Additive Effects of N-Acetylcysteine and [R4W4] Combination Treatment on Mycobacterium avium
Abstract
1. Introduction
2. Results
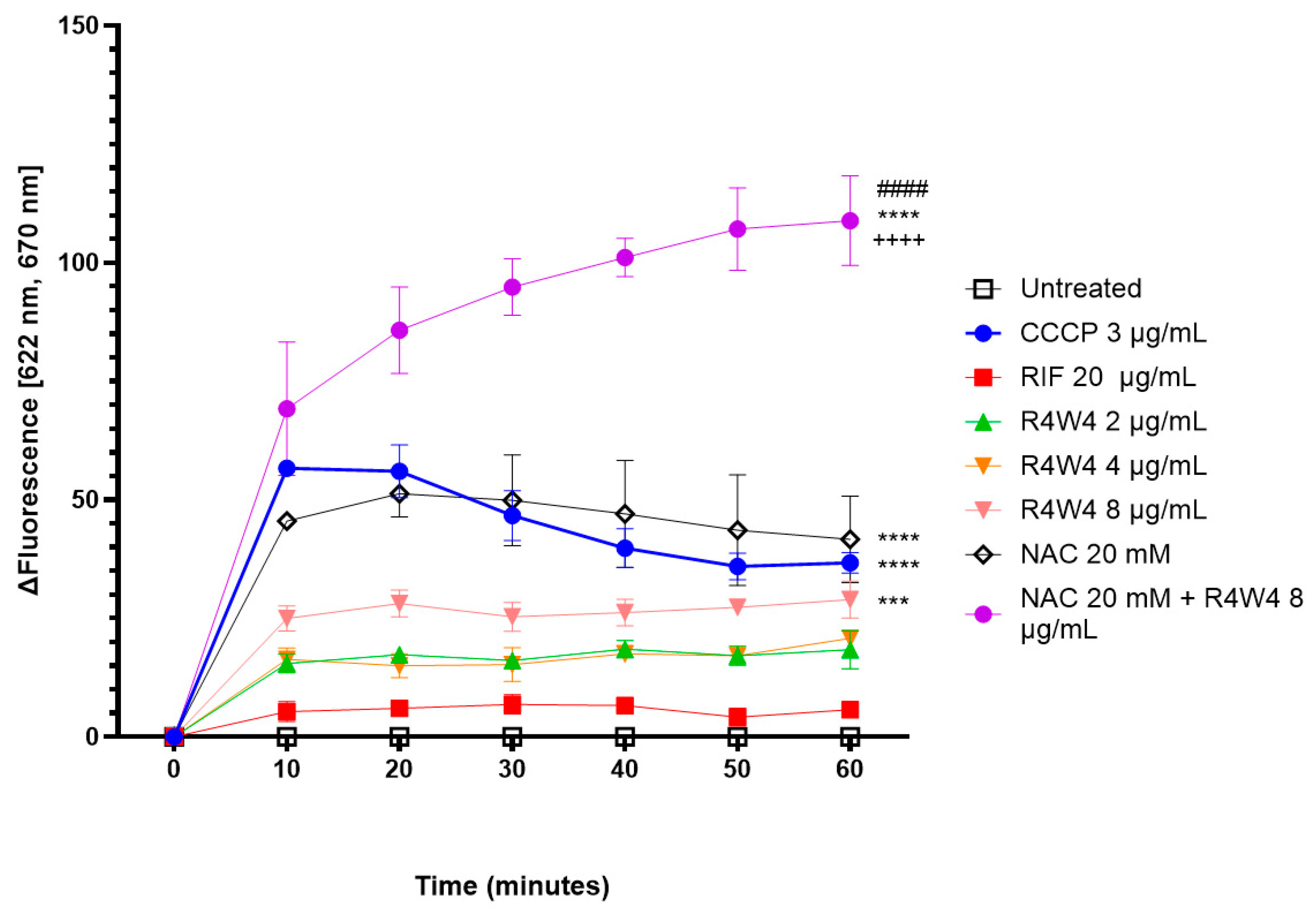
2.1. Membrane Depolarization Potential by [R4W4], NAC, and Their Combination
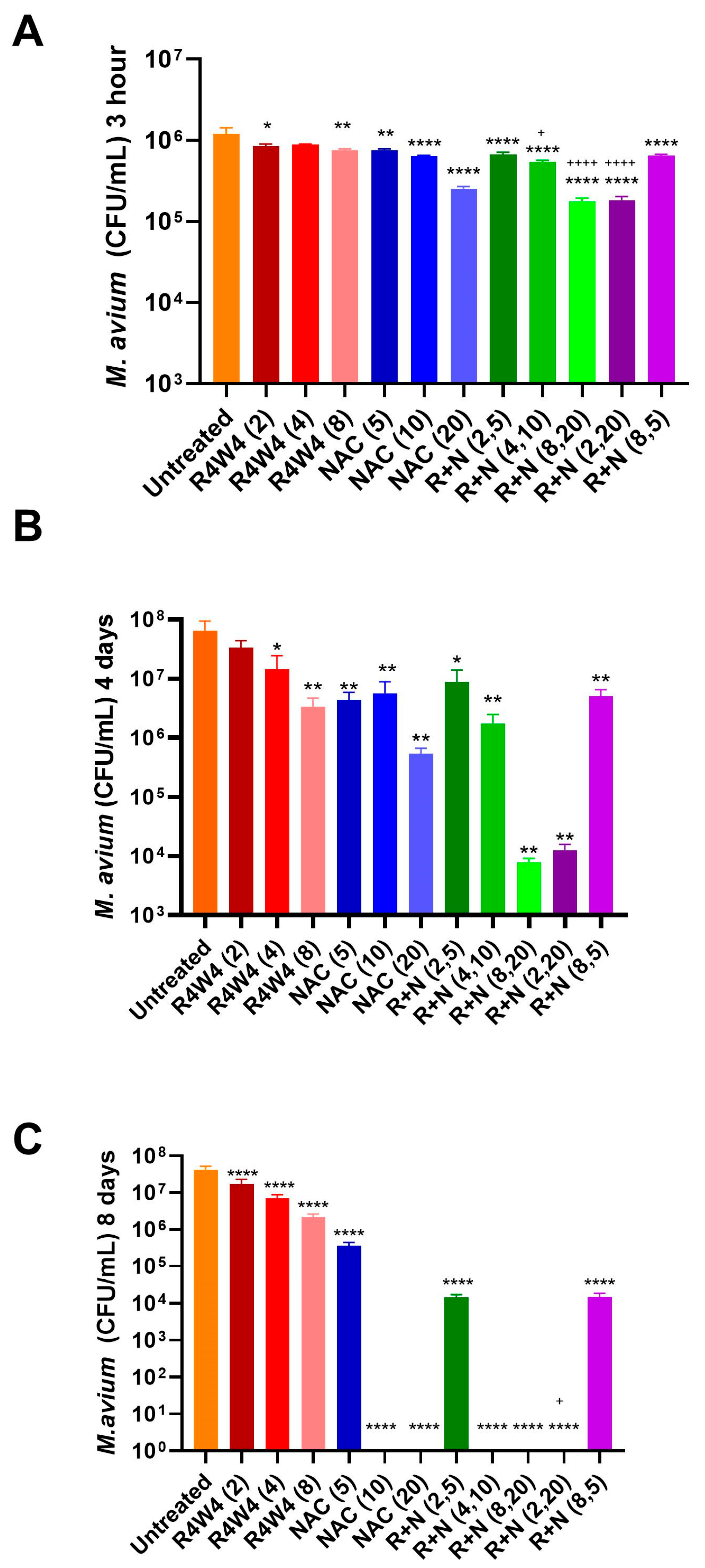
2.2. Combined Antimicrobial Effects of the Combination of [R4W4] and NAC Against M. avium Compared to That of [R4W4] Alone
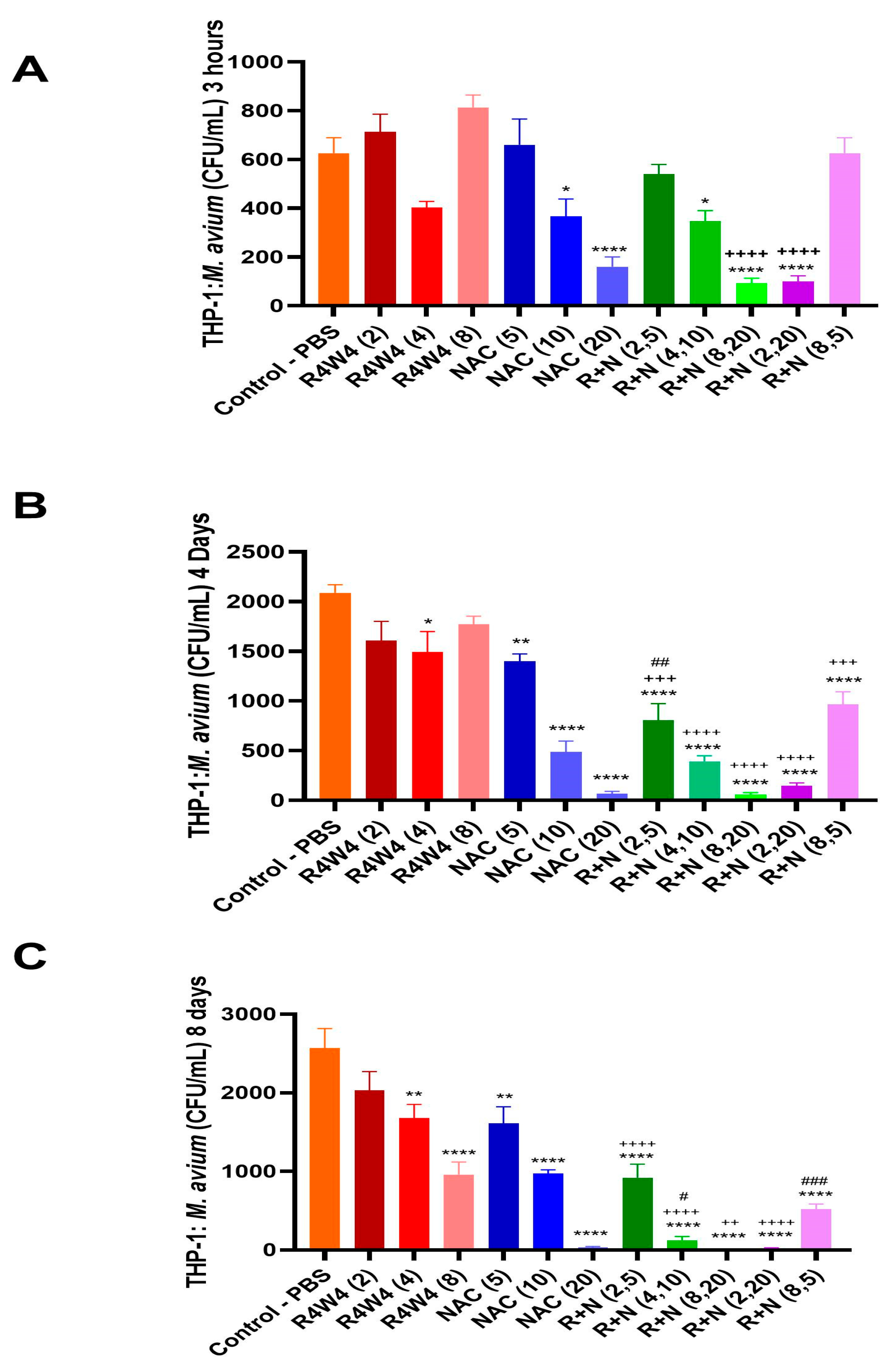
2.3. Additive Antimicrobial Effect of the [R4W4] and NAC Combination Against M. avium Within THP-1 Macrophages Compared to Either [R4W4] or NAC Alone
2.4. Minimum Inhibitory Concentration (MIC) of [R4W4] and NAC Against M. avium
2.5. Checkerboard Synergy Analysis of [R4W4] and NAC
3. Discussion
4. Materials and Methods
4.1. Bacterial and Reagent Processing and Preparation
4.2. Bacterial Cell Culture, Antibiotic Treatment, and CFU Counts
4.3. THP-1 Cell Preparation, Differentiation, and Infection
4.4. THP-1 Macrophage Treatment and Infection Termination
4.5. Membrane Depolarization Assay
4.6. Determination of MIC Against M. avium and Additive Effects of the Combination Treatment
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAC | Mycobacterium avium complex |
| NAC | N-acetylcysteine |
| AMP | Antimicrobial peptide |
| R+N | R4W4 and NAC combination treatment |
| DisC3(5) | 3,3″-dipropylthiadicarbocyanine iodine |
| CFU | Colony-forming unit |
Appendix A

References
- Daley, C.L. Mycobacterium avium Complex Disease. Microbiol. Spectr. 2017, 5, 663–701. [Google Scholar] [CrossRef]
- Kaczmarkowska, A.; Didkowska, A.; Kwiecień, E.; Stefańska, I.; Rzewuska, M.; Anusz, K. The Mycobacterium avium complex—An underestimated threat to humans and animals. Ann. Agric. Environ. Med. 2022, 29, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Holland, S.M.; Prevots, D.R. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am. J. Respir. Crit. Care Med. 2012, 185, 881–886. [Google Scholar] [CrossRef]
- Busatto, C.; Vianna, J.S.; da Silva, L.V.J.; Ramis, I.B.; da Silva, P.E.A. Mycobacterium avium: An overview. Tuberculosis 2019, 114, 127–134. [Google Scholar] [CrossRef]
- Akram, S.M. Mycobacterium avium Complex; StatPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431110/ (accessed on 23 October 2025).
- Faria, S.; Joao, I.; Jordao, L. General Overview on Nontuberculous Mycobacteria, Biofilms, and Human Infection. J. Pathog. 2015, 2015, 809014. [Google Scholar] [CrossRef]
- Park, H.E.; Lee, W.; Choi, S.; Jung, M.; Shin, M.K.; Shin, S.J. Modulating macrophage function to reinforce host innate resistance against Mycobacterium avium complex infection. Front. Immunol. 2022, 13, 931876. [Google Scholar] [CrossRef]
- Diagnosis and Treatment of Disease Caused by Nontuberculous Mycobacteria. Am. J. Respir. Crit. Care Med. 1997, 156, S1–S25. [CrossRef]
- Doffinger, R.; Patel, S.Y.; Kumararatne, D.S. Host genetic factors and mycobacterial infections: Lessons from single gene disorders affecting innate and adaptive immunity. Microbes Infect. 2006, 8, 1141–1150. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jang, S.J.; Ok, T.; Kim, G.U.; Park, H.S.; Leem, J.; Kang, B.H.; Park, S.J.; Oh, D.K.; Kang, B.J.; et al. Disseminated Mycobacterium intracellulare Infection in an Immunocompetent Host. Tuberc. Respir. Dis. 2012, 72, 452–456. [Google Scholar] [CrossRef][Green Version]
- Xu, L.; Matrova, E.; Dietz, N.E. Mycobacterium avium Infection of Nasal Septum in a Diabetic Adult: A Case Report. Head Neck Pathol. 2016, 10, 552–555. [Google Scholar] [CrossRef]
- Sivasubramanian, D.; Kalyanasundaram, B.V.; Palanisamy, N.N.; Nagaraju, S. Chronic Lymphocytic Lymphoma Complicated by Mycobacterium Avium and Scedosporium Apiospermum. Eur. J. Case Rep. Intern. Med. 2025, 12, 005621. [Google Scholar] [CrossRef] [PubMed]
- Welch, A. What Are Mycobacterium avium Complex Infections? Causes, Treatment, Prevention, and More. 6 November 2022. Available online: https://www.everydayhealth.com/rare-diseases/mycobacterium-avium-complex-infections/ (accessed on 18 August 2025).
- Marochi-Telles, J.P.; Muniz, R., Jr.; Sztajnbok, J.; Cosme-de Oliveira, A. Disseminated Mycobacterium avium on HIV/AIDS: Historical and Current Literature Review. AIDS Rev. 2020, 22, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Buchacz, K.; Baker, R.K.; Palella, F.J., Jr.; Chmiel, J.S.; Lichtenstein, K.A.; Novak, R.M.; Wood, K.C.; Brooks, J.T. AIDS-defining opportunistic illnesses in US patients, 1994-2007: A cohort study. Aids 2010, 24, 1549–1559. [Google Scholar] [CrossRef]
- Lai, K.K.; Stottmeier, K.D.; Sherman, I.H.; McCabe, W.R. Mycobacterial Cervical Lymphadenopathy: Relation of Etiologic Agents to Age. JAMA 1984, 251, 1286–1288. [Google Scholar] [CrossRef]
- Christensen, J.B.; Koeppe, J. Mycobacterium avium complex cervical lymphadenitis in an immunocompetent adult. Clin. Vaccine Immunol. 2010, 17, 1488–1490. [Google Scholar] [CrossRef]
- Ito, M.; Koga, Y.; Hachisu, Y.; Murata, K.; Sunaga, N.; Maeno, T.; Hisada, T. Treatment strategies with alternative treatment options for patients with Mycobacterium avium complex pulmonary disease. Respir. Investig. 2022, 60, 613–624. [Google Scholar] [CrossRef]
- Diel, R.; Nienhaus, A.; Ringshausen, F.C.; Richter, E.; Welte, T.; Rabe, K.F.; Loddenkemper, R. Microbiologic Outcome of Interventions Against Mycobacterium avium Complex Pulmonary Disease: A Systematic Review. Chest 2018, 153, 888–921. [Google Scholar] [CrossRef]
- Joo, S.H. Cyclic peptides as therapeutic agents and biochemical tools. Biomol. Ther. 2012, 20, 19–26. [Google Scholar] [CrossRef]
- Oh, D.; Sun, J.; Nasrolahi Shirazi, A.; LaPlante, K.L.; Rowley, D.C.; Parang, K. Antibacterial activities of amphiphilic cyclic cell-penetrating peptides against multidrug-resistant pathogens. Mol. Pharm. 2014, 11, 3528–3536. [Google Scholar] [CrossRef]
- Sajid, M.I.; Lohan, S.; Kato, S.; Tiwari, R.K. Combination of Amphiphilic Cyclic Peptide [R(4)W(4)] and Levofloxacin against Multidrug-Resistant Bacteria. Antibiotics 2022, 11, 416. [Google Scholar] [CrossRef]
- Hernandez, J.; Ashley, D.; Cao, R.; Abrahem, R.; Nguyen, T.; To, K.; Yegiazaryan, A.; Akinwale David, A.; Kumar Tiwari, R.; Venketaraman, V. Cyclic Peptide [R(4)W(4)] in Improving the Ability of First-Line Antibiotics to Inhibit Mycobacterium tuberculosis Inside in vitro Human Granulomas. Front. Immunol. 2020, 11, 1677. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.; Sasaninia, K.; Abnousian, A.; Badaoui, A.; Owens, J.; Beever, A.; Kachour, N.; Tiwari, R.K.; Venketaraman, V. Additive Effects of Cyclic Peptide [R4W4] When Added Alongside Azithromycin and Rifampicin against Mycobacterium avium Infection. Pathogens 2023, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci. 2003, 60, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A.; Herzenberg, L.A. N-Acetylcysteine--a safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007, 7, 355–359. [Google Scholar] [CrossRef]
- Teskey, G.; Cao, R.; Islamoglu, H.; Medina, A.; Prasad, C.; Prasad, R.; Sathananthan, A.; Fraix, M.; Subbian, S.; Zhong, L.; et al. The Synergistic Effects of the Glutathione Precursor, NAC and First-Line Antibiotics in the Granulomatous Response Against Mycobacterium tuberculosis. Front. Immunol. 2018, 9, 2069. [Google Scholar] [CrossRef]
- Schäfer, A.B.; Wenzel, M. A How-To Guide for Mode of Action Analysis of Antimicrobial Peptides. Front. Cell. Infect. Microbiol. 2020, 10, 540898. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef]
- Amaral, E.P.; Conceição, E.L.; Costa, D.L.; Rocha, M.S.; Marinho, J.M.; Cordeiro-Santos, M.; D’Império-Lima, M.R.; Barbosa, T.; Sher, A.; Andrade, B.B. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 2016, 16, 251. [Google Scholar] [CrossRef]
- Alderwick, L.J.; Harrison, J.; Lloyd, G.S.; Birch, H.L. The Mycobacterial Cell Wall--Peptidoglycan and Arabinogalactan. Cold Spring Harb Perspect. Med. 2015, 5, a021113. [Google Scholar] [CrossRef]
- Batt, S.M.; Minnikin, D.E.; Besra, G.S. The thick waxy coat of mycobacteria, a protective layer against antibiotics and the host’s immune system. Biochem. J. 2020, 477, 1983–2006. [Google Scholar] [CrossRef]
- Moon, S.H.; Huang, E. Novel linear lipopeptide paenipeptin C’ binds to lipopolysaccharides and lipoteichoic acid and exerts bactericidal activity by the disruption of cytoplasmic membrane. BMC Microbiol. 2019, 19, 6. [Google Scholar] [CrossRef]
- Povilaitis, S.C.; Fathizadeh, A.; Kogan, M.; Elber, R.; Webb, L.J. Design of Peptides for Membrane Insertion: The Critical Role of Charge Separation. J. Phys. Chem. B 2022, 126, 6454–6463. [Google Scholar] [CrossRef]
- Li, Y.-X.; Pang, H.-B. Macropinocytosis as a cell entry route for peptide-functionalized and bystander nanoparticles. J. Control. Release 2021, 329, 1222–1230. [Google Scholar] [CrossRef]
- Ezeriņa, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H(2)S and Sulfane Sulfur Production. Cell Chem. Biol. 2018, 25, 447–459.e444. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.; Sasaninia, K.; Badaoui, A.; Glassman, I.; Abnousian, A.; Rai, N.; Tiwari, R.K.; Venketaraman, V. The effects of cyclic peptide [R4W4] in combination with first-line therapy on the survival of Mycobacterium avium. Front. Cell. Infect. Microbiol. 2025, 15, 1547376. [Google Scholar] [CrossRef] [PubMed]
- Briffotaux, J.; Xu, Y.; Huang, W.; Hui, Z.; Wang, X.; Gicquel, B.; Liu, S. A Hydrazine-Hydrazone Adamantine Compound Shows Antimycobacterial Activity and Is a Probable Inhibitor of MmpL3. Molecules 2022, 27, 7130. [Google Scholar] [CrossRef] [PubMed]
- Ardalani, H.; Anam, S.; Kromphardt, K.J.K.; Staerk, D.; Kongstad, K.T. Coupling Microplate-Based Antibacterial Assay with Liquid Chromatography for High-Resolution Growth Inhibition Profiling of Crude Extracts: Validation and Proof-of-Concept Study with Staphylococcus aureus. Molecules 2021, 26, 1550. [Google Scholar] [CrossRef]
- Antimicrobial Synergy Testing/Checkerboard Assay. 2025. Available online: https://antiviral.creative-diagnostics.com/antimicrobial-synergy-testing-checkerboard-assay.html (accessed on 16 October 2025).
- Nikolic, I.; Vukovic, D.; Gavric, D.; Cvetanovic, J.; Aleksic Sabo, V.; Gostimirovic, S.; Narancic, J.; Knezevic, P. An Optimized Checkerboard Method for Phage-Antibiotic Synergy Detection. Viruses 2022, 14, 1542. [Google Scholar] [CrossRef]
| Treatment | Mean % Inhibition ± SD | MIC |
|---|---|---|
| R4W4 1 | 76.51 ± 33.12 | 6 µg/mL |
| R4W4 2 | 55.06 ± 18.92 | |
| R4W4 4 | 82.28 ± 45.33 | |
| R4W4 6 | 158.18 ± 11.45 | |
| R4W4 8 | 145.05 ± 13.14 | |
| NAC 2.5 | 89.97 ± 25.97 | 5 mM |
| NAC 5 | 136.68 ± 22.86 | |
| NAC 10 | 94.97 ± 7.95 | |
| NAC 15 | 140.44 ± 3.00 | |
| NAC 20 | 176.00 ± 6.16 |
| Treatment | FIC Index |
|---|---|
| R+N (2,5) | 1.33 |
| R+N (2,20) | 4.33 |
| R+N (4,10) | 2.67 |
| R+N (8,20) | 5.33 |
| R+N (8,5) | 2.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaninia, K.; Era, I.H.; Newman, N.; Melendez, J.; Akif, W.; Sharma, E.; Nikjeh, O.; Glassman, I.; Jiménez, C.; Sharma, N.; et al. Additive Effects of N-Acetylcysteine and [R4W4] Combination Treatment on Mycobacterium avium. Int. J. Mol. Sci. 2025, 26, 10361. https://doi.org/10.3390/ijms262110361
Sasaninia K, Era IH, Newman N, Melendez J, Akif W, Sharma E, Nikjeh O, Glassman I, Jiménez C, Sharma N, et al. Additive Effects of N-Acetylcysteine and [R4W4] Combination Treatment on Mycobacterium avium. International Journal of Molecular Sciences. 2025; 26(21):10361. https://doi.org/10.3390/ijms262110361
Chicago/Turabian StyleSasaninia, Kayvan, Iffat Hasnin Era, Nezam Newman, Jesse Melendez, Wajiha Akif, Eashan Sharma, Omid Nikjeh, Ira Glassman, Cristián Jiménez, Navya Sharma, and et al. 2025. "Additive Effects of N-Acetylcysteine and [R4W4] Combination Treatment on Mycobacterium avium" International Journal of Molecular Sciences 26, no. 21: 10361. https://doi.org/10.3390/ijms262110361
APA StyleSasaninia, K., Era, I. H., Newman, N., Melendez, J., Akif, W., Sharma, E., Nikjeh, O., Glassman, I., Jiménez, C., Sharma, N., Xu, A., Lambros, M., Zhou, M., Tiwari, R., & Venketaraman, V. (2025). Additive Effects of N-Acetylcysteine and [R4W4] Combination Treatment on Mycobacterium avium. International Journal of Molecular Sciences, 26(21), 10361. https://doi.org/10.3390/ijms262110361











