Exploring the Overlap of MASLD and IBD: Insights from a Single-Center Experience
Abstract
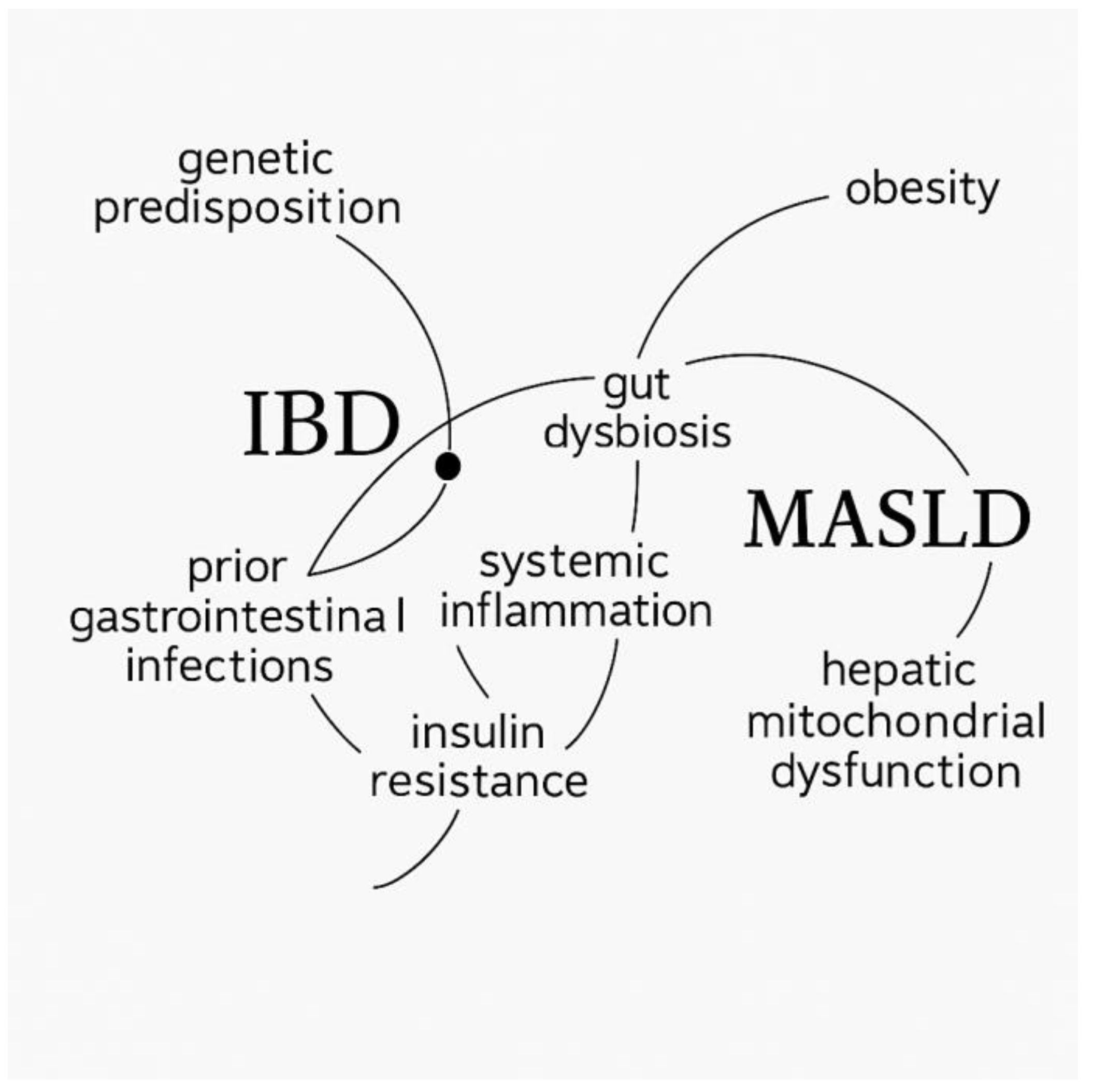
1. Introduction
2. Results
3. Discussion
Limitations
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AP | Alkaline phosphatase |
| APRI | AST-to-platelet ratio index |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| CAP | Controlled attenuation parameter |
| CD | Crohn’s disease |
| CRP | C-reactive protein |
| FIB-4 | Fibrosis-4 score |
| GGT | Gamma-glutamyl transferase |
| Hb | Hemoglobin |
| HbA1c | Glycated hemoglobin |
| HDL | High-density lipoprotein cholesterol |
| HBI | Harvey–Bradshaw Index |
| IBD | Inflammatory bowel disease |
| IC | Indeterminate colitis |
| kPa | Kilopascal |
| LDL-c | Low-density lipoprotein cholesterol |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MRI | Magnetic resonance imaging |
| PIIINP | Procollagen III amino-terminal peptide |
| PLT | Platelets |
| PNPLA3-I148M | Isoleucine-to-methionine substitution at position 148 in the patatin-like phospholipase domain containing 3 protein |
| TB | Total bilirubin |
| TG | Triglycerides |
| TIMP-1 | Tissue inhibitor of metalloproteinases-1 |
| UC | Ulcerative colitis |
| UHRatio | Uric acid-to-HDL cholesterol ratio |
References
- Yamada, T.; Alpers, D.H. (Eds.) Textbook of Gastroenterology, 5th ed.; Blackwell Publishing: Chichester, UK; Hoboken, NJ, USA, 2009. [Google Scholar]
- Maresca, R.; Mignini, I.; Varca, S.; Calvez, V.; Termite, F.; Esposto, G.; Laterza, L.; Scaldaferri, F.; Ainora, M.E.; Gasbarrini, A.; et al. Inflammatory Bowel Diseases and Non-Alcoholic Fatty Liver Disease: Piecing a Complex Puzzle Together. Int. J. Mol. Sci. 2024, 25, 3278. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Li, Y.-Y. Inflammatory Bowel Disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef]
- Mahfouz, M.; Martin, P.; Carrion, A.F. Hepatic Complications of Inflammatory Bowel Disease. Clin. Liver Dis. 2019, 23, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Restellini, S.; Chazouillères, O.; Frossard, J.-L. Hepatic Manifestations of Inflammatory Bowel Diseases. Liver Int. 2017, 37, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The Global Epidemiology of Nonalcoholic Fatty Liver Disease (MASLD) and Nonalcoholic Steatohepatitis (NASH): A Systematic Review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A Position Statement on MASLD/NASH Based on the EASL 2009 Special Conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef]
- Thanapirom, K.; Tsochatzis, E.A. Non-Alcoholic Fatty Liver Disease (MASLD) and the Quest for Effective Treatments. Hepatobiliary Surg. Nutr. 2019, 8, 77–79. [Google Scholar] [CrossRef]
- Zeigerer, A. MASLD—A Rising Metabolic Disease. Mol. Metab. 2021, 50, 101274. [Google Scholar] [CrossRef]
- Kneeman, J.M.; Misdraji, J.; Corey, K.E. Secondary Causes of Nonalcoholic Fatty Liver Disease. Ther. Adv. Gastroenterol. 2012, 5, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.C.; Lübbe, F.; Bressem, K.; Wagner, M.; Hamm, B.; Makowski, M.R. Non-Alcoholic Fatty Liver Disease in Underweight Patients with Inflammatory Bowel Disease: A Case-Control Study. PLoS ONE 2018, 13, e0206450. [Google Scholar] [CrossRef]
- Palumbo, C.S.; Restellini, S.; Chao, C.-Y.; Aruljothy, A.; Lemieux, C.; Wild, G.; Afif, W.; Lakatos, P.L.; Bitton, A.; Cocciolillo, S.; et al. Screening for Nonalcoholic Fatty Liver Disease in Inflammatory Bowel Diseases: A Cohort Study Using Transient Elastography. Inflamm. Bowel Dis. 2018, 25, 124–133. [Google Scholar] [CrossRef]
- Spengler, E.K.; Loomba, R. Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of MASLD and NASH. Mayo Clin. Proc. 2015, 90, 1233–1246. [Google Scholar] [CrossRef]
- Beaton, M.D. Current Treatment Options for Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Can. J. Gastroenterol. 2012, 26, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Domínguez, S.J.; García-Mateo, S.; Laredo, V.; Gargallo-Puyuelo, C.J.; Gallego Llera, B.; López de la Cruz, J.; Gomollón, F. Liver Fibrosis in Non-Alcoholic Fatty Liver Disease and Progression to Hepatocellular Carcinoma in Patients with Inflammatory Bowel Disease: A Systematic Review. Cancers 2023, 15, 3367. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, J.; Luo, M. Association of Inflammatory Cytokines with Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 880298. [Google Scholar] [CrossRef]
- Sun, H.-H.; Tian, F. Inflammatory Bowel Disease and Cardiovascular Disease Incidence and Mortality: A Meta-Analysis. Eur. J. Prev. Cardiol. 2018, 25, 1623–1631. [Google Scholar] [CrossRef]
- Nasir, K.; Acquah, I.; Dey, A.K.; Agrawal, T.; Hassan, S.Z.; Glassner, K.; Abraham, B.; Quigley, E.M.M.; Blankstein, R.; Virani, S.S.; et al. Inflammatory Bowel Disease and Atherosclerotic Cardiovascular Disease in U.S. Adults—A Population-Level Analysis in the National Health Interview Survey. Am. J. Prev. Cardiol. 2022, 9, 100316. [Google Scholar] [CrossRef]
- Negreanu, A.M.; Stemate, A.; Spataru, T.; Negreanu, L.J. Early Atherosclerosis and Acute Vascular Events in Ulcerative Colitis Patients—A Case Series. Mod. Med. 2022, 29, 123–127. [Google Scholar] [CrossRef]
- Ritaccio, G.; Stoleru, G.; Abutaleb, A.; Cross, R.K.; Shetty, K.; Sakiani, S.; Wong, U. Nonalcoholic Fatty Liver Disease Is Common in IBD Patients; However, Progression to Hepatic Fibrosis by Noninvasive Markers Is Rare. Dig. Dis. Sci. 2020, 66, 3186–3191. [Google Scholar] [CrossRef] [PubMed]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The Microbiome and Inflammatory Bowel Disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 2019, 576, 143–148. [Google Scholar] [CrossRef]
- Li, R.; Andreu-Sánchez, S.; Kuipers, F.; Fu, J.; Torres-Fuentes, C.; Zheng, X.; Su, J.; Zhou, L.; Hoekstra, M.; Fu, J.; et al. Bile Acids and Gut Microbiota in Inflammatory Bowel Disease. Front. Physiol. 2022, 13, 833233. [Google Scholar]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. FXR and TGR5 in Metabolic-Associated Fatty Liver Disease. Hepatology 2018, 68, 1418–1432. [Google Scholar]
- De Preter, V.; Machiels, K.; Joossens, M.; Arijs, I.; Matthys, C.; Vermeire, S.; Rutgeerts, P.; Evenepoel, P.; Verbeke, K.; Van Oudenhove, L.; et al. Gut Microbial Metabolism and SCFA in Crohn’s Disease. J. Crohn’s Colitis 2015, 9, 387–394. [Google Scholar]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P.; Miele, L.; Valenti, L.; et al. Intestinal Permeability and Liver Inflammation in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 49–58. [Google Scholar]
- Yeh, J.; Kim, J.; Bae, J.S.; Lee, J.; Kim, B.; Kim, W.; Park, J.; Lee, H.; Choi, Y.; Kim, D.; et al. Cross-Talk between Gut Inflammation and MASLD. Hepatol. Int. 2023, 17, 12–24. [Google Scholar]
- Albillos, A.; Martin-Mateos, R.; Van der Merwe, S.; Wiest, R.; Jalan, R.; Álvarez-Mon, M. Cirrhosis-Associated Immune Dysfunction. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 112–134. [Google Scholar] [CrossRef]
- Likhitsup, A. High Prevalence of Non-Alcoholic Fatty Liver Disease in Patients with Inflammatory Bowel Disease Receiving Anti-Tumor Necrosis Factor Therapy. Ann. Gastroenterol. 2019, 32, 463–468. [Google Scholar] [CrossRef]
- Voss, J.; Schneider, C.V.; Kleinjans, M.; Bruns, T.; Trautwein, C.; Strnad, P. Hepatobiliary Phenotype of Individuals with Chronic Intestinal Disorders. Sci. Rep. 2021, 11, 19954. [Google Scholar] [CrossRef]
- Lin, A.; Roth, H.; Anyane-Yeboa, A.; Rubin, D.T.; Paul, S. Prevalence of Nonalcoholic Fatty Liver Disease in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2020, 27, 947–955. [Google Scholar] [CrossRef]
- Gizard, E.; Ford, A.C.; Bronowicki, J.-P.; Peyrin-Biroulet, L. Systematic Review: The Epidemiology of the Hepatobiliary Manifestations in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2014, 40, 3–15. [Google Scholar] [CrossRef]
- Aggarwal, M.; Garg, R.; Parthasarthy, G.; Nowacki, A.S.; Padival, R.; McCullough, A.; Qazi, T.; Click, B.; Rieder, F.; Cohen, B.L. Crohn’s Disease Is Associated with Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2022, 68, 1006–1015. [Google Scholar] [CrossRef]
- Bessissow, T.; Le, N.H.; Rollet, K.; Afif, W.; Bitton, A.; Sebastiani, G. Incidence and Predictors of Nonalcoholic Fatty Liver Disease by Serum Biomarkers in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Jung, V.; Behnisch, R.; Gauss, A. Prevalence and Risk Factors of Nonalcoholic Fatty Liver Disease in Patients with Inflammatory Bowel Diseases: A Cross-Sectional and Longitudinal Analysis. World J. Gastroenterol. 2020, 26, 7367–7381. [Google Scholar] [CrossRef]
- Principi, M.; Iannone, A.; Losurdo, G.; Mangia, M.; Shahini, E.; Albano, F.; Rizzi, S.F.; La Fortezza, R.F.; Lovero, R.; Contaldo, A.; et al. Nonalcoholic Fatty Liver Disease in Inflammatory Bowel Disease: Prevalence and Risk Factors. Inflamm. Bowel Dis. 2018, 24, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Huang, K.; Zhang, X.; Wu, Z.; Wu, Y.; Chu, J.; Kong, W.; Qian, G. Association of Serum Uric Acid-to-High-Density Lipoprotein Cholesterol Ratio with Non-Alcoholic Fatty Liver Disease in American Adults: A Population-Based Analysis. Front. Med. 2023, 10, 1164096. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Dutta, U.; Sharma, V.; Tiwari, A.; Kedia, S.; Kurrey, L.; Jain, S.; Singh, V.; Sahu, P.; Das, P.; et al. Metabolic Dysfunction—Associated Steatotic Liver Disease in Inflammatory Bowel Disease: Emerging Links and Preventive Strategies. Clin. Gastroenterol. Hepatol. 2023, 21, 2041–2052. [Google Scholar]
- Lazaridis, N.; Tsochatzis, E.A.; Mantzaris, G.J. Hepatic Steatosis in Inflammatory Bowel Disease: Pathogenesis, Clinical Significance, and Management. J. Crohn’s Colitis 2023, 17, 91–101. [Google Scholar]
- Yaccob, A.; Mari, A. Practical Clinical Approach to the Evaluation of Hepatobiliary Disorders in Inflammatory Bowel Disease. Frontline Gastroenterol. 2019, 10, 309–315. [Google Scholar] [CrossRef] [PubMed]
| Steatosis Grade | N | CRP (mg/dL) | Mean Rank | p-Value |
|---|---|---|---|---|
| Grade 1 | 49 | 4.23 ± 1.86 | 31.04 | |
| Grade 3 | 1 | 2.15 ± – | 17.50 | |
| Grade 4 | 8 | 3.52 ± 1.47 | 21.56 | |
| Total | 58 | 0.247 |
| Fibrosis Grade | N | CRP (mg/L, Mean ± SD) | p-Value |
|---|---|---|---|
| Grade 1 | 57 | 1.02 ± 1.34 | |
| Grade 2 | 1 | 0.20 ± – | 0.016 |
| Fibrosis Grade | N | Fecal Calprotectin (µg/g, Mean ± SD) | p-Value |
|---|---|---|---|
| Grade 1 | 57 | 572.47 ± 954.99 | |
| Grade 2 | 1 | 22.00 ± – | 0.022 |
| Steatosis Grade | N | UHRatio (Mean Rank) | Sum of Ranks | p-Value |
|---|---|---|---|---|
| Grade 0 | 49 | 27.84 | 1364.00 | |
| Grade 1 | 9 | 38.56 | 347.00 | 0.044 |
| Total | 58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stemate, A.; Negru-Vodă, D.-I.; Mazurencu-Pele, A.M.P.; Popescu, R.-F.; Spătaru, T.-I.; Negreanu, L. Exploring the Overlap of MASLD and IBD: Insights from a Single-Center Experience. Int. J. Mol. Sci. 2025, 26, 10288. https://doi.org/10.3390/ijms262110288
Stemate A, Negru-Vodă D-I, Mazurencu-Pele AMP, Popescu R-F, Spătaru T-I, Negreanu L. Exploring the Overlap of MASLD and IBD: Insights from a Single-Center Experience. International Journal of Molecular Sciences. 2025; 26(21):10288. https://doi.org/10.3390/ijms262110288
Chicago/Turabian StyleStemate, Ana, Delia-Ionela Negru-Vodă, Ana Maria Patricia Mazurencu-Pele, Remus-Florin Popescu, Teodora-Iulia Spătaru, and Lucian Negreanu. 2025. "Exploring the Overlap of MASLD and IBD: Insights from a Single-Center Experience" International Journal of Molecular Sciences 26, no. 21: 10288. https://doi.org/10.3390/ijms262110288
APA StyleStemate, A., Negru-Vodă, D.-I., Mazurencu-Pele, A. M. P., Popescu, R.-F., Spătaru, T.-I., & Negreanu, L. (2025). Exploring the Overlap of MASLD and IBD: Insights from a Single-Center Experience. International Journal of Molecular Sciences, 26(21), 10288. https://doi.org/10.3390/ijms262110288





