Plasma Galectin-4 and Charcot-Leyden Crystal Protein/Galectin-10 as Emerging Biomarkers of Metabolically Induced Inflammation in Patients with Psoriasis
Abstract
1. Introduction
2. Results
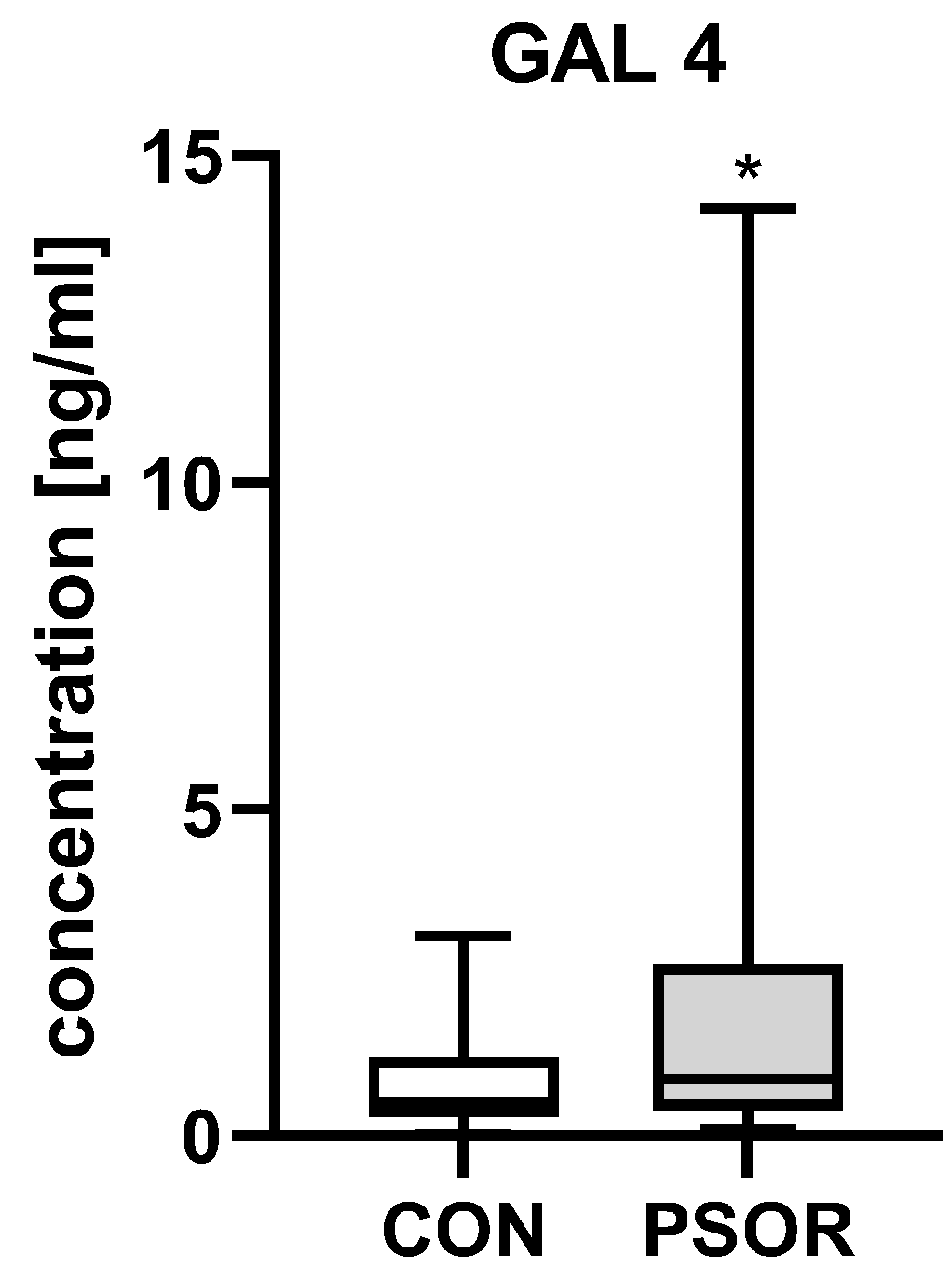
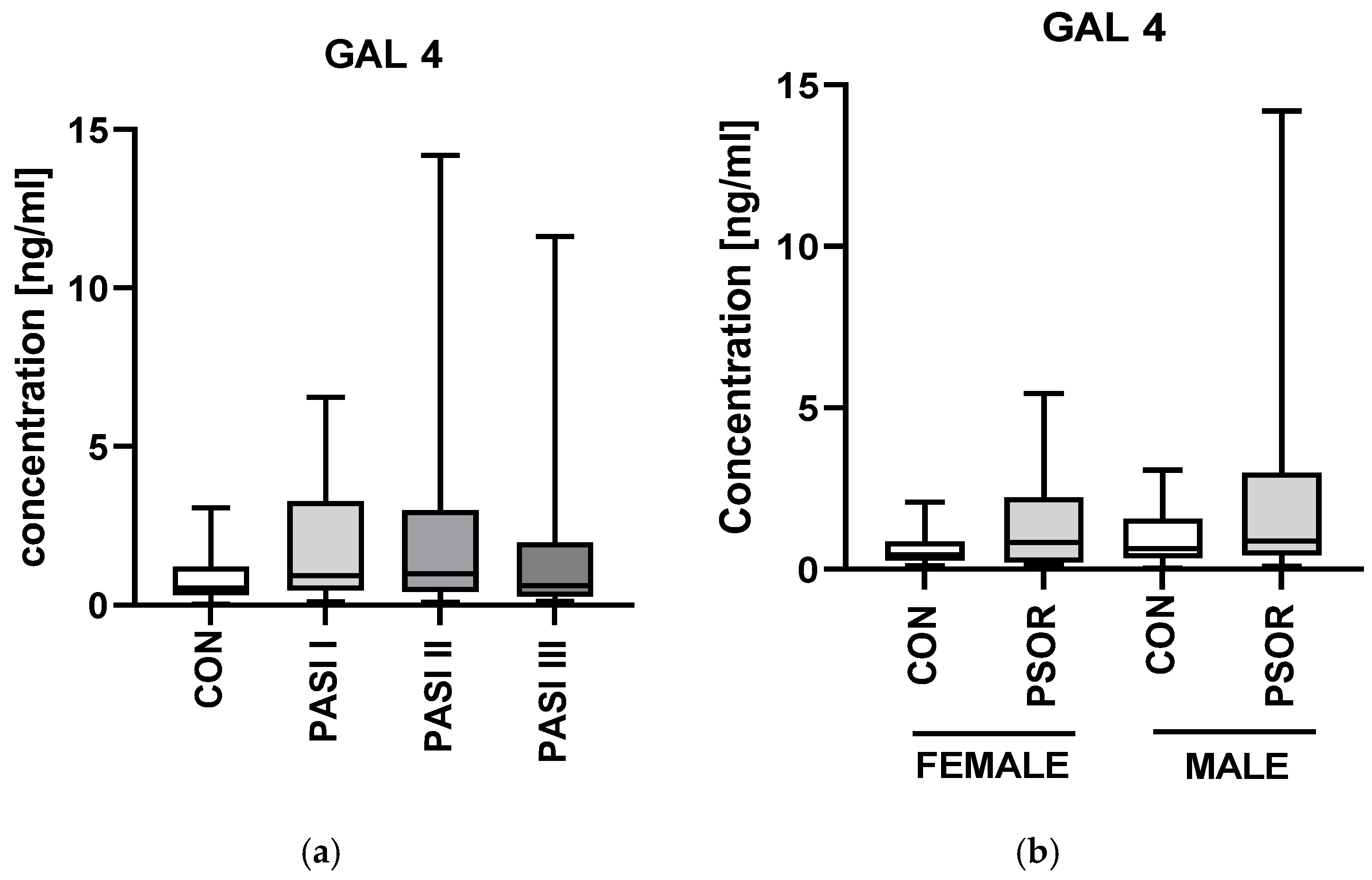
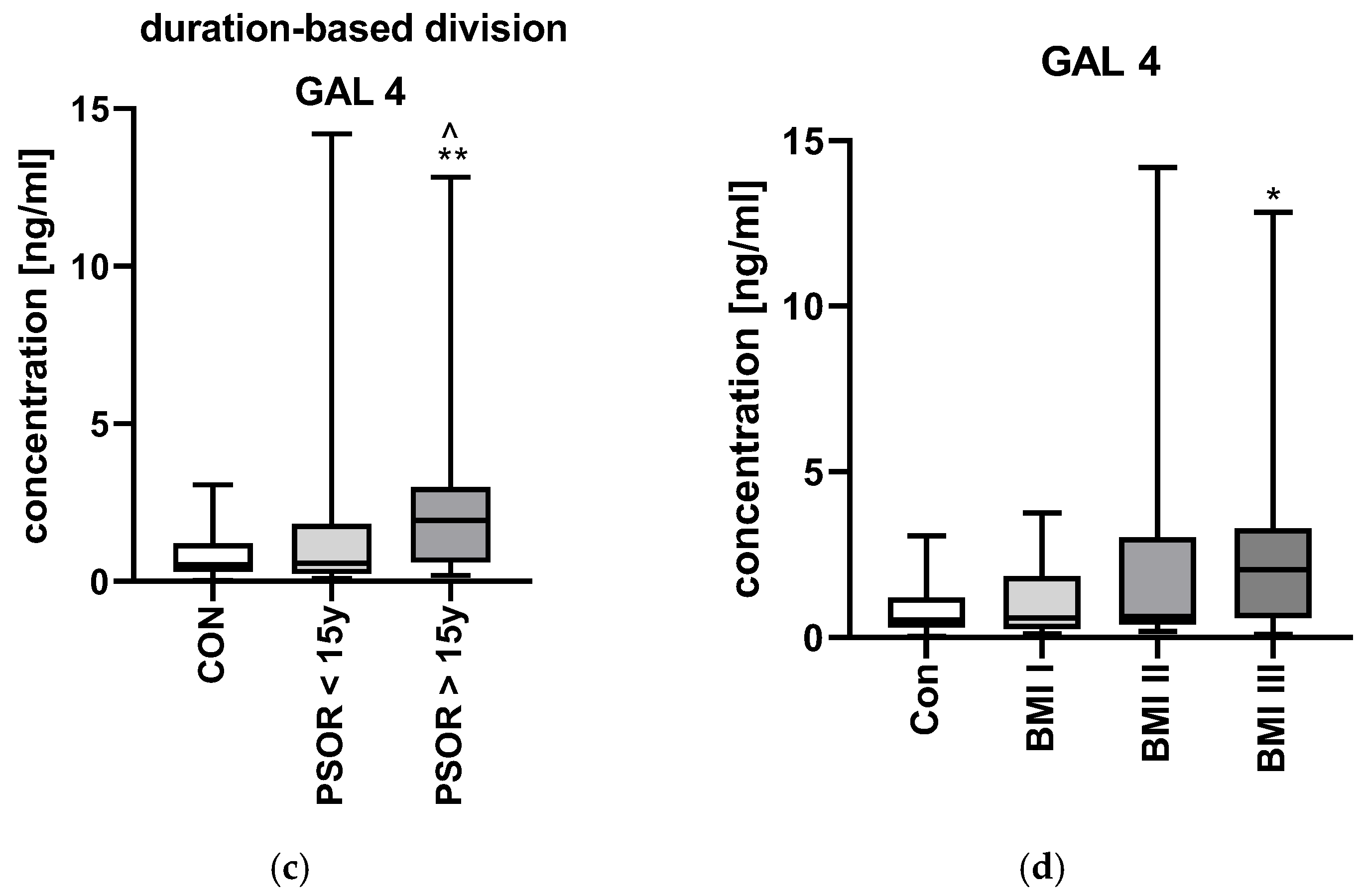
2.1. Galectin-4
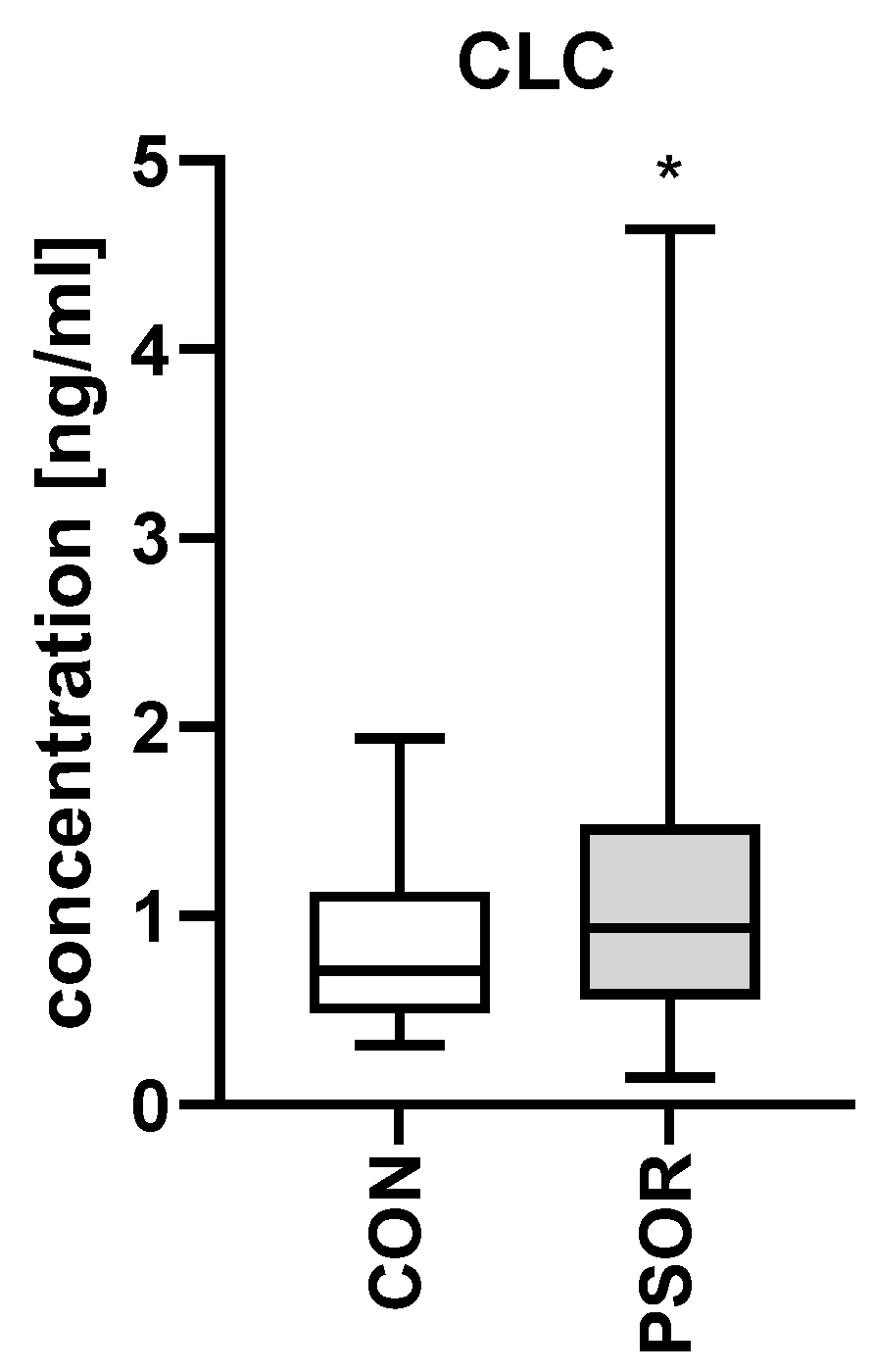
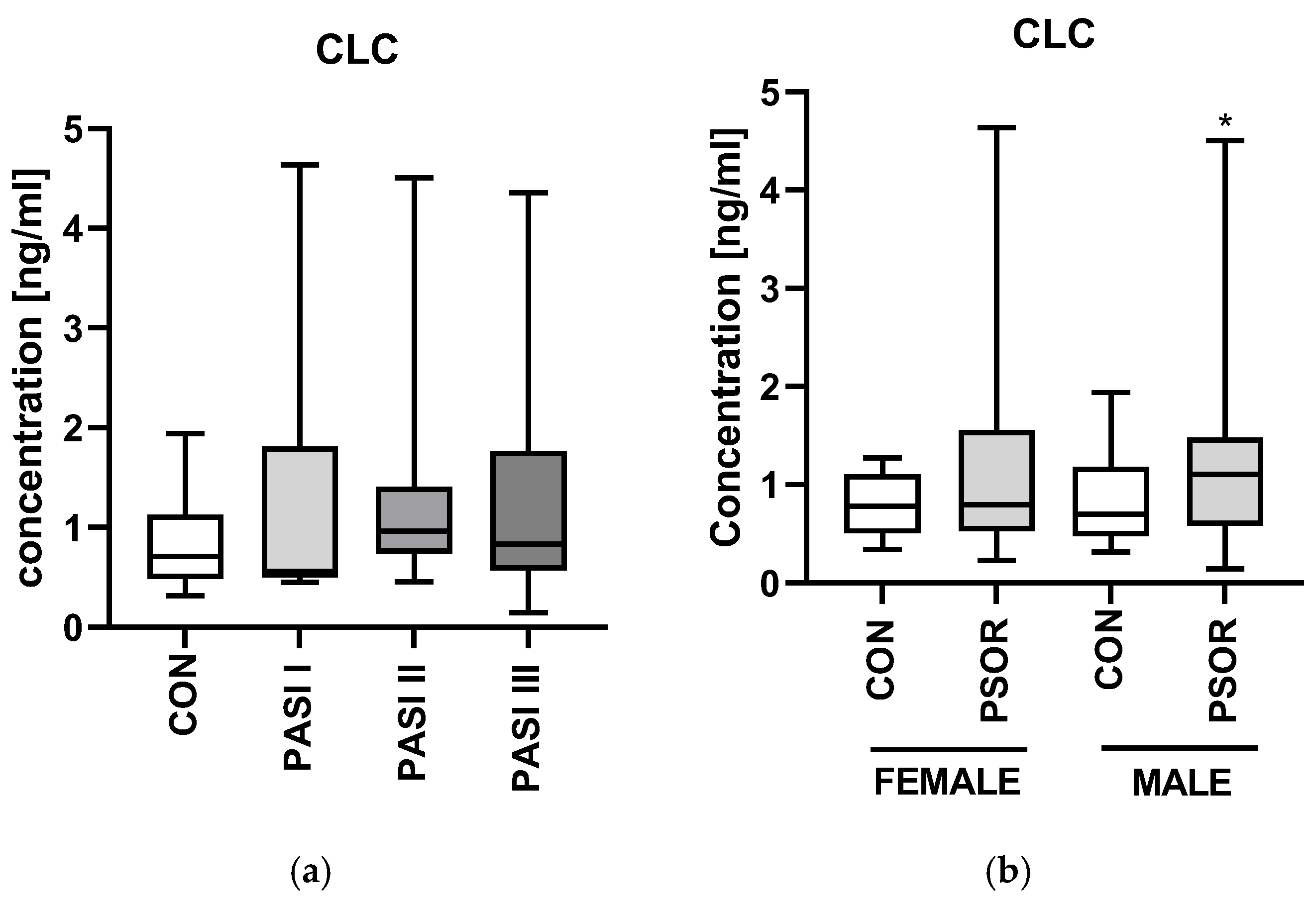
2.2. CLC/Galectin10
3. Discussion
3.1. Galectin-4
3.2. Galectin-10/CLC
4. Materials and Methods
4.1. Plasma Collection
4.2. Statistical Analysis
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TGs | Triglycerides |
| ALT | Alanine aminotransferase |
| AST | Asparagine aminotransferase |
| GLU | Fasting glucose |
| Chol | Total cholesterol |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| RBC | Red blood cells |
| WBC | White blood cells |
| PLT | Platelets |
| HGB | Hemoglobin |
| CR | Creatinine |
References
- Buja, A.; Di Grazia, C.; Garofalo, F.; Bellini, S.; Rossi, M.; Caruso, C.; De Leo, G. The prevalent comorbidome at the onset of psoriasis diagnosis. Dermatol. Ther. 2023, 13, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Kavanaugh, A.; Lebwohl, M.G.; Gniadecki, R.; Merola, J.F. Psoriasis and metabolic syndrome: Implications for the management and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 797–806. [Google Scholar] [CrossRef]
- Adamski, Z.; Kanabaj, K.; Kuźniak, A. The link between psoriasis and other diseases is based on epidemiological and genetic analyses. Adv. Dermatol. Allergol. 2023, 40, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Kovitwanichkanont, T.; Chong, A.H.; Foley, P. Beyond skin deep: Addressing comorbidities in psoriasis. Med. J. Aus. 2020, 212, 528–534. [Google Scholar] [CrossRef]
- Yamazaki, F. Psoriasis: Comorbidities. J. Dermatol. 2021, 48, 732–740. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, Y.P.; Li, L.F. Association between systemic immune-inflammation index and psoriasis, psoriasis comorbidities, and all-cause mortality: A study based on NHANES. Immunol. Inflamm. Dis. 2024, 12, e70050. [Google Scholar] [CrossRef]
- Bänfer, S.; Jacob, R. Galectins. Curr Biol. 2022, 32, R406–R408. [Google Scholar] [CrossRef] [PubMed]
- Dings, R.P.M.; Miller, M.C.; Griffin, R.J.; Mayo, K.H. Galectins as molecular targets for therapeutic intervention. Int. J. Mol. Sci. 2018, 19, 905. [Google Scholar] [CrossRef]
- Brinchmann, M.F.; Patel, D.M.; Iversen, M.H. The role of galectins as modulators of metabolism and inflammation. Med. Inflamm. 2018, 2018, 9186940. [Google Scholar] [CrossRef]
- Arthur, C.M.; Baruffi, M.D.; Cummings, R.D.; Stowell, S.R. Evolving mechanistic insights into galectin functions. Methods Mol. Biol. 2015, 1207, 1–35. [Google Scholar] [CrossRef]
- Baran, A.; Kiluk, P.; Nowowiejska, J.; Kaminski, T.W.; Maciaszek, M.; Flisiak, I. Galectin-3 as a Novel Multifaceted and Not Only Cardiovascular Biomarker in Patients with Psoriasis with Regard to Systemic Treatment—Preliminary Data. Biology 2022, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Nowowiejska, J.; Baran, A.; Hermanowicz, J.M.; Sieklucka, B.; Pawlak, D.; Flisiak, I. Evaluation of Plasma Concentrations of Galectins-1, 2, and 12 in Psoriasis and Their Clinical Implications. Biomolecules 2023, 13, 1472. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.Q.; Guo, X.L. The role of galectin-4 in physiology and diseases. Protein Cell. 2016, 7, 314–324. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). LGALS4 Galectin 4 [Homo Sapiens (Human)]. National Library of Medicine (NIH). Available online: https://www.ncbi.nlm.nih.gov/gene/3960 (accessed on 3 May 2025).
- Ideo, H.; Tsuchida, A.; Takada, Y. Lectin-Based Approaches to Analyze the Role of Glycans and Their Clinical Application in Disease. Int. J. Mol. Sci. 2024, 25, 10231. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.B.; Dodd, S.; Yu, L.-G.; Subramanian, S. Serum galectins as potential biomarkers of inflammatory bowel diseases. PLoS ONE 2020, 15, e0227306. [Google Scholar] [CrossRef]
- Pichler, K.M.; Fischer, A.; Alphonsus, J.; Chiari, C.; Schmidt, S.; Kenn, M.; Schreiner, W.; Weinmann, D.; Rothbauer, M.; Windhager, R.; et al. Galectin Network in Osteoarthritis: Galectin-4 Programs a Pathogenic Signature of Gene and Effector Expression in Human Chondrocytes in Vitro. Histochem. Cell Biol. 2021, 157, 139–151. [Google Scholar] [CrossRef]
- Garcia, T.; Petrera, A.; Hauck, S.M.; Baber, R.; Wirkner, K.; Kirsten, H.; Pott, J.; Tönjes, A.; Henger, S.; Loeffler, M.; et al. Relationship of proteins and subclinical cardiovascular traits in the population-based LIFE-Adult study. Atherosclerosis 2024, 398, 118613. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). Gene: Charcot-Leyden Crystal Galectin [Homo Sapiens (Human)]. National Library of Medicine (NIH). Available online: https://www.ncbi.nlm.nih.gov/gene/1178 (accessed on 5 May 2025).
- Su, J. A Brief History of Charcot-Leyden Crystal Protein/Galectin-10 Research. Molecules 2018, 23, 2931. [Google Scholar] [CrossRef]
- Tomizawa, H.; Yamada, Y.; Arima, M.; Miyabe, Y.; Fukuchi, M.; Hikichi, H.; Melo, R.C.N.; Yamada, T.; Ueki, S. Galectin-10 as a Potential Biomarker for Eosinophilic Diseases. Biomolecules 2022, 12, 1385. [Google Scholar] [CrossRef]
- Gelardi, M.; Giancaspro, R.; Cassano, M. Charcot-Leyden crystals: An ancient but never so current discovery. Am. J. Otolaryngol. 2023, 44, 103844. [Google Scholar] [CrossRef]
- Noh, S.; Jin, S.; Park, C.O.; Yun, K.N.; Kim, J.Y.; Lee, K.H. Elevated Galectin-10 expression of IL-22-producing T cells in patients with atopic dermatitis. J. Investig. Dermatol. 2016, 136, 328–331. [Google Scholar] [CrossRef]
- Wu, N.L.; Liu, F.T. The expression and function of galectins in skin physiology and pathology. Exp. Dermatol. 2018, 27, 475–483. [Google Scholar] [CrossRef]
- Chen, S.; Hsu, D.K. Galectins and cutaneous immunity. J. Chin. Med. Assoc. 2012, 75, 619–624. [Google Scholar] [CrossRef]
- Fu, Y.; Lee, C.H.; Chi, C.C. Association of Psoriasis With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018, 154, 1417–1423. [Google Scholar] [CrossRef]
- Wu, T.; Cheng, H.; Zhuang, J.; Liu, X.; Ouyang, Z.; Qian, R. Risk factors for inflammatory bowel disease: An umbrella review. Front. Cell. Infect. Microbiol. 2025, 14, 1410506. [Google Scholar] [CrossRef]
- Gobbi, R.P.; De Francesco, N.; Bondar, C.; Muglia, C.; Chirdo, F.; Rumbo, M.; Rocca, A.; Toscano, M.A.; Sambuelli, A.; Rabinovich, G.A.; et al. A galectin-specific signature in the gut delineates Crohn’s disease and ulcerative colitis from other human inflammatory intestinal disorders. Biofactors 2016, 42, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Hokama, A.; Mizoguchi, E.; Sugimoto, K.; Shimomura, Y.; Tanaka, Y.; Yoshida, M.; Rietdijk, S.T.; de Jong, Y.P.; Snapper, S.B.; Terhorst, C.; et al. Induced reactivity of intestinal CD4+ T cells with an epithelial cell lectin, Galectin-4, contributes to exacerbation of intestinal inflammation. J. Immunol. 2004, 173, 1231–1237. [Google Scholar] [CrossRef]
- Paclik, D.; Danese, S.; Berndt, U.; Wiedenmann, B.; Dignass, A.; Sturm, A. Galectin-4 Controls Intestinal Inflammation by Selective Regulation of Peripheral and Mucosal T Cell Apoptosis and Cell Cycle. PLoS ONE 2008, 3, e2629. [Google Scholar] [CrossRef]
- Aune, D.; Snekvik, I.; Schlesinger, S.; Norat, T.; Riboli, E.; Vatten, L.J. Body mass index, abdominal fatness, weight gain and the risk of psoriasis: A systematic review and dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2018, 33, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yang, Y.; Liao, Y.; Shi, Y.; Zhang, L.J. Emerging roles of adipose tissue in the pathogenesis of psoriasis and atopic dermatitis in obesity. JID Innov. 2021, 2, 100064. [Google Scholar] [CrossRef] [PubMed]
- Korduner, J.; Holm, H.; Jujic, A.; Melander, O.; Pareek, M.; Molvin, J.; Råstam, L.; Lindblad, U.; Daka, B.; Leosdottir, M.; et al. Galectin-4 levels in hospitalized versus non-hospitalized subjects with obesity: The Malmö Preventive Project. Cardiovasc. Diabetol. 2022, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Dieden, A.; Gudmundsson, P.; Korduner, J.; Molvin, J.; Zaghi, A.; Nezami, Z.; Bachus, E.; Holm, H.; Jujic, A.; Magnusson, M. Galectin-4 is associated with diabetes and obesity in a heart failure population. Sci. Rep. 2023, 13, 20285. [Google Scholar] [CrossRef]
- Molvin, J.; Pareek, M.; Jujic, A.; Melander, O.; Råstam, L.; Lindblad, U.; Daka, B.; Leósdóttir, M.; Nilsson, P.M.; Olsen, M.H.; et al. Using a Targeted Proteomics Chip to Explore Pathophysiological Pathways for Incident Diabetes- The Malmö Preventive Project. Sci. Rep. 2019, 9, 272. [Google Scholar] [CrossRef]
- Molvin, J.; Jujić, A.; Melander, O.; Pareek, M.; Råstam, L.; Lindblad, U.; Daka, B.; Leósdóttir, M.; Nilsson, P.M.; Olsen, M.H.; et al. Proteomic exploration of common pathophysiological pathways in diabetes and cardiovascular disease. ESC Heart Fail. 2020, 7, 4151–4158. [Google Scholar] [CrossRef]
- Mohanty, I.R.; Selvaa Kumar, C.; Maheswari, U. In silico analysis of novel phytocompounds targeting dipeptidyl peptidase 4 enzyme: A new link between prediabetes and its cardio-metabolic complications. Phytomed. Plus 2024, 4, 100665. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Dommasch, E.D.; Shin, D.B.; Azfar, R.S.; Kurd, S.K.; Wang, X.; Troxel, A.B. The Risk of Stroke in Patients with Psoriasis. J. Investig. Dermatol. 2009, 129, 2411–2418. [Google Scholar] [CrossRef]
- Jujic, A.; Vieira, J.P.P.; Matuskova, H.; Nilsson, P.M.; Lindblad, U.; Olsen, M.H.; Duarte, J.M.N.; Meissner, A.; Magnusson, M. Plasma Galectin-4 Levels Are Increased after Stroke in Mice and Humans. Int. J. Mol. Sci. 2023, 24, 10064. [Google Scholar] [CrossRef]
- Gisondi, P.; Barba, E.; Girolomoni, G. Non-alcoholic fatty liver disease fibrosis score in patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Ganzetti, G.; Campanati, A.; Offidani, A. Non-alcoholic fatty liver disease and psoriasis: So far, so near. World J. Hepatol. 2015, 7, 315–326. [Google Scholar] [CrossRef]
- Magdaleno-Tapial, J.; Valenzuela-Oñate, C.; Ortiz-Salvador, J.M.; Martínez-Doménech, Á.; García-Legaz-Martínez, M.; Alonso-Carpio, M.; Tamarit-García, J.J.; Diago-Madrid, M.; Sánchez-Carazo, J.L.; Pérez-Ferriols, A. Prevalence of non-alcoholic fatty liver and liver fibrosis in patients with moderate–severe psoriasis: A cross-sectional cohort study. Australas. J. Dermatol. 2020, 61, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Kłujszo, E.; Parcheta, P.; Witkowska, A.; Kręcisz, B. Non-alcoholic Fatty liver Disease in Patients with Psoriasis—Therapeutic Implications. Adv. Dermatol. Allergol. 2020, 37, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Chiba, T.; Nakahara, T.; Watanabe, K.; Sakai, S.; Noguchi, N.; Noto, M.; Ueki, S.; Kono, M. Eosinophil-derived galectin-10 upregulates matrix metalloproteinase expression in bullous pemphigoid blisters. J. Dermatol. Sci. 2023, 112, 6–14. [Google Scholar] [CrossRef]
- Kamide, Y.; Ueki, S.; Fukuchi, M.; Fujita, N.; Mori, A.; Taniguchi, M.; Sekiya, K. Serum levels of galectin-10 increase in active eosinophilic granulomatosis with polyangiitis. J. Allergy Clin. Immunol. 2022, 149, AB128. [Google Scholar] [CrossRef]
- Buschmann, C.; Unverdorben, L.; Knabl, J.; Hutter, S.; Meister, S.; Beyer, S.; Burgmann, M.; Keilmann, L.; Zati Zehni, A.; Schmoeckel, E.; et al. Galectin-10 expression in placentas of women with gestational diabetes. Curr. Issues Mol. Biol. 2023, 45, 8840–8851. [Google Scholar] [CrossRef] [PubMed]
- Unverdorben, L.; Haufe, T.; Santoso, L.; Hofmann, S.; Jeschke, U.; Hutter, S. Prototype and Chimera-Type Galectins in Placentas with Spontaneous and Recurrent Miscarriages. Int. J. Mol. Sci. 2016, 17, 644. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Controls (n = 30) | Psoriasis (n = 60) |
| Sex (M/F) | 20/10 | 39/21 NS |
| Age [years] | 47 ± 2.5 | 49 ± 2.3 NS |
| Height [cm] | 1.7 ± 0.01 | 1.7 ± 0.01 NS |
| Weight [kg] | 78 ± 3 | 84 ± 2 NS |
| BMI | 25.7 ± 0.77 | 27.6 ± 0.8 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, A.; Nowowiejska, J.; Parzych, J.; Hermanowicz, J.M.; Sieklucka, B.; Pawlak, D.; Flisiak, I. Plasma Galectin-4 and Charcot-Leyden Crystal Protein/Galectin-10 as Emerging Biomarkers of Metabolically Induced Inflammation in Patients with Psoriasis. Int. J. Mol. Sci. 2025, 26, 10339. https://doi.org/10.3390/ijms262110339
Baran A, Nowowiejska J, Parzych J, Hermanowicz JM, Sieklucka B, Pawlak D, Flisiak I. Plasma Galectin-4 and Charcot-Leyden Crystal Protein/Galectin-10 as Emerging Biomarkers of Metabolically Induced Inflammation in Patients with Psoriasis. International Journal of Molecular Sciences. 2025; 26(21):10339. https://doi.org/10.3390/ijms262110339
Chicago/Turabian StyleBaran, Anna, Julia Nowowiejska, Julia Parzych, Justyna Magdalena Hermanowicz, Beata Sieklucka, Dariusz Pawlak, and Iwona Flisiak. 2025. "Plasma Galectin-4 and Charcot-Leyden Crystal Protein/Galectin-10 as Emerging Biomarkers of Metabolically Induced Inflammation in Patients with Psoriasis" International Journal of Molecular Sciences 26, no. 21: 10339. https://doi.org/10.3390/ijms262110339
APA StyleBaran, A., Nowowiejska, J., Parzych, J., Hermanowicz, J. M., Sieklucka, B., Pawlak, D., & Flisiak, I. (2025). Plasma Galectin-4 and Charcot-Leyden Crystal Protein/Galectin-10 as Emerging Biomarkers of Metabolically Induced Inflammation in Patients with Psoriasis. International Journal of Molecular Sciences, 26(21), 10339. https://doi.org/10.3390/ijms262110339










