Switching N-Alkylation Regioselectivity of Trifluoromethylated Pyrazoles Guided by Functional Group Tuning
Abstract
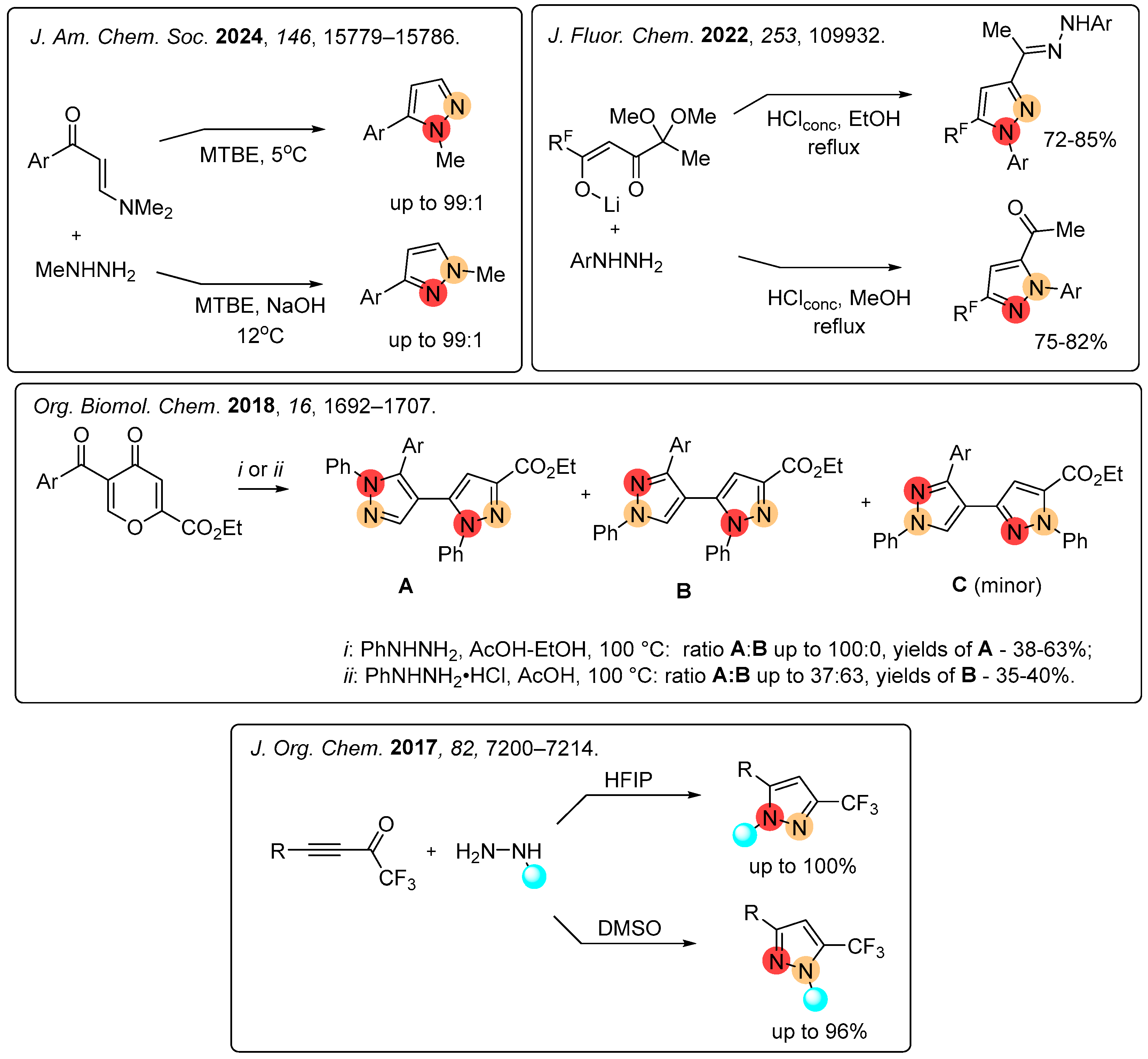
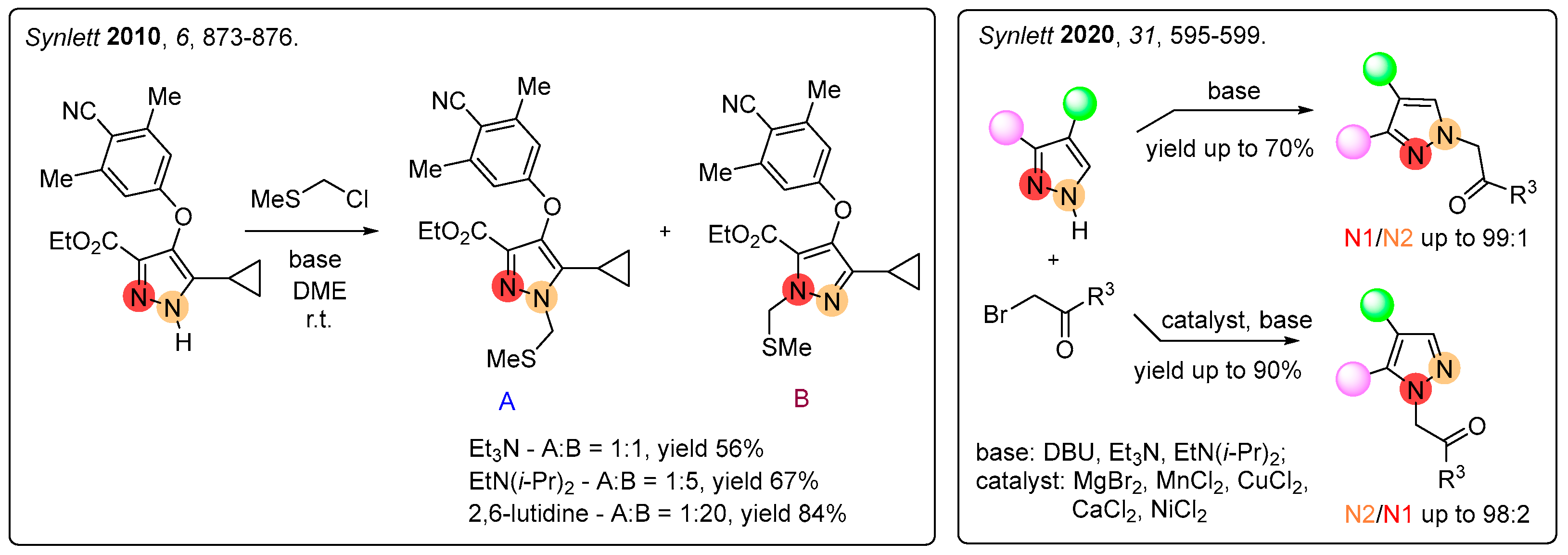
1. Introduction
2. Results
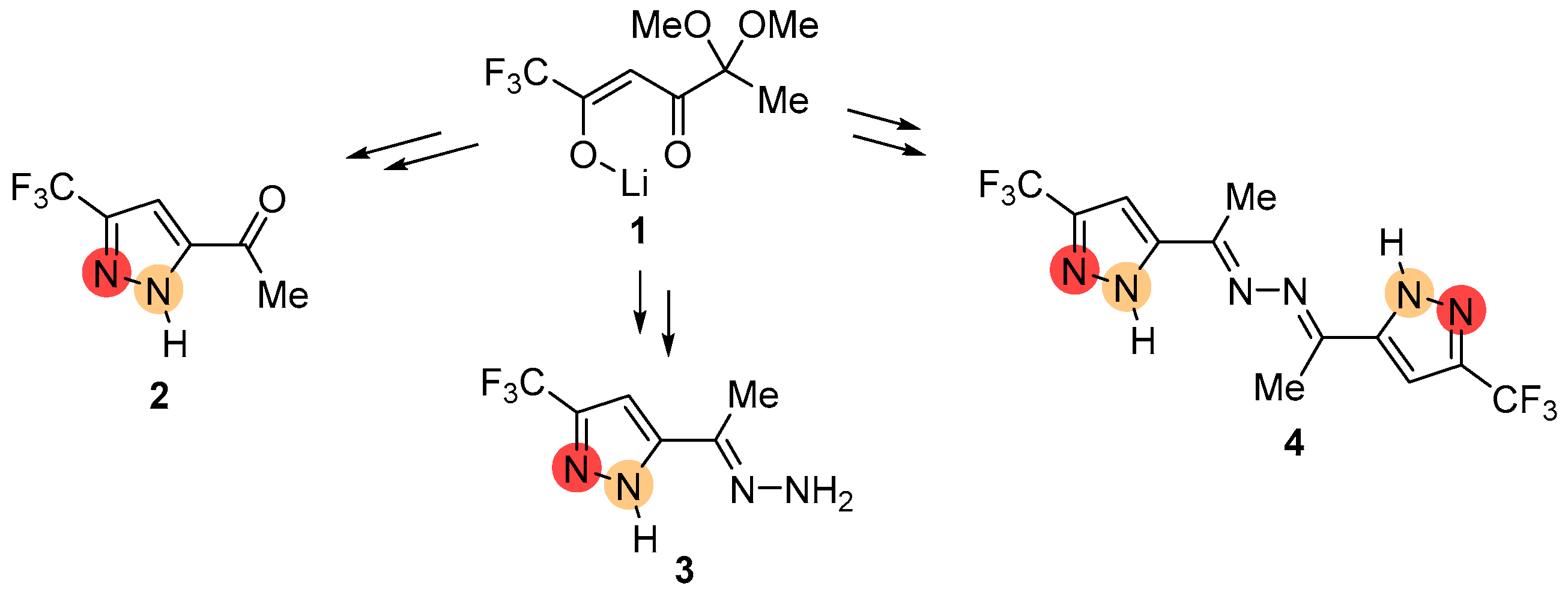
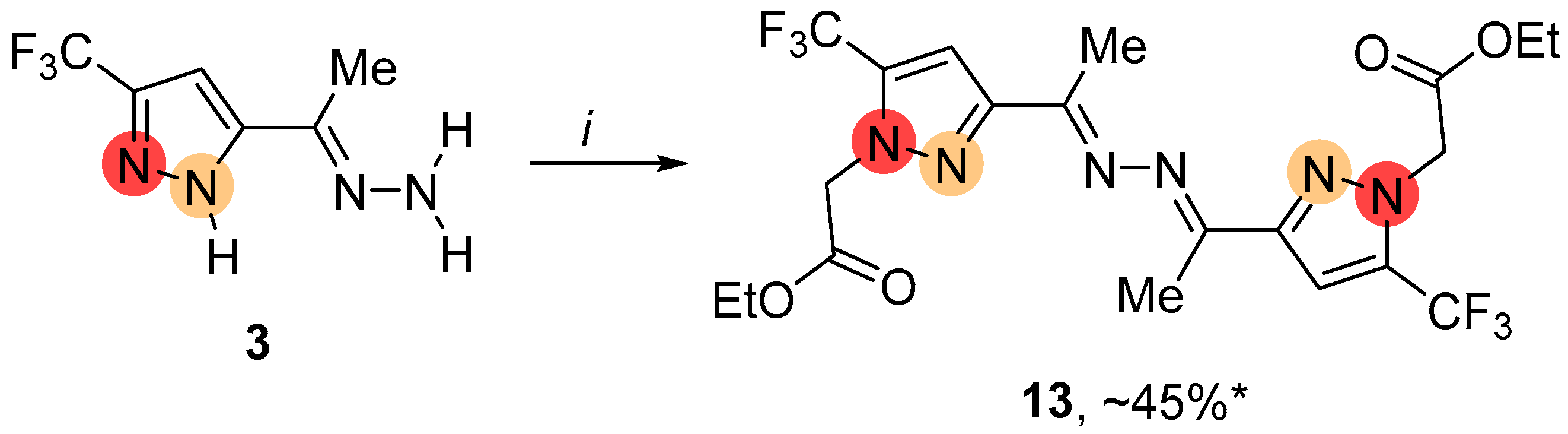
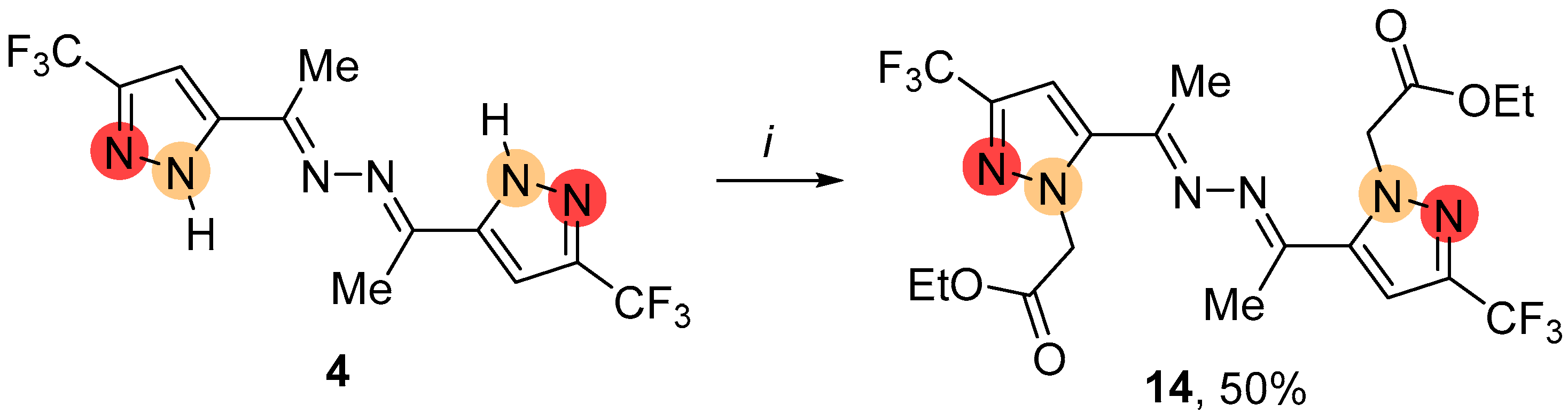
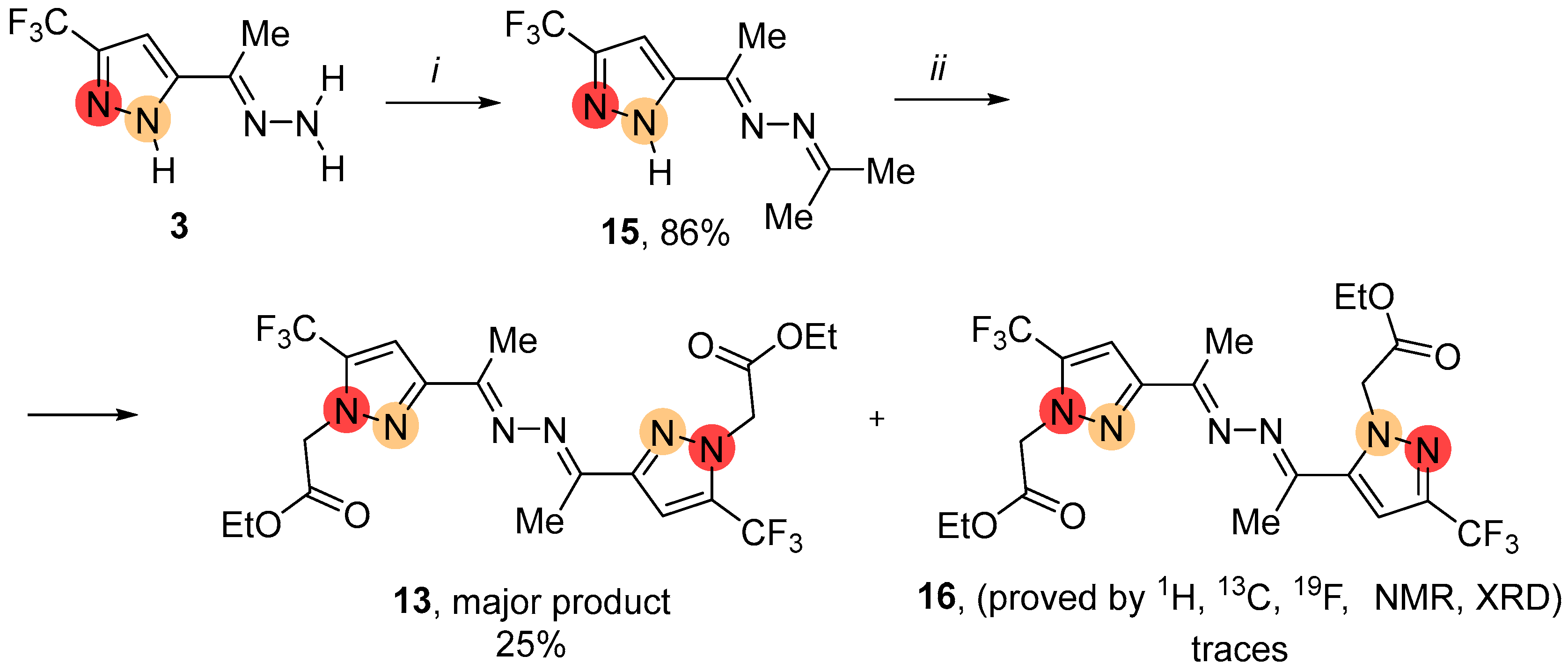
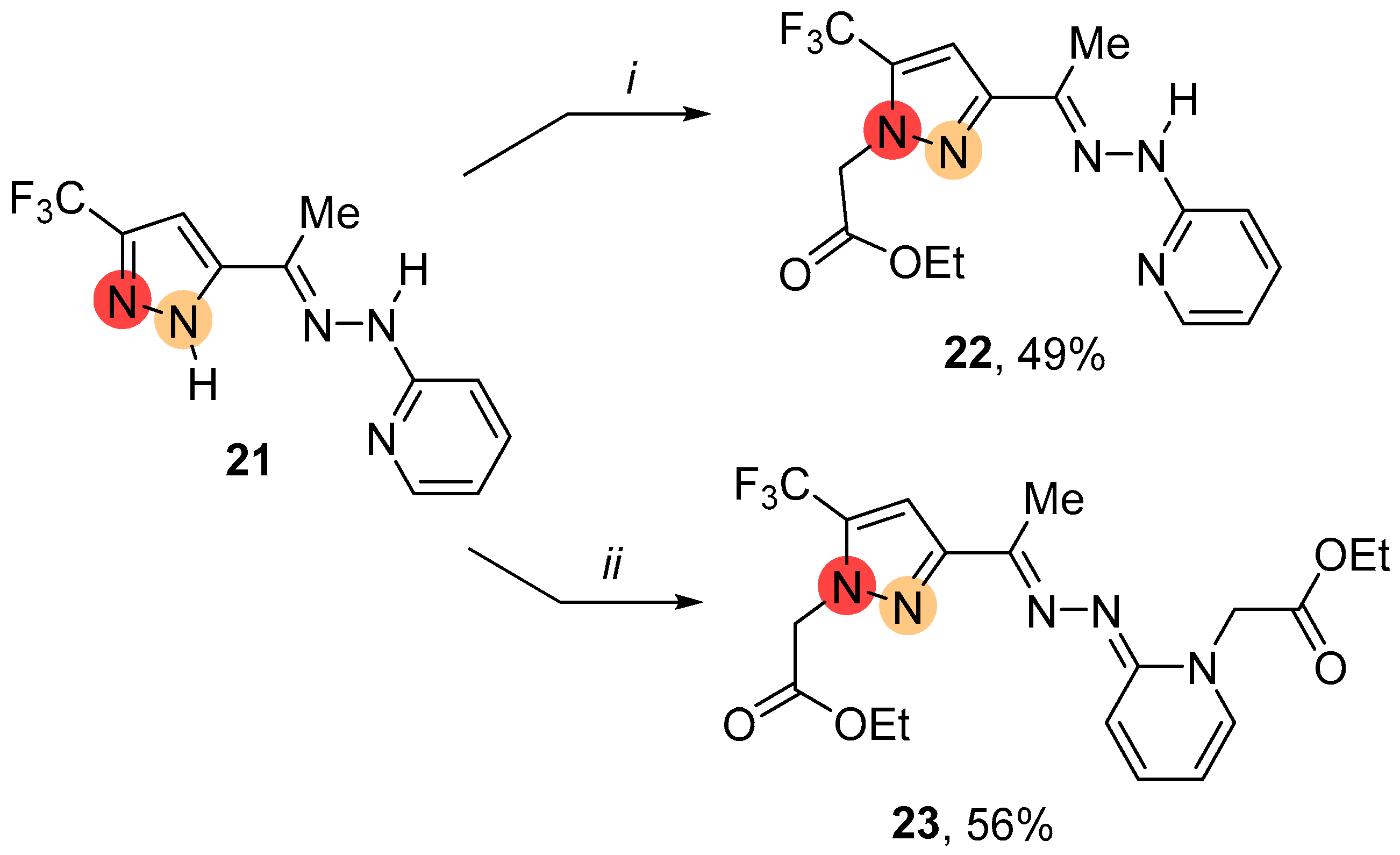
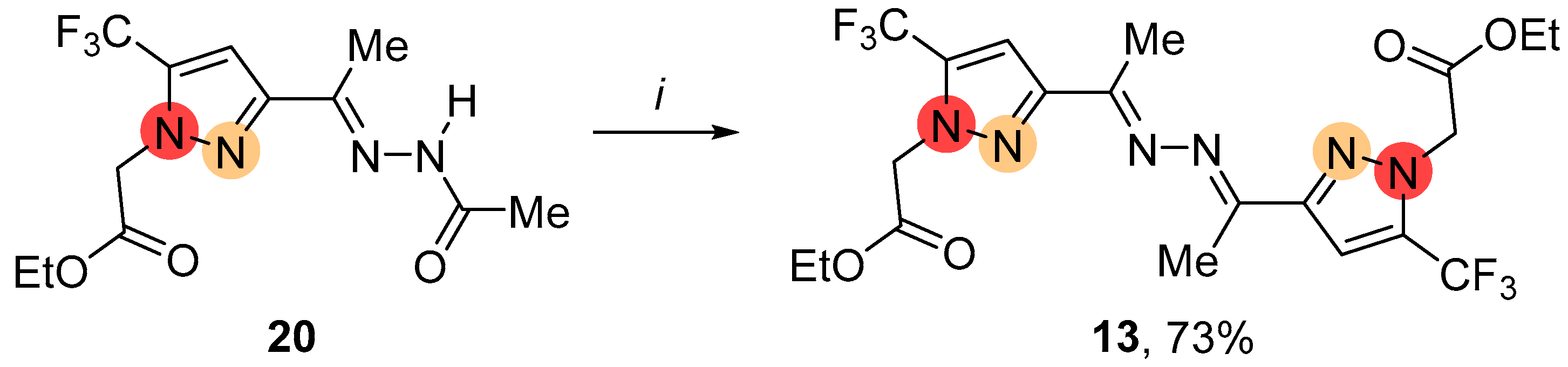
2.1. Regioselective Synthesis of Pyrazoles
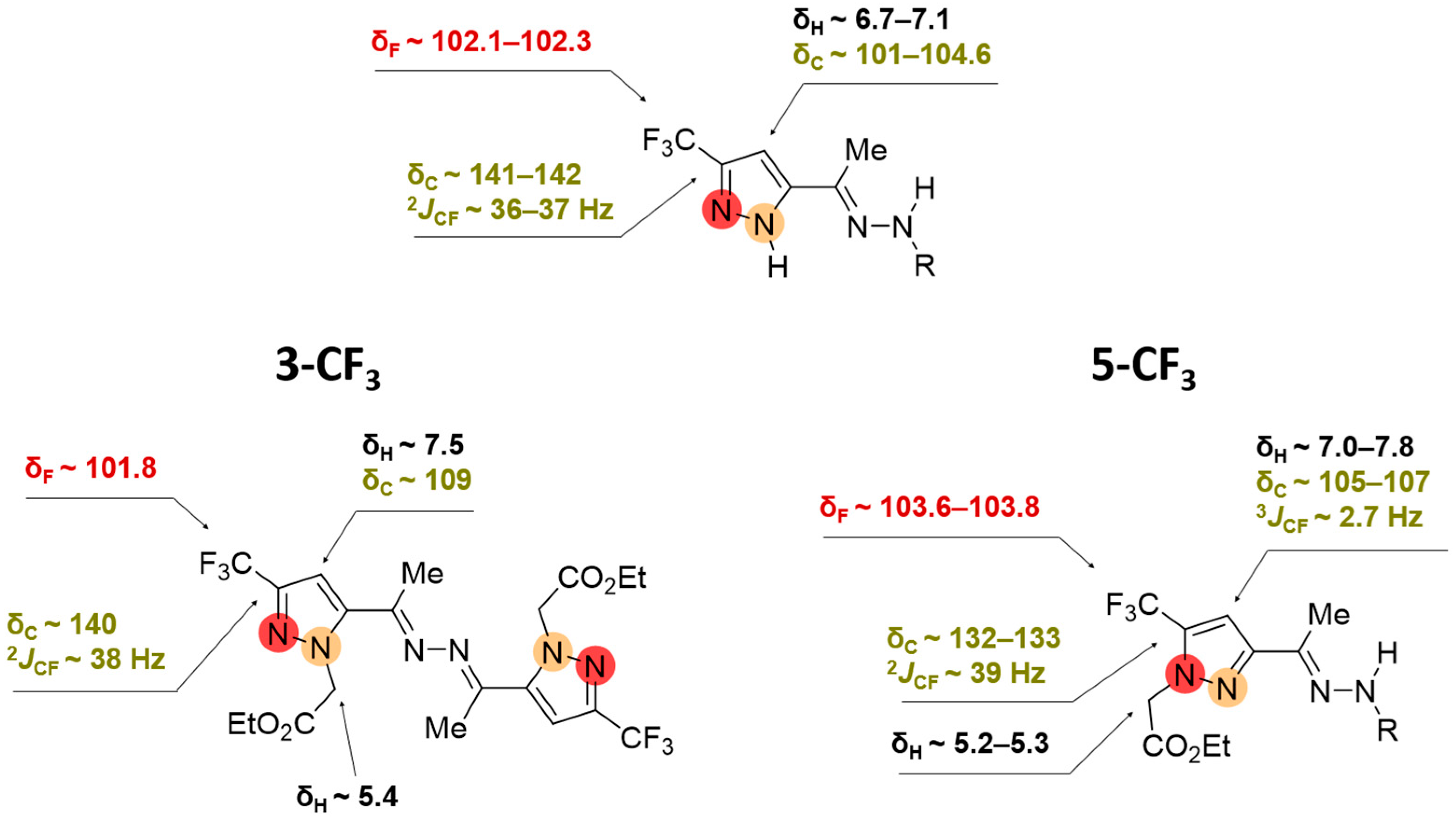
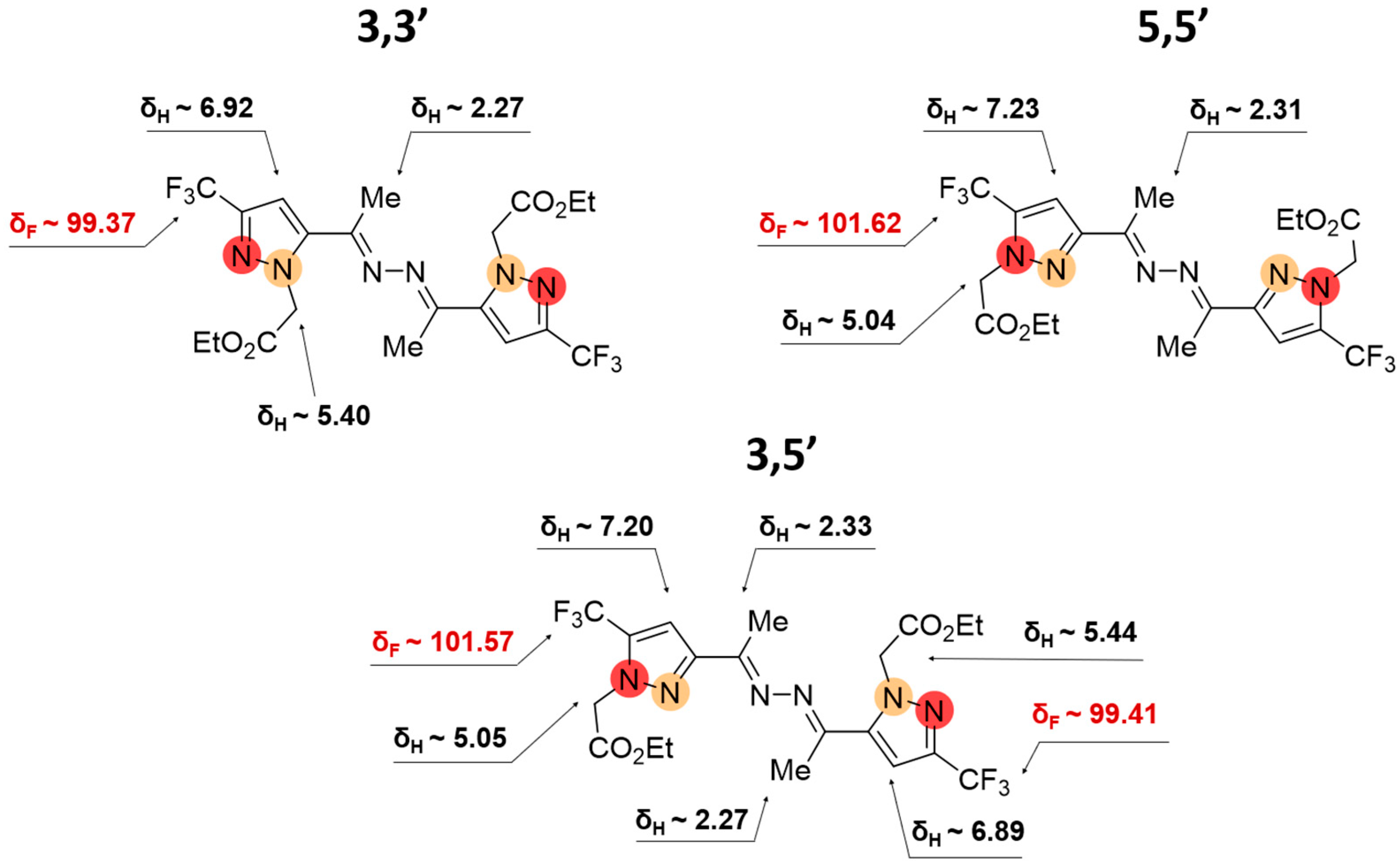
2.2. Regioisomeric Structure Determination
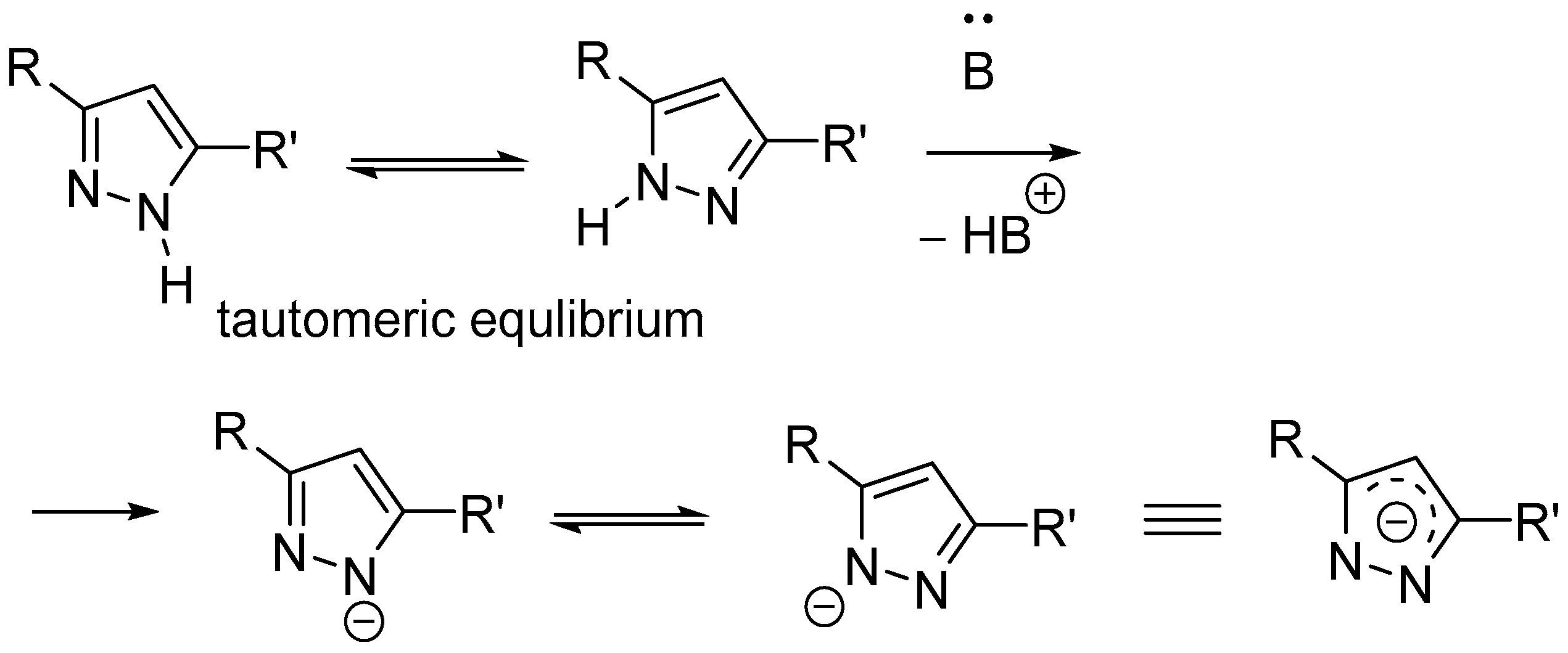
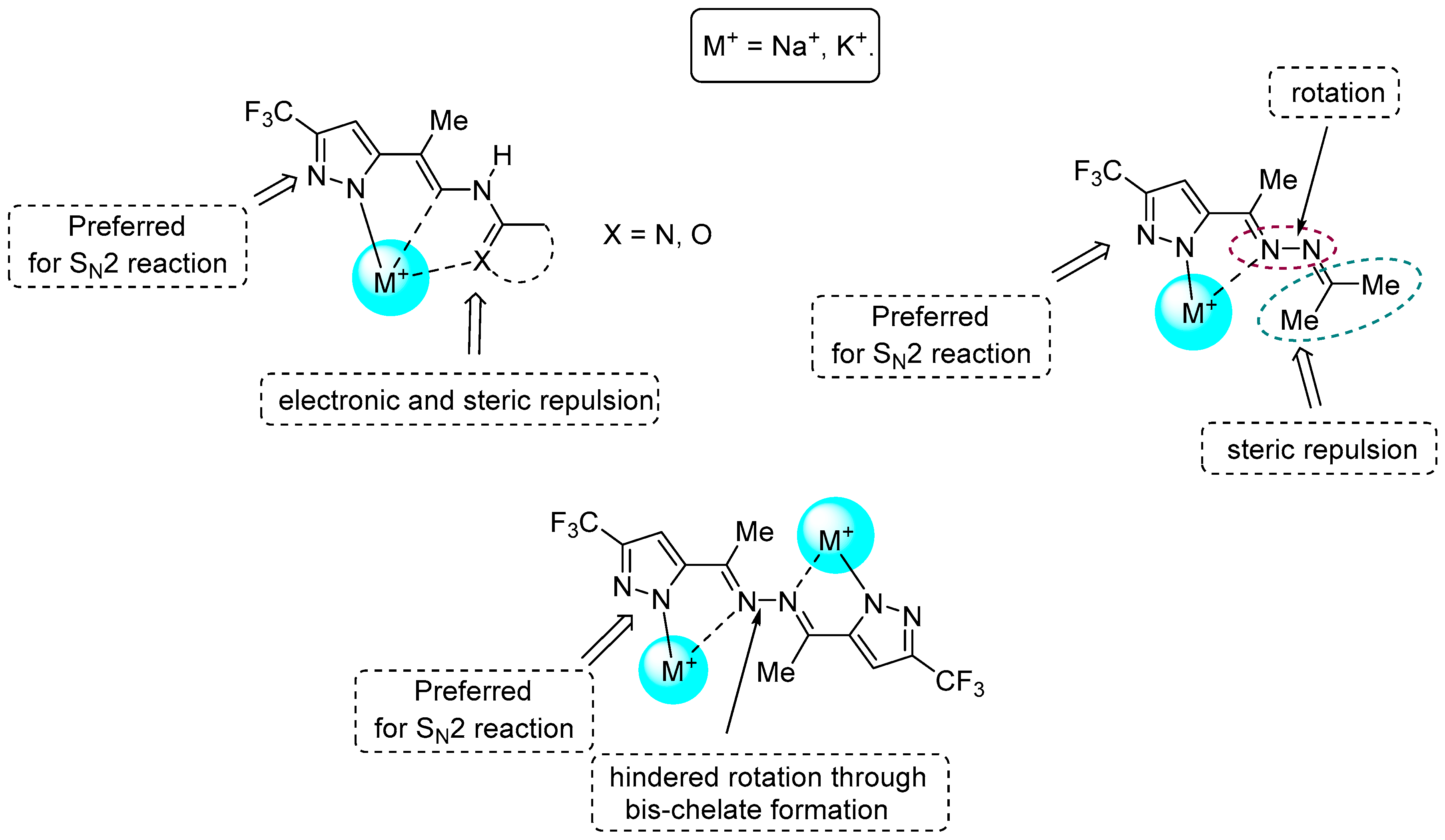
3. Discussion
4. Materials and Methods
4.1. General Chemistry
4.2. Reagents and Solvents
4.3. Experimental Procedures and Characterization Data of the Synthesized Compounds
4.3.1. Synthesis of NH-Pyrazoles 15, 17, 18
4.3.2. Alkylation of NH-Ketazine 4
4.3.3. General Procedure for the Alkylation of NH-Pyrazoles 2, 3, 15, 17, 18 Using K2CO3
4.3.4. General Procedure for the Alkylation of NH-Pyrazole 21 Using NaH
4.3.5. Transamination Reaction
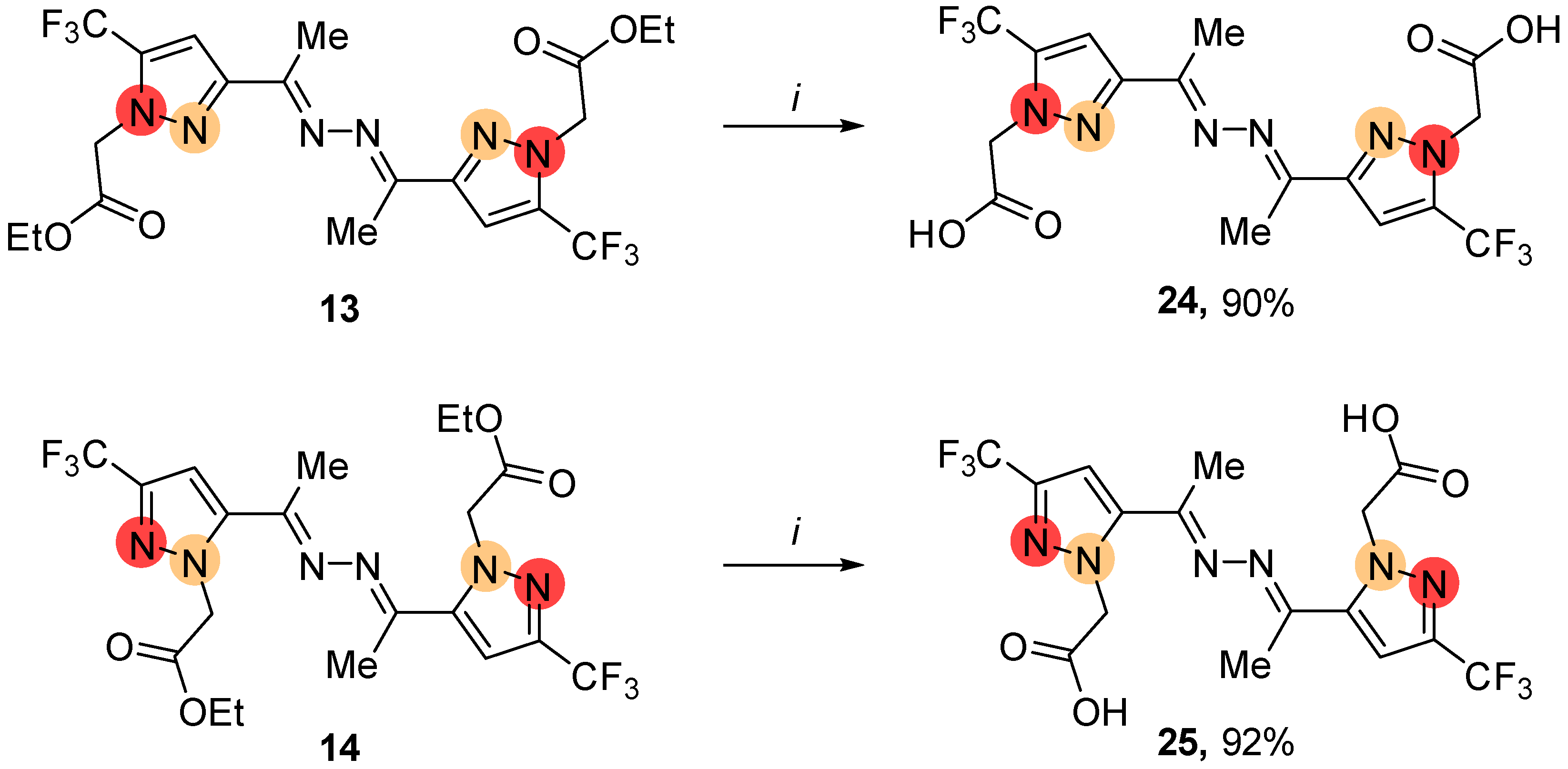
4.3.6. General Procedure for the Hydrolysis of Ester Derivatives 13, 14
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehman, M.U.; He, F.; Shu, X.; Guo, J.; Liu, Z.; Cao, S.; Long, S. Antibacterial and antifungal pyrazoles based on different construction strategies. Eur. J. Med. Chem. 2025, 282, 117081. [Google Scholar] [CrossRef]
- Botella, G.M.; Salituro, F.G.; Harrison, B.L.; Beresis, R.T.; Bai, Z.; Blanco, M.-J.; Belfort, G.M.; Dai, J.; Loya, C.M.; Ackley, M.A.; et al. Neuroactive Steroids. 2. 3α-Hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1’-yl)-19-nor-5β-pregnan-20-one (SAGE-217): A Clinical Next Generation Neuroactive Steroid Positive Allosteric Modulator of the (γ-Aminobutyric Acid)A Receptor. J. Med. Chem. 2017, 60, 7810–7819. [Google Scholar] [CrossRef]
- Zhao, X.; Verma, R.; Sridhara, M.B.; Kumar, K.S.S. Fluorinated azoles as effective weapons in fight against methicillin-resistance staphylococcus aureus (MRSA) and its SAR studies. Bioorg. Chem. 2024, 143, 106975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, C.; Zhang, N.; Fan, R.; Ye, Y.; Xu, J. Recent Advances in the Development of Pyrazole Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 12724. [Google Scholar] [CrossRef]
- Lusardi, M.; Spallarossa, A.; Brullo, C. Amino-Pyrazoles in Medicinal Chemistry: A Review. Int. J. Mol. Sci. 2023, 24, 7834. [Google Scholar] [CrossRef]
- Vahoraa, M.S.; Boruaha, J.J.; Das, S.P. Synthesis and Pharmacological Activities of Pyrazole and Oxadiazole Derivatives: A Review. Russ. J. Org. Chem. 2023, 59, 846–869. [Google Scholar] [CrossRef]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives. Biomedicines 2022, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef]
- Faria, J.V.; Vegi, P.F.; Miguita, A.G.C.; Dos Santos, M.S.; Boechat, N.; Bernardino, A.M.R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 25, 5891–5903. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman, S. Review: Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.; Faouzi, M.E.A. Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorg. Chem. 2020, 97, 103470. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Alam, M.M.; Verma, G.; Akhtar, W.; Akhter, M.; Shaquiquzzaman, M. The therapeutic voyage of pyrazole and its analogs: A review. Eur. J. Med. Chem. 2016, 120, 170–201. [Google Scholar] [CrossRef]
- Verma, R.; Verma, S.K.; Rakesh, K.P.; Girish, Y.R.; Ashrafizadeh, M.; Sharath Kumar, K.S.; Rangappa, K.S. Pyrazole-based analogs as potential antibacterial agents against methicillin-resistance staphylococcus aureus (MRSA) and its SAR elucidation. Eur. J. Med. Chem. 2021, 212, 113134. [Google Scholar] [CrossRef]
- Mykhailiuk, P.K. Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev. 2021, 121, 1670–1715. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Edilova, Y.O.; Burgart, Y.V.; Saloutin, V.I. Fluorinated 1,2,4-triketone analogs: New prospects for heterocyclic and coordination chemistry. Russ. Chem. Bull. 2022, 71, 1321–1341. [Google Scholar] [CrossRef]
- Clemett, D.; Goa, K.L. Celecoxib. Drugs 2000, 59, 957–980. [Google Scholar] [CrossRef]
- Abdelhaleem, E.F.; Kassab, A.E.; El-Nassan, H.B.; Khalil, O.M. Recent advances in the development of celecoxib analogs as anticancer agents: A review. Arch. Pharm. 2022, 355, e2200326. [Google Scholar] [CrossRef]
- Link, J.O.; Rhee, M.S.; Tse, W.C.; Zheng, J.; Somoza, J.R.; Rowe, W.; Begley, R.; Chiu, A.; Mulato, A.; Hansen, D.; et al. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature 2020, 584, 614–618. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Slepukhin, P.A.; Burgart, Y.V.; Malysheva, N.N.; Kozitsina, A.N.; Ivanova, A.V.; Bogomyakov, A.S.; Saloutin, V.I. Dinuclear copper(ii) complex with novel N,N’,N”,O-tetradentate Schiff base ligand containing trifluoromethylpyrazole and hydrazone moieties. Mendeleev Commun. 2018, 28, 202–204. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Onoprienko, A.Y.; Slepukhin, P.A.; Burgart, Y.V.; Saloutin, V.I. Synthesis and tuberculostatic activity of functionalized pyrazoles derived from (trifluoromethyl)pyrazole containing a hydrazone group. Chem. Heterocycl. Compd. 2017, 53, 1324–1329. [Google Scholar] [CrossRef]
- Liu, H.-N.; Zhu, Y.; Chi, Y.; Sun, F.-F.; Shan, L.-S.; Wang, Y.-T.; Dai, B. Synthetic approaches and application of representative clinically approved fluorine-enriched anti-cancer medications. Eur. J. Med. Chem. 2024, 276, 116722. [Google Scholar] [CrossRef]
- Du, T.; Lu, S.; Zhu, Z.; Zhu, M.; Zhang, Y.; Zhang, J.; Chen, J. Pyrazole derivatives: Recent advances in discovery and development of pesticides. Chin. Chem. Lett. 2025, 36, 110912. [Google Scholar] [CrossRef]
- Belousov, A.; Drozdov, A.A.; Taydakov, I.V.; Marchetti, F.; Pettinari, R.; Pettinari, C. Lanthanide azolecarboxylate compounds: Structure, luminescent properties and applications. Coord. Chem. Rev. 2021, 445, 214084. [Google Scholar] [CrossRef]
- Doidge, E.D.; Roebuck, J.W.; Healy, M.R.; Tasker, P.A. Phenolic pyrazoles: Versatile polynucleating ligands. Coord. Chem. Rev. 2015, 288, 98. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, R.-P.; Gong, X.; Xu, X.; Peng, X.; Tang, S. Regioselective pyrazole synthesis via base-mediated [3+2] cycloaddition of 2-alkynyl-1,3-dithianes and sydnones. J. Org. Chem. 2025, 90, 3769–3778. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Wang, Y.; Hua, X.; Zheng, C.; Shi, Q.; Tan, Z.; Zheng, L.; Guo, W. Photocatalytic One-Pot Three-Component Reaction for the Regioselective Synthesis of Bromo-Substituted Pyrazoles. J. Org. Chem. 2024, 89, 16809–16827. [Google Scholar] [CrossRef] [PubMed]
- Enache, B.C.; Hanganu, A.; Tablet, C.; Anghel, C.C.; Popescu, C.C.; Paun, A.; Hădade, N.D.; Mădălan, A.M.; Matache, M. Exploring arylazo-3,5-bis(trifluoromethyl)pyrazole switches. ACS Omega 2022, 7, 39122–39135. [Google Scholar] [CrossRef] [PubMed]
- Edilova, Y.O.; Osipova, E.A.; Slepukhin, P.A.; Saloutin, V.I.; Bazhin, D.N. Exploring three avenues: Chemo- and regioselective transformations of 1,2,4-triketone analogs into pyrazoles and pyridazinones. Int. J. Mol. Sci. 2023, 24, 14234. [Google Scholar] [CrossRef] [PubMed]
- Kudyakova, Y.S.; Onoprienko, A.Y.; Slepukhin, P.A.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Fluorine-containing furan-3(2H)-ones in reactions with binucleophiles: CF3 vs C2F5. Chem. Heterocycl. Comp. 2019, 55, 517–522. [Google Scholar] [CrossRef]
- Shakirova, O.G.; Morozova, T.D.; Kudyakova, Y.S.; Bazhin, D.N.; Kuratieva, N.V.; Klyushova, L.S.; Lavrov, A.N.; Lavrenova, L.G. Synthesis, structure, and properties of a copper(II) binuclear complex based on trifluoromethyl containing bis(pyrazolyl)hydrazone. Int. J. Mol. Sci. 2024, 25, 9414. [Google Scholar] [CrossRef]
- Kuleshova, O.; Khilya, O.; Volovenko, Y.; Mallet-Ladeira, S.; Dyakonenko, V.; Gras, E. Expedited route to fully substituted amino-pyrazole building blocks and their further transformations. ACS Omega 2017, 2, 8911–8927. [Google Scholar] [CrossRef]
- Huang, A.; Wo, K.; Yeun, S.; Lee, C.; Kneitschel, N.; Chang, J.; Zhu, K.; Mello, T.; Bancroft, L.; Norman, N.J.; et al. Regioselective Synthesis, NMR, and Crystallographic Analysis of N1-Substituted Pyrazoles. J. Org. Chem. 2017, 82, 8864–8872. [Google Scholar] [CrossRef]
- Dalton, D.M.; Walroth, R.C.; Rouget-Virbel, C.; Mack, K.A.; Toste, F.D. Utopia Point Bayesian Optimization Finds Condition-Dependent Selectivity for N-Methyl Pyrazole Condensation. J. Am. Chem. Soc. 2024, 146, 15779–15786. [Google Scholar] [CrossRef]
- Edilova, Y.O.; Kudyakova, Y.S.; Kiskin, M.A.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Expanding 1,2,4-triketone toolbox for use as fluorinated building blocks in the synthesis of pyrazoles, pyridazinones and β-diketohydrazones. J. Fluor. Chem. 2022, 253, 109932. [Google Scholar] [CrossRef]
- Obydennov, D.L.; Khammatova, L.R.; Eltsov, O.S.; Sosnovskikh, V.Y. A chemo- and regiocontrolled approach to bipyrazoles and pyridones via the reaction of ethyl 5-acyl-4-pyrone-2-carboxylates with hydrazines. Org. Biomol. Chem. 2018, 16, 1692–1707. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M.; Rulev, A.Y.; Romanov, A.R.; Kondrashov, E.V.; Ushakov, I.A.; Chertkov, V.A.; Nenajdenko, V.G. Selective, metal-free approach to 3- or 5-CF3-pyrazoles: Solvent switchable reaction of CF3-ynones with hydrazines. J. Org. Chem. 2017, 82, 7200–7214. [Google Scholar] [CrossRef]
- Xu, D.; Frank, L.; Nguyen, T.; Stumpf, A.; Russell, D.; Angelaud, R.; Gosselin, F. Magnesium-catalyzed N2-regioselective alkylation of 3-substituted pyrazoles. Synlett 2020, 31, 595–599. [Google Scholar] [CrossRef]
- Bradley, P.A.; de Koning, P.D.; Gibson, K.R.; Lecouturier, Y.C.; MacKenny, M.C.; Morao, I.; Poinsard, C.; Underwood, T.J. Synthetic studies on a nonsteroidal progesterone metabolite: Regioselective N-alkylation of an activated pyrazole. Synlett 2010, 0873–0876. [Google Scholar] [CrossRef]
- Bartholomew, G.L.; Carpaneto, F.; Sarpong, R. Skeletal editing of pyrimidines to pyrazoles by formal carbon deletion. J. Am. Chem. Soc. 2022, 144, 22309–22315. [Google Scholar] [CrossRef]
- Ali, Y.; Chen, L.; Kelly, P.Q.; Bracken, A.J.; Kelly, C.B.; Levin, M.D. Strategic atom replacement enables regiocontrol in pyrazole alkylation. Nature 2025, 641, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Han, H.; Jeong, J.; Kim, D.; Hong, S. Transformation of pyridines into 2D and 3D fused bicyclic heterocycles. J. Am. Chem. Soc. 2025, 147, 21143–21152. [Google Scholar] [CrossRef]
- Rubanov, Z.M.; Koltun, D.S.; Levin, V.V.; Dilman, A.D. Exploring the Radical Addition to Sydnones: A Gateway to Perfluoroalkyl-Substituted Pyrazoles. Org. Lett. 2025, 27, 10241–10245. [Google Scholar] [CrossRef] [PubMed]
- You, G.; Wang, K.; Wang, X.; Wang, G.; Sun, J.; Duan, G.; Xia, C. Visible-light-mediated nickel(II)-catalyzed C–N cross-coupling in water: Green and regioselective access for the synthesis of pyrazole-containing compounds. Org. Lett. 2018, 20, 4005–4009. [Google Scholar] [CrossRef]
- Dwari, S.; Nath, P.P.; Jana, C.K. Ring opening of pyrrolinium ions enabled regioselective synthesis of 4-alkyl N-arylpyrazoles. J. Org. Chem. 2022, 87, 11947–11957. [Google Scholar] [CrossRef]
- Pang, M.; Ramazani, A.; Zhang, Z.; Zhang, G. Reagent-assisted regio-divergent cyclization synthesis of pyrazole. Org. Biomol. Chem. 2025, 23, 2812–2817. [Google Scholar] [CrossRef]
- Fanourakis, A.; Norman, N.J.; Bao, S.T.; Curts, L.; Hui, T.; Zheng, S.-L.; Shou, T.; Zeghibe, A.; Burdick, I.; Fuehrer, H.; et al. Highly Selective N-Alkylation of Pyrazoles: Crystal Structure Evidence for Attractive Interactions. J. Org. Chem. 2022, 87, 10018–10025. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Shao, Y.; Sun, J.; Tang, S. Iridium-catalyzed regio- and enantioselective N-allylation of pyrazoles with dienyl/monoallylic alcohols. Org. Lett. 2024, 26, 3966–3971. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Fang, X.; Yang, Z.; Zhu, C.; Jiang, H. Regioselective Synthesis of 5-Trifluoromethylpyrazoles by [3 + 2] Cycloaddition of Nitrile Imines and 2-Bromo-3,3,3-trifluoropropene. J. Org. Chem. 2021, 86, 2810–2819. [Google Scholar] [CrossRef] [PubMed]
- Bazhin, D.N.; Chizhov, D.L.; Röschenthaler, G.-V.; Kudyakova, Y.S.; Burgart, Y.V.; Slepukhin, P.A.; Saloutin, V.I.; Charushin, V.N. A concise approach to CF3-containing furan-3-ones, (bis)pyrazoles from novel fluorinated building blocks based on 2,3-butanedione. Tetrahedron Lett. 2014, 55, 5714. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Röschenthaler, G.-V.; Burgart, Y.V.; Slepukhin, P.A.; Isenov, M.L.; Saloutin, V.I.; Charushin, V.N. A Convenient Approach to CF3-Containing N-Heterocycles Based on 2-Methoxy-2-methyl-5-(trifluoromethyl)furan-3(2H)-one. Eur. J. Org. Chem. 2015, 2015, 5236–5245. [Google Scholar] [CrossRef]
- Edilova, Y.O.; Kudyakova, Y.S.; Valova, M.S.; Loseva, N.V.; Slepukhin, P.A.; Saloutin, V.I.; Bazhin, D.N. Fluorine-Containing Polydentate Bis(heterocycles) Based on Di- and Triketone Analogs in the Synthesis of Zinc(II) Complexes. Russ. J. Coord. Chem. 2024, 50, 855. [Google Scholar] [CrossRef]
- Khudina, O.G.; Bazhin, D.N.; Makhaeva, G.F.; Burgart, Y.V.; Kovaleva, N.V.; Boltneva, N.P.; Rudakova, E.V.; Skornyakova, T.S.; Saloutin, V.I. Features of Conjugation of Ipidacrine Chloroalkylamides with 5-Acetyl-3-(trifluoromethyl)pyrazole and Its Derivatives. Study of Biological Activity. Russ. J. Org. Chem. 2025, 61, 1112. [Google Scholar] [CrossRef]
- Grover, J.; Dutta, B.; Ghosh, D.; Shee, P.K.; Maiti, S.; Werz, D.B.; Maiti, D. Harnessing C–H acetoxylation: A gateway to oxygen-enriched organic frameworks. Chem. Sci. 2025, 16, 10141–10158. [Google Scholar] [CrossRef]
- Ivanova, A.E.; Burgart, Y.V.; Saloutin, V.I.; Slepukhin, P.A.; Borisevich, S.S.; Khursan, S.L. Ambident polyfluoroalkyl-substituted pyrazoles in the methylation reactions. J. Fluor. Chem. 2017, 195, 47–56. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edilova, Y.O.; Kudyakova, Y.S.; Osipova, E.A.; Slepukhin, P.A.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Switching N-Alkylation Regioselectivity of Trifluoromethylated Pyrazoles Guided by Functional Group Tuning. Int. J. Mol. Sci. 2025, 26, 10335. https://doi.org/10.3390/ijms262110335
Edilova YO, Kudyakova YS, Osipova EA, Slepukhin PA, Burgart YV, Saloutin VI, Bazhin DN. Switching N-Alkylation Regioselectivity of Trifluoromethylated Pyrazoles Guided by Functional Group Tuning. International Journal of Molecular Sciences. 2025; 26(21):10335. https://doi.org/10.3390/ijms262110335
Chicago/Turabian StyleEdilova, Yulia O., Yulia S. Kudyakova, Ekaterina A. Osipova, Pavel A. Slepukhin, Yanina V. Burgart, Victor I. Saloutin, and Denis N. Bazhin. 2025. "Switching N-Alkylation Regioselectivity of Trifluoromethylated Pyrazoles Guided by Functional Group Tuning" International Journal of Molecular Sciences 26, no. 21: 10335. https://doi.org/10.3390/ijms262110335
APA StyleEdilova, Y. O., Kudyakova, Y. S., Osipova, E. A., Slepukhin, P. A., Burgart, Y. V., Saloutin, V. I., & Bazhin, D. N. (2025). Switching N-Alkylation Regioselectivity of Trifluoromethylated Pyrazoles Guided by Functional Group Tuning. International Journal of Molecular Sciences, 26(21), 10335. https://doi.org/10.3390/ijms262110335