Sumac Polyphenols as Pan-Herpesvirus Inhibitors
Abstract
1. Introduction
2. Results
2.1. Selection of Polyphenols
2.2. Preparation of Polyphenols
2.2.1. Rutan and Its Components
2.2.2. Geraniin
2.3. Antiviral Activity of Polyphenols Against Respiratory Viruses and HIV-1
2.4. Activity of Polyphenols Against Viruses of the Family Orthoherpesviridae
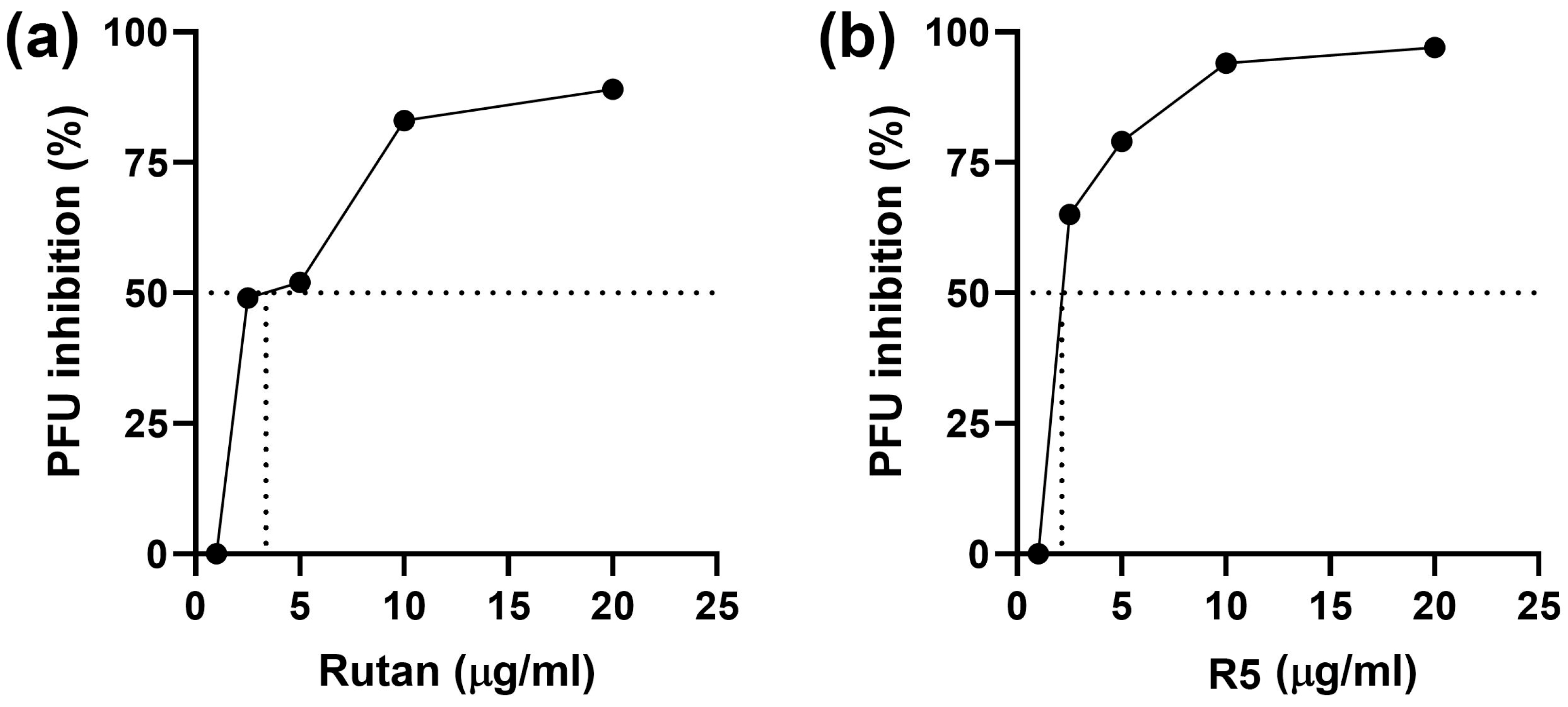
2.4.1. Herpes Simplex Virus
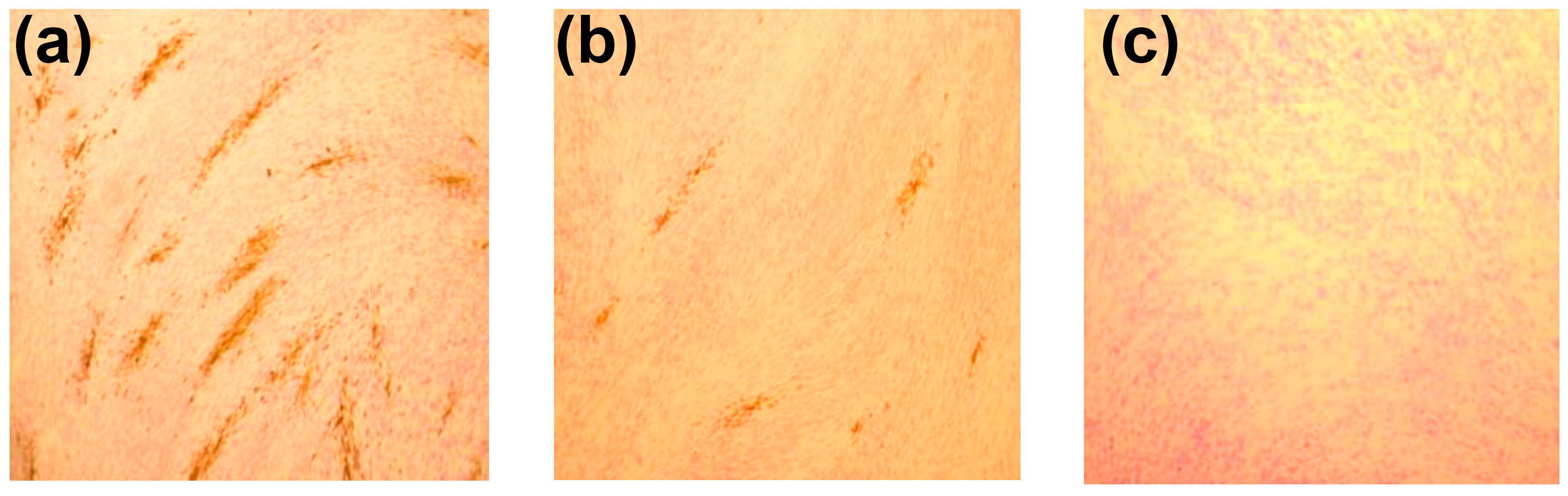
2.4.2. CMV
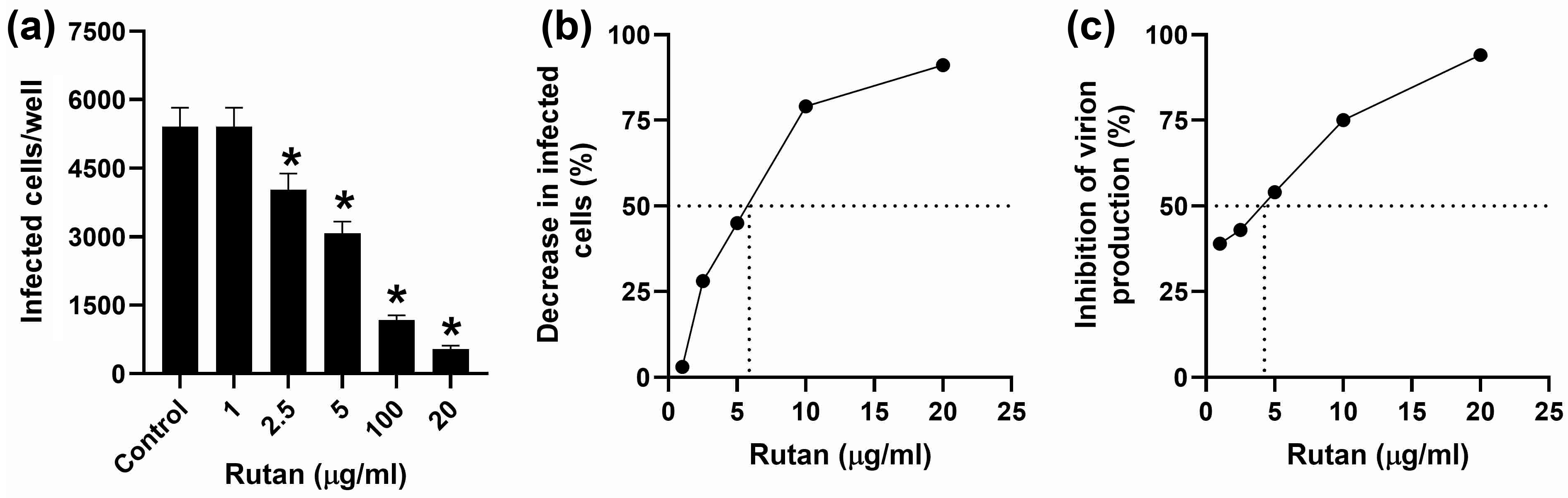
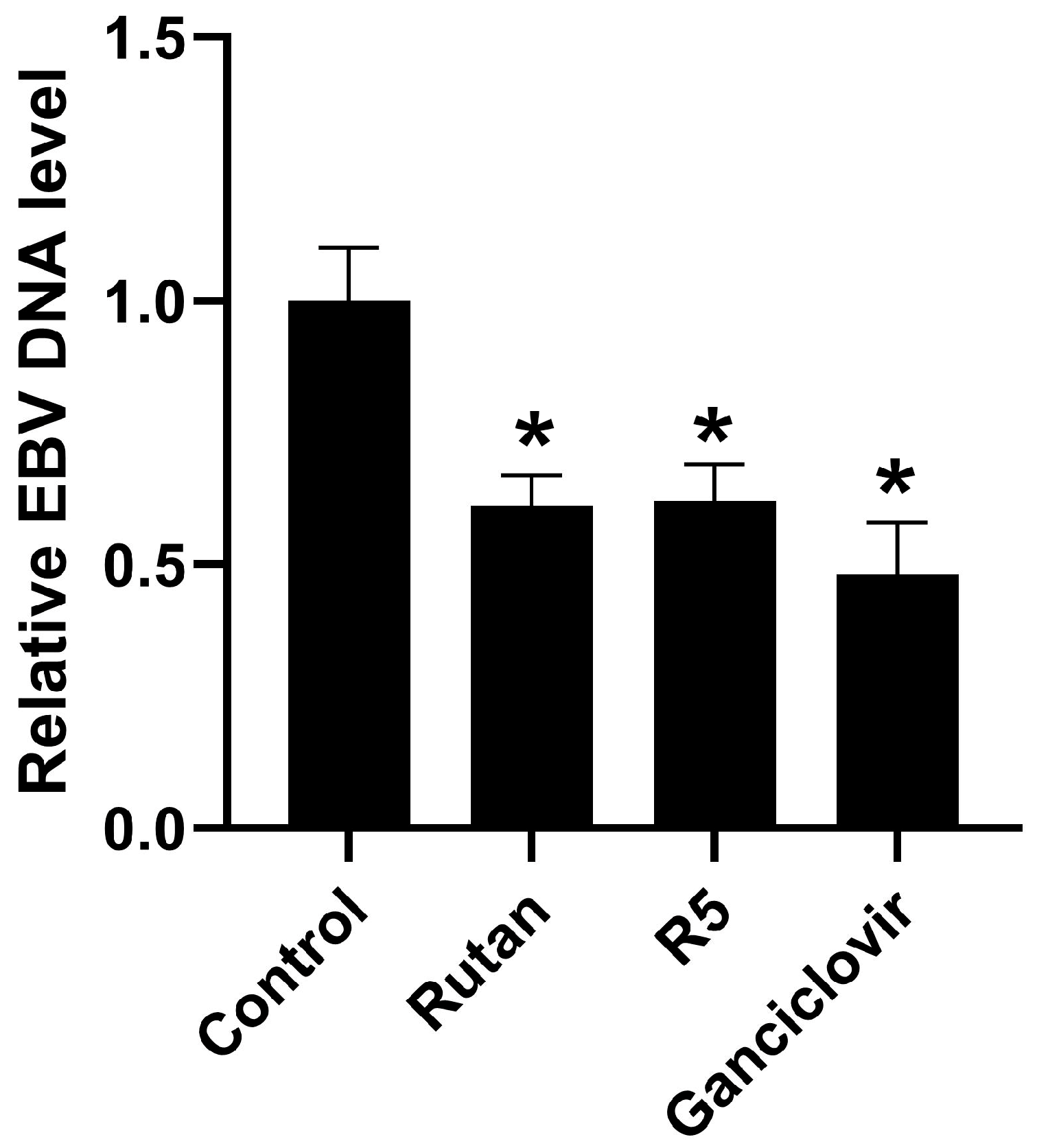
2.4.3. EBV
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Isolation, Purification, and Identification of Polyphenols
4.2.1. Rutan (Sum of Water-Soluble Polyphenols of Sumac Leaves, Rhus coriaria L.)
4.2.2. Geraniin (Ellagitannin of the Aerial Parts of Geranium sanguineum L.)
4.3. Evaluation of In Vitro Antiviral Activity in Permissive Cell Cultures
4.3.1. General Principles
4.3.2. IAV and SARS-CoV-2
4.3.3. HIV-1
4.3.4. HSV-1 and HSV-2
4.3.5. CMV
Cells
Virus
Cytotoxicity Determination
Immunocytochemical Detection of Infected Cells
4.3.6. EBV
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaye, A.D.; Okeagu, C.N.; Pham, A.D.; Silva, R.A.; Hurley, J.J.; Arron, B.L.; Sarfraz, N.; Lee, H.N.; Ghali, G.E.; Gamble, J.W.; et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: International perspectives. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 293–306. [Google Scholar] [CrossRef]
- Robinson, P.C.; Liew, D.F.L.; Tanner, H.L.; Grainger, J.R.; Dwek, R.A.; Reisler, R.B.; Steinman, L.; Feldmann, M.; Ho, L.P.; Hussell, T.; et al. COVID-19 therapeutics: Challenges and directions for the future. Proc. Natl. Acad. Sci. USA 2022, 119, e2119893119. [Google Scholar] [CrossRef] [PubMed]
- Mollentze, N.; Streicker, D.G. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proc. Natl. Acad. Sci. USA 2020, 117, 9423–9430. [Google Scholar] [CrossRef]
- Karim, M.; Lo, C.W.; Einav, S. Preparing for the next viral threat with broad-spectrum antivirals. J. Clin. Investig. 2023, 133, e170236. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.; Daszak, P.; Wolfe, N.D.; Gao, G.F.; Morel, C.M.; Morzaria, S.; Pablos-Mendez, A.; Tomori, O.; Mazet, J.A.K. The Global Virome Project. Science 2018, 359, 872–874. [Google Scholar] [CrossRef]
- Vigant, F.; Santos, N.C.; Lee, B. Broad-spectrum antivirals against viral fusion. Nat. Rev. Microbiol. 2015, 13, 426–437. [Google Scholar] [CrossRef]
- Wang, C.; Xia, S.; Wang, X.; Li, Y.; Wang, H.; Xiang, R.; Jiang, Q.; Lan, Q.; Liang, R.; Li, Q.; et al. Supercoiling Structure-Based Design of a Trimeric Coiled-Coil Peptide with High Potency against HIV-1 and Human beta-Coronavirus Infection. J. Med. Chem. 2022, 65, 2809–2819. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C. Peptide-Based Dual HIV and Coronavirus Entry Inhibitors. Adv. Exp. Med. Biol. 2022, 1366, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhu, Y.; Yan, H.; Wu, T.; Chong, H.; He, Y. Pan-coronavirus fusion inhibitors possess potent inhibitory activity against HIV-1, HIV-2, and simian immunodeficiency virus. Emerg. Microbes Infect. 2021, 10, 810–821. [Google Scholar] [CrossRef]
- Zhao, H.; Meng, X.; Peng, Z.; Lam, H.; Zhang, C.; Zhou, X.; Chan, J.F.; Kao, R.Y.T.; To, K.K.; Yuen, K.Y. Fusion-inhibition peptide broadly inhibits influenza virus and SARS-CoV-2, including Delta and Omicron variants. Emerg. Microbes Infect. 2022, 11, 926–937. [Google Scholar] [CrossRef]
- Borenstein, R.; Hanson, B.A.; Markosyan, R.M.; Gallo, E.S.; Narasipura, S.D.; Bhutta, M.; Shechter, O.; Lurain, N.S.; Cohen, F.S.; Al-Harthi, L.; et al. Ginkgolic acid inhibits fusion of enveloped viruses. Sci. Rep. 2020, 10, 4746. [Google Scholar] [CrossRef]
- Cooper, L.; Schafer, A.; Li, Y.; Cheng, H.; Medegan Fagla, B.; Shen, Z.; Nowar, R.; Dye, K.; Anantpadma, M.; Davey, R.A.; et al. Screening and Reverse-Engineering of Estrogen Receptor Ligands as Potent Pan-Filovirus Inhibitors. J. Med. Chem. 2020, 63, 11085–11099. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Pan, X.; Chen, D.; Xie, X.; Wu, Y.; Shang, W.; Jiang, X.; Sun, Y.; Fan, S.; He, J. Broad-spectrum antivirals of protoporphyrins inhibit the entry of highly pathogenic emerging viruses. Bioorg. Chem. 2021, 107, 104619. [Google Scholar] [CrossRef] [PubMed]
- Shtro, A.A.; Garshinina, A.V.; Alferova, V.A.; Kamzeeva, P.N.; Volok, V.P.; Kolpakova, E.S.; Nikitin, T.D.; Chistov, A.A.; Belyaev, E.S.; Korshun, V.A.; et al. Cationic Perylene Antivirals with Aqueous Solubility for Studies In Vivo. Pharmaceuticals 2022, 15, 1178. [Google Scholar] [CrossRef] [PubMed]
- Stefanik, M.; Bhosale, D.S.; Haviernik, J.; Strakova, P.; Fojtikova, M.; Dufkova, L.; Huvarova, I.; Salat, J.; Bartacek, J.; Svoboda, J.; et al. Diphyllin Shows a Broad-Spectrum Antiviral Activity against Multiple Medically Important Enveloped RNA and DNA Viruses. Viruses 2022, 14, 354. [Google Scholar] [CrossRef]
- Montz, W.E., Jr.; Puyear, R.L.; Brammer, J.D. Identification and quantification of water-soluble hydrocarbons generated by two-cycle outboard motors. Arch. Environ. Contam. Toxicol. 1982, 11, 561–565. [Google Scholar] [CrossRef]
- Low, Z.X.; Kanauchi, O.; AbuBakar, S.; Tiong, V.; Hassandarvish, P. Protocol for Screening Host-Targeting Antivirals (HTAs) Using Human PBMCs and pDCs. Bio-Protocol 2025, 15, e5230. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Panagiotopoulos, A.P.; Siafakas, N.; Tsakris, A. The potential of RNA-binding proteins as host-targeting antivirals against RNA viruses. Int. J. Antimicrob. Agents 2025, 66, 107522. [Google Scholar] [CrossRef]
- Kukhanova, M.K.; Karpenko, I.L.; Ivanov, A.V. DEAD-box RNA Helicase DDX3: Functional Properties and Development of DDX3 Inhibitors as Antiviral and Anticancer Drugs. Molecules 2020, 25, 1015. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, Y.; Deng, J.; Wu, X.; Li, J.; Guo, D.; Xu, K.; Qin, Y.; Chen, M. Peptides targeting RAB11A-FIP2 complex inhibit HPIV3, RSV, and IAV replication as broad-spectrum antivirals. Cell Biosci. 2025, 15, 50. [Google Scholar] [CrossRef]
- Betz, U.A.K.; Garces, R.; Beier, N.; Lindemann, S.; Wolff, K.C.; Riva, L.; Kirkpatrick, M.G.; Gebara-Lamb, A.; McNamara, C.W.; Damoiseaux, R.; et al. Open Source Repurposing Reveals Broad-Spectrum Antiviral Activity of Diphenylureas. Viruses 2025, 17, 385. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Jirillo, E.; Santacroce, L. Current View on Major Natural Compounds Endowed with Antibacterial and Antiviral Effects. Antibiotics 2024, 13, 603. [Google Scholar] [CrossRef]
- Abarova, S.; Alexova, R.; Dragomanova, S.; Solak, A.; Fagone, P.; Mangano, K.; Petralia, M.C.; Nicoletti, F.; Kalfin, R.; Tancheva, L. Emerging Therapeutic Potential of Polyphenols from Geranium sanguineum L. in Viral Infections, Including SARS-CoV-2. Biomolecules 2024, 14, 130. [Google Scholar] [CrossRef]
- Barreca, M.M.; Alessandro, R.; Corrado, C. Effects of Flavonoids on Cancer, Cardiovascular and Neurodegenerative Diseases: Role of NF-kappaB Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 9236. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Sahoo, C.R.; Paidesetty, S.K.; Padhy, R.N. Role of phytocompounds as the potential anti-viral agent: An overview. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2311–2329. [Google Scholar] [CrossRef]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The green tea molecule EGCG inhibits Zika virus entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef]
- Henss, L.; Auste, A.; Schurmann, C.; Schmidt, C.; von Rhein, C.; Muhlebach, M.D.; Schnierle, B.S. The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection. J. Gen. Virol. 2021, 102, 001574. [Google Scholar] [CrossRef]
- Behrendt, P.; Perin, P.; Menzel, N.; Banda, D.; Pfaender, S.; Alves, M.P.; Thiel, V.; Meuleman, P.; Colpitts, C.C.; Schang, L.M.; et al. Pentagalloylglucose, a highly bioavailable polyphenolic compound present in Cortex moutan, efficiently blocks hepatitis C virus entry. Antiviral. Res. 2017, 147, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Haid, S.; Novodomska, A.; Gentzsch, J.; Grethe, C.; Geuenich, S.; Bankwitz, D.; Chhatwal, P.; Jannack, B.; Hennebelle, T.; Bailleul, F.; et al. A plant-derived flavonoid inhibits entry of all HCV genotypes into human hepatocytes. Gastroenterology 2012, 143, 213–222.e215. [Google Scholar] [CrossRef] [PubMed]
- Polyak, S.J.; Ferenci, P.; Pawlotsky, J.M. Hepatoprotective and antiviral functions of silymarin components in hepatitis C virus infection. Hepatology 2013, 57, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Elhassan Taha, M.M.; Makeen, H.A.; Alhazmi, H.A.; Al Bratty, M.; Sultana, S.; Ahsan, W.; Najmi, A.; Khalid, A. Bioactive Natural Antivirals: An Updated Review of the Available Plants and Isolated Molecules. Molecules 2020, 25, 4878. [Google Scholar] [CrossRef] [PubMed]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Mandalari, G.; Sciortino, M.T. Antiviral Activity Exerted by Natural Products against Human Viruses. Viruses 2021, 13, 828. [Google Scholar] [CrossRef]
- Karadeniz, F.; Kang, K.H.; Park, J.W.; Park, S.J.; Kim, S.K. Anti-HIV-1 activity of phlorotannin derivative 8,4’’’-dieckol from Korean brown alga Ecklonia cava. Biosci. Biotechnol. Biochem. 2014, 78, 1151–1158. [Google Scholar] [CrossRef]
- Ahn, M.J.; Yoon, K.D.; Min, S.Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.G.; Huh, H.; Kim, J. Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biol. Pharm. Bull. 2004, 27, 544–547. [Google Scholar] [CrossRef]
- Rani, A.; Saini, V.; Patra, P.; Prashar, T.; Pandey, R.K.; Mishra, A.; Jha, H.C. Epigallocatechin Gallate: A Multifaceted Molecule for Neurological Disorders and Neurotropic Viral Infections. ACS Chem. Neurosci. 2023, 14, 2968–2980. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, Q.; Tao, X.; Yu, P.; Liu, S.; Xie, Y.; Zhu, W. Resveratrol inhibits rabies virus infection in N2a cells by activating the SIRT1/Nrf2/HO-1 pathway. Heliyon 2024, 10, e36494. [Google Scholar] [CrossRef]
- Badia, R.; Garcia-Vidal, E.; Ballana, E. Viral-Host Dependency Factors as Therapeutic Targets to Overcome Antiviral Drug-Resistance: A Focus on Innate Immune Modulation. Front. Virol. 2022, 2, 935933. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, S.; Kumar, R.; Tripathi, B.N.; Barua, S.; Ly, H.; Rouse, B.T. Host-Directed Antiviral Therapy. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Choudhary, S.; Kumar, P.; Tomar, S. Antiviral strategies targeting host factors and mechanisms obliging +ssRNA viral pathogens. Bioorg. Med. Chem. 2021, 46, 116356. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, B.; Deng, L.; Liang, B.; Ping, J. Virus-host interaction networks as new antiviral drug targets for IAV and SARS-CoV-2. Emerg. Microbes Infect. 2022, 11, 1371–1389. [Google Scholar] [CrossRef]
- Eslami, M.; Arjmand, N.; Mahmoudian, F.; Babaeizad, A.; Tahmasebi, H.; Fattahi, F.; Oksenych, V. Deciphering Host-Virus Interactions and Advancing Therapeutics for Chronic Viral Infection. Viruses 2025, 17, 390. [Google Scholar] [CrossRef]
- Kornilaeva, G.V.; Siniavin, A.E.; Schultz, A.; Germann, A.; Moog, C.; von Briesen, H.; Turgiev, A.S.; Karamov, E.V. The Differential Anti-HIV Effect of a New Humic Substance-Derived Preparation in Diverse Cells of the Immune System. Acta Nat. 2019, 11, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Salikhov, S.I.; Mavlyanov, S.M.; Abdulladzhanova, N.G.; Karamov, E.V. Agent with Anti-Influenza Action. Patent UZ IAP 04524, 2012. [Google Scholar]
- Salikhov, S.I.; Abdurakhmanov, I.Y.; Egorov, A.M.; Turaev, A.S.; Oshchepkova, Y.I.; Ziyavitdinov, Z.F.; Berdiev, N.S. Complex of Polyphenolic Compounds with Inhibitory Activity Against 3CL Protease and Method for Its Preparation. Patent UZ IAP 07461, 17 August 2023. [Google Scholar]
- Salikhov, S.I.; Aisa, A.; Shen, J.; Xu, Y.; Xu, H.; Xiao, G.; Jiang, X.; Zhang, L.; Ziyavitdinov, Z.F.; Oshchepkova, Y.I.; et al. An Agent that Blocks Protease 3 CLpro and RNA Polymerase RdRp of RNA Viruses. Patent UZ IAP 06574, 21 September 2021. [Google Scholar]
- Salikhov, S.I.; Abdurakhmonov, I.Y.; Oshchepkova, Y.I.; Ziyavitdinov, J.F.; Berdiev, N.S.; Aisa, H.A.; Shen, J.; Xu, Y.; Xu, H.E.; Jiang, X.; et al. Repurposing of Rutan showed effective treatment for COVID-19 disease. Front. Med. 2023, 10, 1310129. [Google Scholar] [CrossRef]
- Azab, W.; Osterrieder, K. Initial Contact: The First Steps in Herpesvirus Entry. Adv. Anat. Embryol. Cell Biol. 2017, 223, 1–27. [Google Scholar] [CrossRef]
- Chaiyakunapruk, N.; Lee, S.W.H.; Kulchaitanaroaj, P.; Rayanakorn, A.; Lee, H.; Looker, K.J.; Hutubessy, R.; Gottlieb, S.L. Estimated global and regional economic burden of genital herpes simplex virus infection among 15–49 year-olds in 2016. BMC Glob. Public Health 2024, 2, 42. [Google Scholar] [CrossRef]
- de Sousa, R.M.P.; Garcia, L.S.; Lemos, F.S.; de Campos, V.S.; Machado Ferreira, E.; de Almeida, N.A.A.; Maron-Gutierrez, T.; de Souza, E.M.; de Paula, V.S. CRISPR/Cas9 Eye Drop HSV-1 Treatment Reduces Brain Viral Load: A Novel Application to Prevent Neuronal Damage. Pathogens 2024, 13, 1087. [Google Scholar] [CrossRef]
- Huang, Z.; Li, S.; Zhong, L.; Su, Y.; Li, M.; Wang, X.; Wang, Z.; Wang, Z.; Ye, C.; Ren, Z.; et al. Effect of resveratrol on herpesvirus encephalitis: Evidences for its mechanisms of action. Phytomedicine 2024, 127, 155476. [Google Scholar] [CrossRef] [PubMed]
- Antony, F.; Kinha, D.; Nowinska, A.; Rouse, B.T.; Suryawanshi, A. The immunobiology of corneal HSV-1 infection and herpetic stromal keratitis. Clin. Microbiol. Rev. 2024, 37, e0000624. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, N.; Huang, Z.; Wu, J.; Qian, Y. Harmol used for the treatment of herpes simplex virus induced keratitis. Virol. J. 2024, 21, 118. [Google Scholar] [CrossRef]
- Sanami, S.; Shamsabadi, S.; Dayhimi, A.; Pirhayati, M.; Ahmad, S.; Pirhayati, A.; Ajami, M.; Hemati, S.; Shirvani, M.; Alagha, A.; et al. Association between cytomegalovirus infection and neurological disorders: A systematic review. Rev. Med. Virol. 2024, 34, e2532. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liao, Z.; Tan, H.; Wang, K.; Feng, C.; Xing, P.; Zhang, X.; Hua, J.; Jiang, P.; Peng, S.; et al. The association between cytomegalovirus infection and neurodegenerative diseases: A prospective cohort using UK Biobank data. EClinicalMedicine 2024, 74, 102757. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; He, S.; Zhu, W.; Ru, P.; Ge, X.; Govindasamy, K. Human cytomegalovirus in cancer: The mechanism of HCMV-induced carcinogenesis and its therapeutic potential. Front. Cell. Infect. Microbiol. 2023, 13, 1202138. [Google Scholar] [CrossRef]
- Wolacewicz, M.; Becht, R.; Grywalska, E.; Niedzwiedzka-Rystwej, P. Herpesviruses in Head and Neck Cancers. Viruses 2020, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.K.; Arav-Boger, R.; Marr, K.A.; Kraus, E.; Shoham, S.; Lees, L.; Trollinger, B.; Shah, P.; Ambinder, R.; Neofytos, D.; et al. Outcomes in Transplant Recipients Treated With Foscarnet for Ganciclovir-Resistant or Refractory Cytomegalovirus Infection. Transplantation 2016, 100, e74–e80. [Google Scholar] [CrossRef]
- Erice, A. Resistance of human cytomegalovirus to antiviral drugs. Clin. Microbiol. Rev. 1999, 12, 286–297. [Google Scholar] [CrossRef]
- Robinson, W.H.; Steinman, L. Epstein-Barr virus and multiple sclerosis. Science 2022, 375, 264–265. [Google Scholar] [CrossRef]
- Woulfe, J.; Hoogendoorn, H.; Tarnopolsky, M.; Munoz, D.G. Monoclonal antibodies against Epstein-Barr virus cross-react with alpha-synuclein in human brain. Neurology 2000, 55, 1398–1401. [Google Scholar] [CrossRef]
- Rezk, S.A.; Zhao, X.; Weiss, L.M. Epstein-Barr virus (EBV)-associated lymphoid proliferations, a 2018 update. Hum. Pathol. 2018, 79, 18–41. [Google Scholar] [CrossRef]
- Maeda, E.; Akahane, M.; Kiryu, S.; Kato, N.; Yoshikawa, T.; Hayashi, N.; Aoki, S.; Minami, M.; Uozaki, H.; Fukayama, M.; et al. Spectrum of Epstein-Barr virus-related diseases: A pictorial review. Jpn. J. Radiol. 2009, 27, 4–19. [Google Scholar] [CrossRef]
- Dreyfus, D.H. Autoimmune disease: A role for new anti-viral therapies? Autoimmun. Rev. 2011, 11, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Lemus, Y.B.; Martinez, G.A.; Lugo, L.P.; Escorcia, L.G.; Penata, E.Z.; Llanos, N.S.; Bonfanti, A.C.; Acosta-Hoyos, A.J.; Quiroz, E.N. Gene profiling of Epstein-Barr Virus and human endogenous retrovirus in peripheral blood mononuclear cells of SLE patients: Immune response implications. Sci. Rep. 2024, 14, 20236. [Google Scholar] [CrossRef]
- Hoshino, Y.; Katano, H.; Zou, P.; Hohman, P.; Marques, A.; Tyring, S.K.; Follmann, D.; Cohen, J.I. Long-term administration of valacyclovir reduces the number of Epstein-Barr virus (EBV)-infected B cells but not the number of EBV DNA copies per B cell in healthy volunteers. J. Virol. 2009, 83, 11857–11861. [Google Scholar] [CrossRef]
- Jean-Pierre, V.; Lupo, J.; Buisson, M.; Morand, P.; Germi, R. Main Targets of Interest for the Development of a Prophylactic or Therapeutic Epstein-Barr Virus Vaccine. Front. Microbiol. 2021, 12, 701611. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, S.; Xiang, Y.F.; Guo, C.W.; Ge, F.; Yang, C.R.; Zhang, Y.J.; Wang, Y.F.; Kitazato, K. Antiviral activity and possible mechanisms of action of pentagalloylglucose (PGG) against influenza A virus. Arch. Virol. 2011, 156, 1359–1369. [Google Scholar] [CrossRef]
- Ge, H.; Liu, G.; Xiang, Y.F.; Wang, Y.; Guo, C.W.; Chen, N.H.; Zhang, Y.J.; Wang, Y.F.; Kitazato, K.; Xu, J. The mechanism of poly-galloyl-glucoses preventing Influenza A virus entry into host cells. PLoS ONE 2014, 9, e94392. [Google Scholar] [CrossRef]
- Derksen, A.; Hensel, A.; Hafezi, W.; Herrmann, F.; Schmidt, T.J.; Ehrhardt, C.; Ludwig, S.; Kuhn, J. 3-O-galloylated procyanidins from Rumex acetosa L. inhibit the attachment of influenza A virus. PLoS ONE 2014, 9, e110089. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, X.; Huang, L. Anti-Influenza Virus Activity and Constituents. Charact. Paeonia Delavayi Extracts. Mol. 2016, 21, 1133. [Google Scholar] [CrossRef]
- Zhang, T.; Lo, C.Y.; Xiao, M.; Cheng, L.; Pun Mok, C.K.; Shaw, P.C. Anti-influenza virus phytochemicals from Radix Paeoniae Alba and characterization of their neuraminidase inhibitory activities. J. Ethnopharmacol. 2020, 253, 112671. [Google Scholar] [CrossRef]
- Joo, Y.H.; Lee, Y.G.; Lim, Y.; Jeon, H.; Kim, E.H.; Choi, J.; Hong, W.; Jeon, H.; Ahrweiler, M.; Kim, H.; et al. Potent antiviral activity of the extract of Elaeocarpus sylvestris against influenza A virus in vitro and in vivo. Phytomedicine 2022, 97, 153892. [Google Scholar] [CrossRef]
- Lee, Y.G.; Kang, K.W.; Hong, W.; Kim, Y.H.; Oh, J.T.; Park, D.W.; Ko, M.; Bai, Y.F.; Seo, Y.J.; Lee, S.M.; et al. Potent antiviral activity of Agrimonia pilosa, Galla rhois, and their components against SARS-CoV-2. Bioorg. Med. Chem. 2021, 45, 116329. [Google Scholar] [CrossRef]
- Jin, Y.H.; Lee, J.; Jeon, S.; Kim, S.; Min, J.S.; Kwon, S. Natural Polyphenols, 1,2,3,4,6-O-Pentagalloyglucose and Proanthocyanidins, as Broad-Spectrum Anticoronaviral Inhibitors Targeting Mpro and RdRp of SARS-CoV-2. Biomedicines 2022, 10, 1170. [Google Scholar] [CrossRef]
- Chiou, W.C.; Chen, J.C.; Chen, Y.T.; Yang, J.M.; Hwang, L.H.; Lyu, Y.S.; Yang, H.Y.; Huang, C. The inhibitory effects of PGG and EGCG against the SARS-CoV-2 3C-like protease. Biochem. Biophys. Res. Commun. 2022, 591, 130–136. [Google Scholar] [CrossRef]
- Tan, H.; Ma, C.; Wang, J. Invalidation of dieckol and 1,2,3,4,6-pentagalloylglucose (PGG) as SARS-CoV-2 main protease inhibitors and the discovery of PGG as a papain-like protease inhibitor. Med. Chem. Res. 2022, 31, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Pattaro-Junior, J.R.; Araujo, I.G.; Moraes, C.B.; Barbosa, C.G.; Philippsen, G.S.; Freitas-Junior, L.H.; Guidi, A.C.; de Mello, J.C.P.; Peralta, R.M.; Fernandez, M.A.; et al. Antiviral activity of Cenostigma pluviosum var. peltophoroides extract and fractions against SARS-CoV-2. J. Biomol. Struct. Dyn. 2023, 41, 7297–7308. [Google Scholar] [CrossRef] [PubMed]
- Ezell, J.; Al-Horani, R.A. Chemically Synthesized 1,2,3,4,6-Pentakis-O-Galloyl-beta-D-Glucopyranoside Blocks SARS-CoV-2 Spike Interaction with Host ACE-2 Receptor. Med. Chem. 2024, 20, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.H.; Yang, L.J.; Hamdoun, S.; Chung, S.K.; Lam, C.W.; Zhang, K.X.; Guo, X.; Xia, C.; Law, B.Y.K.; Wong, V.K.W. 1,2,3,4,6-Pentagalloyl Glucose, a RBD-ACE2 Binding Inhibitor to Prevent SARS-CoV-2 Infection. Front. Pharmacol. 2021, 12, 634176. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, Y.-G.; Yang, Y.J.; Jeong, Y.J.; Kwon, J.H.; Park, J.-H.; Kim, H.; Kang, S.; Tark, D.; Lee, G.-H.; et al. Anti-SARS-CoV-2 Efficacy of Elaeocarpus sylvestris Extract Verified by in silico, in vitro, Preclinical, and Clinical Studies. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Ngan, L.T.; Jang, M.J.; Kwon, M.J.; Ahn, Y.J. Antiviral activity and possible mechanism of action of constituents identified in Paeonia lactiflora root toward human rhinoviruses. PLoS ONE 2015, 10, e0121629. [Google Scholar] [CrossRef]
- Yeo, S.J.; Yun, Y.J.; Lyu, M.A.; Woo, S.Y.; Woo, E.R.; Kim, S.J.; Lee, H.J.; Park, H.K.; Kook, Y.H. Respiratory syncytial virus infection induces matrix metalloproteinase-9 expression in epithelial cells. Arch. Virol. 2002, 147, 229–242. [Google Scholar] [CrossRef]
- Ahn, M.J.; Kim, C.Y.; Lee, J.S.; Kim, T.G.; Kim, S.H.; Lee, C.K.; Lee, B.B.; Shin, C.G.; Huh, H.; Kim, J. Inhibition of HIV-1 integrase by galloyl glucoses from Terminalia chebula and flavonol glycoside gallates from Euphorbia pekinensis. Planta Med. 2002, 68, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.N.; Kesharwani, A.; Agarwal, A.; Polachira, S.K.; Nair, R.; Gupta, S.K. Herbal Gel Formulation Developed for Anti-Human Immunodeficiency Virus (HIV)-1 Activity Also Inhibits In Vitro HSV-2 Infection. Viruses 2018, 10, 580. [Google Scholar] [CrossRef]
- Tu, Z.; Gong, W.; Zhang, Y.; Feng, Y.; Liu, Y.; Tu, C. Inhibition of Rabies Virus by 1,2,3,4,6-Penta-O-galloyl-beta-d-Glucose Involves mTOR-Dependent Autophagy. Viruses 2018, 10, 201. [Google Scholar] [CrossRef]
- Sharma, N.; Kumar, P.; Giri, R. Polysaccharides like pentagalloylglucose, parishin a and stevioside inhibits the viral entry by binding the Zika virus envelope protein. J. Biomol. Struct. Dyn. 2021, 39, 6008–6020. [Google Scholar] [CrossRef]
- Kullappan, M.; Benedict, B.A.; Rajajagadeesan, A.; Baskaran, P.; Periadurai, N.D.; Ambrose, J.M.; Gandhamaneni, S.H.; Nakkella, A.K.; Agarwal, A.; Veeraraghavan, V.P.; et al. Ellagic Acid as a Potential Inhibitor against the Nonstructural Protein NS3 Helicase of Zika Virus: A Molecular Modelling Study. BioMed Res. Int. 2022, 2022, 2044577. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Harish, D.R.; Charla, R.; Vetrivel, U.; Jalalpure, S.S.; Bhandare, V.V.; Deshpande, S.H.; Hegde, H.V.; Roy, S. Structural insights into modeling of hepatitis B virus reverse transcriptase and identification of its inhibitors from potential medicinal plants of Western Ghats: An in silico and in vitro study. J. Biomol. Struct. Dyn. 2024, 42, 11731–11749. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, H.K.; Jung, M.K.; Mar, W. In vitro antiviral activity of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose against hepatitis B virus. Biol. Pharm. Bull. 2006, 29, 2131–2134. [Google Scholar] [CrossRef]
- Duan, D.; Li, Z.; Luo, H.; Zhang, W.; Chen, L.; Xu, X. Antiviral compounds from traditional Chinese medicines Galla Chinese as inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 2004, 14, 6041–6044. [Google Scholar] [CrossRef]
- Zuo, G.Y.; Li, Z.Q.; Chen, L.R.; Xu, X.J. In vitro anti-HCV activities of Saxifraga melanocentra and its related polyphenolic compounds. Antivir. Chem. Chemother. 2005, 16, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Chen, Z.P.; Ju, H.Q.; Komatsu, M.; Ji, Y.H.; Liu, G.; Guo, C.W.; Zhang, Y.J.; Yang, C.R.; Wang, Y.F.; et al. Autophagy is involved in anti-viral activity of pentagalloylglucose (PGG) against Herpes simplex virus type 1 infection in vitro. Biochem. Biophys. Res. Commun. 2011, 405, 186–191. [Google Scholar] [CrossRef]
- Jin, F.; Ma, K.; Chen, M.; Zou, M.; Wu, Y.; Li, F.; Wang, Y. Pentagalloylglucose Blocks the Nuclear Transport and the Process of Nucleocapsid Egress to Inhibit HSV-1 Infection. Jpn. J. Infect. Dis. 2016, 69, 135–142. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.Y.; Do, M.H.; Lee, C.H.; Song, Y.J. 1,2,3,4,6-Penta-O-galloyl-ss-D-glucose, a bioactive compound in Elaeocarpus sylvestris extract, inhibits varicella-zoster virus replication. Antiviral. Res. 2017, 144, 266–272. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, J.E.; Song, Y.J. Antiviral Activities of Quercetin and Isoquercitrin Against Human Herpesviruses. Molecules 2020, 25, 2379. [Google Scholar] [CrossRef]
- Choi, J.G.; Kim, Y.S.; Kim, J.H.; Chung, H.S. Antiviral activity of ethanol extract of Geranii Herba and its components against influenza viruses via neuraminidase inhibition. Sci. Rep. 2019, 9, 12132. [Google Scholar] [CrossRef] [PubMed]
- Arokiyaraj, S.; Stalin, A.; Kannan, B.S.; Shin, H. Geranii Herba as a Potential Inhibitor of SARS-CoV-2 Main 3CL(pro), Spike RBD, and Regulation of Unfolded Protein Response: An In Silico Approach. Antibiotics 2020, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Chung, H.S.; Noh, S.G.; Lee, B.; Chung, H.Y.; Choi, J.G. Geraniin Inhibits the Entry of SARS-CoV-2 by Blocking the Interaction between Spike Protein RBD and Human ACE2 Receptor. Int. J. Mol. Sci. 2021, 22, 8604. [Google Scholar] [CrossRef] [PubMed]
- Boadu, A.; Agoni, C.; Karpoormath, R.; Soliman, M.; Nlooto, M. Repurposing antiviral phytochemicals from the leaf extracts of Spondias mombin (Linn) towards the identification of potential SARS-CoV-2 inhibitors. Sci. Rep. 2022, 12, 10896. [Google Scholar] [CrossRef]
- Aribisala, J.O.; Aruwa, C.E.; Uthman, T.O.; Nurain, I.O.; Idowu, K.; Sabiu, S. Cheminformatics Bioprospection of Broad Spectrum Plant Secondary Metabolites Targeting the Spike Proteins of Omicron Variant and Wild-Type SARS-CoV-2. Metabolites 2022, 12, 982. [Google Scholar] [CrossRef]
- Hiremath, S.; Kumar, H.D.V.; Nandan, M.; Mantesh, M.; Shankarappa, K.S.; Venkataravanappa, V.; Basha, C.R.J.; Reddy, C.N.L. In silico docking analysis revealed the potential of phytochemicals present in Phyllanthus amarus and Andrographis paniculata, used in Ayurveda medicine in inhibiting SARS-CoV-2. 3 Biotech 2021, 11, 44. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.M.; Zhao, J.; Bai, Y.M.; Yan, C.Q.; Du, G.H.; Zheng, L.S.; Liu, A.L. Fumarprotocetraric acid and geraniin were identified as novel inhibitors of human respiratory syncytial virus infection in vitro. Front. Cell. Infect. Microbiol. 2024, 14, 1484245. [Google Scholar] [CrossRef]
- Notka, F.; Meier, G.R.; Wagner, R. Inhibition of wild-type human immunodeficiency virus and reverse transcriptase inhibitor-resistant variants by Phyllanthus amarus. Antiviral. Res. 2003, 58, 175–186. [Google Scholar] [CrossRef]
- Notka, F.; Meier, G.; Wagner, R. Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antiviral. Res. 2004, 64, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Abdul Ahmad, S.A.; Palanisamy, U.D.; Tejo, B.A.; Chew, M.F.; Tham, H.W.; Syed Hassan, S. Geraniin extracted from the rind of Nephelium lappaceum binds to dengue virus type-2 envelope protein and inhibits early stage of virus replication. Virol. J. 2017, 14, 229. [Google Scholar] [CrossRef]
- Abdul Ahmad, S.A.; Palanisamy, U.D.; Khoo, J.J.; Dhanoa, A.; Syed Hassan, S. Efficacy of geraniin on dengue virus type-2 infected BALB/c mice. Virol. J. 2019, 16, 26. [Google Scholar] [CrossRef]
- Haddad, J.G.; Grauzdyte, D.; Koishi, A.C.; Viranaicken, W.; Venskutonis, P.R.; Nunes Duarte Dos Santos, C.; Despres, P.; Diotel, N.; El Kalamouni, C. The Geraniin-Rich Extract from Reunion Island Endemic Medicinal Plant Phyllanthus phillyreifolius Inhibits Zika and Dengue Virus Infection at Non-Toxic Effect Doses in Zebrafish. Molecules 2020, 25, 2316. [Google Scholar] [CrossRef]
- Huang, R.L.; Huang, Y.L.; Ou, J.C.; Chen, C.C.; Hsu, F.L.; Chang, C. Screening of 25 compounds isolated from Phyllanthus species for anti-human hepatitis B virus in vitro. Phytother. Res. 2003, 17, 449–453. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Zhou, W.; Feng, M.; Zhou, P. Anti-hepatitis B virus activities of Geranium carolinianum L. extracts and identification of the active components. Biol. Pharm. Bull. 2008, 31, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cai, D.; Zhang, L.; Tang, W.; Yan, R.; Guo, H.; Chen, X. Identification of hydrolyzable tannins (punicalagin, punicalin and geraniin) as novel inhibitors of hepatitis B virus covalently closed circular DNA. Antiviral. Res. 2016, 134, 97–107. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Liu, D.; Proksch, P.; Lin, W. Inhibitory effects of polyphenols toward HCV from the mangrove plant Excoecaria agallocha L. Bioorg. Med. Chem. Lett. 2012, 22, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Cheng, H.Y.; Lin, T.C.; Chiang, L.C.; Lin, C.C. The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-beta-D-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J. Ethnopharmacol. 2007, 110, 555–558. [Google Scholar] [CrossRef]
- Siqueira, E.; Lima, T.L.C.; Boff, L.; Lima, S.G.M.; Lourenco, E.M.G.; Ferreira, E.G.; Barbosa, E.G.; Machado, P.R.L.; Farias, K.J.S.; Ferreira, L.S.; et al. Antiviral Potential of Spondias mombin L. Leaves Extract Against Herpes Simplex Virus Type-1 Replication Using in vitro and In Silico Approaches. Planta Med. 2020, 86, 505–515. [Google Scholar] [CrossRef]
- Lee, Y.G.; Park, D.W.; Kwon, J.E.; Kim, H.; Kang, S.C. Elaeocarpus sylvestris var. ellipticus Extract and Its Major Component, Geraniin, Inhibit Herpes Simplex Virus-1 Replication. Plants 2024, 13, 1437. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, M.H.; Chen, Y.; Liu, T. Network Pharmacology-Based Systematic Analysis of Molecular Mechanisms of Geranium wilfordii Maxim for HSV-2 Infection. Evid. Based Complement. Alternat. Med. 2021, 2021, 1009551. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Li, C.; Chen, R.; Liu, T.; Jiang, Y. Antiviral Effect of Polyphenolic Substances in Geranium wilfordii Maxim against HSV-2 Infection Using in vitro and in silico Approaches. Evid. Based Complement. Alternat. Med. 2022, 2022, 7953728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, L.; Ju, F. In vitro and silico studies of geraniin interfering with HSV-2 replication by targeting glycoprotein D. Nat. Prod. Res. 2024, 38, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, L.; Fan, X.; Qin, C.; Liu, J. Antiviral effect of geraniin on human enterovirus 71 in vitro and in vivo. Bioorg. Med. Chem. Lett. 2012, 22, 2209–2211. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Nayeshiro, H. Geraniin, a new ellagitannin from geranium thunbergii. Tetrahedron. Lett. 1976, 17, 3721–3722. [Google Scholar] [CrossRef]
- Salikhov, S.I.; Egorov, A.M.; Karamov, E.V.; Oshchepkova, Y.I.; Turgiev, A.S.; Ziyavitdinov, Z.F.; Kornilaeva, G.V.; Berdiev, N.S.; Fedyakina, I.T.; Larichev, V.F. An Agent with Antiviral Activity Against Coronavirus Infection SARS-CoV-2. Patent UZ FAP 2665, 14 February 2025. [Google Scholar]
- Luger, P.; Weber, M.; Kashino, S.; Amakura, Y.; Yoshida, T.; Okuda, T.; Beurskens, G.; Dauter, Z. Structure of the Tannin Geraniin Based on Conventional X-ray Data at 295 K and on Synchrotron Data at 293 and 120 K. Acta Crystallogr. Sect. B 1998, 54, 687–694. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [CrossRef]
- Zeng, A.H.; Ou, Y.Y.; Guo, M.M.; Dai, X.; Zhou, D.Z.; Chen, R. Human embryonic lung fibroblasts treated with artesunate exhibit reduced rates of proliferation and human cytomegalovirus infection in vitro. J. Thorac. Dis. 2015, 7, 1151–1157. [Google Scholar] [CrossRef]
- Klimova, R.; Andreev, S.; Momotyuk, E.; Demidova, N.; Fedorova, N.; Chernoryzh, Y.; Yurlov, K.; Turetskiy, E.; Baraboshkina, E.; Shershakova, N.; et al. Aqueous fullerene C60 solution suppresses herpes simplex virus and cytomegalovirus infections. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 487–499. [Google Scholar] [CrossRef]
- Cifuentes-Munoz, N.; El Najjar, F.; Dutch, R.E. Viral cell-to-cell spread: Conventional and non-conventional ways. Adv. Virus Res. 2020, 108, 85–125. [Google Scholar] [CrossRef]
- Zhong, P.; Agosto, L.M.; Munro, J.B.; Mothes, W. Cell-to-cell transmission of viruses. Curr. Opin. Virol. 2013, 3, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.J.; Adler, B.; Sampaio, K.L.; Digel, M.; Jahn, G.; Ettischer, N.; Stierhof, Y.D.; Scrivano, L.; Koszinowski, U.; Mach, M.; et al. UL74 of human cytomegalovirus contributes to virus release by promoting secondary envelopment of virions. J. Virol. 2008, 82, 2802–2812. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, E.A. Using Diploid Human Fibroblasts as a Model System to Culture, Grow, and Study Human Cytomegalovirus Infection. Methods Mol. Biol. 2021, 2244, 39–50. [Google Scholar] [CrossRef]
- Miller, G.; Shope, T.; Lisco, H.; Stitt, D.; Lipman, M. Epstein-Barr virus: Transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc. Natl. Acad. Sci. USA 1972, 69, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Hershkovitz, P. Living New World Monkeys (Platyrrhini): With an Introduction to Primates; University of Chicago Press: Chicago, IL, USA, 1977; p. v. [Google Scholar]
- Klupp, B.G.; Mettenleiter, T.C. The Knowns and Unknowns of Herpesvirus Nuclear Egress. Annu. Rev. Virol. 2023, 10, 305–323. [Google Scholar] [CrossRef]
- Sucharita, S.; Krishnagopal, A.; van Drunen Littel-van den Hurk, S. Comprehensive Analysis of the Tegument Proteins Involved in Capsid Transport and Virion Morphogenesis of Alpha, Beta and Gamma Herpesviruses. Viruses 2023, 15, 2058. [Google Scholar] [CrossRef]
- Feire, A.L.; Koss, H.; Compton, T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 2004, 101, 15470–15475. [Google Scholar] [CrossRef]
- Mahmud, J.; Chan, G.C. Analysis of Cytomegalovirus Glycoprotein and Cellular Receptor Interactions. Methods Mol. Biol. 2021, 2244, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.K.; Lee, J.H.; Kim, H.K.; Lee, A.Y.; Lee, S.O.; Kim, Y.S.; Ryu, S.Y.; Kim, S.Y.; Lee, Y.J.; Ko, B.S. Anti-platelet effects of bioactive compounds isolated from the bark of Rhus verniciflua Stokes. J. Ethnopharmacol. 2006, 106, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Yu, Z.Y.; Chen, Y.C.; Hung, S.L. Effects of epigallocatechin-3-gallate and acyclovir on herpes simplex virus type 1 infection in oral epithelial cells. J. Formos. Med. Assoc. 2021, 120, 2136–2143. [Google Scholar] [CrossRef]
- Chen, Y.L.; Tsai, H.L.; Peng, C.W. EGCG debilitates the persistence of EBV latency by reducing the DNA binding potency of nuclear antigen 1. Biochem. Biophys. Res. Commun. 2012, 417, 1093–1099. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Hu, J.; Liu, S.; Luo, X.; Tang, M.; Bode, A.M.; Dong, Z.; Liu, X.; Liao, W.; et al. (−)-Epigallocatechin-3-Gallate Inhibits EBV Lytic Replication via Targeting LMP1-Mediated MAPK Signal Axes. Oncol. Res. 2021, 28, 763–778. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Chen, C.Y.; Chiou, Y.H.; Shyu, H.W.; Lin, K.H.; Chou, M.C.; Huang, M.H.; Wang, Y.F. Epigallocatechin-3-Gallate Suppresses Human Herpesvirus 8 Replication and Induces ROS Leading to Apoptosis and Autophagy in Primary Effusion Lymphoma Cells. Int. J. Mol. Sci. 2017, 19, 16. [Google Scholar] [CrossRef]
- Xu, J.; Gu, W.; Li, C.; Li, X.; Xing, G.; Li, Y.; Song, Y.; Zheng, W. Epigallocatechin gallate inhibits hepatitis B virus via farnesoid X receptor alpha. J. Nat. Med. 2016, 70, 584–591. [Google Scholar] [CrossRef]
- Ciesek, S.; von Hahn, T.; Colpitts, C.C.; Schang, L.M.; Friesland, M.; Steinmann, J.; Manns, M.P.; Ott, M.; Wedemeyer, H.; Meuleman, P.; et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology 2011, 54, 1947–1955. [Google Scholar] [CrossRef]
- Weber, C.; Sliva, K.; von Rhein, C.; Kummerer, B.M.; Schnierle, B.S. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antiviral. Res. 2015, 113, 1–3. [Google Scholar] [CrossRef]
- Porcaro, G.; Pavone-Cossut, M.R.; Moretti, S.; Bilotta, G.; Aragona, C.; Unfer, V. Oral Treatment with EGCG, Folic Acid, Vitamin B12, and Hyaluronic Acid Improves HPV Clearance and Counteracts Its Persistence: A Clinical Study. Int. J. Mol. Sci. 2025, 26, 5251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.C.; Wu, H.; Wang, J.; Lu, M.; Cao, D.; Lin, J.; Chen, H.; Lin, C.; Wang, Y.; Zhang, X.H.; et al. Multi-level inhibition of SARS-CoV-2 invasion by cannabidiol and epigallocatechin gallate. Virology 2025, 610, 110579. [Google Scholar] [CrossRef]
- Yu, P.W.; Fu, P.F.; Zeng, L.; Qi, Y.L.; Li, X.Q.; Wang, Q.; Yang, G.Y.; Li, H.W.; Wang, J.; Chu, B.B.; et al. EGCG Restricts PRRSV Proliferation by Disturbing Lipid Metabolism. Microbiol. Spectr. 2022, 10, e0227621. [Google Scholar] [CrossRef]
- Bery, D.E.; El-Masry, S.A.; Guirgis, A.A.; Zain, A.M.; Khalil, H. Epigallocatechin-3-gallate inhibits replication of influenza A virus via restoring the host methylated genes following infection. In International Microbiology; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Yin, Z.; Henry, E.C.; Gasiewicz, T.A. (−)-Epigallocatechin-3-gallate is a novel Hsp90 inhibitor. Biochemistry 2009, 48, 336–345. [Google Scholar] [CrossRef] [PubMed]
- De Leo, A.; Arena, G.; Lacanna, E.; Oliviero, G.; Colavita, F.; Mattia, E. Resveratrol inhibits Epstein Barr Virus lytic cycle in Burkitt’s lymphoma cells by affecting multiple molecular targets. Antiviral. Res. 2012, 96, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Chan, K.W.K.; Vasudevan, S.G.; Low, J.G. alpha-Glucosidase inhibitors as broad-spectrum antivirals: Current knowledge and future prospects. Antiviral. Res. 2025, 238, 106147. [Google Scholar] [CrossRef]
- Schilling, M.; Bulli, L.; Weigang, S.; Graf, L.; Naumann, S.; Patzina, C.; Wagner, V.; Bauersfeld, L.; Goujon, C.; Hengel, H.; et al. Human MxB Protein Is a Pan-herpesvirus Restriction Factor. J. Virol. 2018, 92, e01056-18. [Google Scholar] [CrossRef]
- Schumann, S.; Jackson, B.R.; Yule, I.; Whitehead, S.K.; Revill, C.; Foster, R.; Whitehouse, A. Targeting the ATP-dependent formation of herpesvirus ribonucleoprotein particle assembly as an antiviral approach. Nat. Microbiol. 2016, 2, 16201. [Google Scholar] [CrossRef] [PubMed]
- Majewska, A.; Mlynarczyk-Bonikowska, B. 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? Int. J. Mol. Sci. 2022, 23, 3431. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. Journal of Applied Crystallography. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards Quantitative Analysis of Intermolecular Interactions with Hirshfeld Surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Ivanova, O.N.; Gavlina, A.V.; Karpenko, I.L.; Zenov, M.A.; Antseva, S.S.; Zakirova, N.F.; Valuev-Elliston, V.T.; Krasnov, G.S.; Fedyakina, I.T.; Vorobyev, P.O.; et al. Polyamine Catabolism Revisited: Acetylpolyamine Oxidase Plays a Minor Role due to Low Expression. Cells 2024, 13, 1134. [Google Scholar] [CrossRef]
- Buloyan, S.; Harutyunyan, A.; Gasparyan, H.; Sakeyan, A.; Shahkhatuni, A.; Zakirova, N.F.; Yusubalieva, G.; Kirillov, I.M.; Fedyakina, I.T.; Solyev, P.N.; et al. Piperazine-Substituted Pyranopyridines Exhibit Antiproliferative Activity and Act as Inhibitors of HBV Virion Production. Int. J. Mol. Sci. 2025, 26, 3991. [Google Scholar] [CrossRef]
- Pannecouque, C.; Daelemans, D.; De Clercq, E. Tetrazolium-based colorimetric assay for the detection of HIV replication inhibitors: Revisited 20 years later. Nat. Protoc. 2008, 3, 427–434. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Siniavin, A.; Karamov, E.; Kosarev, M.; Kovalchuk, S.; Turgiev, A.; Nametkin, S.; Bagrov, V.; Tavtorkin, A.; Ivchenko, P. A New Approach to Developing Long-Acting Injectable Formulations of Anti-HIV Drugs: Poly(Ethylene Phosphoric Acid) Block Copolymers Increase the Efficiency of Tenofovir against HIV-1 in MT-4 Cells. Int. J. Mol. Sci. 2020, 22, 340. [Google Scholar] [CrossRef]
- Sarisky, R.T.; Nguyen, T.T.; Duffy, K.E.; Wittrock, R.J.; Leary, J.J. Difference in incidence of spontaneous mutations between Herpes simplex virus types 1 and 2. Antimicrob. Agents Chemother. 2000, 44, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, V.P.; Andronova, V.L.; Belyavsky, A.V.; Borisevich, S.S.; Galegov, G.A.; Kandarakov, O.F.; Gruzdev, D.A.; Vozdvizhenskaya, O.A.; Levit, G.L. Large Subunit of the Human Herpes Simplex Virus Terminase as a Promising Target in Design of Anti-Herpesvirus Agents. Molecules 2023, 28, 7375. [Google Scholar] [CrossRef] [PubMed]
- Fateev, I.V.; Sasmakov, S.A.; Abdurakhmanov, J.M.; Ziyaev, A.A.; Khasanov, S.S.; Eshboev, F.B.; Ashirov, O.N.; Frolova, V.D.; Eletskaya, B.Z.; Smirnova, O.S.; et al. Synthesis of Substituted 1,2,4-Triazole-3-Thione Nucleosides Using E. coli Purine Nucleoside Phosphorylase. Biomolecules 2024, 14, 745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Rehling, D.; Jemth, A.S.; Throup, A.; Landazuri, N.; Almlof, I.; Gottmann, M.; Valerie, N.C.K.; Borhade, S.R.; Wakchaure, P.; et al. NUDT15-mediated hydrolysis limits the efficacy of anti-HCMV drug ganciclovir. Cell Chem. Biol. 2021, 28, 1693–1702.e1696. [Google Scholar] [CrossRef]
- Pennisi, R.; Trischitta, P.; Costa, M.; Venuti, A.; Tamburello, M.P.; Sciortino, M.T. Update of Natural Products and Their Derivatives Targeting Epstein-Barr Infection. Viruses 2024, 16, 124. [Google Scholar] [CrossRef]
- Ma, L.; Wang, T.M.; He, Y.Q.; Liao, Y.; Yan, X.; Yang, D.W.; Wang, R.H.; Li, F.J.; Jia, W.H.; Feng, L. Multiplex assays reveal anti-EBV antibody profile and its implication in detection and diagnosis of nasopharyngeal carcinoma. Int. J. Cancer 2024, 155, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
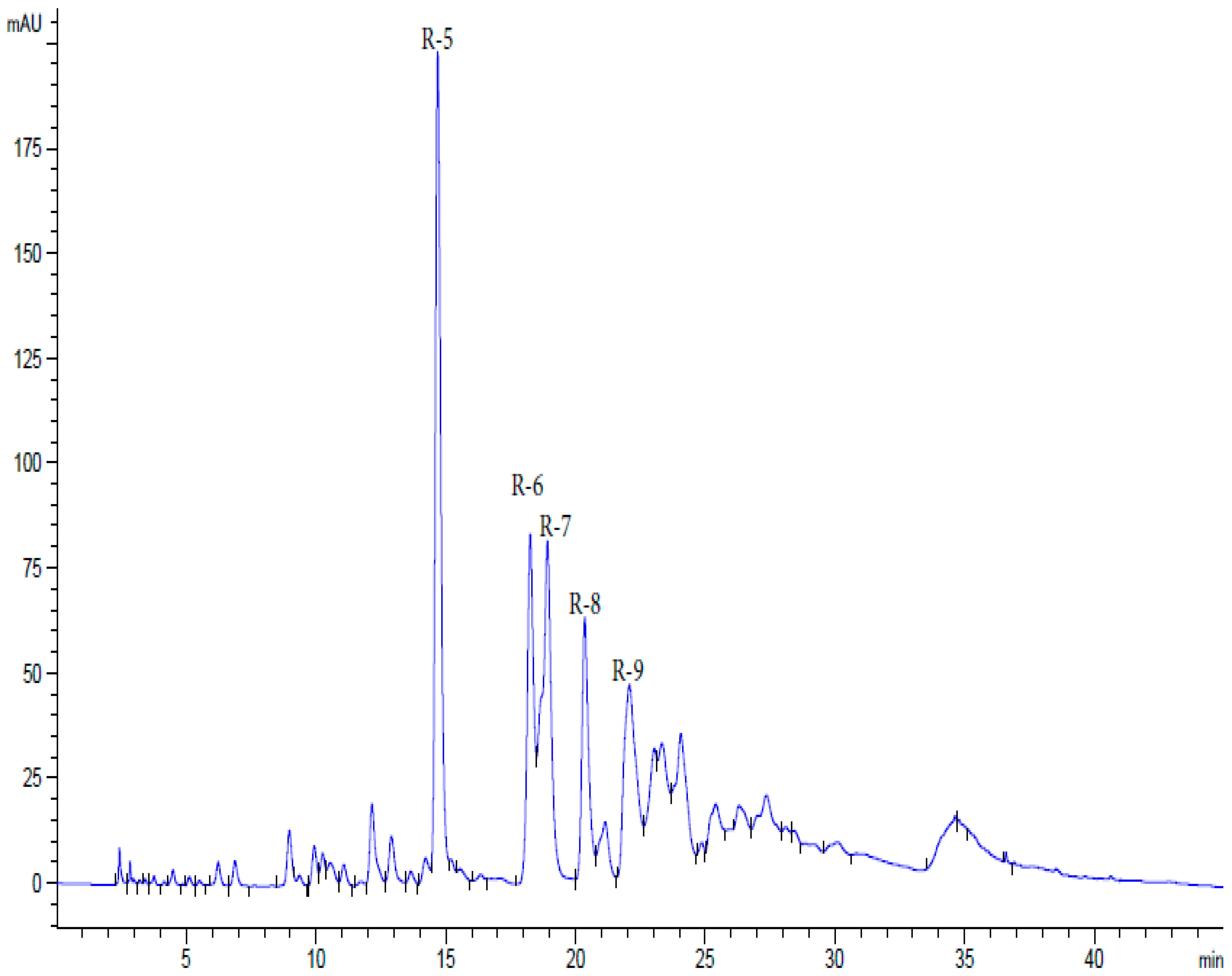


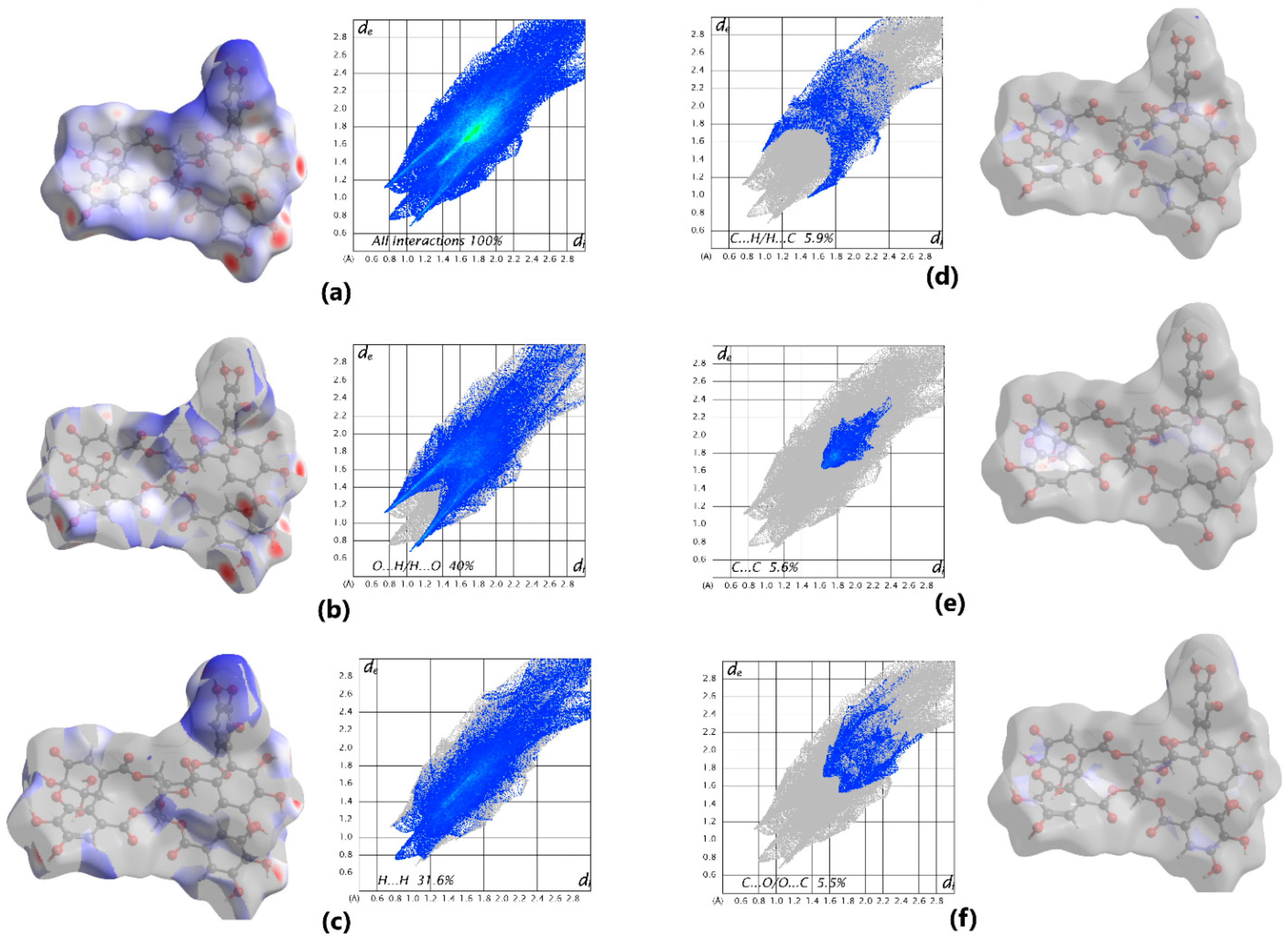
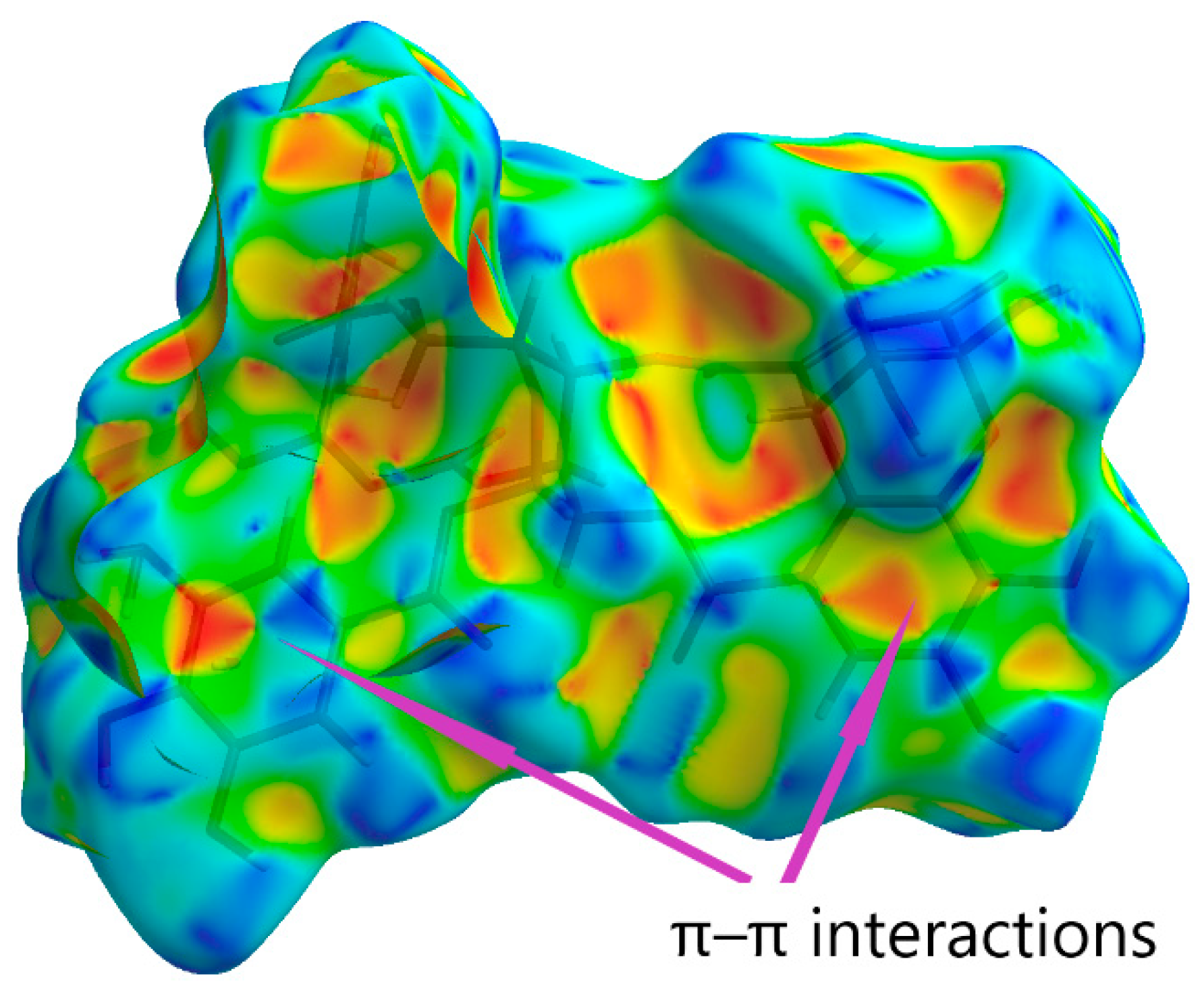


| Substance | Description | Spectrum of Antiviral Activity | Approved for Diseases (Country) |
|---|---|---|---|
| Rutan * | The sum of water-soluble polyphenols of sumac (Rhus coriaria) | Influenza viruses [43], SARS-CoV-2 [44,45,46] | Influenza (Uzbekistan), COVID-19 (Uzbekistan) [46] |
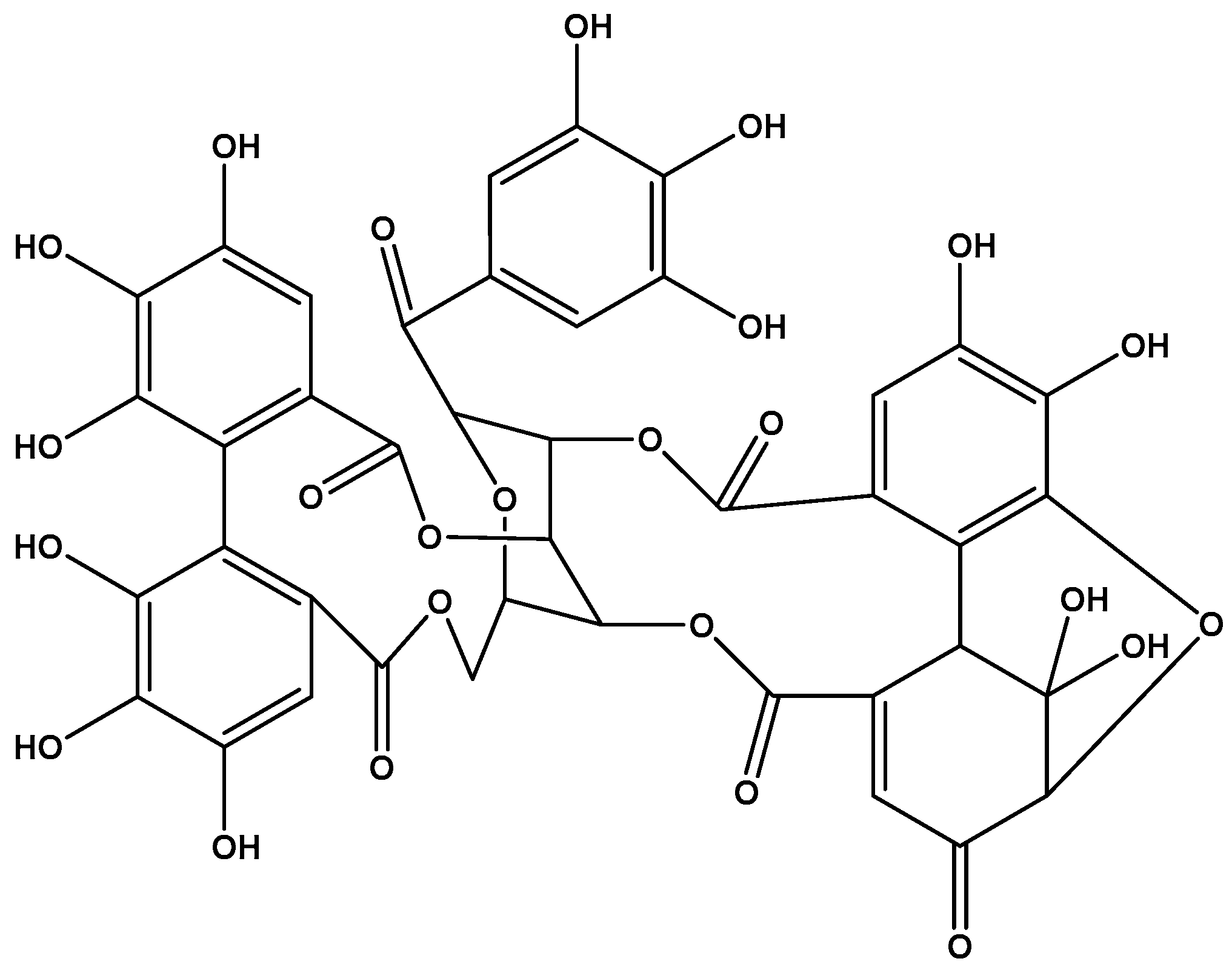
| R5 (Rutan polyphenol) | 1,2,3,4,6-penta-O-galloyl-β-D-glucose | Influenza viruses [67,68,69,70,71,72], SARS-CoV-2 [46,73,74,75,76,77,78,79,80], rhinoviruses [81], respiratory syncytial virus [82], HIV [83,84], rabies virus [85], Zika virus [28,86,87], hepatitis B virus (HBV) [88,89], HCV [28,86,90,91], HSV-1 [92,93], HSV-2 [84], varicella-zoster virus (VZV) [94,95] | - |
| R6 (Rutan polyphenol) | 3-bis-O-galloyl-1,2,4,6-tetra-O-galloyl-β-D-glucose | SARS-CoV-2 [46] | - |
| R7 (Rutan polyphenol) | 2,4-bis-O-galloyl-1,3,6-tri-O-galloyl-β-D-glucose | SARS-CoV-2 [46] | - |
| R8 (Rutan polyphenol) | 2,3,4-bis-O-galloyl-1,6-di-O-galloyl-β-D-glucose | SARS-CoV-2 [46] | - |
| Geraniin | Polyphenol of Geranium sanguineum | Influenza viruses [69,72,96], SARS-CoV-2 [80,97,98,99,100,101], rhinovirus [102], respiratory syncytial virus [102], metapneumovirus [102], HIV [103,104], dengue virus [105,106,107], Zika virus [107], HBV [108,109,110], HCV [111], HSV-1 [112,113,114], HSV-2 [112,115,116,117], enterovirus 71 [118] | Diarrhea (Japan) [119] |
| Substance | Rt | [M-H]− | Fragmentation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| min | m/z. (%) | m/z. (%) | |||||||||
| R5 | 14.54 | 939.05 (5) | 769.09 (100) | 617.08 (35) | 447.04 (16) | 313.02 (5) | 169.01 (12) | 125.0 (5) | |||
| R6 | 18.33 | 1091.2 (3) | 939.17 (45) | 769.12 (100) | 617.09 (33) | 447.03 (27) | 276.9 (16) | 169.01 (35) | 124.9 (12) | ||
| R7 | 1 8.97 | 1243.4 (10) | 1091.8 (5) | 939.4 (100) | 769.13 (45) | 617. 08 (20) | 447.05 (15) | 276.9 (8) | 169.8 (38) | 124.9 (10) | |
| R8 | 20.39 | 1395.6 (5) | 1243.4 (8) | 1091.8 (27) | 939.3 (33) | 769.12 (100) | 601.03 (16) | 447.03 (28) | 276.9 (7) | 169.8 (40) | 124.9 (10) |
| Substance | Δ(lgTCID50)max | IC50, μg/mL | CC50, μg/mL | SI |
|---|---|---|---|---|
| Rutan | 2.5 | 12.44 | 144.3 | 11.6 |
| R5 | 1.5 | 8.85 | 86.34 | 9.8 |
| R6 | 1.0 | >50 | 93.13 | <2 |
| R7 | 2.75 | 2.13 | 191.01 | 89.7 |
| R8 | 4.25 | 0.8 | 233 | 291.3 |
| Geraniin | 4.5 | 10.83 | 288 | 26 |
| Oseltamivir | 6.0 | 0.02 | >10 | >500 |
| Substance | Δ(lgTCID50)max | IC50, μg/mL | CC50, μg/mL | SI |
|---|---|---|---|---|
| Geraniin | 4.75 | 5.2 | 65.4 | 12.6 |
| Nirmatrelvir | 6.0 | 0.1 | >20 | >200 |
| Substance | IC50, μg/mL | CC50, μg/mL | SI |
|---|---|---|---|
| Geraniin | 1.6 | 41 | 25.6 |
| Azidothymidine | 0.002 | 3.6 | 1800 |
| Substance | CC50, μg/mL | HSV-1L2 | HSV-2BH | ||||
|---|---|---|---|---|---|---|---|
| IC50, μg/mL | IC95, μg/mL | SI | IC50, μg/mL | IC95, μg/mL | SI | ||
| Rutan | 172.16 | 6.25 | 12.5 | 28 | 12.5 | 25.0 | 14 |
| R5 | 187.71 | 12.5 | 25.0 | 15 | 12.5 | 25.0 | 15 |
| R6 | 69.62 | 25.0 | >25.0 | 3 | 31.25 | 50.0 | 2 |
| R7 | 165.00 | 5.95 | 8.32 | 28 | 8.32 | 31.25 | 20 |
| R8 | 106.00 | 10.0 | 31.25 | 11 | 31.25 | 62.5 | 3 |
| Geraniin | 65.92 | 1.20 | 1.56 | 55 | 0.39 | 6.25 | 169 |
| Foscarnet | >125 | 15.6 | 62.5 | >8 | 7.80 | 31.25 | >16 |
| Substance | CC50, μg/mL | Post-Inoculation Setup | Pre-Inoculation Setup | ||||
|---|---|---|---|---|---|---|---|
| Inhibition of Intracellular Replication | Inhibition of Infectious Virus Production | ||||||
| IC50, μg/mL | SI | IC50, μg/mL | SI | IC50, μg/mL | SI | ||
| Rutan | 170 | 5.9 | 28.8 | 4.0 | 42.5 | 3.4 | 50 |
| R5 | 150 | 7.4 | 20.5 | 6.4 | 23.4 | 2.2 | 68 |
| Ganciclovir | 185 | 0.22 | 840 | <0.1 | >1850 | No activity | |
| Substance | CC50, μg/mL | Inhibition on EBV Replication | |
|---|---|---|---|
| IC50, μg/mL | SI | ||
| Rutan | 63 | >1 | <63 |
| R5 | 85 | >10 | <8.5 |
| R6 | >20 | - | - |
| R7 | 33 | - | - |
| R8 | >20 | - | - |
| Geraniin | 63 | - | - |
| Ganciclovir | >1000 | 100 | >10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salikhov, S.I.; Oshchepkova, Y.I.; Ziyavitdinov, J.F.; Ashurov, J.M.; Berdiev, N.S.; Kolundin, M.S.; Gaidarov, A.O.; Turgiev, A.S.; Yurlov, K.I.; Larichev, V.F.; et al. Sumac Polyphenols as Pan-Herpesvirus Inhibitors. Int. J. Mol. Sci. 2025, 26, 10398. https://doi.org/10.3390/ijms262110398
Salikhov SI, Oshchepkova YI, Ziyavitdinov JF, Ashurov JM, Berdiev NS, Kolundin MS, Gaidarov AO, Turgiev AS, Yurlov KI, Larichev VF, et al. Sumac Polyphenols as Pan-Herpesvirus Inhibitors. International Journal of Molecular Sciences. 2025; 26(21):10398. https://doi.org/10.3390/ijms262110398
Chicago/Turabian StyleSalikhov, Shavkat I., Yuliya I. Oshchepkova, Jamolitdin F. Ziyavitdinov, Jamshid M. Ashurov, Nodir S. Berdiev, Mikhail S. Kolundin, Akhmed O. Gaidarov, Ali S. Turgiev, Kirill I. Yurlov, Victor F. Larichev, and et al. 2025. "Sumac Polyphenols as Pan-Herpesvirus Inhibitors" International Journal of Molecular Sciences 26, no. 21: 10398. https://doi.org/10.3390/ijms262110398
APA StyleSalikhov, S. I., Oshchepkova, Y. I., Ziyavitdinov, J. F., Ashurov, J. M., Berdiev, N. S., Kolundin, M. S., Gaidarov, A. O., Turgiev, A. S., Yurlov, K. I., Larichev, V. F., Fedyakina, I. T., Andronova, V. L., Fedorova, N. E., Kushch, A. A., Ivanov, A. V., & Karamov, E. V. (2025). Sumac Polyphenols as Pan-Herpesvirus Inhibitors. International Journal of Molecular Sciences, 26(21), 10398. https://doi.org/10.3390/ijms262110398







