Antibiotics Impact the Cytotoxicity and Cytopathic Effect of Helicobacter pylori Extracellular Vesicles Against Gastric Cells
Abstract
1. Introduction
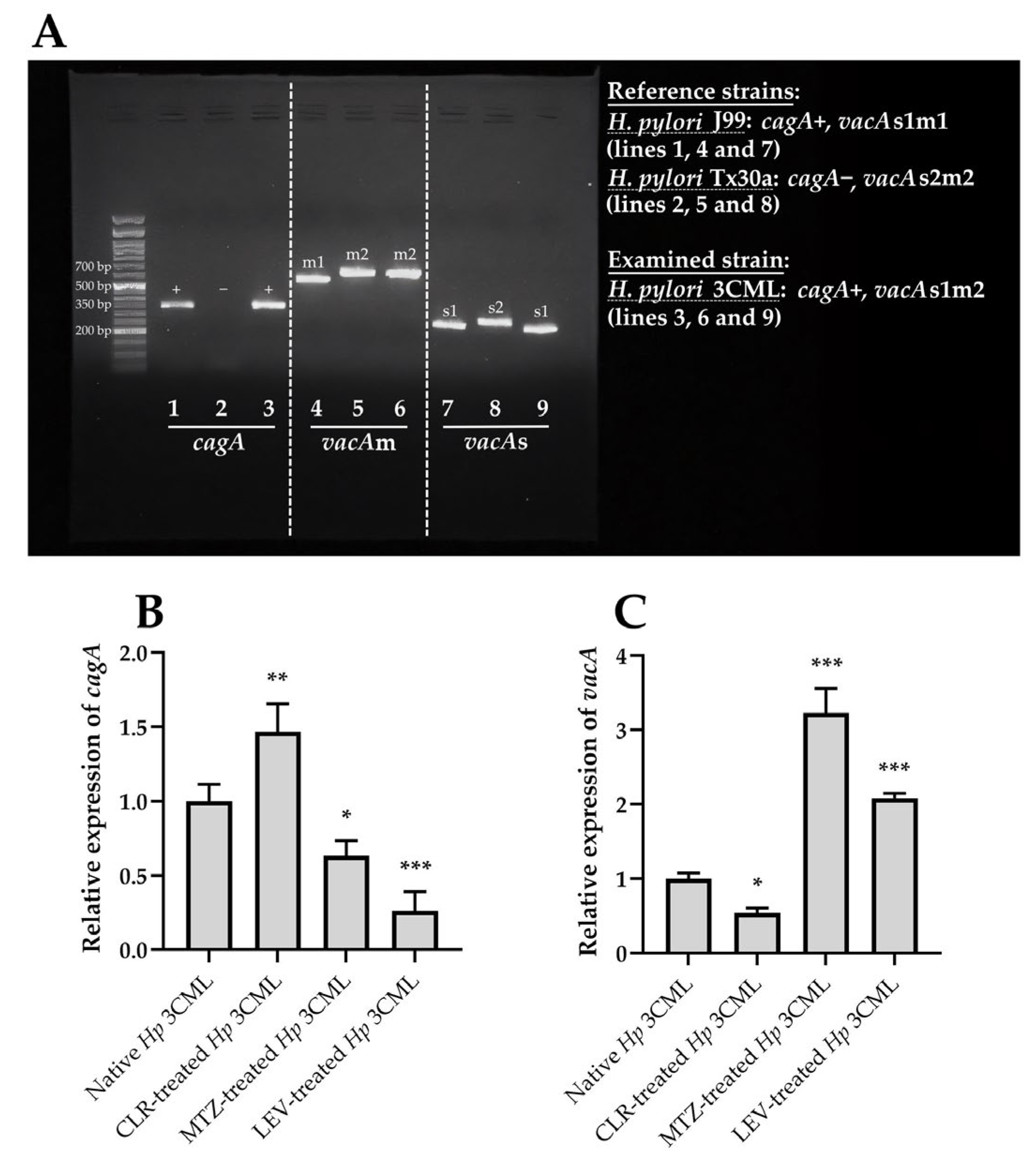
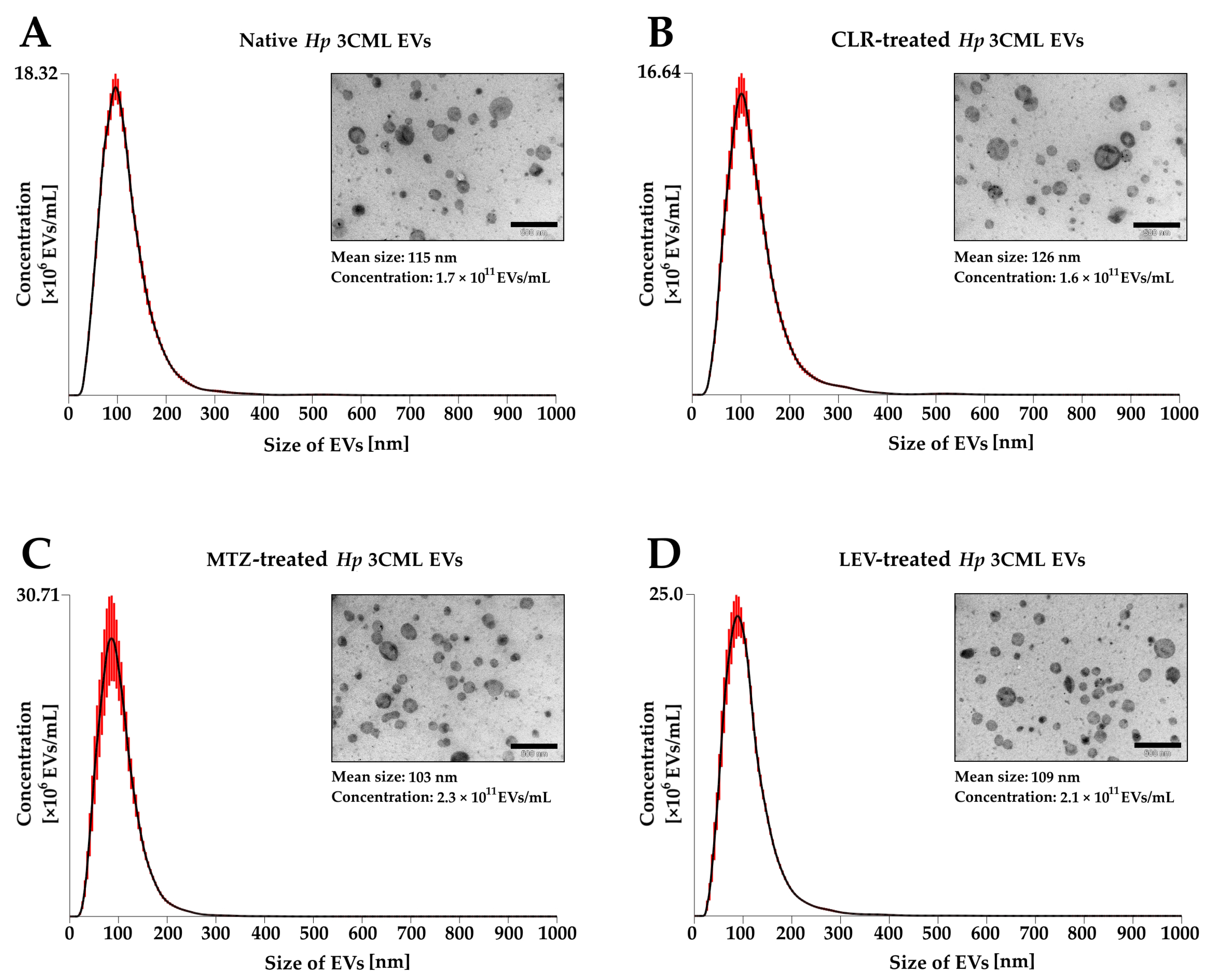
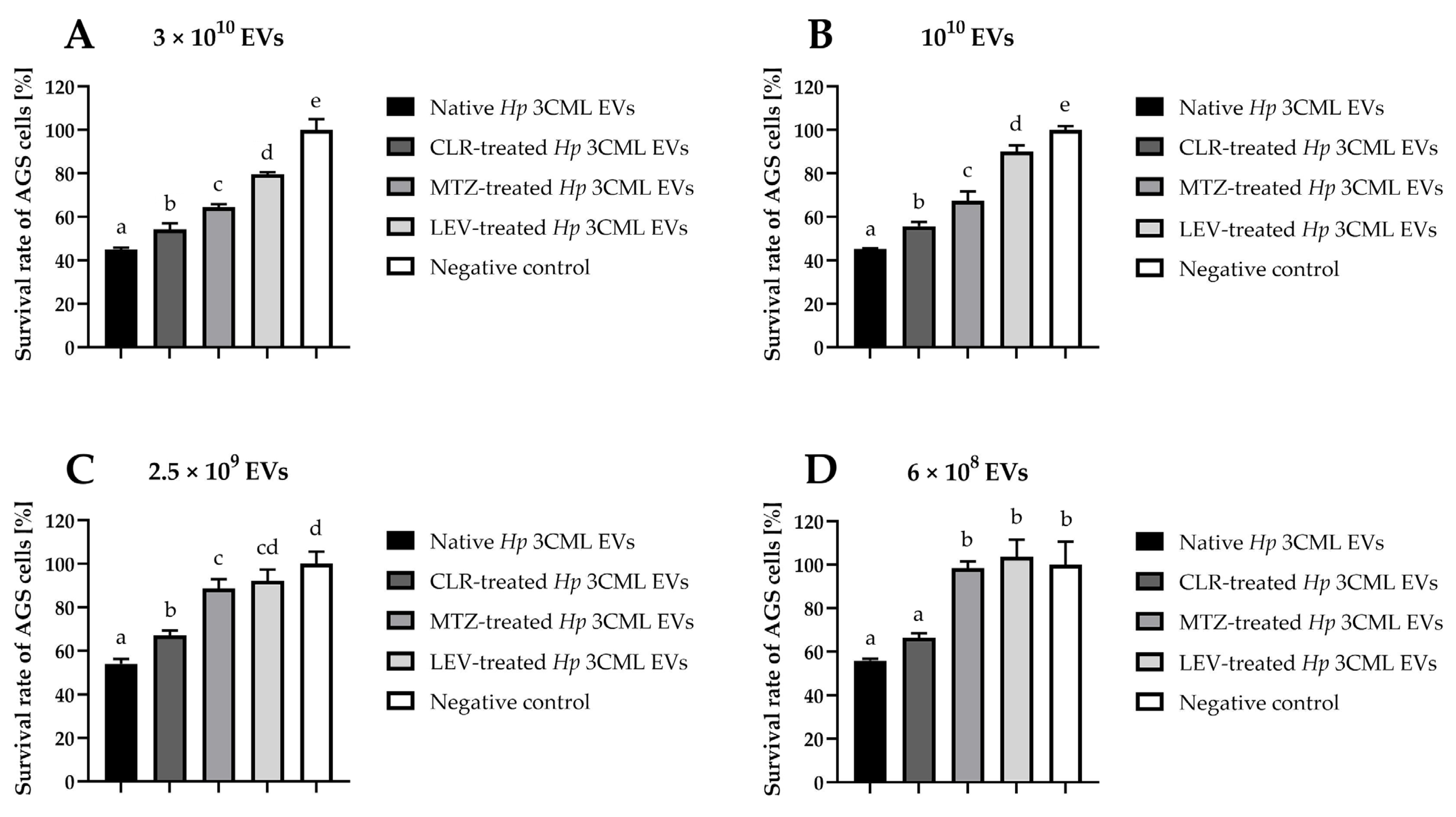
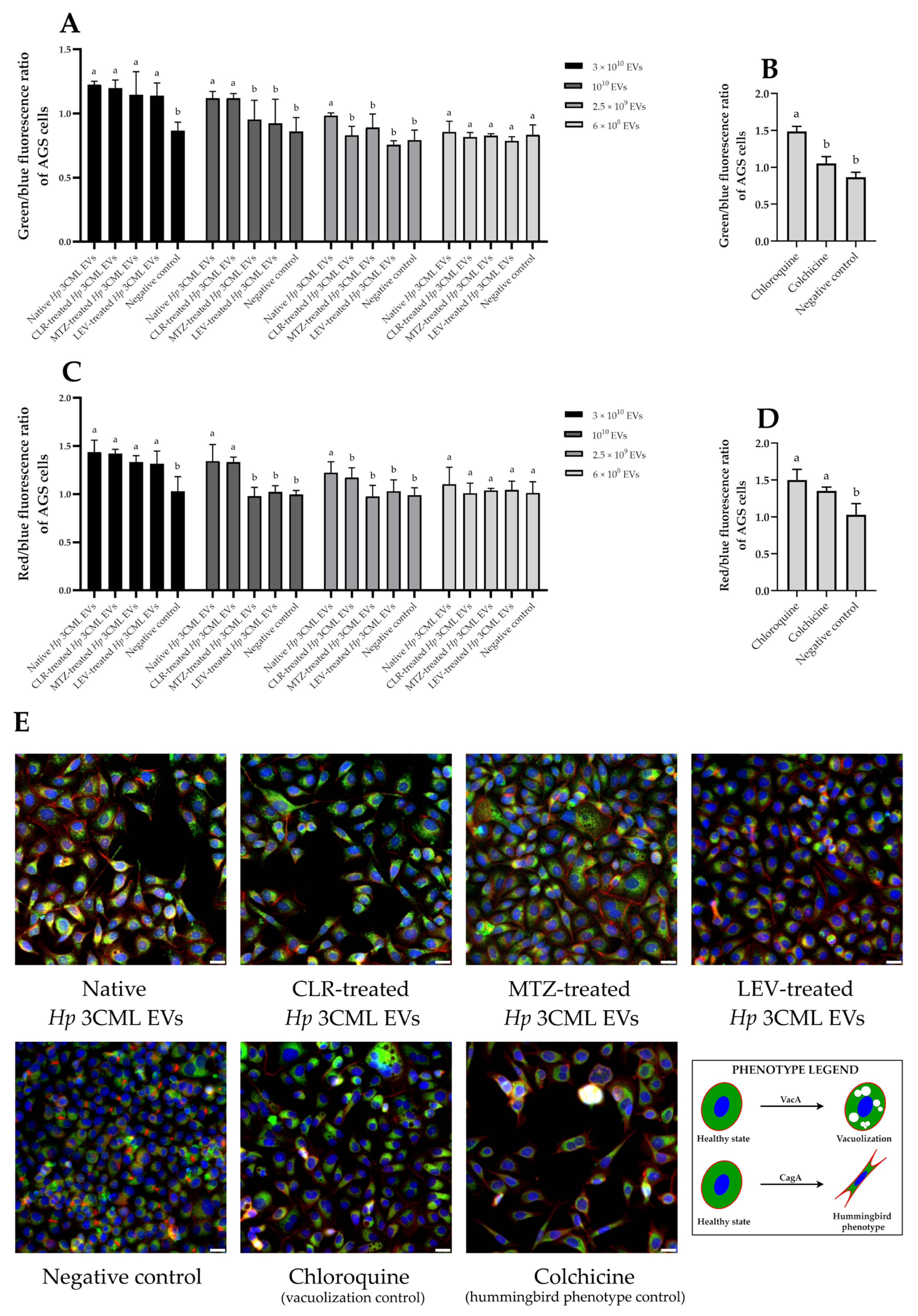
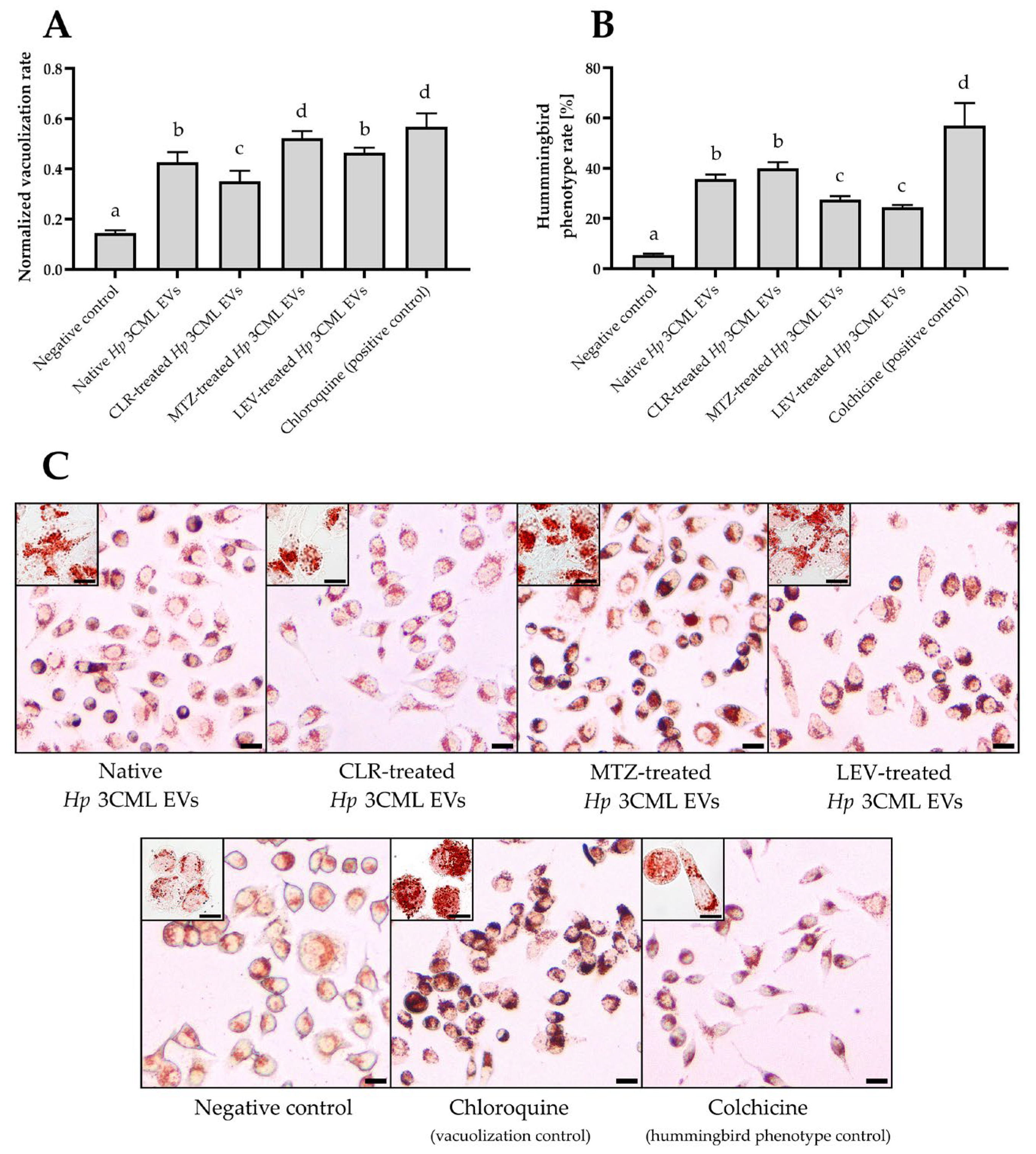
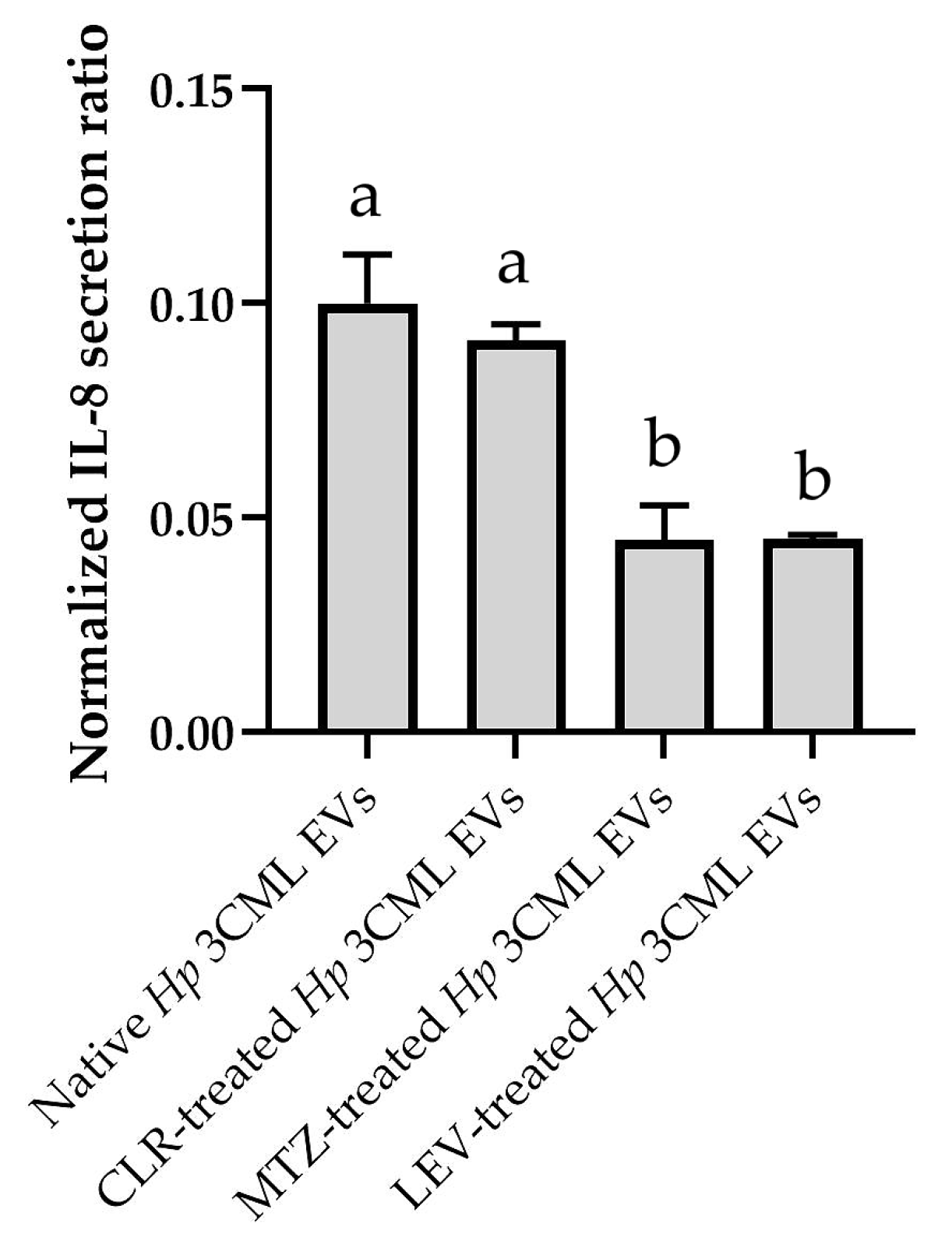
2. Results
3. Discussion
4. Materials and Methods
4.1. Microbiological Experiments
4.1.1. Storage and Revival of Bacteria
4.1.2. Genetic Analyses
4.1.3. Isolation of EVs and Their Characterization
4.2. Cell Line Experiments
4.2.1. Storage and Revival of the Cell Line
4.2.2. Cytotoxicity
4.2.3. Fluorescent Microscopy Observations
4.2.4. Light Microscopy Observations
4.2.5. Stimulation of Proinflammatory Cytokines
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori Infection: The Maastricht VI/Florence Consensus Report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.S.; Ottemann, K.M. Colonization, Localization, and Inflammation: The Roles of H. pylori Chemotaxis In Vivo. Curr. Opin. Microbiol. 2018, 41, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mebuge, D.L.; Noel, R.J.; Gold, B.D. Helicobacter pylori in Children: An Individualized Approach to a Worldwide Disease. Curr. Treat. Options Pediatr. 2025, 11, 13. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori Infection. Nat. Rev. Dis. Prim. 2023, 9, 19. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Aldubaib, M.; Abalkhail, A.; Anagreyyah, S.; Anajirih, N.; Almuzaini, A.M.; Rawway, M.; Alfadhel, A.; Draz, A.; et al. Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges. Antibiotics 2023, 12, 191. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Ouyang, Q.; Li, R.; Li, J.; Chen, W.; Hu, W.; He, L.; Bao, Q.; Li, P.; et al. Helicobacter pylori and Unignorable Extragastric Diseases: Mechanism and Implications. Front. Microbiol. 2022, 13, 972777. [Google Scholar] [CrossRef]
- Chauhan, N.; Tay, A.C.Y.; Marshall, B.J.; Jain, U. Helicobacter pylori VacA, a Distinct Toxin Exerts Diverse Functionalities in Numerous Cells: An Overview. Helicobacter 2019, 24, e12544. [Google Scholar] [CrossRef]
- Foegeding, N.J.; Caston, R.R.; McClain, M.S.; Ohi, M.D.; Cover, T.L. An Overview of Helicobacter pylori VacA Toxin Biology. Toxins 2016, 8, 173. [Google Scholar] [CrossRef]
- Jarzab, M.; Skorko-Glonek, J. There Are No Insurmountable Barriers: Passage of the Helicobacter pylori VacA Toxin from Bacterial Cytoplasm to Eukaryotic Cell Organelle. Membranes 2024, 14, 11. [Google Scholar] [CrossRef]
- Hatakeyama, M. Structure and Function of Helicobacter pylori CagA, the First-Identified Bacterial Protein Involved in Human Cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 196–219. [Google Scholar] [CrossRef]
- Takahashi-Kanemitsu, A.; Knight, C.T.; Hatakeyama, M. Molecular Anatomy and Pathogenic Actions of Helicobacter pylori CagA that Underpin Gastric Carcinogenesis. Cell. Mol. Immunol. 2020, 17, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Virulence Factor Cytotoxin-Associated Gene A (CagA)-Mediated Gastric Pathogenicity. Int. J. Mol. Sci. 2020, 21, 7430. [Google Scholar] [CrossRef] [PubMed]
- Green, E.R.; Mecsas, J. Bacterial Secretion Systems—An Overview. Microbiol. Spectr. 2016, 4, 10.1128/microbiolspec.VMBF-0012–2015. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, S.; Moleleki, L.N.; Motaung, T.E. Bacterial Secretion System Functions: Evidence of Interactions and Downstream Implications. Microbiology 2023, 169, 001326. [Google Scholar] [CrossRef]
- Guerrero-Mandujano, A.; Hernández-Cortez, C.; Ibarra, J.A.; Castro-Escarpulli, G. The Outer Membrane Vesicles: Secretion System Type Zero. Traffic 2017, 18, 425–432. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404, Correction in J. Extracell. Vesicles 2024, 13, e12451. [Google Scholar] [CrossRef]
- Macion, A.; Wyszyńska, A.; Godlewska, R. Delivery of Toxins and Effectors by Bacterial Membrane Vesicles. Toxins 2021, 13, 845. [Google Scholar] [CrossRef]
- Magaña, G.; Harvey, C.; Taggart, C.C.; Rodgers, A.M. Bacterial Outer Membrane Vesicles: Role in Pathogenesis and Host-Cell Interactions. Antibiotics 2024, 13, 32. [Google Scholar] [CrossRef]
- McMillan, H.M.; Kuehn, M.J. The Extracellular Vesicle Generation Paradox: A Bacterial Point of View. EMBO J. 2021, 40, e108174. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Shao, L.; Zhou, A.; Zhao, M.; Li, P.; Zhang, Z.; Wu, J. Both Extracellular Vesicles from Helicobacter pylori-Infected Cells and Helicobacter pylori Outer Membrane Vesicles Are Involved in Gastric/Extragastric Diseases. Eur. J. Med. Res. 2023, 28, 484. [Google Scholar] [CrossRef]
- Jarzab, M.; Posselt, G.; Meisner-Kober, N.; Wessler, S. Helicobacter pylori-Derived Outer Membrane Vesicles (OMVs): Role in Bacterial Pathogenesis? Microorganisms 2020, 8, 1328. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Luo, H.; Xie, Y.; Cao, H.; Mao, L.; Liu, T.; Yue, Y.; Qian, H. Extracellular Vesicles in Helicobacter pylori-Mediated Diseases: Mechanisms and Therapeutic Potential. Cell Commun. Signal. 2025, 23, 79. [Google Scholar] [CrossRef] [PubMed]
- González, M.F.; Díaz, P.; Sandoval-Bórquez, A.; Herrera, D.; Quest, A.F.G. Helicobacter pylori Outer Membrane Vesicles and Extracellular Vesicles from Helicobacter pylori-Infected Cells in Gastric Disease Development. Int. J. Mol. Sci. 2021, 22, 4823. [Google Scholar] [CrossRef] [PubMed]
- Bielaszewska, M.; Bauwens, A.; Kunsmann, L.; Karch, H.; Mellmann, A. Antibiotic-Mediated Modulations of Outer Membrane Vesicles in Enterohemorrhagic Escherichia coli O104:H4 and O157:H7. Antimicrob. Agents Chemother. 2017, 61, e00937-17. [Google Scholar]
- Hadadi-Fishani, M.; Najar-Peerayeh, S.; Siadat, S.D.; Sekhavati, M.; Mobarez, A.M. Isolation and Immunogenicity of Extracted Outer Membrane Vesicles from Pseudomonas aeruginosa under Antibiotics Treatment Conditions. Iran. J. Microbiol. 2021, 13, 824–831. [Google Scholar] [CrossRef]
- Dutta, S.; Iida, K.I.; Takade, A.; Meno, Y.; Nair, G.B.; Yoshida, S.I. Release of Shiga Toxin by Membrane Vesicles in Shigella dysenteriae Serotype 1 Strains and In Vitro Effects of Antimicrobials on Toxin Production and Release. Microbiol. Immunol. 2004, 48, 965–969. [Google Scholar] [CrossRef]
- Lucena, A.C.R.; Ferrarini, M.G.; de Oliveira, W.K.; Marcon, B.H.; Morello, L.G.; Alves, L.R.; Faoro, H. Modulation of Klebsiella pneumoniae Outer Membrane Vesicle Protein Cargo under Antibiotic Treatment. Biomedicines 2023, 11, 1515. [Google Scholar] [CrossRef]
- Torabian, P.; Singh, N.; Crawford, J.; Gonzalez, G.; Burgado, N.; Videva, M.; Miller, A.; Perdue, J.; Dinu, M.; Pietropaoli, A.; et al. Effect of Clinically Relevant Antibiotics on Bacterial Extracellular Vesicle Release from Escherichia coli. Int. J. Antimicrob. Agents 2025, 65, 107384. [Google Scholar] [CrossRef]
- Ran, Z.; Hao, M.; Guo, B.; Wang, J.; Romero-Saavedra, F.; Singh Gondil, V.; Maria Wagner, T. Sub-inhibitory Concentrations of Oxacillin Modulate Biogenesis and Function of Extracellular Vesicles Secreted by Oxacillin-Sensitive Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2025, 16, 1616536. [Google Scholar] [CrossRef]
- Murray, B.O.; Dawson, R.A.; Alsharaf, L.M.; Winter, J.A. Protective Effects of Helicobacter pylori Membrane Vesicles against Stress and Antimicrobial Agents. Microbiology 2020, 166, 751–758. [Google Scholar] [CrossRef]
- Kumar, S.; Schmitt, C.; Gorgette, O.; Marbouty, M.; Duchateau, M.; Gianetto, Q.G.; Matondo, M.; Guigner, J.M.; De Reuse, H. Bacterial Membrane Vesicles as a Novel Strategy for Extrusion of Antimicrobial Bismuth Drug in Helicobacter pylori. MBio 2022, 13, e0163322. [Google Scholar] [CrossRef]
- Krzyżek, P.; Opalińska, A.; Migdał, P.; Tusiewicz, K.; Szpot, P.; Zawadzki, M.; Krzyżanowska, B.; Kulus, M.J.; Podhorska-Okołów, M.; Sobieszczańska, B. Antibiotic Stress Affects the Secretion and Physicochemical Features of Extracellular Vesicles Produced by Helicobacter pylori. J. Antimicrob. Chemother. 2025, 80, 2032–2043. [Google Scholar] [CrossRef]
- Krzyżek, P.; Migdał, P.; Grande, R.; Gościniak, G. Biofilm Formation of Helicobacter pylori in Both Static and Microfluidic Conditions Is Associated With Resistance to Clarithromycin. Front. Cell. Infect. Microbiol. 2022, 12, 868905. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Migdał, P.; Tusiewicz, K.; Zawadzki, M.; Szpot, P. Subinhibitory Concentrations of Antibiotics Affect Development and Parameters of Helicobacter pylori Biofilm. Front. Pharmacol. 2024, 15, 1477317. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Kohli, L.; Klocke, B.J.; Roth, K.A. Chloroquine-Induced Autophagic Vacuole Accumulation and Cell Death in Glioma Cells is p53 Independent. Neuro-Oncology 2010, 12, 481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Tanaka, M.; Peixoto, H.S.; Wink, M. Cucurbitacins: Elucidation of Their Interactions with the Cytoskeleton. PeerJ 2017, 2017, e3357. [Google Scholar] [CrossRef]
- Wang, F.; Xia, P.; Wu, F.; Wang, D.; Wang, W.; Ward, T.; Liu, Y.; Aikhionbare, F.; Guo, Z.; Powell, M.; et al. Helicobacter pylori VacA Disrupts Apical Membrane-Cytoskeletal Interactions in Gastric Parietal Cells. J. Biol. Chem. 2008, 283, 26714–26725. [Google Scholar] [CrossRef]
- Fiorentino, M.; Ding, H.; Blanchard, T.G.; Czinn, S.J.; Sztein, M.B.; Fasanoa, A. Helicobacter pylori-Induced Disruption of Monolayer Permeability and Proinflammatory Cytokine Secretion in Polarized Human Gastric Epithelial Cells. Infect. Immun. 2013, 81, 883. [Google Scholar] [CrossRef]
- Ling, S.S.M.; Khoo, L.H.B.; Hwang, L.A.; Yeoh, K.G.; Ho, B. Instrumental Role of Helicobacter pylori γ-Glutamyl Transpeptidase in VacA-Dependent Vacuolation in Gastric Epithelial Cells. PLoS ONE 2015, 10, e0131460. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Naylor, N.R.; Hasso-Agopsowicz, M.; Kim, C.; Ma, Y.; Frost, I.; Abbas, K.; Aguilar, G.; Fuller, N.; Robotham, J.V.; Jit, M. The Global Economic Burden of Antibiotic-Resistant Infections and the Potential Impact of Bacterial Vaccines: A Modelling Study. BMJ Glob. Health 2025, 10, e016249. [Google Scholar] [CrossRef]
- Schulz, C.; Liou, J.M.; Alboraie, M.; Bornschein, J.; Campos Nunez, C.; Coelho, L.G.; Quach, D.T.; Fallone, C.A.; Chen, Y.C.; Gerhard, M.; et al. Helicobacter pylori Antibiotic Resistance: A Global Challenge in Search of Solutions. Gut 2025, 74, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Muttiah, B.; Wahid, W.; Sukri, A.; Hanafiah, A. Towards Effective Helicobacter pylori Eradication: Emerging Therapies in the Wake of Antibiotic Resistance. Int. J. Mol. Sci. 2025, 26, 6064. [Google Scholar] [CrossRef] [PubMed]
- Megraud, F.; Bruyndonckx, R.; Coenen, S.; Wittkop, L.; Huang, T.D.; Hoebeke, M.; Bénéjat, L.; Lehours, P.; Goossens, H.; Glupczynski, Y. Helicobacter pylori Resistance to Antibiotics in Europe in 2018 and Its Relationship to Antibiotic Consumption in the Community. Gut 2021, 70, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.; Brooks, B.D.; Brooks, A.E. The Complex Relationship between Virulence and Antibiotic Resistance. Genes 2017, 8, 39. [Google Scholar] [CrossRef]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial Resistance and Virulence: A Successful or Deleterious Association in the Bacterial World? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef]
- Davies, J.; Spiegelman, G.B.; Yim, G. The World of Subinhibitory Antibiotic Concentrations. Curr. Opin. Microbiol. 2006, 9, 445–453. [Google Scholar] [CrossRef]
- Bitto, N.J.; Zavan, L.; Johnston, E.L.; Stinear, T.P.; Hill, A.F.; Kaparakis-Liaskos, M. Considerations for the Analysis of Bacterial Membrane Vesicles: Methods of Vesicle Production and Quantification Can Influence Biological and Experimental Outcomes. Microbiol. Spectr. 2021, 9, e01273-21. [Google Scholar] [CrossRef]
- Zavan, L.; Bitto, N.J.; Johnston, E.L.; Greening, D.W.; Kaparakis-Liaskos, M. Helicobacter pylori Growth Stage Determines the Size, Protein Composition, and Preferential Cargo Packaging of Outer Membrane Vesicles. Proteomics 2019, 19, e1800209. [Google Scholar] [CrossRef]
- Ismail, S.; Hampton, M.B.; Keenan, J.I. Helicobacter pylori Outer Membrane Vesicles Modulate Proliferation and Interleukin-8 Production by Gastric Epithelial Cells. Infect. Immun. 2003, 71, 5670–5675. [Google Scholar] [CrossRef]
- Chew, Y.; Chung, H.Y.; Lin, P.Y.; Wu, D.C.; Huang, S.K.; Kao, M.C. Outer Membrane Vesicle Production by Helicobacter pylori Represents an Approach for the Delivery of Virulence Factors CagA, VacA and UreA into Human Gastric Adenocarcinoma (AGS) Cells. Int. J. Mol. Sci. 2021, 22, 3942. [Google Scholar] [CrossRef]
- Federica, D.; Cosimato, I.; Salzano, F.; Mensitieri, F.; Andretta, V.; Santoro, E.; Boccia, G.; Folliero, V.; Franci, G. Adaptations of Bacterial Extracellular Vesicles in Response to Antibiotic Pressure. Int. J. Mol. Sci. 2025, 26, 5025. [Google Scholar] [CrossRef]
- Ribeiro de Freitas, M.C.; Elaine de Almeida, P.; Vieira, W.V.; Ferreira-Machado, A.B.; Resende, J.A.; Lúcia da Silva, V.; Diniz, C.G. Inflammatory Modulation and Outer Membrane Vesicles (OMV) Production Associated to Bacteroides fragilis Response to Subinhibitory Concentrations of Metronidazole during Experimental Infection. Anaerobe 2022, 73, 102504. [Google Scholar] [CrossRef]
- Devos, S.; Van Putte, W.; Vitse, J.; Van Driessche, G.; Stremersch, S.; Van Den Broek, W.; Raemdonck, K.; Braeckmans, K.; Stahlberg, H.; Kudryashev, M.; et al. Membrane Vesicle Secretion and Prophage Induction in Multidrug-Resistant Stenotrophomonas maltophilia in Response to Ciprofloxacin Stress. Environ. Microbiol. 2017, 19, 3930–3937. [Google Scholar] [CrossRef]
- Subsomwong, P.; Asano, K.; Akada, J.; Matsumoto, T.; Nakane, A.; Yamaoka, Y. Proteomic Profiling of Extracellular Vesicles Reveals Potential Biomarkers for Helicobacter pylori Infection and Gastric Cancer. Helicobacter 2025, 30, e70022. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Li, X.; Wang, J.; Wang, Y.; Zhang, C.; Dai, S.; Wang, X.; Deng, X.; Zhao, L.; Shan, B. Outer Membrane Vesicles Secreted by Helicobacter pylori Transmitting Gastric Pathogenic Virulence Factors. ACS Omega 2022, 7, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Erzin, Y.; Koksal, V.; Altun, S.; Dobrucali, A.; Aslan, M.; Erdamar, S.; Dirican, A.; Kocazeybek, B. Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA, babA2 Genotypes and Correlation with Clinical Outcome in Turkish Patients with Dyspepsia. Helicobacter 2006, 11, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Jaber, A.S.; Abbas, F.N. Molecular Detection of the Virulence Gene’s vacA and cagA of Helicobacter pylori by PCR. Iran. J. Ichthyol. 2021, 8, 341–347. [Google Scholar]
- Ramírez-Lázaro, M.J.; Lario, S.; Casalots, A.; Sanfeliu, E.; Boix, L.; García-Iglesias, P.; Sánchez-Delgado, J.; Montserrat, A.; Bella-Cueto, M.R.; Gallach, M.; et al. Real-Time PCR Improves Helicobacter pylori Detection in Patients with Peptic Ulcer Bleeding. PLoS ONE 2011, 6, e20009. [Google Scholar] [CrossRef]
- Krzyżek, P.; Migdał, P.; Krzyżanowska, B.; Duda-Madej, A. Optimization of Helicobacter pylori Biofilm Formation in In Vitro Conditions Mimicking Stomach. Int. J. Mol. Sci. 2024, 25, 9839. [Google Scholar] [CrossRef]
- Kilgore, J.A.; Dolman, N.J. A Review of Reagents for Fluorescence Microscopy of Cellular Compartments and Structures, Part II: Reagents for Non-Vesicular Organelles. Curr. Protoc. 2023, 3, e752. [Google Scholar] [CrossRef]
- Golub, O.; Kilgore, J.A.; Dolman, N.J. A Review of Reagents for Fluorescence Microscopy of Cellular Compartments and Structures, Part III: Reagents for Actin, Tubulin, Cellular Membranes, and Whole Cell and Cytoplasm. Curr. Protoc. 2023, 3, e754. [Google Scholar] [CrossRef]
- Gauthier, N.C.; Ricci, V.; Gounon, P.; Doye, A.; Tauc, M.; Poujeol, P.; Boquet, P. Glycosylphosphatidylinositol-Anchored Proteins and Actin Cytoskeleton Modulate Chloride Transport by Channels Formed by the Helicobacter pylori Vacuolating Cytotoxin VacA in HeLa Cells. J. Biol. Chem. 2004, 279, 9481–9489. [Google Scholar] [CrossRef]
- Chazotte, B. Labeling Nuclear DNA Using DAPI. Cold Spring Harb. Protoc. 2011, 2011, pdb.prot5556. [Google Scholar] [CrossRef] [PubMed]
- Amaral, E.; Guatimosim, S.; Guatimosim, C. Using the Fluorescent Styryl Dye FM1-43 to Visualize Synaptic Vesicles Exocytosis and Endocytosis in Motor Nerve Terminals. Methods Mol. Biol. 2011, 689, 137–148. [Google Scholar]
- Fomina, A.F.; Deerinck, T.J.; Ellisman, M.H.; Cahalan, M.D. Regulation of Membrane Trafficking and Subcellular Organization of Endocytic Compartments Revealed with FM1-43 in Resting and Activated Human T Cells. Exp. Cell Res. 2003, 291, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Emans, N.; Zimmermann, S.; Fischer, R. Uptake of a Fluorescent Marker in Plant Cells is Sensitive to Brefeldin A and Wortmannin. Plant Cell 2002, 14, 71–86. [Google Scholar] [CrossRef]
- Mayr, S.; Hauser, F.; Peterbauer, A.; Tauscher, A.; Naderer, C.; Axmann, M.; Plochberger, B.; Jacak, J. Localization Microscopy of Actin Cytoskeleton in Human Platelets. Int. J. Mol. Sci. 2018, 19, 1150. [Google Scholar] [CrossRef] [PubMed]
- Tohidpour, A.; Gorrell, R.J.; Roujeinikova, A.; Kwok, T.; Editors, A.; Crabtree, J.E.; Wessler, S. The Middle Fragment of Helicobacter pylori CagA Induces Actin Rearrangement and Triggers Its Own Uptake into Gastric Epithelial Cells. Toxins 2017, 9, 237. [Google Scholar] [CrossRef]
- Islam, R.; Corraya, R.M.; Pasovic, L.; Khan, A.Z.; Aass, H.C.D.; Eidet, J.R.; Utheim, T.P. The Effects of Prolonged Storage on ARPE-19 Cells Stored at Three Different Storage Temperatures. Molecules 2020, 25, 5809. [Google Scholar] [CrossRef]
- Parker, H.; Chitcholtan, K.; Hampton, M.B.; Keenan, J.I. Uptake of Helicobacter pylori Outer Membrane Vesicles by Gastric Epithelial Cells. Infect. Immun. 2010, 78, 5054–5061. [Google Scholar] [CrossRef]
- Shimoda, A.; Ueda, K.; Nishiumi, S.; Murata-Kamiya, N.; Mukai, S.A.; Sawada, S.I.; Azuma, T.; Hatakeyama, M.; Akiyoshi, K. Exosomes as Nanocarriers for Systemic Delivery of the Helicobacter pylori Virulence Factor CagA. Sci. Rep. 2016, 6, 18346. [Google Scholar] [CrossRef]
- Choi, M.S.; Ze, E.Y.; Park, J.Y.; Shin, T.S.; Kim, J.G. Helicobacter pylori-Derived Outer Membrane Vesicles Stimulate Interleukin 8 Secretion through Nuclear Factor Kappa B Activation. Korean J. Intern. Med. 2020, 36, 867. [Google Scholar] [CrossRef]
- Choi, H.-I.; Choi, J.-P.; Seo, J.; Kim, B.J.; Rho, M.; Han, J.K.; Kim, J.G. Helicobacter pylori-Derived Extracellular Vesicles Increased in the Gastric Juices of Gastric Adenocarcinoma Patients and Induced Inflammation Mainly via Specific Targeting of Gastric Epithelial Cells. Exp. Mol. Med. 2017, 49, e330. [Google Scholar] [CrossRef]

| Target Gene | Amplicon Size (bp) | Sequences | Annealing Temperature | Reference |
|---|---|---|---|---|
| cagA | 349 | F: 5′ GATAACAGGCAAGCTTTTGAGG 3′ R: 5′ CTGCAAAAGATTGTTTGGCAGA 3′ | 59 °C | [57] |
| vacAs | 256 (s1)/286 (s2) | F: 5′ ATGGAAATACAACAAACACAC 3′ R: 5′ CTGCTTGAATGCGCCAAAC 3′ | 58 °C | [57] |
| vacAm | 570 (m1)/645 (m2) | F: 5′ CAATCTGTCCAATCAAGCGAG 3′ R: 5′ GCGTCTAAATAATTCCAAGG 3′ | 56 °C | [57] |
| Target Gene | Sequences | References |
|---|---|---|
| cagA | F: 5′ TTGACCAACAACCACAAACCGAAG 3′ R: 5′ CTTCCCTTAATTGCGAGATTCC 3′ | [58] |
| vacA | F: 5′ GGTCAAAATGCGGTCATGG 3′ R: 5′ CCATTGGTACCTGTAGAAAC 3′ | [58] |
| 16S rRNA | F: 5′ CTCATTGCGAAGGCGACCT 3′ R: 5′ TCTAATCCTGTTTGCTCCCCA 3′ | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyżek, P.; Chmielarz, M.; Bożemska, E.; Opalińska, A.; Olbromski, M.; Małaszczuk, M.; Krzyżanowska, B.; Haczkiewicz-Leśniak, K.; Podhorska-Okołów, M.; Dzięgiel, P.; et al. Antibiotics Impact the Cytotoxicity and Cytopathic Effect of Helicobacter pylori Extracellular Vesicles Against Gastric Cells. Int. J. Mol. Sci. 2025, 26, 10399. https://doi.org/10.3390/ijms262110399
Krzyżek P, Chmielarz M, Bożemska E, Opalińska A, Olbromski M, Małaszczuk M, Krzyżanowska B, Haczkiewicz-Leśniak K, Podhorska-Okołów M, Dzięgiel P, et al. Antibiotics Impact the Cytotoxicity and Cytopathic Effect of Helicobacter pylori Extracellular Vesicles Against Gastric Cells. International Journal of Molecular Sciences. 2025; 26(21):10399. https://doi.org/10.3390/ijms262110399
Chicago/Turabian StyleKrzyżek, Paweł, Mateusz Chmielarz, Edyta Bożemska, Agnieszka Opalińska, Mateusz Olbromski, Michał Małaszczuk, Barbara Krzyżanowska, Katarzyna Haczkiewicz-Leśniak, Marzenna Podhorska-Okołów, Piotr Dzięgiel, and et al. 2025. "Antibiotics Impact the Cytotoxicity and Cytopathic Effect of Helicobacter pylori Extracellular Vesicles Against Gastric Cells" International Journal of Molecular Sciences 26, no. 21: 10399. https://doi.org/10.3390/ijms262110399
APA StyleKrzyżek, P., Chmielarz, M., Bożemska, E., Opalińska, A., Olbromski, M., Małaszczuk, M., Krzyżanowska, B., Haczkiewicz-Leśniak, K., Podhorska-Okołów, M., Dzięgiel, P., & Sobieszczańska, B. (2025). Antibiotics Impact the Cytotoxicity and Cytopathic Effect of Helicobacter pylori Extracellular Vesicles Against Gastric Cells. International Journal of Molecular Sciences, 26(21), 10399. https://doi.org/10.3390/ijms262110399







