COVID-19 Hijacking of the Host Epigenome: Mechanisms, Biomarkers and Long-Term Consequences
Abstract
1. Introduction
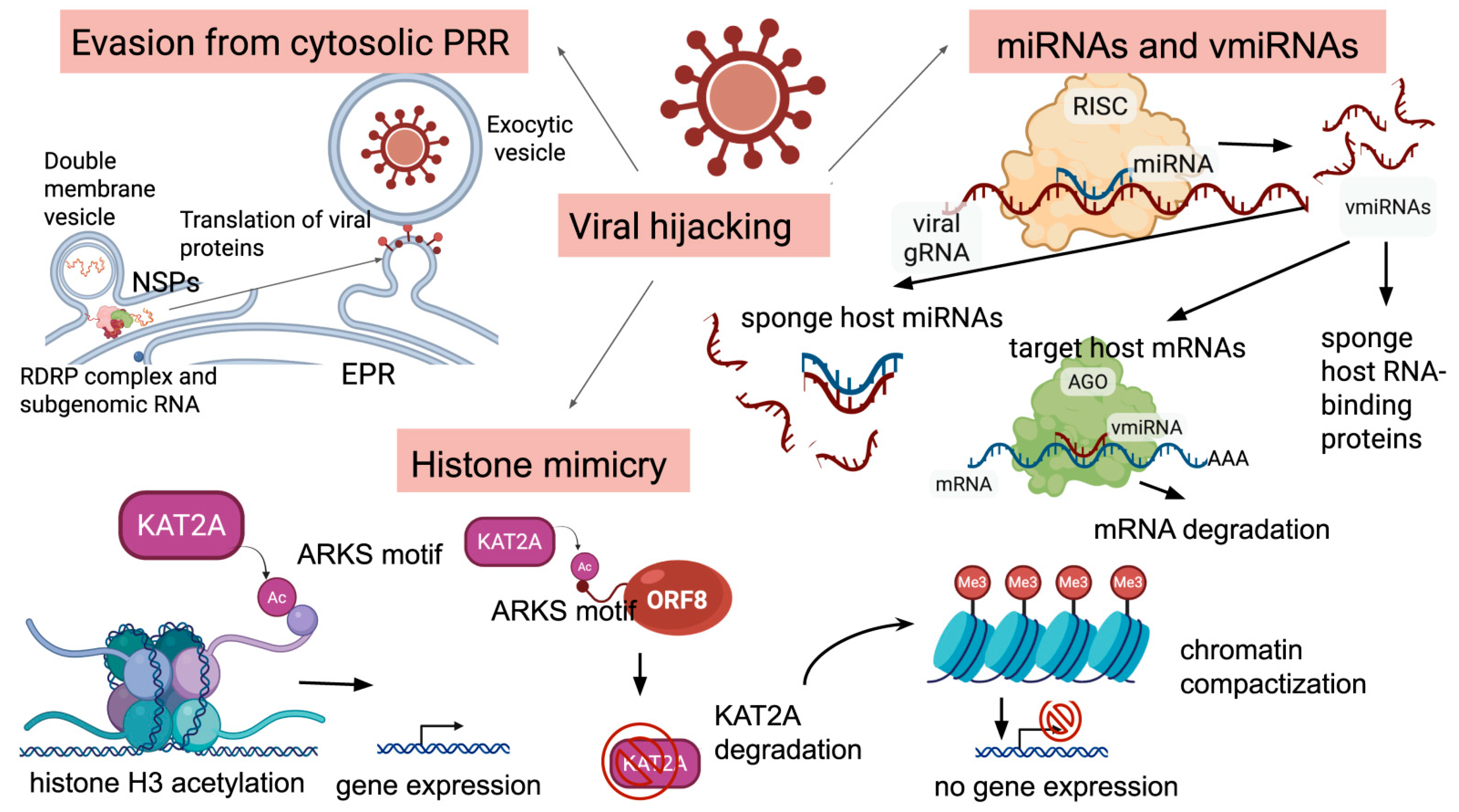
1.1. Viral Invasion and Altered Pathways
1.2. Cell Populations Affected by the Disease
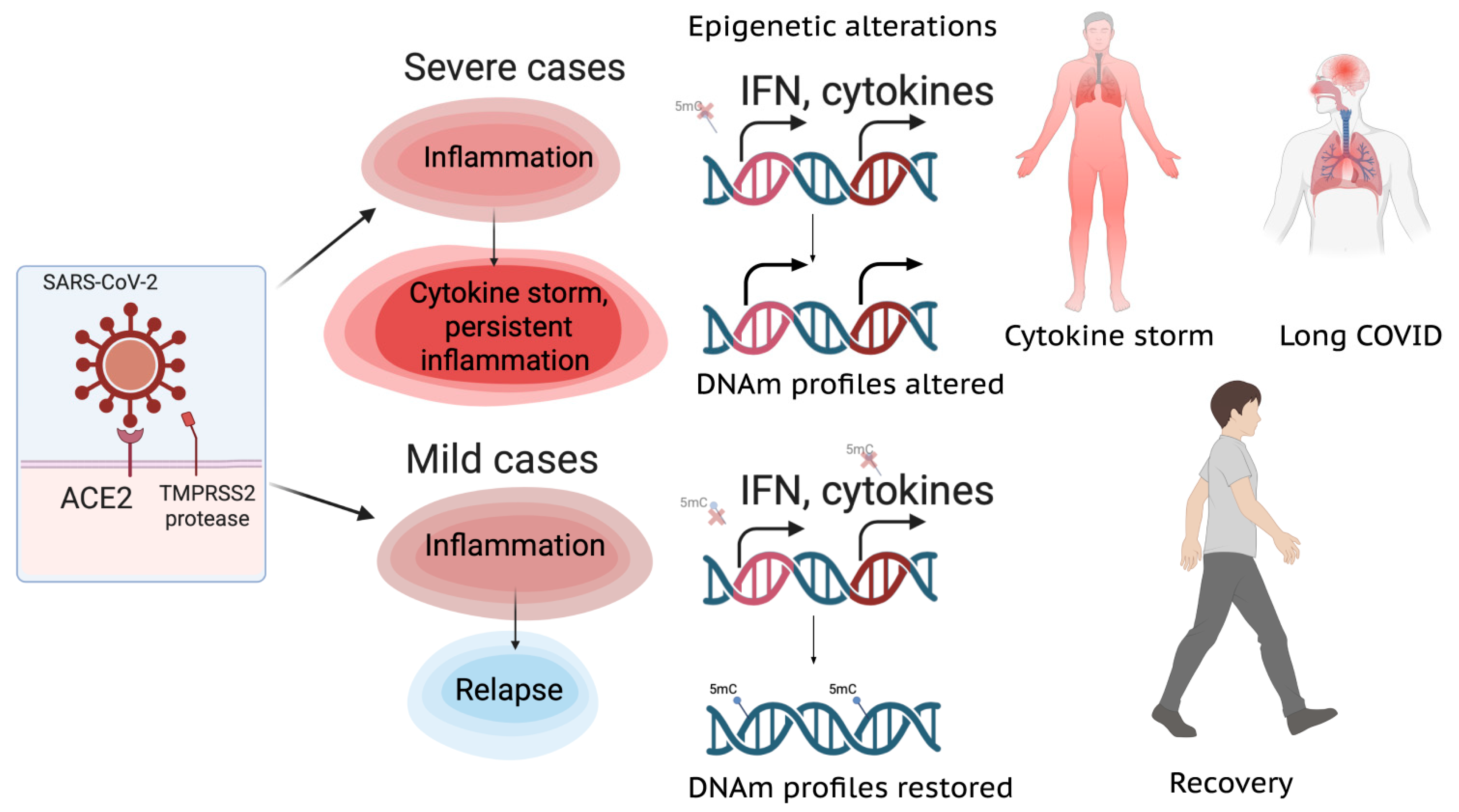
1.3. Cytokine Profiles and Long COVID
2. Epigenetics of COVID-19
2.1. Epigenetic Alterations of Different Cell Populations

2.2. Epigenetic Modifications of the Host Genome Induced by the Virus
2.3. Altered Host miRNome
2.4. Viral miRNA-like RNAs
2.5. Accelerated Epigenetic Aging Associated with COVID-19
Enhanced Epigenetic Drift
2.6. Epimarkers of Disease Severity and Outcome
2.7. Future Directions: Open Questions and Testable Predictions
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID_19) Dashboard. Available online: https://data.who.int/dashboards/covid19/ (accessed on 5 September 2025).
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 Infects Cells after Viral Entry via Clathrin-Mediated Endocytosis. J. Biol. Chem. 2021, 296, 100306. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Narwal, M.; Armache, J.-P.; Edwards, T.J.; Murakami, K.S. SARS-CoV-2 Polyprotein Substrate Regulates the Stepwise Mpro Cleavage Reaction. J. Biol. Chem. 2023, 299, 104697. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus Biology and Replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Lei, X.; Dong, X.; Ma, R.; Wang, W.; Xiao, X.; Tian, Z.; Wang, C.; Wang, Y.; Li, L.; Ren, L.; et al. Activation and Evasion of Type I Interferon Responses by SARS-CoV-2. Nat. Commun. 2020, 11, 3810. [Google Scholar] [CrossRef] [PubMed]
- Kee, J.; Thudium, S.; Renner, D.M.; Glastad, K.; Palozola, K.; Zhang, Z.; Li, Y.; Lan, Y.; Cesare, J.; Poleshko, A.; et al. SARS-CoV-2 Disrupts Host Epigenetic Regulation via Histone Mimicry. Nature 2022, 610, 381–388. [Google Scholar] [CrossRef]
- Santerre, M.; Arjona, S.P.; Allen, C.N.; Shcherbik, N.; Sawaya, B.E. Why Do SARS-CoV-2 NSPs Rush to the ER? J. Neurol. 2021, 268, 2013–2022. [Google Scholar] [CrossRef]
- Hayn, M.; Hirschenberger, M.; Koepke, L.; Nchioua, R.; Straub, J.H.; Klute, S.; Hunszinger, V.; Zech, F.; Prelli Bozzo, C.; Aftab, W.; et al. Systematic Functional Analysis of SARS-CoV-2 Proteins Uncovers Viral Innate Immune Antagonists and Remaining Vulnerabilities. Cell Rep. 2021, 35, 109126. [Google Scholar] [CrossRef]
- Enriquez, J.D.L.C.; Morales, E.R.; Garcia, M.G.R.; Velasco, J.T.; Jimenez, J. SARS-CoV-2 Induces Mitochondrial Dysfunction and Cell Death by Oxidative Stress in Leukocytes of COVID-19 Patients. Free Radic. Res. 2020, 55, 982–995. [Google Scholar] [CrossRef]
- Pan, P.; Shen, M.; Yu, Z.; Ge, W.; Chen, K.; Tian, M.; Xiao, F.; Wang, Z.; Wang, J.; Jia, Y.; et al. SARS-CoV-2 N Protein Promotes NLRP3 Inflammasome Activation to Induce Hyperinflammation. Nat. Commun. 2021, 12, 4664. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Soares, V.C.; De Azevedo-Quintanilha, I.G.; Dias, S.D.S.G.; Fintelman-Rodrigues, N.; Sacramento, C.Q.; Mattos, M.; De Freitas, C.S.; Temerozo, J.R.; Teixeira, L.; et al. SARS-CoV-2 Engages Inflammasome and Pyroptosis in Human Primary Monocytes. Cell Death Discov. 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Plowman, T.; Lagos, D. Non-Coding RNAs in COVID-19: Emerging Insights and Current Questions. Non-Coding RNA 2021, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 Pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Wang, Z.; Gutiérrez-Castrellón, P.; Shi, H. Cell Deaths: Involvement in the Pathogenesis and Intervention Therapy of COVID-19. Signal Transduct. Target. Ther. 2022, 7, 186. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Qiu, Y.; Lu, H. COVID-19: Imbalanced Cell-Mediated Immune Response Drives to Immunopathology. Emerg. Microbes Infect. 2022, 11, 2393–2404. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int. J. Mol. Sci. 2022, 23, 1716. [Google Scholar] [CrossRef]
- Li, C.; He, Q.; Qian, H.; Liu, J. Overview of the Pathogenesis of COVID-19 (Review). Exp. Ther. Med. 2021, 22, 1011. [Google Scholar] [CrossRef]
- Kgatle, M.M.; Lawal, I.O.; Mashabela, G.; Boshomane, T.M.G.; Koatale, P.C.; Mahasha, P.W.; Ndlovu, H.; Vorster, M.; Rodrigues, H.G.; Zeevaart, J.R.; et al. COVID-19 Is a Multi-Organ Aggressor: Epigenetic and Clinical Marks. Front. Immunol. 2021, 12, 752380. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Low, R.N.; Low, R.J.; Akrami, A. A Review of Cytokine-Based Pathophysiology of Long COVID Symptoms. Front. Med. 2023, 10, 1011936. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liu, Q.; Zhang, J.; Xu, S. COVID-19 and Trained Immunity: The Inflammatory Burden of Long Covid. Front. Immunol. 2023, 14, 1294959. [Google Scholar] [CrossRef]
- Pervin, Z.; Tasnim, A.; Ahamed, H.; Hasibuzzaman, M.A. Epigenetic Regulation of the COVID-19 Pathogenesis: Its Impact on the Host Immune Response and Disease Progression. AIMS Allergy Immunol. 2023, 7, 60–81. [Google Scholar] [CrossRef]
- Cheong, J.-G.; Ravishankar, A.; Sharma, S.; Parkhurst, C.N.; Grassmann, S.A.; Wingert, C.K.; Laurent, P.; Ma, S.; Paddock, L.; Miranda, I.C.; et al. Epigenetic Memory of Coronavirus Infection in Innate Immune Cells and Their Progenitors. Cell 2023, 186, 3882–3902.e24. [Google Scholar] [CrossRef]
- Biterge Süt, B. Epigenetic Regulation Mechanisms in Viral Infections: A Special Focus on COVID-19. In Biotechnology to Combat COVID-19; Agrawal, M., Biswas, S., Eds.; IntechOpen: Rijeka, Croatia, 2022; ISBN 978-1-83968-626-9. [Google Scholar]
- Brueggeman, J.M.; Zhao, J.; Schank, M.; Yao, Z.Q.; Moorman, J.P. Trained Immunity: An Overview and the Impact on COVID-19. Front. Immunol. 2022, 13, 837524. [Google Scholar] [CrossRef]
- You, M.; Chen, L.; Zhang, D.; Zhao, P.; Chen, Z.; Qin, E.-Q.; Gao, Y.; Davis, M.M.; Yang, P. Single-Cell Epigenomic Landscape of Peripheral Immune Cells Reveals Establishment of Trained Immunity in Individuals Convalescing from COVID-19. Nat. Cell Biol. 2021, 23, 620–630. [Google Scholar] [CrossRef]
- Spector, B.; Koseva, B.; McLennan, R.; Banerjee, D.; Lankachandra, K.; Bradley, T.; Selvarangan, R.; Grundberg, E. Methylation Patterns of the Nasal Epigenome of Hospitalized SARS-CoV-2 Positive Patients Reveal Insights into Molecular Mechanisms of COVID-19. BMC Med. Genom. 2024, 18, 62. [Google Scholar] [CrossRef]
- Messingschlager, M.; Mackowiak, S.D.; Voelker, M.T.; Bieg, M.; Loske, J.; Chua, R.L.; Liebig, J.; Lukassen, S.; Thürmann, L.; Seegebarth, A.; et al. DNA Methylation Changes during Acute COVID-19 Are Associated with Long-Term Transcriptional Dysregulation in Patients’ Airway Epithelial Cells. EMBO Mol. Med. 2025, 17, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Barbon, S.; Armellin, F.; Passerini, V.; De Angeli, S.; Primerano, S.; Del Pup, L.; Durante, E.; Macchi, V.; De Caro, R.; Parnigotto, P.P.; et al. Innate Immune Response in COVID-19: Single-Cell Multi-Omics Profile of NK Lymphocytes in a Clinical Case Series. Cell Commun. Signal. 2024, 22, 496. [Google Scholar] [CrossRef]
- Corley, M.J.; Pang, A.P.S.; Dody, K.; Mudd, P.A.; Patterson, B.K.; Seethamraju, H.; Bram, Y.; Peluso, M.J.; Torres, L.; Iyer, N.S.; et al. Genome-Wide DNA Methylation Profiling of Peripheral Blood Reveals an Epigenetic Signature Associated with Severe COVID-19. J. Leukoc. Biol. 2021, 110, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug Repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Liu, P.; Hu, J.; Wang, L. SARS-CoV-2 ORF8 Does Not Function in the Nucleus as a Histone Mimic. Protein Cell 2024, 15, 79–82. [Google Scholar] [CrossRef]
- Giroux, N.S.; Ding, S.; McClain, M.T.; Burke, T.W.; Petzold, E.; Chung, H.A.; Rivera, G.O.; Wang, E.; Xi, R.; Bose, S.; et al. Differential Chromatin Accessibility in Peripheral Blood Mononuclear Cells Underlies COVID-19 Disease Severity Prior to Seroconversion. Sci. Rep. 2022, 12, 11714. [Google Scholar] [CrossRef]
- Benoit, J.M.; Dennis, J.H. SARS-CoV-2 Nucleocapsid Uniquely Disrupts Chromatin over Pathophysiologically Relevant Gene Promoters. bioRxiv 2025. [Google Scholar] [CrossRef]
- Beacon, T.H.; Delcuve, G.P.; Davie, J.R. Epigenetic Regulation of ACE2, the Receptor of the SARS-CoV-2 Virus1. Genome 2021, 64, 386–399. [Google Scholar] [CrossRef]
- Wang, C.-W.; Chuang, H.-C.; Tan, T.-H. ACE2 in Chronic Disease and COVID-19: Gene Regulation and Post-Translational Modification. J. Biomed. Sci. 2023, 30, 71. [Google Scholar] [CrossRef] [PubMed]
- Daniel, G.; Paola, A.-R.; Nancy, G.; Fernando, S.-O.; Beatriz, A.; Zulema, R.; Julieth, A.; Claudia, C.; Adriana, R. Epigenetic Mechanisms and Host Factors Impact ACE2 Gene Expression: Implications in COVID-19 Susceptibility. Infect. Genet. Evol. 2022, 104, 105357. [Google Scholar] [CrossRef]
- Tukiainen, T.; Villani, A.-C.; Yen, A.; Rivas, M.A.; Marshall, J.L.; Satija, R.; Aguirre, M.; Gauthier, L.; Fleharty, M.; Kirby, A.; et al. Landscape of X Chromosome Inactivation across Human Tissues. Nature 2017, 550, 244–248. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Li, X.; Li, W.; Liu, X.; Xue, X. The Impact of ACE2 Polymorphisms on COVID-19 Disease: Susceptibility, Severity, and Therapy. Front. Cell. Infect. Microbiol. 2021, 11, 753721. [Google Scholar] [CrossRef]
- Konwar, C.; Asiimwe, R.; Inkster, A.M.; Merrill, S.M.; Negri, G.L.; Aristizabal, M.J.; Rider, C.F.; MacIsaac, J.L.; Carlsten, C.; Kobor, M.S. Risk-Focused Differences in Molecular Processes Implicated in SARS-CoV-2 Infection: Corollaries in DNA Methylation and Gene Expression. Epigenetics Chromatin 2021, 14, 54. [Google Scholar] [CrossRef]
- Foresta, C.; Rocca, M.S.; Di Nisio, A. Gender Susceptibility to COVID-19: A Review of the Putative Role of Sex Hormones and X Chromosome. J. Endocrinol. Investig. 2021, 44, 951–956. [Google Scholar] [CrossRef]
- Muhammad, A.; Forcados, G.E.; Sani, H.; Ndidi, U.S.; Adamu, A.; Katsayal, B.S.; Sadiq, I.Z.; Abubakar, Y.S.; Sulaiman, I.; Abubakar, I.B.; et al. Epigenetic Modifications Associated with Genes Implicated in Cytokine Storm: The Potential Biotherapeutic Effects of Vitamins and Minerals in COVID-19. J. Food Biochem. 2022, 46, e14079. [Google Scholar] [CrossRef]
- Taefehshokr, N.; Lac, A.; Vrieze, A.M.; Dickson, B.H.; Guo, P.N.; Jung, C.; Blythe, E.N.; Fink, C.; Aktar, A.; Dikeakos, J.D.; et al. SARS-CoV-2 NSP5 Antagonizes MHC II Expression by Subverting Histone Deacetylase 2. J. Cell Sci. 2024, 137, jcs262172. [Google Scholar] [CrossRef]
- Hagemeijer, M.C.; Monastyrska, I.; Griffith, J.; Van Der Sluijs, P.; Voortman, J.; Van Bergen En Henegouwen, P.M.; Vonk, A.M.; Rottier, P.J.M.; Reggiori, F.; De Haan, C.A.M. Membrane Rearrangements Mediated by Coronavirus Nonstructural Proteins 3 and 4. Virology 2014, 458–459, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Schubert, K.; Karousis, E.D.; Jomaa, A.; Scaiola, A.; Echeverria, B.; Gurzeler, L.-A.; Leibundgut, M.; Thiel, V.; Mühlemann, O.; Ban, N. SARS-CoV-2 Nsp1 Binds the Ribosomal mRNA Channel to Inhibit Translation. Nat. Struct. Mol. Biol. 2020, 27, 959–966. [Google Scholar] [CrossRef]
- Kumar, A.; Ishida, R.; Strilets, T.; Cole, J.; Lopez-Orozco, J.; Fayad, N.; Felix-Lopez, A.; Elaish, M.; Evseev, D.; Magor, K.E.; et al. SARS-CoV-2 Nonstructural Protein 1 Inhibits the Interferon Response by Causing Depletion of Key Host Signaling Factors. J. Virol. 2021, 95, e00266-21. [Google Scholar] [CrossRef]
- Zhang, K.; Miorin, L.; Makio, T.; Dehghan, I.; Gao, S.; Xie, Y.; Zhong, H.; Esparza, M.; Kehrer, T.; Kumar, A.; et al. Nsp1 Protein of SARS-CoV-2 Disrupts the mRNA Export Machinery to Inhibit Host Gene Expression. Sci. Adv. 2021, 7, eabe7386. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, V.; Wagoner, J.; Athanasiadis, P.; Den Hartigh, A.B.; Sidorova, J.M.; Ianevski, A.; Fink, S.L.; Frigessi, A.; White, J.; Polyak, S.J.; et al. Discovery of Host-Directed Modulators of Virus Infection by Probing the SARS-CoV-2–Host Protein–Protein Interaction Network. Brief. Bioinform. 2022, 23, bbac456. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Vayianos, P.; Nicolaidou, V.; Felekkis, K.; Papaneophytou, C. Alterations in Circulating miRNA Levels after Infection with SARS-CoV-2 Could Contribute to the Development of Cardiovascular Diseases: What We Know So Far. Int. J. Mol. Sci. 2023, 24, 2380. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, G.; Lv, X.; Ren, L. Critical Role of Cellular microRNAs in Virus Infection: Decades of Progress. Anim. Zoonoses 2025. [Google Scholar] [CrossRef]
- Alam, T.; Lipovich, L. miRCOVID-19: Potential Targets of Human miRNAs in SARS-CoV-2 for RNA-Based Drug Discovery. Non-Coding RNA 2021, 7, 18. [Google Scholar] [CrossRef]
- Patel, P.; Ansari, M.Y.; Bapat, S.; Thakar, M.; Gangakhedkar, R.; Jameel, S. The microRNA miR-29a Is Associated with Human Immunodeficiency Virus Latency. Retrovirology 2014, 11, 108. [Google Scholar] [CrossRef]
- Kim, I.S.; Lee, S.-G.; Shin, S.G.; Jeong, H.; Sohn, K.M.; Park, K.-S.; Silwal, P.; Cheon, S.; Kim, J.; Kym, S.; et al. Dysregulated Thrombospondin 1 and miRNA-29a-3p in Severe COVID-19. Sci. Rep. 2022, 12, 21227. [Google Scholar] [CrossRef]
- Ferreira-Gomes, M.; Kruglov, A.; Durek, P.; Heinrich, F.; Tizian, C.; Heinz, G.A.; Pascual-Reguant, A.; Du, W.; Mothes, R.; Fan, C.; et al. SARS-CoV-2 in Severe COVID-19 Induces a TGF-β-Dominated Chronic Immune Response That Does Not Target Itself. Nat. Commun. 2021, 12, 1961. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Becerril, B.; Nava-Quiroz, K.J.; Muñoz-Soria, E.; Camarena, Á.; Fricke-Galindo, I.; Buendia-Roldan, I.; Pérez-Rubio, G.; Chavez-Galán, L.; Pérez-Torres, K.; Téllez-Quijada, F.; et al. High Expression Levels of miR-21-5p in Younger Hospitalized COVID-19 Patients Are Associated with Mortality and Critical Disease. Int. J. Mol. Sci. 2023, 24, 10112. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, M.; Nikolic, D.; Novakovic, I.; Petrovic, B.; Lackovic, M.; Santric-Milicevic, M. miRNAs as a Potential Biomarker in the COVID-19 Infection and Complications Course, Severity, and Outcome. Diagnostics 2023, 13, 1091. [Google Scholar] [CrossRef]
- Singh, M.; Chazal, M.; Quarato, P.; Bourdon, L.; Malabat, C.; Vallet, T.; Vignuzzi, M.; Van Der Werf, S.; Behillil, S.; Donati, F.; et al. A Virus-derived microRNA Targets Immune Response Genes during SARS-CoV-2 Infection. EMBO Rep. 2022, 23, e54341. [Google Scholar] [CrossRef]
- Gao, L.; Kyubwa, E.M.; Starbird, M.A.; Diaz De Leon, J.; Nguyen, M.; Rogers, C.J.; Menon, N. Circulating miRNA Profiles in COVID-19 Patients and Meta-Analysis: Implications for Disease Progression and Prognosis. Sci. Rep. 2023, 13, 21656. [Google Scholar] [CrossRef]
- Mustafa, F.; Ahmad, W.; Gull, B.; Baby, J.; Panicker, N.G.; Khader, T.A.; Baki, H.A.; Rehman, E.; Salim, A.M.; Ahmed, R.L.G.; et al. miRNA Biomarkers for Prognosis and Therapy Monitoring in a Multi-Ethnic Cohort with SARS-CoV-2 Infection. Sci. Rep. 2025, 15, 30815. [Google Scholar] [CrossRef]
- Pius-Sadowska, E.; Kulig, P.; Niedźwiedź, A.; Baumert, B.; Rogińska, D.; Łuczkowska, K.; Sobuś, A.; Parczewski, M.; Kawa, M.; Paczkowska, E.; et al. The Micro-RNA Expression Profile Predicts the Severity of SARS-CoV-2 Infection. Sci. Rep. 2025, 15, 17139. [Google Scholar] [CrossRef]
- Kebriaei, A.; Besharati, R.; Namdar Ahmadabad, H.; Havakhah, S.; Khosrojerdi, M.; Azimian, A. The Relationship between microRNAs and COVID-19 Complications. Non-Coding RNA Res. 2025, 10, 16–24. [Google Scholar] [CrossRef]
- Papadopoulos, K.I.; Papadopoulou, A.; Aw, T.C. MicroRNA-155 Modulation by Renin-Angiotensin System Inhibitors May Underlie Their Enigmatic Role in COVID-19. World J. Exp. Med. 2025, 15, 100748. [Google Scholar] [CrossRef]
- Papadopoulos, K.I.; Papadopoulou, A.; Aw, T.C. Beauty and the Beast: Host microRNA-155 versus SARS-CoV-2. Hum. Cell 2023, 36, 908–922. [Google Scholar] [CrossRef]
- Pawlica, P.; Yario, T.A.; White, S.; Wang, J.; Moss, W.N.; Hui, P.; Vinetz, J.M.; Steitz, J.A. SARS-CoV-2 Expresses a microRNA-like Small RNA Able to Selectively Repress Host Genes. Proc. Natl. Acad. Sci. USA 2021, 118, e2116668118. [Google Scholar] [CrossRef]
- Tucker, E.J.; Wong, S.W.; Marri, S.; Ali, S.; Fedele, A.O.; Michael, M.Z.; Rojas-Canales, D.; Li, J.Y.; Lim, C.K.; Gleadle, J.M. SARS-CoV-2 Produces a microRNA CoV2-miR-O8 in Patients with COVID-19 Infection. iScience 2024, 27, 108719. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Ge, Y.; Xu, Y.; Guo, M.; Mi, K.; Xu, R.; Pei, Y.; Zhang, Q.; Luan, X.; et al. SARS-CoV-2 Encoded microRNAs Are Involved in the Process of Virus Infection and Host Immune Response. J. Biomed. Res. 2021, 35, 216. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Z.; Song, J.; Qian, W.; Gu, X.; Yang, C.; Shen, N.; Xue, F.; Tang, Y. SARS-CoV-2-Encoded MiRNAs Inhibit Host Type I Interferon Pathway and Mediate Allelic Differential Expression of Susceptible Gene. Front. Immunol. 2021, 12, 767726. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Feng, Z.; Wang, Q.; Han, L.; Guan, S.; Liu, L.; Ye, H.; Xu, L.; Han, X. 3’UTR of SARS-CoV-2 Spike Gene Hijack Host miR-296 or miR-520h to Disturb Cell Proliferation and Cytokine Signaling. Front. Immunol. 2022, 13, 924667. [Google Scholar] [CrossRef] [PubMed]
- Hardin, L.T.; Xiao, N. miRNAs: The Key Regulator of COVID-19 Disease. Int. J. Cell Biol. 2022, 2022, 1645366. [Google Scholar] [CrossRef] [PubMed]
- Maranini, B.; Ciancio, G.; Ferracin, M.; Cultrera, R.; Negrini, M.; Sabbioni, S.; Govoni, M. microRNAs and Inflammatory Immune Response in SARS-CoV-2 Infection: A Narrative Review. Life 2022, 12, 288. [Google Scholar] [CrossRef]
- Calzari, L.; Zanotti, L.; Inglese, E.; Scaglione, F.; Cavagnola, R.; Ranucci, F.; Di Blasio, A.M.; Stefanini, G.; Carlo, G.; Parati, G.; et al. Role of Epigenetics in the Clinical Evolution of COVID-19 Disease. Epigenome-Wide Association Study Identifies Markers of Severe Outcome. Eur. J. Med. Res. 2023, 28, 81. [Google Scholar] [CrossRef]
- Calzari, L.; Dragani, D.F.; Zanotti, L.; Inglese, E.; Danesi, R.; Cavagnola, R.; Brusati, A.; Ranucci, F.; Di Blasio, A.M.; Persani, L.; et al. Epigenetic Patterns, Accelerated Biological Aging, and Enhanced Epigenetic Drift Detected 6 Months Following COVID-19 Infection: Insights from a Genome-Wide DNA Methylation Study. Clin. Epigenetics 2024, 16, 112. [Google Scholar] [CrossRef]
- Cao, X.; Li, W.; Wang, T.; Ran, D.; Davalos, V.; Planas-Serra, L.; Pujol, A.; Esteller, M.; Wang, X.; Yu, H. Accelerated Biological Aging in COVID-19 Patients. Nat. Commun. 2022, 13, 2135. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, Y.; Humaira Amanullah, F.; Saad, M.; Taleb, S.; Bradic, M.; Megarbane, A.; Ait Hssain, A.; Abi Khalil, C.; El Hajj, N. Epigenetic Age Acceleration in Surviving versus Deceased COVID-19 Patients with Acute Respiratory Distress Syndrome Following Hospitalization. Clin. Epigenetics 2023, 15, 186. [Google Scholar] [CrossRef]
- Franzen, J.; Nüchtern, S.; Tharmapalan, V.; Vieri, M.; Nikolić, M.; Han, Y.; Balfanz, P.; Marx, N.; Dreher, M.; Brümmendorf, T.H.; et al. Epigenetic Clocks Are Not Accelerated in COVID-19 Patients. Int. J. Mol. Sci. 2021, 22, 9306. [Google Scholar] [CrossRef] [PubMed]
- Konigsberg, I.R.; Barnes, B.; Campbell, M.; Davidson, E.; Zhen, Y.; Pallisard, O.; Boorgula, M.P.; Cox, C.; Nandy, D.; Seal, S.; et al. Host Methylation Predicts SARS-CoV-2 Infection and Clinical Outcome. Commun. Med. 2021, 1, 42. [Google Scholar] [CrossRef]
- Salem, S.; Mosaad, R.; Lotfy, R.; Elbadry, M. Altered Expression of DNA Methyltransferases and Methylation Status of the TLR4 and TNF-α Promoters in COVID-19. Arch. Virol. 2023, 168, 95. [Google Scholar] [CrossRef]
- De Abreu, A.R.; Ibrahim, J.; Lemonidis, V.; Mateiu, L.; Van Camp, G.; Op De Beeck, K. Comparison of Current Methods for Genome-Wide DNA Methylation Profiling. Epigenetics Chromatin 2025, 18, 57. [Google Scholar] [CrossRef]
- Luo, C.; Rivkin, A.; Zhou, J.; Sandoval, J.P.; Kurihara, L.; Lucero, J.; Castanon, R.; Nery, J.R.; Pinto-Duarte, A.; Bui, B.; et al. Robust Single-Cell DNA Methylome Profiling with snmC-Seq2. Nat. Commun. 2018, 9, 3824. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, J.; Tian, W.; Luo, C.; Bartlett, A.; Aldridge, A.; Lucero, J.; Osteen, J.K.; Nery, J.R.; Chen, H.; et al. DNA Methylation Atlas of the Mouse Brain at Single-Cell Resolution. Nature 2021, 598, 120–128. [Google Scholar] [CrossRef]
- Sigurpalsdottir, B.D.; Stefansson, O.A.; Holley, G.; Beyter, D.; Zink, F.; Hardarson, M.Þ.; Sverrisson, S.Þ.; Kristinsdottir, N.; Magnusdottir, D.N.; Magnusson, O.Þ.; et al. A Comparison of Methods for Detecting DNA Methylation from Long-Read Sequencing of Human Genomes. Genome Biol. 2024, 25, 69. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.T.; Workman, R.E.; Zuzarte, P.C.; David, M.; Dursi, L.J.; Timp, W. Detecting DNA Cytosine Methylation Using Nanopore Sequencing. Nat. Methods 2017, 14, 407–410. [Google Scholar] [CrossRef]
- Ahmed, Y.W.; Alemu, B.A.; Bekele, S.A.; Gizaw, S.T.; Zerihun, M.F.; Wabalo, E.K.; Teklemariam, M.D.; Mihrete, T.K.; Hanurry, E.Y.; Amogne, T.G.; et al. Epigenetic Tumor Heterogeneity in the Era of Single-Cell Profiling with Nanopore Sequencing. Clin. Epigenetics 2022, 14, 107. [Google Scholar] [CrossRef]
- Zhang, Z. The Initial COVID-19 Reliable Interactive DNA Methylation Markers and Biological Implications. Biology 2024, 13, 245. [Google Scholar] [CrossRef]
- Castro De Moura, M.; Davalos, V.; Planas-Serra, L.; Alvarez-Errico, D.; Arribas, C.; Ruiz, M.; Aguilera-Albesa, S.; Troya, J.; Valencia-Ramos, J.; Vélez-Santamaria, V.; et al. Epigenome-Wide Association Study of COVID-19 Severity with Respiratory Failure. eBioMedicine 2021, 66, 103339. [Google Scholar] [CrossRef]
- Sharif-zak, M.; Abbasi-Jorjandi, M.; Asadikaram, G.; Ghoreshi, Z.-S.; Rezazadeh-Jabalbarzi, M.; Rashidinejad, H. Influence of Disease Severity and Gender on HLA-C Methylation in COVID-19 Patients. Iran. J. Sci. Technol. Trans. Sci. 2022, 46, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Barturen, G.; Carnero-Montoro, E.; Martínez-Bueno, M.; Rojo-Rello, S.; Sobrino, B.; Porras-Perales, Ó.; Alcántara-Domínguez, C.; Bernardo, D.; Alarcón-Riquelme, M.E. Whole Blood DNA Methylation Analysis Reveals Respiratory Environmental Traits Involved in COVID-19 Severity Following SARS-CoV-2 Infection. Nat. Commun. 2022, 13, 4597. [Google Scholar] [CrossRef] [PubMed]
- Govender, M.; Das, J.; Hopkins, F.R.; Svanberg, C.; Nordgren, J.; Hagbom, M.; Klingström, J.; Nilsdotter-Augustinsson, Å.; Yong, Y.K.; Velu, V.; et al. Altered DNA Methylation Pattern Contributes to Differential Epigenetic Immune Signaling in the Upper Respiratory Airway of COVID-19 Patients. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, Z.; Yang, F.; Zheng, X.; Zong, W.; Li, R.; Bao, Y. Identification of COVID-19-Associated DNA Methylation Variations by Integrating Methylation Array and scRNA-Seq Data at Cell-Type Resolution. Genes 2022, 13, 1109. [Google Scholar] [CrossRef]
- Bowler, S.; Papoutsoglou, G.; Karanikas, A.; Tsamardinos, I.; Corley, M.J.; Ndhlovu, L.C. A Machine Learning Approach Utilizing DNA Methylation as an Accurate Classifier of COVID-19 Disease Severity. Sci. Rep. 2022, 12, 17480. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Molecular Function | Association with COVID-19 Severity | Methylation Status in Severe COVID-19 |
|---|---|---|---|
| AIM2 | Inflammasome component; involved in interferon response to viral infection | Part of a DNA methylation signature (EPICOVID) associated with clinical severity and respiratory failure. | hypo |
| HLA-C | Crucial determinant of immune function and NK cell activity; involved in interferon response to viral infection. | Part of a DNA methylation signature (EPICOVID) associated with clinical severity and respiratory failure. Methylation levels in men were significantly higher in the severe group. | hypo |
| MX1 | Interferon-induced GTP-binding protein; inhibits viral replication by trapping viral components. | Altered DNA methylation profiles are part of a signature with 96.87–100% accuracy in stratifying COVID-19 from other diseases | hypo |
| PARP9 | Poly(ADP-ribose) polymerase involved in DNA repair and interferon signaling. | Altered DNA methylation profiles are part of a signature with 96.87–100% accuracy in stratifying COVID-19 from other diseases | hypo |
| IRF7 | Involved in interferon signaling and viral response. | Differentially methylated sites were significantly enriched in this gene; methylation signature can predict hospitalization, ICU admission, and progression to death. | hypo |
| OAS1 | Involved in interferon signaling and viral response. | Differentially methylated sites were significantly enriched in this gene; methylation signature can predict hospitalization, ICU admission, and progression to death. | hyper |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolotarenko, A.D.; Poghosyan, H.M.; Sheptiy, V.V.; Bruskin, S.A. COVID-19 Hijacking of the Host Epigenome: Mechanisms, Biomarkers and Long-Term Consequences. Int. J. Mol. Sci. 2025, 26, 10372. https://doi.org/10.3390/ijms262110372
Zolotarenko AD, Poghosyan HM, Sheptiy VV, Bruskin SA. COVID-19 Hijacking of the Host Epigenome: Mechanisms, Biomarkers and Long-Term Consequences. International Journal of Molecular Sciences. 2025; 26(21):10372. https://doi.org/10.3390/ijms262110372
Chicago/Turabian StyleZolotarenko, Alena D., Hakob M. Poghosyan, Victoria V. Sheptiy, and Sergey A. Bruskin. 2025. "COVID-19 Hijacking of the Host Epigenome: Mechanisms, Biomarkers and Long-Term Consequences" International Journal of Molecular Sciences 26, no. 21: 10372. https://doi.org/10.3390/ijms262110372
APA StyleZolotarenko, A. D., Poghosyan, H. M., Sheptiy, V. V., & Bruskin, S. A. (2025). COVID-19 Hijacking of the Host Epigenome: Mechanisms, Biomarkers and Long-Term Consequences. International Journal of Molecular Sciences, 26(21), 10372. https://doi.org/10.3390/ijms262110372





