Activin B Regulates Fibroblasts to Promote Granulation Tissue Formation and Angiogenesis During Murine Skin-Wound Healing via the JNK/ERK Signaling Pathway
Abstract
1. Introduction
2. Results
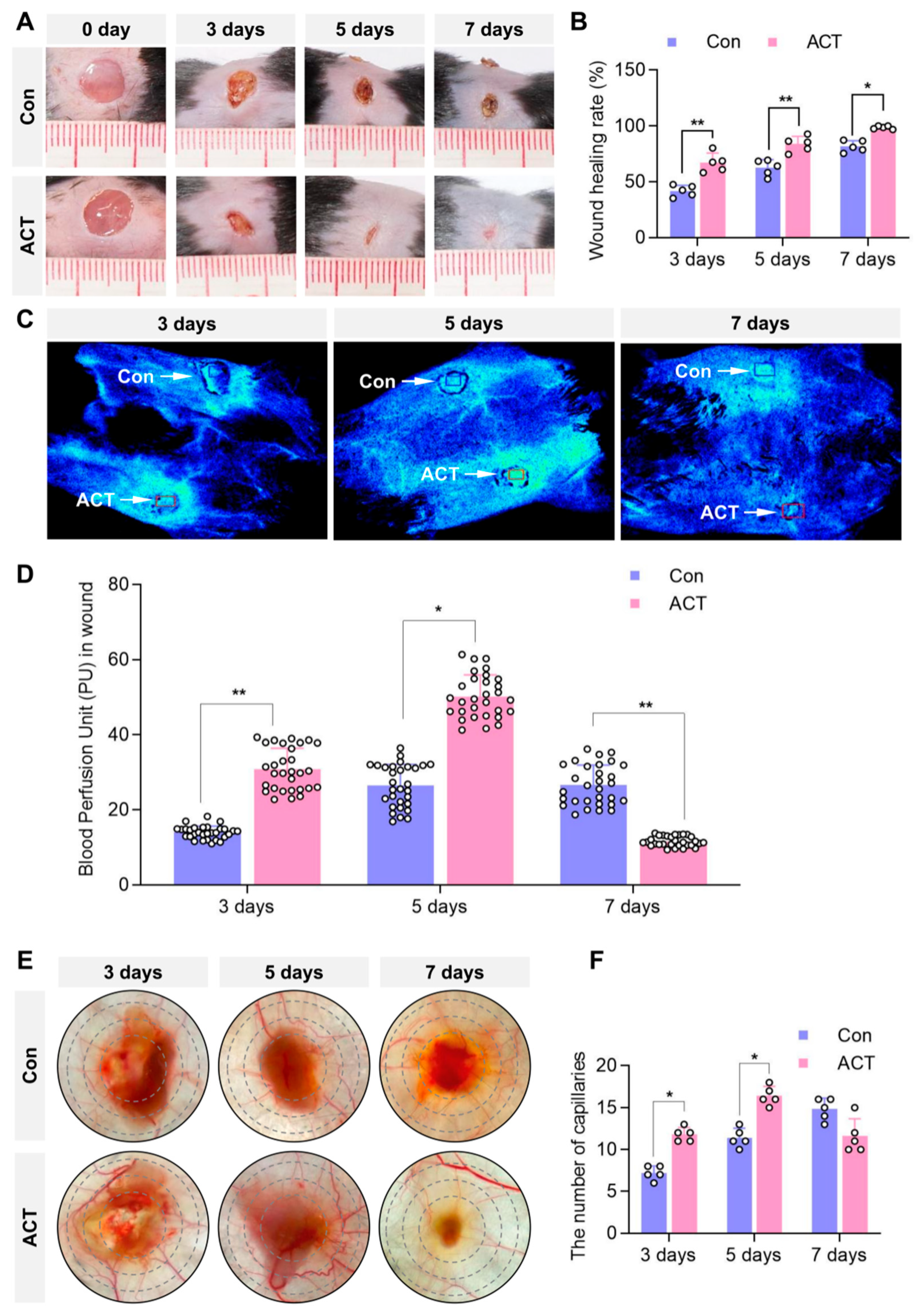
2.1. Activin B Promotes Skin-Wound Closure and Increases Mean Blood Perfusion Volume in Mice
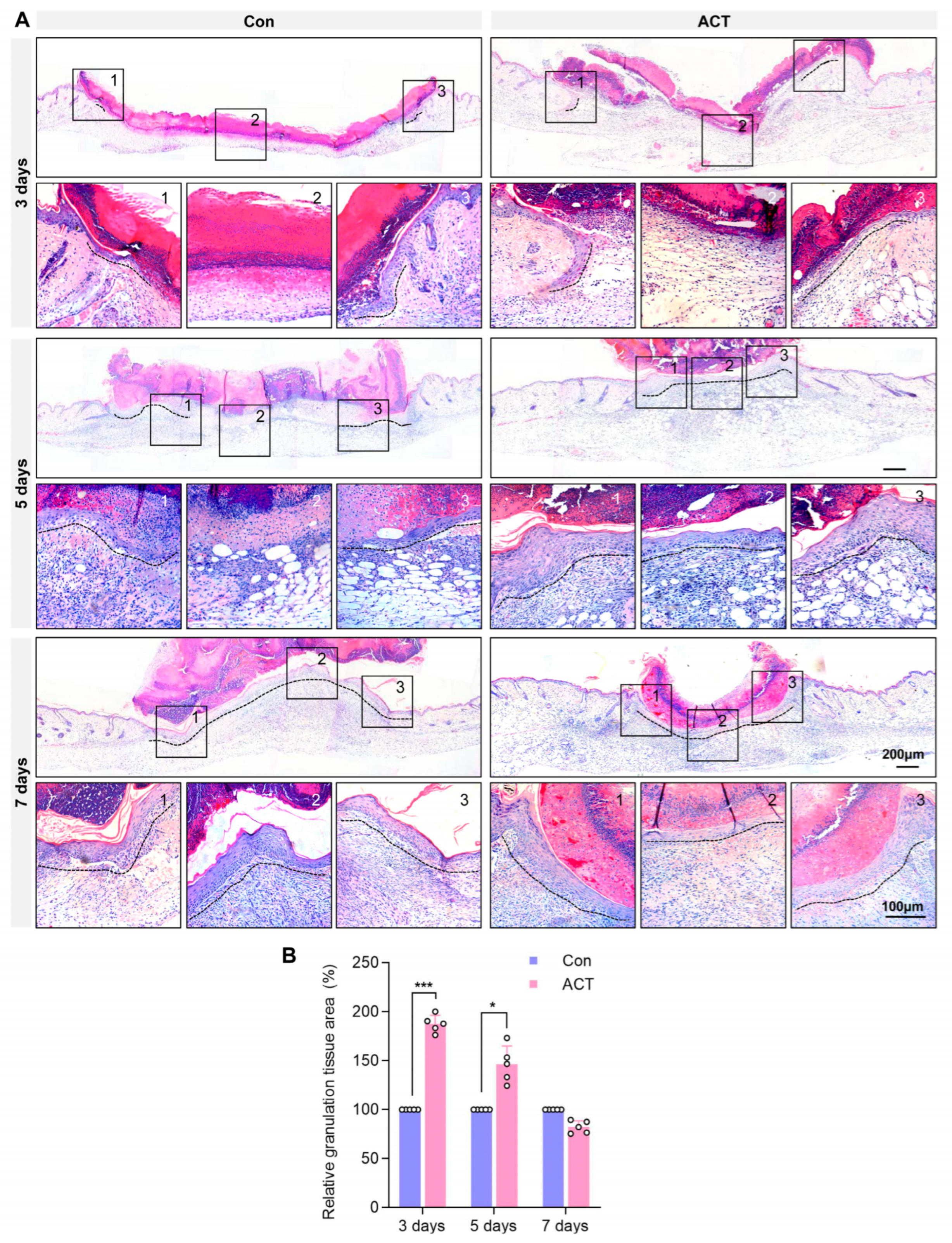
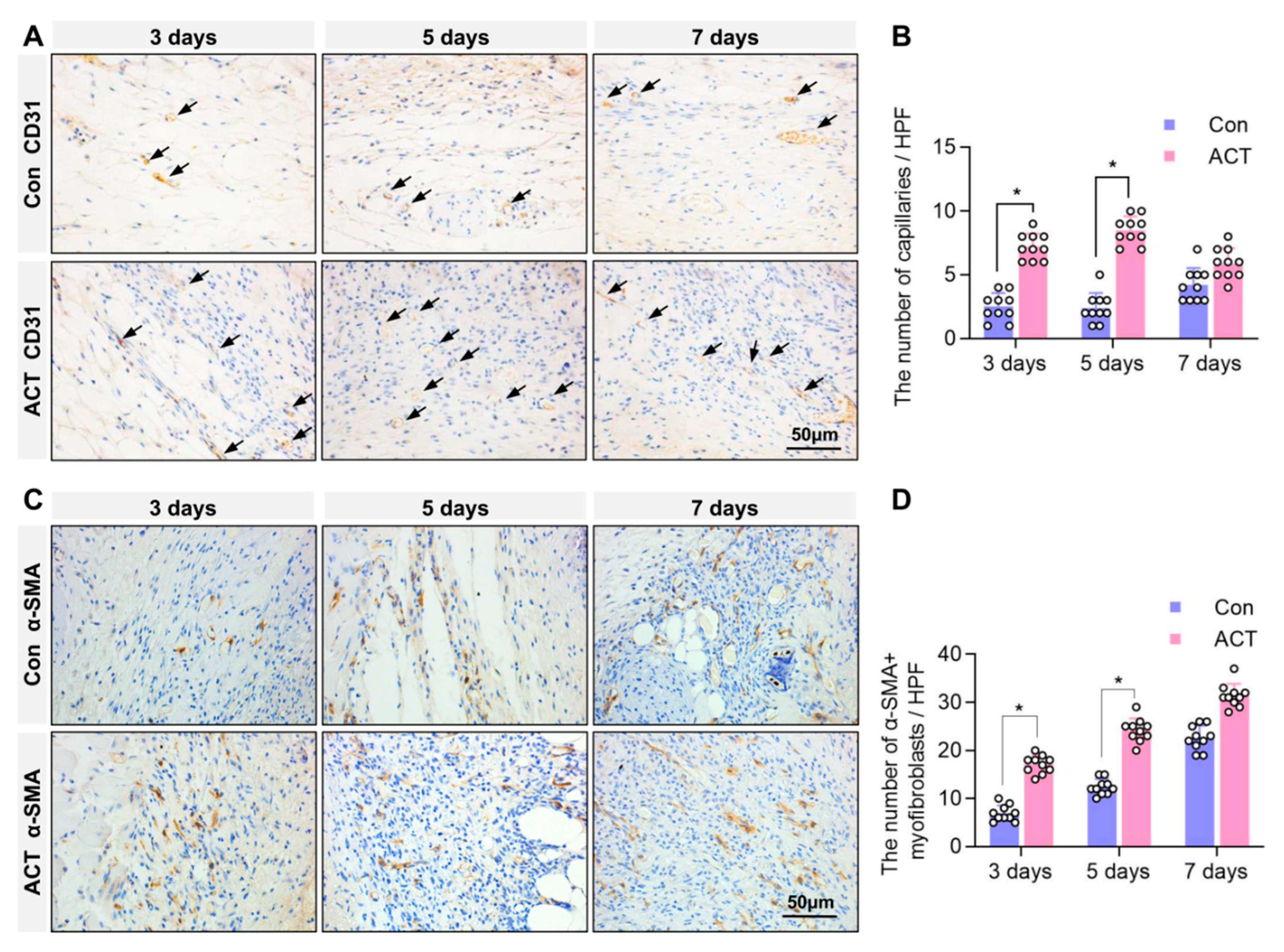
2.2. Activin B Promotes Angiogenesis and Myofibroblast Formation in Wound Granulation Tissue of Mice
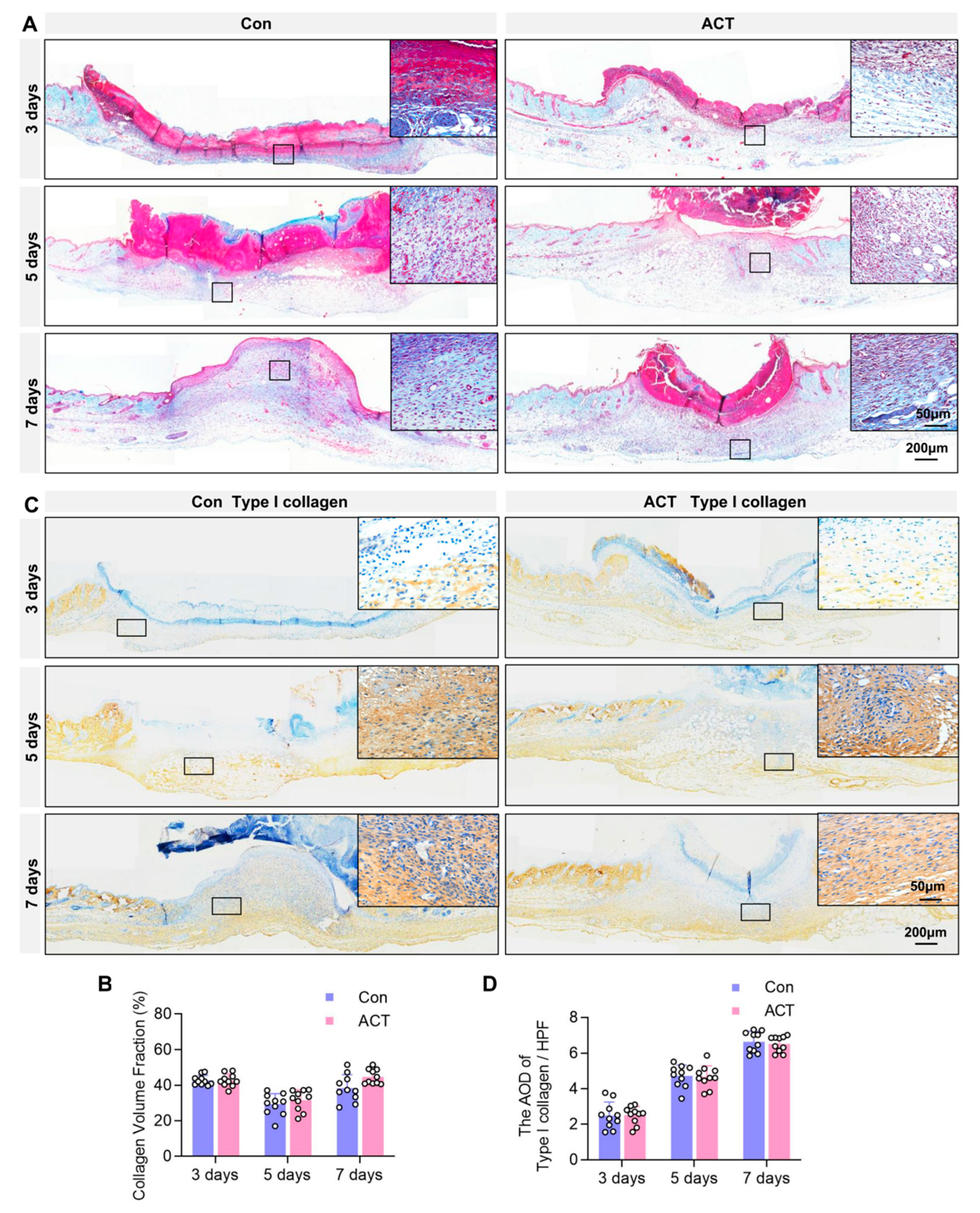
2.3. Activin B Does Not Regulate Collagen Fiber and TYPE I Collagen Synthesis in Wound Granulation Tissue
2.4. Activin B Promotes Fibroblast Proliferation and Migration, but Does Not Regulate Collagen Synthesis
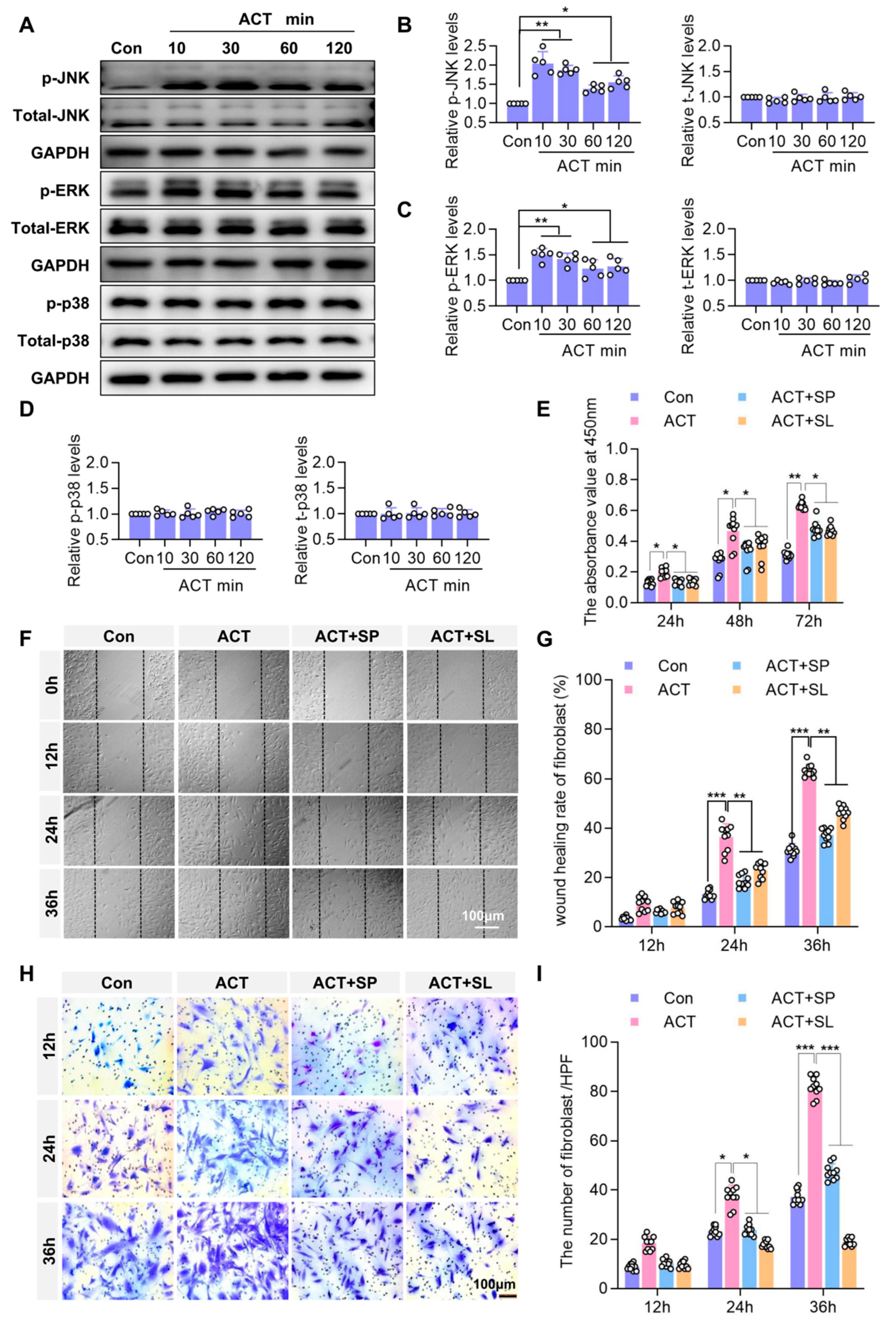
2.5. Activin B Regulates Fibroblast Proliferation and Migration via JNK and ERK Signaling Pathways
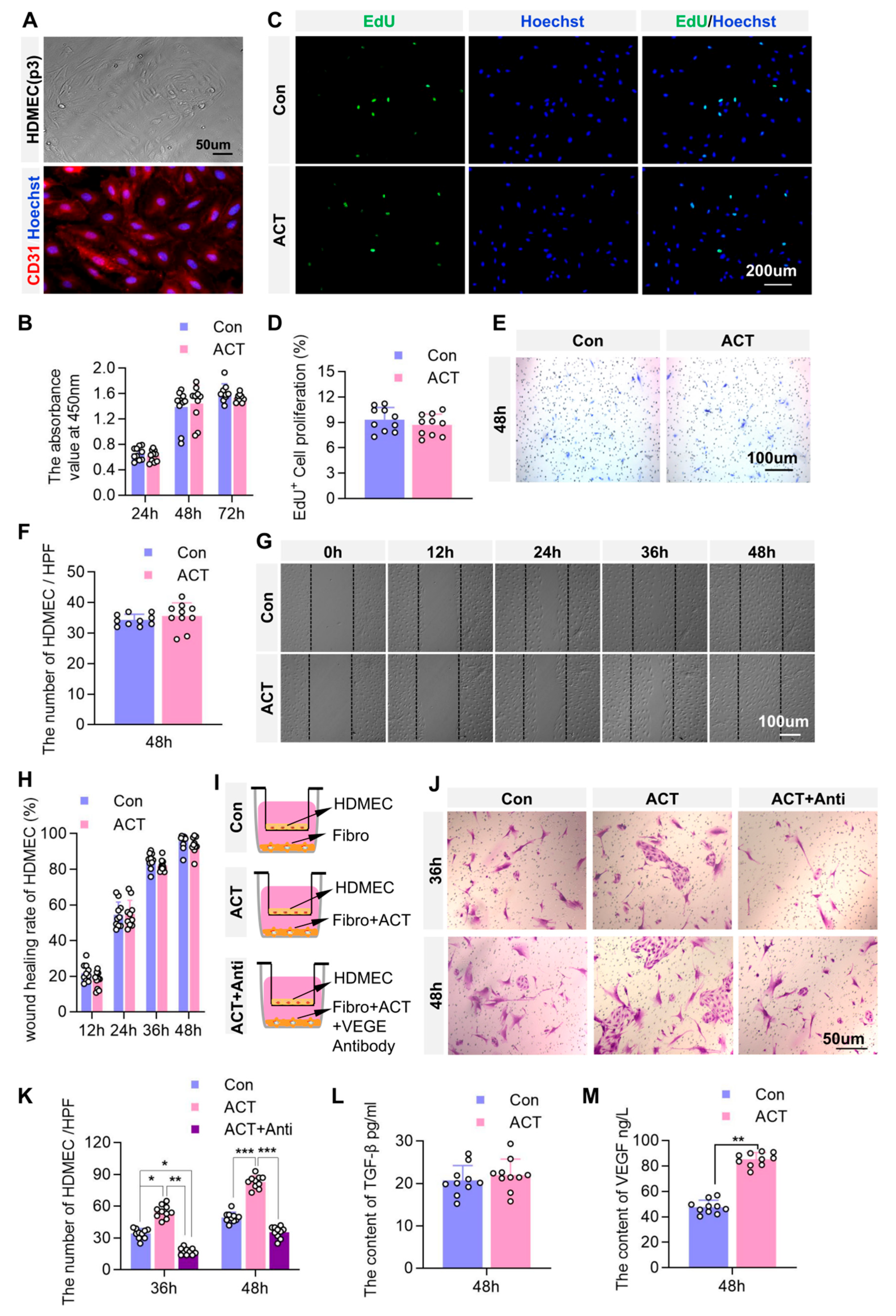
2.6. Activin B May Promote Angiogenesis by Enhancing Fibroblast-Derived Vascular Endothelial Growth Factor (VEGF) Secretion to Stimulate the Proliferation of Dermal Microvascular Endothelial Cells
3. Discussion
3.1. Regulation of Fibroblast Function by Activin B: Induces Collagen Synthesis Deficiency and Does Not Act Directly on Endothelial Cells
3.2. Mechanisms of Activin B in Promoting Wound Blood Perfusion and Angiogenesis, and Its Clinical Translation
3.3. Innovations, Limitations, and Future Directions of the Study
4. Materials and Methods
4.1. Experimental Animals
4.2. Main Reagents and Instruments
4.3. Murine Skin-Wound Model and Grouping Strategy
4.4. Analysis of Mean Blood Perfusion Volume at the Wound Site
4.5. Observation of Wound Vascularization
4.6. Tissue Collection and H&E Staining
4.7. Immunohistochemical Staining
4.8. Masson’s Trichrome Staining
4.9. Cell Culture
4.10. Cell Proliferation Assayed by CCK8 and EdU
4.11. Cell Scratch Assay and Transwell Assay
4.12. ELISA
4.13. RT-PCR Assay
4.14. Western Blot
4.15. Immunofluorescence Staining
4.16. Co-Culture of HDFs and HDMECs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CVF | Collagen volume fraction |
| H&E | Hematoxylin and eosin |
| HDF | Human dermal fibroblasts |
| HDMEC | Human dermal microvascular endothelial cells |
| IACUC | Institutional Animal Care and Use Committee |
| IOD | Integrated optical density |
| PBS | Phosphate-buffered saline |
References
- Overmiller, A.M.; Sawaya, A.P.; Hope, E.D.; Morasso, M.I. Intrinsic Networks Regulating Tissue Repair: Comparative Studies of Oral and Skin Wound Healing. Cold Spring Harb. Perspect. Biol. 2022, 14, a041244. [Google Scholar] [CrossRef]
- Singer, A.J. Healing Mechanisms in Cutaneous Wounds: Tipping the Balance. Tissue Eng. Part B Rev. 2022, 28, 1151–1167. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Scharffetter-Kochanek, K. Mesenchymal Stem Cells Adaptively Respond to Environmental Cues Thereby Improving Granulation Tissue Formation and Wound Healing. Front. Cell Dev. Biol. 2020, 8, 697. [Google Scholar] [CrossRef]
- Lacina, L.; Kolář, M.; Pfeiferová, L.; Gál, P.; Smetana, K. Wound healing: Insights into autoimmunity, ageing, and cancer ecosystems through inflammation and IL-6 modulation. Front. Immunol. 2024, 15, 1403570. [Google Scholar] [CrossRef]
- Knoedler, S.; Broichhausen, S.; Guo, R.; Dai, R.; Knoedler, L.; Kauke-Navarro, M.; Diatta, F.; Pomahac, B.; Machens, H.-G.; Jiang, D.; et al. Fibroblasts—The cellular choreographers of wound healing. Front. Immunol. 2023, 14, 1233800. [Google Scholar] [CrossRef]
- Dong, X.; Xiang, H.; Li, J.; Hao, A.; Wang, H.; Gou, Y.; Li, A.; Rahaman, S.; Qiu, Y.; Li, J.; et al. Dermal fibroblast-derived extracellular matrix (ECM) synergizes with keratinocytes in promoting re-epithelization and scarless healing of skin wounds: Towards optimized skin tissue engineering. Bioact. Mater. 2025, 47, 1–17. [Google Scholar] [CrossRef]
- Jeon, S.; Cho, S.; Yoo, S.; Lee, Y.; Goo, J.; Jeong, Y.J.; Nam, G.-H.; Shin, H.-T.; Park, J.-W.; Jeong, C.; et al. Controlled delivery of HIF-1α via extracellular vesicles with collagen-binding activity for enhanced wound healing. J. Control. Release 2025, 380, 330–347. [Google Scholar] [CrossRef]
- Shaabani, E.; Sharifiaghdam, M.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Gene therapy to enhance angiogenesis in chronic wounds. Mol. Ther. Nucleic Acids 2022, 29, 871–899. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, L.; Yan, C.; Zhou, W.; Endo, Y.; Liu, J.; Hu, L.; Hu, Y.; Mi, B.; Liu, G. Circulating Exosomal miR-20b-5p Inhibition Restores Wnt9b Signaling and Reverses Diabetes-Associated Impaired Wound Healing. Small 2020, 16, e1904044. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.-F.; Wang, Z.-C.; Lou, D.; Fang, Q.-Q.; Hu, Y.-Y.; Zhao, W.-Y.; Zhang, L.-Y.; Wu, L.-H.; Tan, W.-Q. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 2020, 129, 110287. [Google Scholar] [CrossRef]
- Kurosaka, S.; Tanno, H.; Hirose, M.; Kamada, W.; Takayashiki, R.; Sone, I.; Sato, Y.; Watanabe, T.; Ishi, S.; Shoji, M.; et al. Contribution of CARD9 signaling to wound healing in skin promoted by topical administration of heat-killed Enterococcus faecalis strain KH2 and the involvement of Dectin-2. Front. Immunol. 2025, 16, 1550934. [Google Scholar] [CrossRef]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Li, F.; Zhang, C.; Zhong, X.; Li, B.; Zhang, M.; Li, W.; Zheng, L.; Zhu, X.; Chen, S.; Zhang, Y. A 3D radially aligned nanofiber scaffold co-loaded with LL37 mimetic peptide and PDGF-BB for the management of infected chronic wounds. Mater. Today Bio 2024, 28, 101237. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Massagué, J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998, 67, 753–791. [Google Scholar]
- Hübner, G.; Hu, Q.; Smola, H.; Werner, S. Strong induction of activin expression after injury suggests an important role of activin in wound repair. Dev. Biol. 1996, 173, 490–498. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, N.-Y.; Wang, X.-E.; Chen, Y.-H.; Li, Q.-L.; Lu, K.-R.; Sun, L.; Jia, Q.; Zhang, L.; Zhang, L. Activin B promotes epithelial wound healing in vivo through RhoA-JNK signaling pathway. PLoS ONE 2011, 6, e25143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, L.; Wang, X.; Chen, S.; Kong, Y.; Liu, N.; Chen, Y.; Jia, Q.; Zhang, L.; Zhang, L. Activin B Promotes BMSC-Mediated Cutaneous Wound Healing by Regulating Cell Migration via the JNK-ERK Signaling Pathway. Cell Transplant. 2014, 23, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, X.; Zhang, M.; Huang, M.; Yan, Y.; Chen, Y.; Zhang, Y.; Xu, J.; Bu, L.; Fan, R.; et al. Activin B-activated Cdc42 signaling plays a key role in regulating adipose-derived mesenchymal stem cells-mediated skin wound healing. Stem Cell Res. Ther. 2022, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, P.; Guo, F.; Zhang, M.; Yan, Y.; Huang, M.; Chen, Y.; Zhang, L.; Zhang, L. mDia1 and Cdc42 Regulate Activin B-Induced Migration of Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells 2019, 37, 150–162. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, P.; Wang, X.; Zhang, M.; Yan, Y.; Chen, Y.; Zhang, L.; Zhang, L. Activin B regulates adipose-derived mesenchymal stem cells to promote skin wound healing via activation of the MAPK signaling pathway. Int. J. Biochem. Cell Biol. 2017, 87, 69–76. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, M.; Kong, Y.; Chen, S.; Chen, Y.; Wang, X.; Zhang, L.; Lang, W.; Zhang, L.; Zhang, L. Activin B promotes initiation and development of hair follicles in mice. Cells Tissues Organs 2013, 198, 318–326. [Google Scholar] [CrossRef]
- Jin, Y.; Cai, Q.; Wang, L.; Ji, J.; Sun, Y.; Jiang, J.; Wang, C.; Wu, J.; Zhang, B.; Zhao, L.; et al. Paracrine activin B-NF-κB signaling shapes an inflammatory tumor microenvironment in gastric cancer via fibroblast reprogramming. J. Exp. Clin. Cancer Res. 2023, 42, 269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hamang, M.; Culver, A.; Jiang, H.; Yanum, J.; Garcia, V.; Lee, J.; White, E.; Kusumanchi, P.; Chalasani, N.; et al. Activin B promotes the initiation and progression of liver fibrosis. Hepatol. Commun. 2022, 6, 2812–2826. [Google Scholar] [CrossRef] [PubMed]
- Krepinsky, J.C. Activin B, a new player in kidney fibrosis? J. Pathol. 2022, 256, 363–365. [Google Scholar] [CrossRef]
- Ito, S.; Nagata, K. Quality Control of Procollagen in Cells. Annu. Rev. Biochem. 2021, 90, 631–658. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liu, Y. Fibroblast activation and heterogeneity in fibrotic disease. Nat. Rev. Nephrol. 2025, 21, 613–632. [Google Scholar] [CrossRef]
- Arsalan, L.; Leanne, E.F.; Adam, A.D.; Valentina, C.C.; Zeynep, I.; Karen, L.; Francesco, P.; Benjamin, W.M.; Ricky, W.; Derek, J.I.; et al. Microparticles Decorated with Cell-Instructive Surface Chemistries Actively Promote Wound Healing. Adv. Mater. 2022, 36, e2208364. [Google Scholar] [CrossRef]
- Ariella, Z.; Yi-Nan, L.; Neng-Yu, L.; Adrian, S.; Julian, N.; Chih-Wei, C.; Hsiao-Han, H.; Honglin, Z.; Xiao, D.; Jingang, H.; et al. TGFβ promotes fibrosis by MYST1-dependent epigenetic regulation of autophagy. Nat. Commun. 2021, 12, 4404. [Google Scholar] [CrossRef]
- Ruochen, D.; Liqi, W.; Min, N.; Liting, Z.; Xiaoya, G.; Jiao, Y.; Chunming, Z.; Hongliang, L. Activin receptors in human cancer: Functions, mechanisms, and potential clinical applications. Biochem. Pharmacol. 2024, 222, 116061. [Google Scholar] [CrossRef] [PubMed]
- Erich, J.G.; Luisina, O.; Emily, C.K.; Kylie, V.; Elitza, B.; Roselyne, C.; Ravindra, K.; Daniel, J.B.; Thomas, B.T. The orphan ligand, activin C, signals through activin receptor-like kinase 7. eLife 2022, 11, e78197. [Google Scholar] [CrossRef]
- Wietecha, M.S.; Pensalfini, M.; Cangkrama, M.; Müller, B.; Jin, J.; Brinckmann, J.; Mazza, E.; Werner, S. Activin-mediated alterations of the fibroblast transcriptome and matrisome control the biomechanical properties of skin wounds. Nat. Commun. 2020, 11, 2604. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, H.; Ge, J.; Shao, F.; Huang, Z.; Ding, Z.; Dong, L.; Chen, J.; Zhang, J.; Zang, Y. Tubule-derived INHBB promotes interstitial fibroblast activation and renal fibrosis. J. Pathol. 2021, 256, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Egerman, M.A.; Zhang, Y.; Donne, R.; Xu, J.; Gadi, A.; McEwen, C.; Salmon, H.; Xiong, K.; Bai, Y.; Germino, M.; et al. ActRII or BMPR ligands inhibit skeletal myoblast differentiation, and BMPs promote heterotopic ossification in skeletal muscles in mice. Skelet. Muscle 2025, 15, 4. [Google Scholar] [CrossRef]
- Hamang, M.; Yaden, B.; Dai, G. Gastrointestinal pharmacology activins in liver health and disease. Biochem. Pharmacol. 2023, 214, 115668. [Google Scholar] [CrossRef]
- Hu, P.; Rychik, J.; Zhao, J.; Bai, H.; Bauer, A.; Yu, W.; Rand, E.B.; Dodds, K.M.; Goldberg, D.J.; Tan, K.; et al. Single-cell multiomics guided mechanistic understanding of Fontan-associated liver disease. Sci. Transl. Med. 2024, 16, eadk6213. [Google Scholar] [CrossRef]
- Lee, C.; Kim, M.-J.; Kumar, A.; Lee, H.-W.; Yang, Y.; Kim, Y. Vascular endothelial growth factor signaling in health and disease: From molecular mechanisms to therapeutic perspectives. Signal Transduct. Target. Ther. 2025, 10, 170. [Google Scholar] [CrossRef]
- Gharbia, F.Z.; Abouhashem, A.S.; Moqidem, Y.A.; Elbaz, A.A.; Abdellatif, A.; Singh, K.; Sen, C.K.; Azzazy, H.M.E. Adult skin fibroblast state change in murine wound healing. Sci. Rep. 2023, 13, 886. [Google Scholar] [CrossRef]
- Kuai, L.; Zhang, J.-T.; Deng, Y.; Xu, S.; Xu, X.-Z.; Wu, M.-F.; Guo, D.-J.; Chen, Y.; Wu, R.-J.; Zhao, X.-Q.; et al. Sheng-ji Hua-yu formula promotes diabetic wound healing of re-epithelization via Activin/Follistatin regulation. BMC Complement. Altern. Med. 2018, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Qi, Y.; Kong, X.; Wang, R.; Qi, J.; Lin, F.; Cui, X.; Liu, Z. Activin A as a Novel Chemokine Induces Migration of L929 Fibroblasts by ERK Signaling in Microfluidic Devices. Front. Cell Dev. Biol. 2021, 9, 660316. [Google Scholar] [CrossRef]
- Yun, S.O.; Kim, Y.I.; Ahn, H.J.; Min, S.Y. Activin suppresses the expression of inflammatory genes and signaling proteins in human leukemia monocytic THP-1 cells. Cell Mol Biol 2023, 69, 36–40. [Google Scholar] [CrossRef]
- Abarca-Buis, R.F.; Mandujano-Tinoco, E.A.; Cabrera-Wrooman, A.; Krötzsch, E. The complexity of TGFβ/activin signaling in regeneration. J. Cell Commun. Signal. 2021, 15, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Athanasios, D.A.; Stergios, A.P.; Nikolaos, E.R.; Polyzois, M.; Ajay, K.; Bhanu, K.; Christos, S.M. Activins, follistatins and inhibins in postmenopausal osteoporosis: A proof of concept, case-control study. Metabolism 2023, 141, 155397. [Google Scholar] [CrossRef]
- Christophe, G.; Laurent, S.; Athénaïs, B.; Raphaël, T.; Ly, T.; Mina, O.; Christopher, J.R.; Pascal, D.G.; Grégoire, P.; Emmanuel, B.; et al. Serum and Pulmonary Expression Profiles of the Activin Signaling System in Pulmonary Arterial Hypertension. Circulation 2023, 147, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Bong, D.; Sohn, J.; Lee, S.-J.V. Brief guide to RT-qPCR. Mol. Cells 2024, 47, 100141. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wang, X.; Zhao, S.; Chen, X.; Wu, W.; Zhang, Y.; Chen, Q.; Xu, X.; Yang, X.; Zhang, M.; et al. Activin B Regulates Fibroblasts to Promote Granulation Tissue Formation and Angiogenesis During Murine Skin-Wound Healing via the JNK/ERK Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 10284. https://doi.org/10.3390/ijms262110284
Xu J, Wang X, Zhao S, Chen X, Wu W, Zhang Y, Chen Q, Xu X, Yang X, Zhang M, et al. Activin B Regulates Fibroblasts to Promote Granulation Tissue Formation and Angiogenesis During Murine Skin-Wound Healing via the JNK/ERK Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(21):10284. https://doi.org/10.3390/ijms262110284
Chicago/Turabian StyleXu, Jinfu, Xueer Wang, Shan Zhao, Xiaofeng Chen, Wei Wu, Yarui Zhang, Qimei Chen, Xunhong Xu, Xinyu Yang, Min Zhang, and et al. 2025. "Activin B Regulates Fibroblasts to Promote Granulation Tissue Formation and Angiogenesis During Murine Skin-Wound Healing via the JNK/ERK Signaling Pathway" International Journal of Molecular Sciences 26, no. 21: 10284. https://doi.org/10.3390/ijms262110284
APA StyleXu, J., Wang, X., Zhao, S., Chen, X., Wu, W., Zhang, Y., Chen, Q., Xu, X., Yang, X., Zhang, M., & Zhang, L. (2025). Activin B Regulates Fibroblasts to Promote Granulation Tissue Formation and Angiogenesis During Murine Skin-Wound Healing via the JNK/ERK Signaling Pathway. International Journal of Molecular Sciences, 26(21), 10284. https://doi.org/10.3390/ijms262110284






