Evaluation of 161Tb-Labeled Diphosphonates as Potential Bone-Targeting Agents
Abstract
1. Introduction
2. Results
2.1. Cyclic Voltammetry Measurements Results
2.2. Radiolabeling Yield
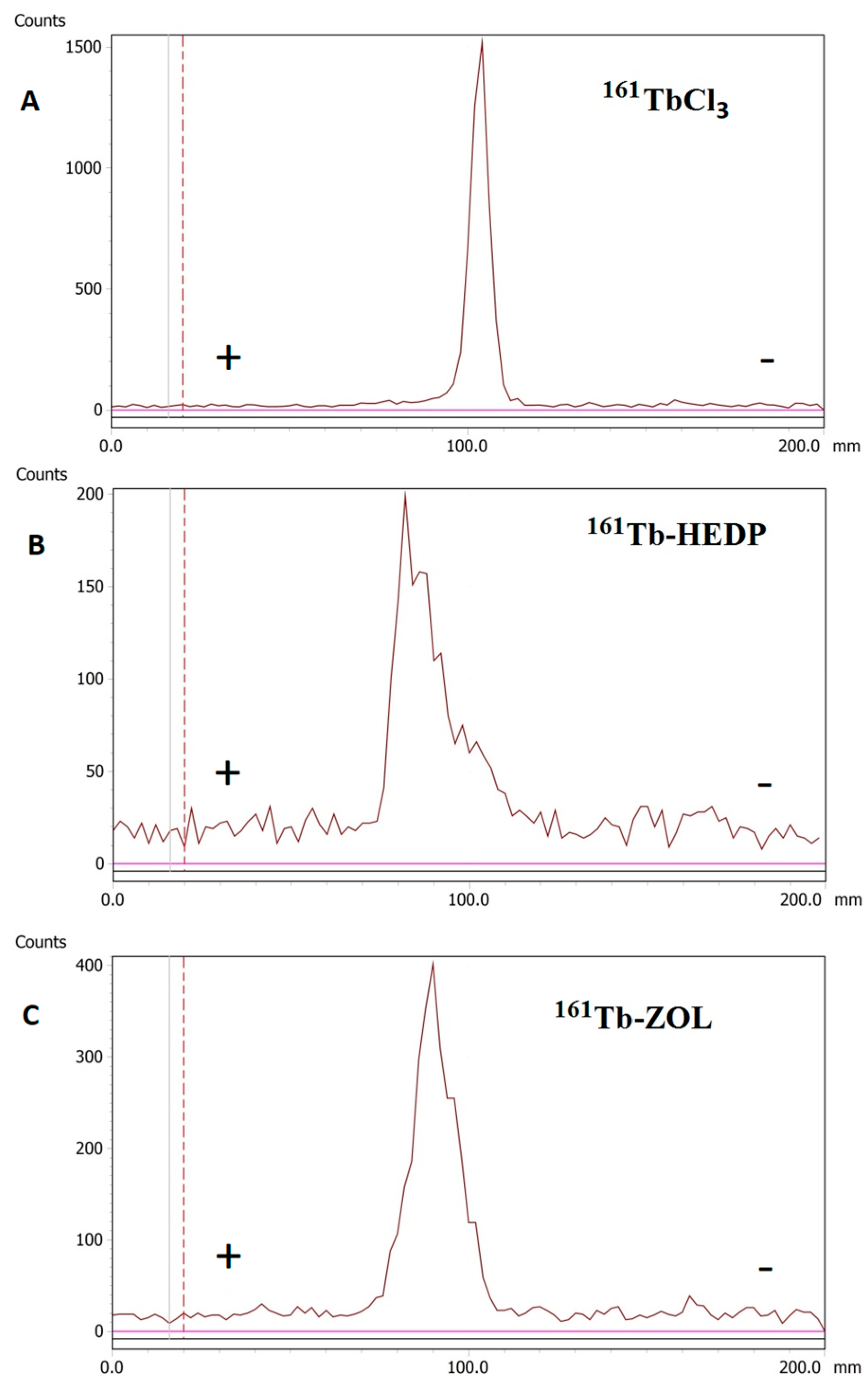
2.3. Radioelectrophoresis Profiles
2.4. Protein Binding and Lipophilicity
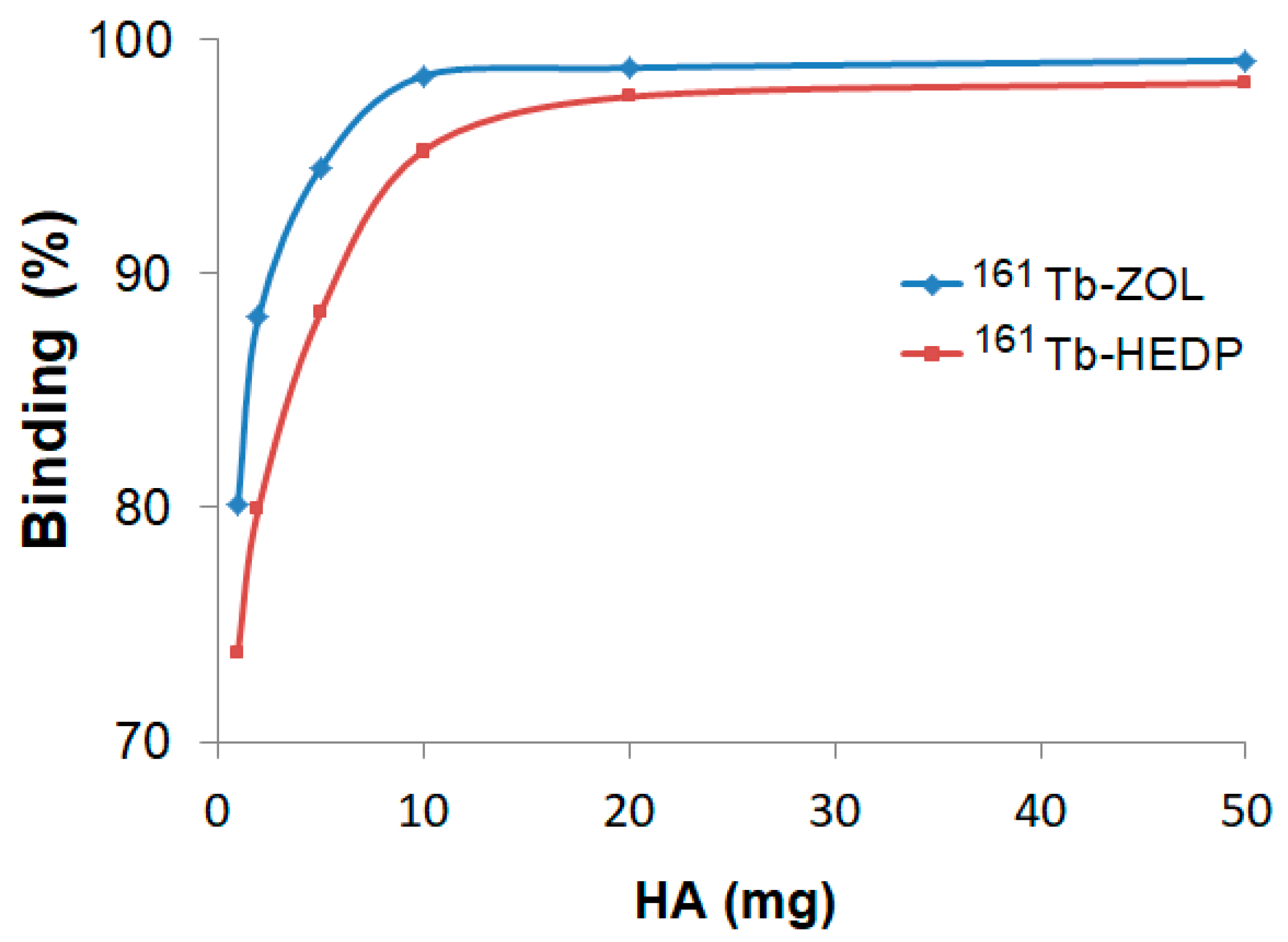
2.5. Hydroxyapatite Binding
2.6. In Vitro Stability
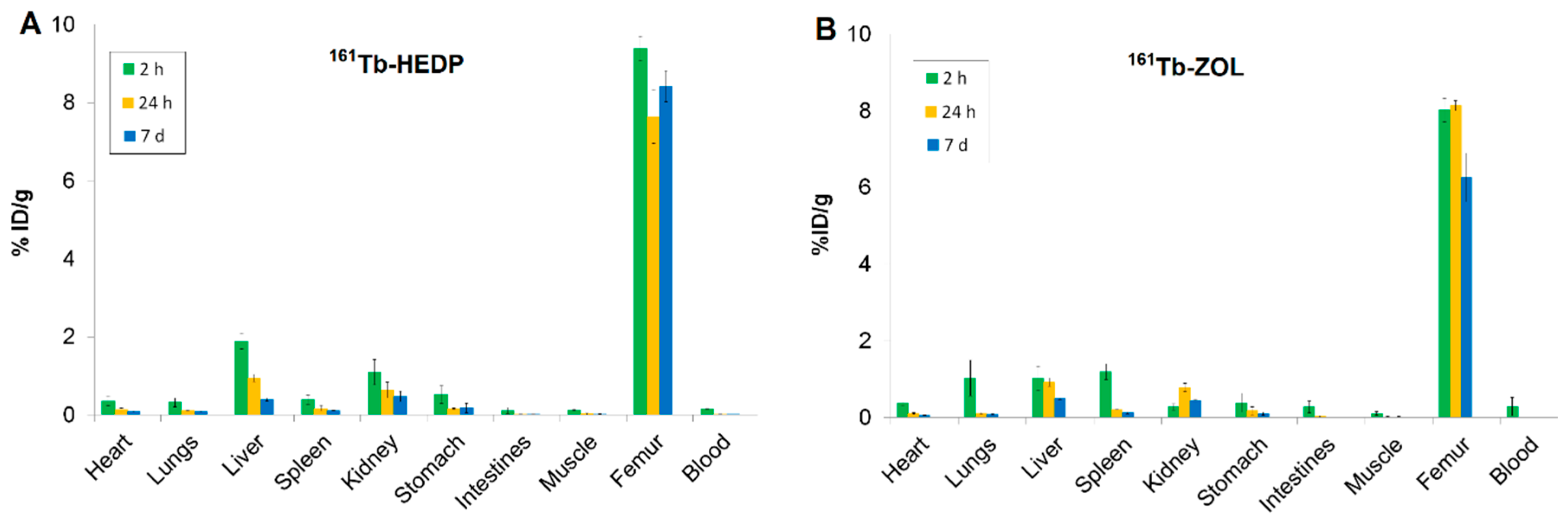
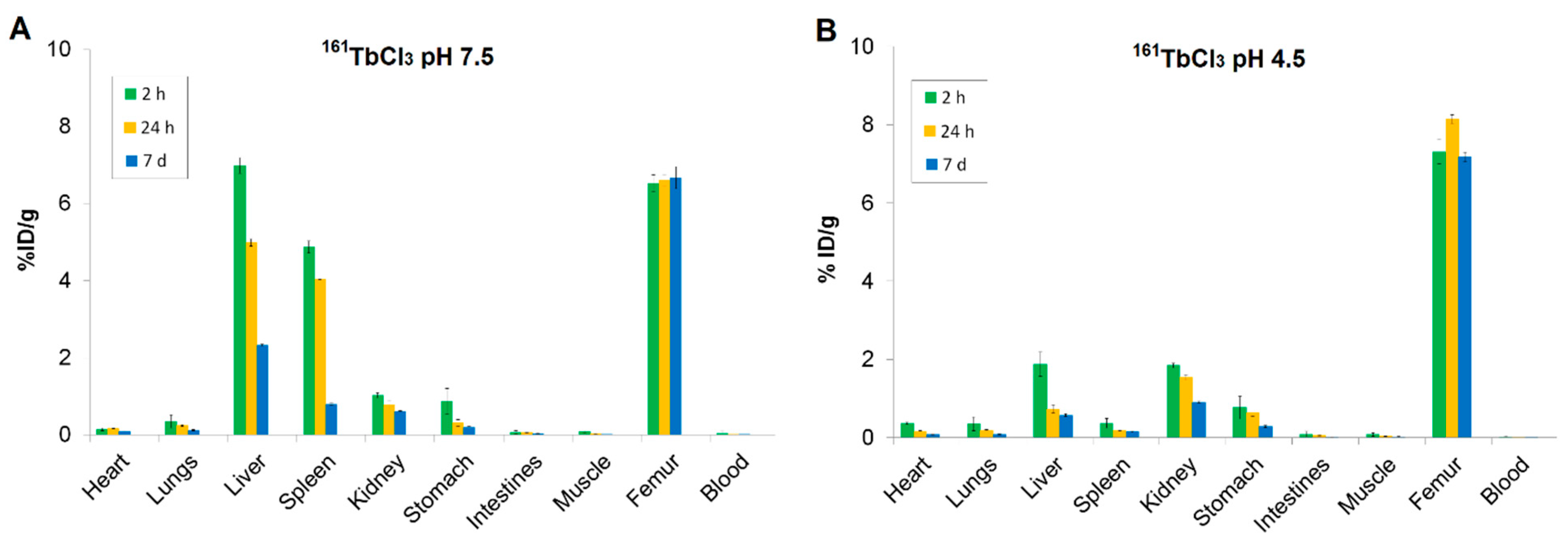
2.7. Biodistribution Profiles
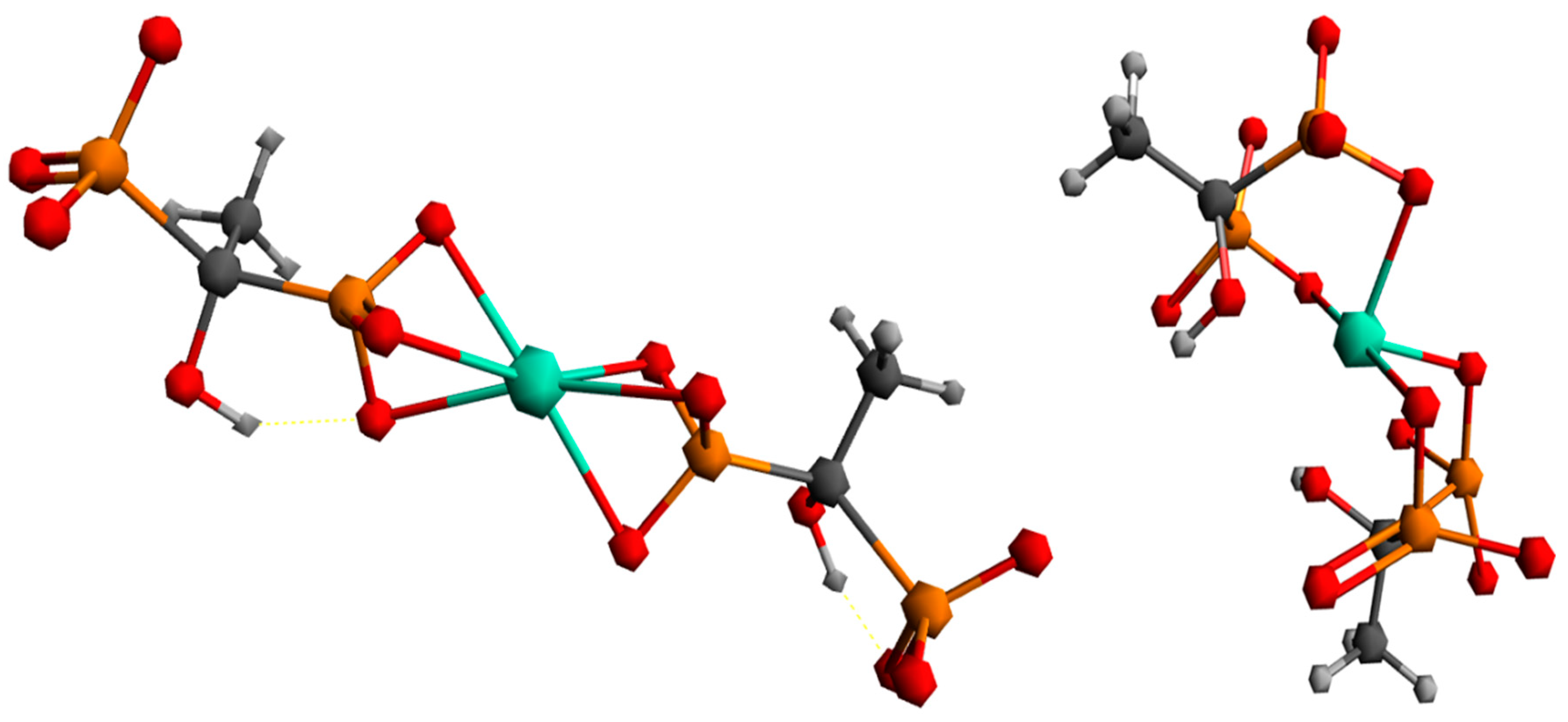
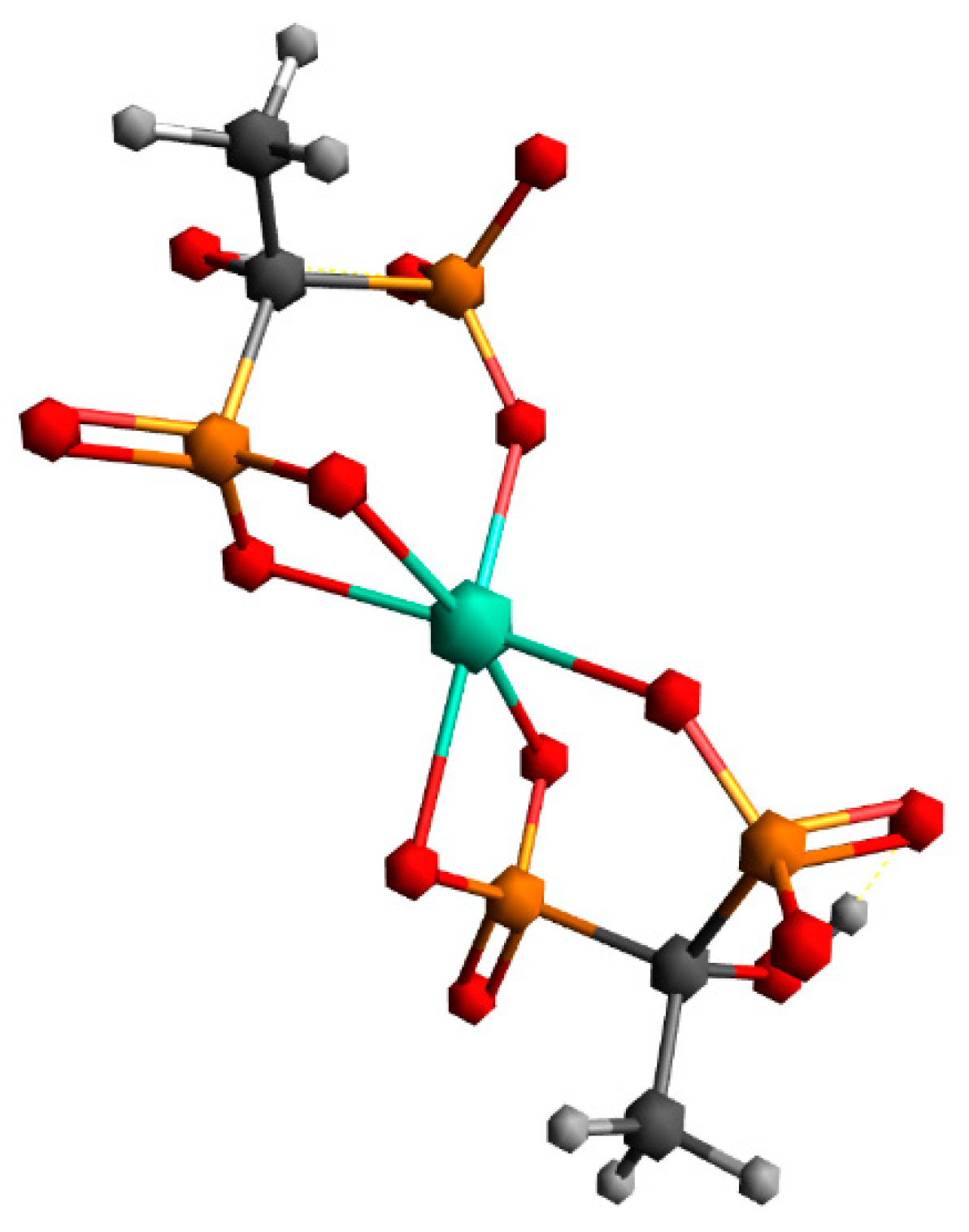
2.8. Results of DFT Calculations
3. Discussion
4. Materials and Methods
4.1. Reagents and Instruments
4.2. Evaluation of Complex Formation by Cyclic Voltammetry
4.3. Radiolabeling of Diphosphonates
4.4. Characterization of 161Tb-Diphosphonate Complexes
4.4.1. Assessment of Radiochemical Purity by Instant Thin-Layer Chromatography (ITLC)
4.4.2. Determination of Complex Charge by Radioelectrophoresis
4.4.3. Determination of Lipophilicity
4.4.4. Protein Binding Determination
4.4.5. Hydroxyapatite Binding Assay
4.5. In Vitro Stability Studies of 161Tb-Diphosphonate Complexes
4.6. Biodistribution Studies
4.7. Computational Details
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coleman, R.E. Clinical Features of Metastatic Bone Disease and Risk of Skeletal Morbidity. Clin. Cancer Res. 2006, 12 Pt 2, 6243s–6249s. [Google Scholar] [CrossRef]
- Silberstein, E.B. Systemic Radiopharmaceutical Therapy of Painful Osteoblastic Metastases. Semin. Radiat. Oncol. 2000, 10, 240–249. [Google Scholar] [CrossRef]
- Manafi-Farid, R.; Masoumi, F.; Divband, G.; Saidi, B.; Ataeinia, B.; Hertel, F.; Schweighofer-Zwink, G.; Morgenroth, A.; Beheshti, M. Targeted Palliative Radionuclide Therapy for Metastatic Bone Pain. J. Clin. Med. 2020, 9, 2622. [Google Scholar] [CrossRef]
- Fischer, M.; Kampen, W.U. Radionuclide Therapy of Bone Metastases. Breast Care 2012, 7, 100–107. [Google Scholar] [CrossRef]
- van Dodewaard-de Jong, J.M.; Bloemendal, H.; de Klerk, J.M.H.; Oprea-Lager, D.E.; Hoekstra, O.S.; van den Berg, H.P.; Los, M.; Beeker, A.; O’Sullivan, J.M.; Verheul, H.M.W.; et al. A Randomized, Phase II Study of Repeated Rhenium-188-HEDP (Rhenium) Combined with Docetaxel versus Docetaxel Alone in Castration Resistant Prostate Cancer (CRPC) Metastatic to Bone: The Taxium II Trial. J. Clin. Oncol. 2016, 34 (Suppl. S15), 5081. [Google Scholar] [CrossRef]
- Kreppel, B.; Gaertner, F.C.; Ahmadzadehfar, H.; Khawar, A.; Roesch, F.; Kürpig, S.; Meisenheimer, M.; Essler, M.; Bundschuh, R.A. Lu-DOTA-Zoledronate Therapy—First Application in a Patient with Primary Osseous Metastatic Bronchial Carcinoma. NuklearMedizin 2020, 59, 281–283. [Google Scholar] [CrossRef]
- Souche, C.; Fouillet, J.; Rubira, L.; Donzé, C.; Deshayes, E.; Fersing, C. Bisphosphonates as Radiopharmaceuticals: Spotlight on the Development and Clinical Use of DOTAZOL in Diagnostics and Palliative Radionuclide Therapy. Int. J. Mol. Sci. 2023, 25, 462. [Google Scholar] [CrossRef] [PubMed]
- Guerra Liberal, F.D.C.; Tavares, A.A.S.; Tavares, J.M.R.S. Comparative Analysis of 11 Different Radioisotopes for Palliative Treatment of Bone Metastases by Computational Methods. Med. Phys. 2014, 41, 114101. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.A.; Schäffer, E.H.; Linzner, U. Studies on Incorporated Short-Lived β-Emitters with Regard to the Induction of Late Effects. Radiat. Environ. Biophys. 1980, 18, 1–11. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, M.R.A.; Knapp, F.F. Lutetium-177 Therapeutic Radiopharmaceuticals: Linking Chemistry, Radiochemistry, and Practical Applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef] [PubMed]
- Mazzarri, S.; Guidoccio, F.; Mariani, G. The Emerging Potential of 177Lu-EDTMP: An Attractive Novel Option for Radiometabolic Therapy of Skeletal Metastases. Clin. Transl. Imaging 2015, 3, 167–168. [Google Scholar] [CrossRef]
- Shinto, A.S.; Shibu, D.; Kamaleshwaran, K.K.; Das, T.; Chakraborty, S.; Banerjee, S.; Thirumalaisamy, P.; Das, P.; Veersekar, G. 177Lu-EDTMP for Treatment of Bone Pain in Patients with Disseminated Skeletal Metastases. J. Nucl. Med. Technol. 2014, 42, 55–61. [Google Scholar] [CrossRef]
- Kálmán, F.K.; Király, R.; Brücher, E. Stability Constants and Dissociation Rates of the EDTMP Complexes of Samarium(III) and Yttrium(III). Eur. J. Inorg. Chem. 2008, 2008, 4719–4727. [Google Scholar] [CrossRef]
- Goeckeler, W.F.; Edwards, B.; Volkert, W.A.; Holmes, R.A.; Simon, J.; Wilson, D. Skeletal Localization of Samarium-153 Chelates: Potential Therapeutic Bone Agents. J. Nucl. Med. 1987, 28, 495–504. [Google Scholar]
- Palma, E.; Correia, J.D.G.; Campello, M.P.C.; Santos, I. Bisphosphonates as Radionuclide Carriers for Imaging or Systemic Therapy. Mol. Biosyst. 2011, 7, 2950–2966. [Google Scholar] [CrossRef] [PubMed]
- Fellner, M.; Riss, P.; Loktionova, N.; Zhernosekov, K.; Thews, O.; Geraldes, C.F.G.C.; Kovacs, Z.; Lukeš, I.; Rösch, F. Comparison of Different Phosphorus-Containing Ligands Complexing 68Ga for PET-Imaging of Bone Metabolism. Radiochim. Acta 2011, 99, 43–51. [Google Scholar] [CrossRef]
- Gralow, J.; Tripathy, D. Managing Metastatic Bone Pain: The Role of Bisphosphonates. J. Pain Symptom Manag. 2007, 33, 462–472. [Google Scholar] [CrossRef]
- Porta-Sales, J.; Garzón-Rodríguez, C.; Llorens-Torromé, S.; Brunelli, C.; Pigni, A.; Caraceni, A. Evidence on the Analgesic Role of Bisphosphonates and Denosumab in the Treatment of Pain Due to Bone Metastases: A Systematic Review within the European Association for Palliative Care Guidelines Project. Palliat. Med. 2017, 31, 5–25. [Google Scholar] [CrossRef]
- Pauwels, E.K.J.; Blom, J.; Camps, J.A.J.; Hermans, J.; Rijke, A.M. A Comparison between the Diagnostic Efficacy of 99mTc-MDP, 99mTc-DPD and 99mTc-HDP for the Detection of Bone Metastases. Eur. J. Nucl. Med. 1983, 8, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.M.; Fulton, R.; Constable, C.; Willowson, K.P.; Kench, P.L. Diagnostic Performance of Whole-Body SPECT/CT in Bone Metastasis Detection Using 99mTc-Labelled Diphosphate: A Systematic Review and Meta-Analysis. Clin. Radiol. 2020, 75, 961.e11–961.e24. [Google Scholar] [CrossRef]
- De Klerk, J.M.H.; Van Dijk, A.; Van het Schip, A.D.; Zonnenberg, B.A.; Van Rijk, P.P. Pharmacokinetics of Rhenium-186 After Administration of Rhenium-186-HEDP to Patients with Bone Metastases. J. Nucl. Med. 1992, 33, 646–651. [Google Scholar]
- Mirković, M.; Milanović, Z.; Stanković, D.; Petrović, Đ.; Vranješ-Đurić, S.; Janković, D.; Radović, M. Investigation of 177Lu-Labeled HEDP, DPD, and IDP as Potential Bone Pain Palliation Agents. J. Radiat. Res. Appl. Sci. 2020, 13, 27–36. [Google Scholar] [CrossRef]
- Nikzad, M.; Jalilian, A.R.; Shirvani-Arani, S.; Bahrami-Samani, A.; Golchoubian, H. Production, Quality Control and Pharmacokinetic Studies of 177Lu-Zoledronate for Bone Pain Palliation Therapy. J. Radioanal. Nucl. Chem. 2013, 298, 1273–1281. [Google Scholar] [CrossRef]
- Yousefnia, H.; Zolghadri, S.; Jalilian, A.R. Absorbed Dose Assessment of 177 Lu-Zoledronate and 177 Lu-EDTMP for Human Based on Biodistribution Data in Rats. J. Med. Phys. 2015, 40, 102–108. [Google Scholar] [CrossRef]
- Müller, C.; van der Meulen, N.P.; Schibli, R. Opportunities and Potential Challenges of Using Terbium-161 for Targeted Radionuclide Therapy in Clinics. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3181–3184. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Ávila, M.E.; Ferreira, A.; Quinto, M.A.; Morgat, C.; Hindié, E.; Champion, C. Radiation Doses from 161Tb and 177Lu in Single Tumour Cells and Micrometastases. EJNMMI Phys. 2020, 7, 33. [Google Scholar] [CrossRef]
- Van Laere, C.; Koole, M.; Deroose, C.M.; Van de Voorde, M.; Baete, K.; Cocolios, T.E.; Duchemin, C.; Ooms, M.; Cleeren, F. Terbium Radionuclides for Theranostic Applications in Nuclear Medicine: From Atom to Bedside. Theranostics 2024, 14, 1720–1743. [Google Scholar] [CrossRef] [PubMed]
- Trejtnar, F.; Bárta, P.; Kozempel, J.; Vlk, M.; Ďurinová, A.; Kuchařová, M.; Pávek, P. Terbium-161 in Nuclear Medicine: Preclinical and Clinical Progress in Comparison with Lutetium-177. Nucl. Med. Biol. 2025, 144–145, 108998. [Google Scholar] [CrossRef]
- Juget, F.; Talip, Z.; Buchillier, T.; Durán, M.T.; Nedjadi, Y.; Desorgher, L.; Bochud, F.; Grundler, P.; van der Meulen, N.P.; Bailat, C. Determination of the gamma and X-ray emission intensities of terbium-161. Appl. Radiat. Isot. 2021, 174, 109770. [Google Scholar] [CrossRef] [PubMed]
- Uygur, E.; Sezgin, C.; Parlak, Y.; Karatay, K.B.; Arikbasi, B.; Avcibasi, U.; Toklu, T.; Barutca, S.; Harmansah, C.; Sozen, T.S.; et al. The Radiolabeling of [161Tb]-PSMA-617 by a Novel Radiolabeling Method and Preclinical Evaluation by In Vitro/In Vivo Methods. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Lange, R.; ter Heine, R.; Knapp, R.F.; de Klerk, J.M.H.; Bloemendal, H.J.; Hendrikse, N.H. Pharmaceutical and Clinical Development of Phosphonate-Based Radiopharmaceuticals for the Targeted Treatment of Bone Metastases. Bone 2016, 91, 159–179. [Google Scholar] [CrossRef]
- Cassells, I.; Ahenkorah, S.; Burgoyne, A.R.; Van de Voorde, M.; Deroose, C.M.; Cardinaels, T.; Bormans, G.; Ooms, M.; Cleeren, F. Radiolabeling of Human Serum Albumin with Terbium-161 Using Mild Conditions and Evaluation of in Vivo Stability. Front. Med. 2021, 8, 675122. [Google Scholar] [CrossRef]
- Zhang, F.; Xue, J.; Shao, J.; Jia, L. Compilation of 222 Drugs’ Plasma Protein Binding Data and Guidance for Study Designs. Drug Discov. Today 2012, 17, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, K.; Álvarez, J.V.; Herreño, A.M.; Otero-Espinar, F.J.; Couce, M.L.; Alméciga-Díaz, C.J.; Tomatsu, S. Bone-Specific Drug Delivery for Osteoporosis and Rare Skeletal Disorders. Curr. Osteoporos. Rep. 2020, 18, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Piert, M. Dynamic Bone Imaging with 99mTc-Labeled Diphosphonates and 18F-NaF: Mechanisms and Applications. J. Nucl. Med. 2013, 54, 590–599. [Google Scholar] [CrossRef]
- Coffman, A.A.; Basta-Pljakic, J.; Guerra, R.M.; Ebetino, F.H.; Lundy, M.W.; Majeska, R.J.; Schaffler, M.B. A Bisphosphonate with a Low Hydroxyapatite Binding Affinity Prevents Bone Loss in Mice After Ovariectomy and Reverses Rapidly With Treatment Cessation. J. Bone Miner. Res. Plus 2021, 5, e10476. [Google Scholar] [CrossRef]
- Zielińska, M.; Pacholak, A.; Burlaga, N.; Chmielewska, E.; Voelkel, A.; Kaczorek, E. Determination of Bisphosphonate Properties in Terms of Bioavailability, Bone Affinity, and Cytotoxicity. Pharmacol. Rep. 2024, 76, 1160–1173. [Google Scholar] [CrossRef]
- Akbar, M.U.; Ahmad, M.R.; Shaheen, A.; Mushtaq, S. A Review on Evaluation of Technetium-99m Labeled Radiopharmaceuticals. J. Radioanal. Nucl. Chem. 2016, 310, 477–493. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, H.; Cai, W.; Qiao, L.; Ying, Y.; Li, W.; Yu, J.; Jiang, L.; Che, S. Reaction Mechanisms of Copper Electrodeposition from 1-Hydroxyethylidene-1,1-Diphosphonic Acid (HEDP) Solution on Glassy Carbon. Mater. Sci. Eng. B 2017, 224, 18–27. [Google Scholar] [CrossRef]
- Amro, A.; Ratrout, S.; Asfour, F. Voltametric Determination of Zoledronic Acid in a Pharmaceutical Formulation. Turk. J. Pharm. Sci. 2021, 18, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Pecequilo, C.V.; Panossian, Z. Study of Copper Electrodeposition Mechanism from a Strike Alkaline Bath Prepared with 1-Hydroxyethane-1,1-Diphosphonic Acid through Cyclic Voltammetry Technique. Electrochim. Acta 2010, 55, 3870–3875. [Google Scholar] [CrossRef]
- Nehra, K.; Dalal, A.; Hooda, A.; Saini, R.K.; Singh, D.; Kumar, S.; Malik, R.S.; Kumar, R.; Kumar, P. Synthesis of Green Emissive Terbium(III) Complexes for Displays: Optical, Electrochemical and Photoluminescent Analysis. Luminescence 2023, 38, 56–63. [Google Scholar] [CrossRef]
- Heller, A.; Senwitz, C.; Foerstendorf, H.; Tsushima, S.; Holtmann, L.; Drobot, B.; Kretzschmar, J. Europium(III) Meets Etidronic Acid (HEDP): A Coordination Study Combining Spectroscopic, Spectrometric, and Quantum Chemical Methods. Molecules 2023, 28, 4469. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, J.; Xue, F.; Wang, J.; Hu, W. Experimental and Computational Study of Zinc Coordinated 1-Hydroxyethylidene-1,1-Diphosphonic Acid Self-Assembled Film on Steel Surface. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126009. [Google Scholar] [CrossRef]
- Zeng, Y.; Sun, L.; Du, D.; He, X.; Shi, L. Lanthanide-Bisphosphonate Coordination Chemistry: Biocompatible Fluorescent Labeling Strategy for Hydrogel. ACS Appl. Bio Mater. 2021, 4, 1057–1064. [Google Scholar] [CrossRef]
- Mao, J.G. Structures and Luminescent Properties of Lanthanide Phosphonates. Coord. Chem. Rev. 2007, 251, 1493–1520. [Google Scholar] [CrossRef]
- Mirković, M.; Janković, D.; Vranješ-Durić, S.; Radović, M.; Stanković, D.; Mijin, D.; Nikolić, N. Novel Tetradentate Diamine Dioxime Ligands: Synthesis, Characterization and in Vivo Behavior of Their 99mTc-Complexes. Appl. Organomet. Chem. 2012, 26, 347–355. [Google Scholar] [CrossRef]
- Milanović, Z.; Janković, D.; Vranješ-Đurić, S.; Radović, M.; Prijović, Ž.; Zavišić, G.; Perić, M.; Stanković, D.; Mirković, M. 177Lu-Doxycycline as Potential Radiopharmaceutical: Electrochemical Characterization, Radiolabeling, and Biodistribution in Tumor-Bearing Mice. Int. J. Radiat. Biol. 2021, 97, 1687–1695. [Google Scholar] [CrossRef]
- Yousefnia, H.; Zolghadri, S.; Sadeghi, H.R.; Naderi, M.; Jalilian, A.R.; Shanehsazzadeh, S. Preparation and Biological Assessment of 177Lu-BPAMD as a High Potential Agent for Bone Pain Palliation Therapy: Comparison with 177Lu-EDTMP. J. Radioanal. Nucl. Chem. 2016, 307, 1243–1251. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 276, 33–79. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 20 October 2025).
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, D.A.; Neese, F. All-Electron Scalar Relativistic Basis Sets for the Lanthanides. J. Chem. Theory Comput. 2009, 5, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, D.A.; Chen, X.Y.; Landis, C.R.; Neese, F. All-Electron Scalar Relativistic Basis Sets for Third-Row Transition Metal Atoms. J. Chem. Theory Comput. 2008, 4, 908–919. [Google Scholar] [CrossRef]
- Pantazis, D.A.; Neese, F. All-Electron Scalar Relativistic Basis Sets for the 6p Elements. Theor. Chem. Acc. 2012, 131, 1292. [Google Scholar] [CrossRef]
- Pantazis, D.A.; Neese, F. All-Electron Scalar Relativistic Basis Sets for the Actinides. J. Chem. Theory Comput. 2011, 7, 677–684. [Google Scholar] [CrossRef]
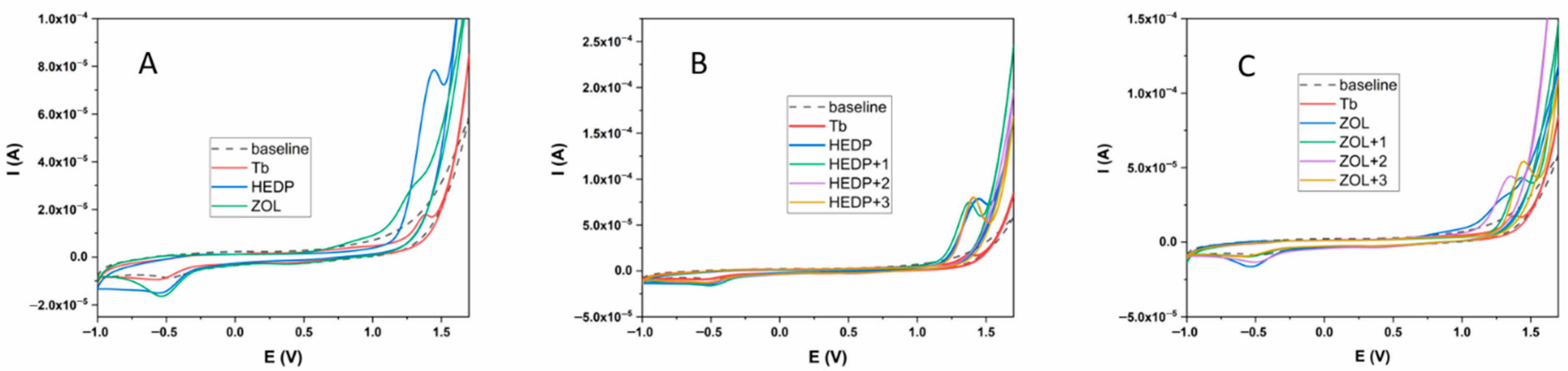

| First Oxidation Pot. (V) | Second Oxidation Pot. (V) | First Reduction Pot. (V) | Second Reduction Pot. (V) | |
|---|---|---|---|---|
| Tb | 1.39 | / | −0.56 | |
| HEDP | 1.45 | / | −0.54 | |
| ZOL | 0.93 | 1.27 | 0.41 | −0.54 |
| Condition | Time (h) | Radiochemical Purity (%) | |
|---|---|---|---|
| 161Tb-HEDP | 161Tb-ZOL | ||
| Room temperature | 0 | 98.64 ± 0.71% | 99.13 ± 1.12% |
| 24 | 97.11 ± 1.05% | 98.74 ± 0.85% | |
| 96 | 95.89 ± 0.94% | 97.53 ± 0.99% | |
| Saline, 37 °C | 24 | 96.78 ± 1.24% | 98.17 ± 0.62% |
| 96 | 95.42 ± 0.83% | 97.25 ± 0.74% | |
| Human serum, 37 °C | 24 | 96.09 ± 0.89% | 97.81 ± 0.96% |
| 96 | 94.85 ± 0.95% | 96.55 ± 0.88% | |
| Parameter | 161Tb-HEDP | 161Tb-ZOL | 161TbCl3 (pH 4.5) |
|---|---|---|---|
| Bone uptake (%ID/g) | 9.39 ± 0.30 | 8.02 ± 0.31 | 7.31 ± 0.32 |
| Blood (%ID/g) | 0.16 ± 0.01 | 0.29 ± 0.14 | 0.02 ± 0.01 |
| Muscle (%ID/g) | 0.12 ± 0.01 | 0.11 ± 0.05 | 0.08 ± 0.05 |
| Kidney (%ID/g) | 1.09 ± 0.33 | 0.28 ± 0.09 | 1.84 ± 0.07 |
| Liver (%ID/g) | 1.89 ± 0.20 | 1.03 ± 0.31 | 1.88 ± 0.31 |
| Other tissues | Minimal uptake | Minimal uptake | Minimal uptake |
| Bone-to-kidney ratio | 8.61 | 28.64 | 3.97 |
| Bone-to-liver ratio | 4.97 | 7.79 | 3.89 |
| Complex | 1 | 2 |
|---|---|---|
| Nuclear repulsion energy | 0 | 273,541.28 |
| Electronic energy | 0 | −273,600.12 |
| ΔEnuc + ΔEelec | 0 | −58.84 |
| Enthalpy | 0 | −57.87 |
| Electronic entropy | 0 | 0.00 |
| Vibrational entropy | 0 | −2.62 |
| Rotational entropy | 0 | −0.16 |
| Translational entropy | 0 | 0.00 |
| Total entropy change | 0 | −2.78 |
| Gibbs free enthalpy change | 0 | −55.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitarica, P.; Vukadinović, A.; Marić, M.; Vranješ-Đurić, S.; Stanković, D.; Perić, M.; Janković, D.; Stanković, D.; Mirković, M.; Radović, M. Evaluation of 161Tb-Labeled Diphosphonates as Potential Bone-Targeting Agents. Int. J. Mol. Sci. 2025, 26, 10392. https://doi.org/10.3390/ijms262110392
Sitarica P, Vukadinović A, Marić M, Vranješ-Đurić S, Stanković D, Perić M, Janković D, Stanković D, Mirković M, Radović M. Evaluation of 161Tb-Labeled Diphosphonates as Potential Bone-Targeting Agents. International Journal of Molecular Sciences. 2025; 26(21):10392. https://doi.org/10.3390/ijms262110392
Chicago/Turabian StyleSitarica, Pavle, Aleksandar Vukadinović, Miloš Marić, Sanja Vranješ-Đurić, Dalibor Stanković, Marko Perić, Drina Janković, Dragana Stanković, Marija Mirković, and Magdalena Radović. 2025. "Evaluation of 161Tb-Labeled Diphosphonates as Potential Bone-Targeting Agents" International Journal of Molecular Sciences 26, no. 21: 10392. https://doi.org/10.3390/ijms262110392
APA StyleSitarica, P., Vukadinović, A., Marić, M., Vranješ-Đurić, S., Stanković, D., Perić, M., Janković, D., Stanković, D., Mirković, M., & Radović, M. (2025). Evaluation of 161Tb-Labeled Diphosphonates as Potential Bone-Targeting Agents. International Journal of Molecular Sciences, 26(21), 10392. https://doi.org/10.3390/ijms262110392







