Histological and Immunohistochemical Methods in Normal and Osteoarthritic Knee Cartilage of Rat and Rabbit Models: A Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Screening Method and Data Extraction
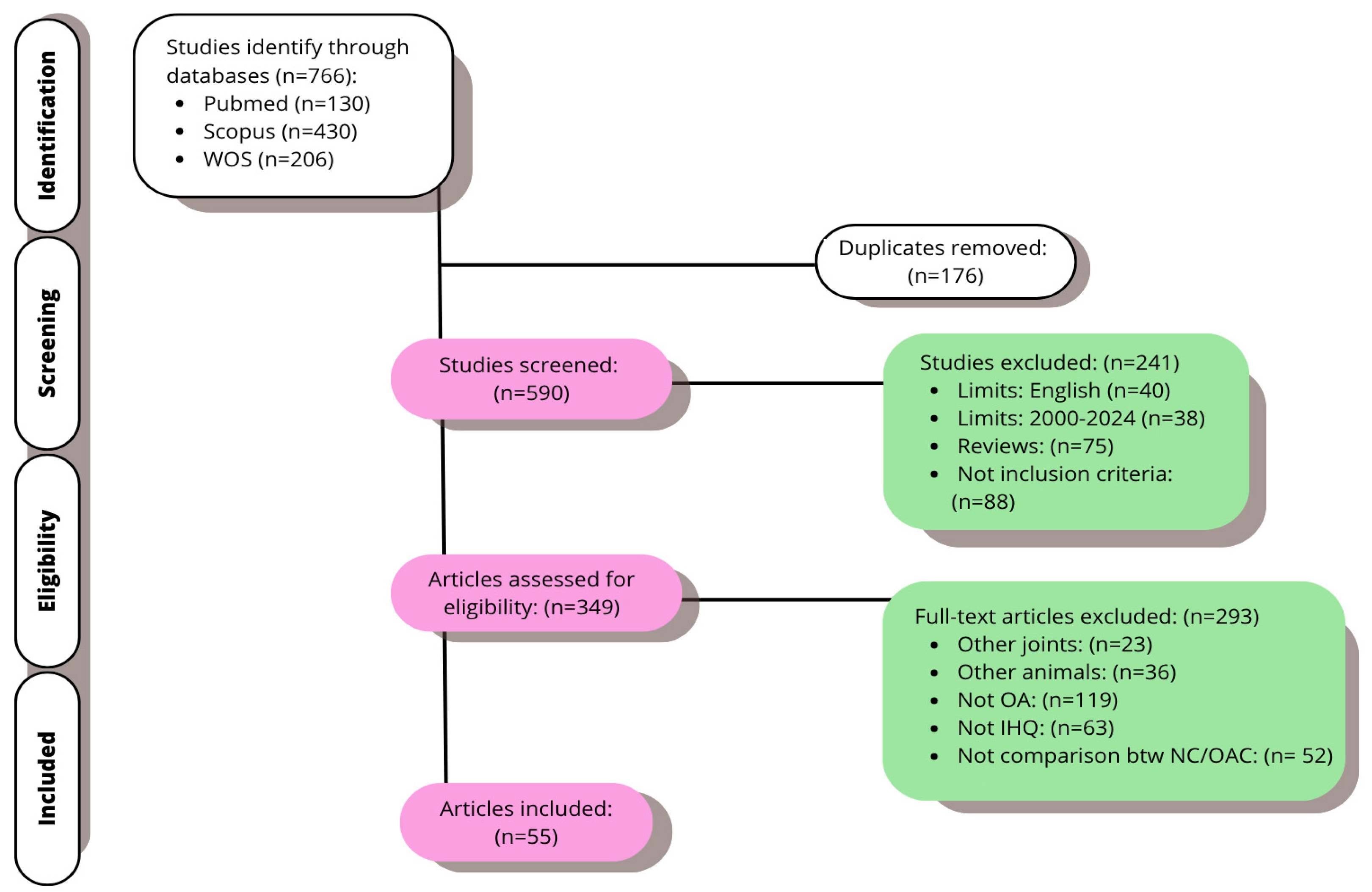
3. Results
3.1. Study Selection
3.2. Main Outcomes
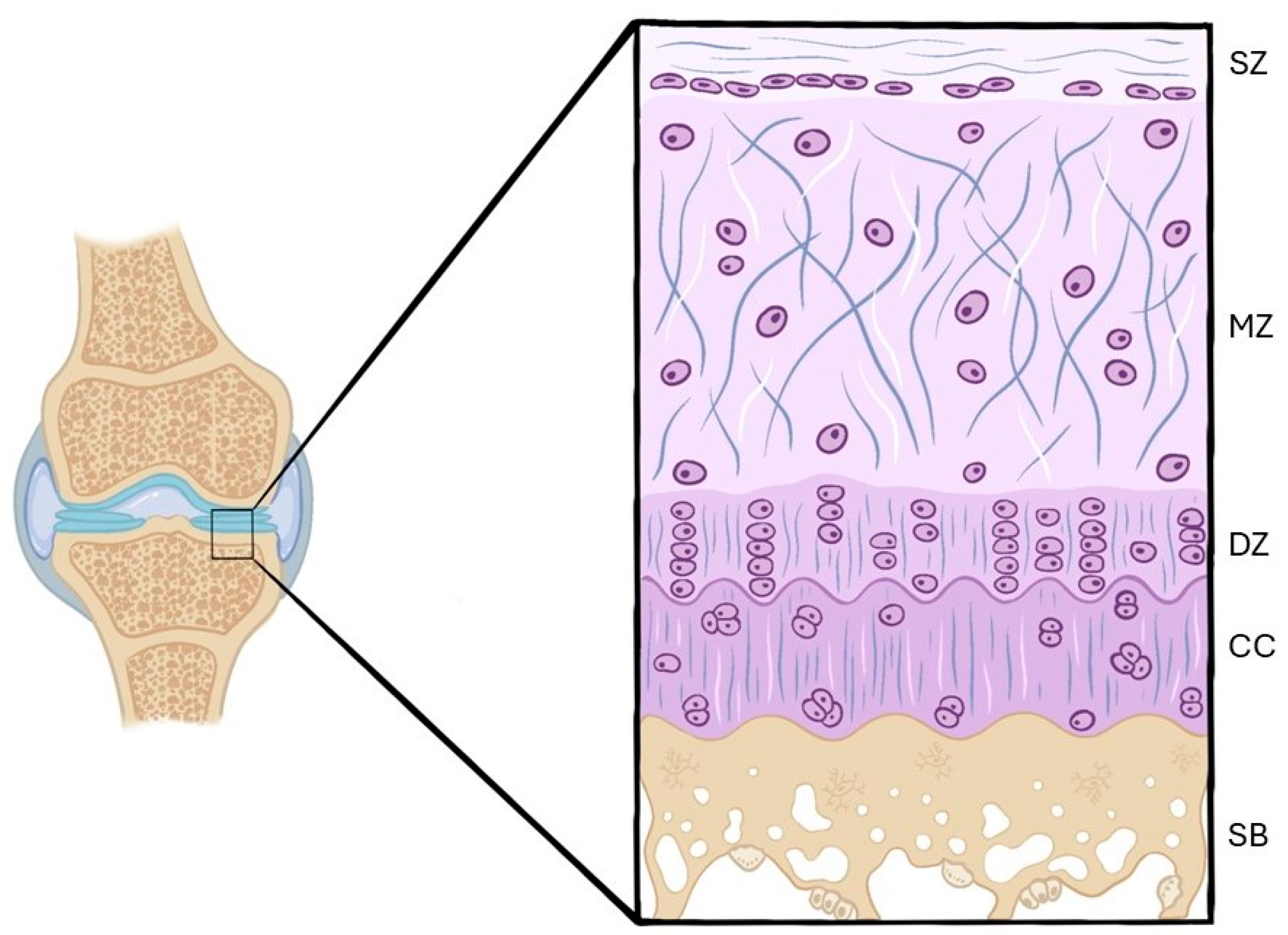
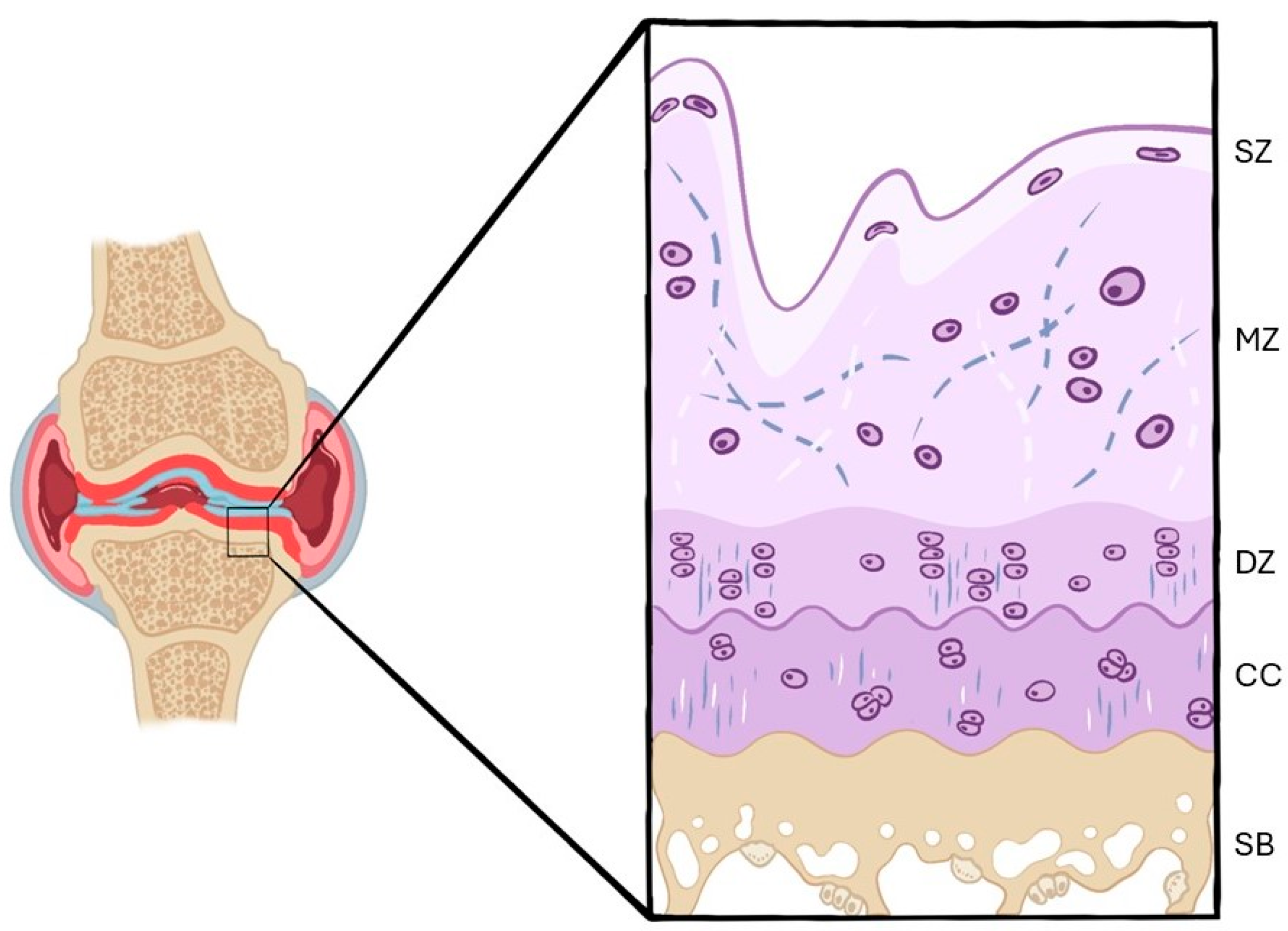
3.2.1. Histological Findings
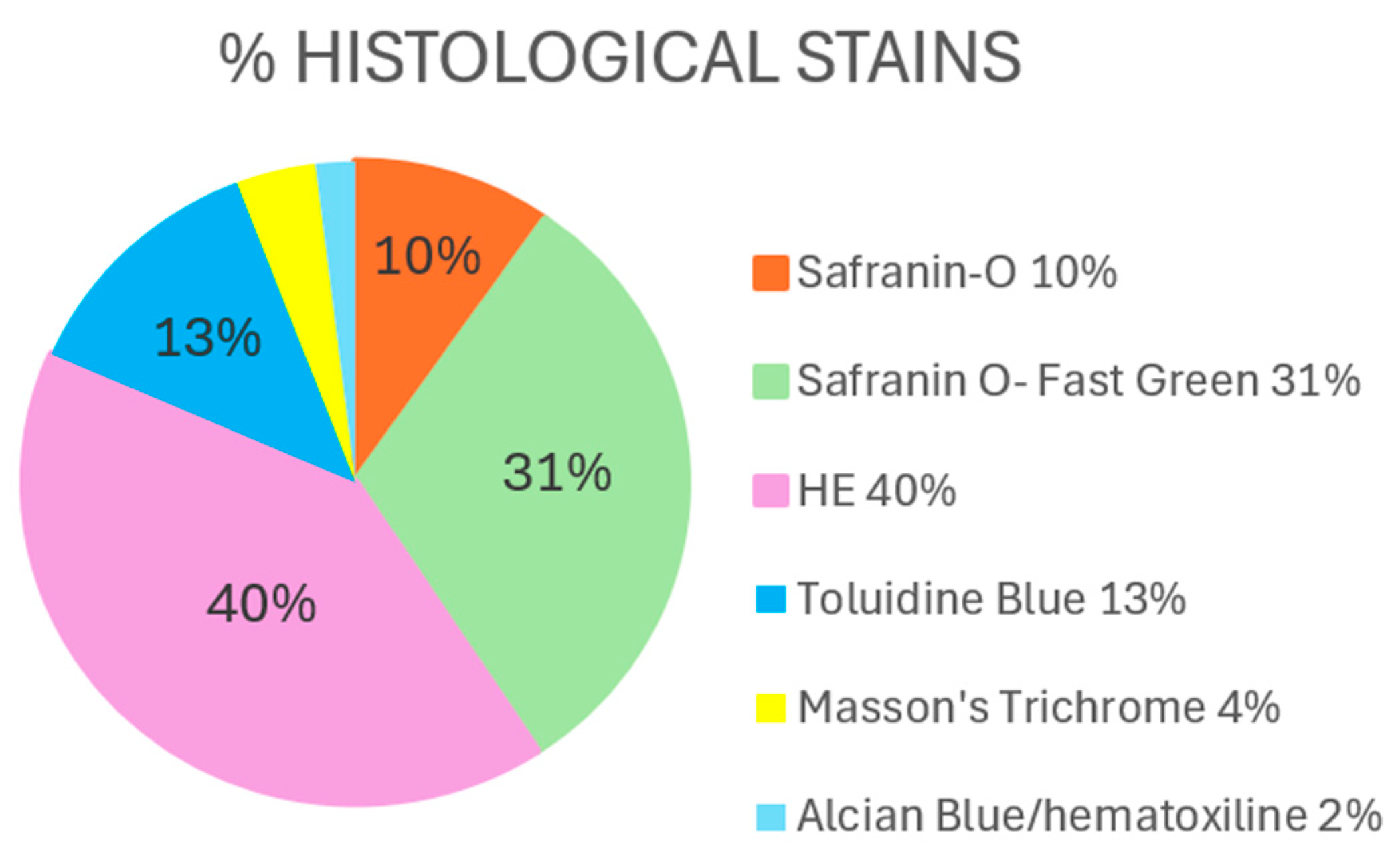
Hematoxylin–Eosin
- Control groups (healthy knees)
- OA groups (OA knees)
Safranin O/Safranin O–Fast Green
- Control groups (healthy knees)
- OA groups (OA knees)
Toluidine Blue
- Control groups (healthy knees)
- OA groups (OA knees)
Others
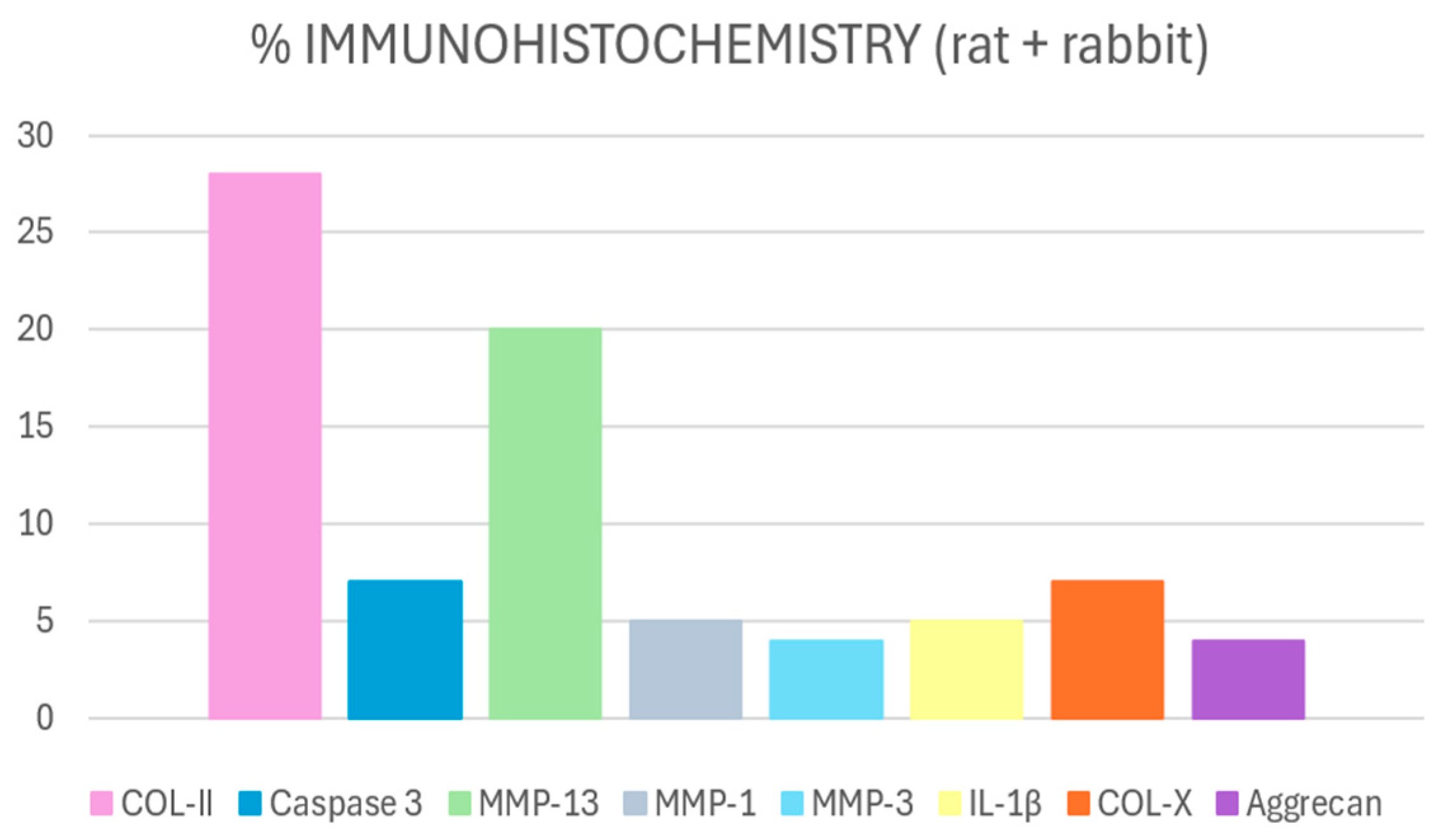
3.2.2. Immunohistochemical Findings
COL-II
MMP-13
Caspase-3
COL-X
Other Markers
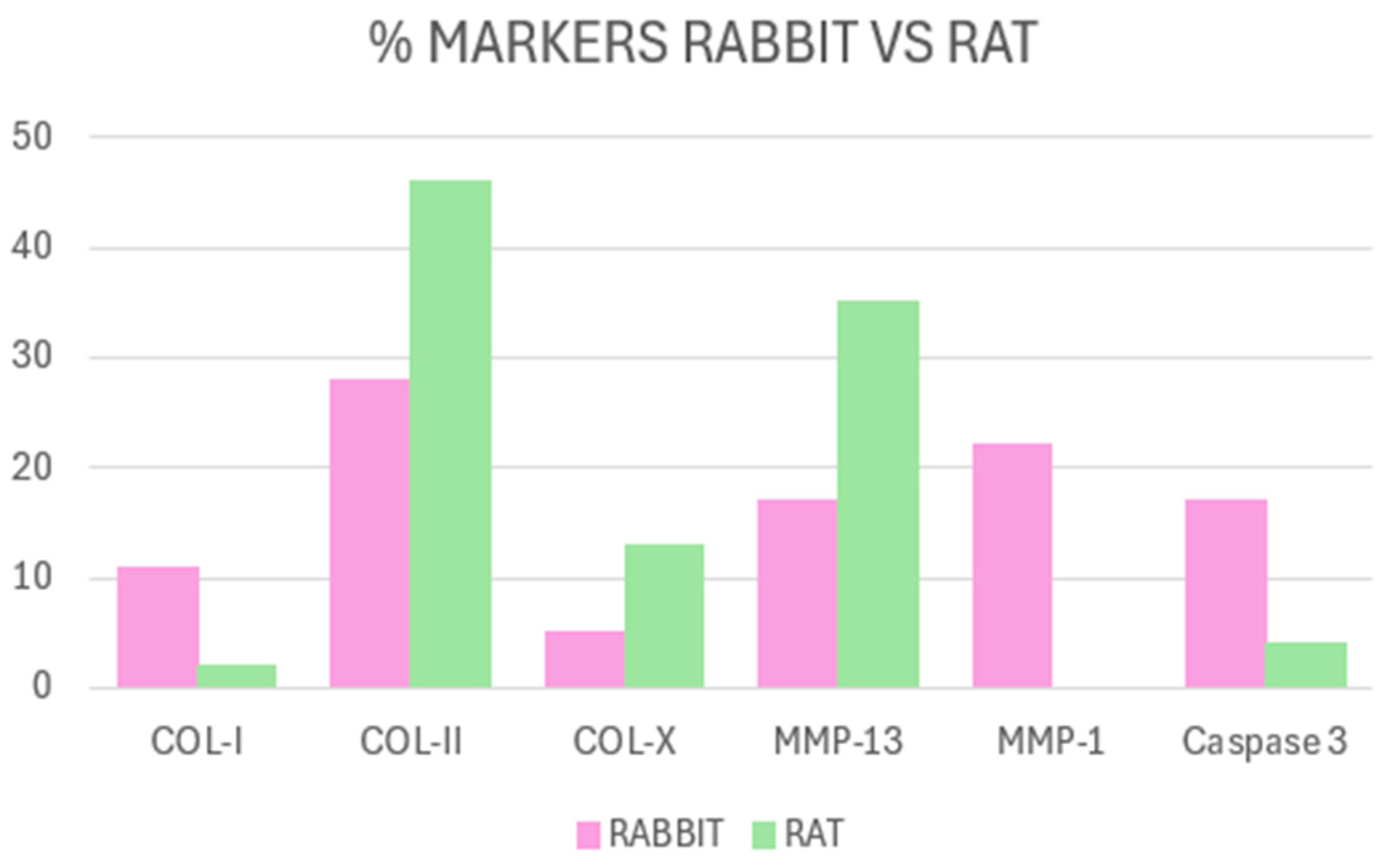
Difference According to the Animal Model Used
4. Discussion
4.1. Histology
4.2. Immunohistochemistry
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Articular Cartilage |
| ECM | Extracellular Matrix |
| COL-II | Type II Collagen |
| SZ | Superficial Zone |
| MZ | Middle Zone |
| DZ | Deep Zone |
| CC | Calcified Cartilage |
| SB | Subchondral Bone |
| OA | Osteoarthritis |
| MMPs | Matrix Metalloproteinases |
| IL-1β | Interleukin 1β |
| TNF-α | Tumor Necrosis Factor α |
| WOS | Web Of Science |
| HE | Hematoxylin–Eosin |
| SO | Safranin O |
| SO-FG | Safranin O–Fast Green |
| TB | Toluidine Blue |
| MT | Masson’s Trichrome |
| COL-X | Type X Collagen |
| iNOS | Inducible Nitric Oxide Synthase |
| RAGE | Receptor for Advanced Glycation End Products |
| NF-κB | Nuclear Factor Kappa |
| BMP-2 | Bone Morphogenetic Protein 2 |
| PACAP | Pituitary Adenylate Cyclase-Activating Polypeptide |
| COL-I | Type I Collagen |
| MMP-8 | Matrix Metalloproteinase 8 |
| MMP-9 | Matrix Metalloproteinase 9 |
| ADAMTS-4/5 | A Disintegrin And Metalloproteinase with Thrombospondin Motifs 4/5 |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| PCNA | Proliferating Cell Nuclear Antigen |
| LC3 | Microtubule-Associated Protein 1A/1B-Light Chain 3 |
| MIA | Sodium Monoiodoacetate |
References
- Martel-Pelletier, J.; Boileau, C.; Pelletier, J.-P.; Roughley, P.J. Cartilage in Normal and Osteoarthritis Conditions. Best Pract. Res. Clin. Rheumatol. 2008, 22, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Peng, Z.; Gorton, D.; Xiao, Y.; Ketheesan, N. Immunohistochemical Analysis of Structural Changes in Collagen for the Assessment of Osteoarthritis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Vaca-González, J.J.; Gutiérrez, M.L.; Garzón-Alvarado, D.A. Cartílago articular: Estructura, patologías y campos eléctricos como alternativa terapéutica. Revisión de conceptos actuales. Rev. Colomb. Ortop. Traumatol. 2017, 31, 202–210. [Google Scholar] [CrossRef]
- Erggelet, C.; Mandelbaum, B.R.; Mrosek, E.H.; Scopp, J.M. Principles of Cartilage Repair; Steinkopff: Heidelberg, Germany, 2008; p. 121. [Google Scholar] [CrossRef]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef]
- Akhtar, N.; Khan, N.M.; Ashruf, O.S.; Haqqi, T.M. Inhibition of Cartilage Degradation and Suppression of PGE2 and MMPs Expression by Pomegranate Fruit Extract in a Model of Posttraumatic Osteoarthritis. Nutrition 2017, 33, 1–13. [Google Scholar] [CrossRef]
- Van den Berg, W. Pathophysiology of Osteoarthritis. Jt. Bone Spine 2000, 67, 555–556. [Google Scholar] [CrossRef]
- Burrage, P.S. Matrix Metalloproteinases: Role in Arthritis. Front. Biosci. 2006, 11, 529. [Google Scholar] [CrossRef]
- Caron, J.P.; Fernandes, J.C.; Martel-Pelletier, J.; Tardif, G.; Mineau, F.; Geng, C.; Pelletier, J.-P. Chondroprotective Effect of Intraarticular Injections of Interleukin-1 Receptor Antagonist in Experimental Osteoarthritis. Suppression of Collagenase-1 Expression. Arthritis Rheum. 1996, 39, 1535–1544. [Google Scholar] [CrossRef]
- Yassin, A.M.; AbuBakr, H.O.; Abdelgalil, A.I.; Khattab, M.S.; El-Behairy, A.M.; Gouda, E.M. COL2A1 and Caspase-3 as Promising Biomarkers for Osteoarthritis Prognosis in an Equus asinus Model. Biomolecules 2020, 10, 354. [Google Scholar] [CrossRef]
- Karpiński, R.; Prus, A.; Baj, J.; Radej, S.; Prządka, M.; Krakowski, P.; Jonak, K. Articular Cartilage: Structure, Biomechanics, and the Potential of Conventional and Advanced Diagnostics. Appl. Sci. 2025, 15, 6896. [Google Scholar] [CrossRef]
- Longo, U.G.; Papalia, R.; De Salvatore, S.; Picozzi, R.; Sarubbi, A.; Denaro, V. Induced Models of Osteoarthritis in Animal Models: A Systematic Review. Biology 2023, 12, 283. [Google Scholar] [CrossRef]
- Mantripragada, V.P.; Piuzzi, N.S.; Zachos, T.; Obuchowski, N.A.; Muschler, G.F.; Midura, R.J. Histopathological Assessment of Primary Osteoarthritic Knees in Large Patient Cohort Reveal the Possibility of Several Potential Patterns of Osteoarthritis Initiation. Curr. Res. Transl. Med. 2017, 65, 133–139. [Google Scholar] [CrossRef]
- Hoemann, C.; Kandel, R.; Roberts, S.; Saris, D.B.F.; Creemers, L.; Mainil-Varlet, P.; Méthot, S.; Hollander, A.; Buschmann, M. International Cartilage Repair Society (ICRS) Recommended Guidelines for Histological Endpoints for Cartilage Repair Studies in Animal Models and Clinical Trials. Cartilage 2011, 2, 153–172. [Google Scholar] [CrossRef]
- Pauli, C.; Whiteside, R.; Heras, F.L.; Nesic, D.; Koziol, J.; Grogan, S.P.; Matyas, J.; Pritzker, K.P.H.; D’Lima, D.D.; Lotz, M.K. Comparison of Cartilage Histopathology Assessment Systems on Human Knee Joints at All Stages of Osteoarthritis Development. Osteoarthr. Cartil. 2012, 20, 476–485. [Google Scholar] [CrossRef]
- Ostergaard, K.; Salter, D.M. Immunohistochemistry in the Study of Normal and Osteoarthritic Articular Cartilage. Prog. Histochem. Cytochem. 1998, 33, III-165. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, J.; Zhao, S.; Wu, J.; Jin, Y.; Yu, L.; Wu, N.; Wu, Z.; Wang, Y.; Lin, M. ADAMTS5 in Osteoarthritis: Biological Functions, Regulatory Network, and Potential Targeting Therapies. Front. Mol. Biosci. 2021, 8, 703110. [Google Scholar] [CrossRef] [PubMed]
- Otterness, I.G.; Swindell, A.C.; Zimmerer, R.O.; Poole, A.R.; Ionescu, M.; Weiner, E. An Analysis of 14 Molecular Markers for Monitoring Osteoarthritis: Segregation of the Markers into Clusters and Distinguishing Osteoarthritis at Baseline. Osteoarthr. Cartil. 2000, 8, 180–185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, L.; Kazezian, Z.; Pitsillides, A.A.; Bull, A.M.J. A Synoptic Literature Review of Animal Models for Investigating the Biomechanics of Knee Osteoarthritis. Front. Bioeng. Biotechnol. 2024, 12, 1408015. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Fernandes, J.C.; Brunet, J.; Moldovan, F.; Schrier, D.; Flory, C.; Martel-Pelletier, J. In Vivo Selective Inhibition of Mitogen-Activated Protein Kinase Kinase 1/2 in Rabbit Experimental Osteoarthritis Is Associated with a Reduction in the Development of Structural Changes. Arthritis Rheum. 2003, 48, 1582–1593. [Google Scholar] [CrossRef]
- Kobayashi, T.; Notoya, K.; Nakamura, A.; Akimoto, K. Fursultiamine, a Vitamin B1 Derivative, Enhances Chondroprotective Effects of Glucosamine Hydrochloride and Chondroitin Sulfate in Rabbit Experimental Osteoarthritis. Inflamm. Res. 2005, 54, 249–255. [Google Scholar] [CrossRef]
- Tibesku, C.O.; Szuwart, T.; Ocken, S.A.; Skwara, A.; Fuchs, S. Increase in the Expression of the Transmembrane Surface Receptor CD44v6 on Chondrocytes in Animals with Osteoarthritis. Arthritis Rheum. 2005, 52, 810–817. [Google Scholar] [CrossRef]
- Jean, Y.H.; Wen, Z.H.; Chang, Y.C.; Hsieh, S.P.; Lin, J.D.; Tang, C.C.; Chen, W.F.; Chou, A.K.; Wong, C.S. Increase in Excitatory Amino Acid Concentration and Transporters Expression in Osteoarthritic Knees of Anterior Cruciate Ligament Transected Rabbits. Osteoarthr. Cartil. 2008, 16, 1442–1449. [Google Scholar] [CrossRef]
- Shirai, T.; Kobayashi, M.; Nishitani, K.; Satake, T.; Kuroki, H.; Nakagawa, Y.; Nakamura, T. Chondroprotective Effect of Alendronate in a Rabbit Model of Osteoarthritis. J. Orthop. Res. 2011, 29, 1572–1577. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, H.; Tian, F.; Song, H.; Zhang, Y. Enhancement of Subchondral Bone Quality by Alendronate Administration for the Reduction of Cartilage Degeneration in the Early Phase of Experimental Osteoarthritis. Clin. Exp. Med. 2011, 11, 235–243. [Google Scholar] [CrossRef]
- Jansen, H.; Meffert, R.H.; Birkenfeld, F.; Petersen, W.; Pufe, T. Detection of Vascular Endothelial Growth Factor (VEGF) in Moderate Osteoarthritis in a Rabbit Model. Ann. Anat.—Anat. Anz. 2012, 194, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wei, B.; Liu, S.; Mao, F.; Zhang, X.; Hu, J.; Zhou, J.; Yao, Q.; Xu, Y.; Wang, L. Cartilage Matrix Changes in Contralateral Mobile Knees in a Rabbit Model of Osteoarthritis Induced by Immobilization. BMC Musculoskelet. Disord. 2015, 16, 224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lin, X.; Xiong, Y.; Jiang, L.; Li, W.; Li, J.; Wu, L. Chondroprotective Effects of Palmatine on Osteoarthritis In Vivo and In Vitro: A Possible Mechanism of Inhibiting the Wnt/β-Catenin and Hedgehog Signaling Pathways. Int. Immunopharmacol. 2016, 34, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Fan, S.; Li, R.; Ouyang, Z.; Liu, Z.; Lin, Q. Effects of Ultrasound on Vascular Endothelial Growth Factor in Cartilage, Synovial Fluid, and Synovium in Rabbit Knee Osteoarthritis. Acta Biochim. Pol. 2020, 67, 379–385. [Google Scholar] [CrossRef]
- Shi, X.; Yu, W.; Wang, T.; Battulga, O.; Wang, C.; Shu, Q.; Yang, X.; Liu, C.; Guo, C. Electroacupuncture Alleviates Cartilage Degradation: Improvement in Cartilage Biomechanics via Pain Relief and Potentiation of Muscle Function in a Rabbit Model of Knee Osteoarthritis. Biomed. Pharmacother. 2020, 123, 109724. [Google Scholar] [CrossRef]
- Fang, L.; Lin, L.; Lv, Y.; Huang, Z.; Lin, X.; Wang, X.; Chen, B. The Mechanism of Aerobic Exercise Combined with Glucosamine Therapy and CircUNK in Improving Knee Osteoarthritis in Rabbits. Life Sci. 2021, 275, 119375. [Google Scholar] [CrossRef]
- Hossain, M.A.; Adithan, A.; Alam, M.J.; Kopalli, S.R.; Kim, B.; Kang, C.W.; Hwang, K.C.; Kim, J.H. IGF-1 Facilitates Cartilage Reconstruction by Regulating PI3K/AKT, MAPK, and NF-κB Signaling in Rabbit Osteoarthritis. J. Inflamm. Res. 2021, 14, 3555–3568. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Wu, Y.L.; Wei, W.; Zhang, Y.L.; Liu, D.; Ma, X.X.; Li, C.; Ma, Y.Y.; Ledda, M. Effect of Warm Acupuncture Combined with Bone Marrow Mesenchymal Stem Cells Transplantation on Cartilage Tissue in Rabbit Knee Osteoarthritis. Evid.-Based Complement. Altern. Med. 2021, 2021, 5523726. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Grad, S.; Wen, C.; Lai, Y.; Alini, M.; Qin, L.; Wang, X. An Impaired Healing Model of Osteochondral Defect in Papain-Induced Arthritis. J. Orthop. Transl. 2021, 26, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Alam, M.J.; Kim, B.; Kang, C.W.; Kim, J.H. Ginsenoside-Rb1 Prevents Bone Cartilage Destruction through Down-Regulation of p-Akt, p-P38, and p-P65 Signaling in Rabbit. Phytomedicine 2022, 100, 154039. [Google Scholar] [CrossRef]
- Chen, P.; Tang, S.; Gao, H.; Zhang, H.; Chen, C.; Fang, Z.; Peng, G.; Weng, H.; Chen, A.; Zhang, C.; et al. Wharton’s Jelly Mesenchymal Stem Cell-Derived Small Extracellular Vesicles as Natural Nanoparticles to Attenuate Cartilage Injury via microRNA Regulation. Int. J. Pharm. 2022, 623, 121952. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, L.; Shen, L.; Fang, Q.; Yang, Z.; Wang, R.; Wu, Q.; Xie, Y. Comparison of the Effects of Autologous and Allogeneic Purified Platelet-Rich Plasma on Cartilage Damage in a Rabbit Model of Knee Osteoarthritis. Front. Surg. 2022, 9, 911468. [Google Scholar] [CrossRef]
- Aktas, E.; Sener, E.; Gocun, P.U. Mechanically Induced Experimental Knee Osteoarthritis Benefits from Anti-Inflammatory and Immunomodulatory Properties of Simvastatin via Inhibition of Matrix Metalloproteinase-3. J. Orthop. Traumatol. 2011, 12, 145–151. [Google Scholar] [CrossRef]
- Moon, S.J.; Woo, Y.J.; Jeong, J.H.; Park, M.K.; Oh, H.J.; Park, J.S.; Kim, E.K.; Cho, M.L.; Park, S.H.; Kim, H.Y.; et al. Rebamipide Attenuates Pain Severity and Cartilage Degeneration in a Rat Model of Osteoarthritis by Downregulating Oxidative Damage and Catabolic Activity in Chondrocytes. Osteoarthr. Cartil. 2012, 20, 1426–1438. [Google Scholar] [CrossRef]
- Lee, J.; Hong, Y.S.; Jeong, J.H.; Yang, E.J.; Jhun, J.Y.; Park, M.K.; Jung, Y.O.; Min, J.K.; Kim, H.Y.; Park, S.H.; et al. Coenzyme Q10 Ameliorates Pain and Cartilage Degradation in a Rat Model of Osteoarthritis by Regulating Nitric Oxide and Inflammatory Cytokines. Edited by Z. Rakonczay. PLoS ONE 2013, 8, e69362. [Google Scholar] [CrossRef]
- Wen, Z.H.; Tang, C.C.; Chang, Y.C.; Huang, S.Y.; Chen, C.H.; Wu, S.C.; Hsieh, S.P.; Hsieh, C.S.; Wang, K.Y.; Lin, S.Y.; et al. Intra-Articular Injection of the Selective Cyclooxygenase-2 Inhibitor Meloxicam (Mobic) Reduces Experimental Osteoarthritis and Nociception in Rats. Osteoarthr. Cartil. 2013, 21, 1976–1986. [Google Scholar] [CrossRef]
- Di Rosa, M.; Szychlinska, M.A.; Tibullo, D.; Malaguarnera, L.; Musumeci, G. Expression of CHI3L1 and CHIT1 in Osteoarthritic Rat Cartilage Model. A Morphological Study. Eur. J. Histochem. 2014, 58, 2423. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Aoyama, T.; Ito, A.; Yamaguchi, S.; Nagai, M.; Tajino, J.; Zhang, X.; Kuroki, H. Effects of Short-Term Gentle Treadmill Walking on Subchondral Bone in a Rat Model of Instability-Induced Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Aoyama, T.; Tajino, J.; Ito, A.; Nagai, M.; Yamaguchi, S.; Zhang, X.; Kiyan, W.; Kuroki, H. Subchondral Plate Porosity Colocalizes with the Point of Mechanical Load during Ambulation in a Rat Knee Model of Post-Traumatic Osteoarthritis. Osteoarthr. Cartil. 2016, 24, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.; Milares, L.P.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.R.; Medalha, C.; Muniz Renno, A.C. Aerobic Exercise Training and Low-Level Laser Therapy Modulate Inflammatory Response and Degenerative Process in an Experimental Model of Knee Osteoarthritis in Rats. Osteoarthr. Cartil. 2016, 24, 169–177. [Google Scholar] [CrossRef]
- Wen, Z.H.; Tang, C.C.; Chang, Y.C.; Huang, S.Y.; Lin, Y.Y.; Hsieh, S.P.; Lee, H.P.; Lin, S.C.; Chen, W.F.; Jean, Y.H. Calcitonin Attenuates Cartilage Degeneration and Nociception in an Experimental Rat Model of Osteoarthritis: Role of TGF-β in Chondrocytes. Sci. Rep. 2016, 6, 28862. [Google Scholar] [CrossRef]
- Murata, K.; Kanemura, N.; Kokubun, T.; Fujino, T.; Morishita, Y.; Onitsuka, K.; Fujiwara, S.; Nakajima, A.; Shimizu, D.; Takayanagi, K. Controlling Joint Instability Delays the Degeneration of Articular Cartilage in a Rat Model. Osteoarthr. Cartil. 2017, 25, 297–308. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Wang, Y.; Bai, L. Transient Receptor Potential Vanilloid 5 Mediates Ca2+ Influx and Inhibits Chondrocyte Autophagy in a Rat Osteoarthritis Model. Cell. Physiol. Biochem. 2017, 42, 319–332. [Google Scholar] [CrossRef]
- Isaka, S.; Someya, A.; Nakamura, S.; Naito, K.; Nozawa, M.; Inoue, N.; Sugihara, I.; Kaneko, K. Evaluation of the Effect of Oral Administration of Collagen Peptides on an Experimental Rat Osteoarthritis Model. Exp. Ther. Med. 2017, 13, 2699–2706. [Google Scholar] [CrossRef]
- Chen, C.H.; Ho, M.L.; Chang, L.H.; Kang, L.; Lin, Y.S.; Lin, S.Y.; Wu, S.C.; Chang, J.K. Parathyroid Hormone-(1–34) Ameliorated Knee Osteoarthritis in Rats via Autophagy. J. Appl. Physiol. 2018, 124, 1177–1185. [Google Scholar] [CrossRef]
- Yan, B.; Zhou, L.; Wang, C.; Wang, R.; Yan, L.; Yu, L.; Liu, F.; Du, W.; Yu, G.; Yuan, Q.; et al. Intra-Articular Injection of Fructus Ligustri Lucidi Extract Attenuates Pain Behavior and Cartilage Degeneration in Mono-Iodoacetate Induced Osteoarthritic Rats. Front. Pharmacol. 2018, 9, 1360. [Google Scholar] [CrossRef]
- Jacer, S.; Shafaei, H.; Soleimani Rad, J. An Investigation on the Regenerative Effects of Intra Articular Injection of Co-Cultured Adipose Derived Stem Cells with Chondron for Treatment of Induced Osteoarthritis. Adv. Pharm. Bull. 2018, 8, 297–306. [Google Scholar] [CrossRef]
- Bei, M.; Tian, F.; Liu, N.; Zheng, Z.; Cao, X.; Zhang, H.; Wang, Y.; Xiao, Y.; Dai, M.; Zhang, L. A Novel Rat Model of Patellofemoral Osteoarthritis Due to Patella Baja, or Low-Lying Patella. Med. Sci. Monit. 2019, 25, 2702–2717. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhou, L.; Xie, D.; Du, W.; Chen, F.; Yuan, Q.; Tong, P.; Shan, L.; Efferth, T. Chondroprotective Effects of Platelet Lysate towards Monoiodoacetate-Induced Arthritis by Suppression of TNF-α-Induced Activation of NF-ĸB Pathway in Chondrocytes. Aging 2019, 11, 2797–2811. [Google Scholar] [CrossRef] [PubMed]
- Ping, S.H.; Tian, F.M.; Liu, H.; Sun, Q.; Shao, L.T.; Lian, Q.Q.; Zhang, L. Raloxifene Inhibits the Overexpression of TGF-β1 in Cartilage and Regulates the Metabolism of Subchondral Bone in Rats with Osteoporotic Osteoarthritis. Bosn. J. Basic Med. Sci. 2021, 21, 284. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, J.; Li, S.; Zhang, M.; He, B. Protective Effect of Ethyl Acetate Fraction from Semen Sojae Germinatum, the Processed Sprout of Chinese Black Soybean, on Rat Experimental Osteoarthritis. BMC Complement. Med. Ther. 2020, 20, 117. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Zhu, S.; Bi, R. Comparison of Early-Stage Changes of Osteoarthritis in Cartilage and Subchondral Bone between Two Different Rat Models. PeerJ 2020, 8, e8934. [Google Scholar] [CrossRef]
- Song, X.; Ma, T.; Hu, H.; Zhao, M.; Bai, H.; Wang, X.; Liu, L.; Li, T.; Sheng, X.; Xu, X.; et al. Chronic Circadian Rhythm Disturbance Accelerates Knee Cartilage Degeneration in Rats Accompanied by the Activation of the Canonical Wnt/β-Catenin Signaling Pathway. Front. Pharmacol. 2021, 12, 760988. [Google Scholar] [CrossRef]
- Luo, S.; Li, W.; Wu, W.; Shi, Q. Elevated Expression of MMP8 and MMP9 Contributes to Diabetic Osteoarthritis Progression in a Rat Model. J. Orthop. Surg. Res. 2021, 16, 64. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Z.; Sun, Z.; Liao, B.; Cai, Y. Recombinant Platelet-Derived Growth Factor-BB Alleviates Osteoarthritis in a Rat Model by Decreasing Chondrocyte Apoptosis In Vitro and In Vivo. J. Cell. Mol. Med. 2021, 25, 7472–7484. [Google Scholar] [CrossRef]
- Chen, Z.; Ge, Y.; Zhou, L.; Li, T.; Yan, B.; Chen, J.; Huang, J.; Du, W.; Lv, S.; Tong, P.; et al. Pain Relief and Cartilage Repair by Nanofat against Osteoarthritis: Preclinical and Clinical Evidence. Stem Cell Res. Ther. 2021, 12, 477. [Google Scholar] [CrossRef]
- Yan, B.; Lv, S.; Tong, P.; Yan, L.; Chen, Z.; Zhou, L.; Yuan, Q.; Guo, L.; Shan, L. Intra-Articular Injection of Adipose-Derived Stem Cells Ameliorates Pain and Cartilage Anabolism/Catabolism in Osteoarthritis: Preclinical and Clinical Evidences. Front. Pharmacol. 2022, 13, 854025. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.B.; Santana, B.H.D.; Martins, B.M.; Porto, E.S.; Severino, P.; Cardoso, J.C.; Souto, E.B.; Albuquerque-Júnior, R. Application of Formononetin for the Treatment of Knee Osteoarthritis Induced by Medial Meniscectomy in a Rodent Model. Appl. Sci. 2022, 12, 8591. [Google Scholar] [CrossRef]
- Emre Gökdemir, C.; Malik Okuyan, H.; Karaboğa, İ.; Yusuf Terzi, M.; Kalacı, A. Comparison of the Protective Effect of the Upper Zone of the Growth Plate and Unique Cartilage Matrix-Associated Protein with Hyaluronic Acid and Corticosteroids on an Experimental Rat Osteoarthritis Model. Arch. Rheumatol. 2024, 39, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, M.; Tang, J.; Ma, T.; Bai, H.; Wang, X.; Liu, L.; Li, T.; Xu, X.; Sheng, X.; et al. Dark-Light Cycle Disrupts Bone Metabolism and Suppresses Joint Deterioration in Osteoarthritic Rats. Arthritis Res. Ther. 2022, 24, 158. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Dai, T.; Jiao, S.; Xue, X.; Jiang, J.; Li, S.; Meng, Q. Pioglitazone-Loaded Cartilage-Targeted Nanomicelles (Pio@C-HA-DOs) for Osteoarthritis Treatment. Int. J. Nanomed. 2023, 18, 5871–5890. [Google Scholar] [CrossRef]
- Sun, Y.; Su, S.; Li, M.; Deng, A. Inhibition of miR-182-5p Targets FGF9 to Alleviate Osteoarthritis. Anal. Cell. Pathol. 2023, 2023, 5911546. [Google Scholar] [CrossRef]
- Han, J.; Zhan, L.N.; Huang, Y.; Guo, S.; Zhou, X.; Kapilevich, L.; Wang, Z.; Ning, K.; Sun, M.; Zhang, X.A. Moderate Mechanical Stress Suppresses Chondrocyte Ferroptosis in Osteoarthritis by Regulating NF-κB p65/GPX4 Signaling Pathway. Sci. Rep. 2024, 14, 5078. [Google Scholar] [CrossRef]
- Department of Anatomy and Embryology, Faculty of Medicine, Cairo University, Egypt; Haleem, N.Y.A.; Nasralla, M.M.; Abd El-Moaty, M.M.; Farid, E.F.; Shuaib, D.M. Possible Therapeutic Effect of Combined Platelet-Rich Plasma and Stem Cells on Induced Knee Osteoarthritis in Adult Male Albino Rat: Histological and Immunohistochemical Study. EJA 2024, 28, 189–205. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, Y.C.; Yang, K.C.; Lin, Y.F.; Wu, A.T.H.; Tseng, C.L. Development and Functional Evaluation of a Hyaluronic Acid Coated Nano-Formulation with Kaempferol as a Novel Intra-Articular Agent for Knee Osteoarthritis Treatment. Biomed. Pharmacother. 2024, 175, 116717. [Google Scholar] [CrossRef]
- Guo, X.; Feng, X.; Yang, Y.; Zhang, H.; Bai, L. Spermidine Attenuates Chondrocyte Inflammation and Cellular Pyroptosis through the AhR/NF-κB Axis and the NLRP3/Caspase-1/GSDMD Pathway. Front. Immunol. 2024, 15, 1462777. [Google Scholar] [CrossRef]
- Giunta, S.; Castorina, A.; Marzagalli, R.; Szychlinska, M.; Pichler, K.; Mobasheri, A.; Musumeci, G. Ameliorative Effects of PACAP against Cartilage Degeneration. Morphological, Immunohistochemical and Biochemical Evi-dence from In Vivo and In Vitro Models of Rat Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 5922–5944, Correction in Int. J. Mol. Sci. 2024, 25, 12148. [Google Scholar] [CrossRef]
- Zhao, Z.; Ji, H.; Jing, R.; Liu, C.; Wang, M.; Zhai, L.; Bai, X.; Xing, G. Extracorporeal Shock-Wave Therapy Reduces Progression of Knee Osteoarthritis in Rabbits by Reducing Nitric Oxide Level and Chondrocyte Apoptosis. Arch. Orthop. Trauma Surg. 2012, 132, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Fang, L.; Lin, L.; Lv, Y.; Huang, Z.; Lin, X.; Wang, X. Aerobic Exercise Combined with Glucosamine Hydrochloride Capsules Inhibited the Apoptosis of Chondrocytes in Rabbit Knee Osteoarthritis by Affecting TRPV5 Expression. Gene 2022, 830, 146465. [Google Scholar] [CrossRef] [PubMed]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI Histopathology Initiative—Recommendations for Histological Assessments of Osteoarthritis in the Rat. Osteoarthr. Cartil. 2010, 18, S24–S34. [Google Scholar] [CrossRef]
- Hyllested, J.L.; Veje, K.; Ostergaard, K. Histochemical Studies of the Extracellular Matrix of Human Articular Cartilage—A Review. Osteoarthr. Cartil. 2002, 10, 333–343. [Google Scholar] [CrossRef]
- Laverty, S.; Girard, C.A.; Williams, J.M.; Hunziker, E.B.; Pritzker, K.P.H. The OARSI Histopathology Initiative—Recommendations for Histological Assessments of Osteoarthritis in the Rabbit. Osteoarthr. Cartil. 2010, 18, S53–S65. [Google Scholar] [CrossRef]
- Cook, J.L.; Kuroki, K.; Visco, D.; Pelletier, J.P.; Schulz, L.; Lafeber, F.P.J.G. The OARSI Histopathology Initiative—Recommendations for Histological Assessments of Osteoarthritis in the Dog. Osteoarthr. Cartil. 2010, 18, S66–S79. [Google Scholar] [CrossRef]
- Lotz, M.; Martel-Pelletier, J.; Christiansen, C.; Brandi, M.L.; Bruyère, O.; Chapurlat, R.; Collette, J.; Cooper, C.; Giacovelli, G.; Kanis, J.A.; et al. Value of Biomarkers in Osteoarthritis: Current Status and Perspectives. Ann. Rheum. Dis. 2013, 72, 1756–1763. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, S.; Su, H.; Cheng, J. Isoliquiritigenin Inhibits IL-1β-Induced Production of Matrix Metalloproteinase in Articular Chondrocytes. Mol. Ther.—Methods Clin. Dev. 2018, 9, 153–159. [Google Scholar] [CrossRef]
- Longo, U.G.; Loppini, M.; Fumo, C.; Rizzello, G.; Khan, W.S.; Maffulli, N.; Denaro, V. Osteoarthritis: New Insights in Animal Models. Open Orthop. J. 2012, 6, 558–563. [Google Scholar] [CrossRef]
- Tang, H.; Qin, K.; Wang, A.; Li, S.; Fang, S.; Gao, W.; Lu, M.; Huang, W.; Zhang, H.; Yin, Z. 3,3′-Diindolylmethane Inhibits LPS-Induced Human Chondrocytes Apoptosis and Extracellular Matrix Degradation by Activating PI3K-Akt-mTOR-Mediated Autophagy. Front. Pharmacol. 2022, 13, 999851. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.M. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet. Pathol. 2015, 52, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Toyoda, F.; Staunton, C.A.; Maeda, T.; Okumura, N.; Matsuura, H.; Matsusue, Y.; Imai, S.; Barrett-Jolley, R. Activation of a Chondrocyte Volume-Sensitive Cl − Conductance Prior to Macroscopic Cartilage Lesion Formation in the Rabbit Knee Anterior Cruciate Ligament Transection Osteoarthritis Model. Osteoarthr. Cartil. 2016, 24, 1786–1794. [Google Scholar] [CrossRef]
- Guo, Y.N.; Cui, S.J.; Tian, Y.J.; Zhao, N.R.; Zhang, Y.D.; Gan, Y.H.; Zhou, Y.H.; Wang, X.D. Chondrocyte Apoptosis in Temporomandibular Joint Osteoarthritis Promotes Bone Resorption by Enhancing Chemotaxis of Osteoclast Precursors. Osteoarthr. Cartil. 2022, 30, 1140–1153. [Google Scholar] [CrossRef]
- Wang, X.D.; Kou, X.X.; He, D.Q.; Zeng, M.M.; Meng, Z.; Bi, R.Y.; Liu, Y.; Zhang, J.N.; Gan, Y.H.; Zhou, Y.H.; et al. Progression of Cartilage Degradation, Bone Resorption and Pain in Rat Temporomandibular Joint Osteoarthritis Induced by Injection of Iodoacetate. PLoS ONE 2012, 7, e45036. [Google Scholar] [CrossRef]
- Wu, H.; Du, J.; Zheng, Q. Expression of MMP-1 in Cartilage and Synovium of Experimentally Induced Rabbit ACLT Traumatic Osteoarthritis: Immunohistochemical Study. Rheumatol. Int. 2008, 29, 31–36. [Google Scholar] [CrossRef]
- He, B.; Wu, J.P.; Kirk, T.B.; Carrino, J.A.; Xiang, C.; Xu, J. High-Resolution Measurements of the Multilayer Ultra-Structure of Articular Cartilage and Their Translational Potential. Arthritis Res. Ther. 2014, 16, 205. [Google Scholar] [CrossRef]
- Wu, J.P.; Swift, B.J.; Becker, T.; Squelch, A.; Wang, A.; Zheng, Y.C.; Zhao, X.; Xu, J.; Xue, W.; Zheng, M.; et al. High-Resolution Study of the 3D Collagen Fibrillary Matrix of Achilles Tendons without Tissue Labelling and Dehydrating. J. Microsc. 2017, 266, 273–287. [Google Scholar] [CrossRef]
- Cigdem, O.; Deniz, C.M. Artificial Intelligence in Knee Osteoarthritis: A Comprehensive Review for 2022. Osteoarthr. Imaging 2023, 3, 100161. [Google Scholar] [CrossRef]
- Khader, A.; Alquran, H. Automated Prediction of Osteoarthritis Level in Human Osteochondral Tissue Using Histopathological Images. Bioengineering 2023, 10, 764. [Google Scholar] [CrossRef]
| Author/Year | Immunohistochemistry | Histology |
|---|---|---|
| Pelletier et al., 2003 [20] | MMP-1 | Safranin O |
| Kobayashi et al., 2005 [21] | MMP-1 | Safranin O–Fast Green |
| Tibesku et al., 2005 [22] | CD44v6 | Safranin O |
| Jean et al., 2008 [23] | GLAST, GLT-1 | HE, Safranin O–Fast Green |
| Shirai et al., 2011 [24] | MMP-13, IL-1β, COL-X, VEGF, RANKL | HE, Safranin O |
| Zhang et al., 2011 [25] | MMP-13, BMP-2 | HE, Safranin O |
| Jansen et al., 2012 [26] | VEGF | HE, Safranin O |
| Zhou et al., 2015 [27] | COL-I, COL-II | Safranin O–Fast Green, Sirius Red |
| Zhou et al., 2016 [28] | β-Catenin, Shh, Gli-2 | HE, Safranin O–Fast Green |
| Akhtar et al., 2017 [6] | Caspase-3, p85 | Safranin O–Fast Green |
| Luo et al., 2020 [29] | VEGF | HE |
| Shi et al., 2020 [30] | COL-II | Safranin O–Fast Green |
| Fang et al., 2021 [31] | COL-II | HE, Toluidine Blue |
| Hossain et al., 2021 [32] | MMP-1 | HE, Safranin O, Masson’s trichrome |
| Liu et al., 2021 [33] | Bax, BcL, Caspase-3 | HE, Safranin O–Fast Green, Toluidine Blue |
| Meng et al., 2021 [34] | COL-I, COL-II, MMP-3 | HE, Safranin O–Fast Green, Toluidine Blue |
| Hossain et al., 2022 [35] | MMP-1 | HE, Safranin O, Masson’s trichrome |
| Chen et al., 2022 [36] | Caspase-3, Caspase-9, COL-II | HE |
| Wang et al., 2022 [37] | BMP-2, SOX-9 | HE |
| Author/Year | Immunohistochemistry | Histology |
|---|---|---|
| Aktas et al., 2011 [38] | MMP-3 | HE |
| Moon et al., 2012 [39] | MMP-13, iNOS, Nitrotyrosine | HE, Safranin O–Fast Green, Toluidine Blue |
| Lee et al., 2013 [40] | MMP-13, iNOS, RAGE | HE, Safranin O–Fast Green, Toluidine Blue |
| Wen et al., 2013 [41] | p38, JNK, phospho-ERK | HE |
| Di Rosa et al., 2014 [42] | CHI3L1, CHIT1 | HE |
| Iijima et al., 2015 [43] | COL-II | Toluidine Blue |
| Iijima et al., 2016 [44] | COL-II | Toluidine Blue |
| Assis et al., 2016 [45] | IL-1β, Caspase-3, MMP-13 | HE |
| Wen et al., 2016 [46] | TGF-β1 | HE, Safranin O–Fast Green |
| Murata et al., 2017 [47] | TNF-α, IL-1β, Caspase-3 | Safranin O–Fast Green, Toluidine Blue |
| Wei et al., 2017 [48] | LC3B | HE, Toluidine Blue |
| Isaka et al., 2017 [49] | COL-II, MMP-13 | Toluidine Blue |
| Chen et al., 2018 [50] | COL-II, COL-X | Safranin O–Fast Green |
| Yan et al., 2018 [51] | MMP-13, COL-II, COL-X | HE, Safranin O–Fast Green, Alcian Blue/Hematoxylin |
| Jacer et al., 2018 [52] | COL-II | HE, Toluidine Blue |
| Bei et al., 2019 [53] | COL-II, MMP-3 | HE, Safranin O–Fast Green |
| Yan et al., 2019 [54] | MMP-13, COL-II | HE, Safranin O–Fast Green, Alcian Blue/Hematoxylin |
| Ping et al., 2020 [55] | MMP-13, ADAMTS-5, COL-X, COL-II, TGF-β1, Aggrecan | Toluidine Blue |
| Wang et al., 2020 [56] | MMP-13, COL-II | HE, Safranin O–Fast Green |
| Yang et al., 2020 [57] | COL-II, MMP-13, RUNX2 | HE, Toluidine Blue |
| Song et al., 2021 [58] | MMP-3, MMP-13, ADAMTS-4, Aggrecan, COL-II | Safranin O–Fast Green |
| Luo et al., 2021 [59] | COL-I, COL-II, MMP-8, MMP-9 | Safranin O–Fast Green |
| Zhu et al., 2021 [60] | COL-II, COL-X | HE, Toluidine Blue |
| Chen et al., 2021 [61] | COL-II, COL-X, MMP-13 | HE, Safranin O–Fast Green |
| Yan et al., 2022 [62] | MMP-13, COL-II, COL-X | HE, Safranin O–Fast Green, Alcian Blue/Hematoxylin |
| Barreto et al., 2022 [63] | COL-I,I IL-1β | Safranin O–Fast Green |
| Gökdemir et al., 2022 [64] | BPM-2, NF-κb | HE, Safranin O–Fast Green, Toluidine Blue |
| Song et al., 2022 [65] | MMP-3, MMP-13 | Safranin O–Fast Green |
| Chen et al., 2022 [36] | COL-II, MMP-13, ADAMTS-5 | HE, Safranin O–Fast Green |
| Chen et al., 2023 [66] | COL-II, MMP-13, Aggrecan, ADAMTS-4 | HE, Safranin O–Fast Green |
| Sun et al., 2023 [67] | Ki67 | HE, Safranin O–Fast Green |
| Han et al., 2024 [68] | COL-II, MMP-13 | HE, Toluidine Blue |
| Haleem et al., 2024 [69] | COL-II, PCNA | HE, Alcian Blue, Masson’s trichrome |
| Lee et al., 2024 [70] | COL-II, MMP-9, Aggrecan, IL-1β | HE, Safranin O–Fast Green |
| Guo et al., 2024 [71] | COL-II, IL-6, NF-κb, p65 | HE, Safranin O–Fast Green, Toluidine Blue |
| Giunta et al., 2024 [72] | PACAP | HE, Toluidine Blue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabucedo-Suárez, A.; Permuy, M.; Muñoz, F.; López-Peña, M. Histological and Immunohistochemical Methods in Normal and Osteoarthritic Knee Cartilage of Rat and Rabbit Models: A Literature Review. Int. J. Mol. Sci. 2025, 26, 10300. https://doi.org/10.3390/ijms262110300
Sabucedo-Suárez A, Permuy M, Muñoz F, López-Peña M. Histological and Immunohistochemical Methods in Normal and Osteoarthritic Knee Cartilage of Rat and Rabbit Models: A Literature Review. International Journal of Molecular Sciences. 2025; 26(21):10300. https://doi.org/10.3390/ijms262110300
Chicago/Turabian StyleSabucedo-Suárez, Ana, María Permuy, Fernando Muñoz, and Mónica López-Peña. 2025. "Histological and Immunohistochemical Methods in Normal and Osteoarthritic Knee Cartilage of Rat and Rabbit Models: A Literature Review" International Journal of Molecular Sciences 26, no. 21: 10300. https://doi.org/10.3390/ijms262110300
APA StyleSabucedo-Suárez, A., Permuy, M., Muñoz, F., & López-Peña, M. (2025). Histological and Immunohistochemical Methods in Normal and Osteoarthritic Knee Cartilage of Rat and Rabbit Models: A Literature Review. International Journal of Molecular Sciences, 26(21), 10300. https://doi.org/10.3390/ijms262110300






