A Celsr3 Mutation Linked to Tourette Disorder Disrupts Cortical Dendritic Patterning and Striatal Cholinergic Interneuron Excitability
Abstract
1. Introduction
2. Results
2.1. Celsr3R774H/R774H Mice Show Sex-Specific Sensorimotor Gating Deficits
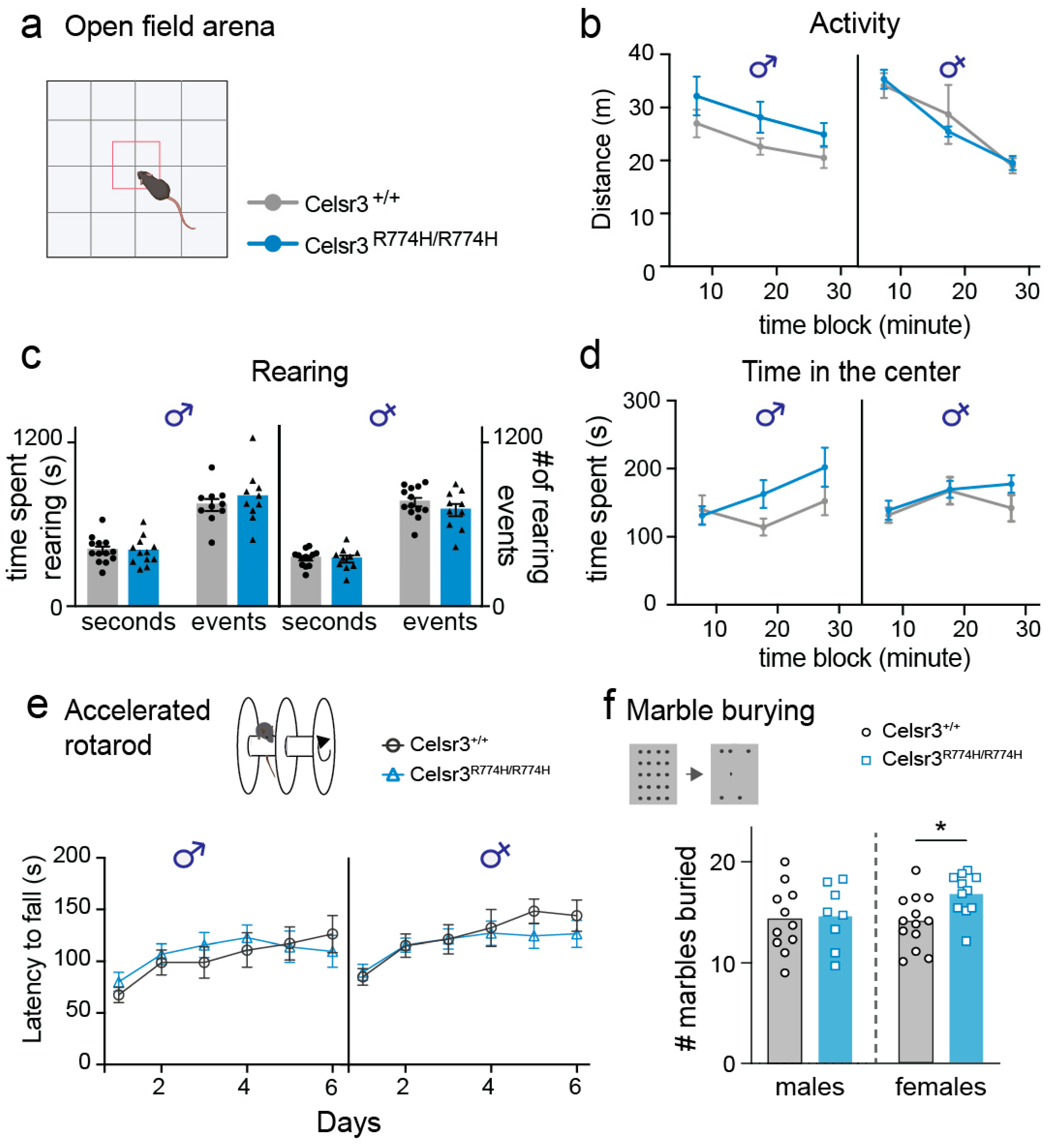
2.2. Celsr3R774H/R774H Mice Do Not Show Hyperactivity or Repetitive Motor Behaviors in the Open Field
2.3. Celsr3R774H/R774H Mice Exhibit Normal Motor Coordination as Measured by Accelerated Rotarod
2.4. Female Celsr3R774H/R774H Mice Show Perseverative Digging Behavior
2.5. Axon Tract Development Is Grossly Normal in Celsr3R774H/R774H Mice
2.6. Celsr3R774H/R774H Mice Have Organized Cortical Layering and Do Not Show Interneuron Loss
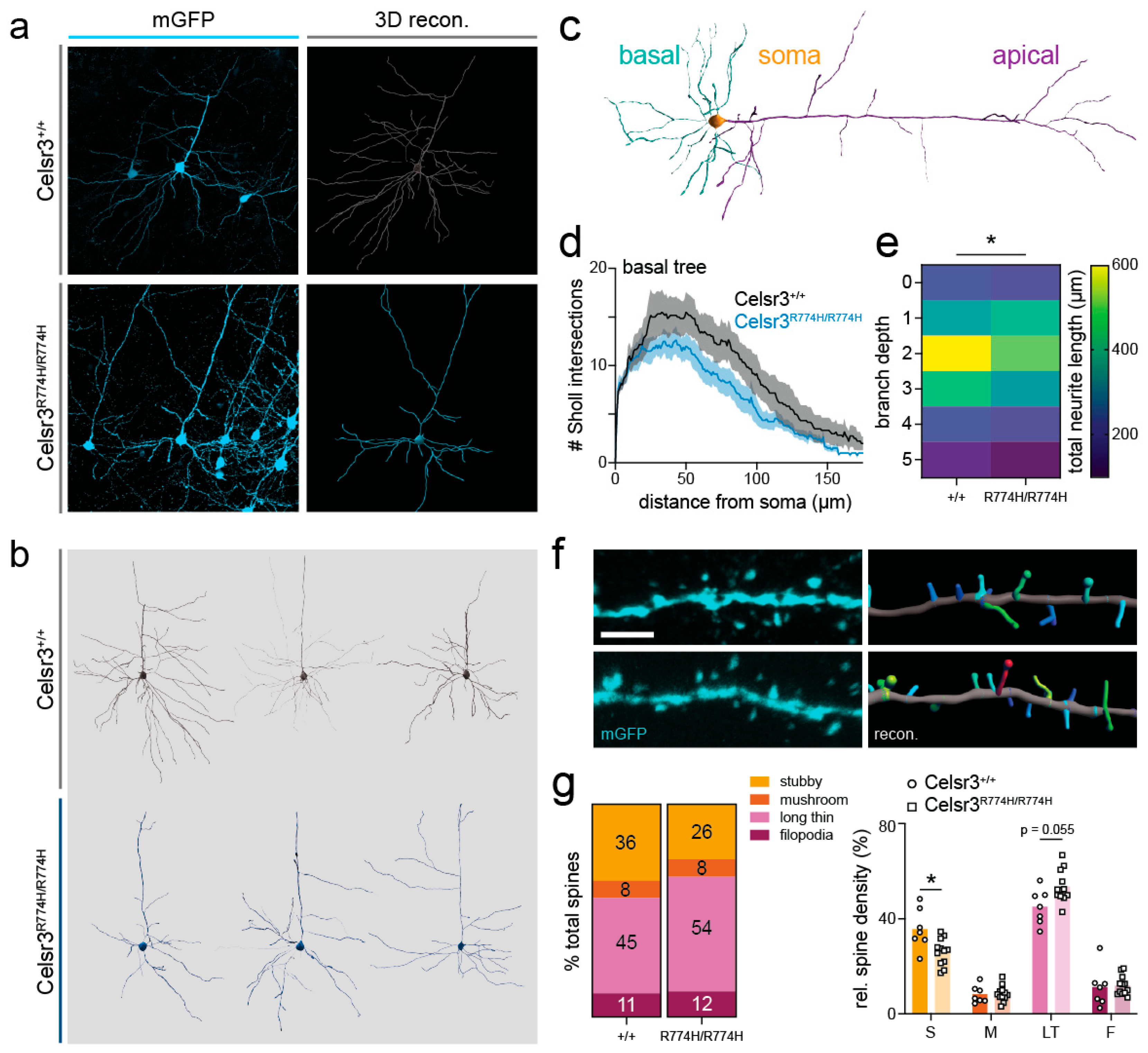
2.7. Cortical Pyramidal Neuron Dendritic Patterning Is Affected in Celsr3R774H/R774H Mice
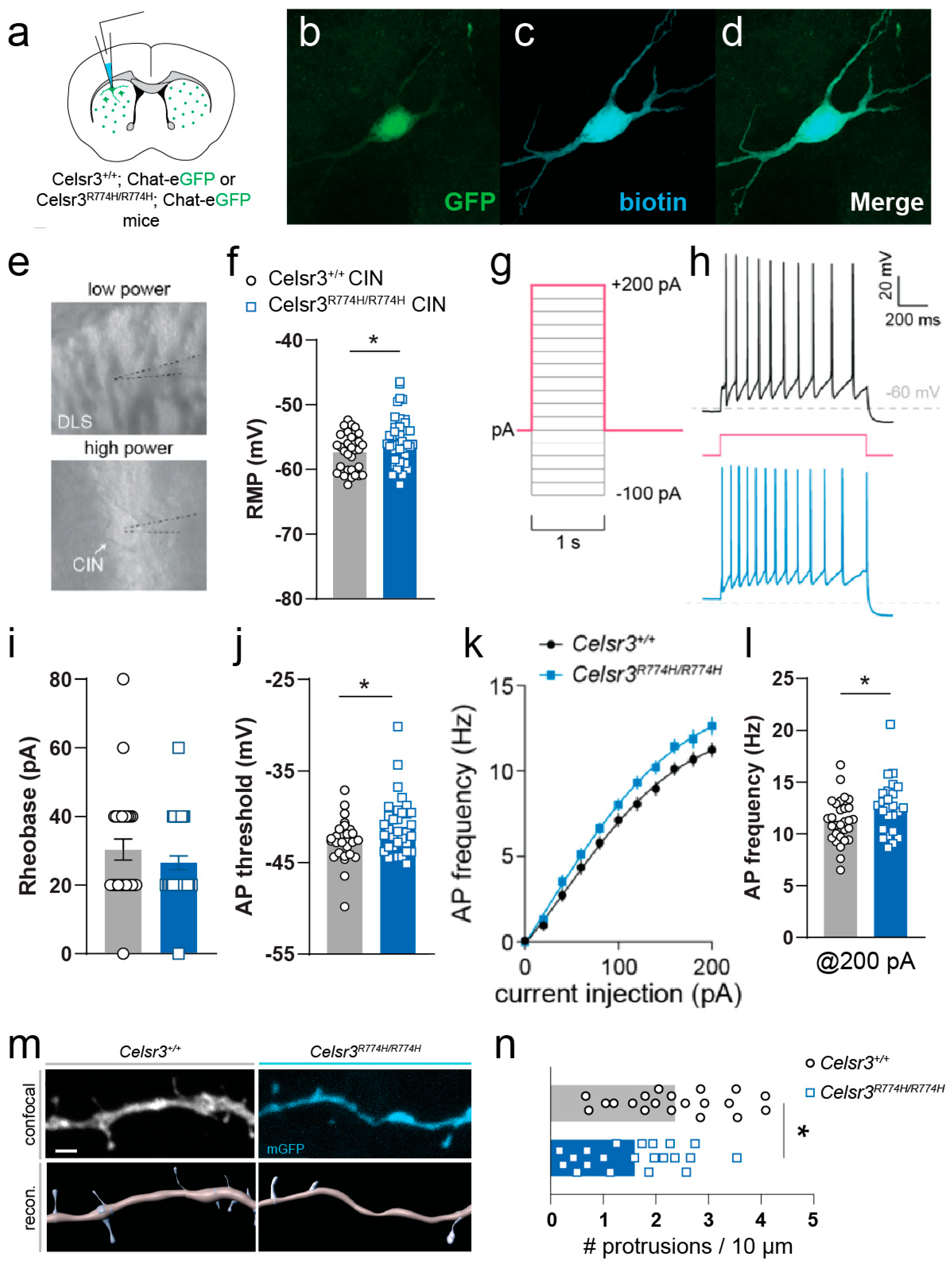
2.8. Celsr3R774H/R774H Cholinergic Interneurons Have Altered Membrane Properties and Spine Density
3. Discussion
4. Materials and Methods
4.1. Mouse Lines
4.2. Histology & Immunostaining
4.2.1. Tissue Collection
4.2.2. Immunofluorescent Labeling
4.3. Western Blot Analysis
4.4. Viral Sparse Cell Labeling
4.5. Microscopy & Image Analysis
4.5.1. Cortical Layer Labeling
4.5.2. Interneuron Counting
4.5.3. Anatomical Recovery of Cortical Pyramidal Neurons
4.5.4. Celsr3 and Interneuron Colocalization
4.6. Behavioral Assays
4.6.1. Prepulse Inhibition of the Acoustic Startle Reflex
4.6.2. Open Field Arena
4.6.3. Accelerated Rotarod Test
4.6.4. Marble Burying Assay
4.7. Ex Vivo Electrophysiology
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TD | Tourette Disorder |
| GABA | Gamma-aminobutyric acid |
| CELSR3 | Cadherin EGF LAG seven-pass G-type receptor 3 |
| NIPBL | Nipped-B-like |
| WWC1 | WW and C2 domain-containing protein 1 |
| CSTC | Cortico-striato-thalamo-cortical (circuit) |
| PPI | Prepulse inhibition |
| μ-OR | μ-opioid receptor |
| NN | Nearest neighbor |
| PV | Parvalbumin |
| PVIN | Parvalbumin-expressing interneuron |
| SSTIN | Somatostatin-expressing interneuron |
| AP | Anteroposterior |
| GFP | Green fluorescent protein |
| YFP | Yellow fluorescent protein |
| RFP | Red fluorescent protein |
| AP | Action potential |
| RMP | Resting membrane potential |
| KO | Knockout (genetically modified organism lacking a specific gene) |
| CIN | Cholinergic interneuron |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| TRE | Tetracycline Response Element |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats (genome editing technology) |
| ROI | Region of interest |
Appendix A
Appendix A.1
| Characteristic | Celsr3+/+ | Celsr3R774H/R774H | p-Value |
|---|---|---|---|
| Input resistance (Rm), MΩ | 0.3218 * | ||
| n | 31 cells, 8 mice | 38 cells, 7 mice | |
| Min, max | 87.18, 282.8 | 107.8, 463.3 | |
| Mean | 184.8 | 207.6 | |
| SD | 49.09 | 73.55 | |
| SEM | 8.817 | 11.93 | |
| Lower 95% CI, upper 95% CI | 166.8, 202.8 | 183.4, 231.8 | |
| Membrane capacitance (Cm), pF | 0.7207 # | ||
| n | 31 cells, 8 mice | 38 cells, 7 mice | |
| Min, max | 22.13, 46.36 | 20.41, 47.34 | |
| Mean | 33.52 | 34.05 | |
| SD | 6.135 | 5.995 | |
| SEM | 1.102 | 0.9725 | |
| Lower 95% CI, upper 95% CI | 31.27, 35.77 | 32.08, 36.02 | |
| Membrane time constant (tau), ms | 0.8948 # | ||
| n | 10 cells, 3 mice | 17 cells, 3 mice | |
| Min, max | 2.114, 3.535 | 1.192, 3.999 | |
| Mean | 2.882 | 2.914 | |
| SD | 0.4947 | 0.6517 | |
| SEM | 0.1564 | 0.1581 | |
| Lower 95% CI, upper 95% CI | 2.528, 3.236 | 2.579, 3.249 | |
| Resting membrane potential, mV | 0.0229 # | ||
| n | 31 cells, 8 mice | 38 cells, 7 mice | |
| Min, max | −62.33, −52.36 | −62.35, −46.46 | |
| Mean | −57.34 | −55.39 | |
| SD | 2.863 | 3.857 | |
| SEM | 0.5142 | 0.6256 | |
| Lower 95% CI, upper 95% CI | −58.39, −56.29 | −56.66, −54.13 | |
| Rheobase, pA | 0.3561 * | ||
| n | 27 cells, 7 mice | 34 cells, 6 mice | |
| Min, max | 0, 80 | 0, 60 | |
| Mean | 30.37 | 26.47 | |
| SD | 16.05 | 11.78 | |
| SEM | 3.089 | 2.020 | |
| Lower 95% CI, upper 95% CI | 24.02, 36.72 | 22.36, 30.58 | |
| AP latency, ms | 0.6189 * | ||
| n | 27 cells, 7 mice | 34 cells, 6 mice | |
| Min, max | 78.76, 783.5 | 30.96, 974.3 | |
| Mean | 294.8 | 283.7 | |
| SD | 191.0 | 220.2 | |
| SEM | 36.75 | 37.77 | |
| Lower 95% CI, upper 95% CI | 219.2, 370.3 | 206.8, 360.5 | |
| AP threshold, mV | 0.0752 * | ||
| n | 27 cells, 7 mice | 34 cells, 6 mice | |
| Min, max | −49.82, −37.08 | −45.14, −30.14 | |
| Mean | −42.65 | −41.08 | |
| SD | 2.405 | 3.120 | |
| SEM | 0.4629 | 0.5350 | |
| Lower 95% CI, upper 95% CI | −43.60, −41.70 | −42.17, −39.99 | |
| AP rise time, ms | 0.1049 # | ||
| n | 27 cells, 7 mice | 34 cells, 6 mice | |
| Min, max | 0.4664, 0.6915 | 0.4326, 0.6723 | |
| Mean | 0.5679 | 0.5428 | |
| SD | 0.06169 | 0.05702 | |
| SEM | 0.01187 | 0.009778 | |
| Lower 95% CI, upper 95% CI | 0.5435, 0.5923 | 0.5229, 0.5627 | |
| AP peak amplitude (from threshold), mV | 0.3684 # | ||
| n | 27 cells, 7 mice | 34 cells, 6 mice | |
| Min, max | 74.18, 92.29 | 75.65, 92.39 | |
| Mean | 83.34 | 84.37 | |
| SD | 4.516 | 4.301 | |
| SEM | 0.8691 | 0.7376 | |
| Lower 95% CI, upper 95% CI | 81.56, 85.13 | 82.87, 85.87 | |
| AP half width, ms | 0.1739 # | ||
| n | 27 cells, 7 mice | 34 cells, 6 mice | |
| Min, max | 1.693, 2.960 | 1.462, 3.210 | |
| Mean | 2.232 | 2.101 | |
| SD | 0.3209 | 0.4034 | |
| SEM | 0.06177 | 0.06919 | |
| Lower 95% CI, upper 95% CI | 2.105, 2.359 | 1.960, 2.241 | |
| AP decay time, ms | 0.5801 # | ||
| n | 27 cells, 7 mice | 34 cells, 6 mice | |
| Min, max | 1.963, 3.425 | 1.677, 2.057 | |
| Mean | 2.614 | 2.545 | |
| SD | 0.3972 | 0.5428 | |
| SEM | 0.07644 | 0.09308 | |
| Lower 95% CI, upper 95% CI | 2.457, 2.771 | 2.355, 2.734 | |
| AHP peak amplitude, mV | 0.5227 # | ||
| n | 24 cells, 7 mice | 33 cells, 6 mice | |
| Min, max | −22.48, −9.902 | −24.66, −9.931 | |
| Mean | −17.13 | −17.70 | |
| SD | 3.499 | 3.223 | |
| SEM | 0.7142 | 0.5610 | |
| Lower 95% CI, upper 95% CI | −18.60, −15.65 | −18.85, −16.56 | |
| AHP time to peak, ms | 0.5649 # | ||
| n | 24 cells, 7 mice | 33 cells, 6 mice | |
| Min, max | 8.848, 82.15 | 9.671, 79.57 | |
| Mean | 40.89 | 43.14 | |
| SD | 15.72 | 13.56 | |
| SEM | 3.209 | 2.360 | |
| Lower 95% CI, upper 95% CI | 34.25, 47.53 | 38.34, 47.95 |
Appendix A.2
| Spine Class | Criteria |
|---|---|
| Stubby | Spine length < 1 μm |
| Mushroom | Spine length < 3 μm and spine head width > spine neck width × 2 |
| Long Thin | Spine head width ≥ spine neck width |
| Filopodia | True |
References
- Leckman, J.F.; Bloch, M.H.; Scahill, L.; King, R.A. Tourette Syndrome: The Self Under Siege. J. Child Neurol. 2006, 21, 642–649. [Google Scholar] [CrossRef]
- Hartmann, A.; Worbe, Y.; Black, K.J. Tourette syndrome research highlights from 2017. F1000Research 2018, 7, 1122. [Google Scholar] [CrossRef]
- Hirschtritt, M.E.; Lee, P.C.; Pauls, D.L.; Dion, Y.; Grados, M.A.; Illmann, C.; King, R.A.; Sandor, P.; McMahon, W.M.; Lyon, G.J.; et al. Lifetime Prevalence, Age of Risk, and Genetic Relationships of Comorbid Psychiatric Disorders in Tourette Syndrome. JAMA Psychiatry 2015, 72, 325. [Google Scholar] [CrossRef]
- Robertson, M.M.; Cavanna, A.E.; Eapen, V. Gilles de la Tourette Syndrome and Disruptive Behavior Disorders: Prevalence, Associations, and Explanation of the Relationships. J. Neuropsychiatry Clin. Neurosci. 2015, 27, 33–41. [Google Scholar] [CrossRef]
- Willsey, A.J.; Morris, M.T.; Wang, S.; Willsey, H.R.; Sun, N.; Teerikorpi, N.; Baum, T.B.; Cagney, G.; Bender, K.J.; Desai, T.A.; et al. The Psychiatric Cell Map Initiative: A Convergent Systems Biological Approach to Illuminating Key Molecular Pathways in Neuropsychiatric Disorders. Cell 2018, 174, 505–520. [Google Scholar] [CrossRef]
- Price, R.A. A Twin Study of Tourette Syndrome. Arch. Gen. Psychiatry 1985, 42, 815. [Google Scholar] [CrossRef] [PubMed]
- Draper, A.; Jackson, S.R. Alterations in structural connectivity may contribute both to the occurrence of tics in Gilles de la Tourette syndrome and to their subsequent control. Brain 2015, 138, 244–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jackson, G.M.; Draper, A.; Dyke, K.; Pépés, S.E.; Jackson, S.R. Inhibition, Disinhibition, and the Control of Action in Tourette Syndrome. Trends Cogn. Sci. 2015, 19, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-Y.; Liu, F.-C. Synaptic Wiring of Corticostriatal Circuits in Basal Ganglia: Insights into the Pathogenesis of Neuropsychiatric Disorders. eNeuro 2019, 6, ENEURO.0076-19.2019. [Google Scholar] [CrossRef]
- Wang, Z.; Maia, T.V.; Marsh, R.; Colibazzi, T.; Gerber, A.; Peterson, B.S. The Neural Circuits That Generate Tics in Tourette’s Syndrome. Am. J. Psychiatry 2011, 168, 1326–1337. [Google Scholar] [CrossRef]
- Worbe, Y.; Malherbe, C.; Hartmann, A.; Pélégrini-Issac, M.; Messé, A.; Vidailhet, M.; Lehéricy, S.; Benali, H. Functional immaturity of cortico-basal ganglia networks in Gilles de la Tourette syndrome. Brain 2012, 135, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Felling, R.J.; Singer, H.S. Neurobiology of Tourette Syndrome: Current Status and Need for Further Investigation. J. Neurosci. 2011, 31, 12387–12395. [Google Scholar] [CrossRef]
- Hashemiyoon, R.; Kuhn, J.; Visser-Vandewalle, V. Putting the Pieces Together in Gilles de la Tourette Syndrome: Exploring the Link Between Clinical Observations and the Biological Basis of Dysfunction. Brain Topogr. 2017, 30, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, T.F.; Wang, R.; Tischfield, M.A. Genetic advances and translational phenotypes in rodent models for Tourette disorder. Curr. Opin. Neurobiol. 2025, 90, 102967. [Google Scholar] [CrossRef]
- Worbe, Y.; Hartmann, A. Neuroimaging of Gilles de la Tourette syndrome. In Magnetic Resonance Imaging in Movement Disorders, 1st ed.; Tuite, P., Dagher, A., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 121–133. [Google Scholar] [CrossRef]
- Draganski, B.; Martino, D.; Cavanna, A.E.; Hutton, C.; Orth, M.; Robertson, M.M.; Critchley, H.D.; Frackowiak, R.S. Multispectral brain morphometry in Tourette syndrome persisting into adulthood. Brain 2010, 133, 3661–3675. [Google Scholar] [CrossRef]
- Fahim, C.; Yoon, U.; Das, S.; Lyttelton, O.; Chen, J.; Arnaoutelis, R.; Rouleau, G.; Sandor, P.; Frey, K.; Brandner, C.; et al. Somatosensory–motor bodily representation cortical thinning in Tourette: Effects of tic severity, age and gender. Cortex 2010, 46, 750–760. [Google Scholar] [CrossRef]
- Tinaz, S.; Belluscio, B.A.; Malone, P.; Van Der Veen, J.W.; Hallett, M.; Horovitz, S.G. Role of the sensorimotor cortex in tourette syndrome using multimodal imaging: Multimodal Neuroimaging in Tourette Syndrome. Hum. Brain Mapp. 2014, 35, 5834–5846. [Google Scholar] [CrossRef]
- Worbe, Y.; Gerardin, E.; Hartmann, A.; Valabrégue, R.; Chupin, M.; Tremblay, L.; Vidailhet, M.; Colliot, O.; Lehéricy, S. Distinct structural changes underpin clinical phenotypes in patients with Gilles de la Tourette syndrome. Brain 2010, 133, 3649–3660. [Google Scholar] [CrossRef]
- Greene, D.J.; Williams, A.C., III; Koller, J.M.; Schlaggar, B.L.; Black, K.J.; The Tourette Association of America Neuroimaging Consortium. Brain structure in pediatric Tourette syndrome. Mol. Psychiatry 2017, 22, 972–980, Erratum in Mol. Psychiatry 2020, 25, 3112. https://doi.org/10.1038/mp.2016.194. [Google Scholar] [CrossRef]
- Neuner, I.; Werner, C.J.; Arrubla, J.; Stöcker, T.; Ehlen, C.; Wegener, H.P.; Schneider, F.; Shah, N.J. Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Front. Hum. Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Silbersweig, D.A.; Chee, K.-Y.; Holmes, A.; Robertson, M.M.; Trimble, M.; Frith, C.D.; Frackowiak, R.S.J.; Dolan, R.J. A Functional Neuroanatomy of Tics in Tourette Syndrome. Arch. Gen. Psychiatry 2000, 57, 741. [Google Scholar] [CrossRef]
- Marsh, R.; Zhu, H.; Wang, Z.; Skudlarski, P.; Peterson, B.S. A Developmental fMRI Study of Self-Regulatory Control in Tourette’s Syndrome. Am. J. Psychiatry 2007, 164, 955–966. [Google Scholar] [CrossRef]
- Tobe, R.H.; Bansal, R.; Xu, D.; Hao, X.; Liu, J.; Sanchez, J.; Peterson, B.S. Cerebellar morphology in Tourette syndrome and obsessive-compulsive disorder. Ann. Neurol. 2010, 67, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.R.; Loayza, J.; Crighton, M.; Sigurdsson, H.P.; Dyke, K.; Jackson, G.M. The role of the insula in the generation of motor tics and the experience of the premonitory urge-to-tic in Tourette syndrome. Cortex 2020, 126, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.S.; Choi, H.A.; Hao, X.; Amat, J.A.; Zhu, H.; Whiteman, R.; Liu, J.; Xu, D.; Bansal, R. Morphologic Features of the Amygdala and Hippocampus in Children and Adults With Tourette Syndrome. Arch. Gen. Psychiatry 2007, 64, 1281. [Google Scholar] [CrossRef] [PubMed]
- Kalanithi, P.S.A.; Zheng, W.; Kataoka, Y.; DiFiglia, M.; Grantz, H.; Saper, C.B.; Schwartz, M.L.; Leckman, J.F.; Vaccarino, F.M. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 13307–13312. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kalanithi, P.S.A.; Grantz, H.; Schwartz, M.L.; Saper, C.; Leckman, J.F.; Vaccarino, F.M. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J. Comp. Neurol. 2010, 518, 277–291. [Google Scholar] [CrossRef]
- Rapanelli, M.; Frick, L.R.; Pittenger, C. The Role of Interneurons in Autism and Tourette Syndrome. Trends Neurosci. 2017, 40, 397–407. [Google Scholar] [CrossRef]
- Wang, Y.; Fasching, L.; Wu, F.; Suvakov, M.; Huttner, A.; Berretta, S.; Roberts, R.; Leckman, J.F.; Fernandez, T.V.; Abyzov, A.; et al. Interneuron Loss and Microglia Activation by Transcriptome Analyses in the Basal Ganglia of Tourette Disorder. Biol. Psychiatry 2025, 98, 260–270. [Google Scholar] [CrossRef]
- Bronfeld, M.; Yael, D.; Belelovsky, K.; Bar-Gad, I. Motor tics evoked by striatal disinhibition in the rat. Front. Syst. Neurosci. 2013, 7, 50. [Google Scholar] [CrossRef]
- McCairn, K.W.; Bronfeld, M.; Belelovsky, K.; Bar-Gad, I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain 2009, 132, 2125–2138. [Google Scholar] [CrossRef]
- Worbe, Y.; Baup, N.; Grabli, D.; Chaigneau, M.; Mounayar, S.; McCairn, K.; Féger, J.; Tremblay, L. Behavioral and Movement Disorders Induced by Local Inhibitory Dysfunction in Primate Striatum. Cereb. Cortex 2009, 19, 1844–1856. [Google Scholar] [CrossRef]
- McCairn, K.W.; Nagai, Y.; Hori, Y.; Ninomiya, T.; Kikuchi, E.; Lee, J.-Y.; Suhara, T.; Iriki, A.; Minamimoto, T.; Takada, M.; et al. A Primary Role for Nucleus Accumbens and Related Limbic Network in Vocal Tics. Neuron 2016, 89, 300–307. [Google Scholar] [CrossRef]
- Sagalajev, B.; Lennartz, L.; Mokhtari, N.; Szpak, M.; Uyar, M.S.; Schüller, T.; Baldermann, J.C.; Andrade, P.; Visser-Vandewalle, V.; Sesia, T. Frequent vocalizations and deep brain stimulation-responsive hyperkinesia in a striatal disinhibition rat model for Tourette syndrome. Int. J. Neuropsychopharmacol. 2025, 28, pyaf039. [Google Scholar] [CrossRef] [PubMed]
- Cadeddu, R.; Van Zandt, M.; Santovito, L.S.; Odeh, K.; Anderson, C.J.; Flanagan, D.; Nordkild, P.; Pinna, G.; Pittenger, C.; Bortolato, M. Prefrontal allopregnanolone mediates the adverse effects of acute stress in a mouse model of tic pathophysiology. Neuropsychopharmacology 2023, 48, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Kobets, A.; Du, J.-C.; Lennington, J.; Li, L.; Banasr, M.; Duman, R.S.; Vaccarino, F.M.; DiLeone, R.J.; Pittenger, C. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 893–898. [Google Scholar] [CrossRef]
- Scharf, J.M.; Miller, L.L.; Gauvin, C.A.; Alabiso, J.; Mathews, C.A.; Ben-Shlomo, Y. Population prevalence of Tourette syndrome: A systematic review and meta-analysis. Mov. Disord. 2015, 30, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Sul, J.H.; Tsetsos, F.; Nawaz, M.S.; Huang, A.Y.; Zelaya, I.; Illmann, C.; Osiecki, L.; Darrow, S.M.; Hirschtritt, M.E.; et al. Interrogating the Genetic Determinants of Tourette’s Syndrome and Other Tic Disorders Through Genome-Wide Association Studies. Am. J. Psychiatry 2019, 176, 217–227. [Google Scholar] [CrossRef]
- Willsey, A.J.; Fernandez, T.V.; Yu, D.; King, R.A.; Dietrich, A.; Xing, J.; Sanders, S.J.; Mandell, J.D.; Huang, A.Y.; Richer, P.; et al. De Novo Coding Variants Are Strongly Associated with Tourette Disorder. Neuron 2017, 94, 486–499.e9. [Google Scholar] [CrossRef]
- Wang, S.; Mandell, J.D.; Kumar, Y.; Sun, N.; Morris, M.T.; Arbelaez, J.; Nasello, C.; Dong, S.; Duhn, C.; Zhao, X.; et al. De Novo Sequence and Copy Number Variants Are Strongly Associated with Tourette Disorder and Implicate Cell Polarity in Pathogenesis. Cell Rep. 2018, 24, 3441–3454, Erratum in Cell Rep. 2018, 25, 3544. https://doi.org/10.1016/j.celrep.2018.12.024. [Google Scholar] [CrossRef]
- Takeichi, M. The cadherin superfamily in neuronal connections and interactions. Nat. Rev. Neurosci. 2007, 8, 11–20. [Google Scholar] [CrossRef]
- Wu, J.; Poppi, L.A.; Tischfield, M.A. Planar cell polarity and the pathogenesis of Tourette Disorder: New hypotheses and perspectives. Dev. Biol. 2022, 489, 14–20. [Google Scholar] [CrossRef]
- Zhou, L.; Bar, I.; Achouri, Y.; Campbell, K.; De Backer, O.; Hebert, J.M.; Jones, K.; Kessaris, N.; De Rouvroit, C.L.; O’Leary, D.; et al. Early Forebrain Wiring: Genetic Dissection Using Conditional Celsr3 Mutant Mice. Science 2008, 320, 946–949. [Google Scholar] [CrossRef]
- Jia, Z.; Guo, Y.; Tang, Y.; Xu, Q.; Li, B.; Wu, Q. Regulation of the Protocadherin Celsr3 Gene and Its Role in Globus Pallidus Development and Connectivity. Mol. Cell. Biol. 2014, 34, 3895–3910. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ying, G.; Wu, S.; Hou, R.; Huang, W.; Capecchi, M.R.; Wu, Q. The Protocadherin Gene Celsr3 Is Required for Interneuron Migration in the Mouse Forebrain. Mol. Cell. Biol. 2009, 29, 3045–3061. [Google Scholar] [CrossRef] [PubMed]
- Tissir, F.; Bar, I.; Jossin, Y.; Goffinet, A.M. Erratum: Corrigendum: Protocadherin Celsr3 is crucial in axonal tract development. Nat. Neurosci. 2005, 8, 451–457, Erratum in Nat. Neurosci. 2006, 9, 147. https://doi.org/10.1038/nn0106-147a. [Google Scholar] [CrossRef]
- Zhou, Q.; Qin, J.; Liang, Y.; Zhang, W.; He, S.; Tissir, F.; Qu, Y.; Zhou, L. Celsr3 is required for Purkinje cell maturation and regulates cerebellar postsynaptic plasticity. iScience 2021, 24, 102812. [Google Scholar] [CrossRef] [PubMed]
- Nasello, C.; Poppi, L.A.; Wu, J.; Kowalski, T.F.; Thackray, J.K.; Wang, R.; Persaud, A.; Mahboob, M.; Lin, S.; Spaseska, R.; et al. Human mutations in high-confidence Tourette disorder genes affect sensorimotor behavior, reward learning, and striatal dopamine in mice. Proc. Natl. Acad. Sci. USA 2024, 121, e2307156121. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Karban, B.; Ploum, Y.; Sharp, R.; Geyer, M.A.; Eastvold, A. Tactile prepuff inhibition of startle in children with Tourette’s syndrome: In search of an “fMRI-friendly” startle paradigm. Biol. Psychiatry 2001, 50, 578–585. [Google Scholar] [CrossRef]
- Castellanos, F.X.; Fine, E.J.; Kaysen, D.; Marsh, W.L.; Rapoport, J.L.; Hallett, M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: Preliminary results. Biol. Psychiatry 1996, 39, 33–41. [Google Scholar] [CrossRef]
- Lv, J.; Liang, S.; Qin, P.; Liu, X.; Ge, X.; Guo, Y.; Xia, S.; Jing, W.; Lu, Y.; Zhang, T.; et al. WWC1 mutation drives dopamine dysregulation and synaptic imbalance in Tourette’s syndrome. Sci. Adv. 2025, 11, eadr4588. [Google Scholar] [CrossRef]
- Baldan, L.C.; Williams, K.A.; Gallezot, J.-D.; Pogorelov, V.; Rapanelli, M.; Crowley, M.; Anderson, G.M.; Loring, E.; Gorczyca, R.; Billingslea, E.; et al. Histidine Decarboxylase Deficiency Causes Tourette Syndrome: Parallel Findings in Humans and Mice. Neuron 2014, 81, 77–90, Erratum in Neuron 2014, 82, 1186–1187. https://doi.org/10.1016/j.neuron.2014.05.023. [Google Scholar] [CrossRef]
- Cadeddu, R.; Branca, C.; Braccagni, G.; Musci, T.; Piras, I.S.; Anderson, C.J.; Capecchi, M.R.; Huentelman, M.J.; Moos, P.J.; Bortolato, M. Tic-related behaviors in Celsr3 mutant mice are contributed by alterations of striatal D3 dopamine receptors. Mol. Psychiatry 2025, 30, 3912–3924. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xu, Y.; Wang, M.; Ruan, Y.; So, K.-F.; Tissir, F.; Goffinet, A.; Zhou, L. A Role for Atypical Cadherin Celsr3 in Hippocampal Maturation and Connectivity. J. Neurosci. 2012, 32, 13729–13743. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, L.; Gall, D.; Qu, Y.; Prigogine, C.; Cheron, G.; Tissir, F.; Schiffmann, S.N.; Goffinet, A.M. Maturation of “Neocortex Isole” In Vivo in Mice. J. Neurosci. 2010, 30, 7928–7939. [Google Scholar] [CrossRef]
- Luo, L.; Ambrozkiewicz, M.C.; Benseler, F.; Chen, C.; Dumontier, E.; Falkner, S.; Furlanis, E.; Gomez, A.M.; Hoshina, N.; Huang, W.-H.; et al. Optimizing Nervous System-Specific Gene Targeting with Cre Driver Lines: Prevalence of Germline Recombination and Influencing Factors. Neuron 2020, 106, 37–65.e5. [Google Scholar] [CrossRef]
- Garris, J.; Quigg, M. The female Tourette patient: Sex differences in Tourette Disorder. Neurosci. Biobehav. Rev. 2021, 129, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Martos, Y.V.; Braz, B.Y.; Beccaria, J.P.; Murer, M.G.; Belforte, J.E. Compulsive Social Behavior Emerges after Selective Ablation of Striatal Cholinergic Interneurons. J. Neurosci. 2017, 37, 2849–2858. [Google Scholar] [CrossRef]
- Schaefer, A.T.; Larkum, M.E.; Sakmann, B.; Roth, A. Coincidence Detection in Pyramidal Neurons is Tuned by Their Dendritic Branching Pattern. J. Neurophysiol. 2003, 89, 3143–3154. [Google Scholar] [CrossRef]
- Constantinople, C.M.; Bruno, R.M. Deep Cortical Layers Are Activated Directly by Thalamus. Science 2013, 340, 1591–1594. [Google Scholar] [CrossRef]
- Gentet, L.J.; Kremer, Y.; Taniguchi, H.; Huang, Z.J.; Staiger, J.F.; Petersen, C.C.H. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat. Neurosci. 2012, 15, 607–612. [Google Scholar] [CrossRef]
- Paulsen, O.; Moser, E. A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends Neurosci. 1998, 21, 273–278. [Google Scholar] [CrossRef]
- Wiecki, T.V.; Frank, M.J. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol. Rev. 2013, 120, 329–355. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Sabatini, B.L. Anatomical and Physiological Plasticity of Dendritic Spines. Annu. Rev. Neurosci. 2007, 30, 79–97. [Google Scholar] [CrossRef]
- Fox, K.; Wong, R.O.L. A Comparison of Experience-Dependent Plasticity in the Visual and Somatosensory Systems. Neuron 2005, 48, 465–477. [Google Scholar] [CrossRef]
- Yuste, R.; Bonhoeffer, T. Genesis of dendritic spines: Insights from ultrastructural and imaging studies. Nat. Rev. Neurosci. 2004, 5, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Tissir, F.; Goffinet, A.M. Expression of planar cell polarity genes during development of the mouse CNS. Eur. J. Neurosci. 2006, 23, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Abdurakhmanova, S.; Chary, K.; Kettunen, M.; Sierra, A.; Panula, P. Behavioral and stereological characterization of Hdc KO mice: Relation to Tourette syndrome. J. Comp. Neurol. 2017, 525, 3476–3487. [Google Scholar] [CrossRef]
- Liu, S.; Tian, M.; He, F.; Li, J.; Xie, H.; Liu, W.; Zhang, Y.; Zhang, R.; Yi, M.; Che, F.; et al. Mutations in ASH1L confer susceptibility to Tourette syndrome. Mol. Psychiatry 2020, 25, 476–490. [Google Scholar] [CrossRef]
- Mainen, Z.F.; Sejnowski, T.J. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature 1996, 382, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Kosillo, P.; Zhang, Y.-F.; Threlfell, S.; Cragg, S.J. Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cereb. Cortex 2016, 26, 4160–4169. [Google Scholar] [CrossRef]
- Zhou, F.; Wilson, C.J.; Dani, J.A. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J. Neurobiol. 2002, 53, 590–605. [Google Scholar] [CrossRef]
- Nelson, A.B.; Bussert, T.G.; Kreitzer, A.C.; Seal, R.P. Striatal Cholinergic Neurotransmission Requires VGLUT3. J. Neurosci. 2014, 34, 8772–8777. [Google Scholar] [CrossRef] [PubMed]
- Taverna, S.; Ilijic, E.; Surmeier, D.J. Recurrent Collateral Connections of Striatal Medium Spiny Neurons Are Disrupted in Models of Parkinson’s Disease. J. Neurosci. 2008, 28, 5504–5512. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Luan, P.; Qiao, Q.; He, Y.; Zatka-Haas, P.; Zhang, G.; Lin, M.Z.; Lak, A.; Jing, M.; Mann, E.O.; et al. An axonal brake on striatal dopamine output by cholinergic interneurons. Nat. Neurosci. 2025, 28, 783–794. [Google Scholar] [CrossRef]
- Chuhma, N.; Tanaka, K.F.; Hen, R.; Rayport, S. Functional Connectome of the Striatal Medium Spiny Neuron. J. Neurosci. 2011, 31, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.A.; Rotstein, H.G.; Alvarez, V.A. Striatal Local Circuitry: A New Framework for Lateral Inhibition. Neuron 2017, 96, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J. Dynamic Dopamine Modulation in the Basal Ganglia: A Neurocomputational Account of Cognitive Deficits in Medicated and Nonmedicated Parkinsonism. J. Cogn. Neurosci. 2005, 17, 51–72. [Google Scholar] [CrossRef]
- Klug, J.R.; Engelhardt, M.D.; Cadman, C.N.; Li, H.; Smith, J.B.; Ayala, S.; Williams, E.W.; Hoffman, H.; Jin, X. Differential inputs to striatal cholinergic and parvalbumin interneurons imply functional distinctions. eLife 2018, 7, e35657. [Google Scholar] [CrossRef]
- Müller-Vahl, K.R.; Kaufmann, J.; Grosskreutz, J.; Dengler, R.; Emrich, H.M.; Peschel, T. Prefrontal and anterior cingulate cortex abnormalities in Tourette Syndrome: Evidence from voxel-based morphometry and magnetization transfer imaging. BMC Neurosci. 2009, 10, 47. [Google Scholar] [CrossRef]
- De Oliveira, R.B.; Graham, B.; Howlett, M.C.H.; Gravina, F.S.; Oliveira, M.W.S.; Imtiaz, M.S.; Callister, R.J.; Lim, R.; Brichta, A.M.; Van Helden, D.F. Ketamine anesthesia helps preserve neuronal viability. J. Neurosci. Methods 2010, 189, 230–232. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasello, C.; Yilmaz, G.D.; Poppi, L.A.; Kowalski, T.F.; Ho-Nguyen, K.T.; Wu, J.; Matrongolo, M.; Thackray, J.K.; Shi, A.; Carayannopoulos, N.L.; et al. A Celsr3 Mutation Linked to Tourette Disorder Disrupts Cortical Dendritic Patterning and Striatal Cholinergic Interneuron Excitability. Int. J. Mol. Sci. 2025, 26, 10307. https://doi.org/10.3390/ijms262110307
Nasello C, Yilmaz GD, Poppi LA, Kowalski TF, Ho-Nguyen KT, Wu J, Matrongolo M, Thackray JK, Shi A, Carayannopoulos NL, et al. A Celsr3 Mutation Linked to Tourette Disorder Disrupts Cortical Dendritic Patterning and Striatal Cholinergic Interneuron Excitability. International Journal of Molecular Sciences. 2025; 26(21):10307. https://doi.org/10.3390/ijms262110307
Chicago/Turabian StyleNasello, Cara, G. Duygu Yilmaz, Lauren A. Poppi, Tess F. Kowalski, K. T. Ho-Nguyen, Junbing Wu, Matthew Matrongolo, Joshua K. Thackray, Anna Shi, Nicolas L. Carayannopoulos, and et al. 2025. "A Celsr3 Mutation Linked to Tourette Disorder Disrupts Cortical Dendritic Patterning and Striatal Cholinergic Interneuron Excitability" International Journal of Molecular Sciences 26, no. 21: 10307. https://doi.org/10.3390/ijms262110307
APA StyleNasello, C., Yilmaz, G. D., Poppi, L. A., Kowalski, T. F., Ho-Nguyen, K. T., Wu, J., Matrongolo, M., Thackray, J. K., Shi, A., Carayannopoulos, N. L., Cheedalla, N., McGinnis, J., Chen, J., Khondker, A., Tissir, F., Heiman, G. A., Tischfield, J. A., & Tischfield, M. A. (2025). A Celsr3 Mutation Linked to Tourette Disorder Disrupts Cortical Dendritic Patterning and Striatal Cholinergic Interneuron Excitability. International Journal of Molecular Sciences, 26(21), 10307. https://doi.org/10.3390/ijms262110307






