The Role of a Newly Synthesized Antimicrobial Peptide (KK)2-KWWW-NH2 in Modulating Phosphatidylinositol Monolayer Properties in the Presence of Ascorbic Acid
Abstract
1. Introduction
2. Results
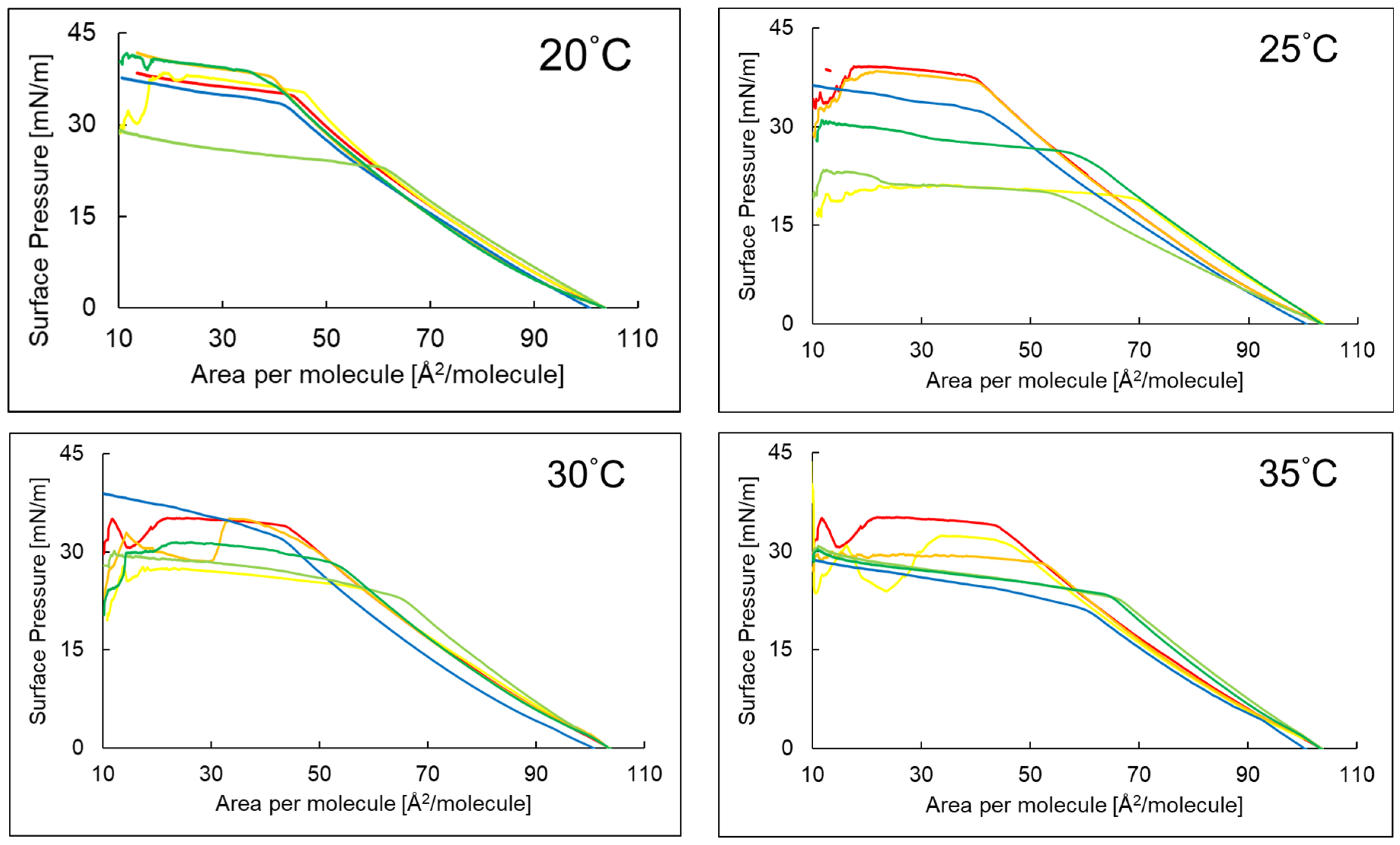
2.1. Compression Isotherms of the PI Monolayer on an Aqueous Subphase Containing Peptide and AA or EAA
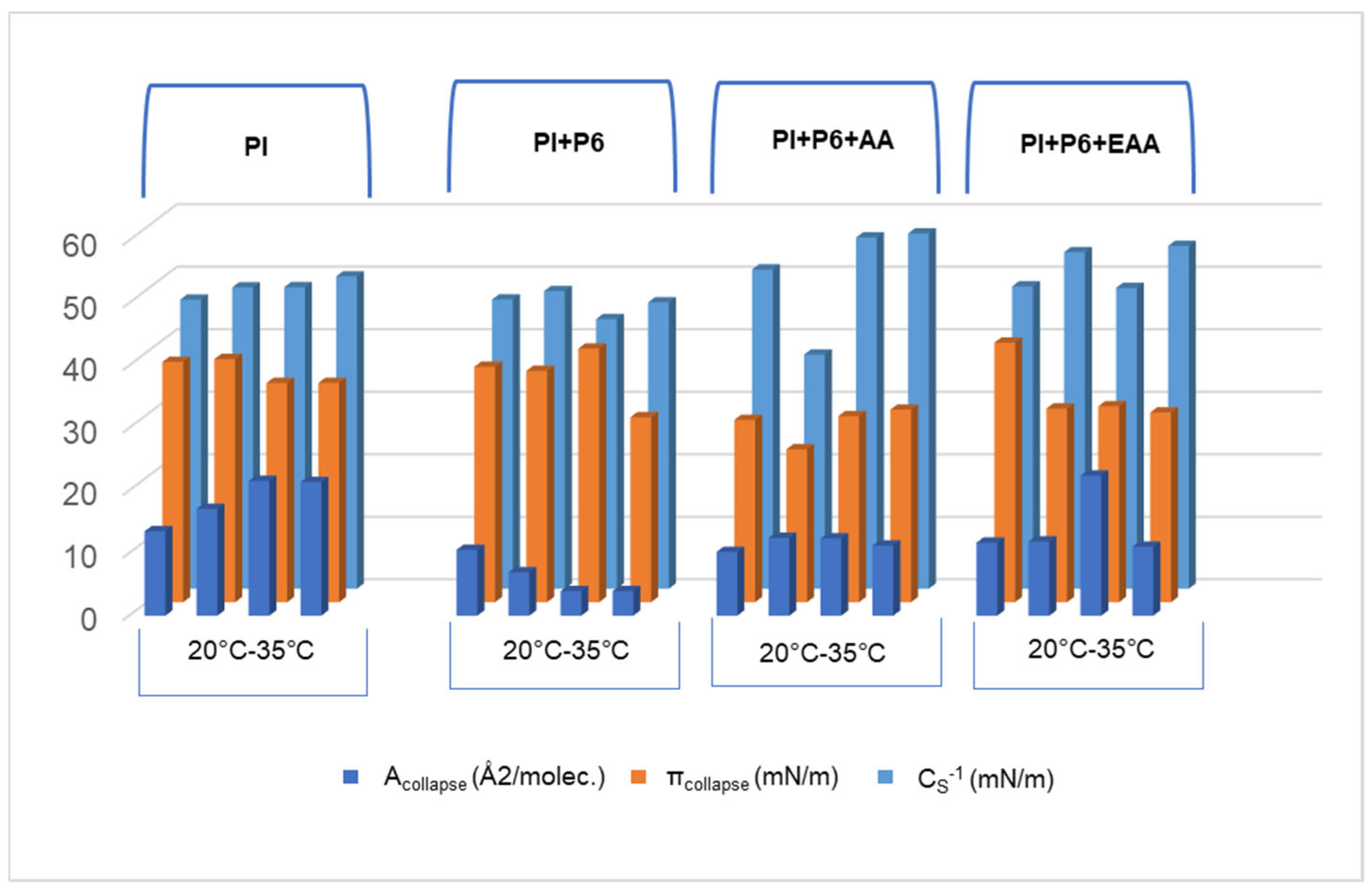
2.2. Compressibility of the PI Monolayer on Aqueous Subphase with Peptide, AA, or EAA
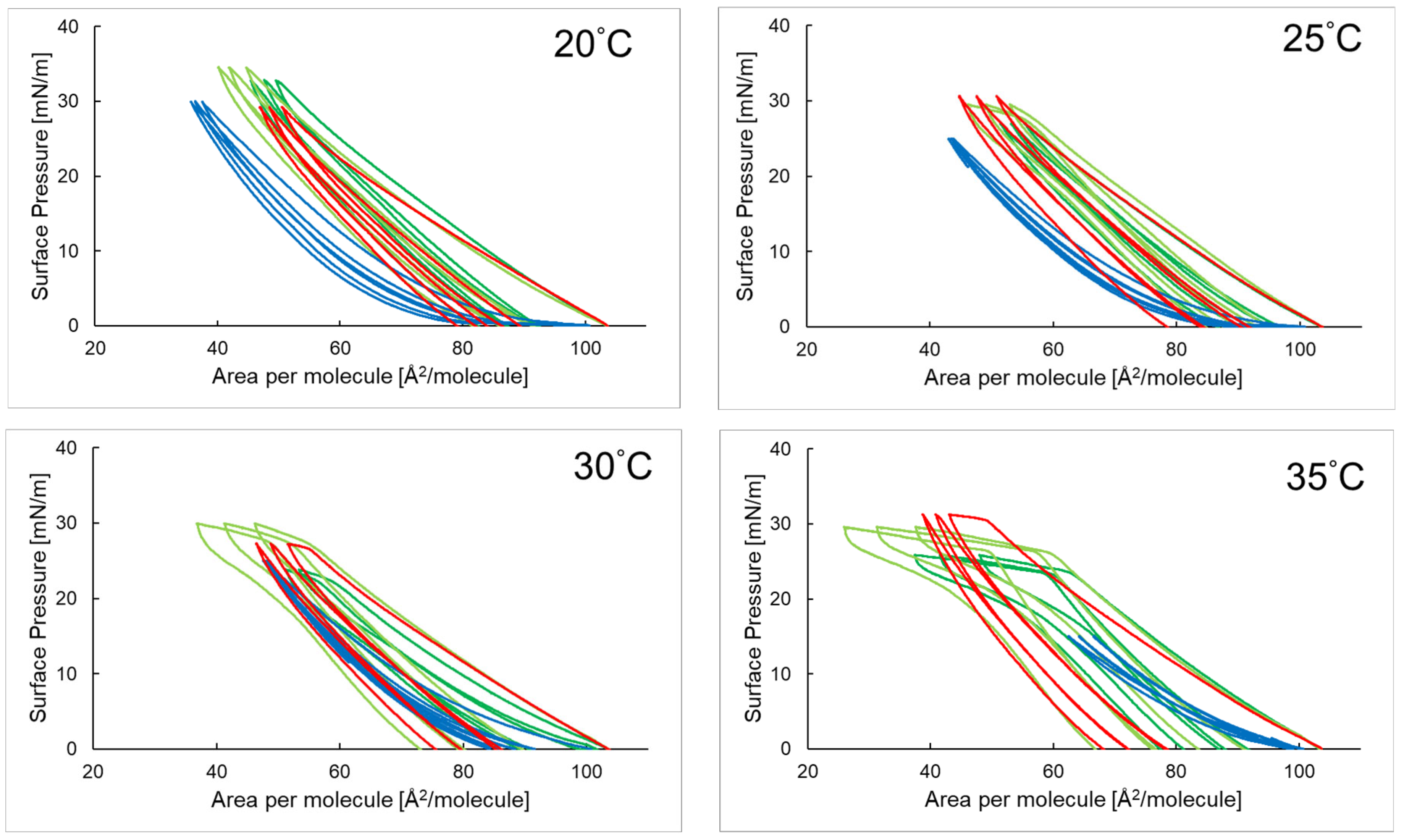
2.3. Compression and Expansion of the PI Monolayer on an Aqueous Subphase Containing Peptide, AA, or EAA
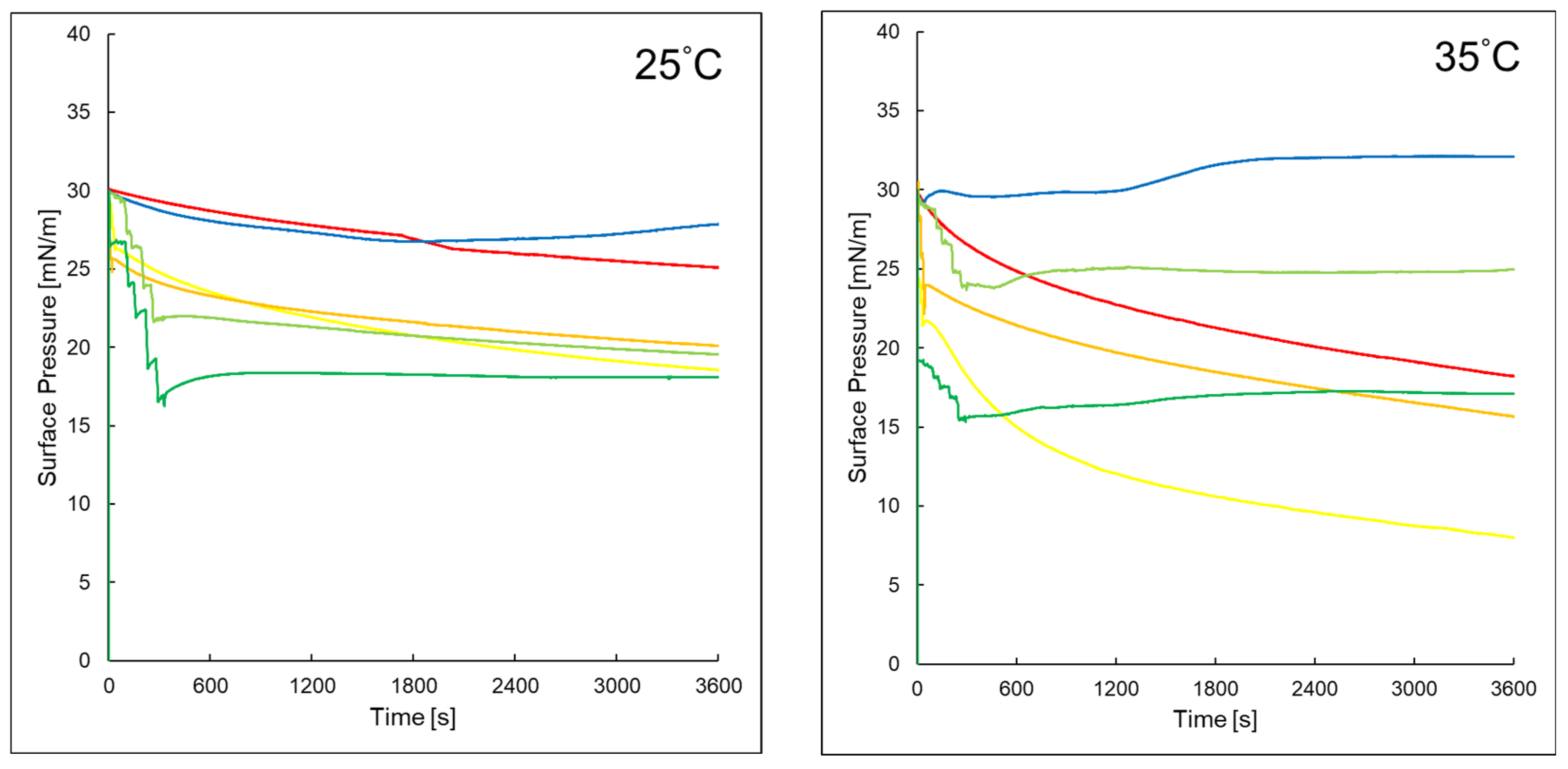
2.4. Time-Dependent Surface Pressure of Evaluated Monolayers in the Studied Systems
3. Discussion
4. Materials and Methods
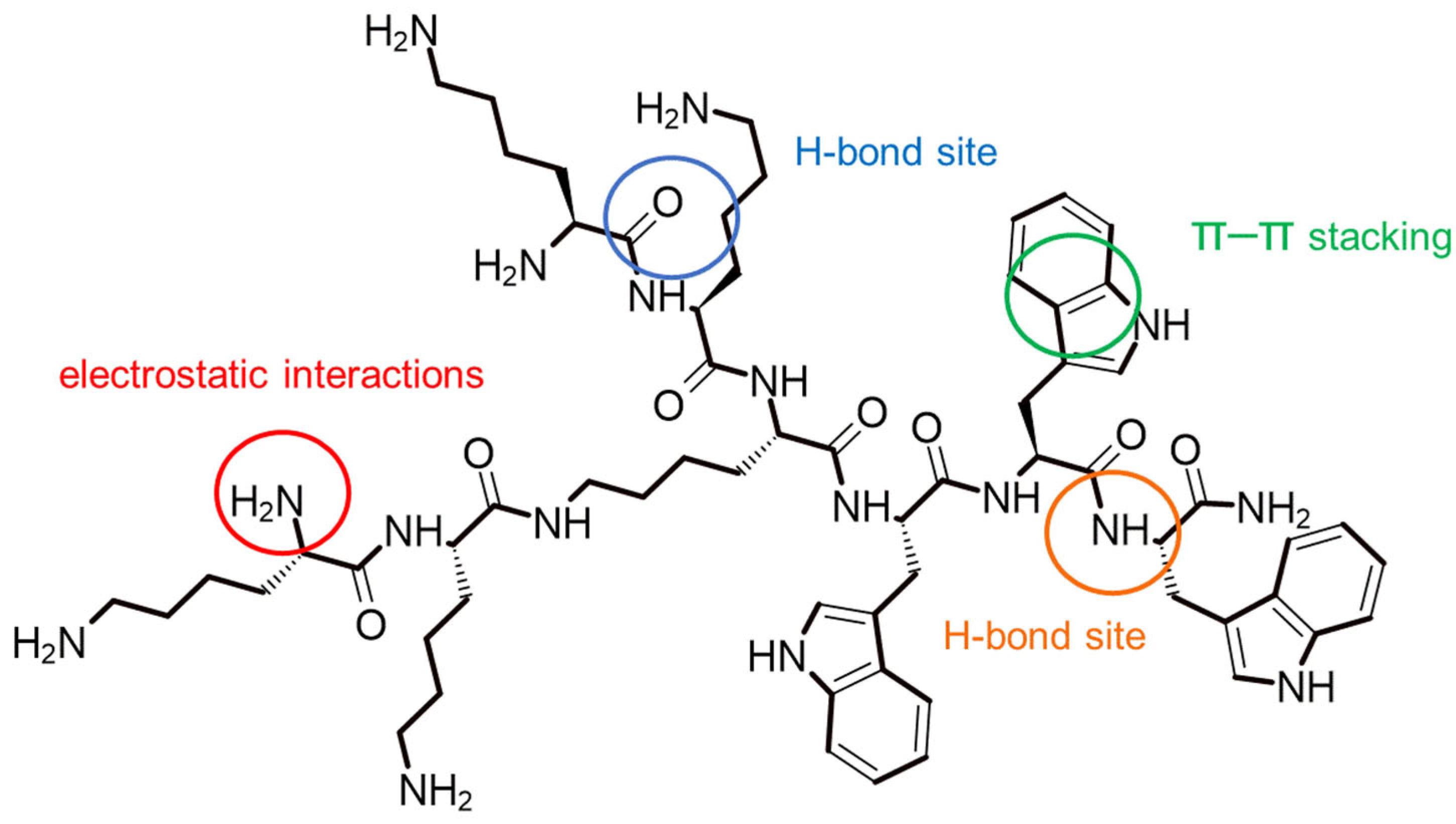
4.1. Synthesis and Characterization of the Peptide
4.2. Langmuir Films
4.3. Hysteresis Measurements
4.4. Compressibility Modulus of the Monolayer
4.5. Surface Pressure Changes over Time
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- García-Beltrán, J.M.; Arizcun, M.; Chaves-Pozo, E. Antimicrobial Peptides from Photosynthetic Marine Organisms with Potential Application in Aquaculture. Mar. Drugs 2023, 21, 290. [Google Scholar] [CrossRef]
- Wang, C.-K.; Shih, L.-Y.; Chang, K.Y. Large-Scale Analysis of Antimicrobial Activities in Relation to Amphipathicity and Charge Reveals Novel Characterization of Antimicrobial Peptides. Molecules 2017, 22, 2037. [Google Scholar] [CrossRef]
- Yudaev, P.; Tupikov, A.; Chistyakov, E. Organocyclophosphazenes and Materials Based on Them for Pharmaceuticals and Biomedicine. Biomolecules 2025, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, W.; Hamza, M.I.; Qayyum, S.; Khan, M.A.; Mukhtar, N.; Kamal, A.; Sarwar, R.; Nazish, M.; Alrefae, A.F.; Almutairi, M.H. Phyto-Synthesis and Characterization of Silver Nanoparticles from Mint Leaf Extract and Their Evaluation in Antimicrobial and Pharmacological Applications. BMC Plant Biol. 2025, 25, 1072. [Google Scholar] [CrossRef] [PubMed]
- Golonka, I.; Greber, K.E.; Oleksy-Wawrzyniak, M.; Paleczny, J.; Dryś, A.; Junka, A.; Sawicki, W.; Musiał, W. Antimicrobial and Antioxidative Activity of Newly Synthesized Peptides Absorbed into Bacterial Cellulose Carrier against Acne vulgaris. Int. J. Mol. Sci. 2021, 22, 7466. [Google Scholar] [CrossRef]
- Golonka, I.; Greber, K.E.; Szyja, B.M.; Petrus, P.P.; Pucułek, J.E.; Musiał, W. Effect of Newly Synthesized Structures of Peptides on the Stability of the Monolayers Formed. Int. J. Mol. Sci. 2023, 24, 4318. [Google Scholar] [CrossRef]
- Xie, F.; Chai, J.K.; Hu, Q.; Yu, Y.; Ma, L.; Liu, L.; Zhang, X.; Li, B.; Zhang, D. Transdermal permeation of drugs with differing lipophilicity: Effect of penetration enhancer camphor. Int. J. Pharm. 2016, 507, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Golonka, I.; Pucułek, J.E.; Greber, K.E.; Dryś, A.; Sawicki, W.; Musiał, W. Evaluation of the Effect of Antibacterial Peptides on Model Monolayers. Int. J. Mol. Sci. 2023, 24, 14861. [Google Scholar] [CrossRef]
- Mordarska, H.; Paściak, M. A simple method for differentiation of Propionibacterium acnes and Propionibacterium propionicum. FEMS Microbiol. Lett. 1994, 123, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Bhate, K.; Williams, H.C. Epidemiology of acne vulgaris. Br. J. Dermatol. 2013, 168, 474–485. [Google Scholar] [CrossRef]
- Golonka, I.; Łukasiewicz, I.W.; Sebastiańczyk, A.; Greber, K.E.; Sawicki, W.; Musiał, W. The Influence of the Amphiphilic Properties of Peptides on the Phosphatidylinositol Monolayer in the Presence of Ascorbic Acid. Int. J. Mol. Sci. 2024, 25, 12484. [Google Scholar] [CrossRef]
- Rustemeyer, J.; Radtke, J.; Bremerich, A. Thermography and thermoregulation of the face. Head Face Med. 2007, 3, 17. [Google Scholar] [CrossRef]
- Tian, X.; Yu, J.; Liu, W. Facial skin temperature and its relationship with overall thermal sensation during winter in Changsha, China. Indoor Air 2022, 32, e13138. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef]
- Parsons, J.B.; Frank, M.W.; Subramanian, C.; Saenkham, P.; Rock, C.O. Metabolic basis for the differential susceptibility of Gram-positive pathogens to fatty acid synthesis inhibitors. Proc. Natl. Acad. Sci. USA 2011, 108, 15378–15383. [Google Scholar] [CrossRef] [PubMed]
- Golonka, I.; Łukasiewicz, I.W.; Sebastiańczyk, A.; Greber, K.E.; Sawicki, W.; Musiał, W. The Role of Temperature and Subphase Components in Shaping Selected Physicochemical Properties of the Phosphatidylinositol Monolayer. Int. J. Mol. Sci. 2025, 26, 3472. [Google Scholar] [CrossRef]
- Jurak, M.; Szafran, K.; Cea, P.; Martín, S. Analysis of Molecular Interactions between Components in Phospholipid-Immunosuppressant-Antioxidant Mixed Langmuir Films. Langmuir 2021, 37, 5601–5616. [Google Scholar] [CrossRef]
- Agudelo, J.; Bossa, G.V.; May, S. Incorporation of Molecular Reorientation into Modeling Surface Pressure-Area Isotherms of Langmuir Monolayers. Molecules 2021, 26, 4372. [Google Scholar] [CrossRef]
- Khemaissa, S.; Sagan, S.; Walrant, A. Tryptophan, an Amino-Acid Endowed with Unique Properties and Its Many Roles in Membrane Proteins. Crystals 2021, 11, 1032. [Google Scholar] [CrossRef]
- Rojewska, M.; Smułek, W.; Kaczorek, E.; Prochaska, K. Langmuir Monolayer Techniques for the Investigation of Model Bacterial Membranes and Antibiotic Biodegradation Mechanisms. Membranes 2021, 11, 707. [Google Scholar] [CrossRef]
- Pastuszak, K.; Jurak, M.; Kowalczyk, B.; Tarasiuk, J.; Wiącek, A.E.; Palusińska-Szysz, M. Susceptibility of Legionella gormanii Membrane-Derived Phospholipids to the Peptide Action of Antimicrobial LL-37—Langmuir Monolayer Studies. Molecules 2024, 29, 1522. [Google Scholar] [CrossRef]
- Mottola, M.; Caruso, B.; Perillo, M.A. Langmuir films at the oil/water interface revisited. Sci. Rep. 2019, 9, 2259. [Google Scholar] [CrossRef]
- Sudheesh, S.; Ahmad, J.; Singh, G.S. Hysteresis of Isotherms of Mixed Monolayers of N-Octadecyl-N′-phenylthiourea and Stearic Acid at Air/Water Interface. J. Chem. 2012, 2012, 835397. [Google Scholar]
- Nag, K.; Perez-Gil, J.; Ruano, M.L.F.; Worthman, L.A.D.; Stewart, J.; Casals, C.; Keough, K.M.W. Phase Transitions in Films of Lung Surfactant at the Air-Water Interface. Biophys. J. 1998, 74, 2983–2995. [Google Scholar] [CrossRef] [PubMed]
- Knobler, C.M.; Desai, R.C. Phase Transitions in Monolayers. Annu. Rev. Phys. Chem. 1992, 43, 207–236. [Google Scholar] [CrossRef]
- Golonka, I.; Kizior, B.; Szyja, B.M.; Damek, M.P.; Musiał, W. Assessment of the Influence of the Selected Range of Visible Light Radiation on the Durability of the Gel with Ascorbic Acid and Its Derivative. Int. J. Mol. Sci. 2022, 23, 8759. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, F.; Hossain, A.S.M.M.A.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. Topical Delivery of 3-O-ethyl l-ascorbic Acid from Complex Solvent Systems. Sci. Pharm. 2020, 88, 19. [Google Scholar] [CrossRef]
- Stamford, N.P. Stability, transdermal penetration, and cutaneous effects of ascorbic acid and its derivatives. J. Cosmet. Dermatol. 2012, 11, 310–317. [Google Scholar] [CrossRef]
- Epand, R.M.; Epand, R.F. Lipid domains in bacterial membranes and the action of an-timicrobial agents. Biochim. Biophys. Acta Biomembr. 2009, 1788, 289–294. [Google Scholar] [CrossRef]
- Matsuzaki, K. Why and how are peptide–lipid interactions utilized for self-defense? Biophys. Chem. 1999, 82, 141–150. [Google Scholar]
- de Planque, M.R.; Killian, J.A. Protein-lipid interactions studied with designed transmembrane peptides: Role of hydrophobic matching and interfacial anchoring. Mol. Membr. Biol. 2003, 20, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Krastev, R.; Lalchev, Z. Surface chemistry study of the interactions of pharmaceutical ingredients with human meibum films. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4605–4615. [Google Scholar] [CrossRef]

| Evaluated Systems | Composition [Number of Molecules] | |||
|---|---|---|---|---|
| Monolayer | Subphase | |||
| PI | AA | EAA | P6 | |
| PI * | 1.20 × 1016 | - | - | - |
| PI + AA * | 2.30 × 1016 | - | - | |
| PI + EAA * | - | 2.30 × 1016 | ||
| PI + P6 | - | - | 2.30 × 1016 | |
| PI + P6 + AA | 2.30 × 1016 | - | 2.30 × 1016 | |
| PI + P6 + EAA | - | 2.30 × 1016 | 2.30 × 1016 | |
| Evaluated Systems | Temperature (°C) | Alift-off (Å2/molec.) | Acollapse (Å2/molec.) | πcollapse (mN/m) | χ | aLE/LC |
|---|---|---|---|---|---|---|
| PI | 20 | 101.35 | 13.54 | 38.43 | 0.13 | −0.571 |
| 25 | 101.27 | 17.09 | 38.89 | 0.17 | −0.594 | |
| 30 | 101.30 | 21.56 | 35.06 | 0.21 | −0.573 | |
| 35 | 101.26 | 21.38 | 35.08 | 0.21 | −0.572 | |
| PI + AA | 20 | 101.53 | 24.11 | 37.96 | 0.24 | −0.588 |
| 25 | 102.80 | 33.73 | 21.23 | 0.33 | −0.552 | |
| 30 | 101.54 | 18.23 | 27.67 | 0.18 | −0.529 | |
| 35 | 101.54 | 34.00 | 32.41 | 0.33 | −0.547 | |
| PI + EAA | 20 | 100.72 | 13.50 | 41.74 | 0.13 | −0.581 |
| 25 | 101.10 | 22.05 | 38.46 | 0.22 | −0.586 | |
| 30 | 101.86 | 33.27 | 35.11 | 0.33 | −0.558 | |
| 35 | 101.60 | 13.68 | 29.97 | 0.13 | −0.547 | |
| PI + P6 | 20 | 98.61 | 10.54 | 37.65 | 0.11 | −0.554 |
| 25 | 98.29 | 6.93 | 37.00 | 0.07 | −0.551 | |
| 30 | 97.90 | 3.94 | 40.56 | 0.04 | −0.554 | |
| 35 | 98.60 | 3.94 | 29.58 | 0.04 | −0.512 | |
| PI + P6 + AA | 20 | 101.68 | 10.19 | 29.16 | 0.10 | −0.537 |
| 25 | 100.76 | 12.44 | 24.44 | 0.12 | −0.411 | |
| 30 | 101.59 | 12.36 | 29.76 | 0.12 | −0.598 | |
| 35 | 101.83 | 11.21 | 30.79 | 0.11 | −0.613 | |
| PI + P6 + EAA | 20 | 100.79 | 11.67 | 41.50 | 0.12 | −0.592 |
| 25 | 101.67 | 11.87 | 30.97 | 0.12 | −0.582 | |
| 30 | 101.54 | 11.41 | 31.34 | 0.11 | −0.558 | |
| 35 | 101.55 | 11.04 | 30.35 | 0.11 | −0.598 |
| Acronym | Temperature | |||
|---|---|---|---|---|
| 20 °C | 25 °C | 30 °C | 35 °C | |
| PI | 46.23 | 48.18 | 48.22 | 49.95 |
| PI + AA | 49.39 | 51.58 | 47.53 | 44.60 |
| PI + EAA | 45.47 | 49.01 | 46.77 | 47.21 |
| PI + P6 | 46.26 | 47.60 | 43.13 | 45.8 |
| PI + P6 + AA | 51.06 | 37.45 | 56,18 | 56.82 |
| PI + P6 + EAA | 48.35 | 53.82 | 48.11 | 54.82 |
| Temperature | Rv (%) Parameter for the Evaluated Systems | ||||||
|---|---|---|---|---|---|---|---|
| PI | PI + AA | PI + EAA | PI + P6 | PI + P6 + AA | PI + P6 + EAA | ||
| 20 °C | loop 1 | 70.77 | 70.13 | 72.84 | 80.59 | 75.80 | 71.42 |
| loop 2 | 73.06 | 81.89 | 80.04 | 88.67 | 83.54 | 80.31 | |
| loop 3 | 72.66 | 83.64 | 82.32 | 96.43 | 85.04 | 81.53 | |
| 25 °C | loop 1 | 74.54 | 73.30 | 72.63 | 87.34 | 68.59 | 99.62 |
| loop 2 | 77.57 | 77.64 | 80.30 | 90.32 | 69.88 | 76.81 | |
| loop 3 | 79.03 | 79.60 | 81.95 | 87.11 | 75.41 | 72.58 | |
| 30 °C | loop 1 | 86.81 | 59.59 | 67.03 | 83.47 | 59.47 | 78.70 |
| loop 2 | 77.23 | 73.46 | 76.80 | 97.06 | 67.58 | 67.88 | |
| loop 3 | 80.80 | 79.49 | 80.61 | 95.94 | 75.68 | 74.88 | |
| 35 °C | loop 1 | 66.43 | 51.74 | 60.53 | 87.34 | 58.77 | 57.83 |
| loop 2 | 79.77 | 69.83 | 74.97 | 93.87 | 65.14 | 56.84 | |
| loop 3 | 86.83 | 74.38 | 78.56 | 97.77 | 90.13 | 63.42 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golonka, I.; Sebastiańczyk, A.; Łukasiewicz, I.W.; Greber, K.E.; Sawicki, W.; Musiał, W. The Role of a Newly Synthesized Antimicrobial Peptide (KK)2-KWWW-NH2 in Modulating Phosphatidylinositol Monolayer Properties in the Presence of Ascorbic Acid. Int. J. Mol. Sci. 2025, 26, 10344. https://doi.org/10.3390/ijms262110344
Golonka I, Sebastiańczyk A, Łukasiewicz IW, Greber KE, Sawicki W, Musiał W. The Role of a Newly Synthesized Antimicrobial Peptide (KK)2-KWWW-NH2 in Modulating Phosphatidylinositol Monolayer Properties in the Presence of Ascorbic Acid. International Journal of Molecular Sciences. 2025; 26(21):10344. https://doi.org/10.3390/ijms262110344
Chicago/Turabian StyleGolonka, Iwona, Aleksandra Sebastiańczyk, Izabela W. Łukasiewicz, Katarzyna E. Greber, Wiesław Sawicki, and Witold Musiał. 2025. "The Role of a Newly Synthesized Antimicrobial Peptide (KK)2-KWWW-NH2 in Modulating Phosphatidylinositol Monolayer Properties in the Presence of Ascorbic Acid" International Journal of Molecular Sciences 26, no. 21: 10344. https://doi.org/10.3390/ijms262110344
APA StyleGolonka, I., Sebastiańczyk, A., Łukasiewicz, I. W., Greber, K. E., Sawicki, W., & Musiał, W. (2025). The Role of a Newly Synthesized Antimicrobial Peptide (KK)2-KWWW-NH2 in Modulating Phosphatidylinositol Monolayer Properties in the Presence of Ascorbic Acid. International Journal of Molecular Sciences, 26(21), 10344. https://doi.org/10.3390/ijms262110344







