Chronic Glomerular Thrombotic Microangiopathy in a 72-Year-Old Patient with B-Cell Chronic Lymphocytic Leukemia and IgG Lambda Paraprotein
Abstract
1. Introduction
2. Case Presentation
2.1. Medical History and Clinical Findings
2.2. Renal Biopsy Evaluation
2.3. Follow-Up and Therapy
2.4. Complement Profile Assessment
2.4.1. Methodology
2.4.2. Results (Table 3)
| Complement Profile | 3 Weeks After the Biopsy | 15 Months Later | 19 Months Later |
|---|---|---|---|
| Total complement activity, classical pathway (hemolytic test; CH50/mL; 48–103) | 1 | 6 | 0 |
| Total complement activity, alternative pathway (WIELISA-ALT; %; 70–125) | 63 | 71 | 77 |
| Total complement activity, MBL lectin pathway (WIELISA-MBL; %; 35–130%) | NT | NT | 0 |
| C1q antigen (mg/L; 60–180; from May 2024 mg/L; 150–320) | 14 | 270 | 268 |
| Complement C3 (g/L; 0.9–1.8) | 0.79 | 1.45 | 1.54 |
| Complement C4 (g/L; 0.15–0.55) | 0.04 | 0.37 | 0.39 |
| Complement Factor H antigen (mg/L; 250–880) | 959 | 499 | NT |
| Complement Factor I antigen (%; 70–130) | 184 | 128 | NT |
| Complement Factor B antigen (%; 70–130) | 120 | 124 | NT |
| Anti-H Factor IgG (Au/mL; <110) | 5 | 3 | NT |
| Anti-C1q IgG (mU/L; <52) | 20 | 2 | 6 |
| SC5B-9 (terminal complement complex (ng/mL; 110–252) | NT | 426 | NT |
| ADAMTS13 activity (%; 67–150) | 70 | 79 | NT |
| Haptoglobin (g/L; 0.3–2.0) | 3.73 | 2.79 | NT |
2.5. Screening for Anti-Endothelial Cell Antibodies (AECAs)
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genest, D.S.; Patriquin, C.J.; Licht, C.; John, R.; Reich, H.N. Renal thrombotic microangiopathy: A review. Am. J. Kidney Dis. 2023, 81, 591–605. [Google Scholar] [CrossRef]
- Laszik, Z. Thrombotic microangiopathies. In Silva’s Diagnostic Renal Pathology, 2nd ed.; Zhou, X.J., Laszik, Z.G., Nadasdy, T., D’Agati, V.D., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 347–384. [Google Scholar]
- Hanna, R.M.; Henriksen, K.; Kalantar-Zadeh, K.; Ferrey, A.; Burwick, R.; Jhaveri, K.D. Thrombotic microangiopathy syndromes-common ground and distinct frontiers. Adv. Chronic Kidney Dis. 2022, 29, 149–160. [Google Scholar] [CrossRef]
- Ravindran, A.; Go, R.S.; Fervenza, F.C.; Sethi, S. Thrombotic microangiopathy associated with monoclonal gammopathy. Kidney Int. 2017, 91, 691–698. [Google Scholar] [CrossRef]
- Yui, J.C.; Garceau, D.; Jhaveri, K.D.; Wanchoo, R.; Bijol, V.; Glezerman, I.; Hassoun, H.; Dispenzieri, A.; Russel, S.J.; Leung, N. Monoclonal gammopathy-associated thrombotic microangiopathy. Am. J. Hematol. 2019, 94, E250–E282. [Google Scholar] [CrossRef]
- Martins, M.; Bridoux, F.; Goujon, J.M.; Meuleman, M.S.; Ribes, D.; Rondeau, E.; Guerry, M.-J.; Delmas, Y.; Levy, B.; Ducloux, D.; et al. Complement activation and thrombotic microangiopathy associated with monoclonal gammopathy: A national French case series. Am. J. Kidney Dis. 2022, 80, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Strati, P.; Nasr, S.H.; Leung, N.; Hanson, C.A.; Chaffee, K.G.; Schwager, S.M.; Achenbach, S.J.; Call, T.G.; Parikh, S.A.; Ding, W.; et al. Renal complications in chronic lymphocytic leukemia and monoclonal B-cell lymphocytosis: The Mayo Clinic experience. Haematologica 2015, 100, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wang, H.; Su, T.; Wang, S. Case report: Chronic lymphocytic leukemia with recurrent complement-mediated thrombotic microangiopathy and C3 glomerulonephritis. Front. Med. 2022, 9, 813439. [Google Scholar] [CrossRef]
- Leung, N.; Bridoux, F.; Batuman, V.; Chaidos, A.; Cockwell, P.; D’Agati, V.; Dispenzieri, A.; Fervenza, F.C.; Fermand, J.-P.; Gibbs, S.; et al. The evaluation of monoclonal gammopathy of renal significance: A consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat. Rev. 2019, 15, 43–59. [Google Scholar] [CrossRef]
- Nasr, K.; Karam, S.; Mazepa, M.; Czyzyk, J.; Klomjit, N. Case Report: Successful treatment of renal-limited thrombotic microangiopathy secondary to chronic lymphocytic leukemia. Front. Nephrol. 2024, 4, 1400027. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Rajkumar, S.V.; D’Agati, V.D. The complexity and heterogeneity of monoclonal immunoglobulin-associated renal diseases. J. Am. Soc. Nephrol. 2018, 29, 1810–1823. [Google Scholar] [CrossRef]
- Iványi, B.; Fahmy, H.; Brown, H.; Szenohradszky, P.; Halloran, P.F.; Solez, K. Peritubular capillaries in chronic renal allograft rejection: A quantitative ultrastructural study. Hum. Pathol. 2000, 31, 1129–1138. [Google Scholar] [CrossRef]
- Gasim, A.H.; Chua, J.S.; Wolterbeek, R.; Schmitz, J.; Weimer, E.; Singh, H.K.; Nickeleit, V. Glomerular C4d deposits can mark structural capillary wall remodelling in thrombotic microangiopathy and transplant glomerulopathy: C4d beyond active antibody-mediated injury: A retrospective study. Transpl. Int. 2017, 30, 519–532. [Google Scholar] [CrossRef]
- Drachenberg, C.B.; Papdimitriou, J.C.; Chandra, P.; Haririan, A.; Mendley, S.; Weir, M.R.; Rubin, M.F. Epidemiology and pathophysiology of glomerular C4d staining in native kidney biopsies. Kidney Int. Rep. 2019, 4, 1555–1567. [Google Scholar] [CrossRef]
- Michelis, R.; Tadmor, T.; Aviv, A.; Stemer, G.; Majdob, R.; Shvidel, L.; Shehadeh, M.; Barhoum, M.; Braester, A. Cell-free IgG-aggregates in plasma of patients with chronic lymphocytic leukemia cause chronic activation of the classical component pathway. PLoS ONE 2020, 15, e0230033. [Google Scholar] [CrossRef]
- Naseraldeen, N.; Michelis, R.; Barhoum, M.; Chezar, J.; Tadmor, T.; Aviv, A.; Shvidel, L.; Litmanovich, A.; Shedaheh, M.; Sterner, G.; et al. The role of alpha 2 macroglobulin in IgG-aggregation and chronic activation of the complement system in patients with chronic lymphocytic leukemia. Front. Immunol. 2021, 11, 603569. [Google Scholar] [CrossRef] [PubMed]
- Michelis, R.; Milhem, L.; Galouk, E.; Stemer, G.; Aviv, A.; Tadmor, T.; Shehadeh, M.; Shvidel, L.; Barhoum, M.; Braester, A. Increased serum level of alpha-2 macroglobulin and its production by B-lymyphocytes in chronic lymphocytic leukemia. Front. Immunol. 2022, 13, 953644. [Google Scholar] [CrossRef] [PubMed]
- Iványi, B.; Hansen, H.E.; Olsen, T.S. Postcapillary venule-like transformation of peritubular capillaries in acute renal allograft reaction. Arch. Pathol. Lab. Med. 1992, 116, 1062–1067. [Google Scholar]
- Jaccard, A.; Pascal, V.; Magy, L.; Roussel, M. POEMS Syndrome. Presse Med. 2025, 54, 104270. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, Y.; Imai, H.; Yasuda, T.; Wakui, H.; Miura, A.B. A spectrum of clinicopathologic features of nephropathy associated with POEMS syndrome. Nephrol. Dial. Transplant. 1999, 14, 2370–2378. [Google Scholar] [CrossRef]
- Ye, W.; Wang, C.; Cai, Q.-Q.; Dai, H.; Duan, M.-H.; Cao, X.-X.; Zhou, D.-B.; Li, Y. Renal impairment in patients with polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy and skin changes syndrome: Incidence, treatment and outcome. Nephrol. Dial. Transplant. 2016, 31, 275–283. [Google Scholar] [CrossRef]
- Pfister, F.; Aman, K.; Daniel, C.; Klewer, M.; Büttner, A.; Büttner-Herold, M. Characteristic morphological changes in anti-VEGF therapy-induced glomerular microangiopathy. Histopathology 2018, 73, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Person, F.; Rinschen, M.M.; Brix, S.R.; Wulf, S.; Noriega, M.d.l.M.; Fehrle, W.; Schmitz, J.; Schwarz, A.; Ivanyi, P.; Steinmetz, O.M.; et al. Bevacizumab-associated glomerular microangiopathy. Mod. Pathol. 2019, 32, 684–700. [Google Scholar] [CrossRef] [PubMed]
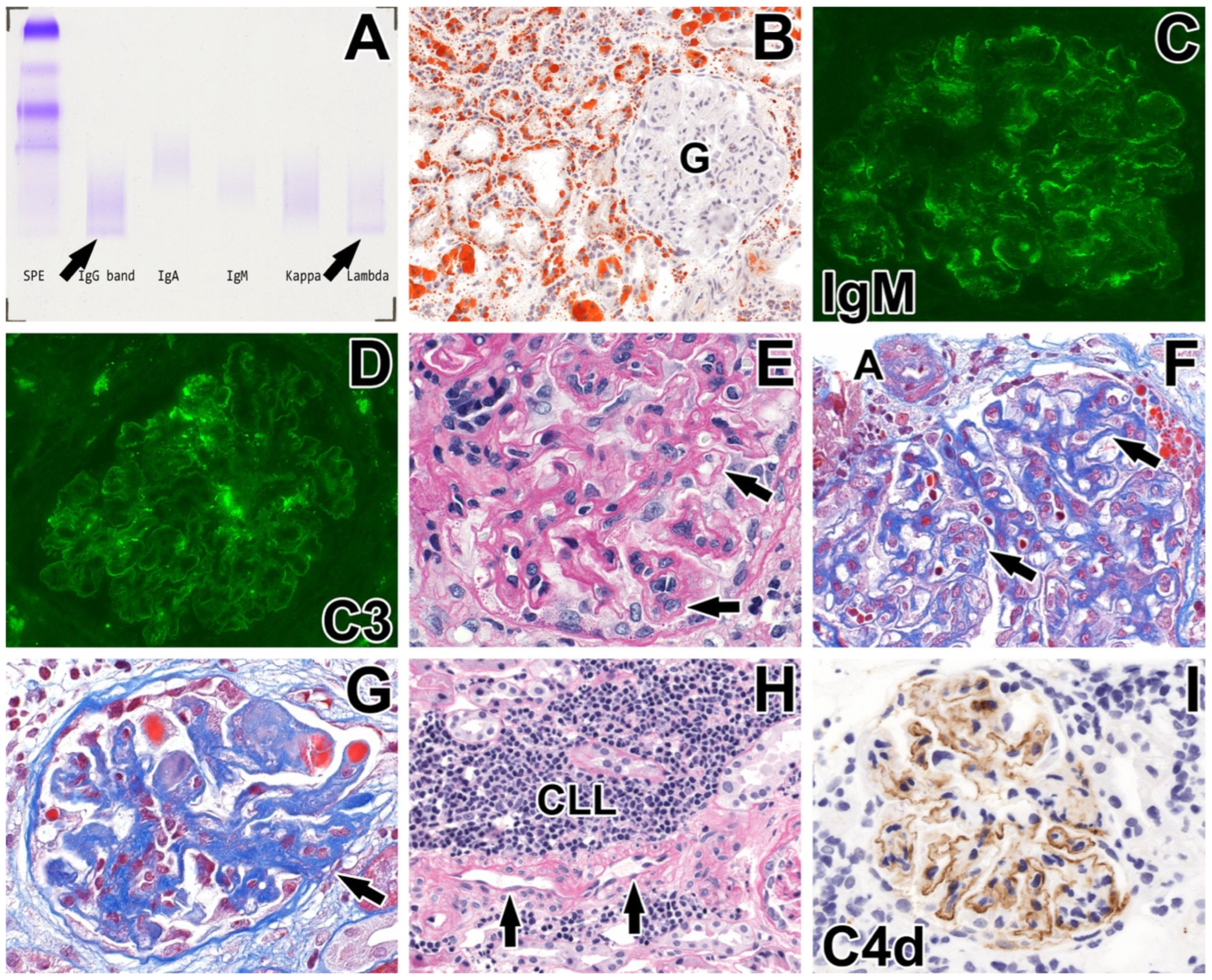
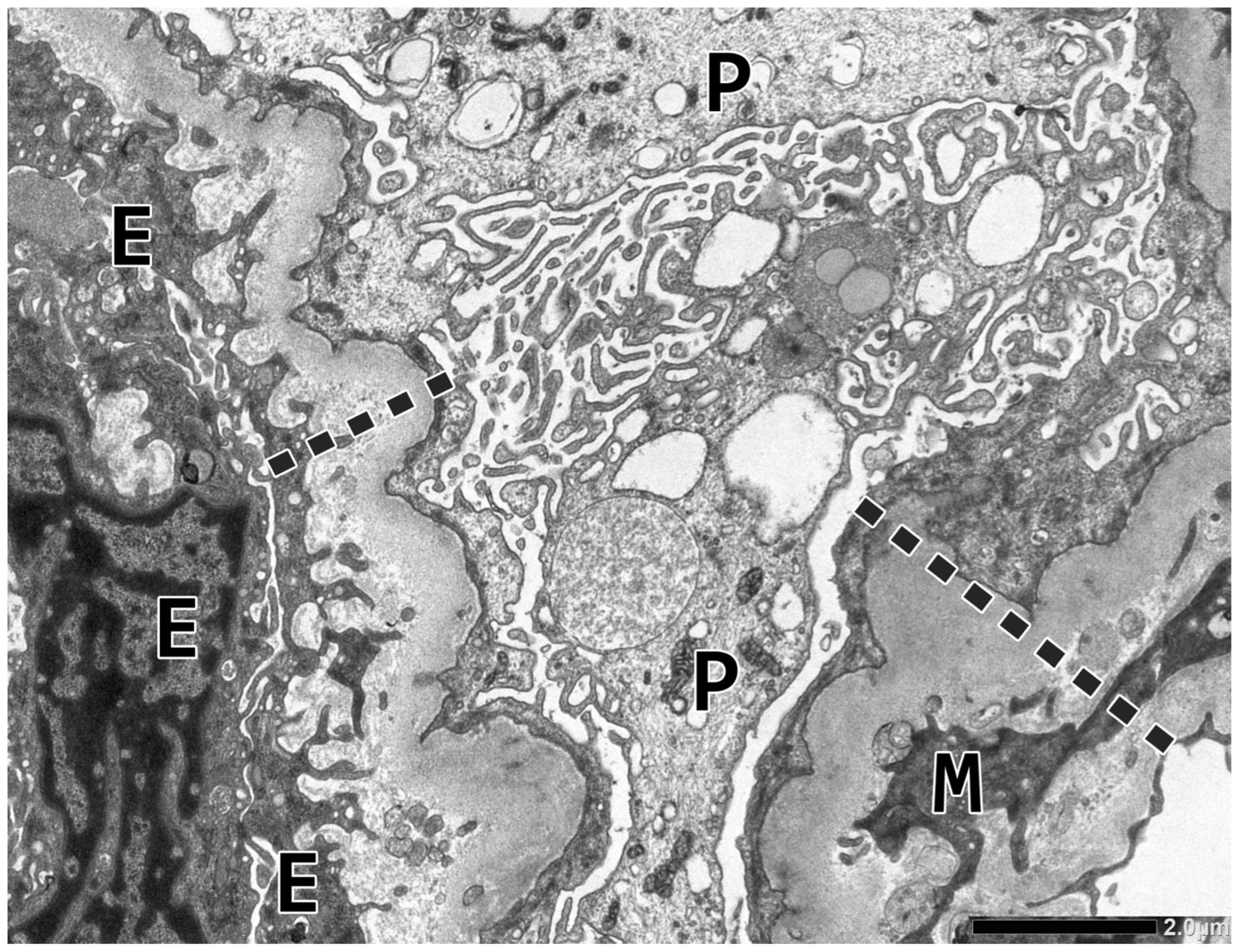

| Blood Test | At Renal Biopsy | 19 Months Later |
|---|---|---|
| Total protein (g/L; 60–87) | 41 | 66 |
| Albumin (g/L; 34–48) | 23 | 46 |
| Total bilirubin (μmol/L; <21.0) | 5.1 | 8,9 |
| Total cholesterol (mmol/L; <5.2) | 4.9 | 5.28 |
| Triglyceride (mmol/L; <1.70) | 1.5 | 1.24 |
| Creatinine (μmol/L; 71–115) | 122 | 114 |
| eGFR (ml/min/1.73 m2; >90) | 50 | 54 |
| White blood cell count (Giga/L; 5.00–10.00) | 7.49 | 8.41 |
| Lymphocyte count (Giga/L; 1.02–3.55) | 4.34 | 1.97 |
| Hemoglobin (g/L; 130–165) | 97 | 133 |
| Platelet (Giga/L; 150–400) | 91 | 123 |
| LDH (U/L) | 238 | NT |
| Serological work-up for autoantibodies, cryoglobulins, and viral panel | Negative | NT |
| Monoclonal protein testing | ||
| SPEP, gamma-globulins (%; 6.2–15.4) | 8.3; faint abnormal band in the gamma region | 7.3; no extrafraction |
| Serum immunofixation | IgG/lambda monoclonal component | IgG/lambda suspected |
| Kappa FLC (mg/L; 6.70–22.40) | 19.4 | 13.7 |
| Lambda FLC (mg/L; 8.3–27.0) | 61.1 | 23.2 |
| Kappa/lambda ratio (0.31–1.56) | 0.31 | 0.59 |
| Urine abnormalities | ||
| Albumin/creatinine ratio (mg/mmol; <2.50) | 648 | 6.5 |
| Dysmorphic red blood cells/visual field | 40–50 | 6–8 |
| No. of Glomeruli/ GS | Glom. Thrombi | MSG | DCs | IFTA (%) | Focal CLL Infiltr. | ATN | Arterial Thrombi/ Sclerosis | Arteriolar Thrombi | Lipids in Tubuli | IH | EM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24/1 | Yes | No | Yes | 15 | Yes | No | No/mild intimal fibrosis in 1 profile out of 10 | No | Yes | IgM, C3, C4d | dFPE, dGBM, MCI, thickened, serrated GECs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitó, L.; Gurbity Pálfi, T.; Jost, K.; Nagy, S.P.; Prohászka, Z.; Iványi, B. Chronic Glomerular Thrombotic Microangiopathy in a 72-Year-Old Patient with B-Cell Chronic Lymphocytic Leukemia and IgG Lambda Paraprotein. Int. J. Mol. Sci. 2025, 26, 10310. https://doi.org/10.3390/ijms262110310
Bitó L, Gurbity Pálfi T, Jost K, Nagy SP, Prohászka Z, Iványi B. Chronic Glomerular Thrombotic Microangiopathy in a 72-Year-Old Patient with B-Cell Chronic Lymphocytic Leukemia and IgG Lambda Paraprotein. International Journal of Molecular Sciences. 2025; 26(21):10310. https://doi.org/10.3390/ijms262110310
Chicago/Turabian StyleBitó, László, Timea Gurbity Pálfi, Krisztina Jost, Simon Péter Nagy, Zoltán Prohászka, and Béla Iványi. 2025. "Chronic Glomerular Thrombotic Microangiopathy in a 72-Year-Old Patient with B-Cell Chronic Lymphocytic Leukemia and IgG Lambda Paraprotein" International Journal of Molecular Sciences 26, no. 21: 10310. https://doi.org/10.3390/ijms262110310
APA StyleBitó, L., Gurbity Pálfi, T., Jost, K., Nagy, S. P., Prohászka, Z., & Iványi, B. (2025). Chronic Glomerular Thrombotic Microangiopathy in a 72-Year-Old Patient with B-Cell Chronic Lymphocytic Leukemia and IgG Lambda Paraprotein. International Journal of Molecular Sciences, 26(21), 10310. https://doi.org/10.3390/ijms262110310





