STK38 Kinase Promotes Cell Migration Induced by Oncogenic Ras via MerTK Activation
Abstract
1. Introduction
2. Results
2.1. STK38 Interacts with MerTK
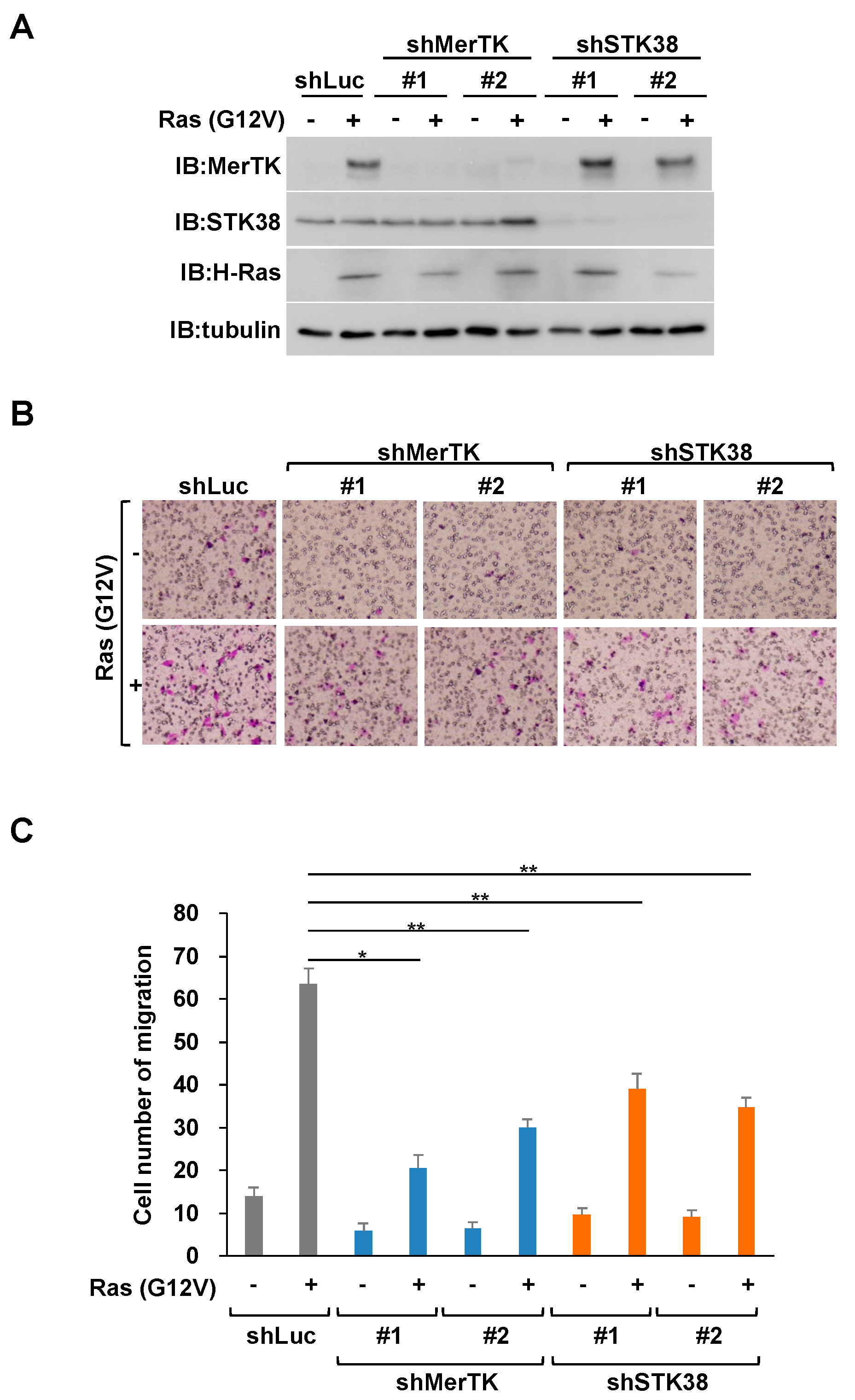
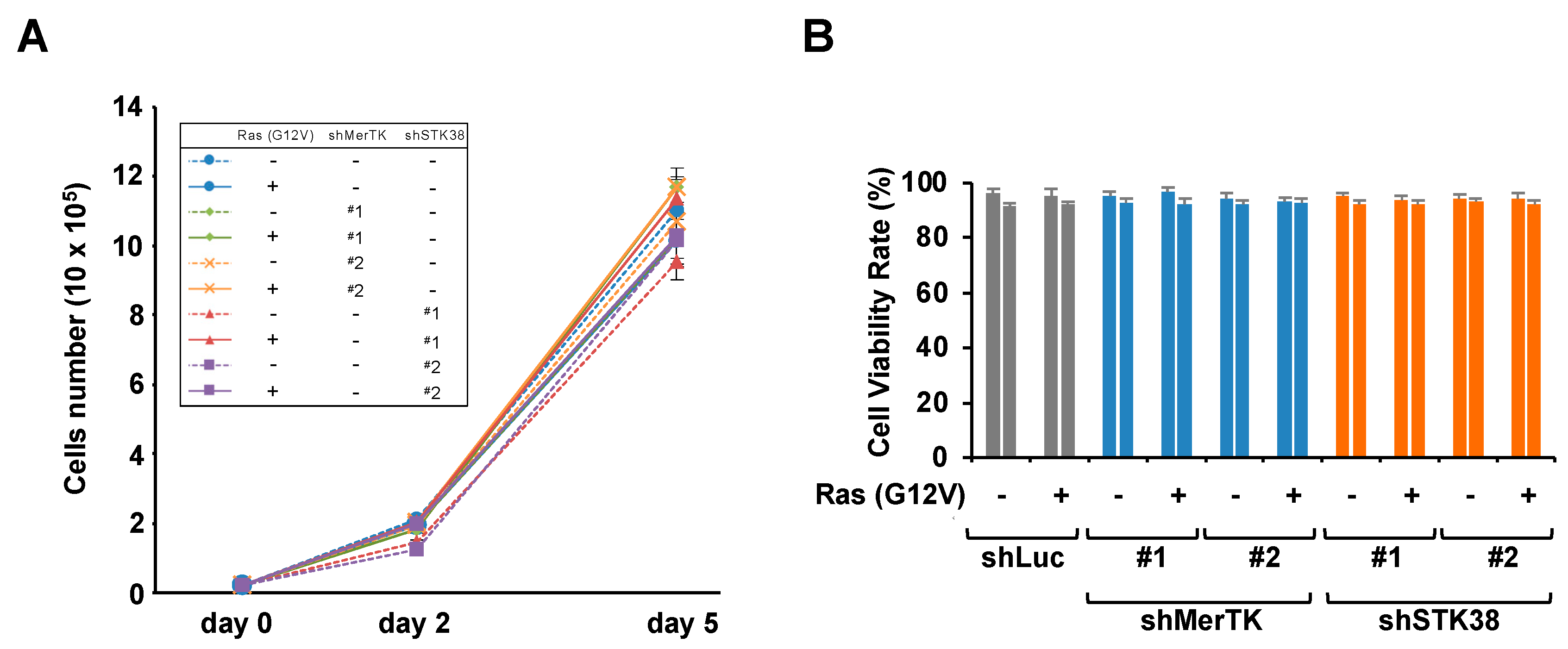
2.2. STK38 Contributes to Ras (G12V)-Induced Cell Migration
2.3. STK38 Kinase Activity Is Necessary for STK38-Mediated Enhancement of Ras (G12V)-Induced Cell Migration
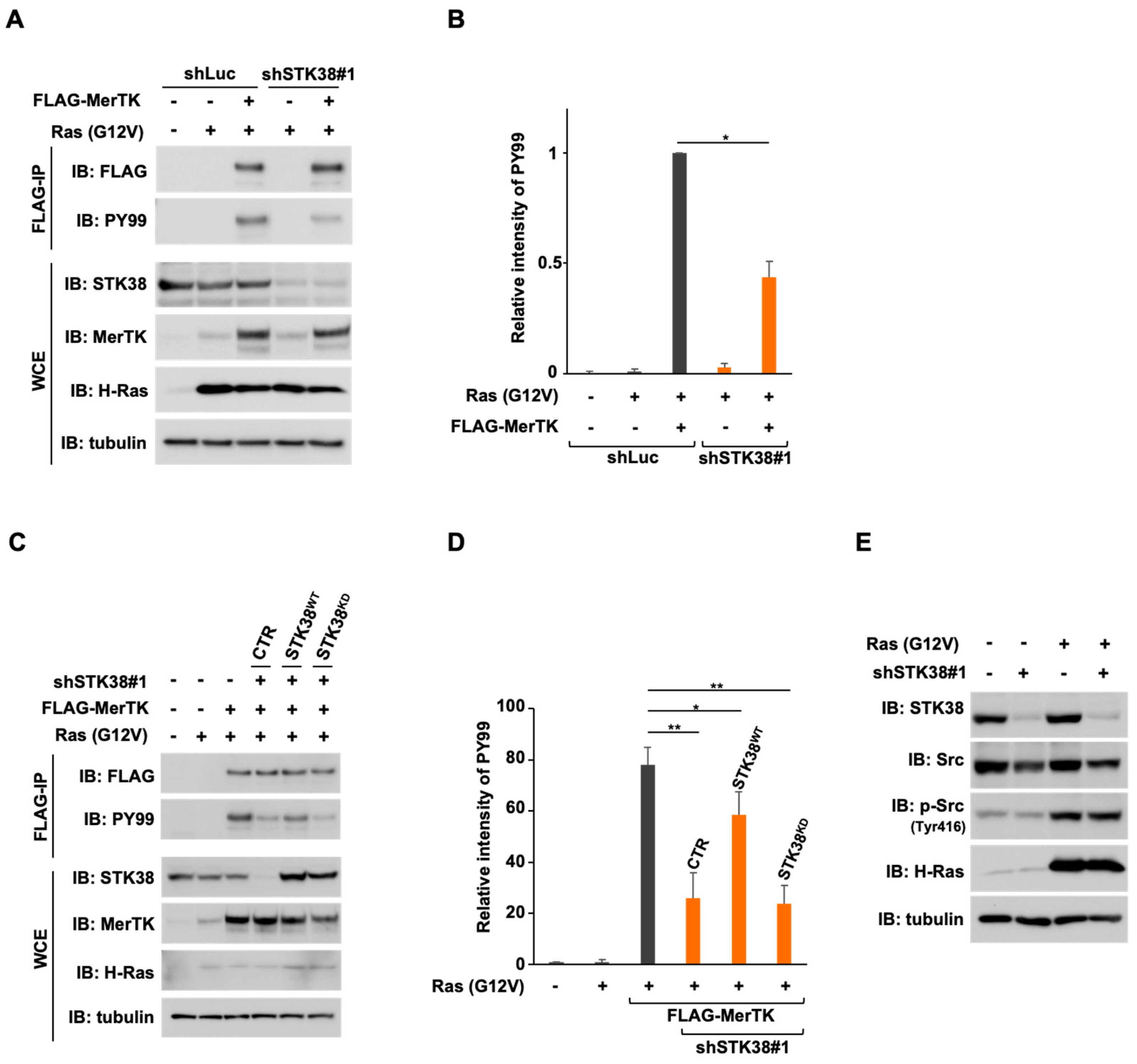
2.4. STK38 Promotes the Tyrosine Phosphorylation of MerTK Depending on Its Kinase Activity
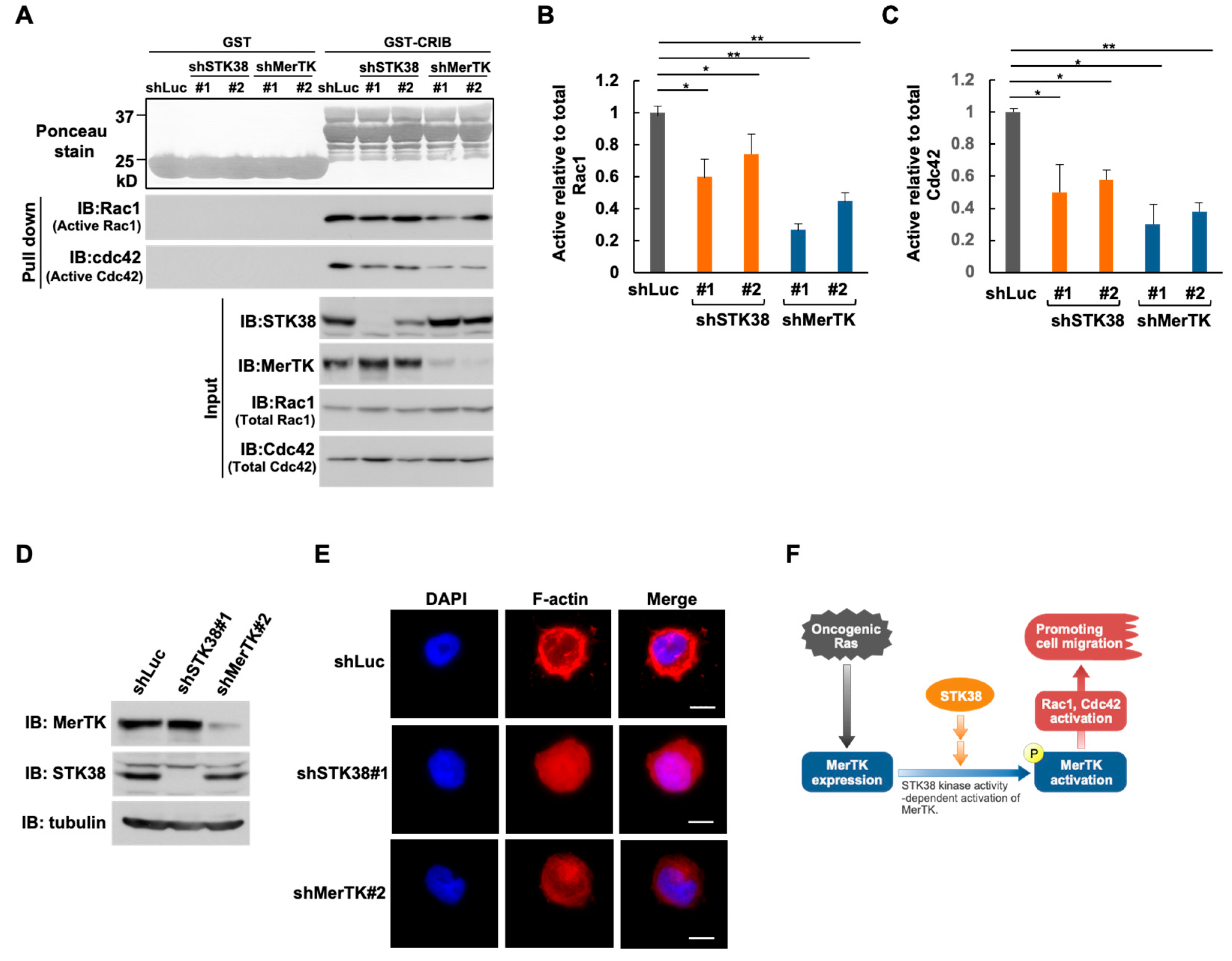
2.5. MerTK and STK38 Promote Rac1 and Cdc42 Activation
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Plasmid Constructs
4.3. Retroviral Gene Expression
4.4. Tandem Affinity Purification of Tagged MerTK Protein and Identification of MerTK-Interacting Proteins
4.5. Cellular Fractionation
4.6. Immunoblotting
4.7. Immunoprecipitation
4.8. RNA Interference for MerTK and STK38
4.9. Immunofluorescence Analysis
4.10. Migration Assay
4.11. Measurement of Cell Proliferation and Cell Viability
4.12. In Vitro Pulldown Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karnoub, A.E.; Weinberg, R.A. Ras oncogenes: Split personalities. Nat. Rev. Mol. Cell Biol. 2008, 9, 517–531. [Google Scholar] [CrossRef]
- Cascone, I.; Selimoglu, R.; Ozdemir, C.; Del, N.E.; Yeaman, C.; White, M.; Camonis, J. Distinct roles of RalA and RalB in the progression of cytokinesis are supported by distinct RalGEFs. EMBO J. 2008, 27, 2375–2387. [Google Scholar] [CrossRef] [PubMed]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death—Apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]
- Buday, L.; Downward, J. Many faces of Ras activation. Biochim. Biophys. Acta Rev. Cancer 2008, 1786, 178–187. [Google Scholar] [CrossRef]
- Spencer-Smith, R.; O’Bryan, J.P. Direct inhibition of RAS: Quest for the Holy Grail? Semin. Cancer Biol. 2019, 54, 138–148. [Google Scholar] [CrossRef]
- Graham, D.K.; Deryckere, D.; Davies, K.D.; Earp, H.S. The TAM family: Phosphatidylserine-sensing receptor tyrosine kinases gone awry in cancer. Nat. Rev. Cancer 2014, 14, 769–785. [Google Scholar] [CrossRef]
- Xie, S.; Li, Y.; Li, X.; Wang, L.; Yang, N.; Wang, Y.; Wei, H. Mer receptor tyrosine kinase is frequently overexpressed in human non-small cell lung cancer, confirming resistance to erlotinib. Oncotarget 2015, 6, 9206–9219. [Google Scholar] [CrossRef] [PubMed]
- Davra, V.; Kumar, S.; Geng, K.; Calianese, D.; Mehta, D.; Gadiyar, V.; Kasikara, C.; Lahey, K.C.; Chang, Y.J.; Wichroski, M.; et al. Axl and Mertk receptors cooperate to promote breast cancer progression by combined oncogenic signaling and evasion of host antitumor immunity. Cancer Res. 2021, 81, 698–712. [Google Scholar] [CrossRef]
- Iida, M.; Crossman, B.E.; Kostecki, K.L.; Glitchev, C.E.; Kranjac, C.A.; Crow, M.T.; Adams, J.M.; Liu, P.; Ong, I.; Yang, D.T.; et al. MerTK drives proliferation and metastatic potential in triple-negative breast cancer. Int. J. Mol. Sci. 2024, 25, 5109. [Google Scholar] [CrossRef] [PubMed]
- Frejno, M.; Zenezini Chiozzi, R.; Wilhelm, M.; Koch, H.; Zheng, R.; Klaeger, S.; Ruprecht, B.; Meng, C.; Kramer, K.; Jarzab, A.; et al. Pharmacoproteomic characterisation of human colon and rectal cancer. Mol. Syst. Biol. 2017, 13, 951. [Google Scholar] [CrossRef]
- Schmitz, R.; Valls, A.F.; Yerbes, R.; von Richter, S.; Kahlert, C.; Loges, S.; Weitz, J.; Schneider, M.; Ruiz de Almodovar, C.; Ulrich, A.; et al. TAM receptors Tyro3 and Mer as novel targets in colorectal cancer. Oncotarget 2016, 7, 56355–56370. [Google Scholar] [CrossRef]
- Wang, Y.; Moncayo, G.; Morin, P.; Xue, G.; Grzmil, M.; Lino, M.M.; Clément-Schatlo, V.; Frank, S.; Merlo, A.; Hemmings, B.A. Mer receptor tyrosine kinase promotes invasion and survival in glioblastoma multiforme. Oncogene 2013, 32, 872–882. [Google Scholar] [CrossRef]
- Knubel, K.H.; Pernu, B.M.; Sufit, A.; Nelson, S.; Pierce, A.M.; Keating, A.K. MerTK inhibition is a novel therapeutic approach for glioblastoma multiforme. Oncotarget 2014, 5, 1338–1351. [Google Scholar] [CrossRef] [PubMed]
- Tworkoski, K.A.; Platt, J.T.; Bacchiocchi, A.; Bosenberg, M.; Boggon, T.J.; Stern, D.F. MERTK controls melanoma cell migration and survival and differentially regulates cell behavior relative to AXL. Pigment. Cell Melanoma Res. 2013, 26, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sherick, A.B.; Eisenman, K.M.; Sather, S.; McGranahan, A.; Armistead, P.M.; McGary, C.S.; Hunsucker, S.A.; Schlegel, J.; Martinson, H.; Cannon, C.; et al. Aberrant Mer receptor tyrosine kinase expression contributes to leukemogenesis in acute myeloid leukemia. Oncogene 2013, 32, 5359–5368, Erratum in Oncogene 2016, 35, 5288. [Google Scholar] [CrossRef]
- Ammoun, S.; Provenzano, L.; Zhou, L.; Barczyk, M.; Evans, K.; Hilton, D.A.; Hafizi, S. Hanemann, C.O. Axl/Gas6/NFκB signalling in schwannoma pathological proliferation, adhesion and survival. Oncogene 2014, 33, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.T.; Deryckere, D.; Earp, H.S.; Graham, D.K. Molecular pathways: MERTK signaling in cancer. Clin. Cancer Res. 2013, 19, 5275–5280. [Google Scholar] [CrossRef]
- Hafizi, S.; Dahlbäck, B. Gas6 and protein S: Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006, 273, 5231–5244. [Google Scholar] [CrossRef]
- Varnum, B.C. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature 1995, 373, 623–626. [Google Scholar] [CrossRef]
- Tanim, K.M.; Holtzhausen, A.; Thapa, A.; Huelse, J.M.; Graham, D.K.; Earp, H.S. MERTK Inhibition as a Targeted Novel Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 7660. [Google Scholar] [CrossRef]
- Ohta, S.; Tago, K.; Funakoshi-Tago, M.; Matsugi, J.; Yanagisawa, K. Intracellular NF-HEV/IL-33 harbors essential roles in Ras-induced cellular transformation by contributing to cyclin D1 protein synthesis. Cell. Signal. 2016, 28, 1025–1036. [Google Scholar] [CrossRef]
- Ohta, S.; Tago, K.; Kuchimaru, T.; Funakoshi-Tago, M.; Horie, H.; Aoki-Ohmura, C.; Matsugi, J.; Yanagisawa, K. Essential roles of MerTK for cell migration accelerated by oncogenic Ras-IL-33 signaling axis. FEBS J. 2022, 289, 1950–1967. [Google Scholar] [CrossRef]
- Cornils, H.; Kohler, R.S.; Hergovich, A.; Hemmings, B.A. Human NDR kinases control G1/S cell cycle transition by directly regulating p21 stability. Mol. Cell. Biol. 2011, 31, 1382–1395. [Google Scholar] [CrossRef]
- Hergovich, A.; Lamla, S.; Nigg, E.A.; Hemmings, B.A. Centrosome-associated NDR kinase regulates centrosome duplication. Mol. Cell. 2007, 25, 625–634. [Google Scholar] [CrossRef]
- Martin, A.P.; Aushev, V.N.; Zalcman, G.; Camonis, J.H. The STK38-XPO1 axis, a new actor in physiology and cancer. Cell. Mol. Life Sci. 2021, 78, 1943–1955. [Google Scholar] [CrossRef]
- Adeyinka, A.; Emberley, E.; Niu, Y.; Snell, L.; Murphy, L.C.; Sowter, H.; Wykoff, C.C.; Harris, A.L.; Watson, P.H. Analysis of gene expression in ductal carcinoma in situ of the breast. Clin. Cancer Res. 2002, 8, 3788–3795. [Google Scholar]
- Bhattacharjee, A.; Richards, W.G.; Staunton, J.; Li, C.; Monti, S.; Vasa, P.; Ladd, C.; Beheshti, J.; Bueno, R.; Gillette, M.; et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc. Natl. Acad. Sci. USA 2001, 98, 13790–13795. [Google Scholar] [CrossRef] [PubMed]
- Garber, M.E.; Troyanskaya, O.G.; Schluens, K.; Petersen, S.; Thaesler, Z.; Pacyna-Gengelbach, M.; van de Rijn, M.; Rosen, G.D.; Perou, C.M.; Whyte, R.I.; et al. Diversity of gene expression in adenocarcinoma of the lung. Proc. Natl. Acad. Sci. USA 2001, 98, 13784–13789, Correction in Proc. Natl. Acad. Sci. USA 2001, 98, 15064. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.B.; Zarrinkar, P.P.; Sapinoso, L.M.; Kern, S.G.; Behling, C.A.; Monk, B.J.; Lockhart, D.J.; Burger, R.A.; Hampton, G.M. Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Bettoun, A.; Joffre, C.; Parrini, M.C.; Gundogdu, R.; Ahmad, A.D.; Gomez, M.; Cascone, I.; Meunier, B.; White, M.A.; Codogno, P.; et al. Mitochondrial clearance by the STK38 kinase supports oncogenic Ras-induced cell transformation. Oncotarget 2016, 7, 44142–44160, Correction in Oncotarget 2018, 9, 22870. [Google Scholar] [CrossRef]
- Hergovich, A.; Bichsel, S.J.; Hemmings, B.A. Human NDR kinases are rapidly activated by MOB proteins through recruitment to the plasma membrane and phosphorylation. Mol. Cell. Biol. 2005, 25, 8259–8272. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Sharif, A.A.D.; Hergovich, A. The NDR/LATS protein kinases in immunology and cancer biology. Semin. Cancer Biol. 2018, 48, 104–114. [Google Scholar] [CrossRef]
- Rastislav, T.; Samuel, J.B.; He´le‘ne, R.; Mario, R.S.; Brian, A.H. Ca2+-mediated regulation of NDR protein kinase through autophosphorylation and phosphorylation by an upstream kinase. J. Biol. Chem. 2003, 278, 6710–6718. [Google Scholar]
- Thomas, A.; Millward, D.H.; Brian, A.H. Ndr protein kinase is regulated by phosphorylation on two conserved sequence motifs. J. Biol. Chem. 1999, 274, 33847–33850. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.-C.; Chen, H.-C. p120RasGAP-Mediated Activation of c-Src is critical for oncogenic Ras to Induce tumor invasion. Cancer Res. 2012, 72, 2405–2415. [Google Scholar] [CrossRef] [PubMed]
- Penuel, E.; Martin, G.S. Transformation by v-Src: Ras-MAPK and PI3K-mTOR mediate parallel pathways. Mol. Biol. Cell. 1999, 10, 1693–1703. [Google Scholar] [CrossRef]
- Wu, Y.; Singh, S.; Georgescu, M.M.; Birge, R.B. A role for Mer tyrosine kinase in αvβ5 integrin-mediated phagocytosis of apoptotic cells. J. Cell Sci. 2005, 118, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Shelby, S.J.; Colwill, K.; Dhe-Paganon, S.; Pawson, T.; Thompson, D.A. MERTK interactions with SH2-Domain proteins in the retinal pigment epithelium. PLoS ONE 2013, 8, e53964. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure and regulation. Biochem. Biophys. Res. Commun. 2004, 324, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Xiquan, L.; Nicole, A.D.; Marilyn, D.R. Signaling from Integrins to Fyn to Rho Family GTPases Regulates Morphologic Differentiation of Oligodendrocytes. J. Neurosci. 2004, 24, 7140–7149. [Google Scholar] [CrossRef]
- Mahajan, N.P.; Earp, H.S. An SH2 Domain-dependent, Phosphotyrosine-independent Interaction between Vav1 and the Mer Receptor Tyrosine Kinase: A mechanism for localizing guanine nucleotide-exchange factor action. J. Biol. Chem. 2003, 278, 42596–42603. [Google Scholar] [CrossRef]
- Tang, F.; Gill, J.; Ficht, X.; Barthlott, T.; Cornils, H.; Schmitz-Rohmer, D.; Hynx, D.; Zhou, D.; Zhang, L.; Xue, G.; et al. The kinases NDR1/2 act downstream of the Hippo homolog MST1 to mediate both egress of thymocytes from the thymus and lymphocyte motility. Sci. Signal. 2015, 8, ra100. [Google Scholar] [CrossRef]
- Cornils, H.; Kohler, R.S.; Hergovich, A.; Hemmings, B.A. Downstream of human NDR kinases: Impacting on c-myc and p21 protein stability to control cell cycle progression. Cell Cycle 2011, 10, 1897–1904. [Google Scholar] [CrossRef]
- Fukasawa, T.; Enomoto, A.; Miyagawa, K. Serine-Threonine Kinase 38 regulates CDC25A stability and the DNA damage-induced G2/M checkpoint. Cell. Signal. 2015, 27, 1569–1575. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, F.; Terracciano, L.; Hynx, D.; Kohler, R.; Bichet, S.; Hess, D.; Cron, P.; Hemmings, B.A.; Hergovich, A.; et al. NDR functions as a physiological YAP1 kinase in the intestinal epithelium. Curr. Biol. 2015, 25, 296–305. [Google Scholar] [CrossRef]
- Martin, A.P.; Jacquemyn, M.; Lipecka, J.; Chhuon, C.; Aushev, V.N.; Meunier, B.; Singh, M.K.; Carpi, N.; Piel, M.; Codogno, P.; et al. STK38 kinase acts as XPO1 gatekeeper regulating the nuclear export of autophagy proteins and other cargoes. EMBO Rep. 2019, 20, e48150. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Ikeda, M.; Katsunuma, K.; Ohashi, K.; Mizuno, K. MST2- and furry-mediated activation of NDR1 kinase is critical for precise alignment of mitotic chromosomes. Curr. Biol. 2009, 19, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Stegert, M.R.; Tamaskovic, R.; Bichsel, S.J.; Hergovich, A.; Hemmings, B.A. Regulation of NDR2 protein kinase by multi-site phosphorylation and the S100B calcium-binding protein. J. Biol. Chem. 2004, 279, 23806–23812. [Google Scholar] [CrossRef]
- Hergovich, A. Regulation and functions of mammalian LATS/NDR kinases: Looking beyond canonical Hippo signal-ling. Cell Biosci. 2013, 3, 32. [Google Scholar] [CrossRef]
- Selimoglu, R.; Bettoun, A.; Joffre, C.; Meunier, B.; Parrini, M.C.; Fesquet, D.; Formstecher, E.; Cascone, I.; Hergovich, A.; Camonis, J. RalA GTPase and MAP4K4 function through NDR1 activation in stress response and apoptotic signaling. HSOA J. Cell. Biol. Cell. Metab. 2014, 1, 1–11. [Google Scholar]
- Wan, L.; Pantel, K.; Kang, Y. Tumor metastasis: Moving new biological insights into the clinic. Nat. Med. 2013, 19, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Peter, D.; Alan, R.H. Signaling Networks that Regulate Cell Migration. Cold Spring Harb. Perspect. Biol. 2015, 7, a005959. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, J.; Sambade, M.J.; Sather, S.; Moschos, S.J.; Tan, A.C.; Winges, A.; DeRyckere, D.; Carson, C.C.; Trembath, D.G.; Tentler, J.J.; et al. MERTK receptor tyrosine kinase is a therapeutic target in melanoma. J. Clin. Investig. 2013, 123, 2257–2267. [Google Scholar] [CrossRef]
- Rogers, A.E.J.; Le, J.P.; Sather, S.; Pernu, B.M.; Graham, D.K.; Pierce, A.M.; Keating, A.K. Mer receptor tyrosine kinase inhibition impedes glioblastoma multiforme migration and alters cellular morphology. Oncogene 2012, 31, 4171–4181. [Google Scholar] [CrossRef]
- Ehrhardt, A.; Ehrhardt, G.R.A.; Guo, X.; Schrader, J.W. Ras and relatives—Job sharing and networking keep an old family together. Exp. Hematol. 2002, 30, 1089–1106. [Google Scholar] [CrossRef]
- Crosas-Molist, E.; Samain, R.; Kohlhammer, L.; Orgaz, J.L.; George, S.L.; Maiques, O.; Barcelo, J.; Sanz-Moreno, V. Rho GTPase signalling in cancer progression and dissemination. Physiol. Rev. 2022, 102, 455–510. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohta, S.; Tago, K.; Kasashima, K.; Ebina, M.; Tominaga, K. STK38 Kinase Promotes Cell Migration Induced by Oncogenic Ras via MerTK Activation. Int. J. Mol. Sci. 2025, 26, 10388. https://doi.org/10.3390/ijms262110388
Ohta S, Tago K, Kasashima K, Ebina M, Tominaga K. STK38 Kinase Promotes Cell Migration Induced by Oncogenic Ras via MerTK Activation. International Journal of Molecular Sciences. 2025; 26(21):10388. https://doi.org/10.3390/ijms262110388
Chicago/Turabian StyleOhta, Satoshi, Kenji Tago, Katsumi Kasashima, Masayuki Ebina, and Kaoru Tominaga. 2025. "STK38 Kinase Promotes Cell Migration Induced by Oncogenic Ras via MerTK Activation" International Journal of Molecular Sciences 26, no. 21: 10388. https://doi.org/10.3390/ijms262110388
APA StyleOhta, S., Tago, K., Kasashima, K., Ebina, M., & Tominaga, K. (2025). STK38 Kinase Promotes Cell Migration Induced by Oncogenic Ras via MerTK Activation. International Journal of Molecular Sciences, 26(21), 10388. https://doi.org/10.3390/ijms262110388





