Impaired Peripheral Blood Mononuclear Cell (PBMC) Mitochondrial Respiration Is Associated with Mortality and Long COVID Syndrome Severity in COVID-19 Patients
Abstract
1. Introduction
2. Results
2.1. Clinical and Biological Characteristics of the Population
2.2. Long-COVID Symptoms Characterization
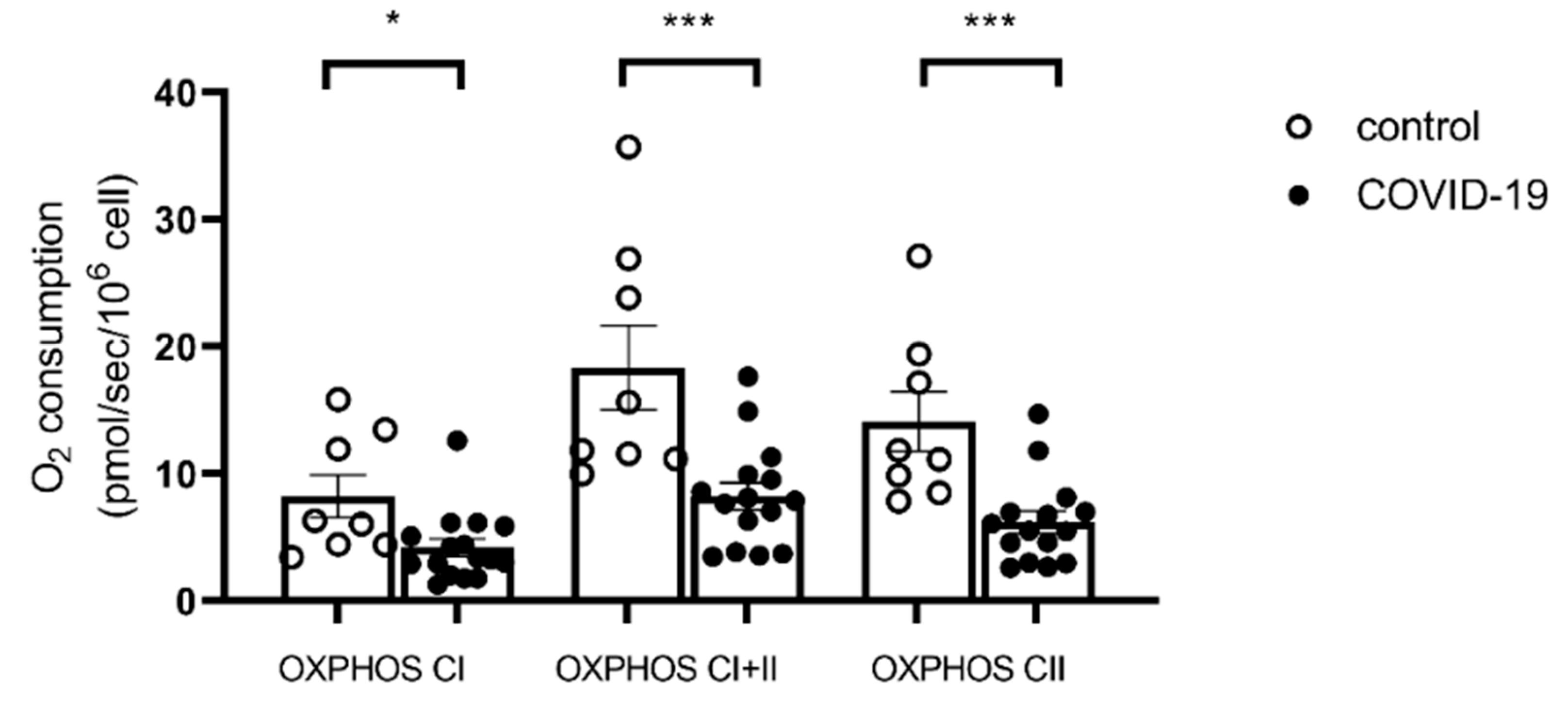
2.3. PBMC Mitochondrial Respiration
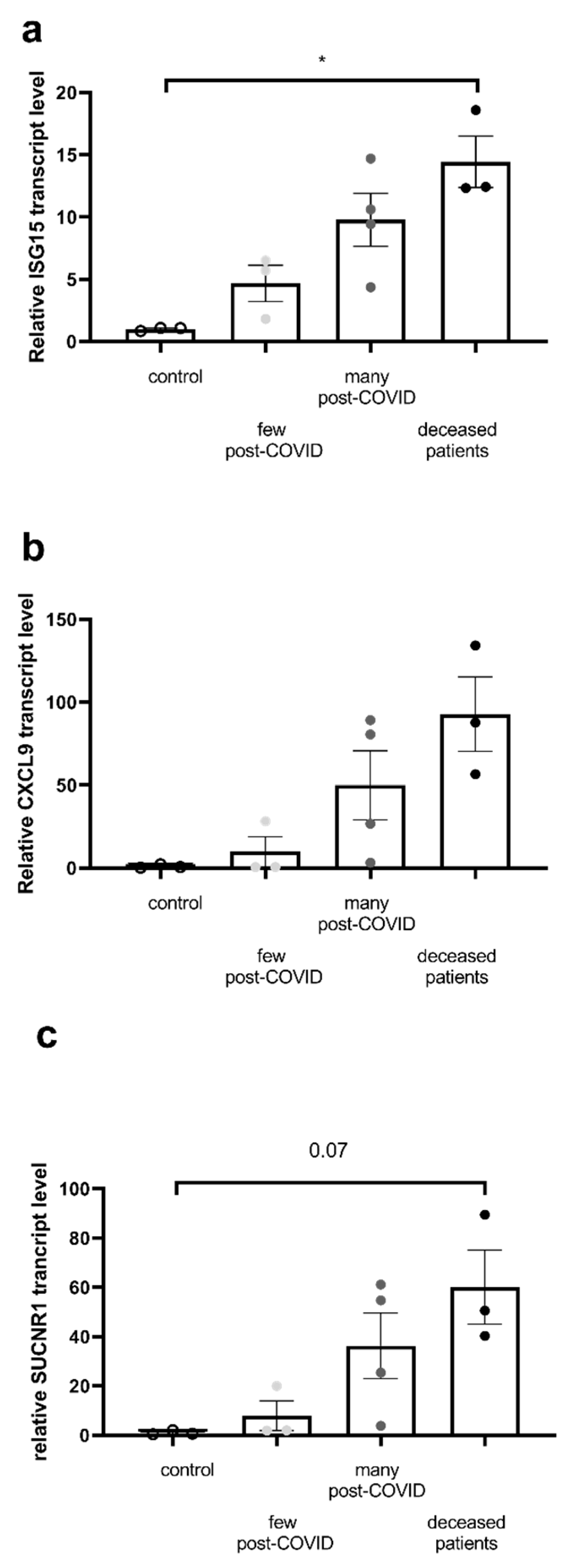
2.4. Inflammatory Marker Transcripts Are Enhanced in the Most Severe COVID Patients
3. Discussion
3.1. PBMC Mitochondrial Respiration Is Impaired in COVID-19 Patients
3.2. Mortality Is Associated with Severe Impairment in PBMC Mitochondrial Respiration
3.3. Severe Long-COVID Symptoms Are Associated with Severe Impairment in PBMC Mitochondrial Respiration
3.4. Limitations of the Study
4. Methods
4.1. Population and Study Design
4.2. Peripheral Blood Mononuclear Cell (PBMC) Mitochondrial Isolation and Respiration
4.2.1. Peripheral Blood Mononuclear Cell (PBMC) Isolation
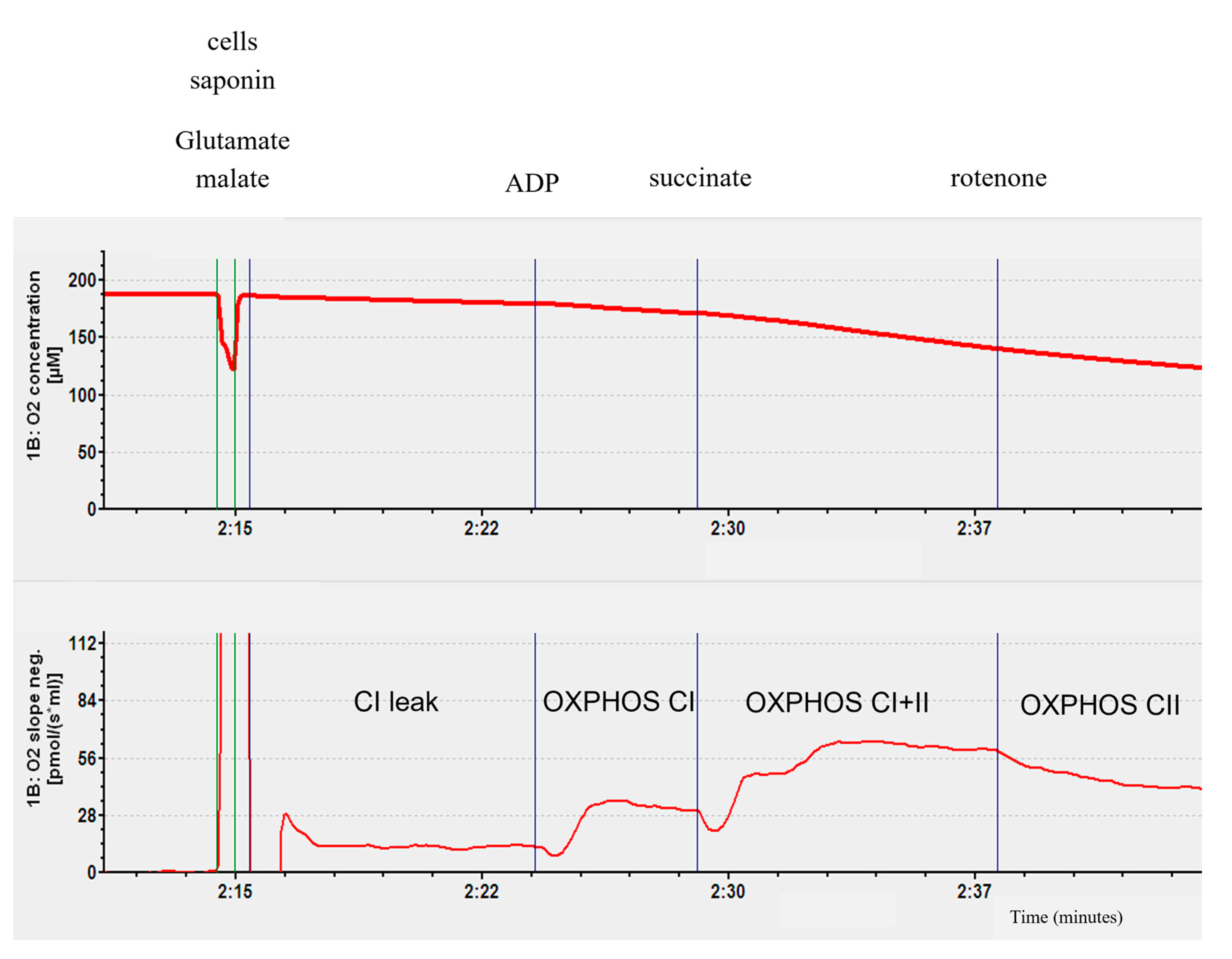
4.2.2. Mitochondrial Respiratory Chain Complex Respiration Assessment
4.3. RT-PCR
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Huang, C.-F.; Tay, C.K.; Zhuang, Y.-F.; Liu, J.; Sewa, D.W. Rationale and Significance of Patient Selection in Awake Prone Positioning for COVID-19 Pneumonia. Eur. Respir. J. 2020, 56, 2002173. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269, Erratum in Nature 2020, 580, E7. [Google Scholar] [CrossRef]
- Worobey, M.; Levy, J.I.; Malpica Serrano, L.; Crits-Christoph, A.; Pekar, J.E.; Goldstein, S.A.; Rasmussen, A.L.; Kraemer, M.U.G.; Newman, C.; Koopmans, M.P.G.; et al. The Huanan Seafood Wholesale Market in Wuhan Was the Early Epicenter of the COVID-19 Pandemic. Science 2022, 377, 951–959. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146, Erratum in Nat. Rev. Microbiol. 2023, 21, 408. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease (COVID-19). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/coronavirus-disease-(Covid-19) (accessed on 19 September 2025).
- Whitaker, M.; Elliott, J.; Chadeau-Hyam, M.; Riley, S.; Darzi, A.; Cooke, G.; Ward, H.; Elliott, P. Persistent COVID-19 Symptoms in a Community Study of 606,434 People in England. Nat. Commun. 2022, 13, 1957. [Google Scholar] [CrossRef]
- Tarazona, V.; Kirouchena, D.; Clerc, P.; Pinsard-Laventure, F.; Bourrion, B. Quality of Life in COVID-19 Outpatients: A Long-Term Follow-Up Study. J. Clin. Med. 2022, 11, 6478. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Nikolic Turnic, T.; Vasiljevic, I.; Stanic, M.; Jakovljevic, B.; Mikerova, M.; Ekkert, N.; Reshetnikov, V.; Jakovljevic, V. Post-COVID-19 Status and Its Physical, Nutritional, Psychological, and Social Effects in Working-Age Adults-A Prospective Questionnaire Study. J. Clin. Med. 2022, 11, 6668. [Google Scholar] [CrossRef] [PubMed]
- Van Wambeke, E.; Bezler, C.; Kasprowicz, A.-M.; Charles, A.-L.; Andres, E.; Geny, B. Two-Years Follow-Up of Symptoms and Return to Work in Complex Post-COVID-19 Patients. J. Clin. Med. 2023, 12, 741. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Post COVID-19 Condition (Long COVID). 2022. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-COVID-19-condition (accessed on 19 September 2025).
- Cervia, C.; Zurbuchen, Y.; Taeschler, P.; Ballouz, T.; Menges, D.; Hasler, S.; Adamo, S.; Raeber, M.E.; Bächli, E.; Rudiger, A.; et al. Immunoglobulin Signature Predicts Risk of Post-Acute COVID-19 Syndrome. Nat. Commun. 2022, 13, 446. [Google Scholar] [CrossRef]
- Pinto, M.D.; Downs, C.A.; Huang, Y.; El-Azab, S.A.; Ramrakhiani, N.S.; Barisano, A.; Yu, L.; Taylor, K.; Esperanca, A.; Abrahim, H.L.; et al. A Distinct Symptom Pattern Emerges for COVID-19 Long-Haul: A Nationwide Study. Sci. Rep. 2022, 12, 15905. [Google Scholar] [CrossRef]
- Ballering, A.V.; van Zon, S.K.R.; Olde Hartman, T.C.; Rosmalen, J.G.M. Lifelines Corona Research Initiative Persistence of Somatic Symptoms after COVID-19 in the Netherlands: An Observational Cohort Study. Lancet Lond. Engl. 2022, 400, 452–461. [Google Scholar] [CrossRef]
- Wanga, V.; Chevinsky, J.R.; Dimitrov, L.V.; Gerdes, M.E.; Whitfield, G.P.; Bonacci, R.A.; Nji, M.A.M.; Hernandez-Romieu, A.C.; Rogers-Brown, J.S.; McLeod, T.; et al. Long-Term Symptoms Among Adults Tested for SARS-CoV-2—United States, January 2020-April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1235–1241. [Google Scholar] [CrossRef]
- Riou, M.; Oulehri, W.; Momas, C.; Rouyer, O.; Lebourg, F.; Meyer, A.; Enache, I.; Pistea, C.; Charloux, A.; Marcot, C.; et al. Reduced Flow-Mediated Dilatation Is Not Related to COVID-19 Severity Three Months after Hospitalization for SARS-CoV-2 Infection. J. Clin. Med. 2021, 10, 1318. [Google Scholar] [CrossRef] [PubMed]
- Riou, M.; Coste, F.; Meyer, A.; Enache, I.; Talha, S.; Charloux, A.; Reboul, C.; Geny, B. Mechanisms of Pulmonary Vasculopathy in Acute and Long-Term COVID-19: A Review. Int. J. Mol. Sci. 2024, 25, 4941. [Google Scholar] [CrossRef] [PubMed]
- Romão, P.R.; Teixeira, P.C.; Schipper, L.; da Silva, I.; Santana Filho, P.; Júnior, L.C.R.; Peres, A.; Gonçalves da Fonseca, S.; Chagas Monteiro, M.; Lira, F.S.; et al. Viral Load Is Associated with Mitochondrial Dysfunction and Altered Monocyte Phenotype in Acute Severe SARS-CoV-2 Infection. Int. Immunopharmacol. 2022, 108, 108697. [Google Scholar] [CrossRef]
- Gibellini, L.; De Biasi, S.; Paolini, A.; Borella, R.; Boraldi, F.; Mattioli, M.; Lo Tartaro, D.; Fidanza, L.; Caro-Maldonado, A.; Meschiari, M.; et al. Altered Bioenergetics and Mitochondrial Dysfunction of Monocytes in Patients with COVID-19 Pneumonia. EMBO Mol. Med. 2020, 12, e13001. [Google Scholar] [CrossRef] [PubMed]
- Ajaz, S.; McPhail, M.J.; Singh, K.K.; Mujib, S.; Trovato, F.M.; Napoli, S.; Agarwal, K. Mitochondrial Metabolic Manipulation by SARS-CoV-2 in Peripheral Blood Mononuclear Cells of Patients with COVID-19. Am. J. Physiol. Cell Physiol. 2021, 320, C57–C65. [Google Scholar] [CrossRef]
- De la Cruz-Enríquez, J.; Rojas-Morales, E.; Ruíz-García, M.G.; Tobón-Velasco, J.C.; Jiménez-Ortega, J.C. SARS-CoV-2 Induces Mitochondrial Dysfunction and Cell Death by Oxidative Stress/Inflammation in Leukocytes of COVID-19 Patients. Free Radic. Res. 2021, 55, 982–995. [Google Scholar] [CrossRef]
- Bizjak, D.A.; Ohmayer, B.; Buhl, J.L.; Schneider, E.M.; Walther, P.; Calzia, E.; Jerg, A.; Matits, L.; Steinacker, J.M. Functional and Morphological Differences of Muscle Mitochondria in Chronic Fatigue Syndrome and Post-COVID Syndrome. Int. J. Mol. Sci. 2024, 25, 1675. [Google Scholar] [CrossRef]
- Nemeikaitė-Čėnienė, A.; Marozienė, A.; Misevičienė, L.; Tamulienė, J.; Yantsevich, A.V.; Čėnas, N. 5Flavoenzyme-Catalyzed Single-Electron Reduction of Nitroaromatic Antiandrogens: Implications for Their Cytotoxicity. Free Radic. Res. 2021, 55, 246–254. [Google Scholar] [CrossRef]
- De Vitis, C.; Capalbo, C.; Torsello, A.; Napoli, C.; Salvati, V.; Loffredo, C.; Blandino, G.; Piaggio, G.; Auciello, F.R.; Pelliccia, F.; et al. Opposite Effect of Thyroid Hormones on Oxidative Stress and on Mitochondrial Respiration in COVID-19 Patients. Antioxidants 2022, 11, 1998. [Google Scholar] [CrossRef]
- Alfatni, A.; Riou, M.; Charles, A.-L.; Meyer, A.; Barnig, C.; Andres, E.; Lejay, A.; Talha, S.; Geny, B. Peripheral Blood Mononuclear Cells and Platelets Mitochondrial Dysfunction, Oxidative Stress, and Circulating mtDNA in Cardiovascular Diseases. J. Clin. Med. 2020, 9, 311. [Google Scholar] [CrossRef]
- Singh, K.K.; Chaubey, G.; Chen, J.Y.; Suravajhala, P. Decoding SARS-CoV-2 Hijacking of Host Mitochondria in COVID-19 Pathogenesis. Am. J. Physiol. Cell Physiol. 2020, 319, C258–C267. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, C.; Rao, Y.; Ngo, C.; Feng, J.J.; Zhao, J.; Zhang, S.; Wang, T.-Y.; Carriere, J.; Savas, A.C.; et al. SARS-CoV-2 Nsp5 Demonstrates Two Distinct Mechanisms Targeting RIG-I and MAVS To Evade the Innate Immune Response. mBio 2021, 12, e0233521. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, P.; Ma, W.; Wang, X.; Wang, H.; Yu, Z.; Chang, H.; Wang, T.; Jin, S.; Wang, X.; et al. SARS-CoV-2 ORF10 Suppresses the Antiviral Innate Immune Response by Degrading MAVS through Mitophagy. Cell. Mol. Immunol. 2022, 19, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug Repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Huynh, T.V.; Rethi, L.; Lee, T.-W.; Higa, S.; Kao, Y.-H.; Chen, Y.-J. Spike Protein Impairs Mitochondrial Function in Human Cardiomyocytes: Mechanisms Underlying Cardiac Injury in COVID-19. Cells 2023, 12, 877. [Google Scholar] [CrossRef]
- Liang, S.; Bao, C.; Yang, Z.; Liu, S.; Sun, Y.; Cao, W.; Wang, T.; Schwantes-An, T.-H.; Choy, J.S.; Naidu, S.; et al. SARS-CoV-2 Spike Protein Induces IL-18-Mediated Cardiopulmonary Inflammation via Reduced Mitophagy. Signal Transduct. Target. Ther. 2023, 8, 108. [Google Scholar] [CrossRef]
- Lee, E.; Ozigbo, A.A.; Varon, J.; Halma, M.; Laezzo, M.; Ang, S.P.; Iglesias, J. Mitochondrial Reactive Oxygen Species: A Unifying Mechanism in Long COVID and Spike Protein-Associated Injury: A Narrative Review. Biomolecules 2025, 15, 1339. [Google Scholar] [CrossRef]
- Refrigeri, M.; Tola, A.; Mogavero, R.; Pietracupa, M.M.; Gionta, G.; Scatena, R. Mechanisms of Mitochondrial Impairment by SARS-CoV-2 Proteins: A Nexus of Pathogenesis with Significant Biochemical and Clinical Implications. Int. J. Mol. Sci. 2025, 26, 9885. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Mathioudakis, A.G.; Nikitara, K.; Stamatelopoulos, K.; Georgiopoulos, G.; Phalkey, R.; Leonardi-Bee, J.; Fernandez, E.; Carnicer-Pont, D.; Vestbo, J.; et al. Prognostic Factors for Mortality, Intensive Care Unit and Hospital Admission Due to SARS-CoV-2: A Systematic Review and Meta-Analysis of Cohort Studies in Europe. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2022, 31, 220098. [Google Scholar] [CrossRef] [PubMed]
- Regolo, M.; Vaccaro, M.; Sorce, A.; Stancanelli, B.; Colaci, M.; Natoli, G.; Russo, M.; Alessandria, I.; Motta, M.; Santangelo, N.; et al. Neutrophil-to-Lymphocyte Ratio (NLR) Is a Promising Predictor of Mortality and Admission to Intensive Care Unit of COVID-19 Patients. J. Clin. Med. 2022, 11, 2235. [Google Scholar] [CrossRef] [PubMed]
- de Boer, E.; Petrache, I.; Goldstein, N.M.; Olin, J.T.; Keith, R.C.; Modena, B.; Mohning, M.P.; Yunt, Z.X.; San-Millán, I.; Swigris, J.J. Decreased Fatty Acid Oxidation and Altered Lactate Production during Exercise in Patients with Post-Acute COVID-19 Syndrome. Am. J. Respir. Crit. Care Med. 2022, 205, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Macnaughtan, J.; Chau, K.-Y.; Brennan, E.; Toffoli, M.; Spinazzola, A.; Hillman, T.; Heightman, M.; Schapira, A.H.V. Mitochondrial Function Is Impaired in Long COVID Patients. Ann. Med. 2025, 57, 2528167. [Google Scholar] [CrossRef]
- Aminzadeh, A.; Azmi-Naei, N.; Teimouri, M.; Rohani-Rasaf, M. FIB-4, APRI, and ALRI as Predictors of COVID-19 Outcomes: Insights from a Large-Scale Study. Diagnostics 2025, 15, 1984. [Google Scholar] [CrossRef]
- Sarkar, L.; Liu, G.; Gack, M.U. ISG15: Its Roles in SARS-CoV-2 and Other Viral Infections. Trends Microbiol. 2023, 31, 1262–1275. [Google Scholar] [CrossRef]
- Riou, M.; Charles, A.-L.; Enache, I.; Evrard, C.; Pistea, C.; Giannini, M.; Charloux, A.; Geny, B. Acute Severe Hypoxia Decreases Mitochondrial Chain Complex II Respiration in Human Peripheral Blood Mononuclear Cells. Int. J. Mol. Sci. 2025, 26, 705. [Google Scholar] [CrossRef]
- Levy, D.; Giannini, M.; Oulehri, W.; Riou, M.; Marcot, C.; Pizzimenti, M.; Debrut, L.; Charloux, A.; Geny, B.; Meyer, A. Long Term Follow-Up of Sarcopenia and Malnutrition after Hospitalization for COVID-19 in Conventional or Intensive Care Units. Nutrients 2022, 14, 912. [Google Scholar] [CrossRef]
- Dirajlal-Fargo, S.; Maison, D.P.; Durieux, J.C.; Andrukhiv, A.; Funderburg, N.; Ailstock, K.; Gerschenson, M.; Mccomsey, G.A. Altered Mitochondrial Respiration in Peripheral Blood Mononuclear Cells of Post-Acute Sequelae of SARS-CoV-2 Infection. Mitochondrion 2024, 75, 101849. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Coelho-Júnior, H.J.; Calvani, R.; Girolimetti, G.; Di Corato, R.; Ciciarello, F.; Galluzzo, V.; Di Mario, C.; Tolusso, B.; Santoro, L.; et al. Mitochondria-Derived Vesicles and Inflammatory Profiles of Adults with Long COVID Supplemented with Red Beetroot Juice: Secondary Analysis of a Randomized Controlled Trial. Int. J. Mol. Sci. 2025, 26, 1224. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.R.; Kadam, S.; Brahme, A.; Agrawal, M.; Apte, K.; Narke, G.; Kekan, K.; Madas, S.; Salvi, S. Systemic Immuno-Metabolic Alterations in Chronic Obstructive Pulmonary Disease (COPD). Respir. Res. 2019, 20, 171. [Google Scholar] [CrossRef]
- Riou, M.; Alfatni, A.; Charles, A.-L.; Andrès, E.; Pistea, C.; Charloux, A.; Geny, B. New Insights into the Implication of Mitochondrial Dysfunction in Tissue, Peripheral Blood Mononuclear Cells, and Platelets during Lung Diseases. J. Clin. Med. 2020, 9, 1253. [Google Scholar] [CrossRef] [PubMed]



| Healthy Controls | COVID Patients | p Value | ||||
|---|---|---|---|---|---|---|
| Total COVID Patients | With Few Post-COVID Symptoms | With Many Post-COVID Symptoms | Deceased Patients | |||
| Gender (M/F) | 5/3 | 14/6 | 5/1 | 3/3 | 3/1 | |
| Age (years) | 59.49 ± 1.10 | 65.69 ± 1.69 | 67.22 ± 3.50 | 63.65 ± 2.30 | 68.75 ± 4.73 | 0.055 |
| Body Mass Index (kg/m2) | 25.0 ± 1.60 | 29.50 ± 1.33 | 27.17 ± 1.08 | 31.33 ± 2.39 | 29.5 ± 3.62 | 0.029 |
| Comorbidities (n, %) | ||||||
| Hypertension | 0 | 9, 45% | 3, 50% | 3, 50% | 2, 50% | |
| Dyslipidaemia | 0 | 8, 40% | 3, 50% | 1, 16.6% | 3, 75% | |
| Diabetes | 0 | 7, 35% | 1, 16.6% | 5, 83.3% | 1, 25% | |
| Chronic cardiac insufficiency | 0 | 6, 30% | 2, 33.3% | 3, 50% | 1, 25% | |
| Chronic respiratory insufficiency | 0 | 4, 20% | 0, 0% | 3, 50% | 0, 0% | |
| Chronic renal insufficiency | 0 | 2, 10% | 1, 16.6% | 1, 16.6% | 0, 0% | |
| Normal Values (Range) | Total COVID Patients | Patients with Few Post-COVID Symptoms | Patients with Many Post-COVID Symptoms | Deceased Patients | |
|---|---|---|---|---|---|
| Creatinine (umol/L) ± SEM | 64–104 | 111.5 ± 16.40 n = 20 | 123.5 ± 25.7 n = 6 | 81.98 ± 31.14 n = 6 | 129.9 ± 20.64 n = 4 |
| HB (hemoglobin level, g/dL) | 12–16 | 8.93 ± 0.20 n = 20 p < 0.0001 | 9.02 ± 0.35 n = 6 p < 0.001 | 8.67 ± 0.27 n = 6 p < 0.0001 | 8.92 ± 0.64 n = 4 p < 0.05 |
| Blood Cell Count (×109/L) | |||||
| Leucocytes | 4–11 | 13.52 ± 1.35 n = 20 p = 0.08 | 13.26 ± 1.09 n = 6 p = 0.09 | 11.77 ± 2.46 n = 6 | 18.33 ± 5.13 n = 4 |
| Lymphocytes | 1–4 | 1.38 ± 0.19 n = 17 | 1.42 ± 0.12 n = 6 | 1.82 ± 0.62 n = 5 | 0.93 ± 0.24 n = 3 |
| Neutrophils | 1.40–7.70 | 8.67 ± 0.82 n = 17 | 9.13 ± 1.05 n = 6 | 8.60 ± 1.97 n = 5 | 9.17 ± 2.25 n = 3 |
| Inflammation | |||||
| CRP (mg/L) ± SEM | <5.0 | 66.38 ± 13.33 n = 12 p < 0.001 | 108.2 ± 33.72 n = 3 p = 0.09 | 60.15 ± 17.33 n = 4 p < 0.05 | 73.43 ± 3.73 n = 3 p < 0.01 |
| NLR | 1.44–1.63 | 7.32 ± 1.11 n = 15 p < 0.001 | 6.66 ± 0.89 n = 6 p < 0.01 | 6.39 ± 1.46 n = 5 p < 0.05 | 13.96 6.54 n = 2 |
| LLR | 2.35–3.81 | 0.11 ± 0.01 n = 17 p < 0.0001 | 0.11 ± 0.01 n = 6 p < 0.0001 | 0.12 ± 0.03 n = 6 p < 0.0001 | 0.07 ± 0.01 n = 20 p < 0.0001 |
| Symptoms Score Mean ± SEM/n | Patients with No or Few COVID Symptoms | Patients with Many COVID Symptoms |
|---|---|---|
| Anosmia/Agueusia | 1/1 | 6.67 ± 0.88/3 |
| Dyspnea (effort/at rest) | 4/1 | 6.5 ± 0.87/4 |
| Tiredness | 6 ± 0/2 | 5.67 ± 0.8/6 |
| Muscle pain | 0/6 | 5.67 ± 1.02/6 |
| Neural trouble | 1.50 ± 0.5/2 | 4.25 ± 0.75/4 |
| Anxiety | 0/6 | 4.67 ± 0.67/3 |
| Thoracic pain | 0/6 | 9/1 |
| Digestive trouble | 0/6 | 7/1 |
| Gene Name | Primer Forward | Primer Reverse |
|---|---|---|
| YWHAZ | 5′-AGGAGATTACTACCGTTACTTGGC-3′ | 5′-AGCTTCTTGGTATGCTTGTTGTG-3′ |
| CXCL9 | 5′-TAAGCGCTAGAGGAAGCAGC-3′ | 5′-TTCACTGAACCTCCCCTGGA-3′ |
| ISG15 | 5′-GATCACCCAGAAGATCGGCG-3′ | 5′-GGATGCTCAGAGGTTCGTCG-3′ |
| SUCNR1 | 5′-CGTTGTGGGAGTCCTTGGAA-3′ | 5′-GGTTGCTTATGCAGAGCACG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charles, A.-L.; Debrut, L.; Oulehri, W.; Vincent, V.; Delagreverie, H.; Asael, P.; Riou, M.; Giannini, M.; Meyer, A.; Geny, B. Impaired Peripheral Blood Mononuclear Cell (PBMC) Mitochondrial Respiration Is Associated with Mortality and Long COVID Syndrome Severity in COVID-19 Patients. Int. J. Mol. Sci. 2025, 26, 10377. https://doi.org/10.3390/ijms262110377
Charles A-L, Debrut L, Oulehri W, Vincent V, Delagreverie H, Asael P, Riou M, Giannini M, Meyer A, Geny B. Impaired Peripheral Blood Mononuclear Cell (PBMC) Mitochondrial Respiration Is Associated with Mortality and Long COVID Syndrome Severity in COVID-19 Patients. International Journal of Molecular Sciences. 2025; 26(21):10377. https://doi.org/10.3390/ijms262110377
Chicago/Turabian StyleCharles, Anne-Laure, Léa Debrut, Walid Oulehri, Véronique Vincent, Héloise Delagreverie, Pauline Asael, Marianne Riou, Margherita Giannini, Alain Meyer, and Bernard Geny. 2025. "Impaired Peripheral Blood Mononuclear Cell (PBMC) Mitochondrial Respiration Is Associated with Mortality and Long COVID Syndrome Severity in COVID-19 Patients" International Journal of Molecular Sciences 26, no. 21: 10377. https://doi.org/10.3390/ijms262110377
APA StyleCharles, A.-L., Debrut, L., Oulehri, W., Vincent, V., Delagreverie, H., Asael, P., Riou, M., Giannini, M., Meyer, A., & Geny, B. (2025). Impaired Peripheral Blood Mononuclear Cell (PBMC) Mitochondrial Respiration Is Associated with Mortality and Long COVID Syndrome Severity in COVID-19 Patients. International Journal of Molecular Sciences, 26(21), 10377. https://doi.org/10.3390/ijms262110377





