Features of Peripheral T Cell Remigration into the Thymus
Abstract
1. Introduction
2. The Thymus: Structure and Functions
3. Remigration of Peripheral T Cells into the Thymus
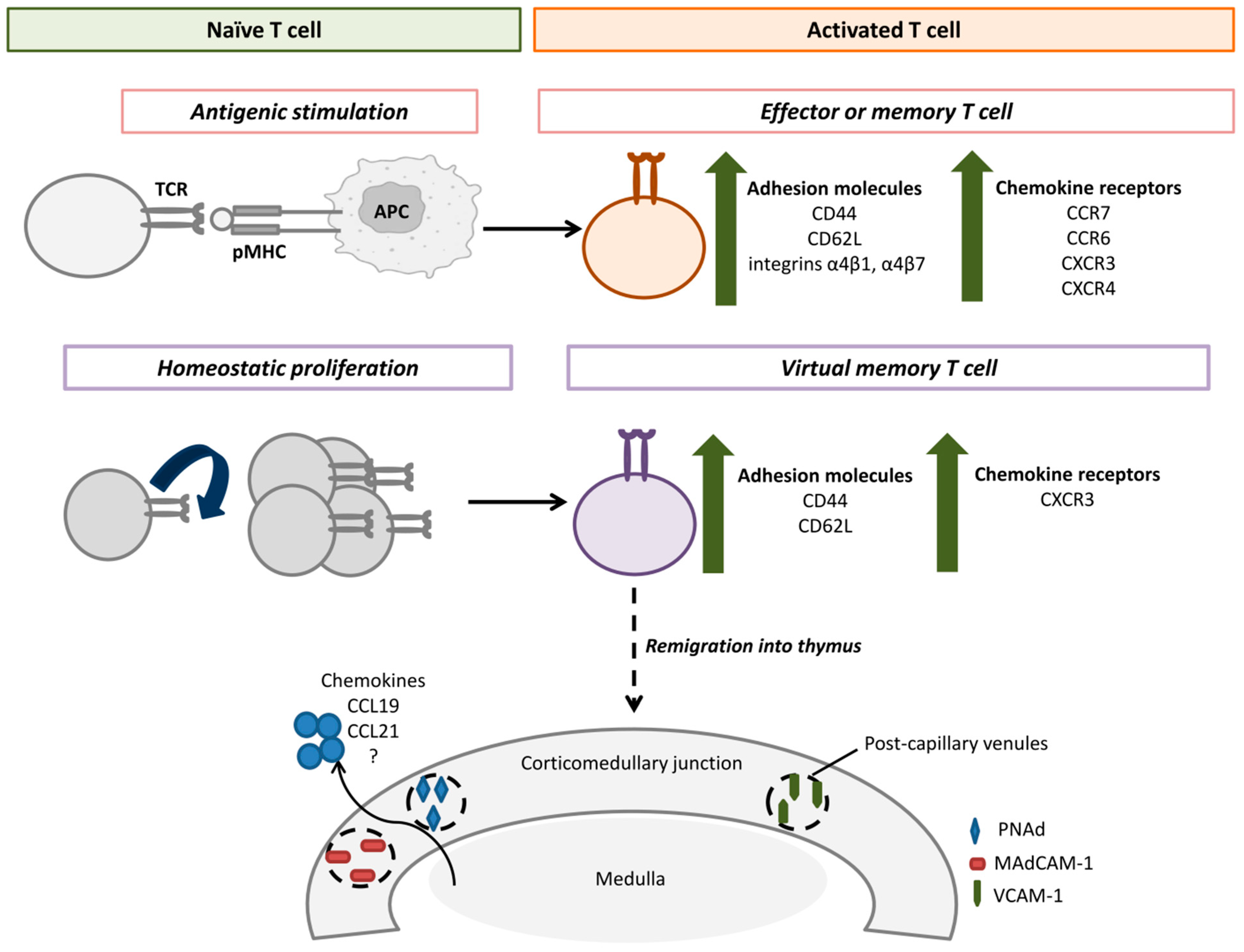
4. Features of Remigrating T Cells
5. The Pathways of Peripheral T Cell Remigration
6. Functions of Remigrated Mature T Cells in the Thymus
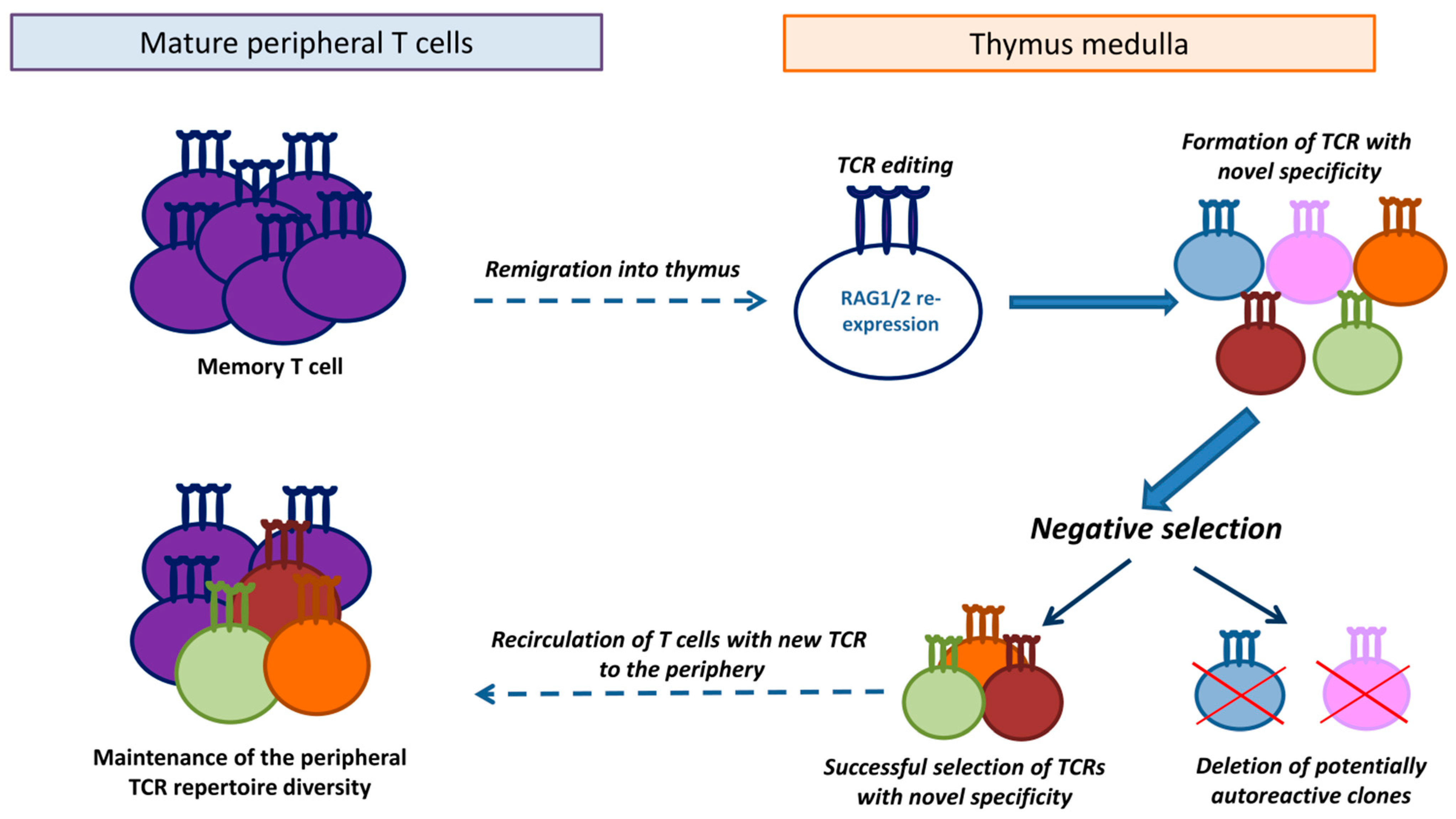
7. A Proposed Hypothesis of TCR Editing in Remigrated T Cells
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Miles, J.J.; Douek, D.C.; Price, D.A. Bias in the αβ T-cell repertoire: Implications for disease pathogenesis and vaccination. Immunol. Cell Biol. 2011, 89, 375–387. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Pérez, M.; Vandenabeele, P.; Tougaard, P. The thymus road to a T cell: Migration, selection, and atrophy. Front. Immunol. 2024, 15, 1443910. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Oschowitzer, A.; Kurzhals, S.R.; Krüger, C.C.; Pircher, H. Thymus-resident memory CD8+ T cells mediate local immunity. Eur. J. Immunol. 2013, 43, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, C.; Nunes-Alves, C.; Cerqueira-Rodrigues, B.; Roque, S.; Barreira-Silva, P.; Behar, S.M.; Correia-Neves, M. T cells home to the thymus and control infection. J. Immunol. 2013, 190, 1646–1658. [Google Scholar] [CrossRef]
- Gossmann, J.; Lohler, J.; Lehmann-Grube, F. Entry of antivirally active T lymphocytes into the thymus of virus-infected mice. J. Immunol. 1991, 146, 293–297. [Google Scholar] [CrossRef]
- Michie, S.A.; Rouse, R.V. Traffic of peripheral B and T lymphocytes to hyperplastic, preneoplastic thymuses of AKR mice. Am. J. Pathol. 1991, 138, 1015–1025. [Google Scholar]
- Levinson, A.I.; Song, D.; Gaulton, G.; Zheng, Y. The intrathymic pathogenesis of myasthenia gravis. Clin. Dev. Immunol. 2004, 11, 215–220. [Google Scholar] [CrossRef]
- Christo, S.N.; Park, S.L.; Mueller, S.N.; Mackay, L.K. The Multifaceted Role of Tissue-Resident Memory T Cells. Annu. Rev. Immunol. 2024, 42, 317–345. [Google Scholar] [CrossRef]
- Michie, S.A.; Kirkpatrick, E.A.; Rouse, R.V. Rare peripheral T cells migrate to and persist in normal mouse thymus. J. Exp. Med. 1988, 168, 1929–1934. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Liotta, F.; Lazzeri, E.; Romagnani, P.; Angeli, R.; Lasagni, L.; Manetti, R.; Marra, F.; Gerard, C.; et al. CXCR3 and alphaEbeta7 integrin identify a subset of CD8+ mature thymocytes that share phenotypic and functional properties with CD8+ gut intraepithelial lymphocytes. Gut 2006, 55, 961–968. [Google Scholar] [CrossRef]
- Cosway, E.J.; James, K.D.; Lucas, B.; Anderson, G.; White, A.J. The thymus medulla and its control of αβT cell development. Semin. Immunopathol. 2021, 43, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Weaver, C.; Berg, L. Janeway’s Immunobiology, 10th ed.; W. W. Norton & Company, Inc.: New York, NY, USA, 2022; p. 960. [Google Scholar]
- Dong, Y.; Guo, H.; Wang, D.; Tu, R.; Qing, G.; Liu, H. Genome-Wide Analysis Identifies Rag1 and Rag2 as Novel Notch1 Transcriptional Targets in Thymocytes. Front. Cell Dev. Biol. 2021, 9, 703338. [Google Scholar] [CrossRef] [PubMed]
- Kieback, E.; Hilgenberg, E.; Stervbo, U.; Lampropoulou, V.; Shen, P.; Bunse, M.; Jaimes, Y.; Boudinot, P.; Radbruch, A.; Klemm, U.; et al. Thymus-derived regulatory T cells are positively selected on natural self-antigen through cognate interactions of high functional avidity. Immunity 2016, 44, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- LeGuern, C.; Germana, S. On the elusive TCR specificity of thymic regulatory T cells. Am. J. Transplant. 2019, 19, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Thiele, D.; La Gruta, N.L.; Nguyen, A.; Hussain, T. Hiding in Plain Sight: Virtually Unrecognizable Memory Phenotype CD8+ T cells. Int. J. Mol. Sci. 2020, 21, 8626. [Google Scholar] [CrossRef]
- Miller, C.H.; Klawon, D.E.; Zeng, S.; Lee, V.; Socci, N.D.; Savage, P.A. Eomes identifies thymic precursors of self-specific memory-phenotype CD8+ T cells. Nat. Immunol. 2020, 21, 567–577. [Google Scholar] [CrossRef]
- Pobezinsky, L.A.; Pobezinskaya, E.L.; Grinenko, T.S.; Chervonskii, A.V.; Kazansky, D.B. Peripheral Pool of CD8+ T-Cells Contains Lymphocytes with Antigen-Specific Receptors That Recognize Syngeneic MHC Class II Molecules. Russ. J. Dev. Biol. 2004, 35, 142–147. [Google Scholar] [CrossRef]
- Pabst, R.; Binns, R.M.; Westermann, J. What is the function of peripheral lymphocytes migrating to the thymus and of B lymphocytes proliferating in the thymus? Thymus 1989, 13, 149–156. [Google Scholar]
- Pabst, R.; Binns, R.M. Heterogeneity of lymphocyte homing physiology: Several mechanisms operate in the control of migration to lymphoid and non-lymphoid organs in vivo. Immunol. Rev. 1989, 108, 83–109. [Google Scholar] [CrossRef]
- Binns, R.M.; Licence, S.T.; Whyte, A.; Wilby, M.; Rothkötter, H.J.; Bacon, M. Genetically determined CD45 variant of value in leucocyte tracing in vivo in the pig. Immunology 1995, 86, 25–33. [Google Scholar]
- Thiault, N.; Darrigues, J.; Adoue, V.; Gros, M.; Binet, B.; Perals, C.; Leobon, B.; Fazilleau, N.; Joffre, O.P.; Robey, E.A.; et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat. Immunol. 2015, 16, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Surh, C.D.; Sprent, J.; Webb, S.R. Exclusion of circulating T cells from the thymus does not apply in the neonatal period. J. Exp. Med. 1993, 177, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.R.; Sprent, J. Induction of neonatal tolerance to Mlsa antigens by CD8+ T cells. Science 1990, 248, 1643–1646. [Google Scholar] [CrossRef] [PubMed]
- Hale, J.S.; Boursalian, T.E.; Turk, G.L.; Fink, P.J. Thymic output in aged mice. Proc. Natl. Acad. Sci. USA 2006, 103, 8447–8452. [Google Scholar] [CrossRef]
- Agus, D.B.; Surh, C.D.; Sprent, J. Reentry of T cells to the adult thymus is restricted to activated T cells. J. Exp. Med. 1991, 173, 1039–1046. [Google Scholar] [CrossRef]
- Bell, E.B.; Sparshott, S.M.; Ager, A. Migration pathways of CD4 T cell subsets in vivo: The CD45RC− subset enters the thymus via α4 integrin-VCAM-1 interaction. Int. Immunol. 1995, 7, 1861–1871. [Google Scholar] [CrossRef]
- Westermann, J.; Smith, T.; Peters, U.; Tschernig, T.; Pabst, R.; Steinhoff, G.; Sparshott, S.M.; Bell, E.B. Both activated and nonactivated leukocytes from the periphery continuously enter the thymic medulla of adult rats: Phenotypes, sources, and magnitude of traffic. Eur. J. Immunol. 1996, 26, 1866–1874. [Google Scholar] [CrossRef]
- Gopinathan, R.; DePaz, H.A.; Oluwole, O.O.; Ali, A.O.; Garrovillo, M.; Engelstad, K.; Hardy, M.A.; Oluwole, S.F. Role of reentry of in vivo alloMHC peptide-activated T cells into the adult thymus in acquired systemic tolerance. Transplantation 2001, 72, 1533–1541. [Google Scholar] [CrossRef]
- Chau, L.A.; Rohekar, S.; Wang, J.J.; Lian, D.; Chakrabarti, S.; Zhang, L.; Zhong, R.; Madrenas, J. Thymic re-entry of mature activated T cells and increased negative selection in vascularized allograft recipients. Clin. Exp. Immunol. 2002, 127, 43–52. [Google Scholar] [CrossRef]
- Kazansky, D.B.; Vagida, M.S.; Silaeva, Y.Y.; Kalinina, A.A.; Zamkova, M.A.; Khromykh, L.M.; Persiyantseva, N.A.; Jolokhava, L.K. Functional capacity of memory cells CD8+ under lymphopenia induced by injection of hydrocortisone. Adv. Mol. Oncol. 2017, 4, 42–48. (In Russian) [Google Scholar] [CrossRef]
- Tian, C.; Bagley, J.; Iacomini, J. Homeostatic expansion permits T cells to re-enter the thymus and deliver antigen in a tolerogenic fashion. Am. J. Transplant. 2007, 7, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, S.L.; Marconi, P.; Brocker, T. Peripheral T cells re-enter the thymus and interfere with central tolerance induction. J. Immunol. 2011, 186, 5612–5619. [Google Scholar] [CrossRef] [PubMed]
- Kirberg, J.; Bosco, N.; Deloulme, J.C.; Ceredig, R.; Agenès, F. Peripheral T lymphocytes recirculating back into the thymus can mediate thymocyte positive selection. J. Immunol. 2008, 181, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.A.; Streeter, P.R.; Butcher, E.C.; Rouse, R.V. L-selectin and alpha 4 beta 7 integrin homing receptor pathways mediate peripheral lymphocyte traffic to AKR mouse hyperplastic thymus. Am. J. Pathol. 1995, 147, 412–421. [Google Scholar]
- Surh, C.D.; Ernst, B.; Sprent, J. Growth of epithelial cells in the thymic medulla is under the control of mature T cells. J. Exp. Med. 1992, 176, 611–616. [Google Scholar] [CrossRef]
- Bosco, N.; Agenes, F.; Rolink, A.G.; Ceredig, R. Peripheral T cell lymphopenia and concomitant enrichment in naturally arising regulatory T cells: The case of the pre-Tα gene-deleted mouse. J. Immunol. 2006, 177, 5014–5023. [Google Scholar] [CrossRef]
- Yang, E.; Zou, T.; Leichner, T.M.; Zhang, S.L.; Kambayashi, T. Both retention and recirculation contribute to long-lived regulatory T-cell accumulation in the thymus. Eur. J. Immunol. 2014, 44, 2712–2720. [Google Scholar] [CrossRef]
- Cowan, J.E.; McCarthy, N.I.; Anderson, G. CCR7 controls thymus recirculation, but not production and emigration, of Foxp3(+) T cells. Cell Rep. 2016, 14, 1041–1048. [Google Scholar] [CrossRef]
- Tian, C.; Bagley, J.; Forman, D.; Iacomini, J. Induction of central tolerance by mature T cells. J. Immunol. 2004, 173, 7217–7222. [Google Scholar] [CrossRef]
- Sprent, J.; Surh, C.D. Re-entry of mature T cells to the thymus: An epiphenomenon? Immunol. Cell Biol. 2009, 87, 46–49. [Google Scholar] [CrossRef]
- Dumont, R.J.; Barrois, R.; Jacobson, E.B. Migration of peripheral T and B cells into the thymus of aging (NZB × SJL)F1 (NS) female mice. Cell Immunol. 1984, 83, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Hale, J.S.; Fink, P.J. Back to the thymus: Peripheral T cells come home. Immunol. Cell Biol. 2009, 87, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Bosco, N.; Kirberg, J.; Ceredig, R.; Agenes, F. Peripheral T cells in the thymus: Have they just lost their way or do they do something? Immunol. Cell Biol. 2009, 87, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, C.C.; Peske, J.D.; Engelhard, V.H. Peripheral tissue homing receptor control of naïve, effector, and memory CD8 T cell localization in lymphoid and non-lymphoid tissues. Front. Immunol. 2013, 4, 241. [Google Scholar] [CrossRef]
- Hardy, C.L.; Godfrey, D.I.; Scollay, R. The effect of antigen stimulation on the migration of mature T cells from the peripheral lymphoid tissues to the thymus. Dev. Immunol. 2001, 8, 123–131. [Google Scholar]
- Martin, M.D.; Badovinac, V.P. Defining Memory CD8 T Cell. Front. Immunol. 2018, 9, 2692. [Google Scholar] [CrossRef]
- Kawabe, T.; Sher, A. Memory-phenotype CD4+ T cells: A naturally arising T lymphocyte population possessing innate immune function. Int. Immunol. 2022, 34, 189–196. [Google Scholar] [CrossRef]
- Pribikova, M.; Moudra, A.; Stepanek, O. Opinion: Virtual memory CD8 T cells and lymphopenia-induced memory CD8 T cells represent a single subset: Homeostatic memory T cells. Immunol. Lett. 2018, 203, 57–61. [Google Scholar] [CrossRef]
- Rodger, B.; Stagg, A.J.; Lindsay, J.O. The role of circulating T cells with a tissue resident phenotype (ex-TRM) in health and disease. Front. Immunol. 2024, 15, 1415914. [Google Scholar] [CrossRef]
- Choi, H.; Song, H.; Jung, Y.W. The Roles of CCR7 for the Homing of Memory CD8+ T Cells into Their Survival Niches. Immune Netw. 2020, 20, e20. [Google Scholar] [CrossRef]
- Campbell, D.J. Control of Regulatory T Cell Migration, Function, and Homeostasis. J. Immunol. 2015, 195, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y. Suppression of the Immune Response by Syngeneic Splenocytes Adoptively Transferred to Sublethally Irradiated Mice. Acta Naturae 2021, 13, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Park, H.J.; Boo, H.J.; Jung, K.C.; Lee, J.I. Characterization of CD8+ virtual memory T cells in IL-4 knockout mice using single-cell RNA sequencing. Biochem. Biophys. Res. Commun. 2024, 738, 150950. [Google Scholar] [CrossRef] [PubMed]
- Naparstek, Y.; Holoshitz, J.; Eisenstein, S.; Reshef, T.; Rappaport, S.; Chemke, J.; Ben-Nun, A.; Cohen, I.R. Effector T lymphocyte line cells migrate to the thymus and persist there. Nature 1982, 300, 262–264. [Google Scholar] [CrossRef]
- Fink, P.J.; Bevan, M.J.; Weissman, I.L. Thymic cytotoxic T lymphocytes are primed in vivo to minor histocompatibility antigens. J. Exp. Med. 1984, 159, 436–451. [Google Scholar] [CrossRef]
- García-León, M.J.; Mosquera, M.; Cela, C.; Alcain, J.; Zuklys, S.; Holländer, G.; Toribio, M.L. Abrogation of Notch Signaling in Embryonic TECs Impacts Postnatal mTEC Homeostasis and Thymic Involution. Front. Immunol. 2022, 13, 867302. [Google Scholar] [CrossRef]
- Baran-Gale, J.; Morgan, M.D.; Maio, S.; Dhalla, F.; Calvo-Asensio, I.; Deadman, M.E.; Handel, A.E.; Maynard, A.; Chen, S.; Green, F.; et al. Ageing compromises mouse thymus function and remodels epithelial cell differentiation. Elife 2020, 9, e56221. [Google Scholar] [CrossRef]
- Srinivasan, J.; Lancaster, J.N.; Singarapu, N.; Hale, L.P.; Ehrlich, L.I.; Richie, E.R. Age-Related Changes in Thymic Central Tolerance. Front. Immunol. 2021, 12, 676236. [Google Scholar] [CrossRef]
- Kazansky, D.B.; Kalinina, A.A.; Khromykh, L.M. Memory T Cells: Investigation of Original Models with Transgenic T Cell Receptors. Biochemistry 2025, 90, 161–172. [Google Scholar] [CrossRef]
| Animals | Experimental Setup | Main Findings | Reference |
|---|---|---|---|
| Mice | LCMV model | Virus-specific T cells were activated on the periphery and migrated into the thymus medulla, where they persisted over one year and possessed features of resident memory cells | [4] |
| Mice | Mycobacterial infection (M. tuberculosis and M. avium) | Activated on the periphery mature antigen-specific T cells migrated into the thymus as the site of infection | [5] |
| Mice | Adoptive transfer of syngeneic CD8+ T cells specific to LCMV antigens | Activated virus-specific donor CD8+ T cells were detected in the thymus medulla of LCMV-infected recipients | [6] |
| Mice | Adoptive transfer of CFSE-labeled CD4+ T cells specific to an influenza hemagglutinin peptide into mice that were preliminarily immunized with β-galactosidase (β-gal) and intrathymically injected with β-gal-encoded MLV-based vectors | Induced inflammation in the thymus medulla facilitated remigration of mature CD4+ T cells with unrelated specificities. Remigrated peripheral CD4+ T cells that escaped the negative selection could contribute to the intrathymic pathogenesis of myasthenia gravis | [8] |
| Thy-1 congenic mice | Adoptive transfer of lymphocytes from the lymph nodes | Mature donor T cells accumulated in the medulla of the recipient’s thymus and possessed features of resident memory cells | [10] |
| Pigs | Adoptive transfer of CD45-congenic lymphocytes | Donor T cells accumulated predominantly in the corticomedullary junction of the thymus 4 h following the adoptive transfer | [22] |
| Rag-GFPFoxp3-Thy-1.1 transgenic mice | Migration in situ of peripheral Tregs. Adoptive transfer of in vitro-activated Tregs from Foxp3-Thy-1.1 transgenic mice into wild type recipients | The intensity of accumulation of mature peripheral GFP− Tregs in the thymus grew with age. Remigrated Tregs suppressed interleukin 2-dependent de novo differentiation of Tregs in the thymus | [23] |
| Thy-1 congenic mice | Adoptive transfer of syngeneic lymphocytes from lymph nodes into 1-day- and 12-week-old recipients | Mature naïve T cells cannot remigrate into the thymus of adult mice (12-week-old) but actively accumulate in the medulla of newborns | [24] |
| Mouse lines with differences in MMTV superantigens | Adoptive transfer of spleen T cells | Mature T cells remigrated into the thymus of newborn mice and mediated the formation of central tolerance to Mlsa superantigens | [25] |
| Rag2p-GFP transgenic mice | Migration in situ of peripheral T cells. Adoptive transfer of syngeneic T cells into Rag2p-GFP mice | Activated T cells can remigrate into the thymus of young mice. Mature T cells can remigrate into the thymus of aged mice without preliminary activation on the periphery | [26] |
| Thy-1 congenic mice | Adoptive transfer of naïve T cells and activated T blasts into irradiated and non-irradiated recipients | Naïve donor T cells rarely migrated into the recipient’s thymus. Irradiation or injections of hydrocortisone into the recipient did not stimulate migration of naïve donor T cells into the recipient’s thymus. Activated T cells actively migrated into the thymus and persisted in the medulla for one month. Irradiation of the recipient before the adoptive transfer enhanced migration of donor activated T cells into the recipient’s thymus | [27] |
| Rats | Adoptive transfer of syngeneic T cells | Predominant remigration of T cells with the phenotype of activated effectors. Remigration of peripheral T cells into the thymus is mediated by interactions of integrin α4 and VCAM-1 | [28] |
| Rats | Adoptive transfer of syngeneic T cells from the spleen and lungs | Predominant migration of antigen-primed CD4+ T cells into the thymus medulla | [29] |
| Rats | Adoptive transfer of radioactively labeled syngeneic alloreactive T cells | Activated T cells migrated into the thymus and were engaged in the induction of central tolerance | [30] |
| Mice with allogeneic heart transplant | Migration in situ of peripheral T cells in the recipient with allotransplant. Adoptive transfer of CFSE-labeled T cells into the recipient with allotransplant | Activated alloreactive T cells migrated only into the thymus of recipients with allotransplants. Remigrated alloreactive T cells died by apoptosis in the thymus medulla | [31] |
| Mice | Immunization of C57BL/6 (H-2b) with allogeneic mastocytoma P815 (H-2d) | Alloreactive memory T cells were detected in the thymus of immunized mice, responded in the secondary immune response in vitro, and were hydrocortisone-resistant | [32] |
| Mice | Adoptive transfer of allogeneic T cells into lethally irradiated recipients | Mature donor T cells could migrate into the recipient’s thymus only after homeostatic proliferation on the periphery. Migrated T cells were engaged in the formation of central tolerance | [33] |
| Mice | Adoptive transfer of syngeneic OT-I CD8+ T cells into C57BL/6 or RIP-mOVA transgenic mice | Remigration of peripheral T cells was enhanced after homeostatic proliferation under lymphopenia. Donor OT-1 T cells eliminated dendritic cells and medullary epithelial cells that express the OVA peptide in the recipient RIP-mOVA thymus. Consequently, autoreactive OVA-specific CD8+ T cells were generated in RIP-mOVA recipients | [34] |
| Mice | Adoptive transfer of H-2Kb+ T cells into H-2Kb− OT-1 transgenic mice | Donor T cells were located in the medulla and, to a lesser extent, the cortex of the recipient’s thymus and participated in positive selection of developing thymocytes by directly presenting the alloantigen (the H-2Kb molecule) | [35] |
| AKR mice | Adoptive transfer of syngeneic lymphocytes from lymph nodes | Enhanced migration of peripheral T cells into the hyperplastic thymus of AKR mice via interactions of the homing receptors L-selectin and integrin α4β7 with addressins PNAd and MAdCAM-1, respectively | [36] |
| Thy-1 congenic mice | Adoptive transfer of mature T cells into SCID mice | Peripheral T cells, including with the phenotype of naïve cells, actively migrated into the thymus of adult SCID mice. Donor T cells accumulated in the medulla of the SCID thymus and regulated the functions of medullary epithelial cells | [37] |
| Mice | Parabiosis between C57BL/6-GFP and pTα knockout mice and between wild type Ly5.1+ mice and Ly5.2+ pTα knockout mice | Donor CD4+ and CD8+ T cells accumulated in the thymus of lymphopenic pTα knockout mice | [38] |
| CD45 congenic mice | Adoptive transfer of CD45.1+ CD4+ T cells into irradiated CD45.2+ wild type mice or non-irradiated CD45.2+ RAG1 knockout mice | Donor Tregs were detected in the thymus of adult recipients. The numbers of donor Tregs in the thymus positively correlated with their numbers in the recipient’s spleen | [39] |
| Rag2p-GFP and Rag2pGFP/ Foxp3RFP transgenic mice | Migration in situ of peripheral Tregs | Peripheral GFP− Tregs accumulated in the thymus and had the phenotype CCR7−CCR6+. CCR7 prevented remigration of Tregs into the thymus | [40] |
| Mice | Adoptive transfer of allogeneic T cells into lethally irradiated recipients | Activated donor T cells migrated into the thymus of irradiated recipients and contributed to the induction of central tolerance | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinina, A.A.; Khromykh, L.M.; Kazansky, D.B. Features of Peripheral T Cell Remigration into the Thymus. Int. J. Mol. Sci. 2025, 26, 10391. https://doi.org/10.3390/ijms262110391
Kalinina AA, Khromykh LM, Kazansky DB. Features of Peripheral T Cell Remigration into the Thymus. International Journal of Molecular Sciences. 2025; 26(21):10391. https://doi.org/10.3390/ijms262110391
Chicago/Turabian StyleKalinina, Anastasiia A., Ludmila M. Khromykh, and Dmitry B. Kazansky. 2025. "Features of Peripheral T Cell Remigration into the Thymus" International Journal of Molecular Sciences 26, no. 21: 10391. https://doi.org/10.3390/ijms262110391
APA StyleKalinina, A. A., Khromykh, L. M., & Kazansky, D. B. (2025). Features of Peripheral T Cell Remigration into the Thymus. International Journal of Molecular Sciences, 26(21), 10391. https://doi.org/10.3390/ijms262110391





