Saparbekova, I.S.; Bazilbayev, S.M.; Doszhanova, G.A.; Medeuova, G. Calorimetric and Thermodynamic Studies of Complex Ferrites in the Temperature Range of 298, 15-673 K. Orient. J. Chem. 2015, 31, 441–445. [Google Scholar] [CrossRef]
- Cao, S.-W.; Zhu, Y.-J.; Cheng, G.-F.; Huang, Y.-H. ZnFe2O4 nanoparticles: Microwave-hydrothermal ionic liquid synthesis and photocatalytic property over phenol. J. Hazard. Mater. 2009, 171, 431–435. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Xue, J.M.; Wang, J.; Chan, H.S.O.; Yu, T.; Shen, Z.X. NiFe2O4 nanoparticles formed in situ in silica matrix by mechanical activation. J. Appl. Phys. 2002, 91, 6015. [Google Scholar] [CrossRef]
- Köseoğlu, Y.; Yıldız, F.; Slazar-Alvarez, G.; Toprak, M.; Muhammed, M.; Aktaş, B. Synthesis, characterization and ESR measurements of CoNiO nanoparticles. Phys. Status Solidi 2005, 42, 1712–1718. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Liu, B.; Wang, J.; Han, G.; Zhang, Y. Characterization and property of magnetic ferrite ceramics with interesting multilayer structure prepared by solid-state reaction. Ceram. Int. 2021, 47, 10927–10939. [Google Scholar] [CrossRef]
- Jabbarzare, S.; Abdellahi, M.; Ghayour, H.; Arpanahi, A.; Khandan, A. A study on the synthesis and magnetic properties of the cerium ferrite ceramic. J. Alloys Compd. 2017, 694, 800–807. [Google Scholar] [CrossRef]
- Samir Ullah, M.; Firoz Uddin, M.; Momin, A.A.; Hakim, M.A. Effect of V2O5 addition on the structural and magnetic properties of Ni–Co–Zn ferrites. Mater. Res. Express. 2021, 8, 016102. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Myslin, M.; Mironyuk, I.; Bououdina, M.; Pędziwiatr, A.T.; Gargula, R.; Bogacz, B.F.; Kurzydło, P. Synthesis, morphology, crystallite size and adsorption properties of nanostructured Mg–Zn ferrites with enhanced porous structure. J. Alloys Compd. 2020, 819, 152945. [Google Scholar] [CrossRef]
- Ghodake, U.R.; Kambale, R.C.; Suryavanshi, S.S. Effect of Mn2+ substitution on structural, electrical transport and dielectric properties of Mg–Zn ferrites. Ceram. Int. 2017, 43, 1129–1134. [Google Scholar] [CrossRef]
- Farid, H.M.T.; Ahmad, I.; Ali, I.; Ramay, S.M.; Mahmood, A.; Murtaza, G. Dielectric and impedance study of praseodymium substituted Mg-based spinel ferrite. J. Magn. Magn. Mater. 2017, 434, 143–150. [Google Scholar] [CrossRef]
- Rathod, V.; Anupama, A.V.; Jali, V.M.; Hiremath, V.A.; Sahoo, B. Combustion synthesis, structure and magnetic properties of Li–Zn ferrite ceramic powders. Ceram. Int. 2017, 43, 14431–14440. [Google Scholar] [CrossRef]
- Phanidhar Varma, P.V.S.K.; Suryanarayana, B.; Raghavendra, V.; Parajuli, D.; Murali, N. Effect of cr substitution on magnetic properties of cocu nano ferrites. Solid State Technol. 2022, 63, 8820–8827. [Google Scholar]
- Kharabe, R.G.; Devan, R.S.; Kanamadi, C.M.; Chougule, B.K. Dielectric properties of mixed Li–Ni–Cd ferrites. Smart Mater. Struct. 2006, 15, N36. [Google Scholar] [CrossRef]
- Sánchez, R.D.; Rivas, J.; Vaqueiro, P.; López-Quintela, M.A.; Caeiro, D. Particle size effects on magnetic properties of yttrium iron garnets prepared by a sol–gel method. J. Magn. Magn. Mater. 2002, 247, 92–98. [Google Scholar] [CrossRef]
- Mataev, M.M.; Abdraimova, M.R.; Saxsena, S.S.; Kezdikbaeva, A.T. Synthesis and X-ray analysis of complex ferrites BiMe3IIFe5O12 (MII = Mg, Ca, Ba). Key Eng. Mater. 2017, 744, 393–398. [Google Scholar] [CrossRef]
- Parajuli, D.; Murali, N.; Samatha, K.; Veeraiah, V. Thermal, structural, morphological, functional group and first cycle charge/discharge study of co substituted LiNi1X-0.02Mg0.02CoXO2 (x = 0.00, 0.02, 0.04,0.06, and 0.08) cathode material for libs. AIP Adv. 2022, 12, 085010. [Google Scholar] [CrossRef]
- Parajuli, D.; Taddesse, P.; Murali, N.; Veeraiah, V.; Samatha, K. Effect of Zn2+ doping on thermal, structural, morphological, functional group, and electrochemical properties of layered LiNi0.8Co0.1Mn0.1O2 cathode material. AIP Adv. 2022, 12, 125012. [Google Scholar] [CrossRef]
- Parajuli, D.; Samatha, K. Structural and cation distribution analysis of Nickel-Copper/Nickel-Magnesium substituted Lithium Ferrites. Bibechana 2024, 21, 74–82. [Google Scholar] [CrossRef]
- Mataev, M.M.; Patrin, G.S.; Abdraymova, M.R.; Tursinova, Z.I.; Yurkin, G.Y. Synthesis and magnetic properties of crystals Bi2BaFe4O10. J. Sib. Fed. Univ.—Math. Phys. 2018, 11, 411–415. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles- a comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Manh, D.H.; Ngoc Nha, T.T.; Hong Phong, L.T.; Nam, P.H.; Thanh, T.D.; Phong, P.T. Determination of the crystalline size of hexagonal La1−xSrxMnO3 (x = 0.3) nanoparticles from X-ray diffraction—A comparative study. RSC Adv. 2023, 13, 25007–25017. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Mohammed, A.K.; Sumaila, A. Comparative study of crystallite size using Williamson-Hall and Debye-Scherrer plots for ZnO nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045013. [Google Scholar] [CrossRef]
- Paswan, S.K.; Kumari, S.; Kar, M.; Singh, A.; Pathak, H.; Borah, J.P.; Kumar, L. Optimization of structure-property relationships in nickel ferrite nanoparticles annealed at different temperature. J. Phys. Chem. Solids 2021, 151, 109928. [Google Scholar] [CrossRef]
- Narang, S.B.; Pubby, K. Nickel spinel ferrites: A review. J. Magn. Magn. Mater. 2021, 519, 167163. [Google Scholar] [CrossRef]
- Okazaki, K. Technology of Ceramic Dielectrics; Energiya: Moscow, Russia, 1976; 256p. [Google Scholar]
- Kasenov, B.K.; Kasenova, S.B.; Sagintaeva, Z.I.; Baisanov, S.; Lu, N.Y.; Nukhuly, A.; Kuanyshbekov, E.E. Heat Capacity and Thermodynamic Functions of Titanium-Manganites of Lanthanum, Lithium and Sodium of LaLi2TiMnO6 and LaNa2TiMnO6. Molecules 2023, 28, 5194. [Google Scholar] [CrossRef] [PubMed]
- Fesenko, E.G. The Perovskite Family and Ferroelectricity; Atomizdat: Moscow, Russia, 1972. [Google Scholar]
- Venevtsev, Y.N.; Politova, E.D.; Ivanov, S.A. Ferroelectric and Antisegnetoelectrics of the Barium Titanate Family; Chemistry: Moscow, Russia, 1985. [Google Scholar]
- Lines, M.; Glass, A. Ferroelectrics and Related Materials; Mir: Moscow, Russia, 1981. [Google Scholar]
- Mataev, M.M.; Abdraimova, M.R.; Nuketaeva, D.Z.; Kezdikbaeva, A.T. YbBiNaFeO5 Compound-Ferrite Extraction Method. Patent of the Republic of Kazakhstan No. 104109, 28 April 2018. [Google Scholar]
- Mataev, M.; Sarsenbaeva, Z.; Keskin, B.; Nurbekova, M.; Meldeshov, A.; Tursyn, Z.; Seitbekova, K. Synthesis, Structure, and Electrophysical and Electrochemical Properties of Novel Composite La0.9MnO3-LaFeO3. Molecules 2025, 30, 132. [Google Scholar] [CrossRef] [PubMed]
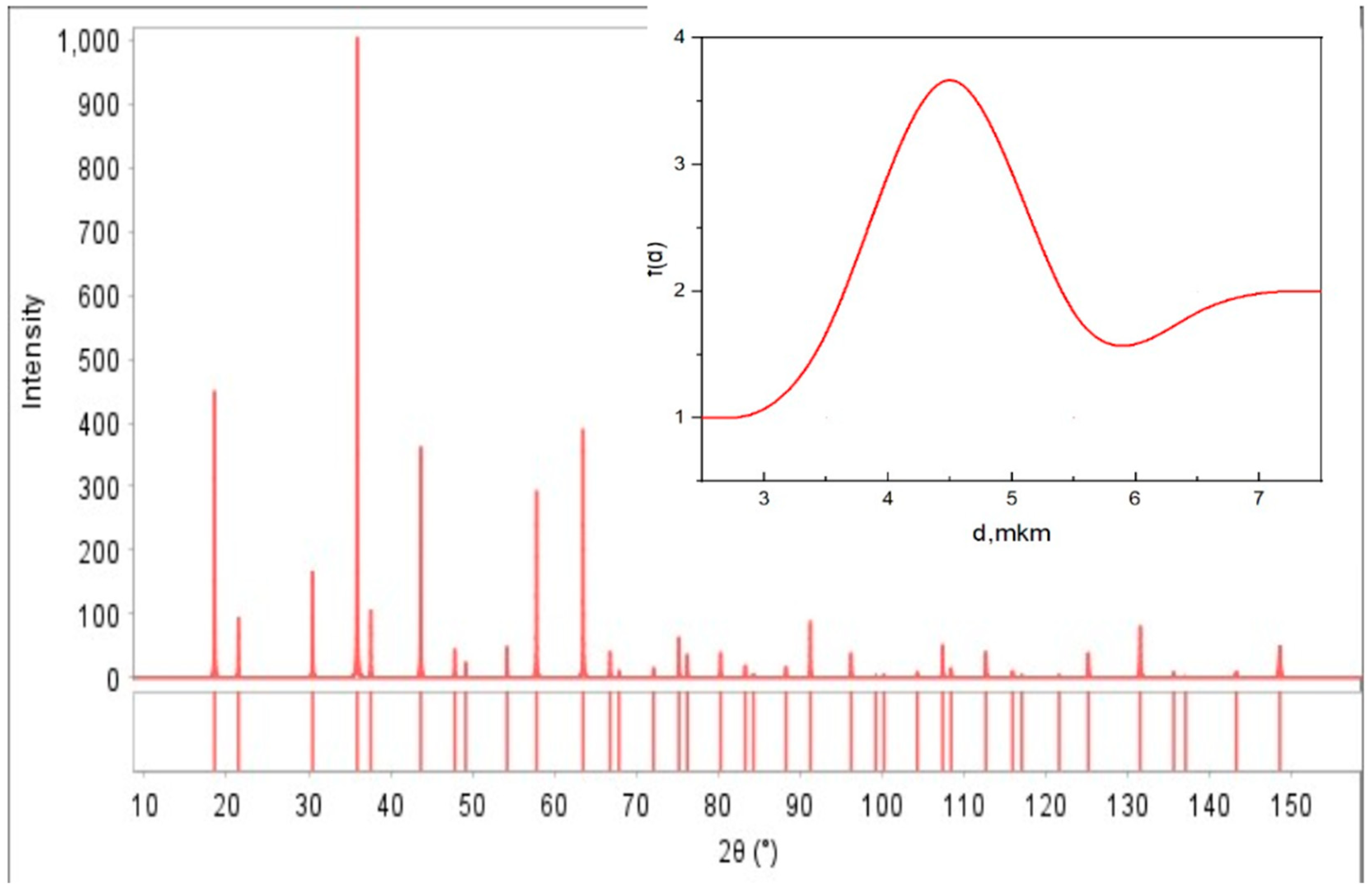
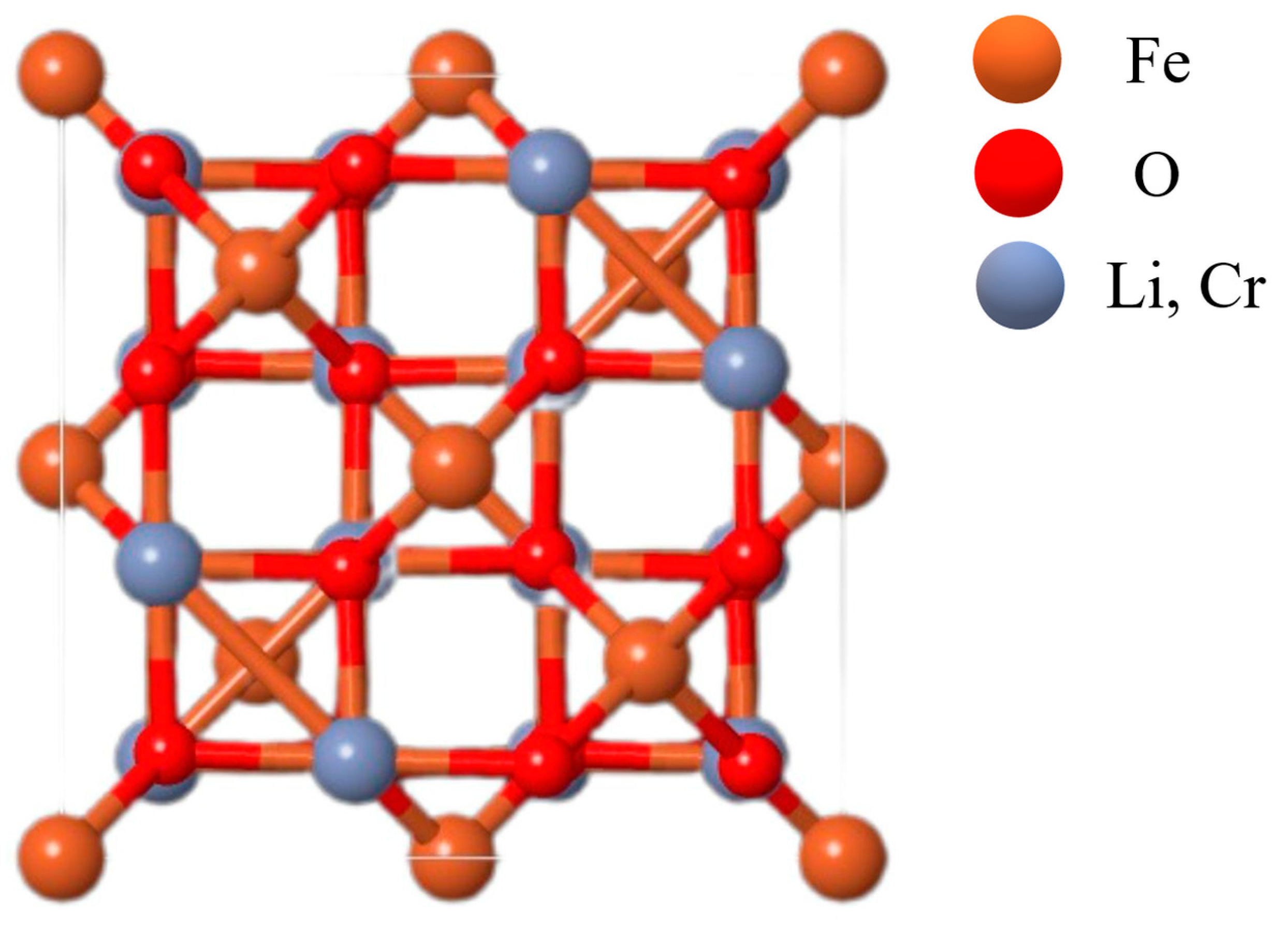
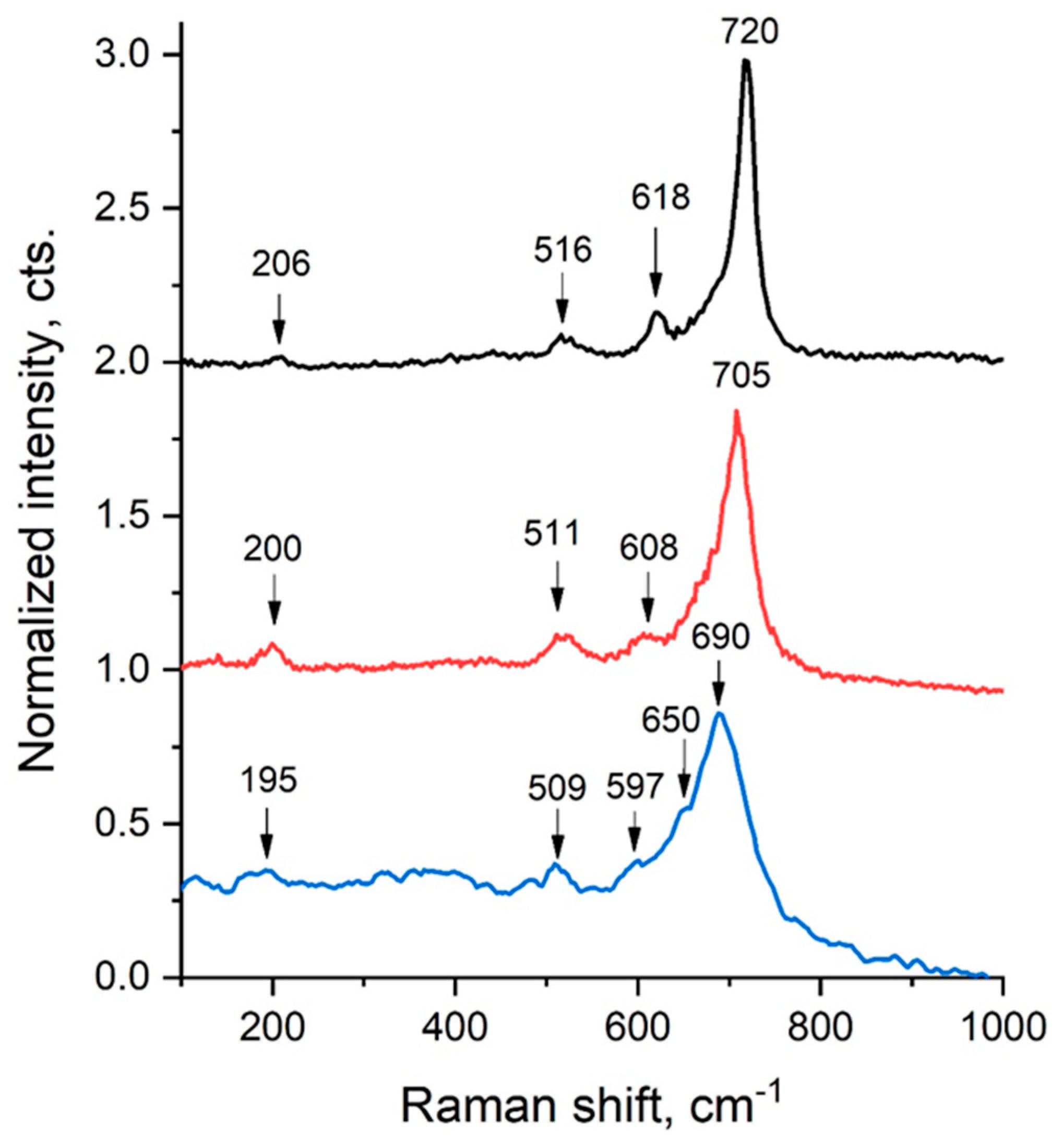
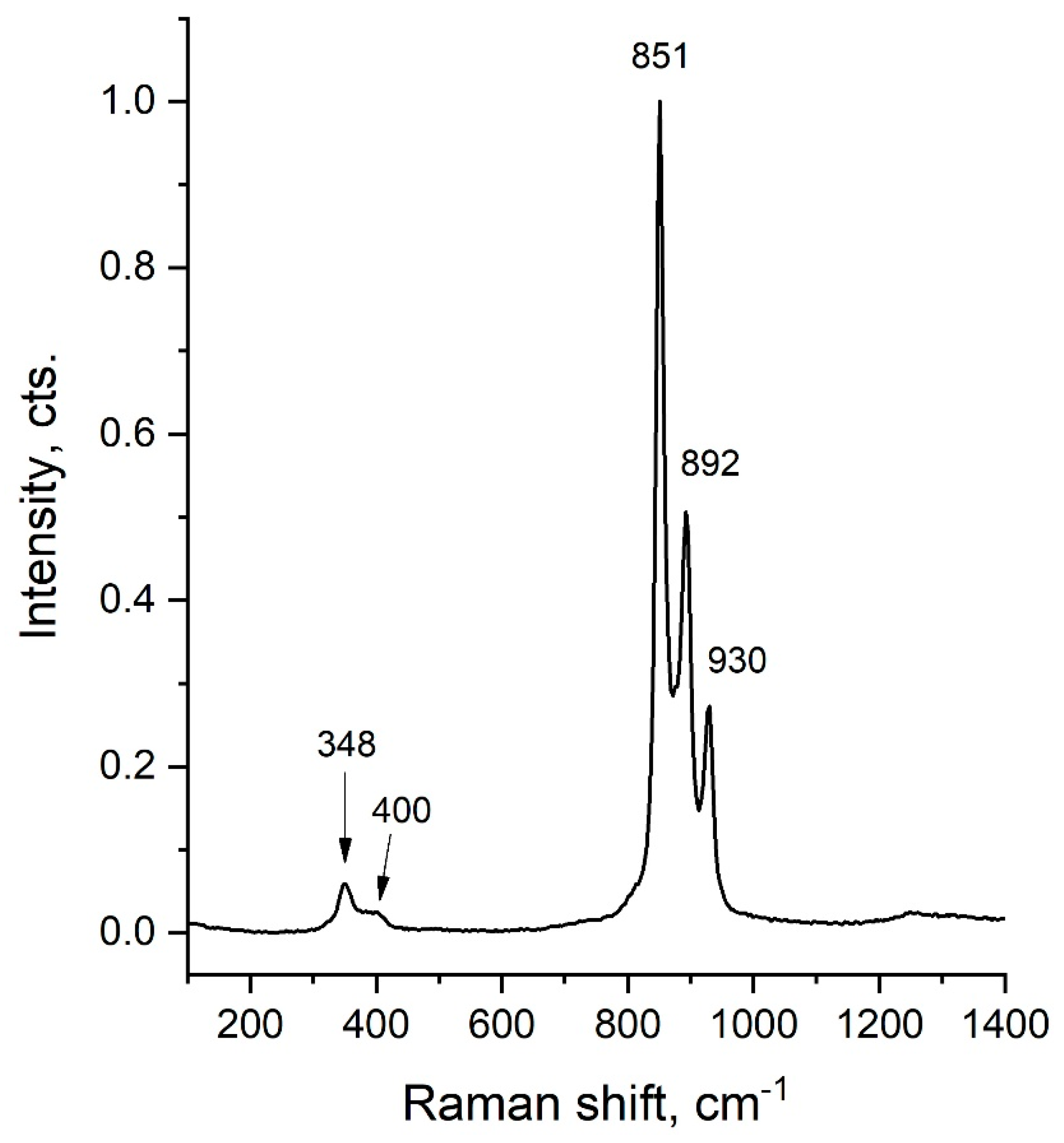
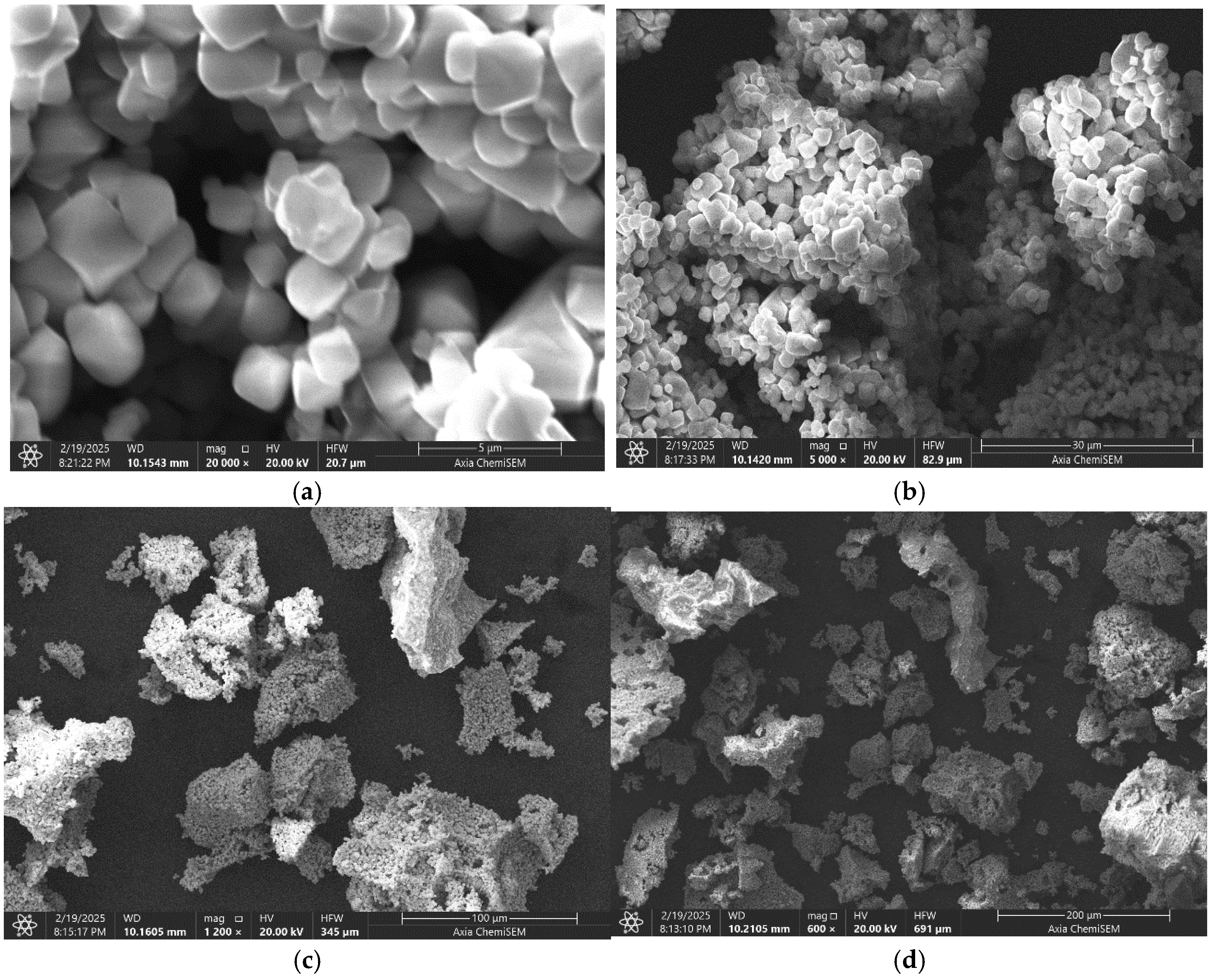
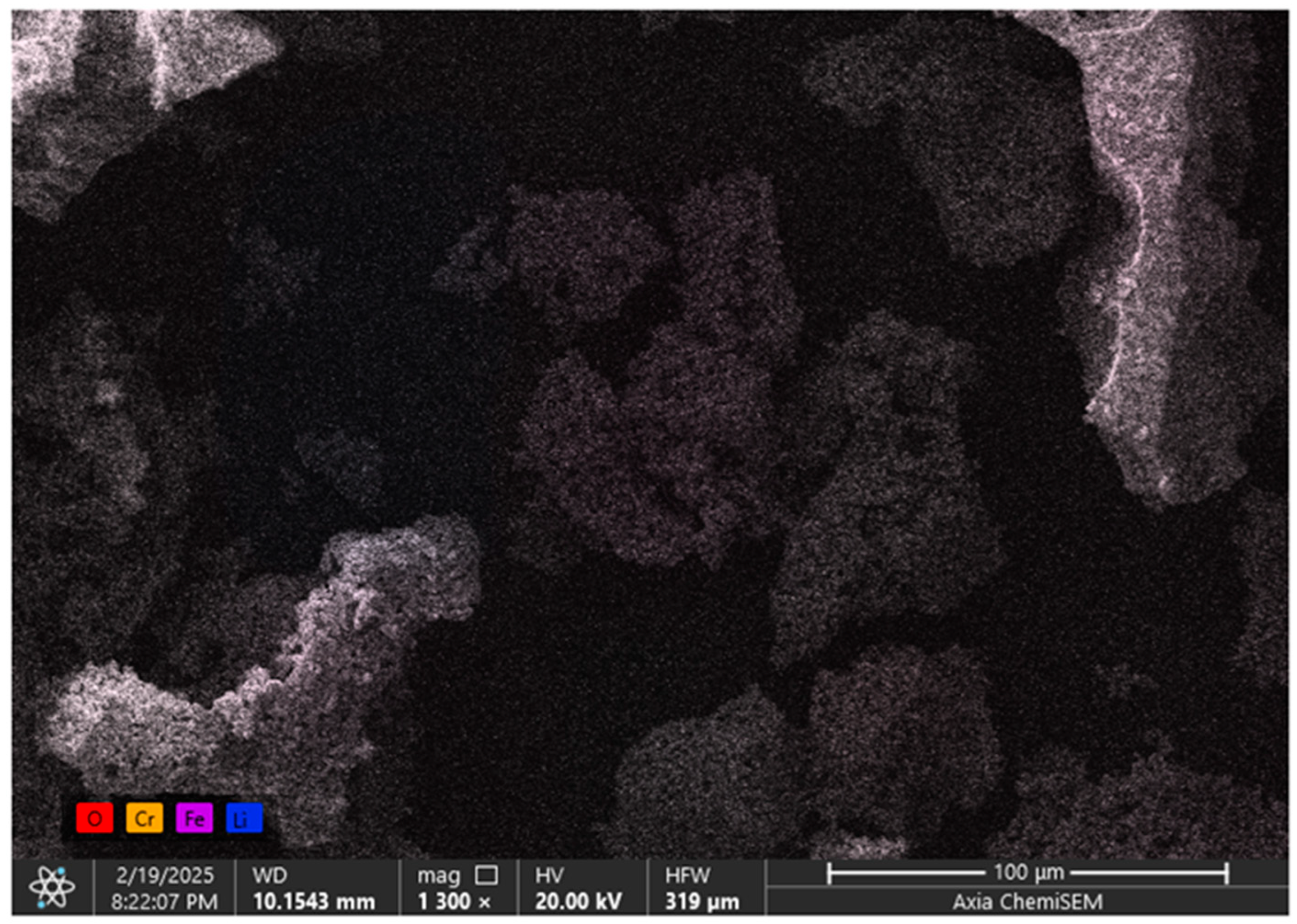
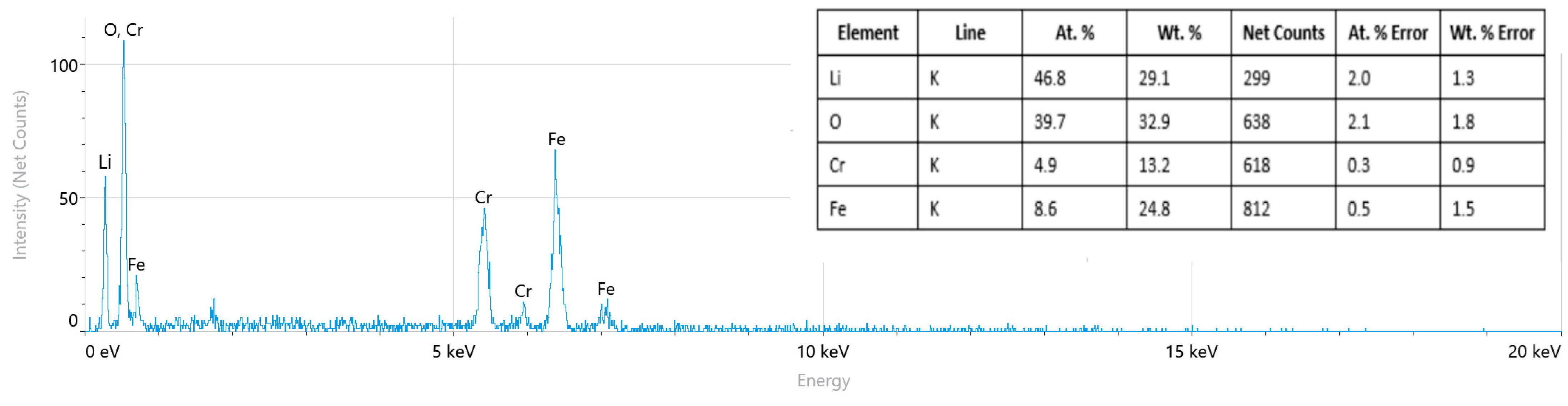

| Sample | LiCr3.4Fe1.6O8 |
|---|---|
| Space Group | , the cubic side is centered |
| Z | 4 |
| Cell Parameter (Å) | |
| a = | 8279 Å |
| b = c = V(Å3) | 8279 Å 8279 Å 57.646 Å3 |
| Agreement factors R-factor I/Ic I/Ic- CW ND α β γ X-ray density (g/cm3) Pycn.density (g/cm3) | 0.036 3.67 0.76 90 90 90 4.694 g/cm3 4.695 g/cm3 |
| Sample | Scherrer Method | Williamson-Hall Method | |
|---|---|---|---|
| D (nm) | D (nm) | ε × 10−3 | |
| LiCr3.4Fe1.6O8 | 5.2 | 9.12 | 1.71 |
| T, K | C, nF | R, Oм | ε | lgε | lgR |
|---|---|---|---|---|---|
| Measurement frequency at 1 kHz | |||||
| 293 303 313 323 333 343 353 363 373 383 393 403 413 423 433 443 453 463 473 483 | 0.27278 0.27426 0.27715 0.28125 0.28772 0.29313 0.29916 0.30751 0.31202 0.31702 0.32255 0.32967 0.3423 0.35119 0.36668 0.38018 0.39802 0.4169 0.43147 0.45456 | 13,400 13,270 12,910 12,560 11,890 11,210 10,290 9383 8831 9061 8814 7881 7098 6902 6153 6317 6010 5584 5149 4656 | 1296 1303 1316 1336 1367 1392 1421 1461 1482 1506 1532 1566 1626 1668 1742 1806 1891 1980 2050 2159 | 3.11 3.11 3.12 3.13 3.14 3.14 3.15 3.16 3.17 3.18 3.19 3.19 3.21 3.22 3.24 3.26 3.28 3.30 3.31 3.33 | 4.13 4.12 4.11 4.10 4.08 4.05 4.01 3.97 3.95 3.96 3.95 3.90 3.85 3.84 3.79 3.80 3.78 3.75 3.71 3.67 |
| Measurement frequency at 5 kHz | |||||
| 293 303 313 323 333 343 353 363 373 383 393 403 413 423 433 443 453 463 473 483 | 0.25678 0.2683 0.2775 0.28638 0.29667 0.30226 0.30787 0.31283 0.31843 0.32148 0.32578 0.32976 0.33303 0.33948 0.35613 0.3713 0.3925 0.41682 0.44245 | 29,630 21,650 13,080 5236 4301 4733 3296 2966 2805 2529 2669 3172 4434 6377 9644 11,520 10,430 8021 5978 3799 | 1220 1274 1318 1360 1389 1409 1436 1462 1486 1513 1527 1547 1566 1582 1613 1692 1764 1864 1980 2102 | 3.09 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.17 3.18 3.18 3.19 3.19 3.20 3.21 3.23 3.25 3.27 3.30 3.32 | 4.47 4.34 4.12 3.72 3.63 3.68 3.52 3.47 3.45 3.40 3.43 3.50 3.65 3.80 3.98 4.06 4.02 3.90 3.78 3.58 |
| Measurement frequency at 10 kHz | |||||
| 293 303 313 323 333 343 353 363 373 383 393 403 413 423 433 443 453 463 473 483 | 0.11814 0.18494 0.22927 0.25954 0.27501 0.28531 0.29302 0.29988 0.30652 0.31215 0.31667 0.32294 0.32779 0.33406 0.34256 0.35658 0.378 0.39475 0.41687 0.44203 | 152,300 70,790 32,200 11,870 4842 3312 2689 2257 1946 1689 1737 3130 5945 8231 8805 8052 5967 4604 3343 2353 | 561 878 1089 1233 1306 1355 1392 1424 1456 1483 1504 1534 1557 1587 1627 1694 1796 1875 1980 2100 | 2.75 2.94 3.04 3.09 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.19 3.20 3.21 3.23 3.25 3.27 3.30 3.32 | 5.18 4.85 4.51 4.07 3.69 3.52 3.43 3.35 3.29 3.23 3.24 3.50 3.77 3.92 3.94 3.91 3.78 3.66 3.52 3.37 |
| T, K | C, nF | R, Oм | ε | Lgε | lgR |
|---|---|---|---|---|---|
| Measurement frequency at 1 kHz | |||||
| 293 303 313 323 333 343 353 363 373 383 393 403 413 423 433 443 453 463 473 483 | 7.7977 10.302 15.68 12.489 8.1684 14.516 86.933 176.65 265.14 378.56 495.27 312.87 11.039 1.8105 0.95809 0.68585 0.70009 0.89737 1.3483 2.1329 | 165,700 135,800 101,700 131,900 203,600 121,200 32,510 19,300 14,110 10,520 8184 10,720 85,500 379,400 649,900 843,600 874,900 796,100 666,100 511,600 | 22,448 29,658 45,140 35,954 23,515 41,789 250,266 508,547 763,295 1,089,813 1,425,802 900,702 31,779 5212 2758 1974 2015 2583 3882 6140 | 4.35 4.47 4.65 4.56 4.37 4.62 5.40 5.71 5.88 6.04 6.15 5.95 4.50 3.72 3.44 3.30 3.30 3.41 3.59 3.79 | 5.22 5.13 5.01 5.12 5.31 5.08 4.51 4.29 4.15 4.02 3.91 4.03 4.93 5.58 5.81 5.93 5.94 5.90 5.82 5.71 |
| Measurement frequency at 5 kHz | |||||
| 293 303 313 323 333 343 353 363 373 383 393 403 413 423 433 443 453 463 473 483 | 1.3926 1.8886 2.728 1.517 0.9064 2.5719 15.47 31.031 45.909 64.608 82.944 40.501 1.7403 0.28075 0.13233 0.09429 0.09084 0.10483 0.13985 0.19866 | 140,100 115,400 89,690 128,100 184,900 93,070 29,090 17,900 13,060 9799 7702 11,420 79,660 293,500 455,500 533,500 555,900 536,400 477,500 401,900 | 4009 5437 7853 4367 2609 7404 44,536 89,333 132,165 185,996 238,782 116,596 5010 808 381 271 262 302 403 572 | 3.60 3.74 3.90 3.64 3.42 3.87 4.65 4.95 5.12 5.27 5.38 5.07 3.70 2.91 2.58 2.43 2.42 2.48 2.60 2.76 | 5.15 5.06 4.95 5.11 5.27 4.97 4.46 4.25 4.12 3.99 3.89 4.06 4.90 5.47 5.66 5.73 5.74 5.73 5.68 5.60 |
| Measurement frequency at 10 kHz | |||||
| 293 303 313 323 333 343 353 363 373 383 393 403 413 423 433 443 453 463 473 483 | 0.62512 0.85581 1.188 0.50394 0.3238 1.2082 7.2162 14.584 21.676 30.192 39.355 14.483 0.64942 0.12128 0.06145 0.04865 0.04694 0.05137 0.06369 0.08415 | 126,300 103,700 83,510 127,500 169,200 76,460 26,640 16,680 12,440 9304 7426 12,940 83,960 243,700 329,700 351,900 362,500 360,400 339,200 306,100 | 1800 2464 3420 1451 932 3478 20,774 41,985 62,402 86,918 113,297 41,694 1870 349 177 140 135 148 183 242 | 3.26 3.39 3.53 3.16 2.97 3.54 4.32 4.62 4.80 4.94 5.05 4.62 3.27 2.54 2.25 2.15 2.13 2.17 2.26 2.38 | 5.10 5.02 4.92 5.11 5.23 4.88 4.43 4.22 4.09 3.97 3.87 4.11 4.92 5.39 5.52 5.55 5.56 5.56 5.53 5.49 |
| (a) | |
|---|---|
| T, K | lg R |
| 293 | 4.41 |
| 313 | 4.22 |
| (b) | |
| T, K | lg R |
| 343 | 6.10 |
| 363 | 3.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mataev, M.; Madiyarova, A.; Abdraimova, M.; Nurbekova, M.; Seibekova, K.; Tursyn, Z.; Kezdykbayeva, A.; Ramachandran, K.; Keskin, B. Novel Spinel Li–Cr Nano-Ferrites: Structure, Morphology, and Electrical/Dielectric Properties. Int. J. Mol. Sci. 2025, 26, 10409. https://doi.org/10.3390/ijms262110409
Mataev M, Madiyarova A, Abdraimova M, Nurbekova M, Seibekova K, Tursyn Z, Kezdykbayeva A, Ramachandran K, Keskin B. Novel Spinel Li–Cr Nano-Ferrites: Structure, Morphology, and Electrical/Dielectric Properties. International Journal of Molecular Sciences. 2025; 26(21):10409. https://doi.org/10.3390/ijms262110409
Chicago/Turabian StyleMataev, Mukhametkali, Altynai Madiyarova, Moldir Abdraimova, Marzhan Nurbekova, Karima Seibekova, Zhanar Tursyn, Assel Kezdykbayeva, Krishnamoorthy Ramachandran, and Bahadir Keskin. 2025. "Novel Spinel Li–Cr Nano-Ferrites: Structure, Morphology, and Electrical/Dielectric Properties" International Journal of Molecular Sciences 26, no. 21: 10409. https://doi.org/10.3390/ijms262110409
APA StyleMataev, M., Madiyarova, A., Abdraimova, M., Nurbekova, M., Seibekova, K., Tursyn, Z., Kezdykbayeva, A., Ramachandran, K., & Keskin, B. (2025). Novel Spinel Li–Cr Nano-Ferrites: Structure, Morphology, and Electrical/Dielectric Properties. International Journal of Molecular Sciences, 26(21), 10409. https://doi.org/10.3390/ijms262110409







