Sustained Activation of Myeloperoxidase Is Associated with Oxidative Stress and Inflammation in People Living with the Human Immunodeficiency Virus at Risk of Cardiovascular Disease
Abstract
1. Introduction
2. General Overview of Myeloperoxidase and Its Potential Pathological Role
2.1. The Discovery of Myeloperoxidase
2.2. The Pathological Link Between Myeloperoxidase and Endothelial Dysfunction
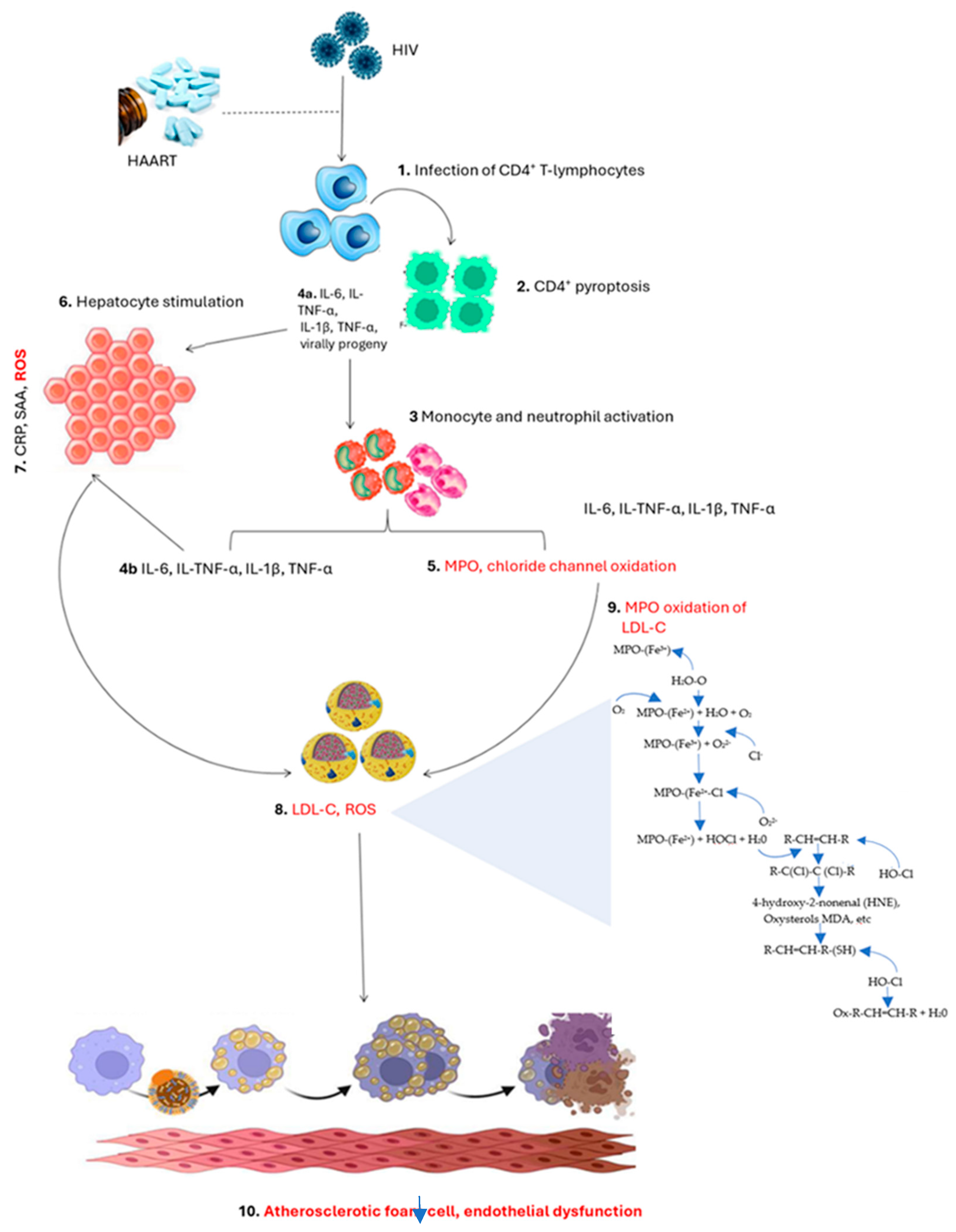
2.3. Pathological Implications of Myeloperoxidase in PLWH
3. Systematic Review Evidence Linking Myeloperoxidase Activity with Oxidative Stress and Inflammation-Associated Cardiovascular Complications in PLWH
3.1. An Overview of the Methodological Approach for a Systematic Review
3.2. Characteristic Features of the Included Studies
3.3. Abnormally Increased Low-Density Lipoprotein Levels Are Associated with Increased Myeloperoxidase Activity and Cardiovascular Disease Risk in PLWH
3.4. Pro-Inflammatory Markers Associate Myeloperoxidase Activity with Endothelial Dysfunction in PLWH
3.5. Evidence Showing the Potential Influence of HAART on Myeloperoxidase Activity in PLWH
3.6. Myeloperoxidase Activity and Endothelial Dysfunction in Individuals Exposed to HAART for Less than Three-Years
3.7. Myeloperoxidase Activity and Endothelial Dysfunction Following Three 3-Years or More Exposure Duration to HAART
3.8. Quality of Evidence and Risk of Bias of the Included Studies
4. Discussion
5. Study Limitations and Strengths
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guedes, M.C.D.S.; Carvalho-Silva, W.H.V.; Andrade-Santos, J.L.; Brelaz-De-Castro, M.C.A.; Souto, F.O.; Guimarães, R.L. Thymic exhaustion and increased immune activation are the main mechanisms involved in impaired immunological recovery of HIV-positive patients under ART. Viruses 2023, 15, 440. [Google Scholar] [CrossRef]
- Perkins, M.V.; Joseph, S.B.; Dittmer, D.P.; Mackman, N. Cardiovascular disease and thrombosis in HIV infection. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 175–191. [Google Scholar] [CrossRef]
- Mokoena, H.; Mabhida, S.E.; Choshi, J.; Dludla, P.V.; Nkambule, B.B.; Mchiza, Z.J.; Ndwandwe, D.E.; Kengne, A.P.; Hanser, S. Endothelial dysfunction and cardiovascular diseases in people living with HIV on specific highly active antiretroviral therapy regimen: A systematic review of clinical studies. Atheroscler. Plus 2024, 55, 47–54. [Google Scholar] [CrossRef]
- Nou, E.; Lo, J.; Grinspoon, S.K. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS 2016, 30, 1495–1509. [Google Scholar] [CrossRef]
- Wei, S.; Evans, P.C.; Strijdom, H.; Xu, S. HIV infection, antiretroviral therapy and vascular dysfunction: Effects, mechanisms and treatments. Pharmacol. Res. 2025, 217, 107812. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, L.; Kress, T.C.; Belin de Chantemèle, E.J. HIV, combination antiretroviral therapy, and vascular diseases in men and women. Basic Transl. Sci. 2022, 7, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J.; Greene, J.N. The epidemiology, mechanisms, diagnosis and treatment of cardiovascular disease in adult patients with HIV. Am. J. Cardiovasc. Dis. 2023, 13, 101. [Google Scholar]
- Meuwese, M.C.; Stroes, E.S.; Hazen, S.L.; van Miert, J.N.; Kuivenhoven, J.A.; Schaub, R.G.; Wareham, N.J.; Luben, R.; Kastelein, J.J.; Khaw, K.T.; et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: The EPIC-Norfolk Prospective Population Study. J. Am. Coll. Cardiol. 2007, 50, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta Int. J. Clin. Chem. 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Borato, D.C.K.; Parabocz, G.C.; Ribas, S.R.W.; Kalva-Filho, C.A.; Borba, L.M.; Ito, C.A.S.; Bail, L.; dos Santos, F.A.; Vellosa, J.C.R. Changes of metabolic and inflammatory markers in HIV infection: Glucose, lipids, serum Hs-CRP and myeloperoxidase. Metabolism 2012, 61, 1353–1360. [Google Scholar] [CrossRef]
- Kargapolova, Y.; Geißen, S.; Zheng, R.; Baldus, S.; Winkels, H.; Adam, M. The Enzymatic and Non-Enzymatic Function of Myeloperoxidase (MPO) in Inflammatory Communication. Antioxidants 2021, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; and Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Crabtree, M.J.; Brixey, R.; Batchelor, H.; Hale, A.B.; Channon, K.M. Integrated redox sensor and effector functions for tetrahydrobiopterin- and glutathionylation-dependent endothelial nitric-oxide synthase uncoupling. J. Biol. Chem. 2013, 288, 561–569. [Google Scholar] [CrossRef]
- Ross, A.C.; Armentrout, R.; O’Riordan, M.A.; Storer, N.; Rizk, N.; Harrill, D.; El Bejjani, D.; McComsey, G.A. Endothelial activation markers are linked to HIV status and are independent of antiretroviral therapy and lipoatrophy. JAIDS J. Acquir. Immune Defic. Syndr. 2008, 49, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.L.; Ford, D.A. MPO (Myeloperoxidase) Caused Endothelial Dysfunction: Not So Positive It Is About the Bleach, It May Be a Fatal Attraction. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1676–1677. [Google Scholar] [CrossRef]
- Arnhold, J. The Dual Role of Myeloperoxidase in Immune Response. Int. J. Mol. Sci. 2020, 21, 8057. [Google Scholar] [CrossRef]
- Siraki, A.G. The many roles of myeloperoxidase: From inflammation and immunity to biomarkers, drug metabolism and drug discovery. Redox Biol. 2021, 46, 102109. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Myeloperoxidase: Mechanisms, reactions and inhibition as a therapeutic strategy in inflammatory diseases. Pharmacol. Ther. 2021, 218, 107685. [Google Scholar] [CrossRef]
- Paumann-Page, M.; Ashby, L.V.; Khalilova, I.; Magon, N.J.; Hofbauer, S.; Paton, L.N.; Furtmüller, P.G.; Obinger, C.; Kettle, A.J. Hypochlorous acid inactivates myeloperoxidase inside phagocytosing neutrophils. Redox Biochem. Chem. 2023, 5, 100008, Correction in Redox Biochem. Chem. 2023, 12, 100053. [Google Scholar] [CrossRef]
- Rizo-Téllez, S.A.; Sekheri, M.; Filep, J.G. Myeloperoxidase: Regulation of Neutrophil Function and Target for Therapy. Antioxidants 2022, 11, 2302. [Google Scholar] [CrossRef]
- Shao, B.; Heinecke, J.W. HDL, lipid peroxidation, and atherosclerosis. J. Lipid Res. 2009, 50, 599–601. [Google Scholar] [CrossRef]
- Zhang, R.; Brennan, M.L.; Shen, Z.; MacPherson, J.C.; Schmitt, D.; Molenda, C.E.; Hazen, S.L. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J. Biol. Chem. 2002, 277, 46116–46122. [Google Scholar] [CrossRef]
- Ito, F.; Ito, T. High-Density Lipoprotein (HDL) Triglyceride and Oxidized HDL: New Lipid Biomarkers of Lipoprotein-Related Atherosclerotic Cardiovascular Disease. Antioxidants 2020, 9, 362. [Google Scholar] [CrossRef]
- Frangie, C.; Daher, J. Role of myeloperoxidase in inflammation and atherosclerosis (Review). Biomed. Rep. 2022, 16, 53. [Google Scholar] [CrossRef]
- Hajieva, P.; Abrosimov, R.; Kunath, S.; Moosmann, B. Antioxidant and prooxidant modulation of lipid peroxidation by integral membrane proteins. Free Radic. Res. 2023, 57, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Hawkins, C.L. The role of myeloperoxidase in biomolecule modification, chronic inflammation, and disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase, modified lipoproteins, and atherogenesis. J. Lipid. Res. 2009, 50, S346–S351. [Google Scholar] [CrossRef] [PubMed]
- Mabhida, S.E.; Mchiza, Z.J.; Mokgalaboni, K.; Hanser, S.; Choshi, J.; Mokoena, H.; Ziqubu, K.; Masilela, C.; Nkambule, B.B.; Ndwandwe, D.E.; et al. High-sensitivity C-reactive protein among people living with HIV on highly active antiretroviral therapy: A systemic review and meta-analysis. BMC Infect. Dis. 2024, 24, 160. [Google Scholar] [CrossRef]
- Baldus, S.; Heitzer, T.; Eiserich, J.P.; Lau, D.; Mollnau, H.; Ortak, M.; Petri, S.; Goldmann, B.; Duchstein, H.J.; Berger, J.; et al. Myeloperoxidase enhances nitric oxide catabolism during myocardial ischemia and reperfusion. Free Radic. Biol. Med. 2004, 37, 902–911. [Google Scholar] [CrossRef]
- Vita, J.A.; Brennan, M.L.; Gokce, N.; Mann, S.A.; Goormastic, M.; Shishehbor, M.H.; Penn, M.S.; Keaney, J.F., Jr.; Hazen, S.L. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation 2004, 110, 1134–1139. [Google Scholar] [CrossRef]
- Fernández, A.; Imaz, A. Clinical considerations when switching antiretroviral therapy. Expert Rev. Clin. Pharmacol. 2024, 17, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Suleman, M.; Khan, S.U.; Hussain, T.; Khan, M.U.; Shamsul Hassan, S.; Majid, M.; Khan, S.U.; Shehzad Khan, M.; Shan Ahmad, R.U.; Arif, M.; et al. Cardiovascular challenges in the era of antiretroviral therapy for AIDS/ HIV: A comprehensive review of research advancements, pathophysiological insights, and future directions. Curr. Probl. Cardiol. 2024, 49, 102353. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, version 6.3; Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 15 January 2024).
- Masiá, M.; Padilla, S.; Bernal, E.; Almenar, M.V.; Molina, J.; Hernández, I.; Graells, M.L.; Gutiérrez, F. Influence of antiretroviral therapy on oxidative stress and cardiovascular risk: A prospective cross-sectional study in HIV-infected patients. Clin. Ther. 2007, 29, 1448–1455. [Google Scholar] [CrossRef]
- Knudsen, A.; Katzenstein, T.L.; Benfield, T.; Jørgensen, N.R.; Kronborg, G.; Gerstoft, J.; Obel, N.; Kjær, A.; Lebech, A.M. Plasma plasminogen activator inhibitor-1 predicts myocardial infarction in HIV-1-infected individuals. Aids 2014, 28, 1171–1179. [Google Scholar] [CrossRef]
- Hileman, C.O.; Turner, R.; Funderburg, N.T.; Semba, R.D.; McComsey, G.A. Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. Aids 2016, 30, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.T.; Oektedalen, O.; Shawarira-Bote, S.; Stray-Pedersen, B. Changes in coronary heart disease risk profiles of HIV patients in Zimbabwe over 9 months: A follow-up study. HIV/AIDS-Res. Palliat. Care 2016, 8, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Zungsontiporn, N.; Ndhlovu, L.C.; Mitchell, B.I.; Stein, J.H.; Kallianpur, K.J.; Nakamoto, B.; Keating, S.M.; Norris, P.J.; Souza, S.A.; Shikuma, C.M.; et al. Serum amyloid P (SAP) is associated with impaired brachial artery flow-mediated dilation in chronically HIV-1 infected adults on stable antiretroviral therapy. HIV Clin. Trials 2015, 16, 228–235. [Google Scholar] [CrossRef]
- Kelesidis, T.; Oda, M.N.; Borja, M.S.; Yee, Y.; Ng, K.F.; Huynh, D.; Elashoff, D.; Currier, J.S. Predictors of impaired HDL function in HIV-1 infected compared to uninfected individuals. JAIDS J. Acquir. Immune Defic. Syndr. 2017, 75, 354–363. [Google Scholar] [CrossRef]
- Lui, G.; Ma, R.C.; Chook, P.; Wong, C.K.; Tam, C.H.; Chan, M.H.; Lee, S.S.; Wong, R.Y.; Cheung, C.S.; Choi, K.W.; et al. Brief report: Progression of atherosclerosis in HIV-infected individuals—Prospective data from an Asian cohort. JAIDS J. Acquir. Immune Defic. Syndr. 2017, 75, 198–202. [Google Scholar] [CrossRef]
- Weke, K.; Amayo, A.; Wandolo, G.; Ndiangui, F. Levels of serum biomarkers for risk of cardiovascular disease in patients on highly active antiretroviral therapy in Homa-Bay County Referral Hospital, Kenya. Columbia Univ. J. Glob. Health 2018, 8. [Google Scholar] [CrossRef]
- Gangcuangco, L.M.A.; Mitchell, B.I.; Siriwardhana, C.; Kohorn, L.B.; Chew, G.M.; Bowler, S.; Kallianpur, K.J.; Chow, D.C.; Ndhlovu, L.C.; Gerschenson, M.; et al. Mitochondrial oxidative phosphorylation in peripheral blood mononuclear cells is decreased in chronic HIV and correlates with immune dysregulation. PLoS ONE 2020, 15, e0231761, Erratum in PLoS ONE 2021, 16, e0249428. [Google Scholar] [CrossRef]
- Mitchell, B.I.; Laws, E.I.; Chow, D.C.; Sah Bandar, I.N.; Gangcuangco, L.M.A.; Shikuma, C.M.; Ndhlovu, L.C. Increased monocyte inflammatory responses to oxidized LDL are associated with insulin resistance in HIV-infected individuals on suppressive antiretroviral therapy. Viruses 2020, 12, 1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lifshitz, L.; Gellatly, K.; Vinton, C.L.; Busman-Sahay, K.; McCauley, S.; Vangala, P.; Kim, K.; Derr, A.; Jaiswal, S.; et al. HIV-1-induced cytokines deplete homeostatic innate lymphoid cells and expand TCF7-dependent memory NK cells. Nat. Immunol. 2020, 21, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Borato, D.C.K.; Kalva-Filho, C.A.; Machado, E.P.; Barbosa, C.R.; Vellosa, J.C.R. Effect of non-nucleoside reverse transcriptase inhibitors and protease inhibitors on serum levels of myeloperoxidase and C-reactive protein in HIV-infected individuals. Braz. J. Pharm. Sci. 2022, 58, e18780. [Google Scholar] [CrossRef]
- Marques de Menezes, E.G.; Deng, X.; Liu, J.; Bowler, S.A.; Shikuma, C.M.; Stone, M.; Hunt, P.W.; Ndhlovu, L.C.; Norris, P.J. Plasma CD16+ Extracellular Vesicles Associate with Carotid Artery Intima-Media Thickness in HIV+ Adults on Combination Antiretroviral Therapy. mBio 2022, 13, e0300521. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ayala, P.; Alanis-Sánchez, G.A.; Álvarez-Zavala, M.; Sánchez-Reyes, K.; Ruiz-Herrera, V.V.; Cabrera-Silva, R.I.; González-Hernández, L.A.; Ramos-Becerra, C.; Cardona-Muñoz, E.; Andrade-Villanueva, J.F. Effect of antiretroviral therapy on decreasing arterial stiffness, metabolic profile, vascular and systemic inflammatory cytokines in treatment-naïve HIV: A one-year prospective study. PLoS ONE 2023, 18, e0282728. [Google Scholar] [CrossRef]
- Nkambule, B.B.; Mxinwa, V.; Mkandla, Z.; Mutize, T.; Mokgalaboni, K.; Nyambuya, T.M.; Dludla, P.V. Platelet activation in adult HIV-infected patients on antiretroviral therapy: A systematic review and meta-analysis. BMC Med. 2020, 18, 357. [Google Scholar] [CrossRef]
- Nyambuya, T.M.; Dludla, P.V.; Mxinwa, V.; Nkambule, B.B. The Effect of Successful Antiretroviral Therapy on Immune Activation and Reconstitution in HIV Infected Adults: A Systematic Review and Meta-Analysis. Aids Rev. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Baseri, M.; Heidari, R.; Mahaki, B.; Hajizadeh, Y.; Momenizadeh, A.; Sadeghi, M. Myeloperoxidase levels predicts angiographic severity of coronary artery disease in patients with chronic stable angina. Adv. Biomed. Res. 2014, 25, 139. [Google Scholar] [CrossRef]
- Ross, A.C.; Rizk, N.; O’Riordan, M.A.; Dogra, V.; El-Bejjani, D.; Storer, N.; Harrill, D.; Tungsiripat, M.; Adell, J.; McComsey, G.A. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin. Infect. Dis. 2009, 49, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health (NIH). Study Quality Assessment Tools. 2021. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 11 June 2025).
- Smith, W.P. Negative Lifestyle Factors Specific to Aging Persons Living with HIV and Multimorbidity. J. Int. Assoc. Provid. AIDS Care 2024, 23, 23259582241245228. [Google Scholar] [CrossRef] [PubMed]
- Hadavandsiri, F.; Shafaati, M.; Mohammad Nejad, S.; Ebrahimzadeh Mousavi, M.; Najafi, A.; Mirzaei, M.; Narouee, S.; Akbarpour, S. Non-communicable disease comorbidities in HIV patients: Diabetes, hypertension, heart disease, and obstructive sleep apnea as a neglected issue. Sci. Rep. 2023, 13, 12730. [Google Scholar] [CrossRef] [PubMed]
| Authors, Year | Region of Study | Study Design | Population | Intervention | Key Observations |
|---|---|---|---|---|---|
| Masiá et al., 2007 [35] | Spain | Cross-sectional | People living with the human immunodeficiency virus (PLWH) (n = 181) with an average age of 40-years, predominately males (79%) of Caucasian (97%) ethnicity. | Non-nucleoside reverse transcriptase inhibitor (NNRTI), protease inhibitor (PI), nucleotide reverse transcriptase inhibitor (NRTI), or NNRTI plus PI. Participants were on highly active antiretroviral therapy (HAART) for at least two months. | Plasma peroxide levels were positively associated with high-sensitivity C-reactive protein (hsCRP) and low-density lipoprotein-cholesterol (LDL-C) but negatively associated with traditional cardiovascular disease (CVD)-risk factors such as age and body mass index (BMI). NNRTI-based regimen significantly lowered peroxide levels as indicators of CVD-risk when compared to PIs. |
| Knudsen et al., 2014 [36] | Denmark | Case–control | PLWH (n = 108) with an average age of 49-years, strictly males (100%) of Caucasian (100%) ethnicity. | NNRTI, PI, NRTI, or NNRTI plus PI. Participants were on HAART for ten years. | Plasma peroxide inducer myeloperoxidase (MPO) and markers of endothelial dysfunction, including sE-selectin, soluble intercellular- and vascular adhesion molecules (sICAM-1 and sVCAM-1), were not associated with myocardial infarction. |
| Hileman et al., 2016 [37] | USA | Randomized controlled trial | PLWH (n = 147) with an average age of 45-years, predominately males (78%) of African (68%) ethnicity. | PIs plus 10 mg of rosuvastatin daily. Participants on HAART were monitored for six months. | Plasma-oxidized LDL-C (oxLDL-C) was positively linked with hsCRP and changes in endothelial function as measured by carotid intima-media thickness (cIMT). |
| Zhou et al., 2016 [38] | Zimbabwe | Observational | PLWH (n = 147) with an average age of 41-years, predominately females (76.7%) of African (100%) ethnicity. | NNRTI or PIs. Participants were on HAART for at least one month but were allowed to change treatment during the nine months of the study period. | Serum elevated MPO levels were linked with LDL-C but not coronary artery disease. |
| Zungsontiporn et al., 2016 [39] | USA | Cross-sectional | PLWH (n = 135) with an average age of 50- years, predominately males (88.1%) of Caucasian (58.5%) ethnicity. | NRTI, NNRTI, PIs, or integrase strand transfer inhibitors (INSTIs). Participants were on HAART for three years or more. | Plasma MPO levels and markers of endothelial dysfunction including sE-selectin, sICAM-1, and sVCAM-1 were not linked with brachial artery flow mediated dilation (FMD). |
| Kelesidis et al., 2017 [40] | USA | Cross-sectional | PLWH (n = 116) with an average age of 48- years, predominately males (100%) of Caucasian (67%) ethnicity. | NNRTIs, PIs, or INSTIs. Participants were on HAART for at least six months. | Plasma-oxidized high-density lipoprotein cholesterol (oxHDL-C) was associated with a higher BMI, lower apolipoprotein-AI, and CVD-risk. |
| Lui et al., 2017 [41] | China | Observational | PLWH (n = 61) with an average age of 50-years, predominately males (89%) of Chinese (97%) ethnicity. | NNRTIs or PIs. Participants were on HAART for four years. | Plasma MPO was independently associated with endothelial dysfunction marked by reduced cIMT. However, LDL-C subclass pattern type B and adiponectin levels were the best predictors of reduced cIMT progression. |
| Weke et al., 2018 [42] | Kenya | Cross-sectional | PLWH (n = 120) with an average age of 34-years, predominately females (64.2%) of African (100%) ethnicity. | HAART-regimen not disclosed. Participants were on HAART for at least six months to five years. | Serum MPO and lipoprotein-associated phospholipase A2 (Lp-PLA2) were elevated and predicted early events of developing CVDs. |
| Gangcuangco et al., 2020 [43] | USA | Cross-sectional | PLWH (n = 149) with an average age of 51-years, predominately males (88.6%) of Caucasian (57.7%) ethnicity. | NRTIs. Participants were on HAART for three months or more. | Plasma MPO levels were elevated and linked with low mitochondrial oxidative phosphorylation, and current use of NRTIs. Moreover, low mitochondrial phosphorylation was linked with sICAM-1, a marker of endothelial dysfunction. |
| Mitchell et al., 2020 [44] | USA | Observational | PLWH (n = 33) with an average age of 53-years, predominately males (88%) of Caucasian (67%) ethnicity. | NRTI, NNRTI, or PIs. Participants were on HAART for three months or more. | Plasma oxLDL-C was associated with impaired LDL-C, HDL-C, triglycerides, and total cholesterol, known CVD-risk factors. |
| Wang et al., 2020 [45] | USA | Observational | PLWH (=50). The demographic data of the study population was not disclosed. | PIs. Participant’s HAART duration not disclosed. | Plasma MPO levels were elevated and consistent with an increase in inflammation, marked by plasma CD14, Interleukin-15, and interferon gamma. |
| Borato et al., 2022 [46] | Brazil | Cross-sectional | PLWH (n = 104) with an average age of 41-years, predominately females (62%) of Caucasian (100%) ethnicity. | NRTIs, NNRTIs, or PIs. Participants were on HAART for six years. | Serum MPO and hsCRP as predictors of CVD-risk were elevated. MPO was elevated in the presence of NNRTIs and PIs, with NRTIs (abacavir) intensifying these levels following three months of administration. |
| De Menezes et al., 2022 [47] | USA | Observational | PLWH (n = 74) having existing CVDs with an average age of 50-years, predominately males (92%) of Caucasian (100%) ethnicity. | HAART not disclosed. Participants were monitored for five years. | Plasma MPO was associated with CD141, and CD14 extracellular vesicles, a predictor of CVDs. Moreover, total extracellular vesicle count was elevated. The direct effects of HAART in modulating these markers were not disclosed. |
| Martínez-Ayala et al., 2023 [48] | Mexico | Cross-sectional | PLWH (n = 20) with an average age of 35-years, predominately males (95%) of Hispanic (100%) ethnicity. | NNRTIs or PIs. Participants were on HAART for four years. | Serum MPO and markers of endothelial dysfunction including sICAM-1, and sVCAM-1 were not significantly different between groups. HAART had a greater impact in reducing MPO, sICAM-1, and sVCAM-1. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokoena, H.; Choshi, J.; Hanser, S.; Mabhida, S.E.; Steel, H.C.; Mokgalaboni, K.; Phoswa, W.N.; Maarman, G.; Nkambule, B.B.; Dludla, P.V. Sustained Activation of Myeloperoxidase Is Associated with Oxidative Stress and Inflammation in People Living with the Human Immunodeficiency Virus at Risk of Cardiovascular Disease. Int. J. Mol. Sci. 2025, 26, 10285. https://doi.org/10.3390/ijms262110285
Mokoena H, Choshi J, Hanser S, Mabhida SE, Steel HC, Mokgalaboni K, Phoswa WN, Maarman G, Nkambule BB, Dludla PV. Sustained Activation of Myeloperoxidase Is Associated with Oxidative Stress and Inflammation in People Living with the Human Immunodeficiency Virus at Risk of Cardiovascular Disease. International Journal of Molecular Sciences. 2025; 26(21):10285. https://doi.org/10.3390/ijms262110285
Chicago/Turabian StyleMokoena, Haskly, Joel Choshi, Sidney Hanser, Sihle E. Mabhida, Helen C. Steel, Kabelo Mokgalaboni, Wendy N. Phoswa, Gerald Maarman, Bongani B. Nkambule, and Phiwayinkosi V. Dludla. 2025. "Sustained Activation of Myeloperoxidase Is Associated with Oxidative Stress and Inflammation in People Living with the Human Immunodeficiency Virus at Risk of Cardiovascular Disease" International Journal of Molecular Sciences 26, no. 21: 10285. https://doi.org/10.3390/ijms262110285
APA StyleMokoena, H., Choshi, J., Hanser, S., Mabhida, S. E., Steel, H. C., Mokgalaboni, K., Phoswa, W. N., Maarman, G., Nkambule, B. B., & Dludla, P. V. (2025). Sustained Activation of Myeloperoxidase Is Associated with Oxidative Stress and Inflammation in People Living with the Human Immunodeficiency Virus at Risk of Cardiovascular Disease. International Journal of Molecular Sciences, 26(21), 10285. https://doi.org/10.3390/ijms262110285






