EPY001, a Novel Monoclonal Antibody Against Pseudomonas aeruginosa Targeting OprF
Abstract
1. Introduction
2. Results
2.1. Affinity, Specificity and Epitope Mapping of EPY001
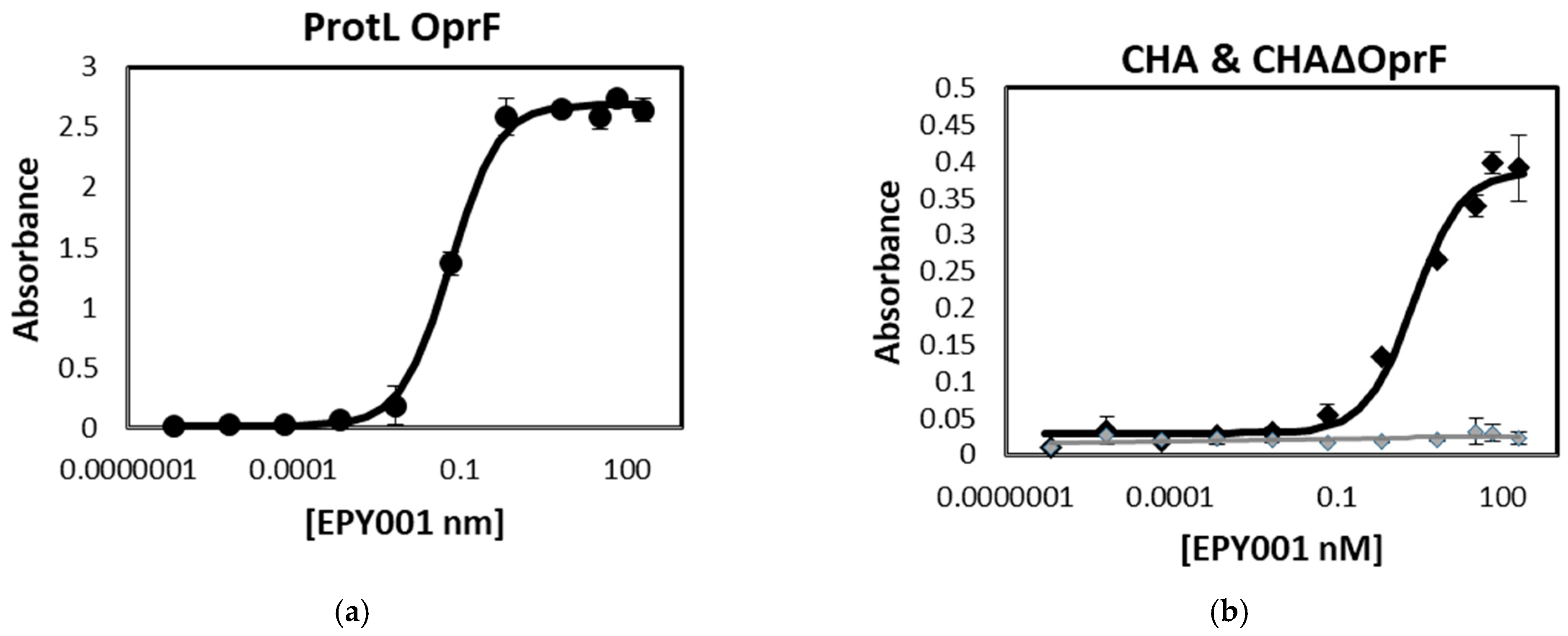
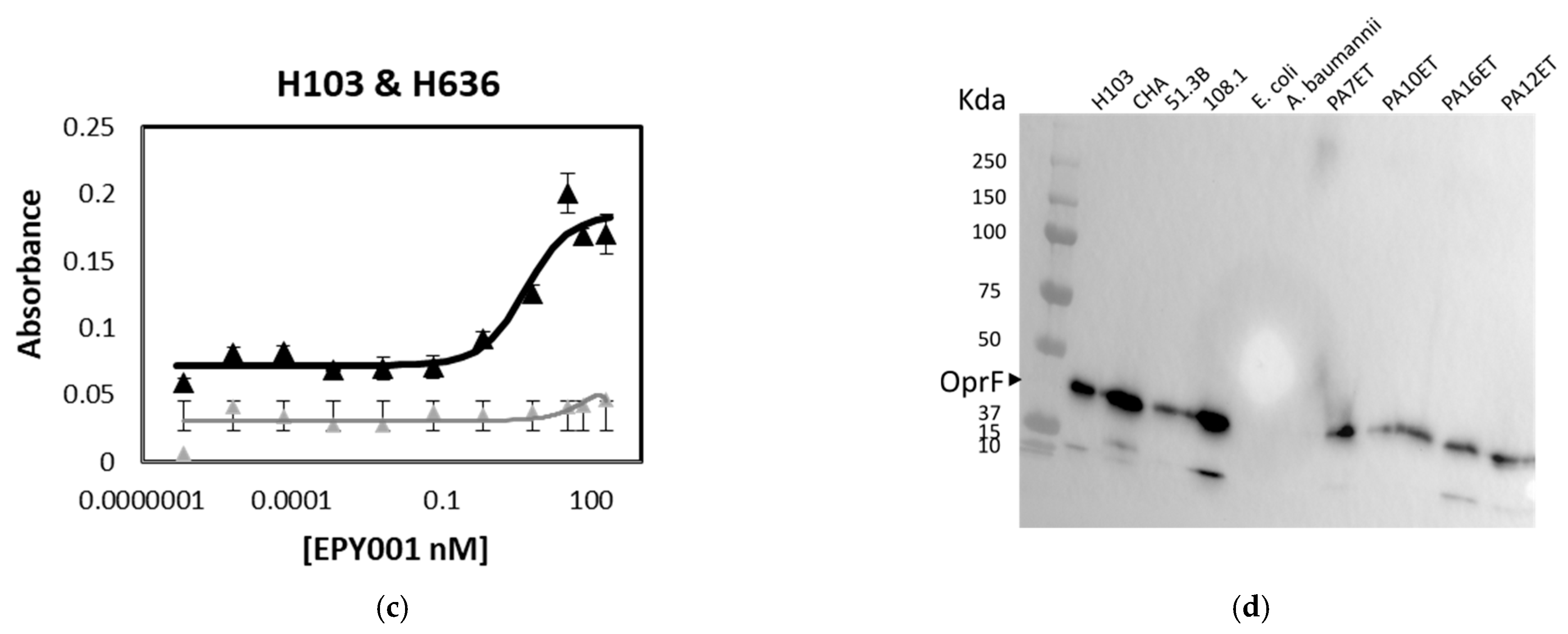
2.1.1. Evaluation of the Binding Affinity and Specificity of the Anti-OprF mAb EPY001
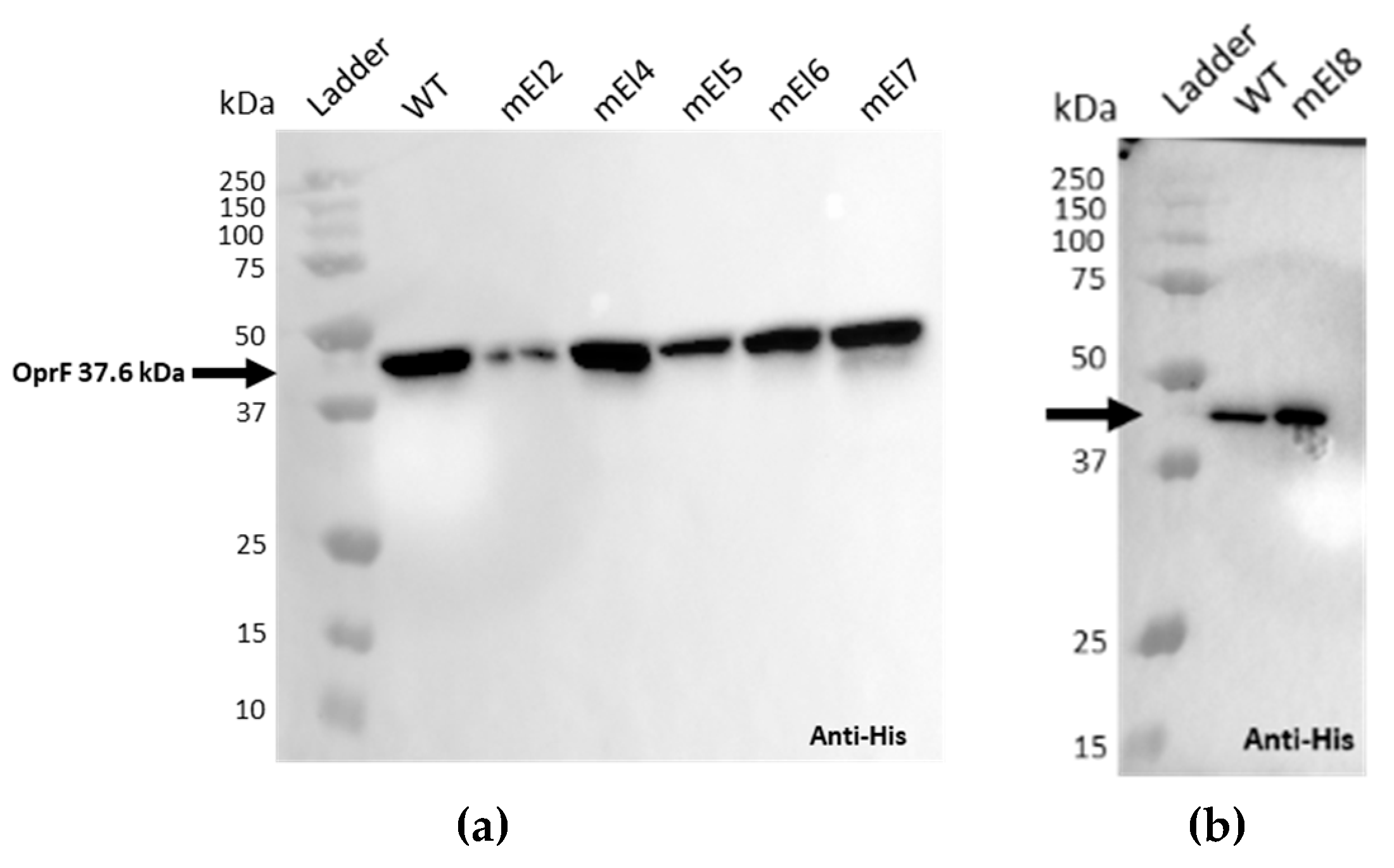
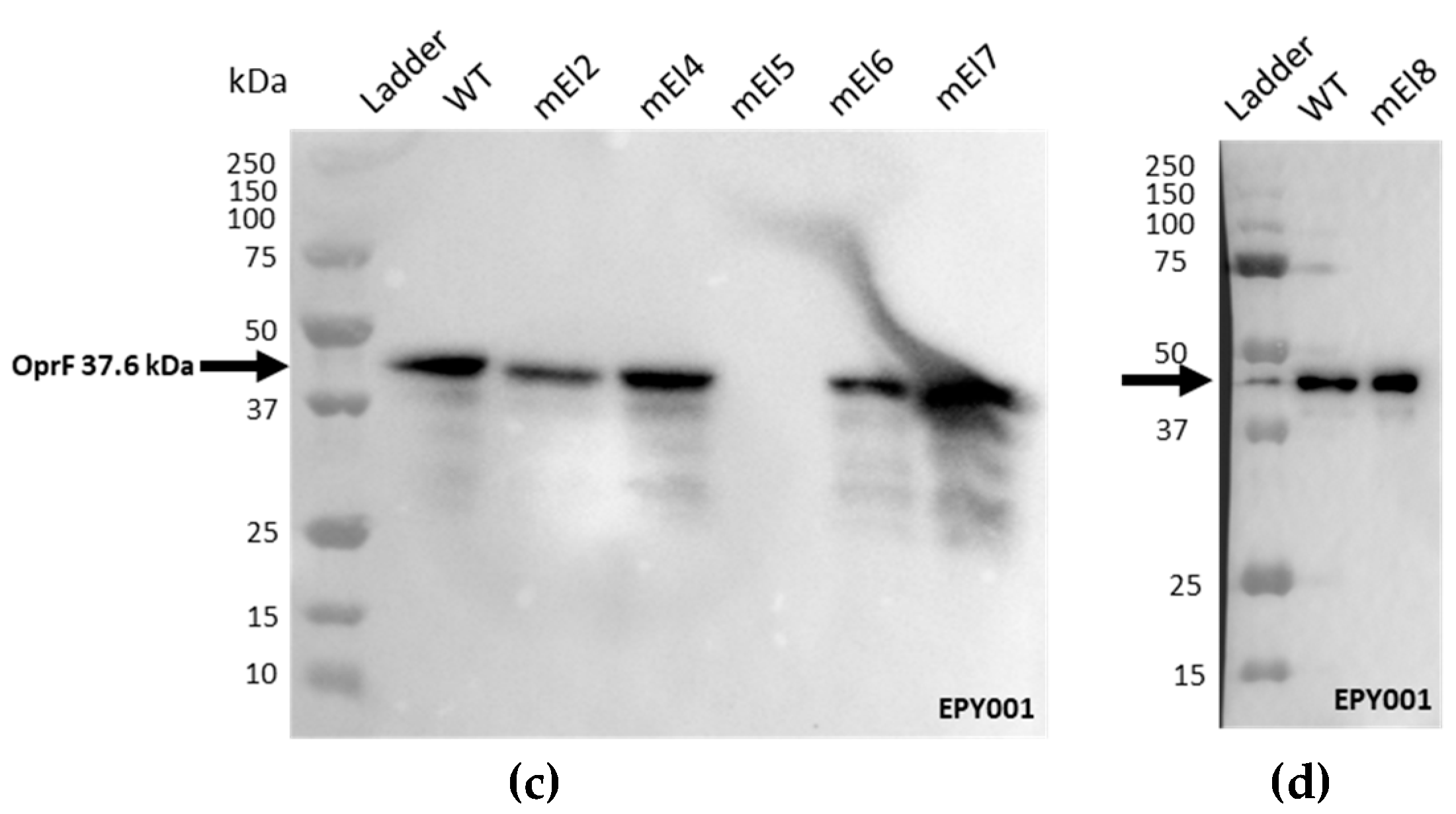
2.1.2. Epitope Mapping of the EPY001 Antibody
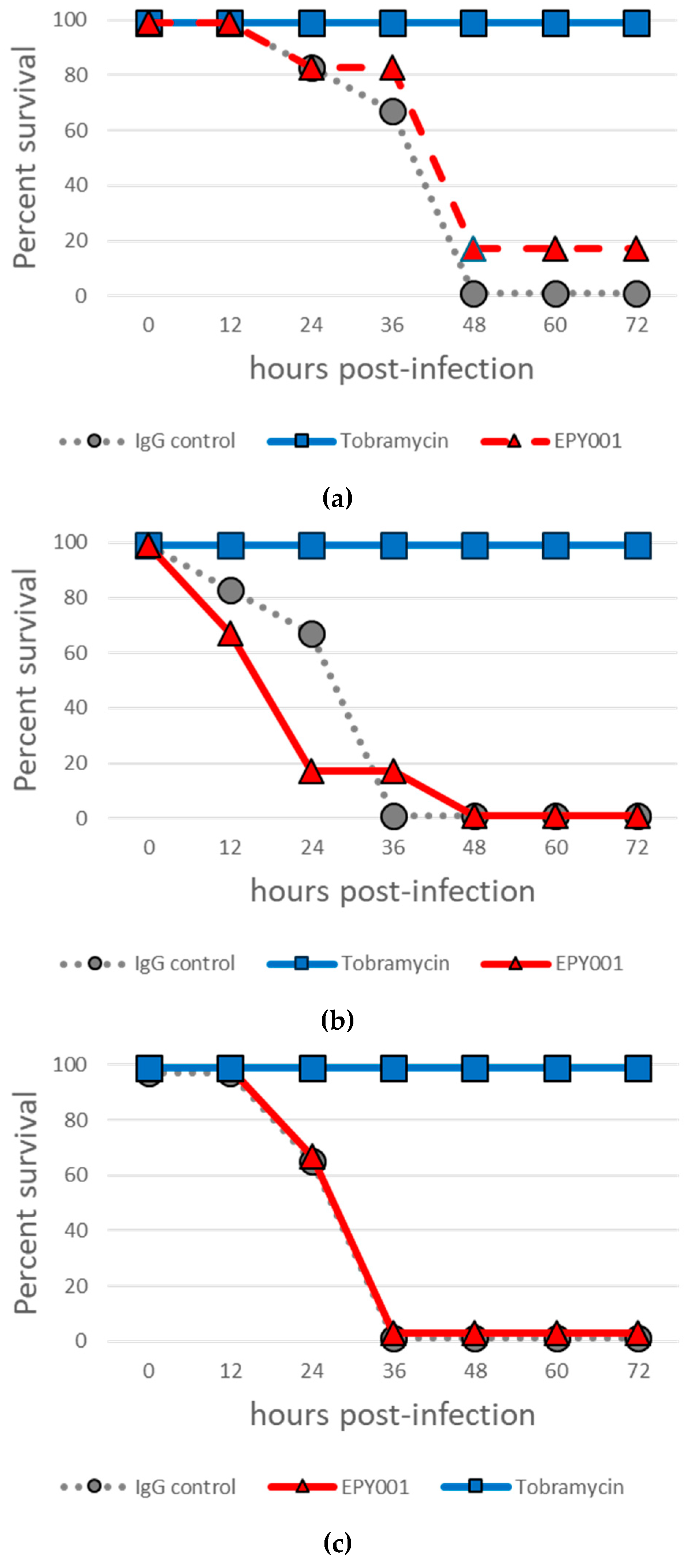
2.2. In Vivo Evaluation of EPY001 Does Not Demonstrate Protective Efficacy Against Acute Pulmonary P. aeruginosa Infection
2.3. In Vitro Efficacy of EPY001
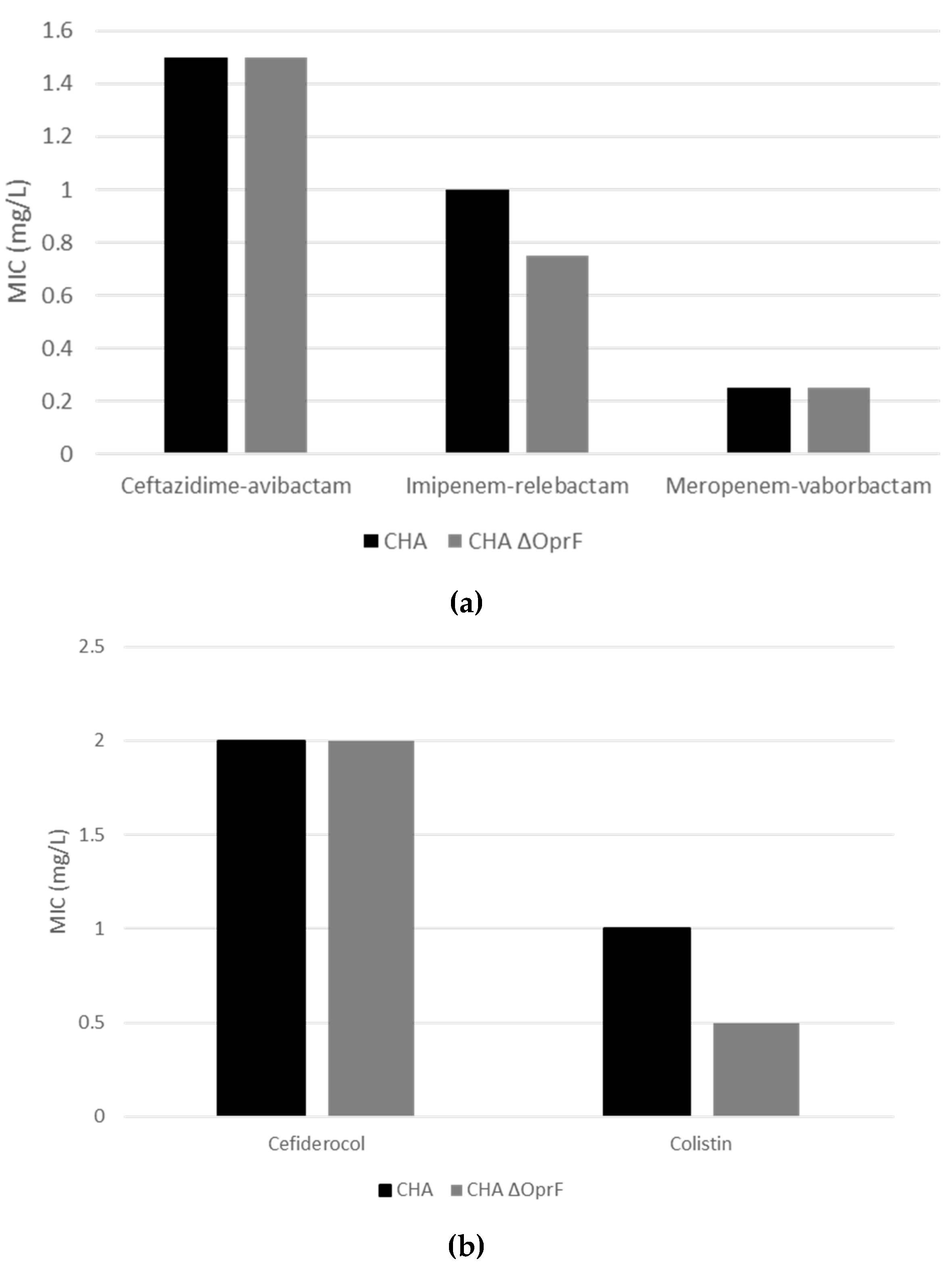
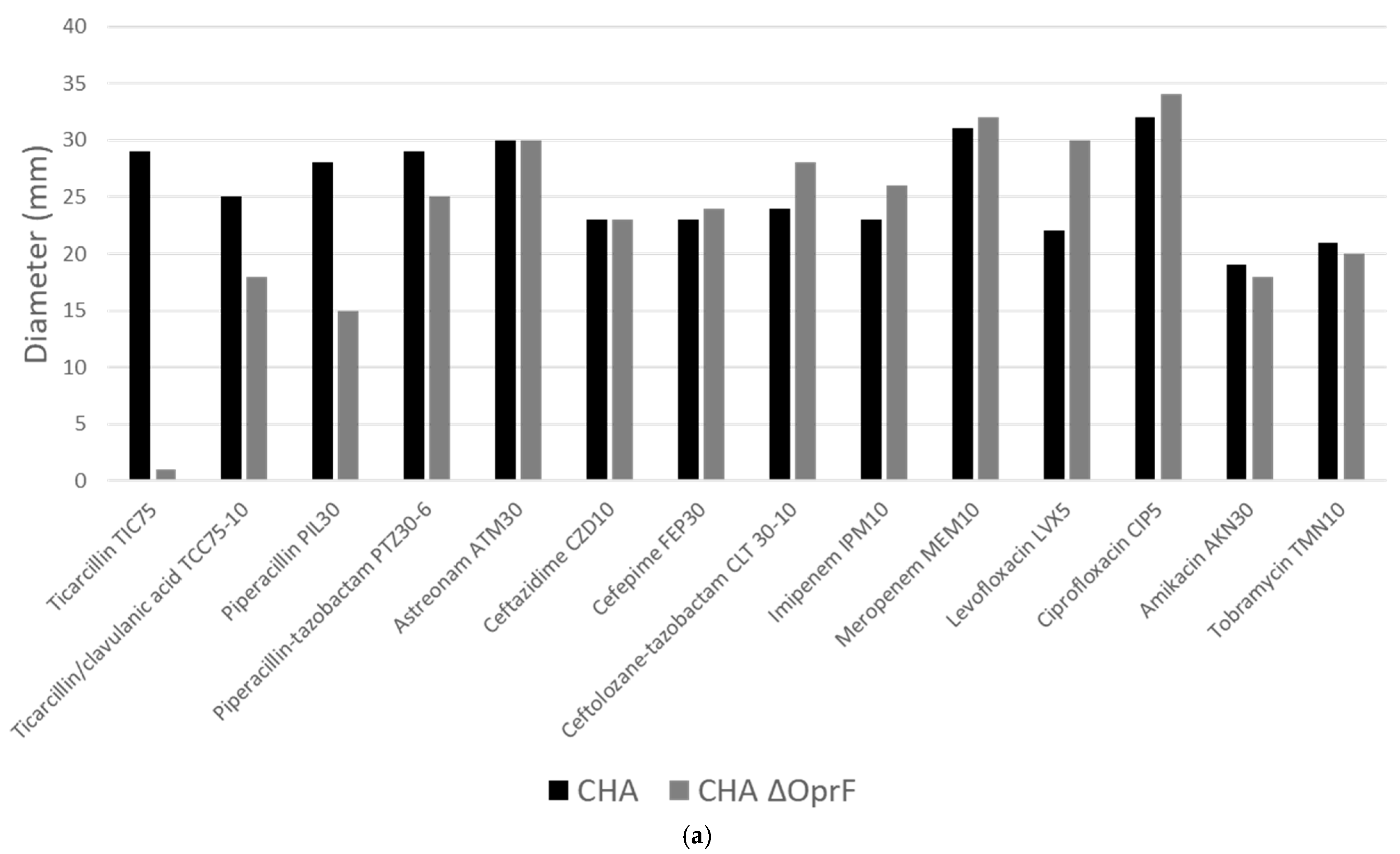
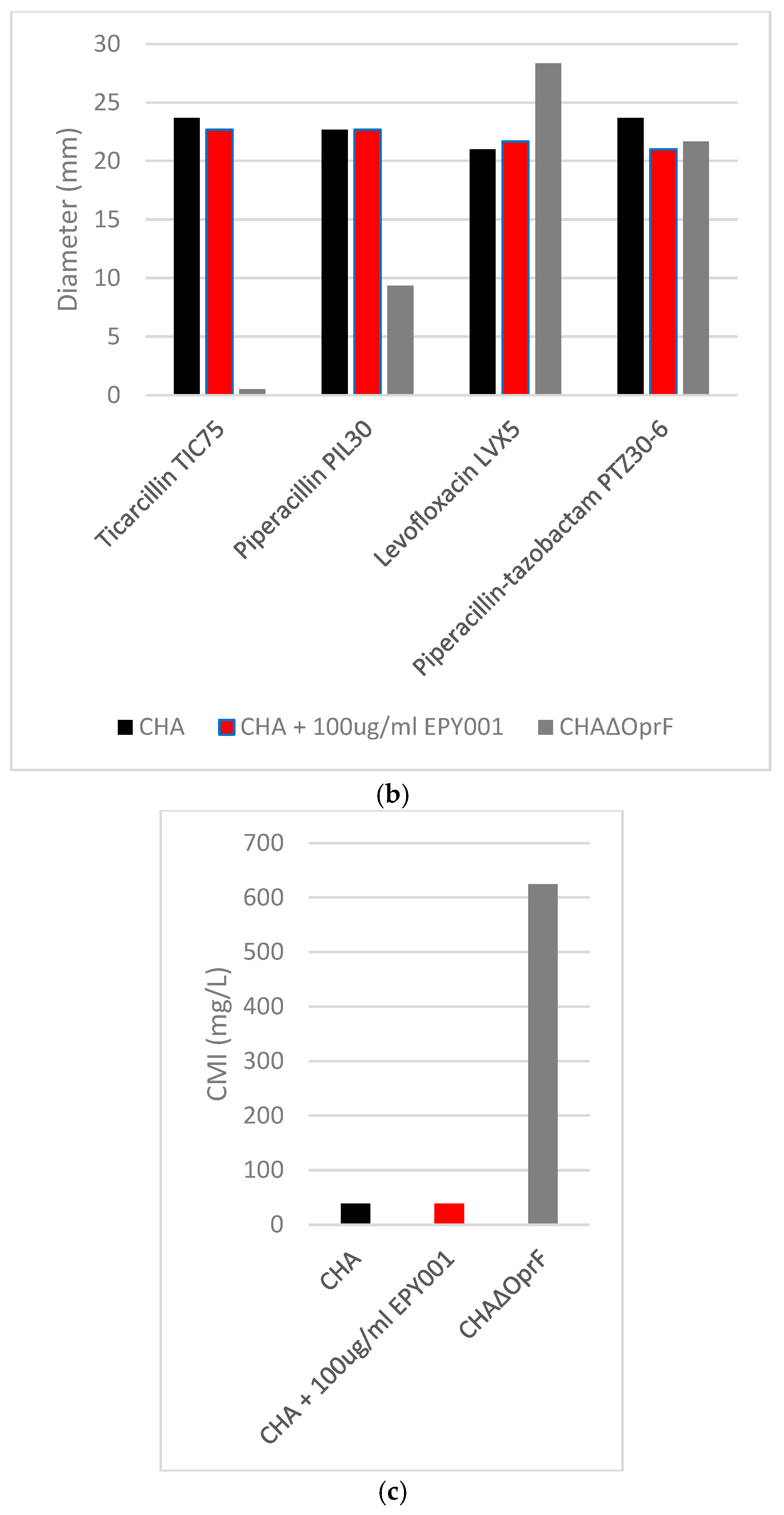
2.3.1. Deletion of OprF Alters Antibiotic Susceptibility, but EPY001 Does Not Modulate Resistance in P. aeruginosa
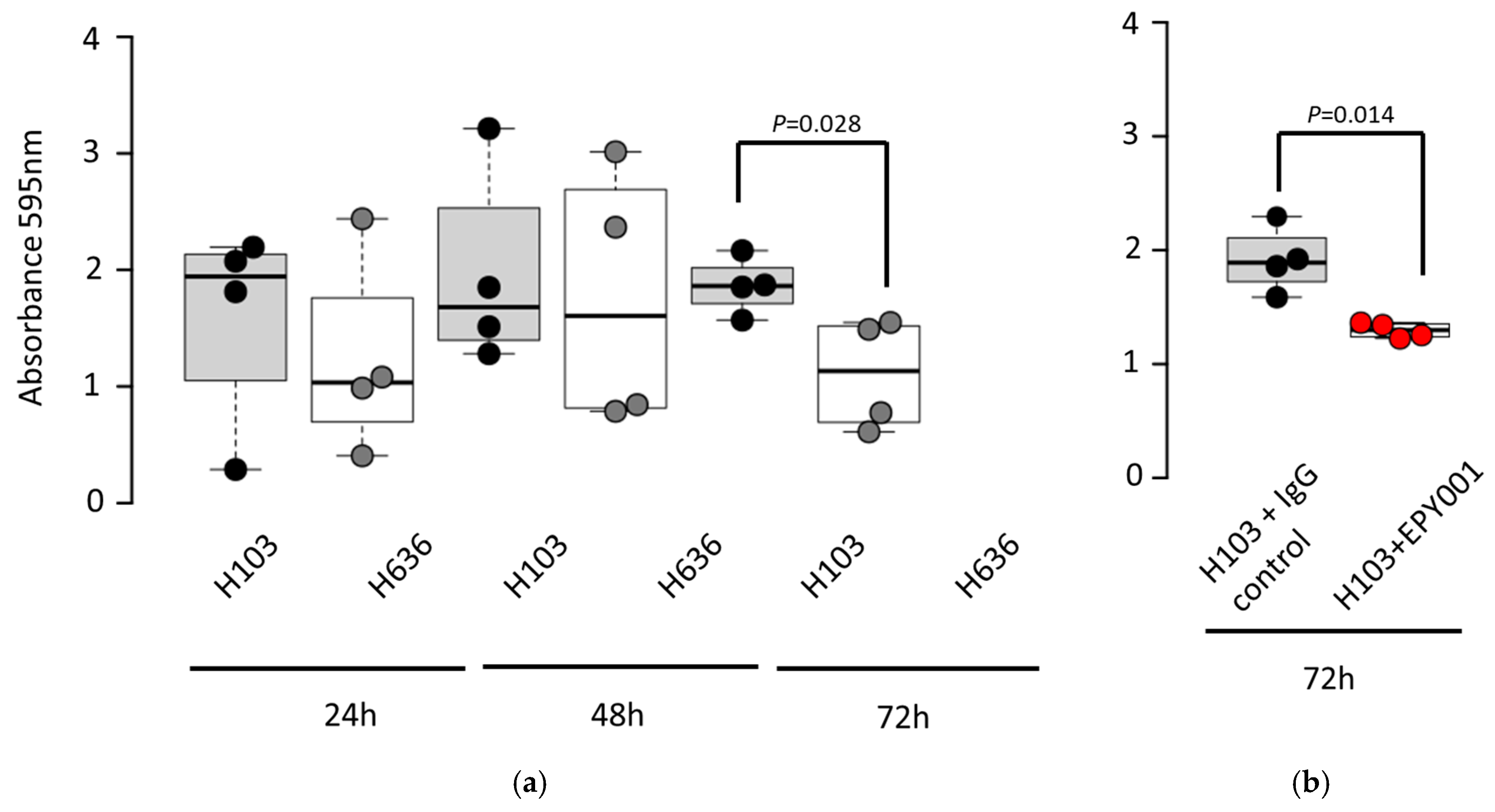
2.3.2. EPY001 Slightly Reduces Biofilm Formation in P. aeruginosa H103
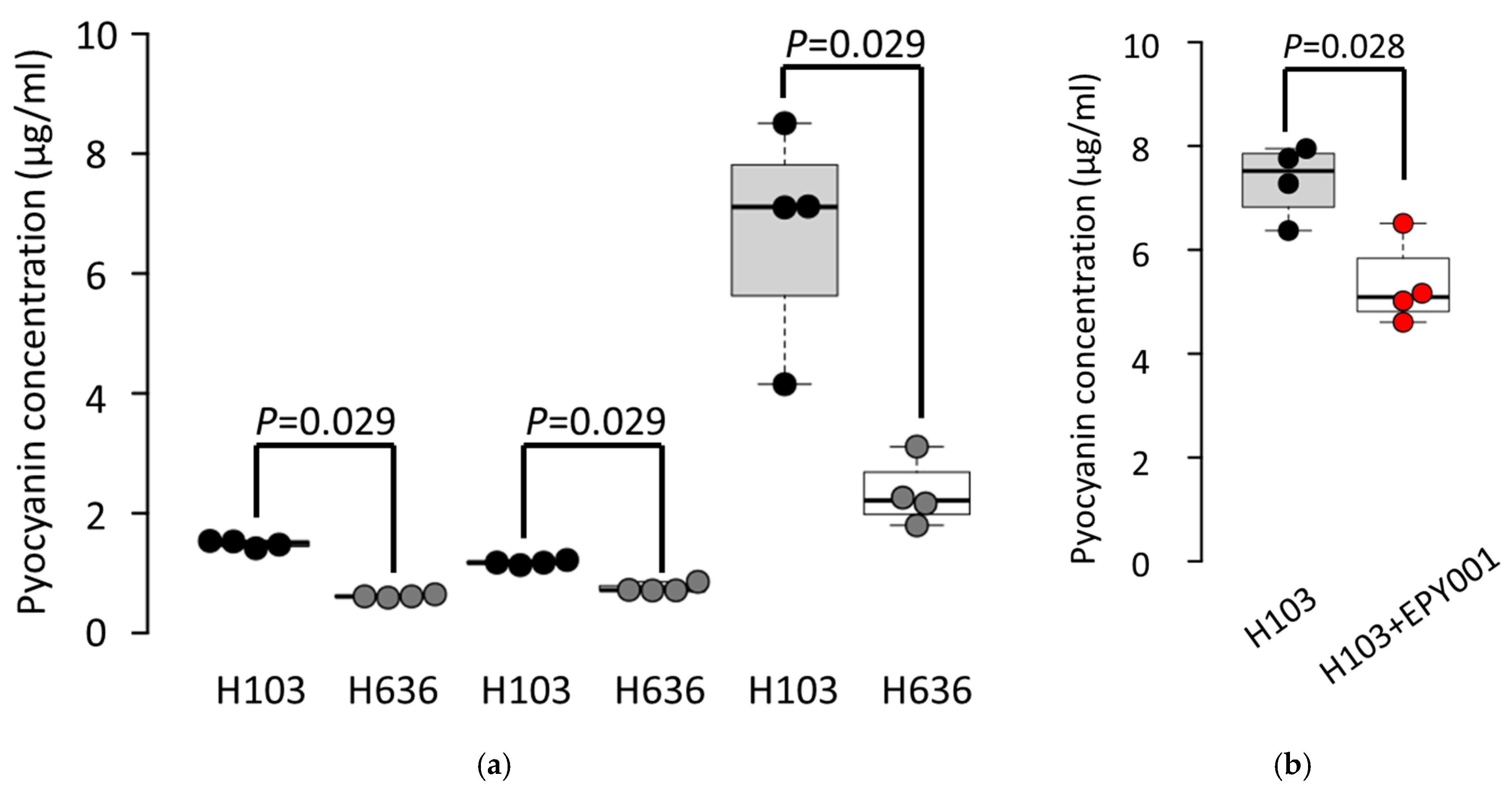
2.3.3. EPY001 Moderately Reduces Pyocyanin Production in P. aeruginosa H103
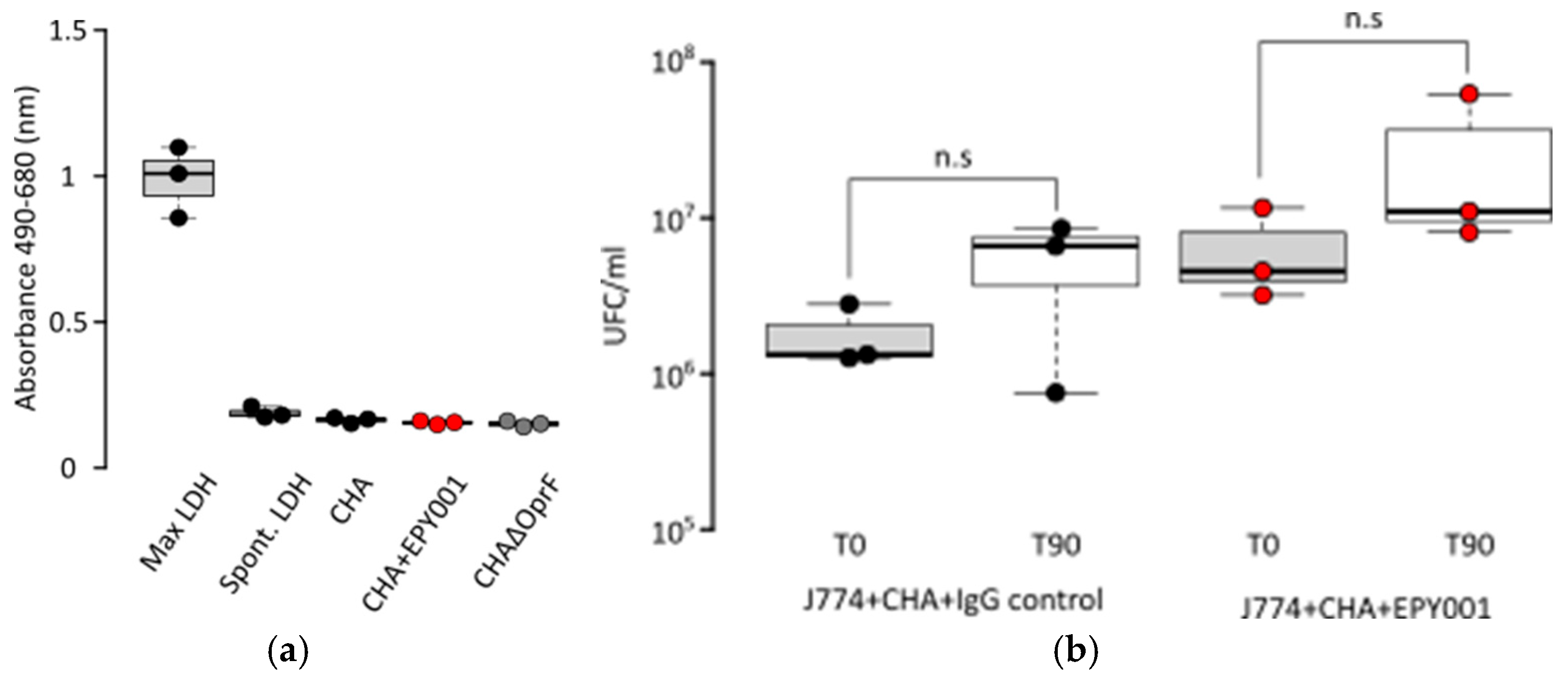
2.3.4. Evaluation of Complement-Dependent Cytotoxicity (CDC) Assay Activity of EPY001 on the CHA Strain
2.3.5. EPY001 Does Not Promote Antibody-Dependent Cellular Phagocytosis (ADCP)
3. Discussion
3.1. Proof of Concept for Generating High-Specificity, High-Affinity Antibodies Against Membrane Proteins via Macaque Immunization with Proteoliposomes
3.2. Impact of OprF on β-Lactam Resistance in P. aeruginosa: Implications for Antibiotic Uptake and Membrane Permeability
3.3. Challenges in EPY001 Efficacy: Murine Lung Infection and FcγR-Mediated Phagocytosis
3.4. Context-Dependent Role of OprF in P. aeruginosa Biofilm Development and Its Modulation by the EPY001 Antibody
3.5. Targeting OprF to Modulate Pyocyanin-Mediated Virulence in P. aeruginosa
3.6. Relevance of mAbs Targeting Bacterial Membrane Proteins in the Fight Against Infections
3.7. Exploring OprF as a Therapeutic Target in P. aeruginosa Infections: Challenges and Future Directions
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Production and Purification of OprF Proteoliposomes
4.3. Coomassie Stained SDS–PAGE Gel
4.4. Production and Purification of Antibodies
4.5. Affinity of the mAb EPY001
4.6. Animal Investigation Protocol
4.7. Mice Acute Pneumonia Model
4.8. Antibiotic Susceptibility Testing
4.9. Biofilm Formation Assays
4.10. Pyocyanin Production Assays
4.11. CDC Activation Assay
4.12. Cytotoxicity Assay (LDH Release)
4.13. ADCP Activation Assay
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mel | mutated extracellular loop |
| mAbs | Monoclonal antibodies |
| CFU | Colony Forming Unit |
| WT | Wild-Type |
| OMPs | Outer Membrane Proteins |
| QS | Quorum-Sensing |
| MIC | Minimal inhibitory concentration |
| IgG | Immunoglobulin G |
| CV | crystal violet solution |
| CDC | Complement-dependent cytotoxicity |
| ADCP | Antibody-Dependent Cellular Phagocytosis |
| CGP | Guinea Pig Complement |
| dCGP | Heat-Inactivated Guinea Pig Complement |
Appendix A
References
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet Lond. Engl. 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Suh, H.Y.; Peck, C.C.; Yu, K.-S.; Lee, H. Determination of the starting dose in the first-in-human clinical trials with monoclonal antibodies: A systematic review of papers published between 1990 and 2013. Drug Des. Devel. Ther. 2016, 10, 4005–4016. [Google Scholar] [CrossRef]
- Lau, W.Y.V.; Taylor, P.K.; Brinkman, F.S.L.; Lee, A.H.Y. Pathogen-associated gene discovery workflows for novel antivirulence therapeutic development. eBioMedicine 2023, 88, 104429. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Morris, S. Approval of Raxibacumab for the Treatment of Inhalation Anthrax Under the US Food and Drug Administration “Animal Rule. ” Front. Microbiol. 2015, 6, 1320. [Google Scholar] [CrossRef]
- Nagy, C.F.; Leach, T.S.; King, A.; Guttendorf, R. Safety, Pharmacokinetics, and Immunogenicity of Obiltoxaximab After Intramuscular Administration to Healthy Humans. Clin. Pharmacol. Drug Dev. 2018, 7, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Greig, S.L. Obiltoxaximab: First Global Approval. Drugs 2016, 76, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.F.; Leach, T.S.; Hoffman, J.H.; Czech, A.; Carpenter, S.E.; Guttendorf, R. Pharmacokinetics and Tolerability of Obiltoxaximab: A Report of 5 Healthy Volunteer Studies. Clin. Ther. 2016, 38, 2083–2097.e7. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, B.J.; Shadiack, A.M.; Carpenter, S.; Sanford, D.; Henning, L.N.; O’Connor, E.; Gonzales, N.; Mondick, J.; French, J.; Stark, G.V.; et al. Efficacy Projection of Obiltoxaximab for Treatment of Inhalational Anthrax across a Range of Disease Severity. Antimicrob. Agents Chemother. 2016, 60, 5787–5795. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Askar, S.F.; Kenney, R.M.; Tariq, Z.; Conner, R.; Williams, J.; Ramesh, M.; Alangaden, G.J. Bezlotoxumab for Prevention of Recurrent Clostridioides difficile Infection with a Focus on Immunocompromised Patients. J. Pharm. Pract. 2023, 36, 584–587. [Google Scholar] [CrossRef]
- Chastre, J.; François, B.; Bourgeois, M.; Komnos, A.; Ferrer, R.; Rahav, G.; De Schryver, N.; Lepape, A.; Koksal, I.; Luyt, C.-E.; et al. Safety, efficacy, and pharmacokinetics of gremubamab (MEDI3902), an anti-Pseudomonas aeruginosa bispecific human monoclonal antibody, in P. aeruginosa-colonised, mechanically ventilated intensive care unit patients: A randomised controlled trial. Crit. Care 2022, 26, 355. [Google Scholar] [CrossRef]
- Ali, S.O.; Yu, X.Q.; Robbie, G.J.; Wu, Y.; Shoemaker, K.; Yu, L.; DiGiandomenico, A.; Keller, A.E.; Anude, C.; Hernandez-Illas, M.; et al. Phase 1 study of MEDI3902, an investigational anti–Pseudomonas aeruginosa PcrV and Psl bispecific human monoclonal antibody, in healthy adults. Clin. Microbiol. Infect. 2019, 25, 629.e1–629.e6. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Beckett, V.V.; Konstan, M.W.; Accurso, F.J.; Burns, J.L.; Mayer-Hamblett, N.; Milla, C.; VanDevanter, D.R.; Chmiel, J.F. KB001-A, a novel anti-inflammatory, found to be safe and well-tolerated in cystic fibrosis patients infected with Pseudomonas aeruginosa. J. Cyst. Fibros. 2018, 17, 484–491. [Google Scholar] [CrossRef]
- Lazar, H.; Horn, M.P.; Zuercher, A.W.; Imboden, M.A.; Durrer, P.; Seiberling, M.; Pokorny, R.; Hammer, C.; Lang, A.B. Pharmacokinetics and Safety Profile of the Human Anti-Pseudomonas aeruginosa Serotype O11 Immunoglobulin M Monoclonal Antibody KBPA-101 in Healthy Volunteers. Antimicrob. Agents Chemother. 2009, 53, 3442–3446. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.V.; Hall, V.L.; McOsker, C.C. Crumbling the Castle: Targeting DNABII Proteins for Collapsing Bacterial Biofilms as a Therapeutic Approach to Treat Disease and Combat Antimicrobial Resistance. Antibiotics 2022, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Loos, A.; Weich, N.; Woo, J.; Lalonde, G.; Yee, L.; Dummer, W.; Truong, V.L. 674. Pre-Clinical and Phase I Safety Data for Anti-Pseudomonas aeruginosa Human Monoclonal Antibody AR-105. Open Forum Infect. Dis. 2019, 6 (Suppl. S2), S307–S308. [Google Scholar] [CrossRef]
- Mateu-Borrás, M.; Dublin, S.R.; Kang, J.; Monroe, H.L.; Sen-Kilic, E.; Miller, S.J.; Witt, W.T.; Chapman, J.A.; Pyles, G.M.; Nallar, S.C.; et al. Novel broadly reactive monoclonal antibody protects against Pseudomonas aeruginosa infection. Infect. Immun. 2025, 93, e00330-24. [Google Scholar] [CrossRef]
- Horspool, A.M.; Sen-Kilic, E.; Malkowski, A.C.; Breslow, S.L.; Mateu-Borras, M.; Hudson, M.S.; Nunley, M.A.; Elliott, S.; Ray, K.; Snyder, G.A.; et al. Development of an anti-Pseudomonas aeruginosa therapeutic monoclonal antibody WVDC-5244. Front. Cell. Infect. Microbiol. 2023, 13, 1117844. [Google Scholar] [CrossRef]
- Hale, M.; Takehara, K.K.; Thouvenel, C.D.; Moustafa, D.A.; Repele, A.; Fontana, M.F.; Netland, J.; McNamara, S.; Gibson, R.L.; Goldberg, J.B.; et al. Monoclonal antibodies derived from B cells in subjects with cystic fibrosis reduce Pseudomonas aeruginosa burden in mice. eLife 2024, 13, RP98851. [Google Scholar] [CrossRef]
- Desveaux, J.-M.; Faudry, E.; Contreras-Martel, C.; Cretin, F.; Dergan-Dylon, L.S.; Amen, A.; Bally, I.; TardivyCasemajor, V.; Chenavier, F.; Fouquenet, D.; et al. Neutralizing human monoclonal antibodies that target the PcrV component of the Type III Secretion System of Pseudomonas aeruginosa act through distinct mechanisms. eLife 2025, 14, RP105195. [Google Scholar] [CrossRef]
- Kazemi Moghaddam, E.; Owlia, P.; Jahangiri, A.; Rasooli, I.; Rahbar, M.R.; Aghajani, M. Conserved OprF as a Selective Immunogen against Pseudomonas aeruginosa. Iran. J. Pathol. 2017, 12, 165–170. [Google Scholar] [CrossRef]
- Woodruff, W.A.; Hancock, R.E. Pseudomonas aeruginosa outer membrane protein F: Structural role and relationship to the Escherichia coli OmpA protein. J. Bacteriol. 1989, 171, 3304–3309. [Google Scholar] [CrossRef]
- Rawling, E.G.; Martin, N.L.; Hancock, R.E. Epitope mapping of the Pseudomonas aeruginosa major outer membrane porin protein OprF. Infect. Immun. 1995, 63, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Azghani, A.O.; Idell, S.; Bains, M.; Hancock, R.E.W. Pseudomonas aeruginosa outer membrane protein F is an adhesin in bacterial binding to lung epithelial cells in culture. Microb. Pathog. 2002, 33, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Estrada, O.; Zaborina, O.; Bains, M.; Shen, L.; Kohler, J.E.; Patel, N.; Musch, M.W.; Chang, E.B.; Fu, Y.-X.; et al. Recognition of Host Immune Activation by Pseudomonas aeruginosa. Science 2005, 309, 774–777. [Google Scholar] [CrossRef]
- Fito-Boncompte, L.; Chapalain, A.; Bouffartigues, E.; Chaker, H.; Lesouhaitier, O.; Gicquel, G.; Bazire, A.; Madi, A.; Connil, N.; Véron, W.; et al. Full Virulence of Pseudomonas aeruginosa Requires OprF. Infect. Immun. 2011, 79, 1176–1186. [Google Scholar] [CrossRef]
- Wessel, A.K.; Liew, J.; Kwon, T.; Marcotte, E.M.; Whiteley, M. Role of Pseudomonas aeruginosa Peptidoglycan-Associated Outer Membrane Proteins in Vesicle Formation. J. Bacteriol. 2013, 195, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Alhede, M.; Bjarnsholt, T.; Givskov, M.; Alhede, M. Pseudomonas aeruginosa biofilms: Mechanisms of immune evasion. Adv. Appl. Microbiol. 2014, 86, 1–40. [Google Scholar] [CrossRef]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef]
- Moussouni, M.; Berry, L.; Sipka, T.; Nguyen-Chi, M.; Blanc-Potard, A.-B. Pseudomonas aeruginosa OprF plays a role in resistance to macrophage clearance during acute infection. Sci. Rep. 2021, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Nunley, M.; Padden, E.; Wiit, W.; Chapman, J.; Boehm, D.; Barbier, M.; Damron, H.; Sen-Kilic, E. Cross-Reactive Ant-OprF Monoclonal Antibodies Against Gram-Negative Pathogens. Available online: https://symposium.foragerone.com/8th-annual-spring-undergraduate-research-symposium/presentations/61474 (accessed on 2 October 2025).
- Spinozzi, F.; Alcaraz, J.-P.; Ortore, M.G.; Gayet, L.; Radulescu, A.; Martin, D.K.; Maccarini, M. Small-Angle Neutron Scattering Reveals the Nanostructure of Liposomes with Embedded OprF Porins of Pseudomonas aeruginosa. Langmuir ACS J. Surf. Colloids 2022, 38, 15026–15037. [Google Scholar] [CrossRef]
- Mayeux, G.; Gayet, L.; Liguori, L.; Odier, M.; Martin, D.K.; Cortès, S.; Schaack, B.; Lenormand, J.-L. Cell-free expression of the outer membrane protein OprF of Pseudomonas aeruginosa for vaccine purposes. Life Sci. Alliance 2021, 4, e202000958. [Google Scholar] [CrossRef]
- Lenormand, J.-L.; Gayet, L.; Mayeux, G. Antibody Against the oprf Protein of Pseudomonas aeruginosa, Use Thereof as a Medicament and Pharmaceutical Composition Containing Same. Apllication Granted Date: 28 January 2021, WO 2021/013904 A1. Available online: https://patents.google.com/patent/WO2021013904A1/en (accessed on 28 January 2021).
- Sall, K.M.; Casabona, M.G.; Bordi, C.; Huber, P.; de Bentzmann, S.; Attrée, I.; Elsen, S. A gacS deletion in Pseudomonas aeruginosa cystic fibrosis isolate CHA shapes its virulence. PLoS ONE 2014, 9, e95936. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.J.; Lazar, G.A. Next generation antibody drugs: Pursuit of the “high-hanging fruit”. Nat. Rev. Drug Discov. 2018, 17, 197–223. [Google Scholar] [CrossRef]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Orr, C.M.; Chan, H.T.C.; James, S.; Penfold, C.A.; Kim, J.; Inzhelevskaya, T.; Mockridge, C.I.; Cox, K.L.; Essex, J.W.; et al. Reducing affinity as a strategy to boost immunomodulatory antibody agonism. Nature 2023, 614, 539–547. [Google Scholar] [CrossRef]
- Blackwood, C.B.; Mateu-Borrás, M.; Sen-Kilic, E.; Pyles, G.M.; Miller, S.J.; Weaver, K.L.; Witt, W.T.; Huckaby, A.B.; Kang, J.; Chandler, C.E.; et al. Bordetella pertussis whole cell immunization protects against Pseudomonas aeruginosa infections. NPJ Vaccines 2022, 7, 143. [Google Scholar] [CrossRef]
- Rahbar, M.R.; Mubarak, S.M.H.; Hessami, A.; Khalesi, B.; Pourzardosht, N.; Khalili, S.; Zanoos, K.A.; Jahangiri, A. A unique antigen against SARS-CoV-2, Acinetobacter baumannii, and Pseudomonas aeruginosa. Sci. Rep. 2022, 12, 10852. [Google Scholar] [CrossRef]
- Yeganeh, O.; Shabani, M.; Pakzad, P.; Mosaffa, N.; Hashemi, A. Evaluation the reactivity of a peptide-based monoclonal antibody derived from OmpA with drug resistant pulsotypes of Acinetobacter baumannii as a potential therapeutic approach. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 30. [Google Scholar] [CrossRef]
- Woodruff, W.A.; Hancock, R.E. Construction and characterization of Pseudomonas aeruginosa protein F-deficient mutants after in vitro and in vivo insertion mutagenesis of the cloned gene. J. Bacteriol. 1988, 170, 2592–2598. [Google Scholar] [CrossRef]
- Bouffartigues, E.; Gicquel, G.; Bazire, A.; Bains, M.; Maillot, O.; Vieillard, J.; Feuilloley, M.G.J.; Orange, N.; Hancock, R.E.W.; Dufour, A.; et al. Transcription of the oprF gene of Pseudomonas aeruginosa is dependent mainly on the SigX sigma factor and is sucrose induced. J. Bacteriol. 2012, 194, 4301–4311. [Google Scholar] [CrossRef]
- Serio, A.W.; Keepers, T.; Andrews, L.; Krause, K.M. Aminoglycoside Revival: Review of a Historically Important Class of Antimicrobials Undergoing Rejuvenation. EcoSal Plus 2018, 8, 10–1128. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2010, 13, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Deb, J.K. Molecular understanding of aminoglycoside action and resistance. Appl. Microbiol. Biotechnol. 2006, 70, 140–150. [Google Scholar] [CrossRef]
- Wohlleben, W.; Arnold, W.; Bissonnette, L.; Pelletier, A.; Tanguay, A.; Roy, P.H.; Gamboa, G.C.; Barry, G.F.; Aubert, E.; Davies, J. On the evolution of Tn21-like multiresistance transposons: Sequence analysis of the gene (aacC1) for gentamicin acetyltransferase-3-I(AAC(3)-I), another member of the Tn21-based expression cassette. Mol. Gen. Genet. MGG 1989, 217, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, E.; Yunusov, T.; Shenhav, L.; Schornack, S. N-acetyltransferase AAC(3)-I confers gentamicin resistance to Phytophthora palmivora and Phytophthora infestans. BMC Microbiol. 2019, 19, 265. [Google Scholar] [CrossRef]
- Tabor, D.E.; Oganesyan, V.; Keller, A.E.; Yu, L.; McLaughlin, R.E.; Song, E.; Warrener, P.; Rosenthal, K.; Esser, M.; Qi, Y.; et al. Pseudomonas aeruginosa PcrV and Psl, the Molecular Targets of Bispecific Antibody MEDI3902, Are Conserved Among Diverse Global Clinical Isolates. J. Infect. Dis. 2018, 218, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- DiGiandomenico, A.; Rao, J.; Harcher, K.; Zaidi, T.S.; Gardner, J.; Neely, A.N.; Pier, G.B.; Goldberg, J.B. Intranasal immunization with heterologously expressed polysaccharide protects against multiple Pseudomonas aeruginosa infections. Proc. Natl. Acad. Sci. USA 2007, 104, 4624–4629. [Google Scholar] [CrossRef]
- Meynet, E.; Laurin, D.; Lenormand, J.L.; Camara, B.; Toussaint, B.; Le Gouëllec, A. Killed but metabolically active Pseudomonas aeruginosa-based vaccine induces protective humoral- and cell-mediated immunity against Pseudomonas aeruginosa pulmonary infections. Vaccine 2018, 36, 1893–1900. [Google Scholar] [CrossRef]
- Thanabalasuriar, A.; Surewaard, B.G.; Willson, M.E.; Neupane, A.S.; Stover, C.K.; Warrener, P.; Wilson, G.; Keller, A.E.; Sellman, B.R.; DiGiandomenico, A.; et al. Bispecific antibody targets multiple Pseudomonas aeruginosa evasion mechanisms in the lung vasculature. J. Clin. Investig. 2017, 127, 2249–2261. [Google Scholar] [CrossRef]
- Hassan, R.; El-Naggar, W.; Abd El-Aziz, A.M.; Shaaban, M.; Kenawy, H.I.; Ali, Y.M. Immunization with outer membrane proteins (OprF and OprI) and flagellin B protects mice from pulmonary infection with mucoid and nonmucoid Pseudomonas aeruginosa. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi 2018, 51, 312–320. [Google Scholar] [CrossRef]
- Bruhns, P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012, 119, 5640–5649. [Google Scholar] [CrossRef]
- Zuo, Y.; Deng, G.-M. Fc Gamma Receptors as Regulators of Bone Destruction in Inflammatory Arthritis. Front. Immunol. 2021, 12, 688201. [Google Scholar] [CrossRef]
- Galvez-Cancino, F.; Simpson, A.P.; Costoya, C.; Matos, I.; Qian, D.; Peggs, K.S.; Litchfield, K.; Quezada, S.A. Fcγ receptors and immunomodulatory antibodies in cancer. Nat. Rev. Cancer 2024, 24, 51–71. [Google Scholar] [CrossRef]
- Mukherjee, S.; Lee, S.C.; Casadevall, A. Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect. Immun. 1995, 63, 573–579. [Google Scholar] [CrossRef]
- Ralph, P.; Nakoinz, I. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature 1975, 257, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Kern, N.; Dong, R.; Douglas, S.M.; Vale, R.D.; Morrissey, M.A. Tight nanoscale clustering of Fcγ receptors using DNA origami promotes phagocytosis. eLife 2011, 10, e68311. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, P.; White, D.; Sulchek, T. Effects of Microparticle Size and Fc Density on Macrophage Phagocytosis. PLoS ONE 2013, 8, e60989. [Google Scholar] [CrossRef]
- Gallo, P.; Gonçalves, R.; Mosser, D.M. The influence of IgG Density and Macrophage Fc (gamma) Receptor Cross-linking on Phagocytosis and IL-10 Production. Immunol. Lett. 2010, 133, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Bakalar, M.H.; Joffe, A.M.; Schmid, E.M.; Son, S.; Podolski, M.; Fletcher, D.A. Size-dependent segregation controls macrophage phagocytosis of antibody-opsonized targets. Cell 2018, 174, 131–142.e13. [Google Scholar] [CrossRef]
- Anttila, M.; Eskola, J.; Ahman, H.; Käyhty, H. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine 1999, 17, 1970–1977. [Google Scholar] [CrossRef]
- Lee, L.H.; Frasch, C.E.; Falk, L.A.; Klein, D.L.; Deal, C.D. Correlates of immunity for pneumococcal conjugate vaccines. Vaccine 2003, 21, 2190–2196. [Google Scholar] [CrossRef]
- Usinger, W.R.; Lucas, A.H. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect. Immun. 1999, 67, 2366–2370. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, C.M.; Mijacika, A.; Somoza, B.; Osorio, J.C. Protocol for assessing antibody-dependent cellular phagocytosis by primary murine and human macrophages. STAR Protoc. 2025, 6, 103787. [Google Scholar] [CrossRef] [PubMed]
- Cassin, E.K.; Tseng, B.S. Pushing beyond the Envelope: The Potential Roles of OprF in Pseudomonas aeruginosa Biofilm Formation and Pathogenicity. J. Bacteriol. 2019, 201, e00050-19. [Google Scholar] [CrossRef]
- Bukhari, S.I.; Aleanizy, F.S. Association of OprF mutant and disturbance of biofilm and pyocyanin virulence in Pseudomonas aeruginosa. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2020, 28, 196–200. [Google Scholar] [CrossRef]
- Cassin, E.K.; Araujo-Hernandez, S.A.; Baughn, D.S.; Londono, M.C.; Rodriguez, D.Q.; Al-Otaibi, N.S.; Picard, A.; Bergeron, J.R.C.; Tseng, B.S. OprF Impacts Pseudomonas aeruginosa Biofilm Matrix eDNA Levels in a Nutrient-Dependent Manner. J. Bacteriol. 2023, 205, e0008023. [Google Scholar] [CrossRef] [PubMed]
- Bouffartigues, E.; Moscoso, J.A.; Duchesne, R.; Rosay, T.; Fito-Boncompte, L.; Gicquel, G.; Maillot, O.; Bénard, M.; Bazire, A.; Brenner-Weiss, G.; et al. The absence of the Pseudomonas aeruginosa OprF protein leads to increased biofilm formation through variation in c-di-GMP level. Front. Microbiol. 2015, 6, 630. [Google Scholar] [CrossRef]
- Yoon, S.S.; Hennigan, R.F.; Hilliard, G.M.; Ochsner, U.A.; Parvatiyar, K.; Kamani, M.C.; Allen, H.L.; DeKievit, T.R.; Gardner, P.R.; Schwab, U.; et al. Pseudomonas aeruginosa anaerobic respiration in biofilms: Relationships to cystic fibrosis pathogenesis. Dev. Cell 2002, 3, 593–603. [Google Scholar] [CrossRef]
- Song, F.; Wang, H.; Sauer, K.; Ren, D. Cyclic-di-GMP and oprF Are Involved in the Response of Pseudomonas aeruginosa to Substrate Material Stiffness during Attachment on Polydimethylsiloxane (PDMS). Front. Microbiol. 2018, 9, 110. [Google Scholar] [CrossRef]
- Barati, H.; Fekrirad, Z.; Jalali Nadoushan, M.; Rasooli, I. Anti-OmpA antibodies as potential inhibitors of Acinetobacter baumannii biofilm formation, adherence to, and proliferation in A549 human alveolar epithelial cells. Microb. Pathog. 2024, 186, 106473. [Google Scholar] [CrossRef]
- Muller, M. Pyocyanin induces oxidative stress in human endothelial cells and modulates the glutathione redox cycle. Free Radic. Biol. Med. 2002, 33, 1527–1533. [Google Scholar] [CrossRef]
- Reszka, K.J.; O’Malley, Y.; McCormick, M.L.; Denning, G.M.; Britigan, B.E. Oxidation of pyocyanin, a cytotoxic product from Pseudomonas aeruginosa, by microperoxidase 11 and hydrogen peroxide. Free Radic. Biol. Med. 2004, 36, 1448–1459. [Google Scholar] [CrossRef]
- Lau, G.W.; Hassett, D.J.; Ran, H.; Kong, F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004, 10, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, C.C.; Chen, Y.; Goetzmann, H.S.; Hao, Y.; Borchers, M.T.; Hassett, D.J.; Young, L.R.; Mavrodi, D.; Thomashow, L.; Lau, G.W. Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis. Am. J. Pathol. 2009, 175, 2473–2488. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S.; Iglewski, B.H.P. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 2003, 6, 56–60. [Google Scholar] [CrossRef]
- Wagner, V.E.; Frelinger, J.G.; Barth, R.K.; Iglewski, B.H. Quorum sensing: Dynamic response of Pseudomonas aeruginosa to external signals. Trends Microbiol. 2006, 14, 55–58. [Google Scholar] [CrossRef]
- Hajjar, H.; Berry, L.; Wu, Y.; Touqui, L.; Vergunst, A.C.; Blanc-Potard, A.-B. Contribution of intramacrophage stages to Pseudomonas aeruginosa infection outcome in zebrafish embryos: Insights from mgtC and oprF mutants. Sci. Rep. 2024, 14, 6297. [Google Scholar] [CrossRef] [PubMed]
- Farsani, H.H.; Rasooli, I.; Gargari, S.L.M.; Nazarian, S.; Astaneh, S.D.A. Recombinant outer membrane protein F-B subunit of LT protein as a prophylactic measure against Pseudomonas aeruginosa burn infection in mice. World J. Methodol. 2015, 5, 230–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, L.; Zhao, X.; Jiang, X.; Qin, J.; Wang, Y.; Yu, X. Enhanced protective efficacy of an OprF/PcrV bivalent DNA vaccine against Pseudomonas aeruginosa using a hydrogel delivery system. Biomed. Pharmacother. Biomed. Pharmacother 2024, 172, 116264. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Rcheulishvili, N.; Papukashvili, D.; Xie, F.; Zhao, J.; Hu, X.; Yu, K.; Yang, N.; Pan, X.; et al. Strong immune responses and protection of PcrV and OprF-I mRNA vaccine candidates against Pseudomonas aeruginosa. NPJ Vaccines 2023, 8, 76. [Google Scholar] [CrossRef]
- Shaikh, M.O.F.; Schaefers, M.M.; Merakou, C.; DiBlasi, M.; Bonney, S.; Liao, T.; Zurakowski, D.; Kehl, M.; Tabor, D.E.; DiGiandomenico, A.; et al. Multicomponent Pseudomonas aeruginosa Vaccines Eliciting Th17 Cells and Functional Antibody Responses Confer Enhanced Protection against Experimental Acute Pneumonia in Mice. Infect. Immun. 2022, 90, e0020322. [Google Scholar] [CrossRef]
- Katseff, A.S.; Toumanios, C.; Barahim, E.; McCormick, A.A.; Arnaboldi, P.M. PcrV in an intranasal adjuvanted tobacco mosaic virus conjugate vaccine mediates protection from Pseudomonas aeruginosa via an early Th1/Th17 skewed localized and systemic immune response. Vaccine 2025, 60, 127306. [Google Scholar] [CrossRef]
- Weimer, E.T.; Lu, H.; Kock, N.D.; Wozniak, D.J.; Mizel, S.B. A fusion protein vaccine containing OprF epitope 8, OprI, and type A and B flagellins promotes enhanced clearance of nonmucoid Pseudomonas aeruginosa. Infect. Immun. 2009, 77, 2356–2366. [Google Scholar] [CrossRef]
- Bahnan, W.; Happonen, L.; Khakzad, H.; Kumra Ahnlide, V.; de Neergaard, T.; Wrighton, S.; André, O.; Bratanis, E.; Tang, D.; Hellmark, T.; et al. A human monoclonal antibody bivalently binding two different epitopes in streptococcal M protein mediates immune function. EMBO Mol. Med. 2023, 15, e16208. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.P.; DiVenere, A.M.; Amengor, D.; Maynard, J.A. Antibodies binding diverse pertactin epitopes protect mice from Bordetella pertussis infection. J. Biol. Chem. 2022, 298, 101715. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, R.C.; Adekunle, O.; Yu, H.; Cho, A.; Nyhoff, L.E.; Kelly, M.; Harris, J.B.; Bhuiyan, T.R.; Qadri, F.; Calderwood, S.B.; et al. Impact of Immunoglobulin Isotype and Epitope on the Functional Properties of Vibrio cholerae O-Specific Polysaccharide-Specific Monoclonal Antibodies. mBio 2021, 12, e03679-20. [Google Scholar] [CrossRef] [PubMed]
- Freschi, L.; Vincent, A.T.; Jeukens, J.; Emond-Rheault, J.-G.; Kukavica-Ibrulj, I.; Dupont, M.-J.; Charette, S.J.; Boyle, B.; Levesque, R.C. The Pseudomonas aeruginosa Pan-Genome Provides New Insights on Its Population Structure, Horizontal Gene Transfer, and Pathogenicity. Genome Biol. Evol. 2019, 11, 109–120. [Google Scholar] [CrossRef]
- Johnson, K.; Delaney, J.C.; Guillard, T.; Reffuveille, F.; Varin-Simon, J.; Li, K.; Wollacott, A.; Frapy, E.; Mong, S.; Tissire, H.; et al. Development of an antibody fused with an antimicrobial peptide targeting Pseudomonas aeruginosa: A new approach to prevent and treat bacterial infections. PLoS Pathog. 2023, 19, e1011612. [Google Scholar] [CrossRef]
- Mimoto, F.; Kuramochi, T.; Katada, H.; Igawa, T.; Hattori, K. Fc Engineering to Improve the Function of Therapeutic Antibodies. Curr. Pharm. Biotechnol. 2016, 17, 1298–1314. [Google Scholar] [CrossRef]
- Liu, R.; Oldham, R.J.; Teal, E.; Beers, S.A.; Cragg, M.S. Fc-Engineering for Modulated Effector Functions-Improving Antibodies for Cancer Treatment. Antibodies Basel Switz. 2020, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.A.; Chan, K.F.; Lin, P.C.; Song, Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. mAbs 2018, 10, 693–711. [Google Scholar] [CrossRef]
- Wilhelm, O.; Jordan, C.; Kek, H.; Brunton-O’Sullivan, M.M.; Rikard-Bell, L.; Ramanathan, P.; Chung, A.W.; Poumbourios, P.; Wines, B.D.; Jaworowski, A.; et al. Afucosylated broadly neutralizing antibodies targeting the HIV envelope elicit enhanced NK-cell-mediated cytotoxicity against HIV-infected CD4+ T-cell and macrophage targets. J. Leukoc. Biol. 2025, 117, qiaf033. [Google Scholar] [CrossRef]
- Liu, S.D.; Chalouni, C.; Young, J.C.; Junttila, T.T.; Sliwkowski, M.X.; Lowe, J.B. Afucosylated antibodies increase activation of FcγRIIIa-dependent signaling components to intensify processes promoting ADCC. Cancer Immunol. Res. 2015, 3, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.S.; Gunn, B.M.; Lu, L.L. Engineering the supernatural: Monoclonal antibodies for challenging infectious diseases. Curr. Opin. Biotechnol. 2022, 78, 102818. [Google Scholar] [CrossRef]
- Sause, W.E.; Buckley, P.T.; Strohl, W.R.; Lynch, A.S.; Torres, V.J. Antibody-Based Biologics and Their Promise to Combat Staphylococcus aureus Infections. Trends Pharmacol. Sci. 2016, 37, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; Hammond, S.A.; Oberst, M.; Wilkinson, R.W. The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J. Immunother. Cancer 2014, 2, 29. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005, 310, 1510–1512. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, B.; Delic-Attree, I.; Vignais, P.M. Pseudomonas aeruginosa contains an IHF-like protein that binds to the algD promoter. Biochem. Biophys. Res. Commun. 1993, 196, 416–421. [Google Scholar] [CrossRef] [PubMed]
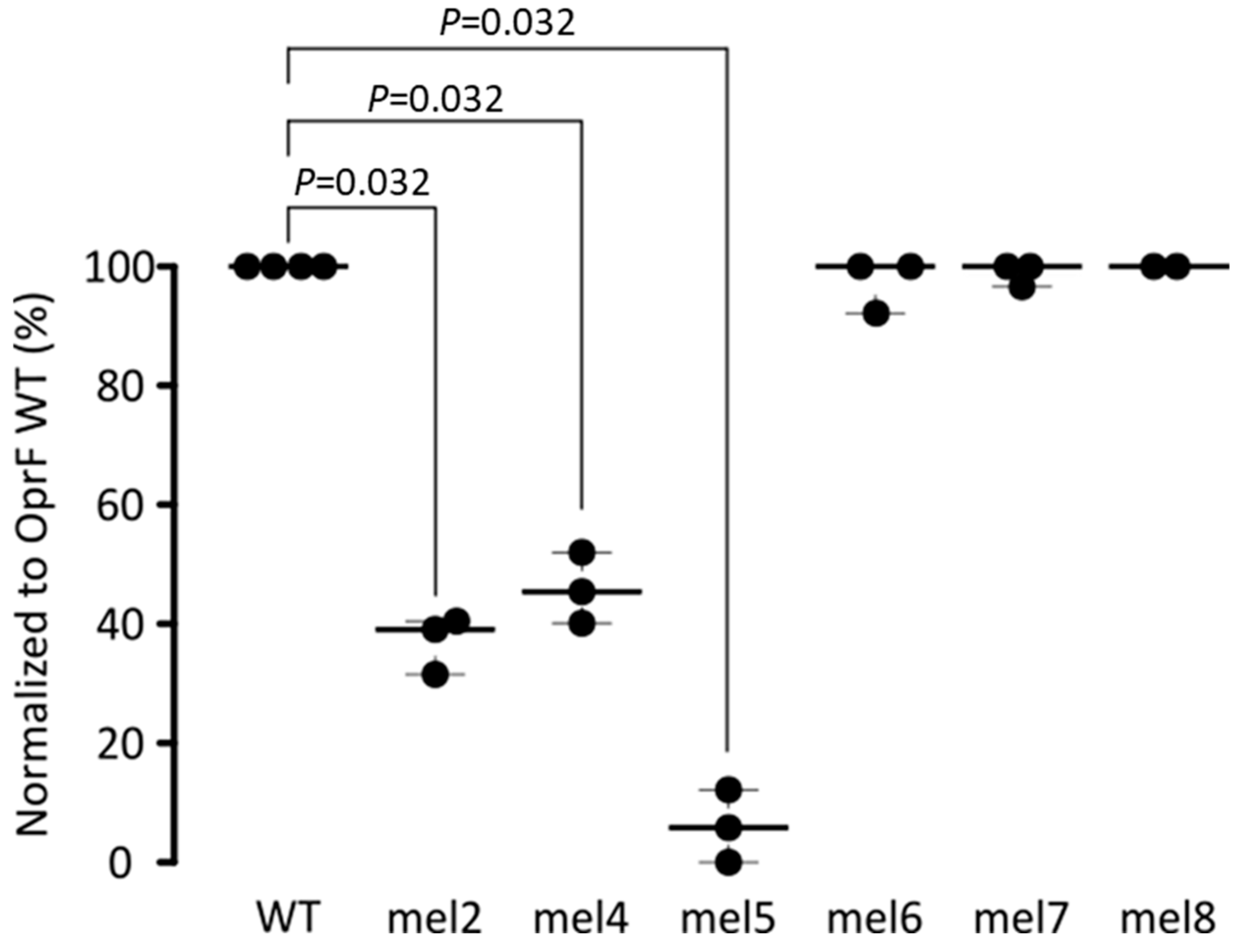

| Mutated Extracellular Loop OprF | Mutations |
|---|---|
| mel2 | Y94A E95A N98A K100A |
| mel4 | D180A H183A Q184A E1861 |
| mel5 | D202A D206A V208A N211A |
| mel6 | K263A K265A E2661 N267A |
| mel7 | Y301A N302A K304A E307A |
| Mel8 | E322A R324A V326E N329A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacroix, G.; Lenormand, J.-L. EPY001, a Novel Monoclonal Antibody Against Pseudomonas aeruginosa Targeting OprF. Int. J. Mol. Sci. 2025, 26, 10380. https://doi.org/10.3390/ijms262110380
Lacroix G, Lenormand J-L. EPY001, a Novel Monoclonal Antibody Against Pseudomonas aeruginosa Targeting OprF. International Journal of Molecular Sciences. 2025; 26(21):10380. https://doi.org/10.3390/ijms262110380
Chicago/Turabian StyleLacroix, Guillaume, and Jean-Luc Lenormand. 2025. "EPY001, a Novel Monoclonal Antibody Against Pseudomonas aeruginosa Targeting OprF" International Journal of Molecular Sciences 26, no. 21: 10380. https://doi.org/10.3390/ijms262110380
APA StyleLacroix, G., & Lenormand, J.-L. (2025). EPY001, a Novel Monoclonal Antibody Against Pseudomonas aeruginosa Targeting OprF. International Journal of Molecular Sciences, 26(21), 10380. https://doi.org/10.3390/ijms262110380






