Normal Hematopoietic Stem Cells in Leukemic Bone Marrow Environment Undergo Morphological Changes Identifiable by Artificial Intelligence
Abstract
1. Introduction
2. Results
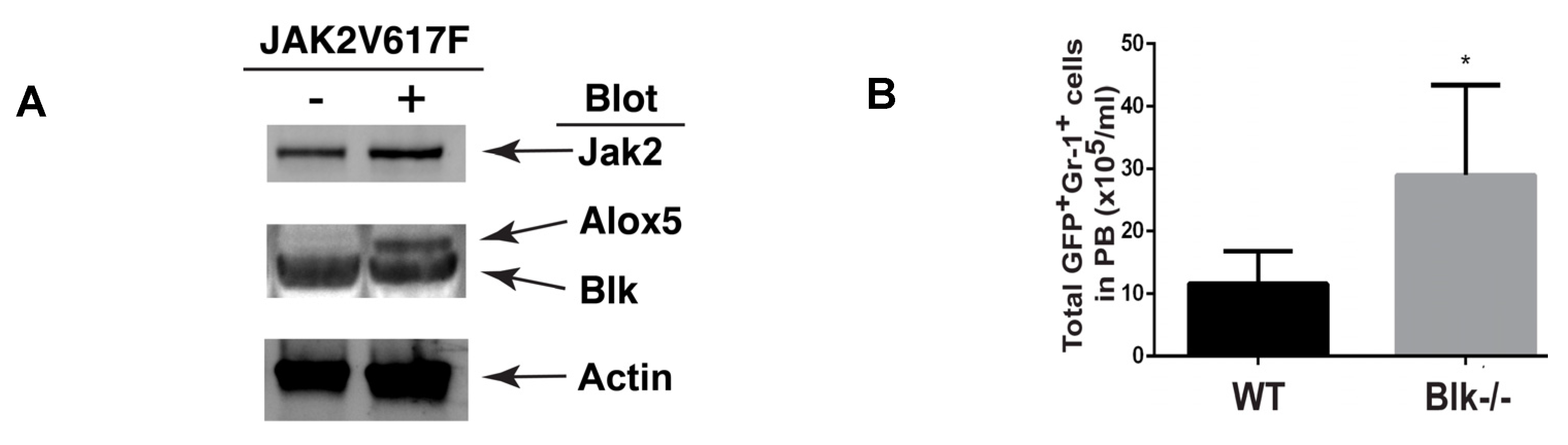
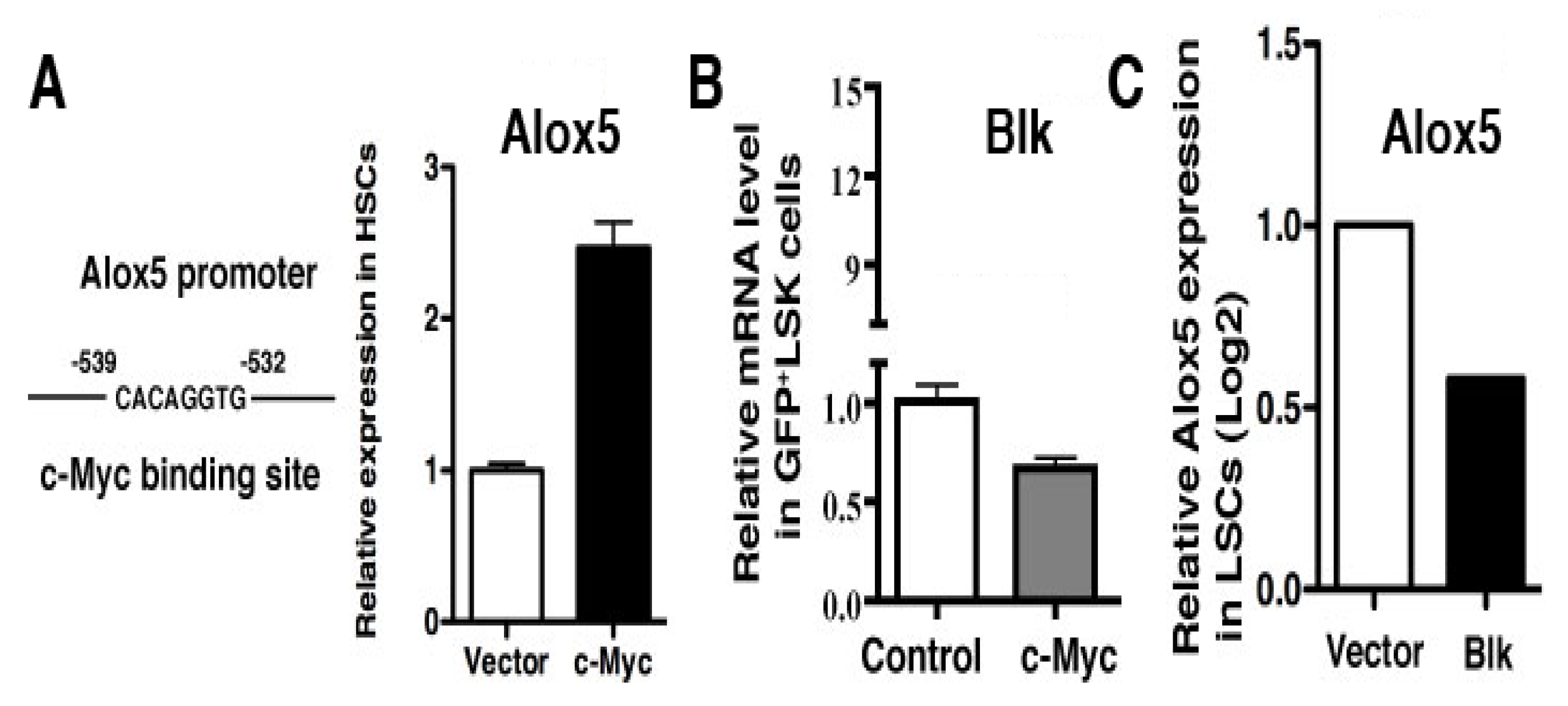
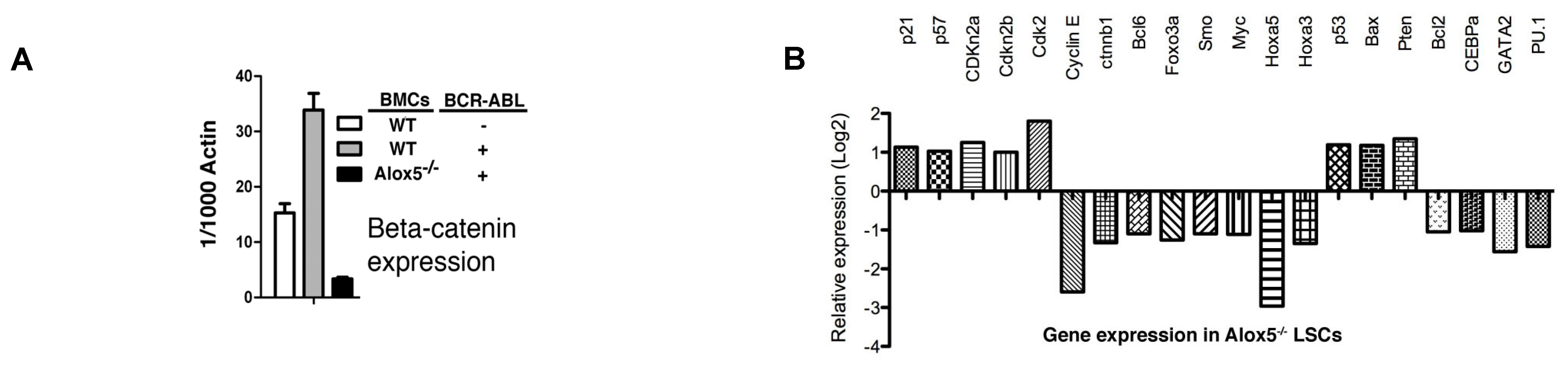
2.1. Molecular Changes in LSCs Reflect Cellular Functions
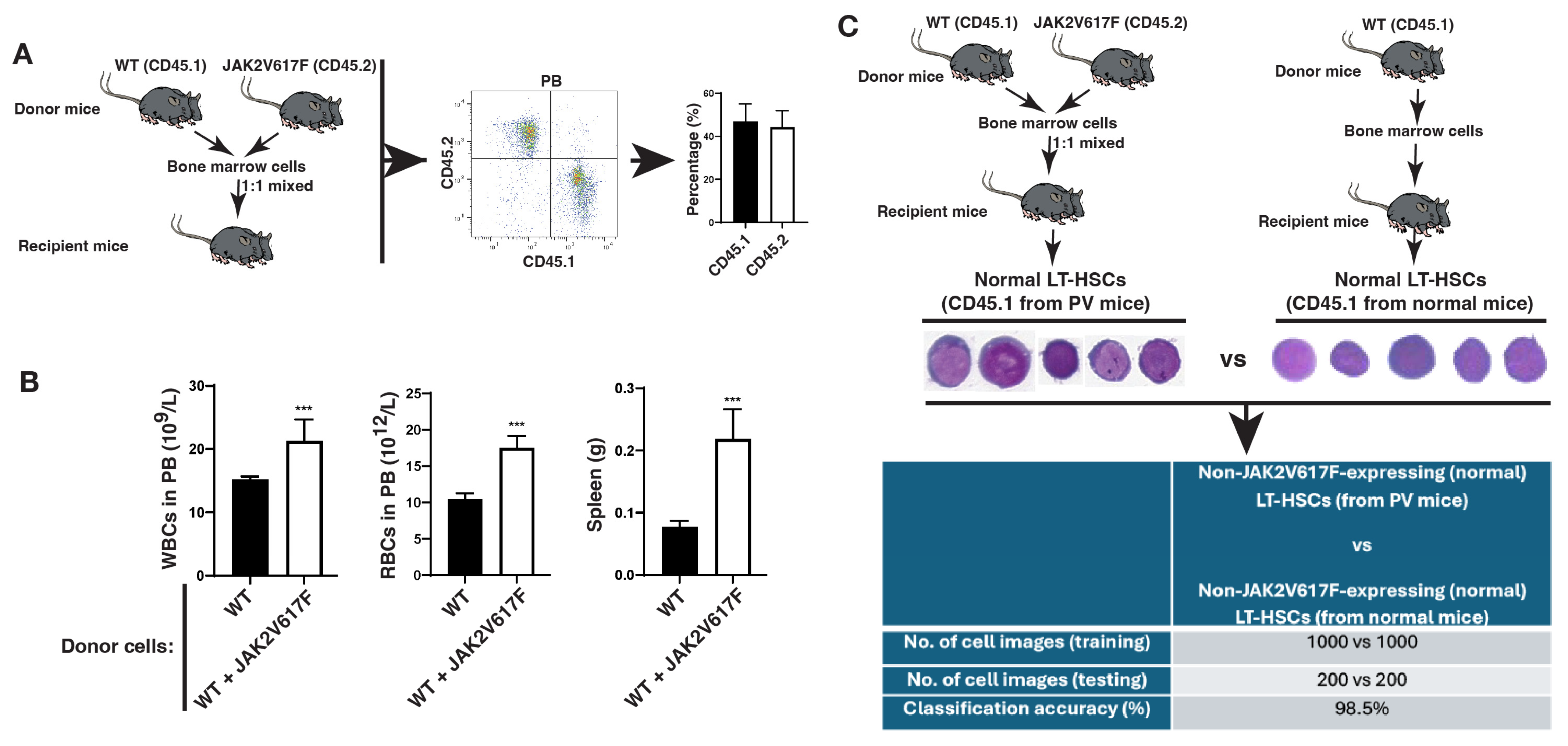
2.2. Normal Hematopoietic Stem Cells (HSCs) in the Leukemic Bone Marrow Environment Undergo Morphological Changes
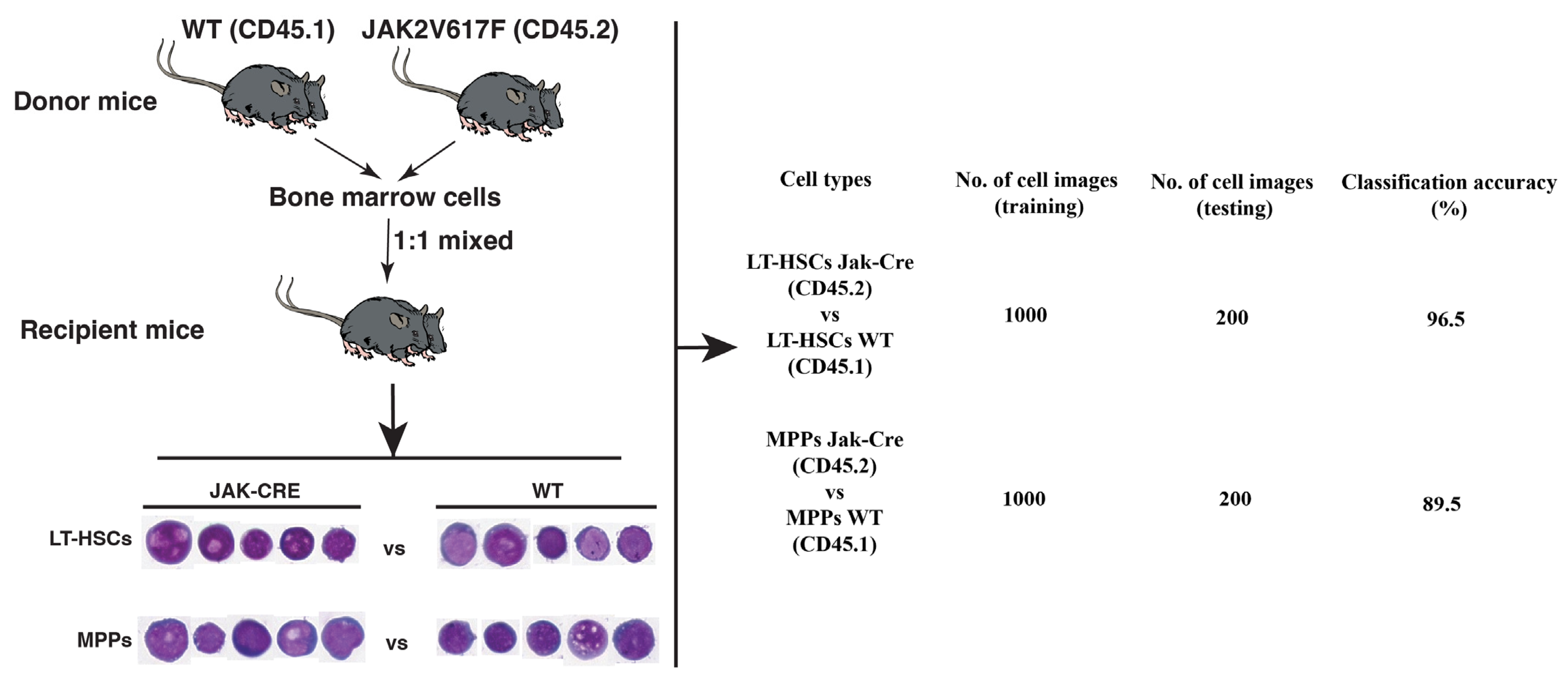
2.3. The LSC Population Is Morphologically Distinguishable from Its Normal Stem Cell Counterpart in the Same Bone Marrow Environment
3. Discussion
4. Materials and Methods
4.1. Mice
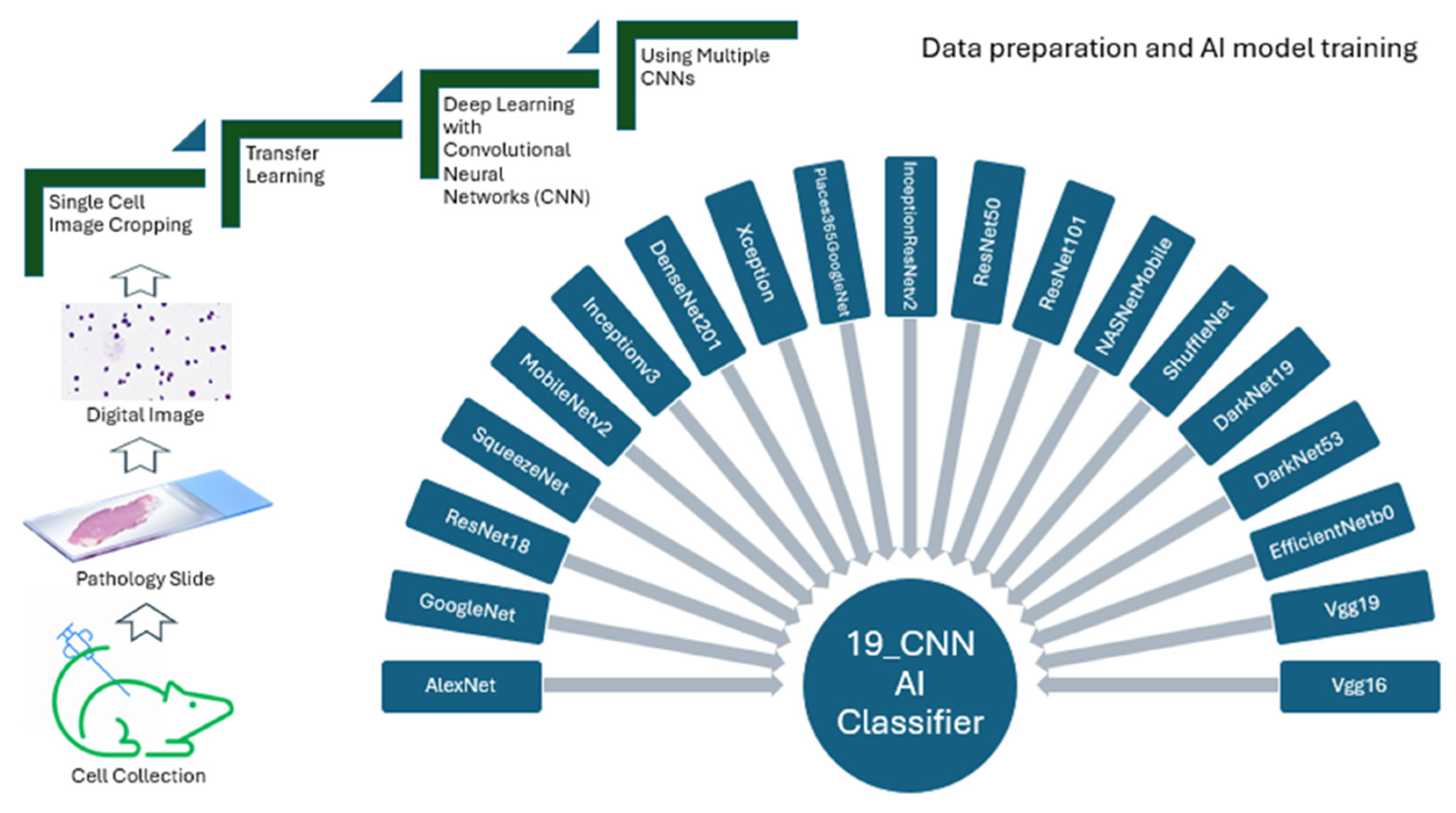
4.2. Single-Cell Image Preparation
4.3. Digital Cell Image Analysis
4.4. Transfer Deep Learning
4.5. Algorithms and Training Options
4.6. Hardware and Software
4.7. Code and Data Availability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PV | polycythemia vera |
| AI | artificial intelligence |
| CNN | convolutional neural networks |
| HSCs | hematopoietic stem cells |
| MPPs | multipotent progenitors |
| LSCs | leukemia stem cells |
References
- Basu, S.; Dong, Y.; Kumar, R.; Jeter, C.; Tang, D.G. Slow-cycling (dormant) cancer cells in therapy resistance, cancer relapse and metastasis. Semin. Cancer Biol. 2022, 78, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Long, N.A.; Golla, U.; Sharma, A.; Claxton, D.F. Acute Myeloid Leukemia Stem Cells: Origin, Characteristics, and Clinical Implications. Stem Cell Rev. Rep. 2022, 18, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Vetrie, D.; Helgason, G.V.; Copland, M. The leukaemia stem cell: Similarities, differences and clinical prospects in CML and AML. Nat. Rev. Cancer 2020, 20, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Dellorusso, P.V.; Olson, O.C.; Passegué, E. Dysregulated haematopoietic stem cell behaviour in myeloid leukaemogenesis. Nat. Rev. Cancer 2020, 20, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; DeSouza, N.; Nguyen, K.; Li, S. Leukaemia Stem Cells and Their Normal Stem Cell Counterparts Are Morphologically Distinguishable by Artificial Intelligence. J. Cell. Mol. Med. 2025, 29, e70564. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Bledsoe, J.R.; Zeng, Y.; Liu, W.; Hu, Y.; Bi, K.; Liang, A.; Li, S. A deep learning diagnostic platform for diffuse large B-cell lymphoma with high accuracy across multiple hospitals. Nat. Commun. 2020, 11, 6004. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Fialkow, P.J.; Faguet, G.B.; Jacobson, R.J.; Vaidya, K.; Murphy, S. Evidence that essential thrombocythemia is a clonal disorder with origin in a multipotent stem cell. Blood 1981, 58, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Prchal, J.F.; Axelrad, A.A. Letter: Bone-marrow responses in polycythemia vera. N. Engl. J. Med. 1974, 290, 1382. [Google Scholar] [PubMed]
- Yumori, Y.; Sugiyama, H.; Takahashi, T.; Haebara, H.; Hoshino, T. An autopsy case of acute myelodysplasia with myelofibrosis--cytogenetically proved evidence of a clonal disorder with origin in a multipotent stem cell. Rinsho Ketsueki 1986, 27, 519–525. [Google Scholar] [PubMed]
- Druker, B.J.; Sawyers, C.L.; Kantarjian, H.; Resta, D.J.; Reese, S.F.; Ford, J.M.; Capdeville, R.; Talpaz, M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 2001, 344, 1038–1042, Correction in: N. Engl. J. Med. 2001, 345, 232. [Google Scholar] [CrossRef]
- Ren, R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat. Rev. Cancer 2005, 5, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Y.; Zhang, H.; Peng, C.; Li, S. Loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia. Nat. Genet. 2009, 41, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shan, Y.; Lu, M.; DeSouza, N.; Guo, Z.; Hoffman, R.; Liang, A.; Li, S. Alox5 Blockade Eradicates JAK2V617F-Induced Polycythemia Vera in Mice. Cancer Res. 2017, 77, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Korn, C.; Méndez-Ferrer, S. Myeloid malignancies and the microenvironment. Blood 2017, 129, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Miraki-Moud, F.; Anjos-Afonso, F.; Hodby, K.A.; Griessinger, E.; Rosignoli, G.; Lillington, D.; Jia, L.; Davies, J.K.; Cavenagh, J.; Smith, M.; et al. Acute myeloid leukemia does not deplete normal hematopoietic stem cells but induces cytopenias by impeding their differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, 13576–13581. [Google Scholar] [CrossRef] [PubMed]
- Akada, H.; Yan, D.; Zou, H.; Fiering, S.; Hutchison, R.E.; Mohi, M.G. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood 2010, 115, 3589–3597. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, C.H.M.; Gotlib, J.; Durocher, J.A.; Chao, M.P.; Mariappan, M.R.; Lay, M.; Jones, C.; Zehnder, J.L.; Lilleberg, S.L.; Weissman, I.L. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc. Natl. Acad. Sci. USA 2006, 103, 6224–6229. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Akada, H.; Nath, D.; Hutchison, R.E.; Mohi, G. Loss of Ezh2 cooperates with Jak2V617F in the development of myelofibrosis in a mouse model of myeloproliferative neoplasm. Blood 2016, 127, 3410–3423. [Google Scholar] [CrossRef] [PubMed]
| No. | Name of the Pretrained CNNs | Input Image Sizes (Pixels) |
|---|---|---|
| 1 | GoogleNet | 224 × 224 |
| 2 | Vgg16 | 224 × 224 |
| 3 | Vgg19 | 224 × 224 |
| 4 | ResNet18 | 224 × 224 |
| 5 | ResNet50 | 224 × 224 |
| 6 | ResNet101 | 224 × 224 |
| 7 | MobileNetv2 | 224 × 224 |
| 8 | DenseNet201 | 224 × 224 |
| 9 | Places365GoogleNet | 224 × 224 |
| 10 | NASNetMobile | 224 × 224 |
| 11 | ShuffleNet | 224 × 224 |
| 12 | EfficientNetb0 | 224 × 224 |
| 13 | AlexNet | 227 × 227 |
| 14 | SqueezeNet | 227 × 227 |
| 15 | DarkNet19 | 256 × 256 |
| 16 | DarkNet53 | 256 × 256 |
| 17 | Inceptionv3 | 299 × 299 |
| 18 | Xception | 299 × 299 |
| 19 | InceptionResNetv2 | 299 × 299 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, A.; DeSouza, N.; Li, S. Normal Hematopoietic Stem Cells in Leukemic Bone Marrow Environment Undergo Morphological Changes Identifiable by Artificial Intelligence. Int. J. Mol. Sci. 2025, 26, 10354. https://doi.org/10.3390/ijms262110354
Li D, Li A, DeSouza N, Li S. Normal Hematopoietic Stem Cells in Leukemic Bone Marrow Environment Undergo Morphological Changes Identifiable by Artificial Intelligence. International Journal of Molecular Sciences. 2025; 26(21):10354. https://doi.org/10.3390/ijms262110354
Chicago/Turabian StyleLi, Dongguang, Athena Li, Ngoc DeSouza, and Shaoguang Li. 2025. "Normal Hematopoietic Stem Cells in Leukemic Bone Marrow Environment Undergo Morphological Changes Identifiable by Artificial Intelligence" International Journal of Molecular Sciences 26, no. 21: 10354. https://doi.org/10.3390/ijms262110354
APA StyleLi, D., Li, A., DeSouza, N., & Li, S. (2025). Normal Hematopoietic Stem Cells in Leukemic Bone Marrow Environment Undergo Morphological Changes Identifiable by Artificial Intelligence. International Journal of Molecular Sciences, 26(21), 10354. https://doi.org/10.3390/ijms262110354





