Characterization of a Novel Galactia lindenii Lectin and Its Effects on Lepidopteran Midgut Cells
Abstract
1. Introduction
2. Results and Discussion
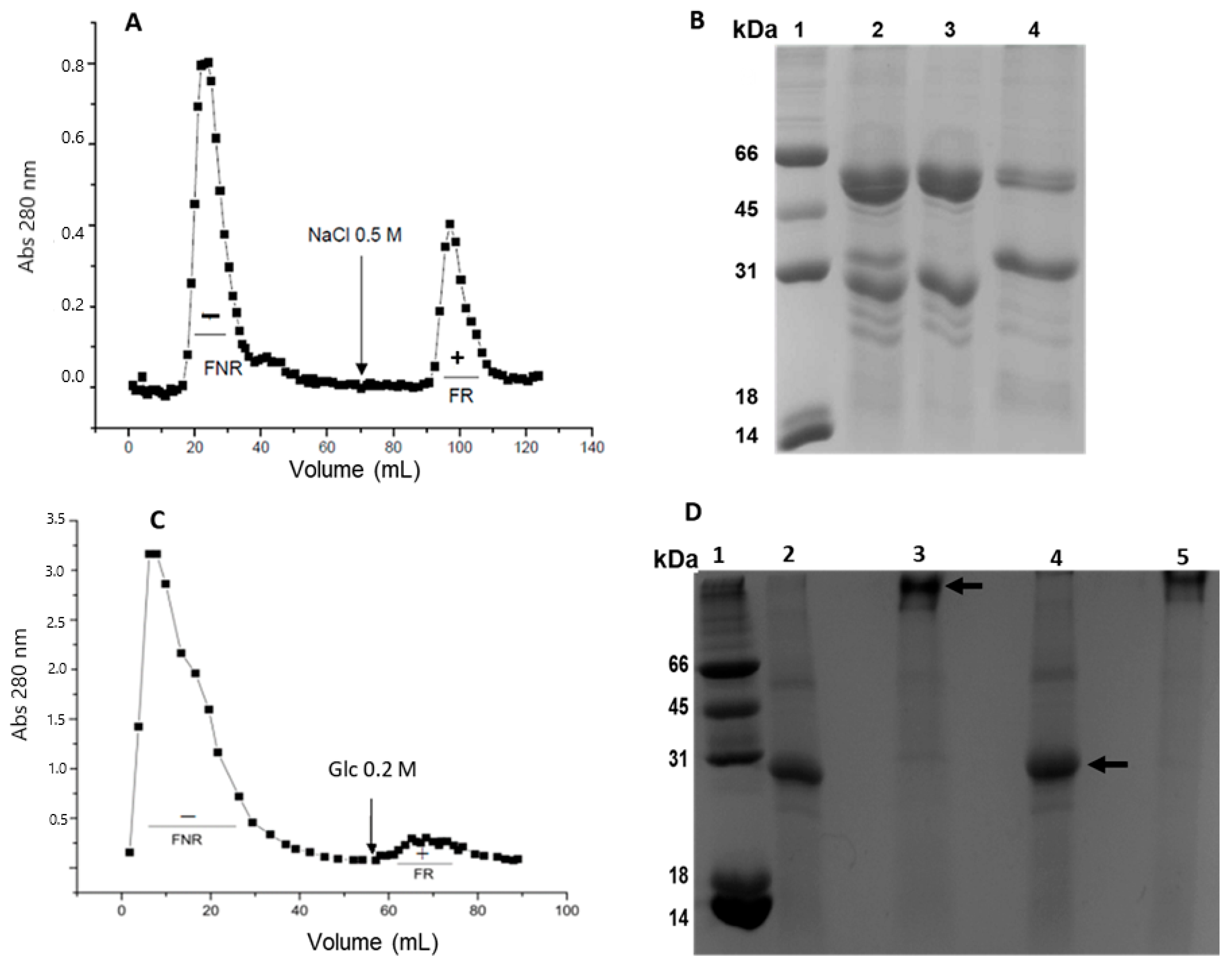
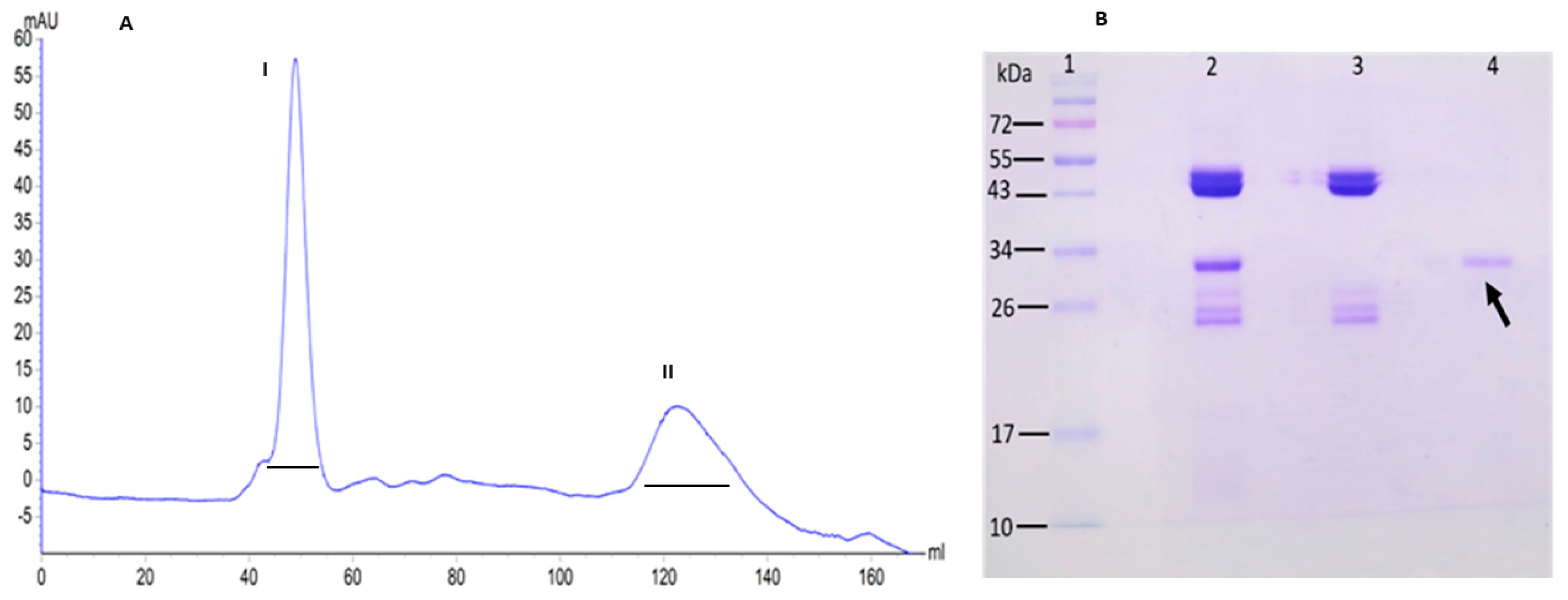
2.1. Extraction and Purification
| Purification Step | Concentration (mg/mL) 1 | Volume 2 (mL) | Total Protein (mg) | Specific Titer 3 Erythrocytes O+ | Specific Titer 4 Erythrocytes A+ | Total Yield (mg/g) 5 |
|---|---|---|---|---|---|---|
| Total extract 6 | 6.75 | 199 | 1343.2 | 1.18 | N.D | 268.6 |
| DEAE-FR 7 | 51.6 | 7 | 361.2 | 1.24 | 2.48 | 72.2 |
| FR S200 8 | 0.478 | 0.3 | 0.143 | 33.47 | 66.94 | 0.029 |
| FPLC-II 9 | 0.510 | 1.7 | 0.867 | 15.68 | 31.37 | - |
2.2. Carbohydrate Inhibition
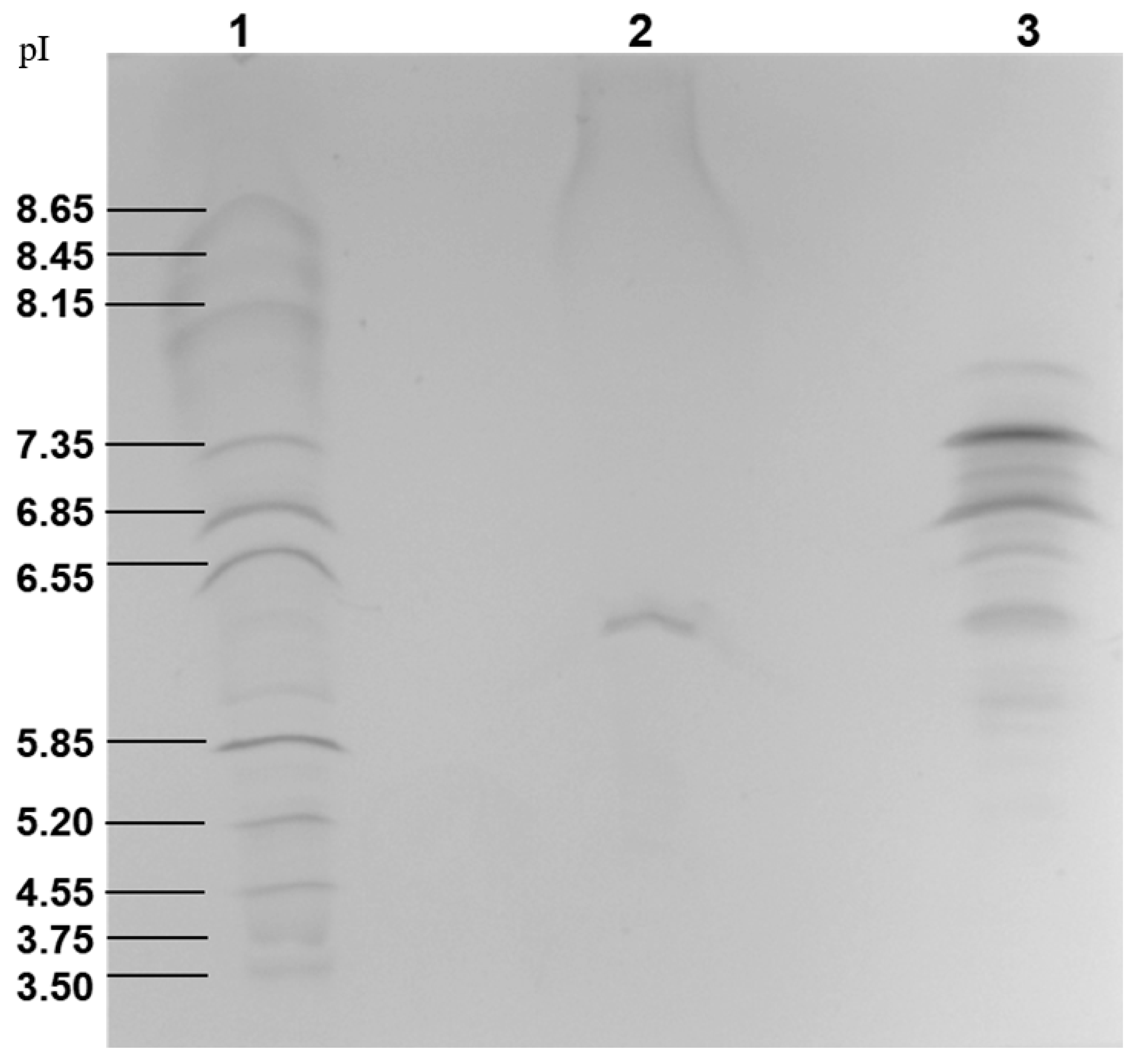
2.3. Determination of the Isoelectric Point and Glycosylation
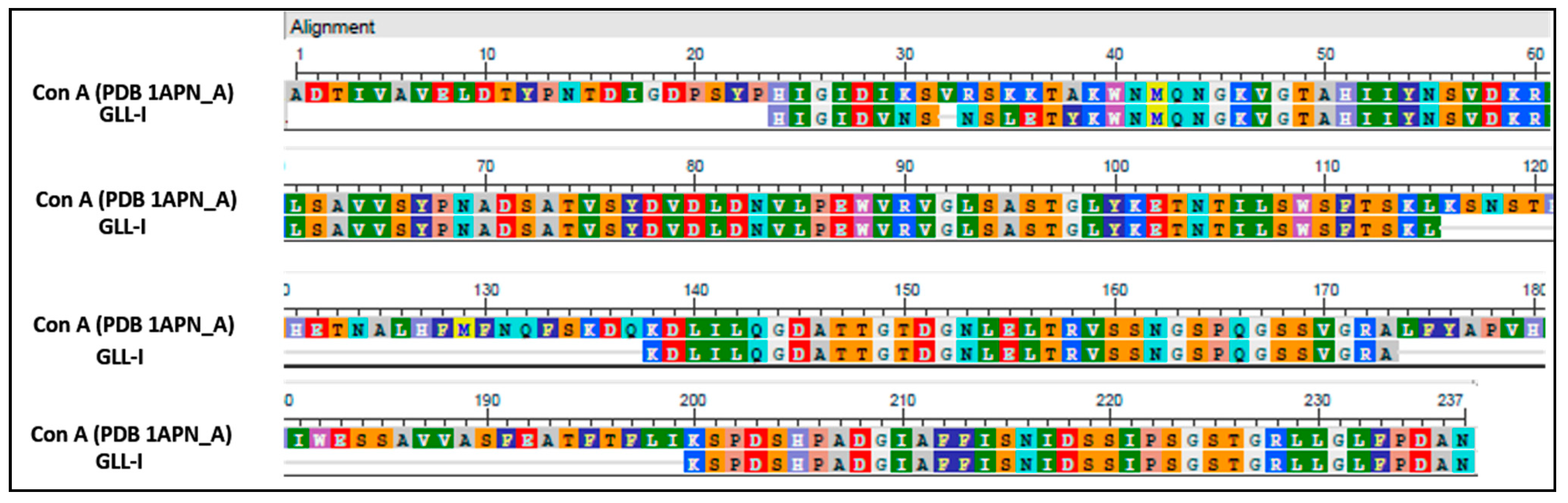
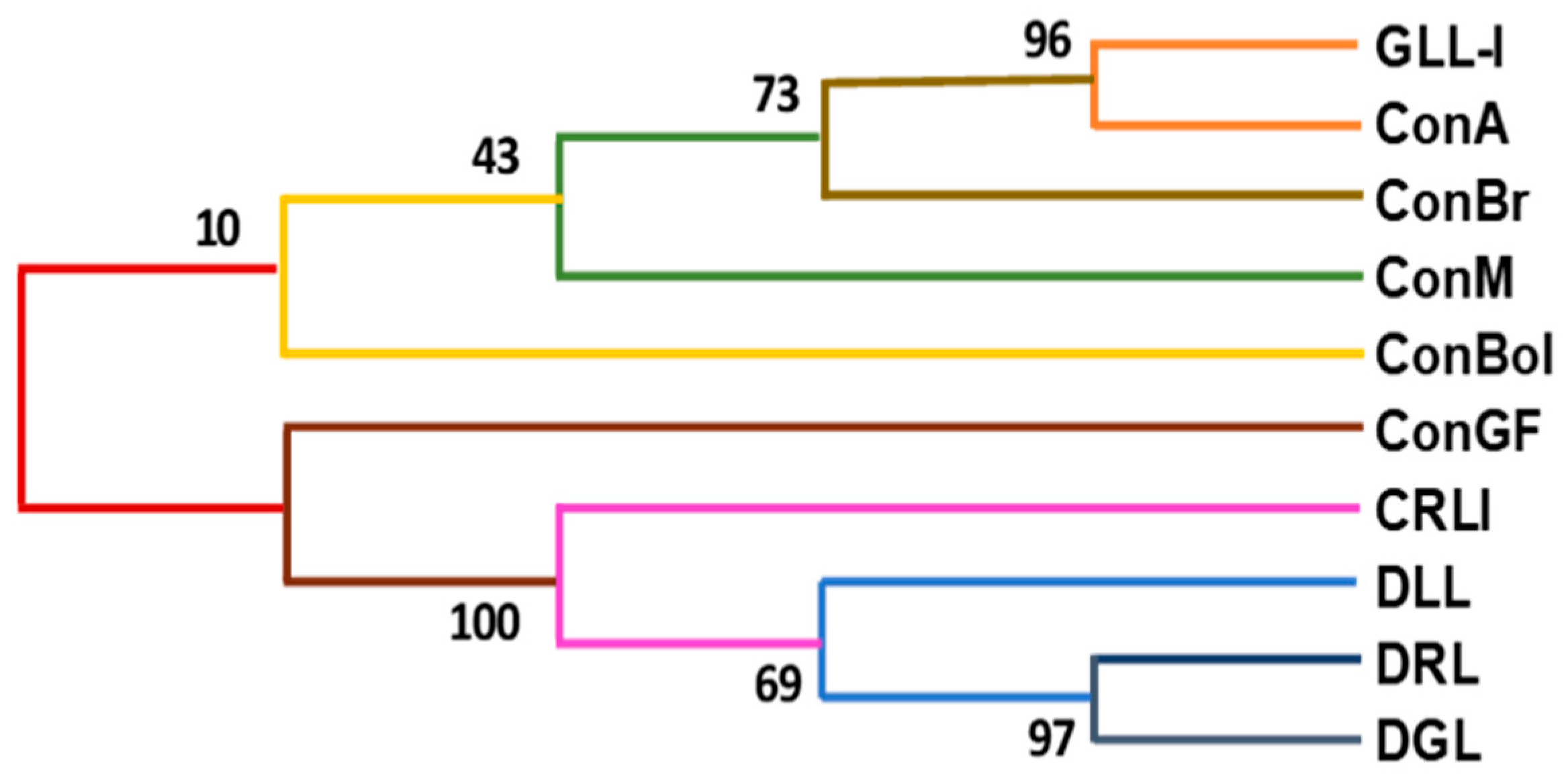
2.4. Analysis of Peptide Sequences and Determination of the Primary Structure of GLL-I in Comparison with Reported Lectins
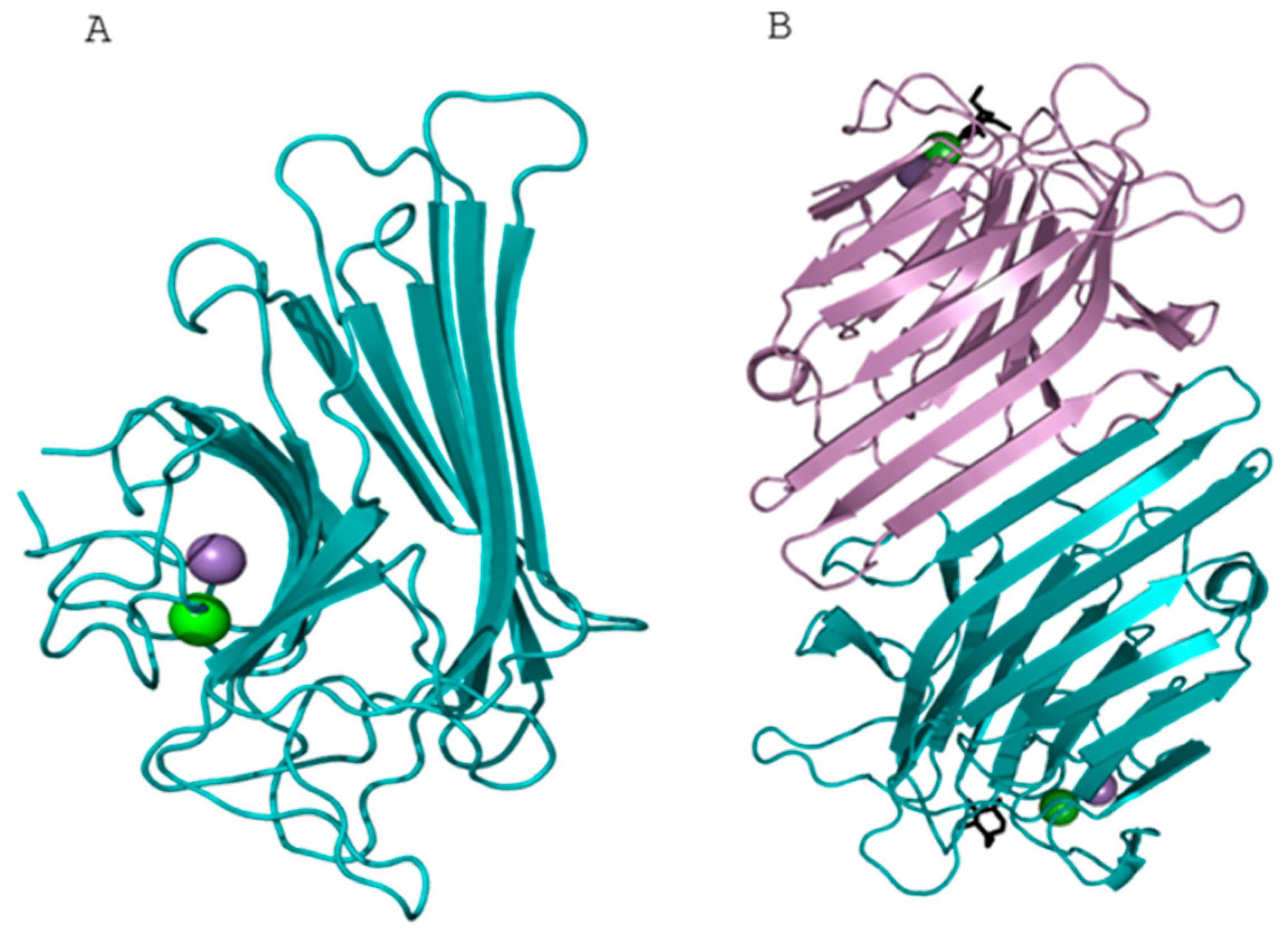
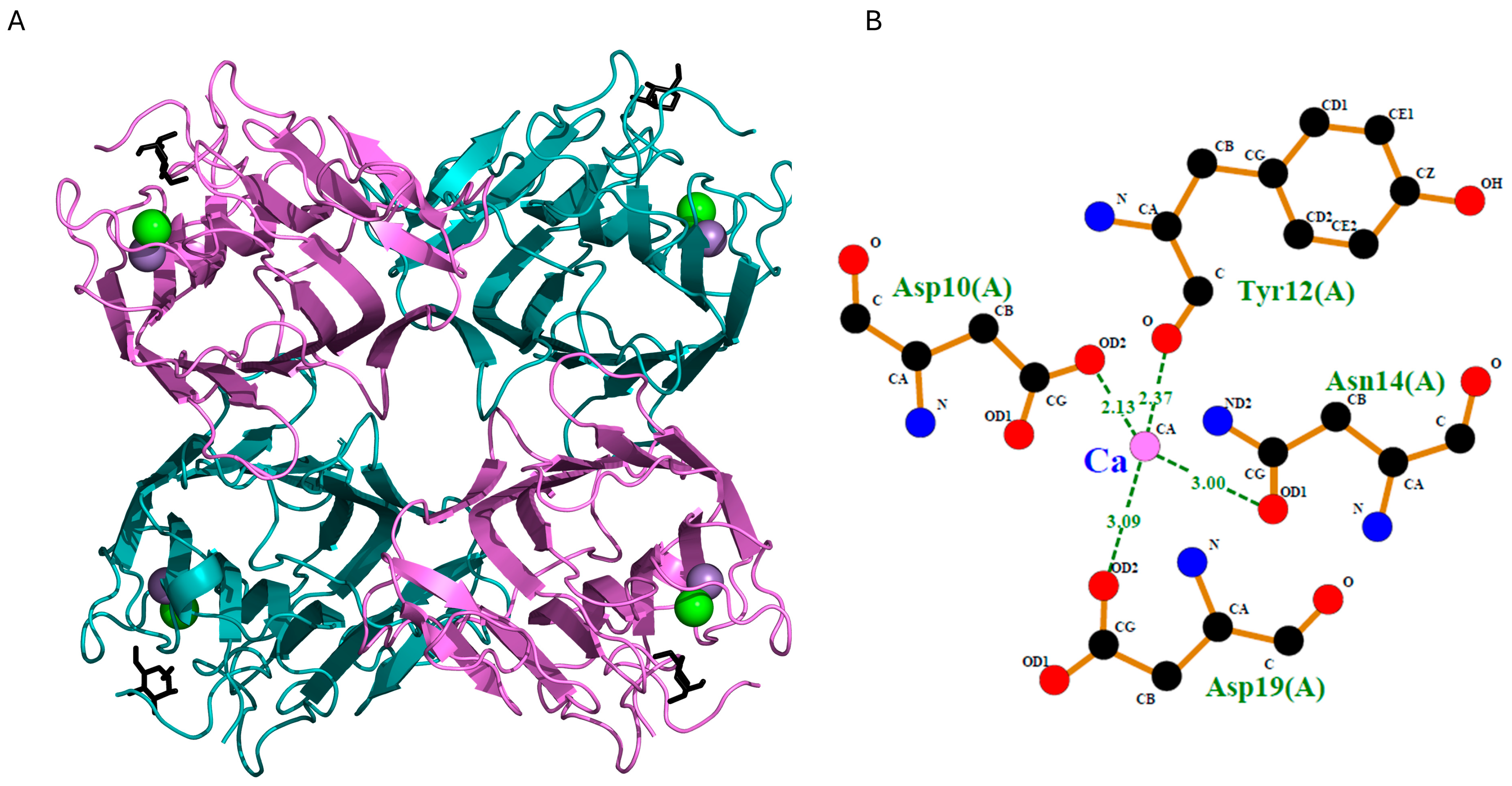
2.5. Prediction of the 3D Structure of Lectin GLL-I
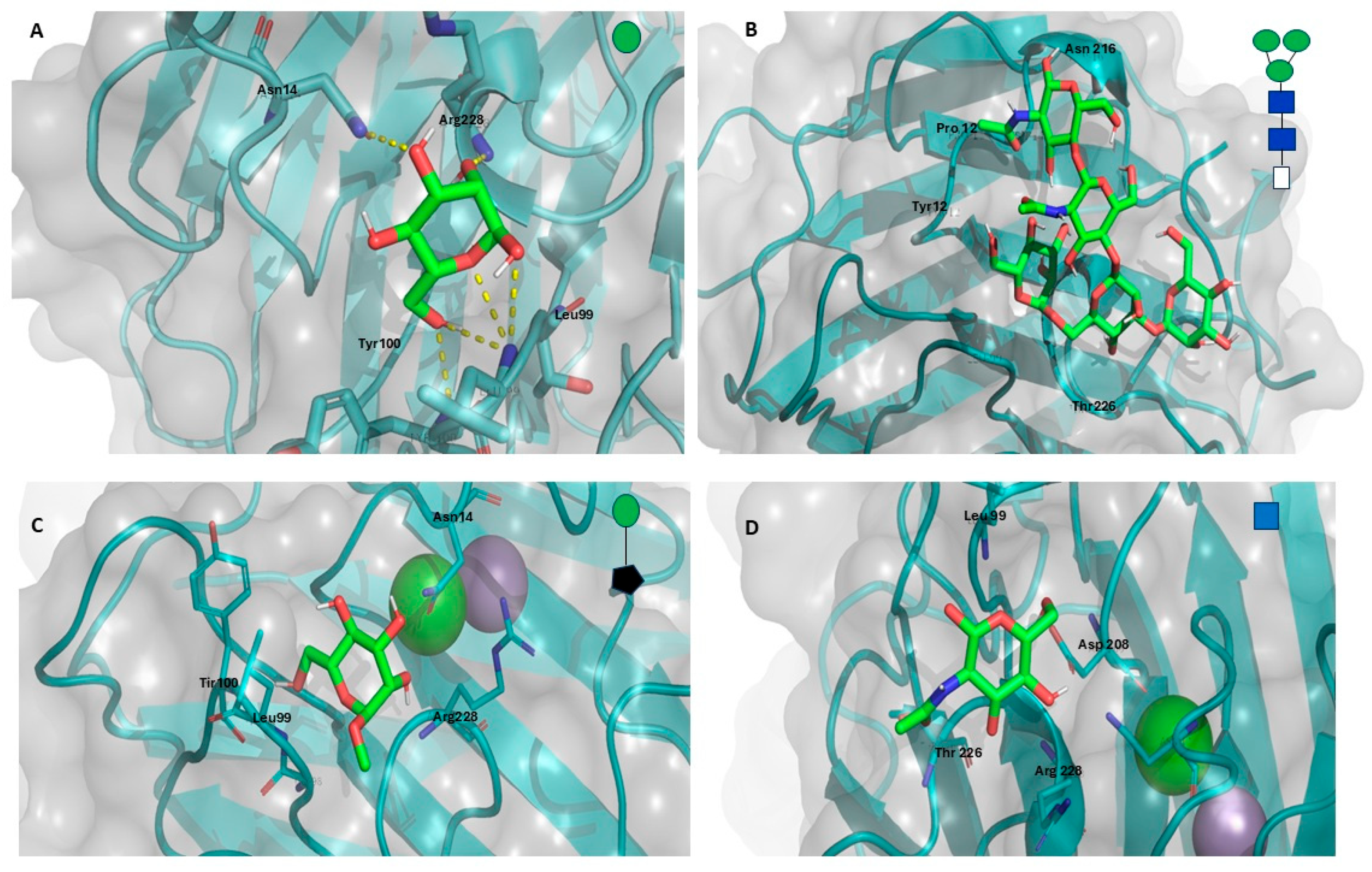
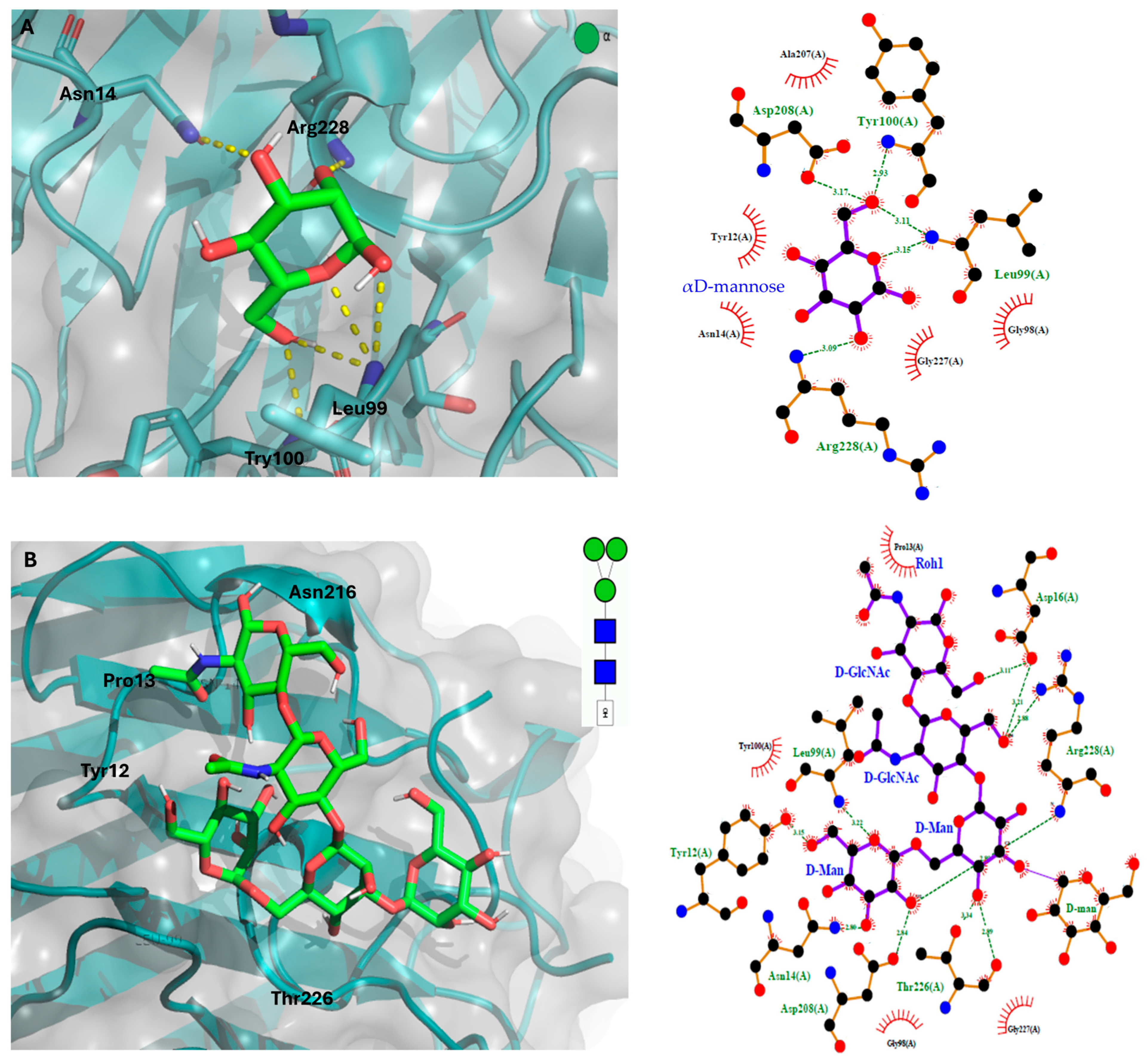
Molecular Docking of the GLL-I Lectin
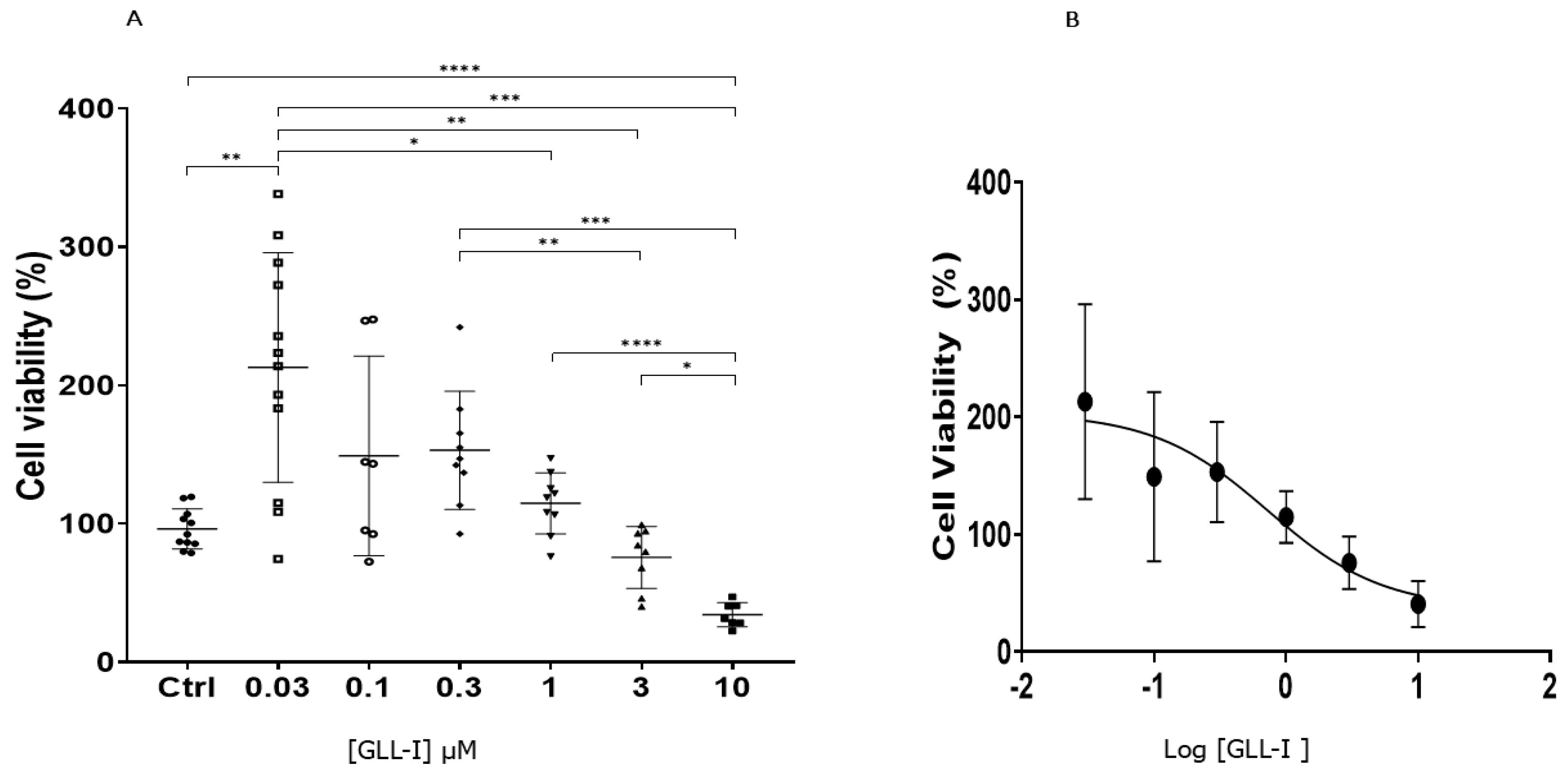
2.6. Cytotoxicity of Lectin GLL-I Against the Cf-203 Cell Line
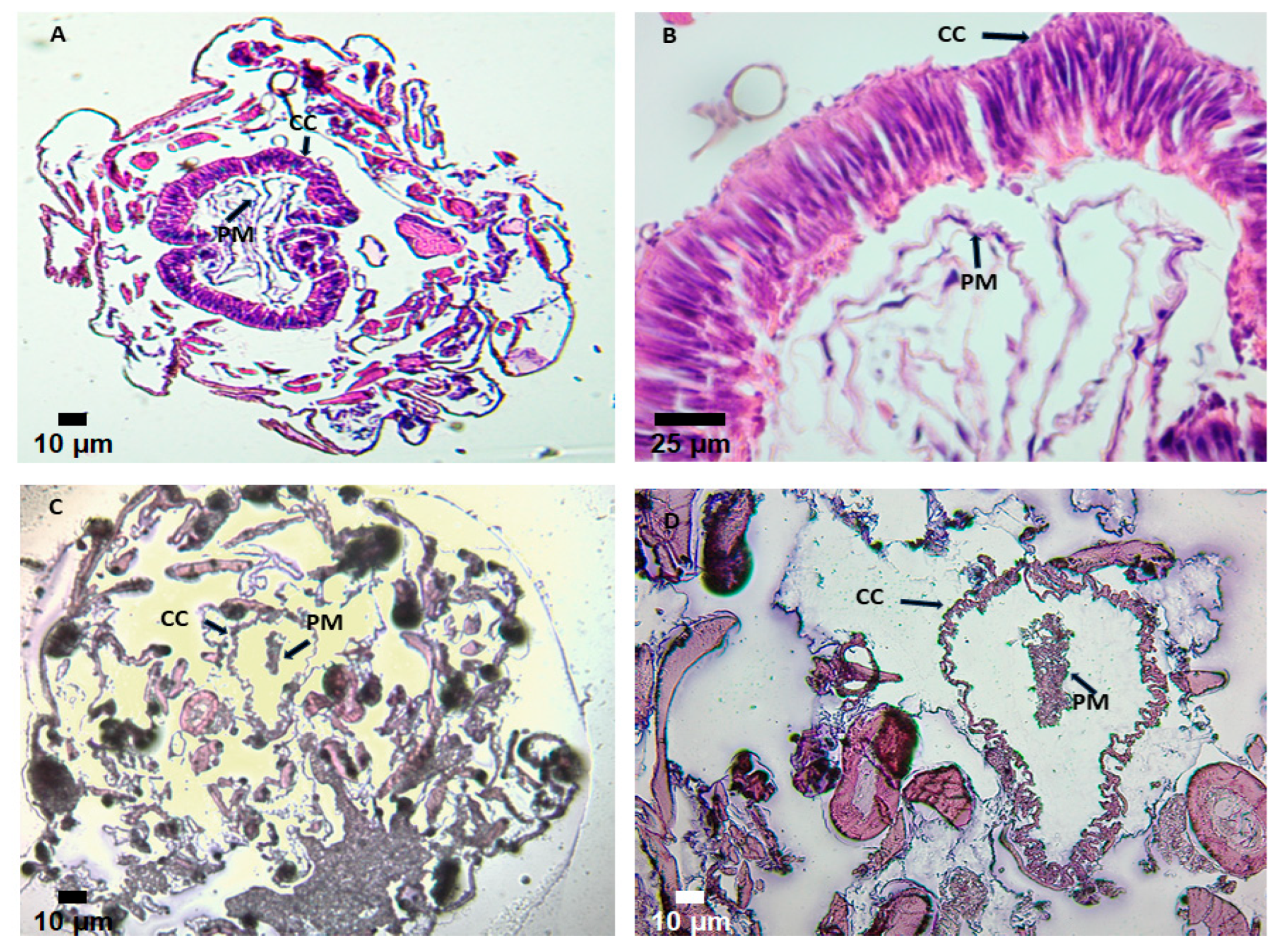
2.7. Binding of GLL-I Lectin to the Digestive Tract of Spodoptera Frugiperda
3. Materials and Methods
3.1. Collection of Plant Material
3.2. Extraction and Purification of Galactia Lindenii Type I Lectin (GLL-I)
3.3. Hemagglutination Inhibition by Different Carbohydrates
3.4. Isoelectric Focusing and Glycosylation
3.5. Amino Acid Sequence
3.5.1. Sample for Peptide Mapping
3.5.2. Multiple Sequence Alignments of GLL-I with Lectins from the Sutribe Diolceinae
3.5.3. Molecular Docking Studies of the GLL-I
3.6. Cytotoxicity Assays of GLL-I Against the CF-203 Cell
3.7. Binding of the GLL-I Lectin to the Digestive Tract of Spodoptera
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GLL-I | Type I Lectin from Galactia lindenii |
| GLL-II | Type II Lectin from Galactia lindenii |
| Glc | Glucose |
| Man | Mannose |
| kDa | Kilodaltons |
| CF203 | Midgut cell line of Choristoneura fumiferana |
| GalNac | N-acetylgalactosamine |
| ConBr | Lectin from Canavalia brasiliensis |
| DGL | Dioclea grandiflora lectin |
| DguiL | Dioclea guianensis lectin |
| ConA | Lectin from Canavalia ensiformis |
| DEAE | Diethylaminomethyl |
| FR S200 | Sephacryl S200 retained fraction |
| DTT | Dithiothreitol |
| ConGF | Lectin from Canavalia grandiflora |
| DrfL | Lectin from Dioclea reflexa |
| DBL | Dioclea bicolor lectin |
| MCI | Minimum inhibitory concentration |
| CRL-I | Cymbosema roseum lectin |
| pI | Isoelectric point |
| DSL-I | Dioclea sericea lectin |
| ConM | Lectin from Canavalia maritima (=Canavalia rosea) |
| ConBol | Lectin from Canavalia boliviana |
| DLL | Dioclea lasiocarpa lectin |
| DRL | Dioclea rostrata (=Macropsychanthus bicolor) lectin |
| DVL | Dioclea virgata lectin |
| CFL | Cratylia floribunda lectin |
| RSA | Agglutinin from Rhizoctonia solani |
| SNA-I | Lectin I from Sambucus nigra |
| SNA-II | Lectin II from Sambucus nigra |
| CRD | Carbohydrate recognition domain |
| ALL | Artocarpus lingnanensis |
| ZFL | Zebrafish liver cell line |
| PNA | Arachis hypogaea |
| PHA | Phaseolus vulgaris lectin |
| PSA | Pisum sativum lectin |
| PM | Peritrophic membrane |
| CC | Columnar cells |
| WSMoL | Lectin from Moringa oleifera |
| FNR − | Unretained fraction |
| FNR + | Retained fraction |
References
- Chettri, D.; Boro, M.; Sarkar, L.; Verma, A.K. Lectins: Biological Significance to Biotechnological Application. Carbohydr. Res. 2021, 506, 108367. [Google Scholar] [CrossRef] [PubMed]
- Konozy, E.H.E.; Osman, M.E.M. Plant Lectin: A Promising Future Anti-Tumor Drug. Biochimie 2022, 202, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Konozy, E.; Osman, M.; Dirar, A. Plant Lectins as Potent Anti-Coronaviruses, Anti-Inflammatory, Antinociceptive and Antiulcer Agents. Saudi J. Biol. Sci. 2022, 29, 103301. [Google Scholar] [CrossRef]
- Tsaneva, M.; Van Damme, E.J.M. 130 Years of Plant Lectin Research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef]
- Fujimoto, Z.; Tateno, H.; Hirabayashi, J. Lectin Structures: Classification Based on the 3-D Structures. Methods Mol. Biol. 2014, 1200, 579–606. [Google Scholar] [CrossRef]
- Prabu, M.M.; Suguna, K.; Vijayan, M. Variability in Quaternary Association of Proteins with the Same Tertiary Fold: A Case Study and Rationalization Involving Legume Lectins. Proteins 1999, 35, 58–69. [Google Scholar] [CrossRef]
- Cavada, B.S.; Pinto-Junior, V.R.; Osterne, V.J.S.; Nascimento, K.S. ConA-like Lectins: High Similarity Proteins as Models to Study Structure/Biological Activities Relationships. Int. J. Mol. Sci. 2018, 20, 30. [Google Scholar] [CrossRef]
- Queiroz, L.P.; Oliveira, A.C.S.; Snak, C. Disentangling the Taxonomy of the Galactia-Camptosema-Collaea Complex with New Generic Circumscriptions in the Galactia Clade (Leguminosae, Diocleae). Neodiversity 2020, 13, 56–94. [Google Scholar] [CrossRef]
- Queiroz, L.P.d.; Fortunato, R.H.; Giulietti, A.M. Phylogeny of the Diocleinae (Papilionoideae: Phaseoleae) Based on Morphological Characters. Adv. Legume Syst. Part 2003, 10, 303–324. [Google Scholar]
- de Queiroz, L.P.; Pastore, J.F.B.; Cardoso, D.; Snak, C.; Lima, A.L.d.C.; Gagnon, E.; Vatanparast, M.; Holland, A.E.; Egan, A.N. A Multilocus Phylogenetic Analysis Reveals the Monophyly of a Recircumscribed Papilionoid Legume Tribe Diocleae with Well-Supported Generic Relationships. Mol. Phylogenet Evol. 2015, 90, 1–19. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, L.P.; Snak, C. Revisiting the Taxonomy of Dioclea and Related Genera (Leguminosae, Papilionoideae), with New Generic Circumscriptions. PhytoKeys 2020, 164, 67–114. [Google Scholar] [CrossRef]
- Osterne, V.J.S.; Santiago, M.Q.; Pinto-Junior, V.R.; Cajazeiras, J.B.; Correia, J.L.A.; Leitão, C.C.F.; Carneiro, R.F.; Pereira-Junior, F.N.; Vasconcelos, M.A.; Rocha, B.A.M. Purification, Partial Characterization, and CNBr-Sepharose Immobilization of a Vasorelaxant Glucose/Mannose Lectin from Canavalia virosa Seeds. Appl. Biochem. Biotechnol. 2014, 172, 3342–3353. [Google Scholar] [CrossRef]
- Osterne, V.J.S.; Silva-Filho, J.C.; Santiago, M.Q.; Pinto-Junior, V.R.; Almeida, A.C.; Barreto, A.A.G.C.; Wolin, I.A.V.; Nascimento, A.P.M.; Amorim, R.M.F.; Rocha, B.A.M. Structural Characterization of a Lectin from Canavalia virosa Seeds with Inflammatory and Cytotoxic Activities. Int. J. Biol. Macromol. 2017, 94, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, T.T.A.; Anderson Matias da Rocha, B.; Alves Carneiro, V.; Vassiliepe Sousa Arruda, F.; Fernandes do Nascimento, A.S.; Cardoso Sá, N.; Do Nascimento, K.S.; Sousa Cavada, B.; Holanda Teixeira, E. Effect of Lectins from Diocleinae Subtribe against Oral Streptococci. Molecules 2011, 16, 3530–3543. [Google Scholar] [CrossRef]
- Oliveira, C.T.; Kunz, D.; Silva, C.P.; Macedo, M.L.R. Entomotoxic Properties of Dioclea violacea Lectin and Its Effects on Digestive Enzymes of Anagasta kuehniella (Lepidoptera). J. Insect Physiol. 2015, 81, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Vandenborre, G.; Smagghe, G.; Van Damme, E.J.M. Plant Lectins as Defense Proteins against Phytophagous Insects. Phytochemistry 2011, 72, 1538–1550. [Google Scholar] [CrossRef]
- Reyes-Montaño, E.; Vega Castro, N. Plant Lectins with Insecticidal and Insectistatic Activities. In Insecticides-Agriculture and Toxicology; InTech Open: Rijeka, Croatia, 2018; Chapter 2; ISBN 978-1-78923-166-3. [Google Scholar] [CrossRef]
- Macedo, M.L.R.; Oliveira, C.F.R.; Oliveira, C.T. Insecticidal Activity of Plant Lectins and Potential Application in Crop Protection. Molecules 2015, 20, 2014–2033. [Google Scholar] [CrossRef]
- Katoch, R.; Tripathi, A. Research advances and prospects of legume lectins. J. Biosci. 2021, 46, 104. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Korbou, G.; Magita, A.; Eliopoulos, P.A.; Poulas, K. Leguminous Seeds Powder Diet Reduces the Survival and Development of the Khapra beetle, Trogoderma granarium Everts (Coleoptera: Dermestidae). Biology 2020, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Michiels, K.; Van Damme, E.J.; Smagghe, G. Plant-insect interactions: What can we learn from plant lectins? Arch. Insect Biochem. Physiol 2010, 73, 193–212. [Google Scholar] [CrossRef]
- Fitches, E.; Wiles, D.; Douglas, A.E.; Hinchliffe, G.; Audsley, N.; Gatehouse, J.A. The insecticidal activity of recombinant garlic lectins towards aphids. Insect Biochem. Mol. Biol. 2008, 38, 905–915. [Google Scholar] [CrossRef]
- Sauvion, N.; Nardon, C.; Febvay, G.; Gatehouse, A.M.; Rahbé, Y. Binding of the insecticidal lectin Concanavalin A in pea aphid, Acyrthosiphon pisum (Harris) and induced effects on the structure of midgut epithelial cells. J. Insect Physiol. 2004, 50, 1137–1150. [Google Scholar] [CrossRef]
- Shukla, S.; Arora, R. Biological activity of soybean trypsin inhibitor and plant lectins against cotton bollworm/legume pod borer, Helicoverpa armigera. Plant Biotechnol. 2005, 22, 1–6. [Google Scholar] [CrossRef]
- Sprawka, I.; Goławska, S.; Goławski, A.; Chrzanowski, G.; Czerniewicz, P.; Sytykiewicz, H. Entomotoxic action of jackbean lectin (Con A) in bird cherry-oat aphid through the effect on insect enzymes. J. Plant Interact. 2013, 9, 425–433. [Google Scholar] [CrossRef]
- Babu, R.M.; Sajeena, A.; Seetharaman, K.; Reddy, M.S. Advances in genetically engineered (transgenic) plants in pest management—An overview. Crop Prot. 2003, 22, 1071–1086. [Google Scholar] [CrossRef]
- Barre, A.; Van Damme, E.J.M.; Klonjkowski, B.; Simplicien, M.; Sudor, J.; Benoist, H.; Rougé, P. Legume Lectins with Different Specificities as Potential Glycan Probes for Pathogenic Enveloped Viruses. Cells 2022, 11, 339. [Google Scholar] [CrossRef]
- Fitches, E.; Edwards, M.G.; Mee, C.; Grishin, E.; Gatehouse, A.M.R.; Edwards, J.P.; Gatehouse, J.A. Fusion proteins containing insect-specific toxins as pest control agents: Snowdrop lectin delivers fused insecticidal spider venom toxin to insect haemolymph following oral ingestion. J. Insect Physiol. 2004, 50, 61–71. [Google Scholar] [CrossRef]
- Tajne, S.; Boddupally, D.; Sadumpati, V.; Vudem, D.R.; Khareedu, V.R. Synthetic fusion-protein containing domains of Bt Cry1Ac and Allium sativum lectin (ASAL) conferred enhanced insecticidal activity against major lepidopteran pests. J. Biotechnol. 2014, 171, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Pyati, P.; Fitches, E.; Gatehouse, J.A. A recombinant fusion protein containing a spider toxin specific for the insect voltage-gated sodium ion channel shows oral toxicity towards insects of different orders. Insect Biochem. Mol. Biol. 2014, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, S. Tsetse peritrophic matrix influences for trypanosome transmission. J. Insect Physiol. 2018, 118, 103919. [Google Scholar] [CrossRef] [PubMed]
- Erlandson, M.A.; Toprak, U.; Hegedus, D.D. Role of the peritrophic matrix in insect-pathogen interactions. J. Insect physiol. 2019, 117, 103894. [Google Scholar] [CrossRef]
- Konno, K.; Mitsuhashi, W. The peritrophic membrane as a target of proteins that play important roles in plant defense and microbial attack. J. Insect Physiol. 2019, 117, 103912. [Google Scholar] [CrossRef]
- Sadlova, J.; Homola, M.; Myskova, J.; Jancarova, M.; Volf, P. Refractoriness of Sergentomyia schwetzi to Leishmania spp. is mediated by the peritrophic matrix. PLoS Negl. Trop. Dis. 2018, 12, e0006382. [Google Scholar] [CrossRef]
- Sede, S.M.; Tosto, D.S.; Gottlieb, A.M.; Poggio, L.; Fortunato, R.H. %J P. Systematics Genetic Relationships in the Galactia–Camptosema–Collaea Complex (Leguminosae) Inferred from AFLP Markers. Plant Syst. Evol. 2008, 276, 261–270. [Google Scholar] [CrossRef]
- Burkart, A. El Género Galactia (Legum.-Phaseoleae) En Sudamérica Con Especial Referencia a La Argentina y Paises Vecinos. J. Darwiniana 1971, 16, 663–796. Available online: https://www.jstor.org/stable/23213905 (accessed on 16 September 2025).
- Moreira Rde, A.; Ainouz, I.L.; De Oliveira, J.T.; Cavada, B.S. Plant Lectins, Chemical and Biological Aspects. Mem. Inst. Oswaldo Cruz 1991, 86 (Suppl. 2), 211–218. [Google Scholar] [CrossRef]
- Rozwarski, D.A.; Swami, B.M.; Brewer, C.F.; Sacchettini, J.C. Crystal Structure of the Lectin from Dioclea grandiflora Complexed with Core Trimannoside of Asparagine-Linked Carbohydrates. J. Biol. Chem. 1998, 273, 32818–32825. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Aparicio, J.; Hermoso, J.; Grangeiro, T.B.; Calvete, J.J.; Cavada, B.S. The Crystal Structure of Canavalia brasiliensis Lectin Suggests a Correlation between Its Quaternary Conformation and Its Distinct Biological Properties from Concanavalin A. FEBS Lett. 1997, 405, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Wah, D.A.; Romero, A.; Gallego del Sol, F.; Cavada, B.S.; Ramos, M.V.; Grangeiro, T.B.; Sampaio, A.H.; Calvete, J.J. Crystal Structure of Native and Cd/Cd-Substituted Dioclea guianensis Seed Lectin. A Novel Manganese-Binding Site and Structural Basis of Dimer-Tetramer Association. J. Mol. Biol. 2001, 310, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo, L.M.; Vega, N.; Pérez, G. Isolation and characterization of novel lectins from Canavalia ensiformis DC and Dioclea grandiflora Mart. Ex Benth. seeds. Braz. J. Plant Physiol. 2005, 17, 315–324. [Google Scholar] [CrossRef]
- Moreira, R.D.A.; Cordeiro, E.D.F.; Ramos, V.; Grangeiro, T.B.; Martins, J.L.; De Oliveira, T.A.; Cavada, B.S.; De Ciências, C.; Federal, U. Isolation and partial characterization of a lectin from seeds of Dioclea violacea. Rev. Bras. Fisiol. Veg. 1996, 8, 23–29. [Google Scholar]
- Bezerra, M.J.; Rodrigues, N.V.; Pires Ade, F.; Bezerra, G.A.; Nobre, C.B.; Alencar, K.L.; Soares, P.M.; do Nascimento, K.S.; Nagano, C.S.; Martins, J.L.; et al. Crystal Structure of Dioclea violacea Lectin and a Comparative Study of Vasorelaxant Properties with Dioclea rostrata Lectin. Int. J. Biochem. Cell Biol. 2013, 45, 807–815. [Google Scholar] [CrossRef]
- Loris, R.; Van Walle, I.; De Greve, H.; Beeckmans, S.; Deboeck, F.; Wyns, L.; Bouckaert, J. Structural Basis of Oligomannose Recognition by the Pterocarpus angolensis seed lectin. J. Mol. Biol. 2004, 335, 1227–1240. [Google Scholar] [CrossRef]
- Cortázar, T.M.; Wilson, I.B.H.; Hykollari, A.; Reyes, E.A.; Vega, N.A. Differential Recognition of Natural and Remodeled Glycotopes by Three Diocleae Lectins. Glycoconj. J. 2018, 35, 205–216. [Google Scholar] [CrossRef]
- Almanza, M.; Vega, N.; Pérez, G. Isolating and Characterising a Lectin from Galactia lindenii Seeds That Recognises Blood Group H Determinants. Arch. Biochem. 2004, 429, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, C.; Mendes Lopes, M.L.; Fialho, E.; Valente-Mesquita, V.L. Polyphenol Oxidase: Characteristics and Mechanisms of Browning Control. Food Rev. Int. 2008, 24, 361–375. [Google Scholar] [CrossRef]
- Cortázar, T.M. Estudio Del Efecto de Lectinas Vegetales Sobre Los Procesos de Migración y Proliferación Celular En Queratinocitos Epidérmicos. Ph.D. Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2019. [Google Scholar]
- Fukuda, N.; Yoshimaru, A.; Hidaka, T.; Ohta, H.; Yamamoto, K.; Yomo, H. Isolation and Characterization of N-Acetylgalactosamine-Specific Lectin from Galactia tashiroi Seeds. Biosci. Biotechnol. Biochem. 1994, 58, 423–424. [Google Scholar] [CrossRef]
- Le Pendu, J.; Gérard, G.; Lambert, F.; Mollicone, R.; Oriol, R. A New Anti-H Lectin from the Seeds of Galactia tenuiflora. Glycoconj. J. 1986, 3, 203–216. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Ceccatto, V.M.; Cavada, B.S.; Nunes, E.P.; Nogueira, N.A.P.; Grangeiro, M.B.; Moreno, F.; Teixeira, E.H.; Sampaio, A.H.; Alves, M.A.O.; Ramos, M.V. Purification and Partial Characterization of a Lectin from Canavalia grandiflora Benth. Seeds. Protein Pept. Lett. 2002, 9, 67–73. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B. Isolation and Characterization of a Glucose/Mannose/Rhamnose-Specific Lectin from the Knife Bean Canavalia gladiata. Arch. Biochem. Biophys. 2005, 439, 91–98. [Google Scholar] [CrossRef]
- Carrington, D.M.; Auffret, A.; Hanke, D.E. Polypeptide Ligation Occurs during Post-Translational Modification of Concanavalin A. Nature 1985, 313, 64–67. [Google Scholar] [CrossRef]
- Pinto-Junior, V.R.; Osterne, V.J.; Santiago, M.Q.; Correia, J.L.; Pereira-Junior, F.N.; Leal, R.B.; Pereira, M.G.; Chicas, L.S.; Nagano, C.S.; Rocha, B.A.; et al. Structural Studies of a Vasorelaxant Lectin from Dioclea reflexa Hook Seeds: Crystal Structure, Molecular Docking and Dynamics. Int. J. Biol. Macromol. 2017, 98, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Reis, W.F.; Silva, M.E.S.; Gondim, A.C.S.; Torres, R.C.F.; Carneiro, R.F.; Nagano, C.S.; Sampaio, A.H.; Teixeira, C.S.; Gomes, L.C.B.F.; Sousa, B.L.; et al. Glucose-Binding Dioclea bicolor Lectin (DBL): Purification, Characterization, Structural Analysis, and Antibacterial Properties. Protein J. 2024, 43, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.T.S.; Coelho, L.C.B.B. Purification of a Glucose/Mannose Specific Lectin, Isoform 1, from Seeds of Cratylia mollis Mart.(Camaratu Bean). Appl. Biochem. Biotechnol. 1995, 55, 261–273. [Google Scholar] [CrossRef]
- Grangeiro, T.B.; Gatehouse, J.A.; Pereira, M.N.; Cavada, B.S. Investigation on the Origin of the Naturally Occurring Fragments of Cratylia floribunda Lectin. Braz. J. Plant Physiol. 1997, 9, 9–13. [Google Scholar]
- Cavada, B.S.; Marinho, E.S.; Souza, E.P.; Benevides, R.G.; Delatorre, P.; Souza, L.A.; Nascimento, K.S.; Sampaio, A.H.; Moreno, F.B.; Rustiguel, J.K.; et al. Purification, Partial Characterization and Preliminary X-Ray Diffraction Analysis of a Mannose-Specific Lectin from Cymbosema roseum Seeds. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 62, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N. Lectins: Carbohydrate-Specific Reagents and Biological Recognition Molecules. J. Biol. Chem. 2007, 282, 2753–2764. [Google Scholar] [CrossRef]
- Sierra, A.; Pérez, G. Extracción, Purificación y Caracterización de Dos Lectinas En Semillas de Dioclea sericea. Rev. Acad. Colomb. Cienc. 1999, 23, 445–454. [Google Scholar] [CrossRef]
- Dam, T.K.; Cavada, B.S.; Nagano, C.S.; Rocha, B.A.M.; Benevides, R.G.; Nascimento, K.S.; De Sousa, L.A.G.; Oscarson, S.; Brewer, C.F. Fine Specificities of Two Lectins from Cymbosema roseum Seeds: A Lectin Specific for High-Mannose Oligosaccharides and a Lectin Specific for Blood Group H Type II Trisaccharide. Glycobiology 2011, 21, 925–933. [Google Scholar] [CrossRef]
- Dam, T.K.; Cavada, B.S.; Grangeiro, T.B.; Santos, C.F.; De Sousa, F.A.M.; Oscarson, S.; Brewer, C.F. Diocleinae Lectins Are a Group of Proteins with Conserved Binding Sites for the Core Trimannoside of Asparagine-Linked Oligosaccharides and Differential Specificities for Complex Carbohydrates. J. Biol. Chem. 1998, 273, 12082–12088. [Google Scholar] [CrossRef]
- Pérez, G. Isolation and Characterization of a Novel Lectin from Dioclea lehmannii (Fabaceae) Seeds. Int. J. Biochem. Cell Biol. 1998, 30, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.; Perez, C.; Sousa-Cavada, B.; Moreira, R.; Richardson, M. Comparison of the Amino Acid Sequences of the Lectins from Seeds of Dioclea lehmannii and Canavalia maritima. Phytochemistry 1991, 30, 2619–2621. [Google Scholar] [CrossRef]
- Moreira, R.A.; Monteiro, A.C.O.; Horta, A.C.G.; Oliveira, J.T.A.; Cavada, B.S. Isolation and Characterization of Dioclea altissima var. megacarpa Seed Lectin. Phytochemistry 1997, 46, 139–144. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB Bioinformatics Resource Portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]
- Delatorre, P.; Rocha, B.A.M.; Souza, E.P.; Oliveira, T.M.; Bezerra, G.A.; Moreno, F.B.M.B.; Freitas, B.T.; Santi-Gadelha, T.; Sampaio, A.H.; Azevedo, W.F. Structure of a Lectin from Canavalia gladiata Seeds: New Structural Insights for Old Molecules. BMC Struct. Biol. 2007, 7, 52. [Google Scholar] [CrossRef]
- André, S.; Kaltner, H.; Manning, J.C.; Murphy, P.V.; Gabius, H.J. Lectins: Getting Familiar with Translators of the Sugar Code. Molecules 2015, 20, 1788–1823. [Google Scholar] [CrossRef]
- Loris, R. Principles of Structures of Animal and Plant Lectins. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2002, 1572, 198–208. [Google Scholar] [CrossRef]
- Loris, R.; Hamelryck, T.; Bouckaert, J.; Wyns, L. Legume Lectin Structure. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1998, 1383, 9–36. [Google Scholar] [CrossRef]
- Smith, C.K.; Regan, L. Construction and Design of β-Sheets. Acc. Chem. Res. 1997, 30, 153–161. [Google Scholar] [CrossRef]
- Lagarda-Diaz, I.; Guzman-Partida, A.; Vazquez-Moreno, L. Legume Lectins: Proteins with Diverse Applications. Int. J. Mol. Sci. 2017, 18, 1242. [Google Scholar] [CrossRef]
- Chandra, N.; Prabu, M.; Suguna, K.; Vijayan, M. Structural Similarity and Functional Diversity in Proteins Containing the Legume Lectin Fold. Protein Eng. 2001, 14, 857–866. [Google Scholar] [CrossRef]
- de Oliveira, T.M.; Delatorre, P.; da Rocha, B.A.M.; de Souza, E.P.; Nascimento, K.S.; Bezerra, G.A.; Moura, T.R.; Benevides, R.G.; Bezerra, E.H.S.; Moreno, F.B.M.B.; et al. Crystal Structure of Dioclea rostrata Lectin: Insights into Understanding the pH-Dependent Dimer-Tetramer Equilibrium and the Structural Basis for Carbohydrate Recognition in Diocleinae Lectins. J. Struct. Biol. 2008, 164, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.R.; Reddy, G.B.; Ahmad, N.; Swaminathan, C.P.; Mitra, N.; Surolia, A. Legume Lectin Family, the “Natural Mutants of the Quaternary State”, Provide Insights into the Relationship between Protein Stability and Oligomerization. Biochim. Biophys. Acta 2001, 1527, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Nagano, C.S.; Calvete, J.J.; Barettino, D.; Pérez, A.; Cavada, B.S.; Sanz, L. Insights into the Structural Basis of the PH-Dependent Dimer–Tetramer Equilibrium through Crystallographic Analysis of Recombinant Diocleinae Lectins. Biochem. J. 2008, 409, 417–428. [Google Scholar] [CrossRef] [PubMed]
- El-Baba, T.J.; Clemmer, D.E. Solution Thermochemistry of Concanavalin A Tetramer Conformers Measured by Variable-Temperature ESI-IMS-MS. Int. J. Mass. Spectrom. 2019, 443, 93–100. [Google Scholar] [CrossRef]
- Goldstein, I.J.; Poretz, R.D. Isolation, Physicochemical Characterization, and Carbohydrate-Binding Specificity of Lectins. In The Lectins; Liener, I.E., Sharon, N., Goldstein, I.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 33–247. [Google Scholar]
- Ramos, M.V.; Moreira, R.A.; Cavada, B.S.; de Oliveira, J.T.A.; Rouge, P. Interaction of Lectins from the Sub-Tribe Diocleinae with Specific Ligands. R. Bras. Fisiol. Veg. 1996, 8, 193–1999. [Google Scholar]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef]
- Gupta, D.; Oscarson, S.; Raju, T.S.; Stanley, P.; Toone, E.J.; Brewer, C.F. A Comparison of the Fine Saccharide-binding Specificity of Dioclea grandiflora Lectin and Concanavalin A. Eur. J. Biochem. 1996, 242, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.; Campos, F.D.A.P.; Moreira, R.A.; Ainouz, I.L.; Begbie, R.; Watt, W.B.; Pusztai, A. The Complete Amino Acid Sequence of the Major α Subunit of the Lectin from the Seeds of Dioclea grandiflora (Mart). Eur. J. Biochem. 1984, 144, 101–111. [Google Scholar] [CrossRef]
- Barroso-Neto, I.L.; Delatorre, P.; Teixeira, C.S.; Correia, J.L.A.; Cajazeiras, J.B.; Pereira, R.I.; Nascimento, K.S.; Laranjeira, E.P.P.; Pires, A.F.; Assreuy, A.M.; et al. Structural Analysis of a Dioclea sclerocarpa Lectin: Study on the Vasorelaxant Properties of Dioclea Lectins. J. Int. J. Biol. 2016, 82, 464–470. [Google Scholar] [CrossRef]
- Naismith, J.H.; Field, R.A. Structural basis of trimannoside recognition by concanavalin A. J. Biol. Chem. 1996, 271, 972–976. [Google Scholar] [CrossRef]
- Hamshou, M.; Van Damme, E.J.M.; Caccia, S.; Cappelle, K.; Vandenborre, G.; Ghesquière, B.; Gevaert, K.; Smagghe, G. High Entomotoxicity and Mechanism of the Fungal GalNAc/Gal-Specific Rhizoctonia solani Lectin in Pest Insects. J. Insect Physiol. 2013, 59, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Candy, L.; Peumans, W.J.; Menu-Bouaouiche, L.; Astoul, C.H.; Van Damme, J.; Van Damme, E.J.M.; Erard, M.; Rougé, P. The Gal/GalNAc-Specific Lectin from the Plant Pathogenic Basidiomycete Rhizoctonia solani Is a Member of the Ricin-B Family. Biochem. Biophys. Res. Commun. 2001, 282, 655–661. [Google Scholar] [CrossRef]
- Vranken, A.M.; Van Damme, E.J.M.; Allen, A.K.; Peumans, W.J. Purification and Properties of an N-acetylgalactosamine Specific Lectin from the Plant Pathogenic Fungus Rhizoctonia solani. FEBS Lett. 1987, 216, 67–72. [Google Scholar] [CrossRef]
- Shahidi-Noghabi, S.; Van Damme, E.J.M.; Iga, M.; Smagghe, G. Exposure of Insect Midgut Cells to Sambucus nigra L. Agglutinins I and II Causes Cell Death via Caspase-Dependent Apoptosis. J. Insect Physiol. 2010, 56, 1101–1107. [Google Scholar] [CrossRef]
- Shahidi-Noghabi, S.; Van Damme, E.J.M.; Smagghe, G. Carbohydrate-Binding Activity of the Type-2 Ribosome-Inactivating Protein SNA-I from Elderberry (Sambucus nigra) Is a Determining Factor for Its Insecticidal Activity. Phytochemistry 2008, 69, 2972–2978. [Google Scholar] [CrossRef]
- Hamshou, M.; Van Damme, E.J.M.; Vandenborre, G.; Ghesquière, B.; Trooskens, G.; Gevaert, K.; Smagghe, G. GalNAc/Gal-Binding Rhizoctonia solani Agglutinin Has Antiproliferative Activity in Drosophila melanogaster S2 Cells via MAPK and JAK/STAT Signaling. PLoS ONE 2012, 7, e33680. [Google Scholar] [CrossRef]
- Tamura, T.; Sadakata, N.; Oda, T.; Muramatsu, T. Role of Zinc Ions in Ricin-Induced Apoptosis in U937 Cells. Toxicol. Lett. 2002, 132, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.N.; Lindsay, C.D.; Griffiths, G.D. Morphology of Ricin and Abrin Exposed Endothelial Cells Is Consistent with Apoptotic Cell Death. Hum. Exp. Toxicol. 1996, 15, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Wolin, I.A.V.; Heinrich, I.A.; Nascimento, A.P.M.; Welter, P.G.; Sosa, L.d.V.; De Paul, A.L.; Zanotto-Filho, A.; Nedel, C.B.; Lima, L.D.; Osterne, V.J.S.; et al. ConBr Lectin Modulates MAPKs and Akt Pathways and Triggers Autophagic Glioma Cell Death by a Mechanism Dependent upon Caspase-8 Activation. Biochimie 2021, 180, 186–204. [Google Scholar] [CrossRef]
- de Oliveira Silva, F.; das Neves Santos, P.; de Melo, C.M.L.; Teixeira, E.H.; de Sousa Cavada, B.; Pereira, V.A.R.; Porto, A.L.F.; Cajazeiras, J.B.; Arruda, F.V.S.; Almeida, A.C. Immunostimulatory Activity of ConBr: A Focus on Splenocyte Proliferation and Proliferative Cytokine Secretion. Cell Tissue Researcn 2011, 346, 237–244. [Google Scholar] [CrossRef]
- Cui, B.; Li, L.; Zeng, Q.; Lin, F.; Yin, L.; Liao, L.; Huang, M.; Wang, J. A Novel Lectin from Artocarpus lingnanensis Induces Proliferation and Th1/Th2 Cytokine Secretion through CD45 Signaling Pathway in Human T Lymphocytes. J. Nat. Med. 2017, 71, 409–421. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.; Hou, Y.; Zhou, H.; Wang, X.; Mai, K.; He, G. Differential Apoptotic and Mitogenic Effects of Lectins in Zebrafish. Front. Endocrinol. 2019, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Valentiner, U.; Fabian, S.; Schumacher, U.; Leathem, A.J. The Influence of Dietary Lectins on the Cell Proliferation of Human Breast Cancer Cell Lines in Vitro. Anticancer. Res. 2003, 23, 1197–1206. [Google Scholar] [PubMed]
- Maciel, E.V.M.; Araújo-Filho, V.S.; Nakazawa, M.; Gomes, Y.M.; Coelho, L.C.B.B.; Correia, M.T.S. Mitogenic Activity of Cratylia mollis Lectin on Human Lymphocytes. Biologicals 2004, 32, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Pani, G.; Colavitti, R.; Borrello, S.; GaleottiI, T. Endogenous Oxygen Radicals Modulate Protein Tyrosine Phosphorylation and JNK-1 Activation in Lectin-Stimulated Thymocytes. Biochem. J. 2000, 347, 173–181. [Google Scholar] [CrossRef]
- Barral-Netto, M.; Santos, S.B.; Barral, A.; Moreira, L.I.M.; Santos, C.F.; Moreira, R.A.; Oliveira, J.T.A.; Cavada, B.S. Human Lymphocyte Stimulation by Legume Lectins from the Diocleae Tribe. J. Immunol. Investig. 1992, 21, 297–303. [Google Scholar] [CrossRef]
- Zha, X.-L.; Wang, H.; Sun, W.; Zhang, H.-Y.; Wen, J.; Huang, X.-Z.; Lu, C.; Shen, Y.-H. Characteristics of the Peritrophic Matrix of the Silkworm, Bombyx mori and Factors Influencing Its Formation. Insect 2021, 12, 516. [Google Scholar] [CrossRef]
- Rodríguez-de la Noval, C.; Rodríguez-Cabrera, L.; Izquierdo, L.; Espinosa, L.A.; Hernandez, D.; Ponce, M.; Moran-Bertot, I.; Tellez-Rodríguez, P.; Borras-Hidalgo, O.; Huang, S. Functional Expression of a Peritrophin A-like SfPER Protein Is Required for Larval Development in Spodoptera frugiperda (Lepidoptera: Noctuidae). Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- de Oliveira, C.F.R.; de Moura, M.C.; Napoleão, T.H.; Paiva, P.M.G.; Coelho, L.C.B.B.; Macedo, M.L.R. A Chitin-Binding Lectin from Moringa oleifera Seeds (WSMoL) Impairs the Digestive Physiology of the Mediterranean Flour Larvae, Anagasta kuehniella. Pestic. Biochem. Physiol. 2017, 142, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Latimer, G.W., Jr. Microchemical Determination of Nitrogen: Micro-Kjeldahl Method. In Official Methods of Analysis of AOAC International; Oxford University Press: New York, NY, USA, 2023. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Perez, G. Isolation and Characterization of a Lectin from the Seeds of Erythrina edulis. J. Phytochem. 1984, 23, 1229–1232. [Google Scholar] [CrossRef]
- Bollag, D.M.; Edelstein, S.J. Chapter 7: Isoelectric Focusing and Two Dimensional Gel Electrophoresis. In Protein Methods; Wiley-Liss, Inc.: New York, NY, USA, 1994; pp. 162–171. [Google Scholar]
- Mouchahoir, T.; Schiel, J.E. Development of an LC-MS/MS Peptide Mapping Protocol for the NISTmAb. Anal. Bioanal. Chem. 2018, 410, 2111–2126. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering Key Features in Protein Structures with the New ENDscript Server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Walski, T.; Van Damme, E.J.M.; Smagghe, G. Penetration through the Peritrophic Matrix Is a Key to Lectin Toxicity against Tribolium castaneum. J. Insect Physiol. 2014, 70, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Hsu, S.-C.; Shih, H.; Lai, M.-Z. Nuclear Factor of Activated T Cells c Is a Target of P38 Mitogen-Activated Protein Kinase in T Cells. Mol. Cell Biol. 2003, 23, 6442–6454. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, G.A.; Viertlmayr, R.; Moura, T.R.; Delatorre, P.; Rocha, B.A.M.; do Nascimento, K.S.; Figueiredo, J.G.; Bezerra, I.G.; Teixeira, C.S.; Simões, R.C.; et al. Structural Studies of an Anti-Inflammatory Lectin from Canavalia boliviana Seeds in Complex with Dimannosides. PLoS ONE 2014, 9, e97015. [Google Scholar] [CrossRef] [PubMed]
- De Souza, G.A.; Oliveira, P.S.; Trapani, S.; Santos, A.C.O.; Rosa, J.C.; Laure, H.J.; Faça, V.M.; Correia, M.T.; Tavares, G.A.; Oliva, G.; et al. Amino acid sequence and tertiary structure of Cratylia mollis seed lectin. Glycobiology 2003, 13, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Gallego Del Sol, F.; Cavada, B.; Calvete, J. Crystal structures of Cratylia floribunda seed lectin at acidic and basic pHs. Insights into the structural basis of the pH-dependent dimer–tetramer transition. J. Struct. Biol. 2007, 158, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Souza Teixeira, C.; da Silva, H.C.; de Moura, T.R.; Pereira-Júnior, F.N.; Nascimento, K.S.D.; Nagano, C.S.; Sampaio, A.H.; Delatorre, P.; Rocha, B.A.M.; Cavada, B.S. Crystal structure of the lectin of Camptosema pedicellatum: Implications of a conservative substitution at the hydrophobic subsite. J. Biochem. 2012, 152, 87–98. [Google Scholar] [CrossRef]


| Carbohydrate | Initial Concentration (mM) | Minimum Inhibitory Concentration (MIC) (mM) | Relative Inhibitory Activity ** |
|---|---|---|---|
| D-Manose | 300 | 150 | 1 |
| p-Nitrophenyl-α-D-mannopyranoside | 37 | 37 | 4 |
| p-Nitrophenyl-β-D-manopyranoside | 37 | 9.5 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Corredor, Z.; Reyes-Montaño, E.; Vega-Castro, N.; Quintero, M.; Hidalgo-Roa, D.; Fernández-Alonso, J.L. Characterization of a Novel Galactia lindenii Lectin and Its Effects on Lepidopteran Midgut Cells. Int. J. Mol. Sci. 2025, 26, 10359. https://doi.org/10.3390/ijms262110359
Casas-Corredor Z, Reyes-Montaño E, Vega-Castro N, Quintero M, Hidalgo-Roa D, Fernández-Alonso JL. Characterization of a Novel Galactia lindenii Lectin and Its Effects on Lepidopteran Midgut Cells. International Journal of Molecular Sciences. 2025; 26(21):10359. https://doi.org/10.3390/ijms262110359
Chicago/Turabian StyleCasas-Corredor, Zulma, Edgar Reyes-Montaño, Nohora Vega-Castro, Mónica Quintero, Deisy Hidalgo-Roa, and José Luis Fernández-Alonso. 2025. "Characterization of a Novel Galactia lindenii Lectin and Its Effects on Lepidopteran Midgut Cells" International Journal of Molecular Sciences 26, no. 21: 10359. https://doi.org/10.3390/ijms262110359
APA StyleCasas-Corredor, Z., Reyes-Montaño, E., Vega-Castro, N., Quintero, M., Hidalgo-Roa, D., & Fernández-Alonso, J. L. (2025). Characterization of a Novel Galactia lindenii Lectin and Its Effects on Lepidopteran Midgut Cells. International Journal of Molecular Sciences, 26(21), 10359. https://doi.org/10.3390/ijms262110359







