IVF and Thermal Manipulation at the First Cleavage Stage Alter Offspring Circadian Phenotype, Sleep, and Brain Epigenetics
Abstract
1. Introduction
2. Results
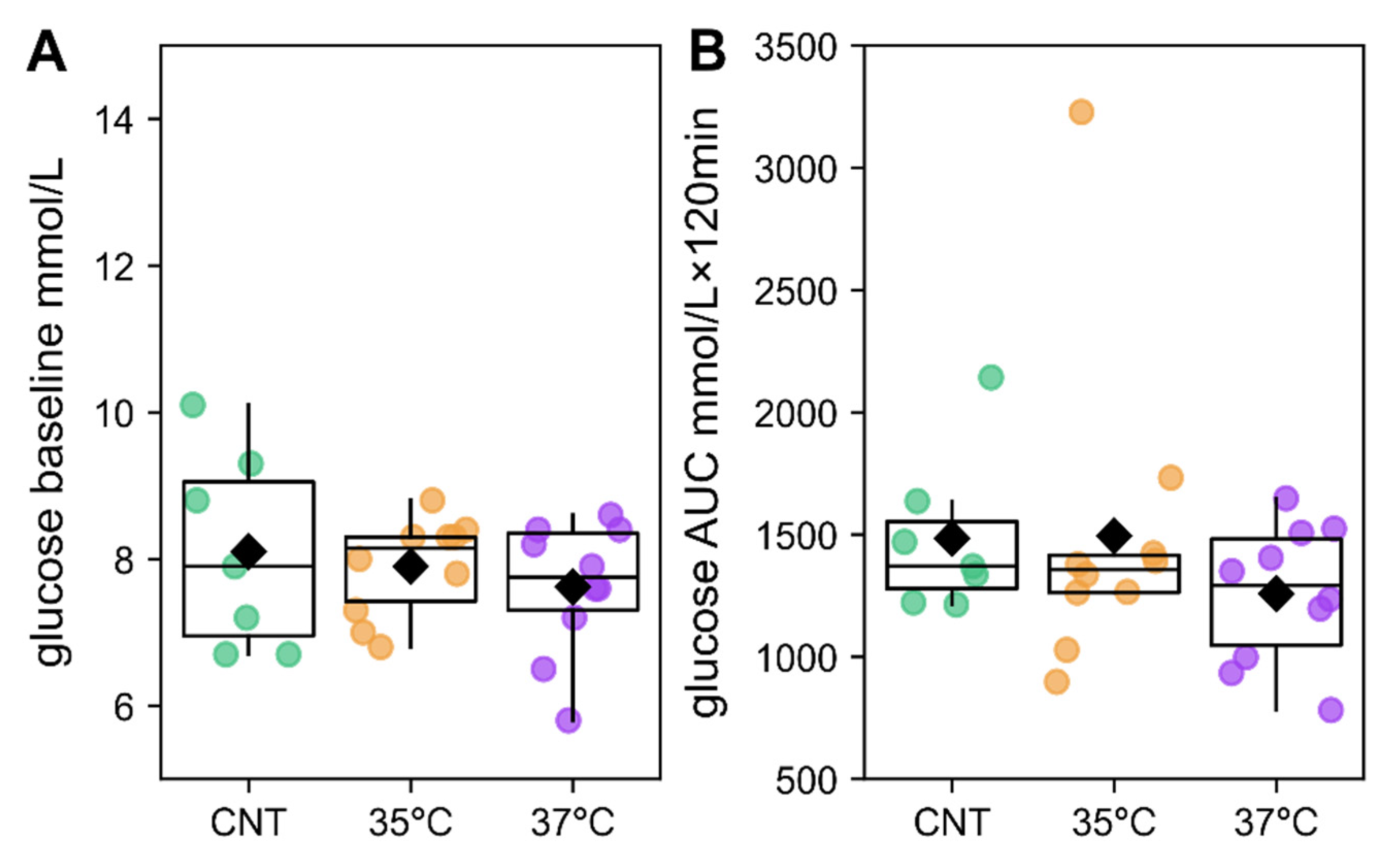
2.1. IVF Offspring Exhibit Increased Body Mass Without Glucose Intolerance
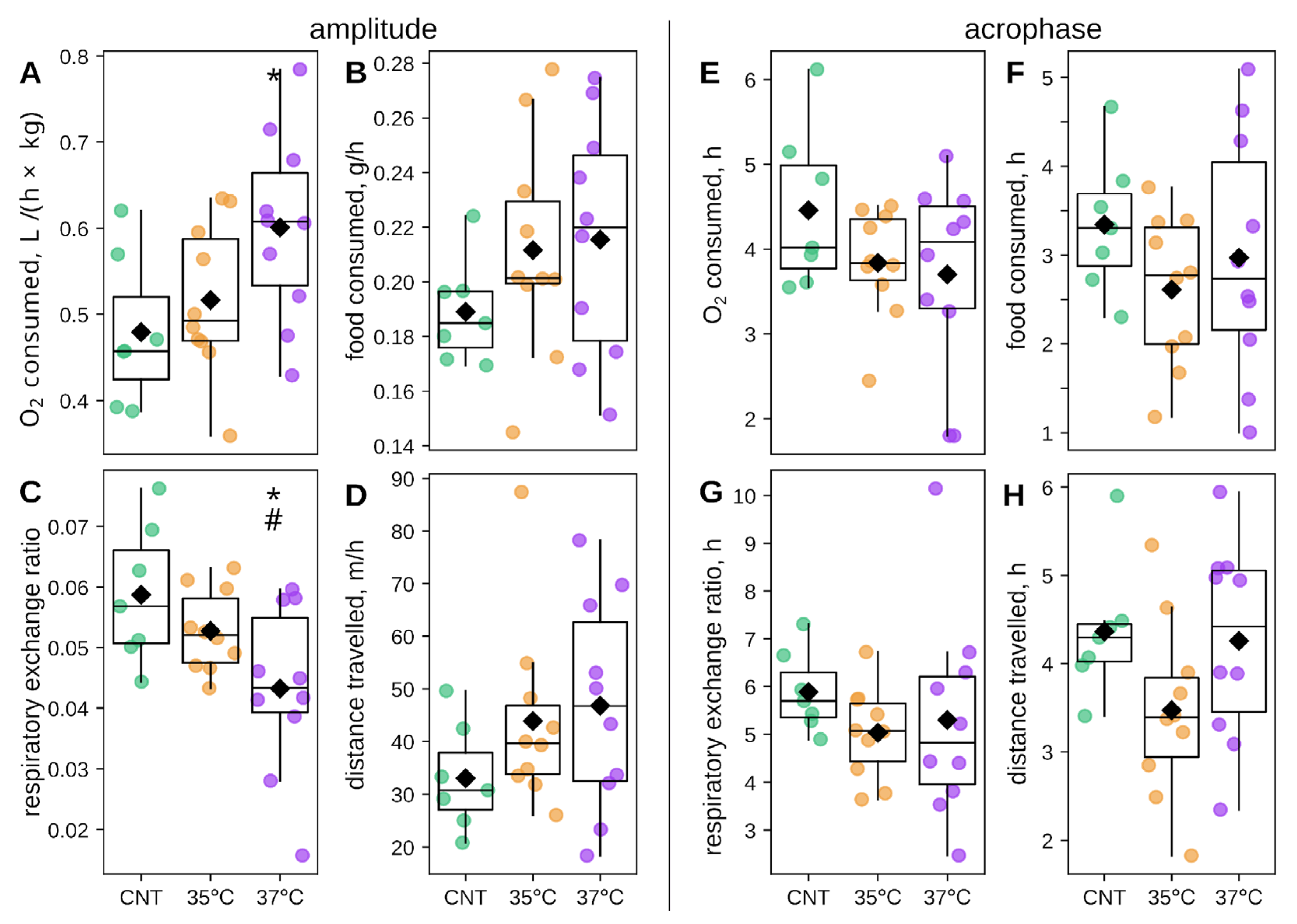
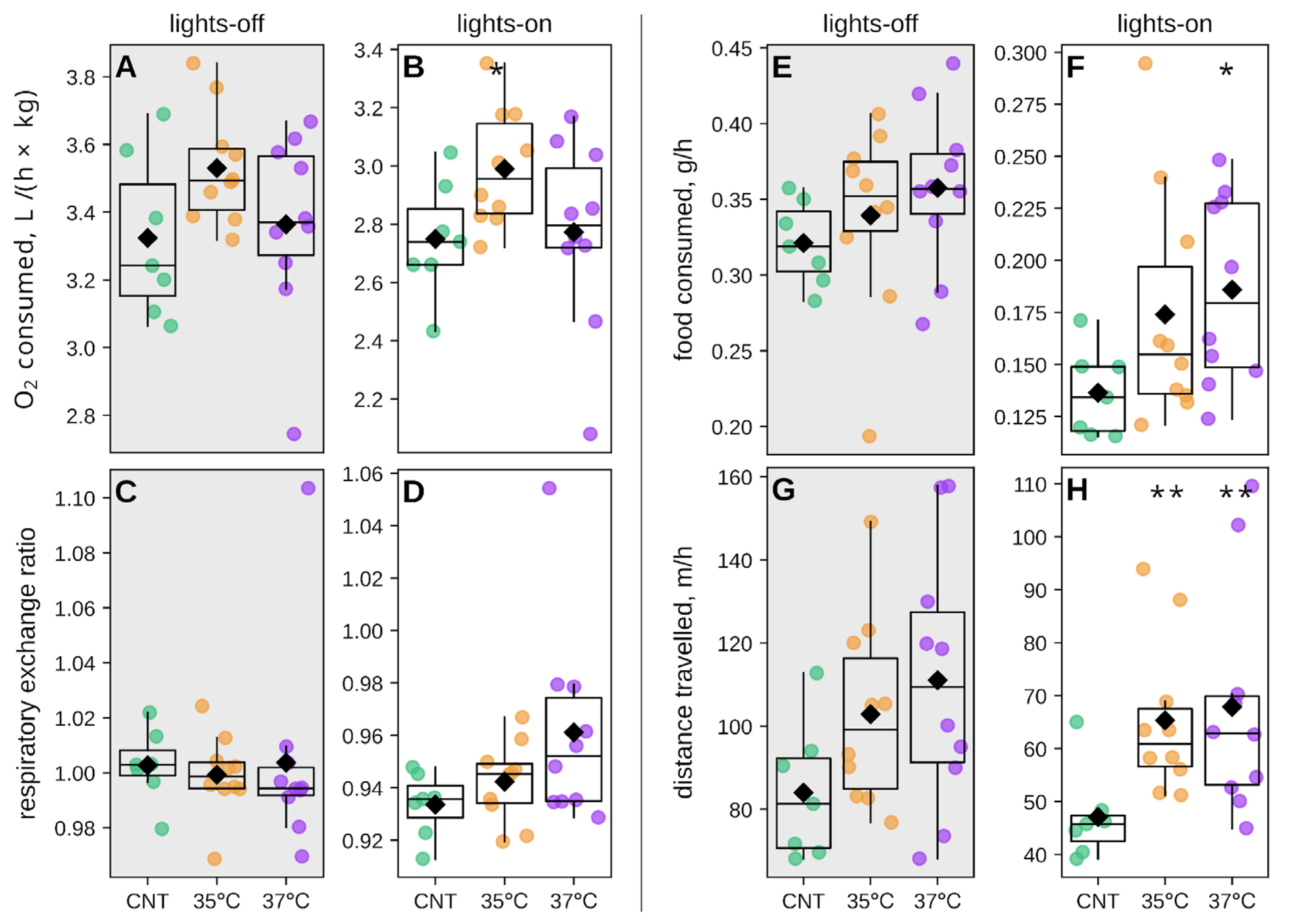
2.2. Metabolic and Behavioral Phenotyping of IVF-Conceived Offspring
2.3. Impact of IVF and Incubation Temperature at the First Cleavage on Offspring Circadian Phenotype
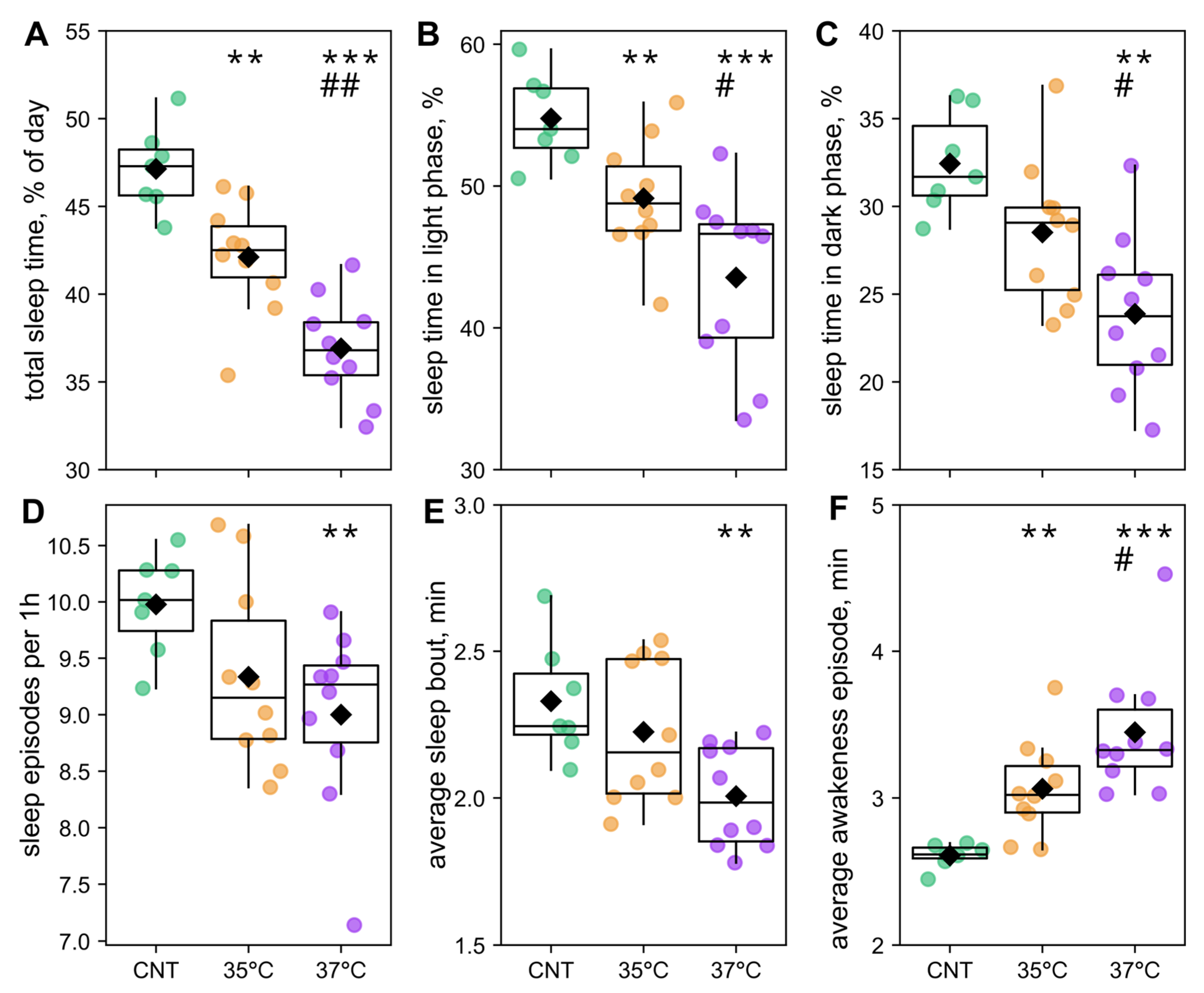
2.4. The Effects of IVF and First-Cleavage Incubation Temperature on Offspring Sleep
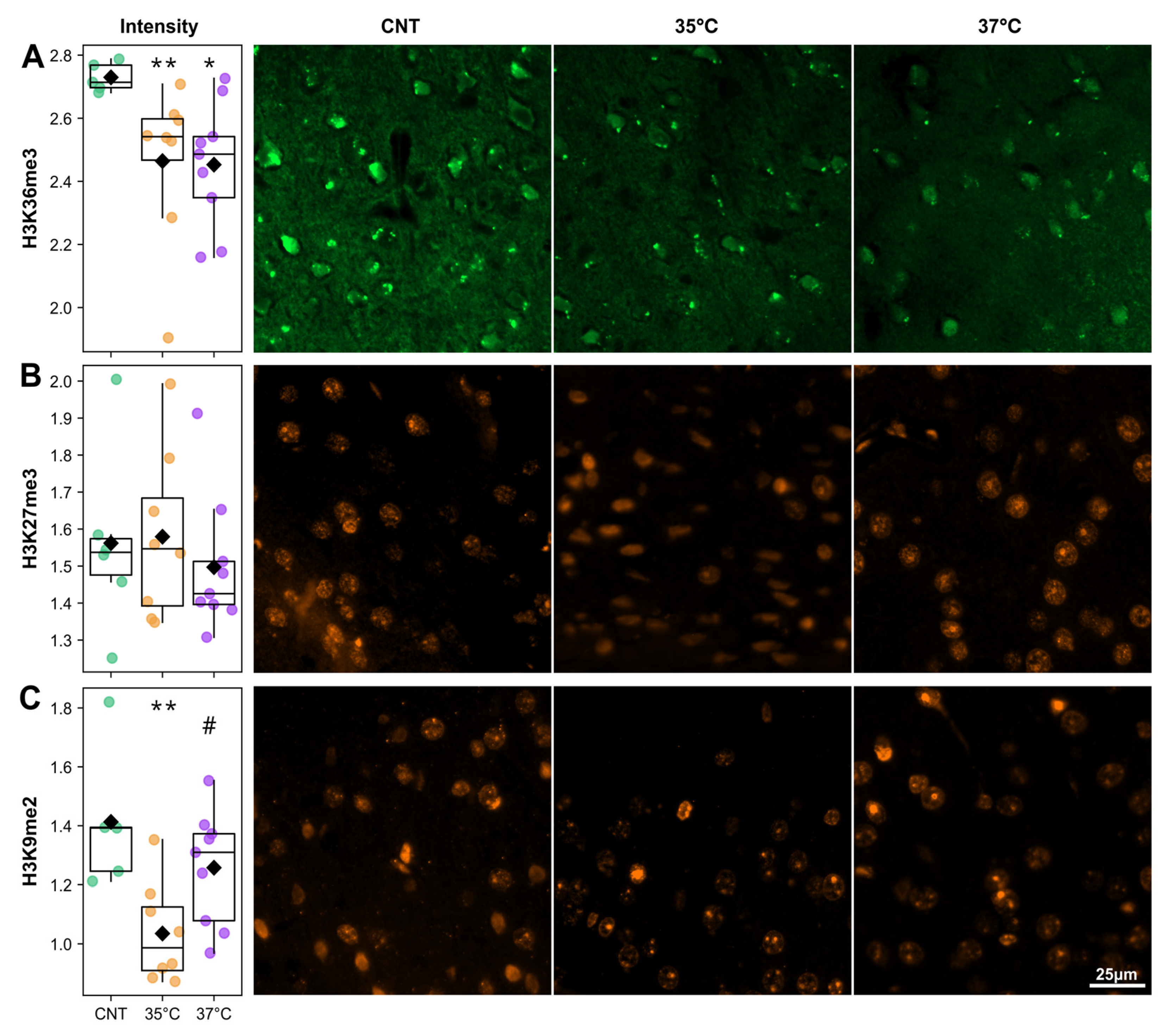
2.5. IVF Mice Have Altered Histone Modification
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. In Vitro Fertilization, Embryo Transfer, and Offspring Weaning
- control (CNT)—(n = 7),
- 35 °C IVF group—(n = 10),
- 37 °C IVF group—(n = 10).
4.3. Glucose Tolerance
4.4. Circadian Phenotyping
4.5. Immunofluorescent Analysis of Histone Modifications in the Somatosensory Cortex
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Assisted Reproductive Technologies |
| COCs | Cumulus-Oocyte Complexes |
| IVF | In Vitro Fertilization |
| H3K9me2 | Di-methylation at the 9th lysine residue of the histone H3 protein |
| H3K27me3 | Tri-methylation at the 27th lysine residue of the histone H3 protein |
| H3K36me3 | Tri-methylation at the 36th lysine residue of the histone H3 protein |
| RER | Respiratory Exchange Ratio (the ratio of VCO2 to VO2) |
| VCO2 | Volume carbon dioxide production |
| VO2 | Volume oxygen consumption |
| ZGA | Zygotic Genome Activation |
| Statistical abbreviations | |
| ANCOVA | Analysis of covariance |
| AUC | Area Under the Curve |
| LSD | Fisher’s Least Significant Difference test |
References
- Liu, Y.; Chen, X.; Deng, X.; Yang, F.; Zheng, J.; Zhou, T.; Xu, L.; Xie, X.; Ju, Z.; Wang, B.; et al. Association of NAD+ Levels with Metabolic Disease in a Community-Based Study. Front. Endocrinol. 2023, 14, 1164788. [Google Scholar] [CrossRef]
- Heber, M.F.; Ptak, G.E. The Effects of Assisted Reproduction Technologies on Metabolic Health and Disease†. Biol. Reprod. 2021, 104, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Aghebati-Maleki, L.; Rashidiani, S.; Csabai, T.; Nnaemeka, O.B.; Szekeres-Bartho, J. Long-Term Effects of ART on the Health of the Offspring. Int. J. Mol. Sci. 2023, 24, 13564. [Google Scholar] [CrossRef]
- Anisimova, M.V.; Gon, Y.; Kontsevaya, G.V.; Romashchenko, A.V.; Khotskin, N.V.; Stanova, A.K.; Gerlinskaya, L.A.; Moshkin, M.P. Body Composition as an Indicator of Metabolic Changes in Mice Obtained by in Vitro Fertilization. Vavilovskii Zhurnal Genet. Sel. 2023, 27, 357–365. [Google Scholar] [CrossRef]
- Bokor, S.; Vass, R.A.; Funke, S.; Ertl, T.; Molnár, D. Epigenetic Effect of Maternal Methyl-Group Donor Intake on Offspring’s Health and Disease. Life 2022, 12, 609. [Google Scholar] [CrossRef]
- Takahashi, S.; Kyogoku, H.; Hayakawa, T.; Miura, H.; Oji, A.; Kondo, Y.; Takebayashi, S.; Kitajima, T.S.; Hiratani, I. Embryonic Genome Instability upon DNA Replication Timing Program Emergence. Nature 2024, 633, 686–694. [Google Scholar] [CrossRef]
- Miura, H.; Takahashi, S.; Poonperm, R.; Tanigawa, A.; Takebayashi, S.; Hiratani, I. Single-Cell DNA Replication Profiling Identifies Spatiotemporal Developmental Dynamics of Chromosome Organization. Nat. Genet. 2019, 51, 1356–1368. [Google Scholar] [CrossRef] [PubMed]
- Sjöblom, C.; Roberts, C.T.; Wikland, M.; Robertson, S.A. Granulocyte-Macrophage Colony-Stimulating Factor Alleviates Adverse Consequences of Embryo Culture on Fetal Growth Trajectory and Placental Morphogenesis. Endocrinology 2005, 146, 2142–2153. [Google Scholar] [CrossRef]
- Scott, K.A.; Yamazaki, Y.; Yamamoto, M.; Lin, Y.; Melhorn, S.J.; Krause, E.G.; Woods, S.C.; Yanagimachi, R.; Sakai, R.R.; Tamashiro, K.L.K. Glucose Parameters Are Altered in Mouse Offspring Produced by Assisted Reproductive Technologies and Somatic Cell Nuclear Transfer1. Biol. Reprod. 2010, 83, 220–227. [Google Scholar] [CrossRef]
- Feuer, S.K.; Donjacour, A.; Simbulan, R.K.; Lin, W.; Liu, X.; Maltepe, E.; Rinaudo, P.F. Sexually Dimorphic Effect of In Vitro Fertilization (IVF) on Adult Mouse Fat and Liver Metabolomes. Endocrinology 2014, 155, 4554–4567. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, M.; Van Weissenbruch, M.M.; Roos, J.C.; Vermeiden, J.P.W.; Van Leeuwen, F.E.; Delemarre-van De Waal, H.A. Body Composition in Children and Adolescents Born after in Vitro Fertilization or Spontaneous Conception. J. Clin. Endocrinol. Metab. 2007, 92, 3417–3423. [Google Scholar] [CrossRef]
- Cui, L.; Zhou, W.; Xi, B.; Ma, J.; Hu, J.; Fang, M.; Hu, K.; Qin, Y.; You, L.; Cao, Y.; et al. Increased Risk of Metabolic Dysfunction in Children Conceived by Assisted Reproductive Technology. Diabetologia 2020, 63, 2150–2157. [Google Scholar] [CrossRef]
- Elhakeem, A.; Taylor, A.E.; Inskip, H.M.; Huang, J.Y.; Mansell, T.; Rodrigues, C.; Asta, F.; Blaauwendraad, S.M.; Håberg, S.E.; Halliday, J.; et al. Long-Term Cardiometabolic Health in People Born after Assisted Reproductive Technology: A Multi-Cohort Analysis. Eur. Heart J. 2023, 44, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Van Der Poorten, D.; Milner, K.; Hui, J.; Hodge, A.; Trenell, M.I.; Kench, J.G.; London, R.; Peduto, T.; Chisholm, D.J.; George, J. Visceral Fat: A Key Mediator of Steatohepatitis in Metabolic Liver Disease†. Hepatology 2008, 48, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Hoshino, K.; Yokota, K.; Itoh, Y.; Tajima, N. Differences in the Pathology of the Metabolic Syndrome With or Without Visceral Fat Accumulation. ENDO 2006, 29, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Covassin, N.; Singh, P.; McCrady-Spitzer, S.K.; St Louis, E.K.; Calvin, A.D.; Levine, J.A.; Somers, V.K. Effects of Experimental Sleep Restriction on Energy Intake, Energy Expenditure, and Visceral Obesity. J. Am. Coll. Cardiol. 2022, 79, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans That Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef]
- Panda, S. Circadian Physiology of Metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef]
- Wilkinson, R.T. The Relationship Between Body Temperature and Performance Across Circadian Phase Shifts. In Rhythmic Aspects of Behavior; Routledge: Abingdon, UK, 1982; ISBN 978-1-003-04615-8. [Google Scholar]
- Takahashi, J.S. Transcriptional Architecture of the Mammalian Circadian Clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Stanton, D.; Justin, H.S.; Reitzel, A.M. Step in Time: Conservation of Circadian Clock Genes in Animal Evolution. Integr. Comp. Biol. 2022, 62, 1503–1518. [Google Scholar] [CrossRef]
- Morris, A.R.; Stanton, D.L.; Roman, D.; Liu, A.C. Systems Level Understanding of Circadian Integration with Cell Physiology. J. Mol. Biol. 2020, 432, 3547–3564. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, J.; Montellier, E.; Sassone-Corsi, P. Molecular Cogs: Interplay between Circadian Clock and Cell Cycle. Trends Cell Biol. 2018, 28, 368–379. [Google Scholar] [CrossRef]
- Leuck, M.; Levandovski, R.; Harb, A.; Quiles, C.; Hidalgo, M.P. Circadian Rhythm of Energy Expenditure and Oxygen Consumption. J. Parenter. Enter. Nutr. 2014, 38, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Sancar, A.; Lindsey-Boltz, L.A.; Gaddameedhi, S.; Selby, C.P.; Ye, R.; Chiou, Y.-Y.; Kemp, M.G.; Hu, J.; Lee, J.H.; Ozturk, N. Circadian Clock, Cancer, and Chemotherapy. Biochemistry 2015, 54, 110–123. [Google Scholar] [CrossRef]
- Zhu, Q.; Belden, W.J. Molecular Regulation of Circadian Chromatin. J. Mol. Biol. 2020, 432, 3466–3482. [Google Scholar] [CrossRef]
- Reinke, H.; Asher, G. Crosstalk between Metabolism and Circadian Clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241. [Google Scholar] [CrossRef]
- Papazyan, R.; Zhang, Y.; Lazar, M.A. Genetic and Epigenomic Mechanisms of Mammalian Circadian Transcription. Nat. Struct. Mol. Biol. 2016, 23, 1045–1052. [Google Scholar] [CrossRef]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the Clock Components CLOCK and BMAL1 Leads to Hypoinsulinaemia and Diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef]
- Guan, D.; Lazar, M.A. Interconnections between Circadian Clocks and Metabolism. J. Clin. Investig. 2021, 131, e148278. [Google Scholar] [CrossRef] [PubMed]
- Gachon, F.; Bugianesi, E.; Castelnuovo, G.; Oster, H.; Pendergast, J.S.; Montagnese, S. Potential Bidirectional Communication between the Liver and the Central Circadian Clock in MASLD. npj Metab. Health Dis. 2025, 3, 15. [Google Scholar] [CrossRef]
- Stanova, A.; Kontsevaya, G.; Romashchenko, A.; Zuev, D.; Silvanovich, E.; Moshkin, Y.; Gerlinskaya, L.; Moshkin, M. The Temperature of the First Cleavage Impacts Preimplantation Development and Newborn Viability. Int. J. Mol. Sci. 2025, 26, 3745. [Google Scholar] [CrossRef] [PubMed]
- Cortessis, V.K.; Azadian, M.; Buxbaum, J.; Sanogo, F.; Song, A.Y.; Sriprasert, I.; Wei, P.C.; Yu, J.; Chung, K.; Siegmund, K.D. Comprehensive Meta-Analysis Reveals Association between Multiple Imprinting Disorders and Conception by Assisted Reproductive Technology. J. Assist. Reprod. Genet. 2018, 35, 943–952. [Google Scholar] [CrossRef]
- Buonfiglio, D.; Hummer, D.L.; Armstrong, A.; Christopher Ehlen, J.; DeBruyne, J.P. Angelman Syndrome and Melatonin: What Can They Teach Us about Sleep Regulation. J. Pineal Res. 2020, 69, e12697. [Google Scholar] [CrossRef]
- Yeung, E.H.; Mendola, P.; Sundaram, R.; Zeng, X.; Guan, W.; Tsai, M.Y.; Robinson, S.L.; Stern, J.E.; Ghassabian, A.; Lawrence, D.; et al. Conception by Fertility Treatment and Offspring Deoxyribonucleic Acid Methylation. Fertil. Steril. 2021, 116, 493–504. [Google Scholar] [CrossRef]
- Stanton, D.L.; Graf, A.; Maia, T.S.; Blum, H.; Jiang, Z.; Hansen, P.J. Absence of a Molecular Circadian Clock in the Preimplantation Embryo Is a Conserved Characteristic in the Mammal. Reproduction 2023, 166, 199–207. [Google Scholar] [CrossRef]
- Zeng, F.; Schultz, R.M. RNA Transcript Profiling during Zygotic Gene Activation in the Preimplantation Mouse Embryo. Dev. Biol. 2005, 283, 40–57. [Google Scholar] [CrossRef]
- Fischl, H.; McManus, D.; Oldenkamp, R.; Schermelleh, L.; Mellor, J.; Jagannath, A.; Furger, A. Cold-induced Chromatin Compaction and Nuclear Retention of Clock mRNAs Resets the Circadian Rhythm. EMBO J. 2020, 39, e105604. [Google Scholar] [CrossRef] [PubMed]
- Chereji, R.V.; Kan, T.-W.; Grudniewska, M.K.; Romashchenko, A.V.; Berezikov, E.; Zhimulev, I.F.; Guryev, V.; Morozov, A.V.; Moshkin, Y.M. Genome-Wide Profiling of Nucleosome Sensitivity and Chromatin Accessibility in Drosophila Melanogaster. Nucleic Acids Res. 2016, 44, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.H.F. Temperature Gradients in Female Reproductive Tissues. Reprod. Biomed. Online 2012, 24, 377–380. [Google Scholar] [CrossRef]
- Kontsevaya, G.; Romashchenko, A.; Babochkina, T.; Sugatova, D.; Shevelev, O.; Sharapova, M.; Moshkin, Y.; Moshkin, M.; Gerlinskaya, L. Induction of Stress Granules and Developmental Instability of Offspring Phenotype Due to Hypothermia During First Mouse Embryo Cleavage. Int. J. Mol. Sci. 2025, 26, 8060. [Google Scholar] [CrossRef]
- Babochkina, T.I.; Gerlinskaya, L.A.; Anisimova, M.V.; Kontsevaya, G.V.; Feofanova, N.A.; Stanova, A.K.; Moshkin, M.P.; Moshkin, Y.M. Mother–Fetus Immune Cross-Talk Coordinates “Extrinsic”/“Intrinsic” Embryo Gene Expression Noise and Growth Stability. Int. J. Mol. Sci. 2022, 23, 12467. [Google Scholar] [CrossRef] [PubMed]
- Phengchat, R.; Takata, H.; Morii, K.; Inada, N.; Murakoshi, H.; Uchiyama, S.; Fukui, K. Calcium Ions Function as a Booster of Chromosome Condensation. Sci. Rep. 2016, 6, 38281. [Google Scholar] [CrossRef]
- Radwan, B.; Jansen, G.; Chaudhury, D. Sleep-Wake Dynamics Pre- and Post-Exposure to Chronic Social Stress. iScience 2021, 24, 103204. [Google Scholar] [CrossRef] [PubMed]
- Kontsevaya, G.V.; Gerlinskaya, L.A.; Moshkin, Y.M.; Anisimova, M.V.; Stanova, A.K.; Babochkina, T.I.; Moshkin, M.P. The Effects of Sperm and Seminal Fluid of Immunized Male Mice on In Vitro Fertilization and Surrogate Mother–Embryo Interaction. Int. J. Mol. Sci. 2021, 22, 10650. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.M.; Harper, J.; Roberts, S.A.; O’Neill, H.C.; Johnstone, E.D.; Brison, D.R. The Impact of Selected Embryo Culture Conditions on ART Treatment Cycle Outcomes: A UK National Study. Hum. Reprod. Open 2020, 2020, hoz031. [Google Scholar] [CrossRef]
- Dumoulin, J.C.; Land, J.A.; Van Montfoort, A.P.; Nelissen, E.C.; Coonen, E.; Derhaag, J.G.; Schreurs, I.L.; Dunselman, G.A.; Kester, A.D.; Geraedts, J.P.; et al. Effect of in Vitro Culture of Human Embryos on Birthweight of Newborns. Hum. Reprod. 2010, 25, 605–612. [Google Scholar] [CrossRef]
- Wale, P.L.; Gardner, D.K. The Effects of Chemical and Physical Factors on Mammalian Embryo Culture and Their Importance for the Practice of Assisted Human Reproduction. Hum. Reprod. Update 2016, 22, 2–22. [Google Scholar] [CrossRef]
- McEvoy, T.G.; Sinclair, K.D.; Young, L.E.; Wilmut, I.; Robinson, J.J. Large Offspring Syndrome and Other Consequences of Ruminant Embryo Culture in Vitro: Relevance to Blastocyst Culture in Human ART. Hum. Fertil. 2000, 3, 238–246. [Google Scholar] [CrossRef]
- Canovas, S.; Ross, P.J.; Kelsey, G.; Coy, P. DNA Methylation in Embryo Development: Epigenetic Impact of ART (Assisted Reproductive Technologies). BioEssays 2017, 39, 1700106. [Google Scholar] [CrossRef]
- Cagnone, G.; Sirard, M.-A. The Embryonic Stress Response to in Vitro Culture: Insight from Genomic Analysis. Reproduction 2016, 152, R247–R261. [Google Scholar] [CrossRef]
- Chason, R.J.; Csokmay, J.; Segars, J.H.; DeCherney, A.H.; Armant, D.R. Environmental and Epigenetic Effects upon Preimplantation Embryo Metabolism and Development. Trends Endocrinol. Metab. 2011, 22, 412–420. [Google Scholar] [CrossRef]
- McBrian, M.A.; Behbahan, I.S.; Ferrari, R.; Su, T.; Huang, T.-W.; Li, K.; Hong, C.S.; Christofk, H.R.; Vogelauer, M.; Seligson, D.B.; et al. Histone Acetylation Regulates Intracellular pH. Mol. Cell 2013, 49, 310–321. [Google Scholar] [CrossRef]
- Elhakeem, A.; Taylor, A.E.; Inskip, H.M.; Huang, J.; Tafflet, M.; Vinther, J.L.; Asta, F.; Erkamp, J.S.; Gagliardi, L.; Guerlich, K.; et al. Association of Assisted Reproductive Technology With Offspring Growth and Adiposity From Infancy to Early Adulthood. JAMA Netw. Open 2022, 5, e2222106. [Google Scholar] [CrossRef] [PubMed]
- Van Montfoort, A.P.A.; Hanssen, L.L.P.; De Sutter, P.; Viville, S.; Geraedts, J.P.M.; De Boer, P. Assisted Reproduction Treatment and Epigenetic Inheritance. Hum. Reprod. Update 2012, 18, 171–197. [Google Scholar] [CrossRef]
- Narapareddy, L.; Rhon-Calderon, E.A.; Vrooman, L.A.; Baeza, J.; Nguyen, D.K.; Mesaros, C.; Lan, Y.; Garcia, B.A.; Schultz, R.M.; Bartolomei, M.S. Sex-specific Effects of in Vitro Fertilization on Adult Metabolic Outcomes and Hepatic Transcriptome and Proteome in Mouse. FASEB J. 2021, 35, e21523. [Google Scholar] [CrossRef] [PubMed]
- Donjacour, A.; Liu, X.; Lin, W.; Simbulan, R.; Rinaudo, P.F. In Vitro Fertilization Affects Growth and Glucose Metabolism in a Sex-Specific Manner in an Outbred Mouse Model1. Biol. Reprod. 2014, 90, 80-1. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy Balance and Obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef]
- Santos-Pinto, J.; Luz, M.A.; Griggio, F.N. Energy Expenditure of Rats Subjected to Long-Term Food Restriction. Int. J. Food Sci. Nutr. 2001, 52, 193–200. [Google Scholar] [CrossRef]
- Pickel, L.; Sung, H.-K. Feeding Rhythms and the Circadian Regulation of Metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef]
- Challet, E. The Circadian Regulation of Food Intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef]
- Imterat, M.; Wainstock, T.; Sheiner, E.; Landau, D.; Walfisch, A.; Harlev, A. Fertility Treatments and the Risk of Pediatric Obstructive Sleep Apnea in the Offspring—Results from a Population-based Cohort Study. Pediatr. Pulmonol. 2019, 54, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- González-Suárez, M.; Aguilar-Arnal, L. Histone Methylation: At the Crossroad between Circadian Rhythms in Transcription and Metabolism. Front. Genet. 2024, 15, 1343030. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, J.; Xu, X.; Li, F.; Wu, K.; Jiang, Y.; Rao, Y.; Zhao, C.; Chen, W.; Wang, X. Restoration of H3k27me3 Modification Epigenetically Silences Cry1 Expression and Sensitizes Leptin Signaling to Reduce Obesity-Related Properties. Adv. Sci. 2021, 8, 2004319. [Google Scholar] [CrossRef]
- Jasinska, M.; Grzegorczyk, A.; Woznicka, O.; Jasek, E.; Kossut, M.; Barbacka-Surowiak, G.; Litwin, J.A.; Pyza, E. Circadian Rhythmicity of Synapses in Mouse Somatosensory Cortex. Eur. J. Neurosci. 2015, 42, 2585–2594. [Google Scholar] [CrossRef]
- Jasińska, M.; Jasek-Gajda, E.; Ziaja, M.; Litwin, J.A.; Lis, G.J.; Pyza, E. Light-Modulated Circadian Synaptic Plasticity in the Somatosensory Cortex: Link to Locomotor Activity. Int. J. Mol. Sci. 2024, 25, 12870. [Google Scholar] [CrossRef] [PubMed]
- Bering, T.; Carstensen, M.B.; Wörtwein, G.; Weikop, P.; Rath, M.F. The Circadian Oscillator of the Cerebral Cortex: Molecular, Biochemical and Behavioral Effects of Deleting the Arntl Clock Gene in Cortical Neurons. Cereb. Cortex 2017, 28, 644–657. [Google Scholar] [CrossRef]
- Ripperger, J.A.; Schibler, U. Rhythmic CLOCK-BMAL1 Binding to Multiple E-Box Motifs Drives Circadian Dbp Transcription and Chromatin Transitions. Nat. Genet. 2006, 38, 369–374. [Google Scholar] [CrossRef]
- Koike, N.; Yoo, S.-H.; Huang, H.-C.; Kumar, V.; Lee, C.; Kim, T.-K.; Takahashi, J.S. Transcriptional Architecture and Chromatin Landscape of the Core Circadian Clock in Mammals. Science 2012, 338, 349–354. [Google Scholar] [CrossRef]
- Duong, H.A.; Weitz, C.J. Temporal Orchestration of Repressive Chromatin Modifiers by Circadian Clock Period Complexes. Nat. Struct. Mol. Biol. 2014, 21, 126–132. [Google Scholar] [CrossRef] [PubMed]


| Catalog No. | Manufacturer | Dilution | Host Species | Target |
|---|---|---|---|---|
| 05-1951 | Millipore (Waltham, MA, USA) | 1:500 | Mouse | H3K27me3 |
| 4909 | Cell Signaling (Waltham, MA, USA) | 1:200 | Rabbit | H3K36me3 |
| ab1220 | Abcam (Fremont, CA, USA) | 1:200 | Mouse | H3K9me2 |
| ab150077 | Abcam (Fremont, CA, USA) | 1:600 | Goat | Alexa Fluor 488 anti-rabbit IgG |
| 2253917 | Invitrogen (Carlsbad, CA, USA) | 1:250 | Goat | Alexa Fluor 555 anti-mouse IgG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuev, D.; Stanova, A.; Kontsevaya, G.; Romashchenko, A.; Khotskin, N.; Sharapova, M.; Moshkin, M.; Gerlinskaya, L.; Moshkin, Y. IVF and Thermal Manipulation at the First Cleavage Stage Alter Offspring Circadian Phenotype, Sleep, and Brain Epigenetics. Int. J. Mol. Sci. 2025, 26, 10360. https://doi.org/10.3390/ijms262110360
Zuev D, Stanova A, Kontsevaya G, Romashchenko A, Khotskin N, Sharapova M, Moshkin M, Gerlinskaya L, Moshkin Y. IVF and Thermal Manipulation at the First Cleavage Stage Alter Offspring Circadian Phenotype, Sleep, and Brain Epigenetics. International Journal of Molecular Sciences. 2025; 26(21):10360. https://doi.org/10.3390/ijms262110360
Chicago/Turabian StyleZuev, Daniil, Aliya Stanova, Galina Kontsevaya, Alexander Romashchenko, Nikita Khotskin, Marina Sharapova, Mikhail Moshkin, Ludmila Gerlinskaya, and Yuri Moshkin. 2025. "IVF and Thermal Manipulation at the First Cleavage Stage Alter Offspring Circadian Phenotype, Sleep, and Brain Epigenetics" International Journal of Molecular Sciences 26, no. 21: 10360. https://doi.org/10.3390/ijms262110360
APA StyleZuev, D., Stanova, A., Kontsevaya, G., Romashchenko, A., Khotskin, N., Sharapova, M., Moshkin, M., Gerlinskaya, L., & Moshkin, Y. (2025). IVF and Thermal Manipulation at the First Cleavage Stage Alter Offspring Circadian Phenotype, Sleep, and Brain Epigenetics. International Journal of Molecular Sciences, 26(21), 10360. https://doi.org/10.3390/ijms262110360







