Comparative Analysis of Cholinergic Machinery in Carcinomas: Discovery of Membrane-Tethered ChAT as Evidence for Surface-Based ACh Synthesis in Neuroblastoma Cells
Abstract
1. Introduction
2. Results
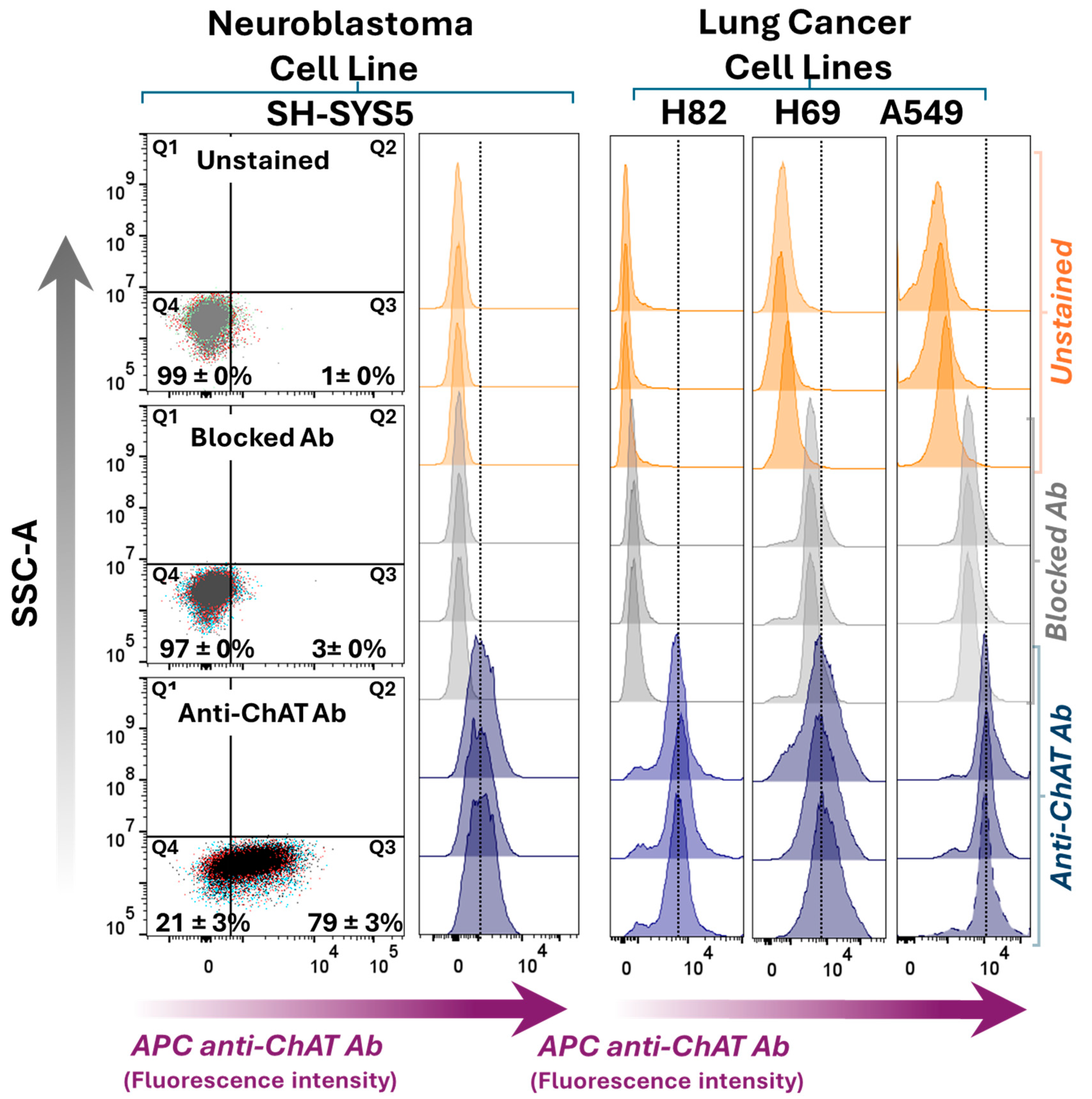
2.1. Differential ChAT Localization in a Neuroblastoma Cell Line Compared with a Lung Adenocarcinoma and Two Small Cell Lung Carcinoma Cells Lines
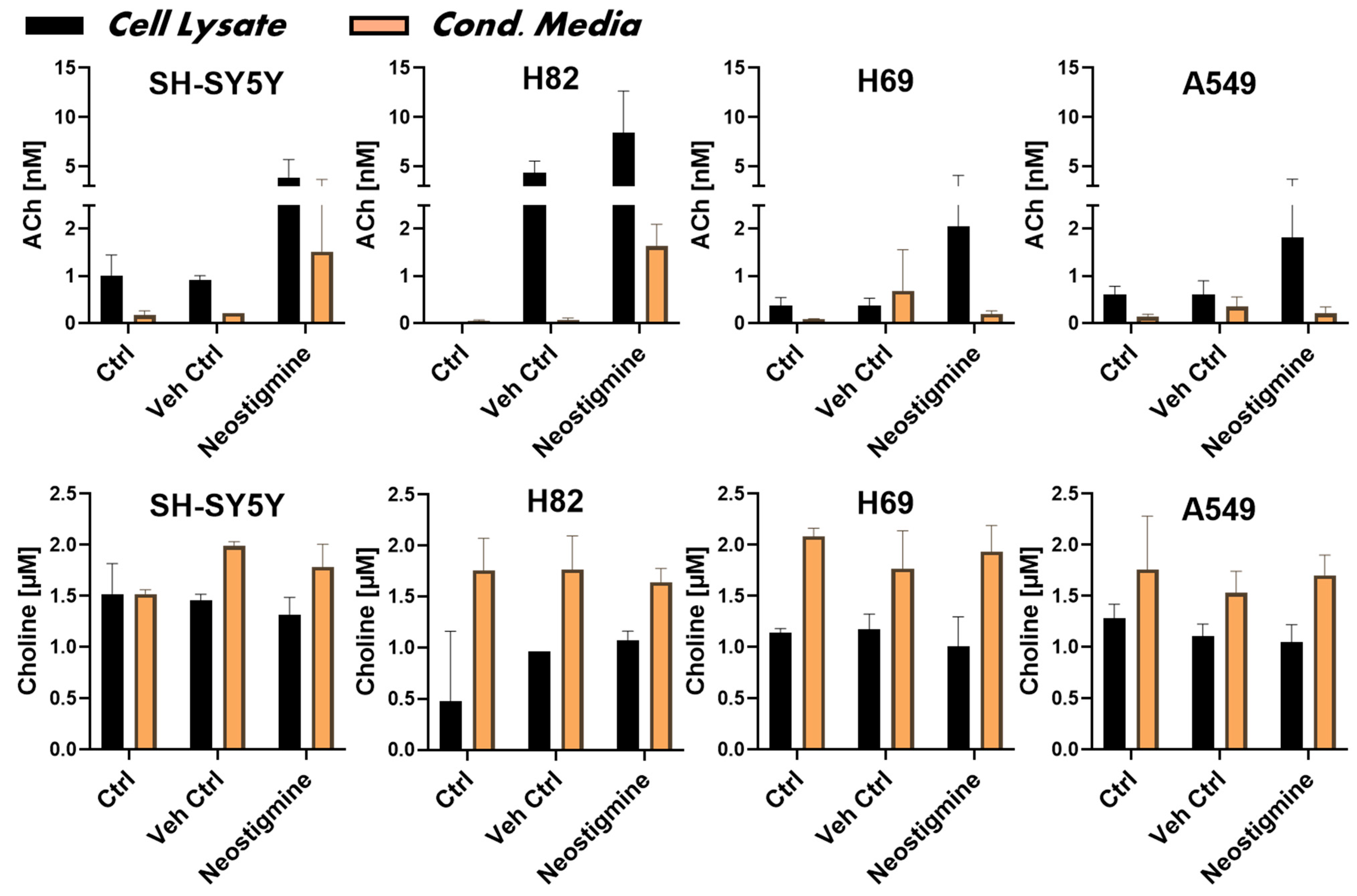
2.2. ChAT Is Functionally Intact in Both Neuroblastoma and Lung Cancer Cells
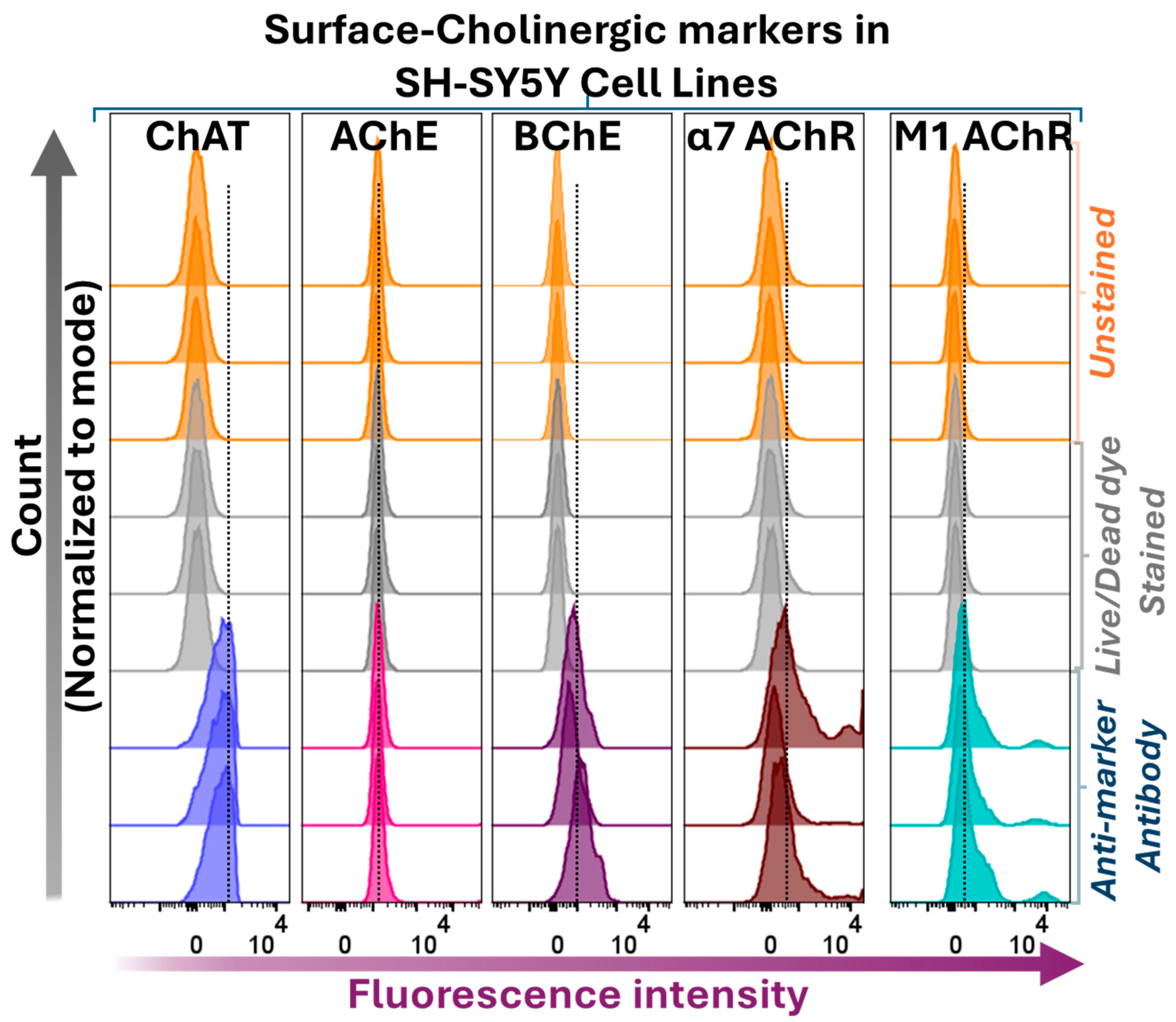
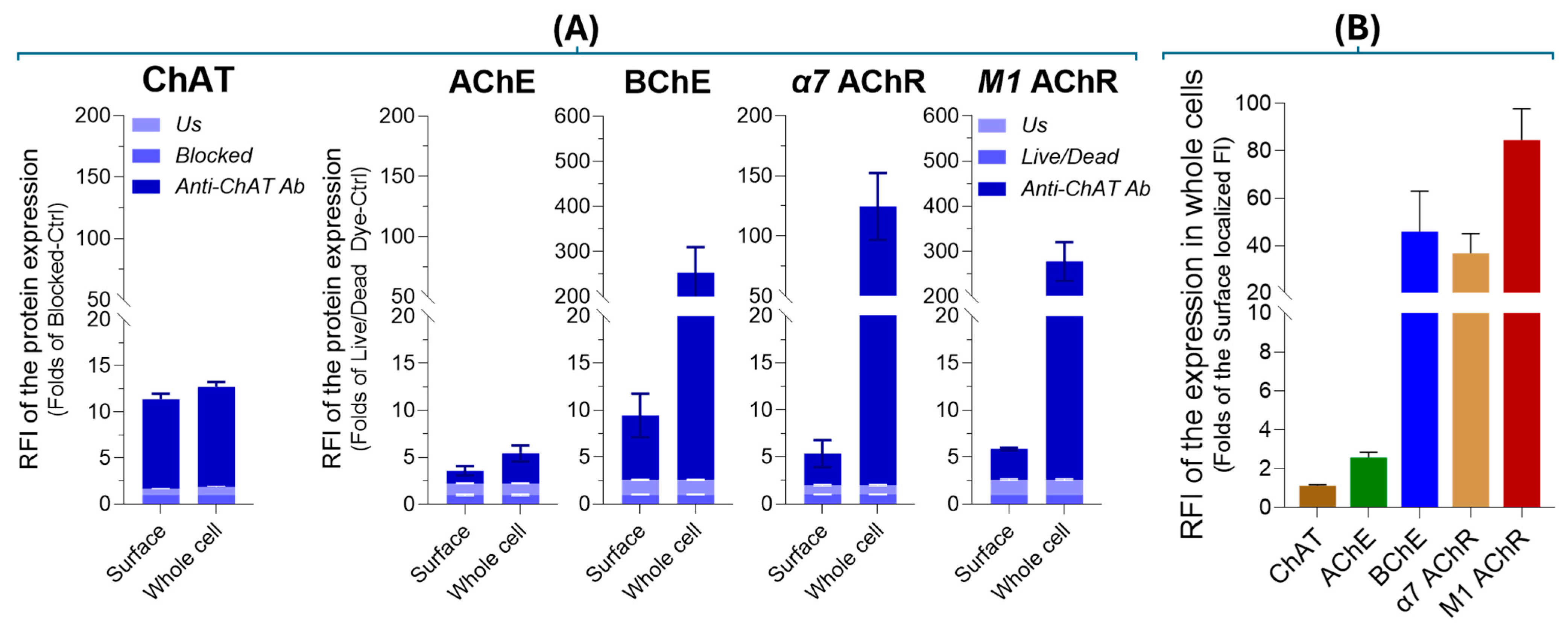
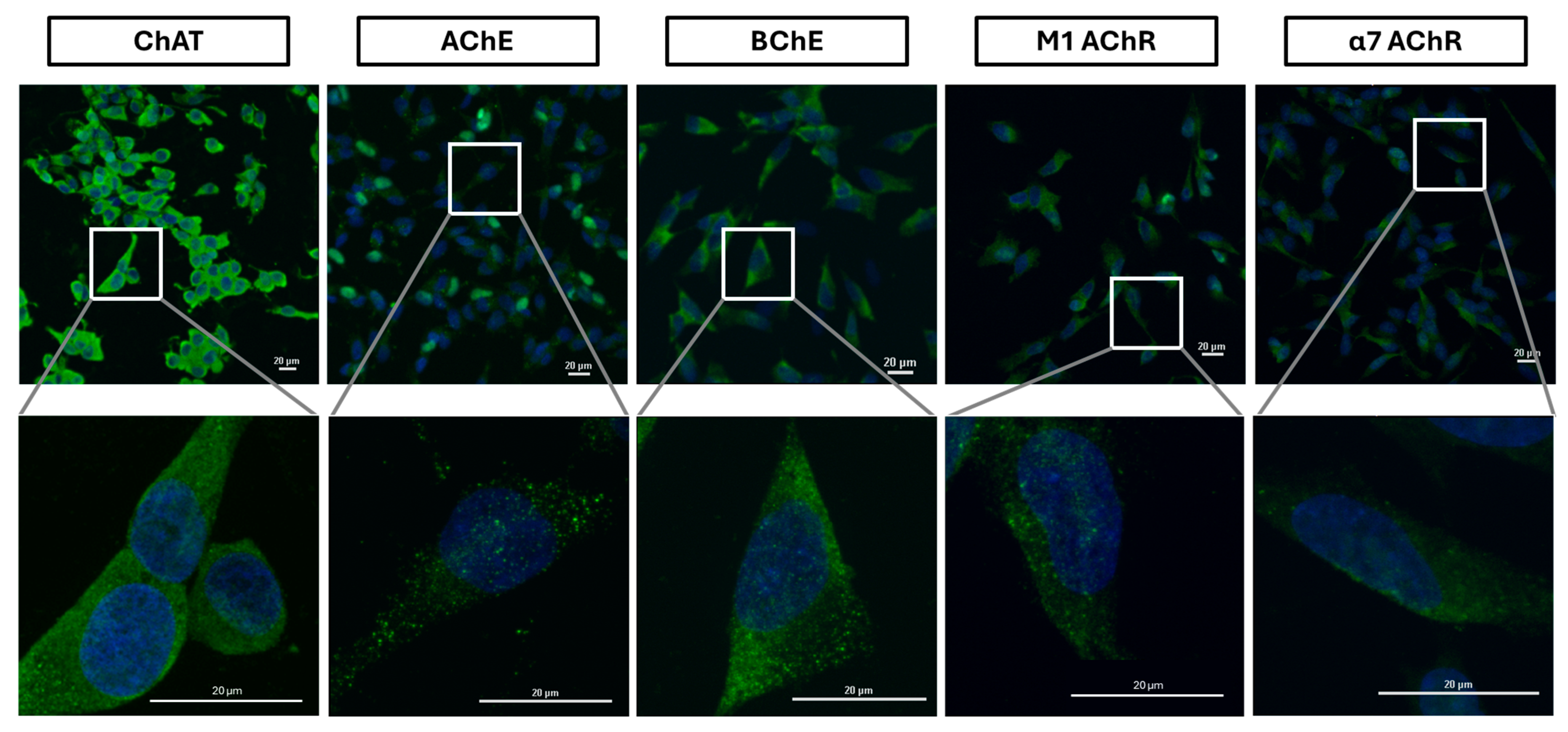
2.3. Surface Localization Analysis of ChAT and the Related Cholinergic Markers in Neuroblastoma Cells
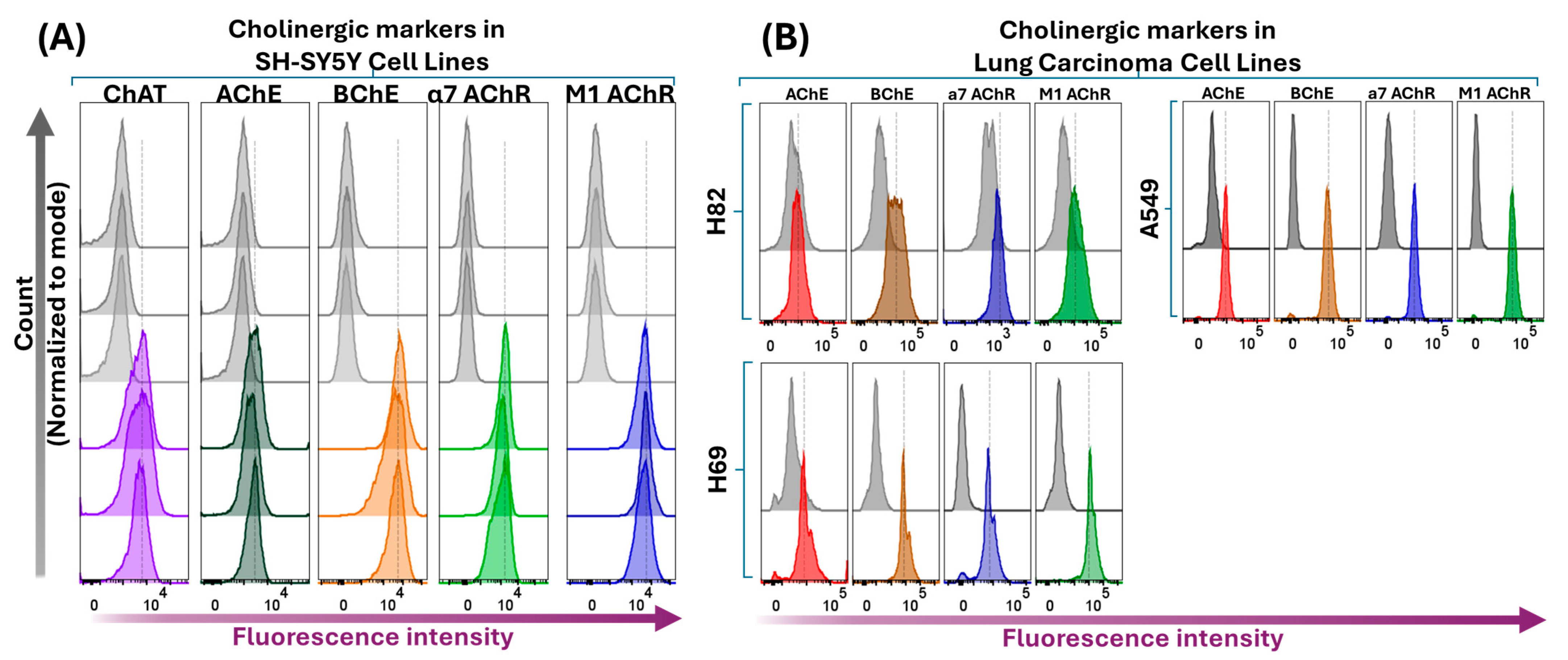
2.4. Intracellular Expression Analysis of ChAT and Related Cholinergic Markers in Neuroblastoma and Lung Cancer Cells
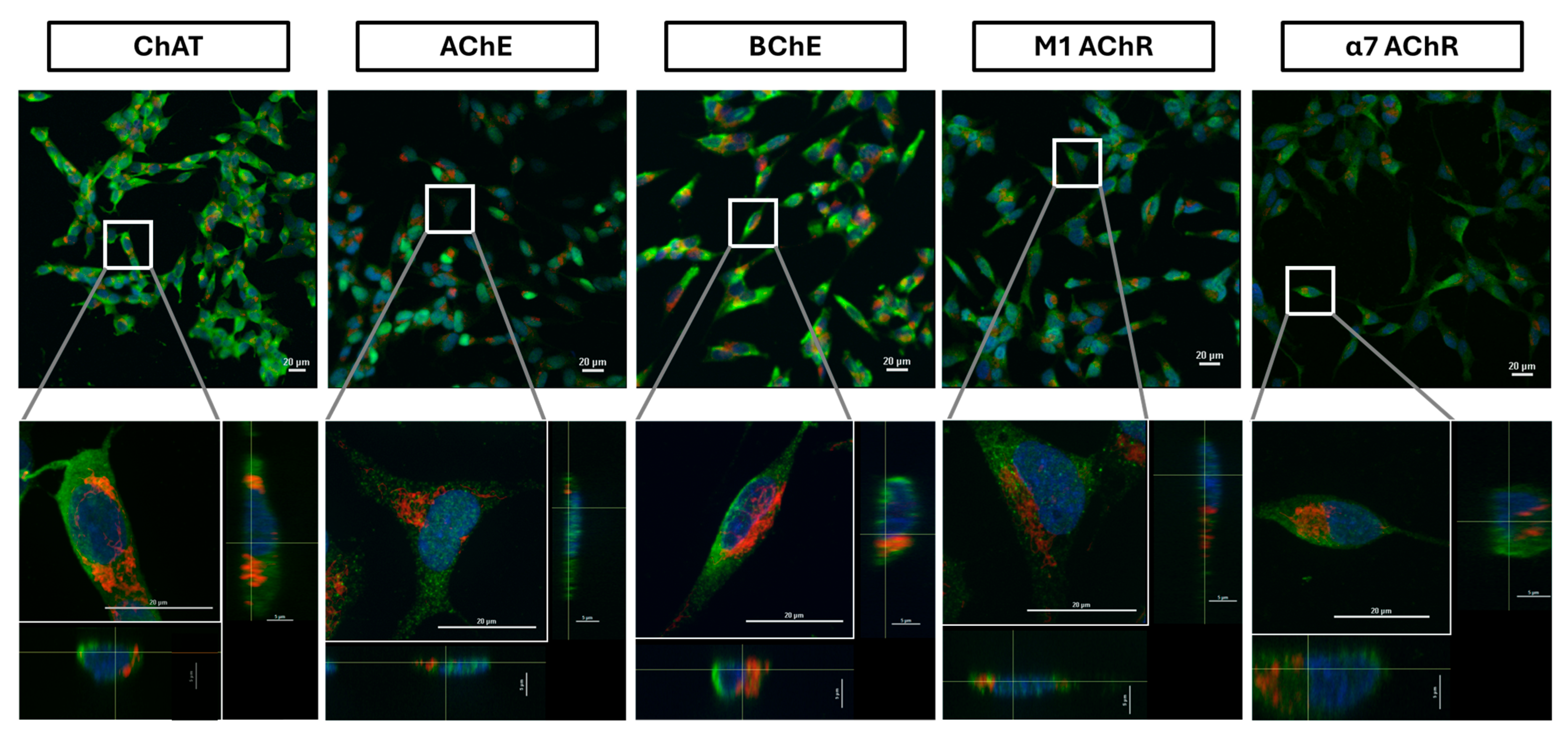
2.5. Confocal Microscopy Confirms Extracellular Expression of ChAT Cholinergic in Neuroblastoma
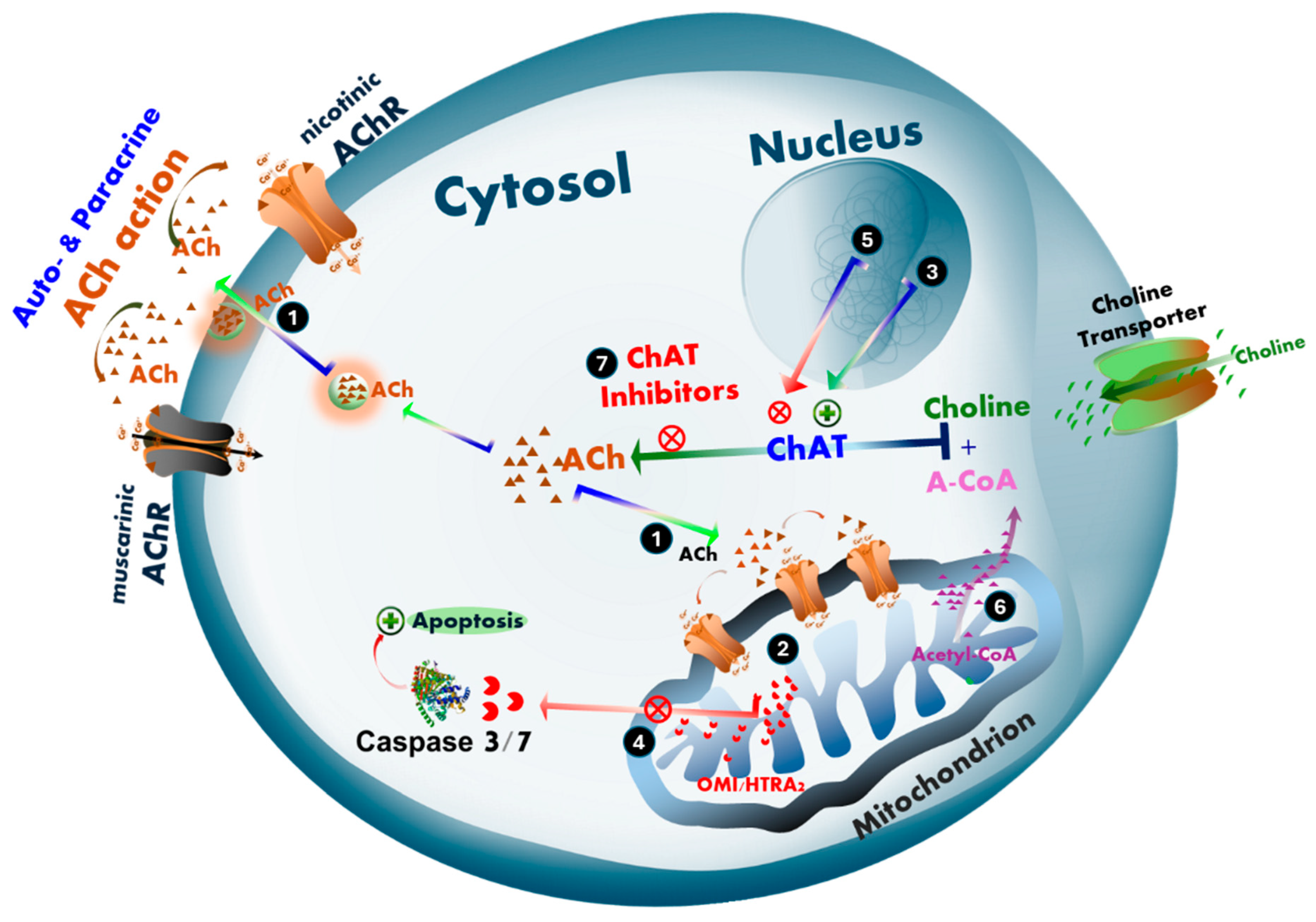
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Lines
4.3. Assessment of Cholinergic Markers by Flow Cytometry
4.4. Assessment of ACh Levels by High-Performance Liquid Chromatography (HPLC)
4.5. Immunofluorescence Staining of SH-SY5Y Cells for ChAT and Related Cholinergic Markers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| AChRs | ACh receptors |
| A-CoA | Acetyl-Coenzyme A |
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| AF647 | Alexa Fluor 647 |
| AF648 | Alexa Fluor 648 |
| APC | Allophycocyanin |
| BChE | Butyrylcholinesterase |
| BDNF | Brain derived neurotrophic factor |
| BSA | bovine serum albumin |
| ChAT | Choline acetyltransferase |
| DMSO | Dimethyl sulfoxide. |
| ENS | Enteric nervous system |
| FBS | Fetal bovine serum |
| FITC | Fluorescein isothiocyanate |
| HPLC-MS/MS | high-performance liquid chromatography-tandem mass spectrometry |
| nAChRs | Nicotinic AChRs |
| NGF | Nerve growth factor |
| NSCLC | Non-small cell lung cancer |
| mAChRs | Muscarinic AChRs |
| PE | Phycoerythrin |
| PPIs | Proton pump inhibitors |
| SCLC | Small cell lung cancer |
| RFI | Relative Fluorescence Intensity |
References
- Vijayaraghavan, S.; Karami, A.; Aeinehband, S.; Behbahani, H.; Grandien, A.; Nilsson, B.; Ekdahl, K.N.; Lindblom, R.P.; Piehl, F.; Darreh-Shori, T. Regulated Extracellular Choline Acetyltransferase Activity- The Plausible Missing Link of the Distant Action of Acetylcholine in the Cholinergic Anti-Inflammatory Pathway. PLoS ONE 2013, 8, e65936. [Google Scholar] [CrossRef]
- Thakur, B.; Hasooni, L.P.; Gera, R.; Mitra, S.; Bjorndahl, L.; Darreh-Shori, T. Presence of key cholinergic enzymes in human spermatozoa and seminal fluiddagger. Biol. Reprod. 2024, 110, 63–77. [Google Scholar] [CrossRef]
- Mesulam, M.M. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J. Comp. Neurol. 2013, 521, 4124–4144. [Google Scholar] [CrossRef]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, B.; Sun, Q.; Zhang, Y.; Ren, M.; Zhang, X.; Li, A.; Yuan, J.; Madisen, L.; Luo, Q.; et al. Generation of a whole-brain atlas for the cholinergic system and mesoscopic projectome analysis of basal forebrain cholinergic neurons. Proc. Natl. Acad. Sci. USA 2018, 115, 415–420. [Google Scholar] [CrossRef]
- Wilcock, G.K.; Esiri, M.M.; Bowen, D.M.; Smith, C.C. Alzheimer’s disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J. Neurol. Sci. 1982, 57, 407–417. [Google Scholar] [CrossRef]
- Esiri, M.M.; Pearson, R.C.; Steele, J.E.; Bowen, D.M.; Powell, T.P. A quantitative study of the neurofibrillary tangles and the choline acetyltransferase activity in the cerebral cortex and the amygdala in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1990, 53, 161–165. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Kaufer, D.I.; Ivanco, L.S.; Lopresti, B.; Koeppe, R.A.; Davis, J.G.; Mathis, C.A.; Moore, R.Y.; DeKosky, S.T. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: An in vivo positron emission tomographic study. Arch Neurol. 2003, 60, 1745–1748. [Google Scholar] [CrossRef]
- Mukaetova-Ladinska, E.B.; Andras, A.; Milne, J.; Abdel-All, Z.; Borr, I.; Jaros, E.; Perry, R.H.; Honer, W.G.; Cleghorn, A.; Doherty, J.; et al. Synaptic proteins and choline acetyltransferase loss in visual cortex in dementia with Lewy bodies. J. Neuropathol. Exp. Neurol. 2013, 72, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.M.; Simpson, J.; Maloney, A.F.; Gordon, A.; Reid, A.H. Alzheimer-like cholinergic deficiency in Down syndrome. Lancet 1980, 2, 979. [Google Scholar] [CrossRef] [PubMed]
- Kanel, P.; Spears, C.C.; Roytman, S.; Koeppe, R.A.; Frey, K.A.; Scott, P.J.H.; Albin, R.L.; Bohnen, N.I. Differential cholinergic systems’ changes in progressive supranuclear palsy versus Parkinson’s disease: An exploratory analysis. J. Neural. Transm. 2022, 129, 1469–1479. [Google Scholar] [CrossRef]
- Warren, N.M.; Piggott, M.A.; Perry, E.K.; Burn, D.J. Cholinergic systems in progressive supranuclear palsy. Brain 2005, 128, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Shinotoh, H.; Namba, H.; Yamaguchi, M.; Fukushi, K.; Nagatsuka, S.; Iyo, M.; Asahina, M.; Hattori, T.; Tanada, S.; Irie, T. Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson’s disease and progressive supranuclear palsy. Ann. Neurol. 1999, 46, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, M.; Javoy-Agid, F.; Hirsch, E.; Scatton, B.; Lheureux, R.; Hauw, J.J.; Duyckaerts, C.; Gray, F.; Morel-Maroger, A.; Rascol, A.; et al. Dopaminergic and cholinergic lesions in progressive supranuclear palsy. Ann. Neurol. 1985, 18, 523–529. [Google Scholar] [CrossRef]
- Oda, Y.; Imai, S.; Nakanishi, I.; Ichikawa, T.; Deguchi, T. Immunohistochemical study on choline acetyltransferase in the spinal cord of patients with amyotrophic lateral sclerosis. Pathol. Int. 1995, 45, 933–939. [Google Scholar] [CrossRef]
- Gillberg, P.G.; Aquilonius, S.M.; Eckernas, S.A.; Lundqvist, G.; Winblad, B. Choline acetyltransferase and substance P-like immuno-reactivity in the human spinal cord: Changes in amyotrophic lateral sclerosis. Brain Res. 1982, 250, 394–397. [Google Scholar] [CrossRef]
- Harrington, A.M.; Hutson, J.M.; Southwell, B.R. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Prog. Histochem. Cytochem. 2010, 44, 173–202. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Gershon, M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. [Google Scholar] [CrossRef]
- Wessler, I.; Kilbinger, H.; Bittinger, F.; Unger, R.; Kirkpatrick, C.J. The non-neuronal cholinergic system in humans: Expression, function and pathophysiology. Life Sci. 2003, 72, 2055–2061. [Google Scholar] [CrossRef]
- Song, P.; Sekhon, H.S.; Proskocil, B.; Blusztajn, J.K.; Mark, G.P.; Spindel, E.R. Synthesis of acetylcholine by lung cancer. Life Sci. 2003, 72, 2159–2168. [Google Scholar] [CrossRef]
- Chernyavsky, A.I.; Shchepotin, I.B.; Galitovkiy, V.; Grando, S.A. Mechanisms of tumor-promoting activities of nicotine in lung cancer: Synergistic effects of cell membrane and mitochondrial nicotinic acetylcholine receptors. BMC Cancer 2015, 15, 152. [Google Scholar] [CrossRef]
- Cheng, K.; Samimi, R.; Xie, G.; Shant, J.; Drachenberg, C.; Wade, M.; Davis, R.J.; Nomikos, G.; Raufman, J.P. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am. J. physiology. Gastrointest. Liver Physiol. 2008, 295, G591–G597. [Google Scholar] [CrossRef]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Nolan, N.A.; Akers, A.T.; Lau, J.K.; Robateau, Z.R.; Miles, S.L.; Dasgupta, P. Acetylcholine signaling system in progression of lung cancers. Pharmacol. Ther. 2019, 194, 222–254. [Google Scholar] [CrossRef]
- Olianas, M.C.; Dedoni, S.; Onali, P. Protection from interferon-beta-induced neuronal apoptosis through stimulation of muscarinic acetylcholine receptors coupled to ERK1/2 activation. Br. J. Pharmacol. 2016, 173, 2910–2928. [Google Scholar] [CrossRef]
- Song, P.; Sekhon, H.S.; Jia, Y.; Keller, J.A.; Blusztajn, J.K.; Mark, G.P.; Spindel, E.R. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003, 63, 214–221. [Google Scholar] [PubMed]
- Wang, S.; Hu, Y. alpha7 nicotinic acetylcholine receptors in lung cancer. Oncol. Lett. 2018, 16, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.P.; Carroll, P.T. A comparison of solubilized and membrane bound forms of choline-O-acetyltransferase (EC 2.3.1.6) in mouse brain nerve endings. Brain Res. 1980, 185, 363–371. [Google Scholar] [CrossRef]
- Carroll, P.T. Membrane-bound choline-O-acetyltransferase in rat hippocampal tissue is associated with synaptic vesicles. Brain Res. 1994, 633, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Pahud, G.; Salem, N.; van de Goor, J.; Medilanski, J.; Pellegrinelli, N.; Eder-Colli, L. Study of subcellular localization of membrane-bound choline acetyltransferase in Drosophila central nervous system and its association with membranes. Eur. J. Neurosci. 1998, 10, 1644–1653. [Google Scholar] [CrossRef]
- Dante, D.; Jangra, J.; Baidya, A.T.K.; Kumar, R.; Darreh-Shori, T. Micellar Choline-Acetyltransferase Complexes Exhibit Ultra-Boosted Catalytic Rate for Acetylcholine Synthesis-Mechanistic Insights for Development of Acetylcholine-Enhancing Micellar Nanotherapeutics. Int. J. Mol. Sci. 2024, 25, 13602. [Google Scholar] [CrossRef]
- Resendes, M.C.; Dobransky, T.; Ferguson, S.S.; Rylett, R.J. Nuclear localization of the 82-kDa form of human choline acetyltransferase. J. Biol. Chem. 1999, 274, 19417–19421. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Bhattacharya, M.; Ferguson, S.S.; Rylett, R.J. Identification of a novel nuclear localization signal common to 69- and 82-kDa human choline acetyltransferase. J. Biol. Chem. 2003, 278, 20217–20224. [Google Scholar] [CrossRef]
- Redin, E.; Quintanal-Villalonga, A.; Rudin, C.M. Small cell lung cancer profiling: An updated synthesis of subtypes, vulnerabilities, and plasticity. Trends Cancer 2024, 10, 935–946. [Google Scholar] [CrossRef]
- Kumar, A.; Lana, E.; Kumar, R.; Lithner, C.U.; Darreh-Shori, T. Soluble Abeta42 Acts as Allosteric Activator of the Core Cholinergic Enzyme Choline Acetyltransferase. Front. Mol. Neurosci. 2018, 11, 327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, A.; Nordberg, A.; Langstrom, B.; Darreh-Shori, T. Proton pump inhibitors act with unprecedented potencies as inhibitors of the acetylcholine biosynthesizing enzyme-A plausible missing link for their association with incidence of dementia. Alzheimers Dement 2020, 16, 1031–1042. [Google Scholar] [CrossRef]
- Inazu, M.; Yamada, T.; Kubota, N.; Yamanaka, T. Functional expression of choline transporter-like protein 1 (CTL1) in small cell lung carcinoma cells: A target molecule for lung cancer therapy. Pharmacol. Res. 2013, 76, 119–131. [Google Scholar] [CrossRef]
- Huang, Z.; Rui, J.; Li, X.; Meng, X.; Liu, Q. Use of (1)(1)C-Choline positron emission tomography/computed tomography to investigate the mechanism of choline metabolism in lung cancer. Mol. Med. Rep. 2015, 11, 3285–3290. [Google Scholar] [CrossRef]
- Silman, I. The multiple biological roles of the cholinesterases. Prog. Biophys Mol. Biol. 2021, 162, 41–56. [Google Scholar] [CrossRef]
- Grisaru, D.; Sternfeld, M.; Eldor, A.; Glick, D.; Soreq, H. Structural roles of acetylcholinesterase variants in biology and pathology. Eur. J. Biochem. 1999, 264, 672–686. [Google Scholar] [CrossRef]
- Christopoulos, C.G.; Kelsey, H.C.; Machin, S.J. A flow-cytometric approach to quantitative estimation of platelet surface immunoglobulin G. Vox Sang 1993, 64, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Sekhon, H.S.; Lu, A.; Arredondo, J.; Sauer, D.; Gravett, C.; Mark, G.P.; Grando, S.A.; Spindel, E.R. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res. 2007, 67, 3936–3944. [Google Scholar] [CrossRef]
- de Medeiros, L.M.; De Bastiani, M.A.; Rico, E.P.; Schonhofen, P.; Pfaffenseller, B.; Wollenhaupt-Aguiar, B.; Grun, L.; Barbe-Tuana, F.; Zimmer, E.R.; Castro, M.A.A.; et al. Cholinergic Differentiation of Human Neuroblastoma SH-SY5Y Cell Line and Its Potential Use as an In vitro Model for Alzheimer’s Disease Studies. Mol. Neurobiol. 2019, 56, 7355–7367. [Google Scholar] [CrossRef]
- Chen, V.P.; Luk, W.K.; Chan, W.K.; Leung, K.W.; Guo, A.J.; Chan, G.K.; Xu, S.L.; Choi, R.C.; Tsim, K.W. Molecular Assembly and Biosynthesis of Acetylcholinesterase in Brain and Muscle: The Roles of t-peptide, FHB Domain, and N-linked Glycosylation. Front. Mol. Neurosci. 2011, 4, 36. [Google Scholar] [CrossRef]
- Garcia-Ayllon, M.S.; Saez-Valero, J.; Munoz-Delgado, E.; Vidal, C.J. Identification of hybrid cholinesterase forms consisting of acetyl- and butyrylcholinesterase subunits in human glioma. Neuroscience 2001, 107, 199–208. [Google Scholar] [CrossRef]
- Zakut, H.; Even, L.; Birkenfeld, S.; Malinger, G.; Zisling, R.; Soreq, H. Modified properties of serum cholinesterases in primary carcinomas. Cancer 1988, 61, 727–737. [Google Scholar] [CrossRef]
- Wang, N.; Yao, M.; Xu, J.; Quan, Y.; Zhang, K.; Yang, R.; Gao, W.Q. Autocrine Activation of CHRM3 Promotes Prostate Cancer Growth and Castration Resistance via CaM/CaMKK-Mediated Phosphorylation of Akt. Clin. Cancer Res. 2015, 21, 4676–4685. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhi, X.; Zhang, Q.; Wei, S.; Li, Z.; Zhou, J.; Jiang, J.; Zhu, Y.; Yang, L.; Xu, H.; et al. Muscarinic receptor M3 mediates cell proliferation induced by acetylcholine and contributes to apoptosis in gastric cancer. Tumor Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 2105–2117. [Google Scholar] [CrossRef] [PubMed]
- Darreh-Shori, T.; Rezaeianyazdi, S.; Lana, E.; Mitra, S.; Gellerbring, A.; Karami, A.; Bogdanovic, N.; Lithner, C.U.; Winblad, B.; Behbahani, H. Increased Active OMI/HTRA2 Serine Protease Displays a Positive Correlation with Cholinergic Alterations in the Alzheimer’s Disease Brain. Mol. Neurobiol. 2019, 56, 4601–4619. [Google Scholar] [CrossRef] [PubMed]
- Tendler, S.; Zhan, Y.; Pettersson, A.; Lewensohn, R.; Viktorsson, K.; Fang, F.; De Petris, L. Treatment patterns and survival outcomes for small-cell lung cancer patients–A Swedish single center cohort study. Acta Oncol. 2020, 59, 388–394. [Google Scholar] [CrossRef]
- Vernay, A.; Cosson, P. Immunofluorescence labeling of cell surface antigens in Dictyostelium. BMC Res. Notes 2013, 6, 317. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, B.; Tarazi, S.; Doležalová, L.; Behbahani, H.; Darreh-Shori, T. Comparative Analysis of Cholinergic Machinery in Carcinomas: Discovery of Membrane-Tethered ChAT as Evidence for Surface-Based ACh Synthesis in Neuroblastoma Cells. Int. J. Mol. Sci. 2025, 26, 10311. https://doi.org/10.3390/ijms262110311
Thakur B, Tarazi S, Doležalová L, Behbahani H, Darreh-Shori T. Comparative Analysis of Cholinergic Machinery in Carcinomas: Discovery of Membrane-Tethered ChAT as Evidence for Surface-Based ACh Synthesis in Neuroblastoma Cells. International Journal of Molecular Sciences. 2025; 26(21):10311. https://doi.org/10.3390/ijms262110311
Chicago/Turabian StyleThakur, Banita, Samar Tarazi, Lada Doležalová, Homira Behbahani, and Taher Darreh-Shori. 2025. "Comparative Analysis of Cholinergic Machinery in Carcinomas: Discovery of Membrane-Tethered ChAT as Evidence for Surface-Based ACh Synthesis in Neuroblastoma Cells" International Journal of Molecular Sciences 26, no. 21: 10311. https://doi.org/10.3390/ijms262110311
APA StyleThakur, B., Tarazi, S., Doležalová, L., Behbahani, H., & Darreh-Shori, T. (2025). Comparative Analysis of Cholinergic Machinery in Carcinomas: Discovery of Membrane-Tethered ChAT as Evidence for Surface-Based ACh Synthesis in Neuroblastoma Cells. International Journal of Molecular Sciences, 26(21), 10311. https://doi.org/10.3390/ijms262110311





