ERO1α as a Potential Drug Target for Breast Cancer: A Systematic Review of Current Evidence
Abstract
1. Introduction
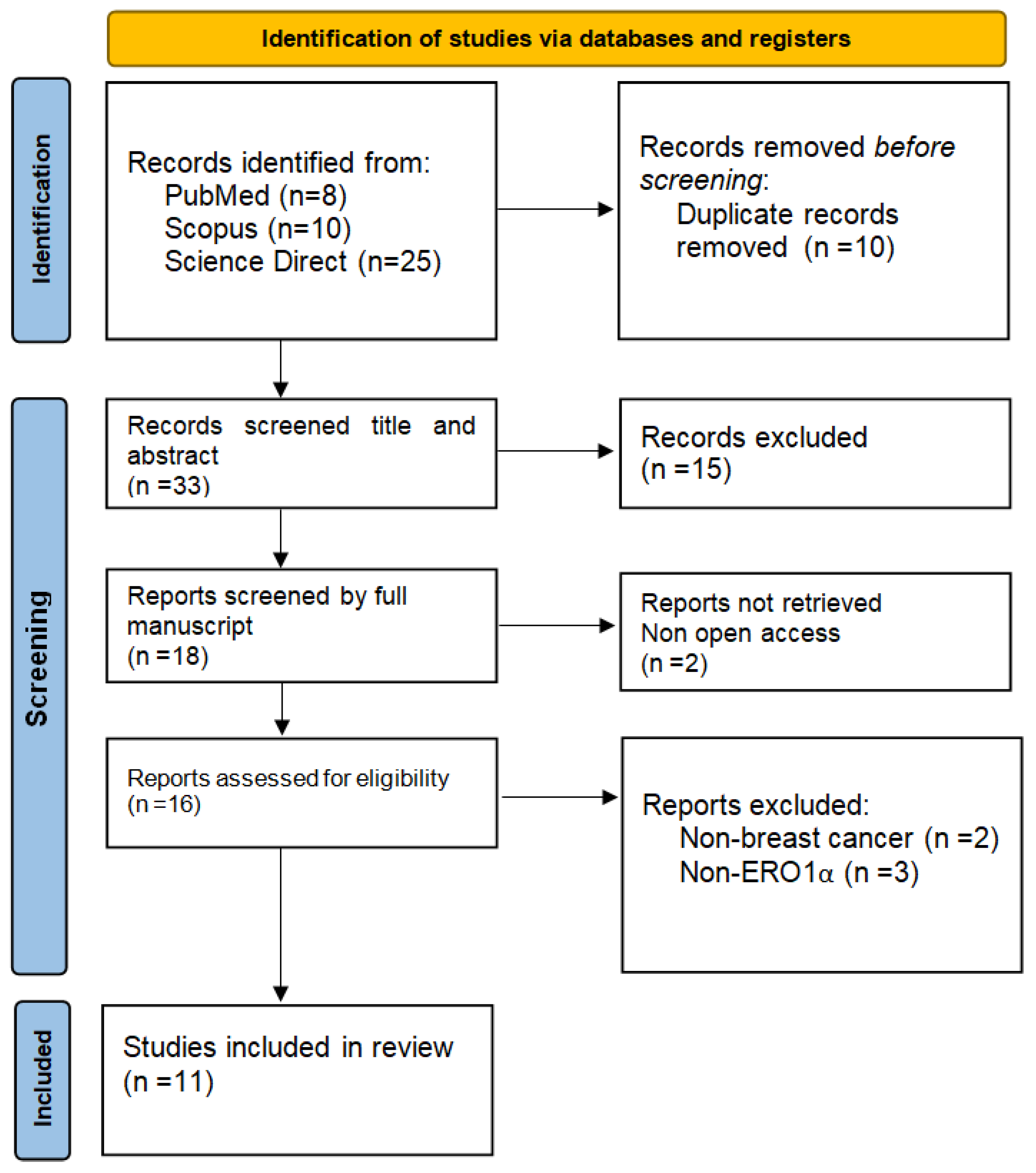
2. Methods
- Search strategy:
- Eligibility criteria:
- Primary research articles focused on ERO1α in breast cancer.
- In vitro and in vivo studies.
- Articles published in English.
- Articles published in languages other than English.
- Reviews and meta-analyses.
- Studies not related to ERO1α or breast cancer.
- Data collection process:
- Types of outcome:
- Data extraction:
3. Results
3.1. Overexpression of ERO1α in Breast Cancer Cells Compared to Normal Cells
3.2. Hypoxia Is a Major Inducer of ERO1α Expression
3.3. ERO1α Stimulates Expression of VEGF-A Leading to Increased Angiogenesis and Metastatic Potential
3.4. ERO1α Mediates mTORC1 Activated Ferroptosis Resistance
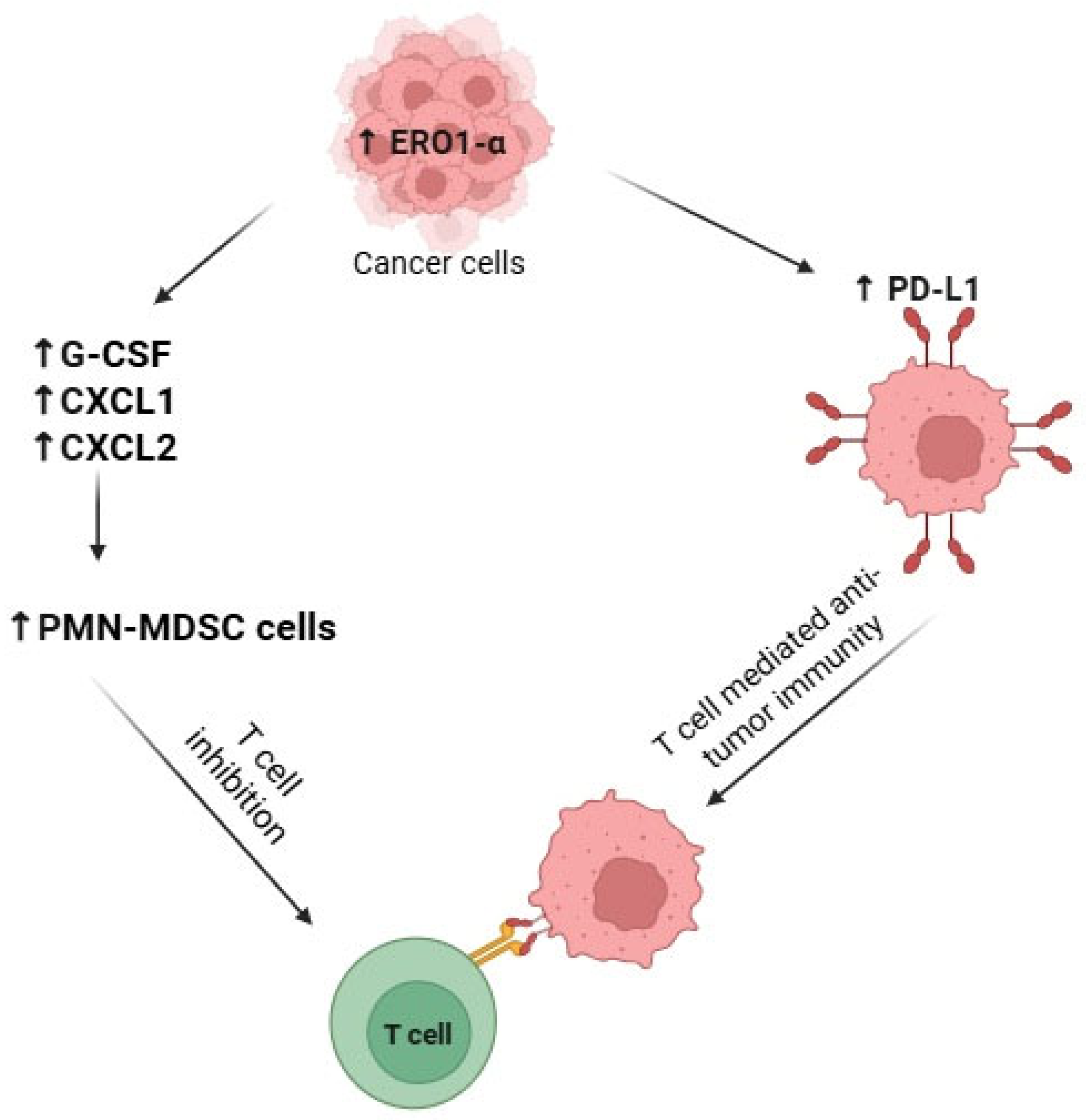
3.5. ERO1α Expression Modulates Immune Response and Tumor Microenvironment
3.5.1. By Inhibiting T-Cell Response via Recruitment of Myeloid-Derived Suppressor Cells (MDSCs)
3.5.2. By Stimulating Expression of PD-L1 and Decreasing Anti-Tumor Immunity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BIP/GRP78 | Binding Immunoglobulin Protein/Glucose-Regulated Protein 78 |
| Breg | Regulatory B Cell |
| CpG | Cytosine-phosphate-Guanine |
| CXCL1/CXCL2 | C-X-C Motif Chemokine Ligand 1/2 |
| CXCR2 | C-X-C Chemokine Receptor Type 2 |
| CCA | Cholangiocarcinoma |
| ER | Endoplasmic Reticulum |
| ERO1α | Endoplasmic Reticulum Oxidoreductin 1 Alpha |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GPX4 | Glutathione Peroxidase 4 |
| HIF-1α | Hypoxia-Inducible Factor 1 Alpha |
| MDSC | Myeloid-Derived Suppressor Cell |
| M-MDSC | Monocytic Myeloid-Derived Suppressor Cell |
| mRNA | Messenger Ribonucleic Acid |
| mTORC1 | Mechanistic Target of Rapamycin Complex 1 |
| NK Cell | Natural Killer Cell |
| PDI | Protein Disulfide Isomerase |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PMN-MDSC | Polymorphonuclear Myeloid-Derived Suppressor Cell |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ROS | Reactive Oxygen Species |
| SLC7A11 | Solute Carrier Family 7 Member 11 |
| TME | Tumor Microenvironment |
| Treg | Regulatory T Cell |
| TSC1/TSC2 | Tuberous Sclerosis Complex 1/2 |
| UPR | Unfolded Protein Response |
| VEGF-A | Vascular Endothelial Growth Factor A |
| VEGF121 | Vascular Endothelial Growth Factor 121 Isoform |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
References
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast Cancer Development and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Moura, T.; Laranjeira, P.; Caramelo, O.; Gil, A.M.; Paiva, A. Breast Cancer and Tumor Microenvironment: The Crucial Role of Immune Cells. Curr. Oncol. 2025, 32, 143. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Wang, J.; Guan, R.; Sun, J.; Jin, P.; Shen, J. Role of Oxidative Stress in the Occurrence, Development, and Treatment of Breast Cancer. Antioxidants 2025, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Capatina, A.L.; Malcolm, J.R.; Stenning, J.; Moore, R.L.; Bridge, K.S.; Brackenbury, W.J.; Holding, A.N. Hypoxia-Induced Epigenetic Regulation of Breast Cancer Progression and the Tumour Microenvironment. Front. Cell Dev. Biol. 2024, 12, 1421629. [Google Scholar] [CrossRef]
- Di Carlo, E.; Sorrentino, C. Oxidative Stress and Age-Related Tumors. Antioxidants 2024, 13, 1109. [Google Scholar] [CrossRef]
- Santos, C.X.C.; Tanaka, L.Y.; Wosniak, J.; Laurindo, F.R.M. Mechanisms and Implications of Reactive Oxygen Species Generation During the Unfolded Protein Response: Roles of Endoplasmic Reticulum Oxidoreductases, Mitochondrial Electron Transport, and NADPH Oxidase. Antioxid. Redox Signal. 2009, 11, 2409–2427. [Google Scholar] [CrossRef]
- Frand, A.R.; Kaiser, C.A. Ero1p Oxidizes Protein Disulfide Isomerase in a Pathway for Disulfide Bond Formation in the Endoplasmic Reticulum. Mol. Cell 1999, 4, 469–477. [Google Scholar] [CrossRef]
- Shergalis, A.G.; Hu, S.; Bankhead, A., 3rd; Neamati, N. Role of the ERO1-PDI Interaction in Oxidative Protein Folding and Disease. Pharmacol. Ther. 2020, 210, 107525. [Google Scholar] [CrossRef]
- Tu, B.P.; Weissman, J.S. Oxidative Protein Folding in Eukaryotes: Mechanisms and Consequences. J. Cell Biol. 2004, 164, 341–346. [Google Scholar] [CrossRef]
- Princiotta, M.F.; Finzi, D.; Qian, S.-B.; Gibbs, J.; Schuchmann, S.; Buttgereit, F.; Bennink, J.R.; Yewdell, J.W. Quantitating Protein Synthesis, Degradation, and Endogenous Antigen Processing. Immunity 2003, 18, 343–354. [Google Scholar] [CrossRef]
- Gupta, N.; Park, J.E.; Tse, W.; Low, J.K.; Kon, O.L.; McCarthy, N.; Sze, S.K. ERO1α Promotes Hypoxic Tumor Progression and Is Associated with Poor Prognosis in Pancreatic Cancer. Oncotarget 2019, 10, 5970–5982. [Google Scholar] [CrossRef]
- Yang, S.; Yang, C.; Yu, F.; Ding, W.; Hu, Y.; Cheng, F.; Zhang, F.; Guan, B.; Wang, X.; Lu, L.; et al. Endoplasmic Reticulum Resident Oxidase ERO1-Lalpha Promotes Hepatocellular Carcinoma Metastasis and Angiogenesis through the S1PR1/STAT3/VEGF-A Pathway. Cell Death Dis. 2018, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Varone, E.; Decio, A.; Chernorudskiy, A.; Minoli, L.; Brunelli, L.; Ioli, F.; Piotti, A.; Pastorelli, R.; Fratelli, M.; Gobbi, M.; et al. The ER Stress Response Mediator ERO1 Triggers Cancer Metastasis by Favoring the Angiogenic Switch in Hypoxic Conditions. Oncogene 2021, 40, 1721–1736. [Google Scholar] [CrossRef] [PubMed]
- Voronkova, M.A.; Johnson, B.; Gandhi, N.; Koomen, J.M.; Patrick, M.; Bhupathi, S.S.; Wu, V.M.; Elliott, A.; Vanderwalde, A.; Halmos, B.; et al. ERO1A Levels Are a Prognostic Indicator in EGFR Mutated Non Small Cell Lung Cancer. Npj Precis. Oncol. 2024, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.D.; Geldenhuys, W.J.; Hazlehurst, L.A. The Role of ERO1α in Modulating Cancer Progression and Immune Escape. J. Cancer Immunol. 2020, 2, 103–115. [Google Scholar] [CrossRef]
- Chen, P.; Sharma, A.; Weiher, H.; Schmidt-Wolf, I.G.H. Biological Mechanisms and Clinical Significance of Endoplasmic Reticulum Oxidoreductase 1 Alpha (ERO1α) in Human Cancer. J. Exp. Clin. Cancer Res. 2024, 43, 71. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Open Science Framework. ERO1α as a Potential Drug Target for Breast Cancer: A Systematic Review of Current Evidence. Available online: https://osf.io/sx3u2/?view_only=c5412e1a180844c6aab8e56f5d5c911a (accessed on 4 October 2025).
- Zhang, Y.; Li, T.; Zhang, L.; Shangguan, F.; Shi, G.; Wu, X.; Cui, Y.; Wang, X.; Wang, X.; Liu, Y.; et al. Targeting the Functional Interplay between Endoplasmic Reticulum Oxidoreductin-1α and Protein Disulfide Isomerase Suppresses the Progression of Cervical Cancer. EBioMedicine 2019, 41, 408–419. [Google Scholar] [CrossRef]
- Zhang, N.; Zhu, L.; Wu, X.; Yan, R.; Yang, S.; Jiang, X.; Liu, X.; Liu, X.; Yan, N.; Cong, G.; et al. The Regulation of Ero1-Alpha in Homocysteine-Induced Macrophage Apoptosis and Vulnerable Plaque Formation in Atherosclerosis. Atherosclerosis 2021, 334, 39–47. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Yang, C.; Chen, D.; Wang, T.; Liu, T.; Yan, W.; Su, Z.; Peng, B.; Ren, X. Cordycepin Reprogramming Lipid Metabolism to Block Metastasis and EMT via ERO1A/mTOR/SREBP1 Axis in Cholangiocarcinoma. Life Sci. 2023, 327, 121698. [Google Scholar] [CrossRef]
- Albassam, H.; Mehta, C.H.; Nayak, U.Y. Identification of Novel Small Molecule Inhibitors for Endoplasmic Reticulum Oxidoreductase 1α (ERO1α) Enzyme: Structure-Based Molecular Docking and Molecular Dynamic Simulation Studies. J. Biomol. Struct. Dyn. 2022, 40, 13218–13232. [Google Scholar] [CrossRef]
- Lee, S.; Lee, E.; Ko, E.; Ham, M.; Lee, H.M.; Kim, E.-S.; Koh, M.; Lim, H.K.; Jung, J.; Park, S.Y.; et al. Tumor-Associated Macrophages Secrete CCL2 and Induce the Invasive Phenotype of Human Breast Epithelial Cells through Upregulation of ERO1-α and MMP-9. Cancer Lett. 2018, 437, 25–34. [Google Scholar] [CrossRef]
- Kutomi, G.; Tamura, Y.; Tanaka, T.; Kajiwara, T.; Kukita, K.; Ohmura, T.; Shima, H.; Takamaru, T.; Satomi, F.; Suzuki, Y.; et al. Human Endoplasmic Reticulum Oxidoreductin 1-α Is a Novel Predictor for Poor Prognosis of Breast Cancer. Cancer Sci. 2013, 104, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kajiwara, T.; Torigoe, T.; Okamoto, Y.; Sato, N.; Tamura, Y. Cancer-Associated Oxidoreductase ERO1-α Drives the Production of Tumor-Promoting Myeloid-Derived Suppressor Cells via Oxidative Protein Folding. J. Immunol. 2015, 194, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kutomi, G.; Kajiwara, T.; Kukita, K.; Kochin, V.; Kanaseki, T.; Tsukahara, T.; Hirohashi, Y.; Torigoe, T.; Okamoto, Y.; et al. Cancer-Associated Oxidoreductase ERO1-α Drives the Production of VEGF via Oxidative Protein Folding and Regulating the mRNA Level. Br. J. Cancer 2016, 114, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kutomi, G.; Kajiwara, T.; Kukita, K.; Kochin, V.; Kanaseki, T.; Tsukahara, T.; Hirohashi, Y.; Torigoe, T.; Okamoto, Y.; et al. Cancer-Associated Oxidoreductase ERO1-α Promotes Immune Escape through up-Regulation of PD-L1 in Human Breast Cancer. Oncotarget 2017, 8, 24706–24718. [Google Scholar] [CrossRef]
- Takei, N.; Yoneda, A.; Kosaka, M.; Sakai-Sawada, K.; Tamura, Y. ERO1α Is a Novel Endogenous Marker of Hypoxia in Human Cancer Cell Lines. BMC Cancer 2019, 19, 510. [Google Scholar] [CrossRef]
- Varone, E.; Decio, A.; Barbera, M.C.; Bolis, M.; Di Rito, L.; Pisati, F.; Giavazzi, R.; Zito, E. Endoplasmic Reticulum Oxidoreductin 1-Alpha Deficiency and Activation of Protein Translation Synergistically Impair Breast Tumour Resilience. Br. J. Pharmacol. 2022, 179, 5180–5195. [Google Scholar] [CrossRef]
- Varone, E.; Chernorudskiy, A.; Cherubini, A.; Cattaneo, A.; Bachi, A.; Fumagalli, S.; Erol, G.; Gobbi, M.; Lenardo, M.J.; Borgese, N.; et al. ERO1 Alpha Deficiency Impairs Angiogenesis by Increasing N-Glycosylation of a Proangiogenic VEGFA. Redox Biol. 2022, 56, 102455. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, H.; Liu, W.; Lin, W.; Sun, A.; Ding, Z.; Chen, X.; Wan, X.; Liu, Y.; Hu, Z.; et al. Augmented ERO1α upon mTORC1 Activation Induces Ferroptosis Resistance and Tumor Progression via Upregulation of SLC7A11. J. Exp. Clin. Cancer Res. 2024, 43, 112. [Google Scholar] [CrossRef]
- Hermawan, A.; Putri, H.; Fatimah, N.; Prasetio, H.H. Transcriptomics Analysis Reveals Distinct Mechanism of Breast Cancer Stem Cells Regulation in Mammospheres from MCF-7 and T47D Cells. Heliyon 2024, 10, e24356. [Google Scholar] [CrossRef]
- Varone, E.; Retini, M.; Cherubini, A.; Chernorudskiy, A.; Marrazza, A.; Guidarelli, A.; Cagnotto, A.; Beeg, M.; Gobbi, M.; Fumagalli, S.; et al. Small Molecule-Mediated Inhibition of the Oxidoreductase ERO1A Restrains Aggressive Breast Cancer by Impairing VEGF and PD-L1 in the Tumor Microenvironment. Cell Death Dis. 2025, 16, 105. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Li, X.-F. Hypoxia and the Tumor Microenvironment. Technol. Cancer Res. Treat. 2021, 20, 15330338211036304. [Google Scholar] [CrossRef]
- Muz, B.; De La Puente, P.; Azab, F.; Azab, A.K. The Role of Hypoxia in Cancer Progression, Angiogenesis, Metastasis, and Resistance to Therapy. Hypoxia 2015, 83, 83–92. [Google Scholar] [CrossRef]
- Takei, N.; Yoneda, A.; Sakai-Sawada, K.; Kosaka, M.; Minomi, K.; Tamura, Y. Hypoxia-Inducible ERO1α Promotes Cancer Progression through Modulation of Integrin-Β1 Modification and Signalling in HCT116 Colorectal Cancer Cells. Sci. Rep. 2017, 7, 9389. [Google Scholar] [CrossRef]
- Wang, M.; Wey, S.; Zhang, Y.; Ye, R.; Lee, A.S. Role of the Unfolded Protein Response Regulator GRP78/BiP in Development, Cancer, and Neurological Disorders. Antioxid. Redox Signal. 2009, 11, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Cellosaurus CAMA-1 (CVCL_1115). Available online: https://www.cellosaurus.org/CVCL_1115 (accessed on 29 September 2025).
- van Horssen, R.; Hollestelle, A.; Rens, J.A.P.; Eggermont, A.M.M.; Schutte, M.; Hagen, T.L.M.T. E-Cadherin Promotor Methylation and Mutation Are Inversely Related to Motility Capacity of Breast Cancer Cells. Breast Cancer Res. Treat. 2012, 136, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in Cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes. Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Razak, S.R.A.; Han, T.; Ahmad, N.H.; Li, X. Ferroptosis as a Potential Target for Cancer Therapy. Cell Death Dis. 2023, 14, 460. [Google Scholar] [CrossRef]
- Zhu, W.-W.; Liu, Y.; Yu, Z.; Wang, H.-Q. SLC7A11-Mediated Cell Death Mechanism in Cancer: A Comparative Study of Disulfidptosis and Ferroptosis. Front. Cell Dev. Biol. 2025, 13, 1559423. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Z.; Zhang, Y.; Yang, Y.; Yang, Y.; Zu, G.; Yu, X.; Chen, W.; Qin, Y.; Xu, X.; et al. IL15RA-STAT3-GPX4/ACSL3 Signaling Leads to Ferroptosis Resistance in Pancreatic Cancer. Acta Biochim. Biophys. Sin. 2025, 57, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-Tolerant Persister Cancer Cells Are Vulnerable to GPX4 Inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Motallebnezhad, M.; Jadidi-Niaragh, F.; Qamsari, E.S.; Bagheri, S.; Gharibi, T.; Yousefi, M. The Immunobiology of Myeloid-Derived Suppressor Cells in Cancer. Tumor Biol. 2016, 37, 1387–1406. [Google Scholar] [CrossRef]
- Lasser, S.A.; Kurt, F.G.O.; Arkhypov, I.; Utikal, J.; Umansky, V. Myeloid-Derived Suppressor Cells in Cancer and Cancer Therapy. Nat. Rev. Clin. Oncol. 2024, 21, 147–164. [Google Scholar] [CrossRef]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef]
- Li, C.; Xue, Y.; Yinwang, E.; Ye, Z. The Recruitment and Immune Suppression Mechanisms of Myeloid-Derived Suppressor Cells and Their Impact on Bone Metastatic Cancer. Cancer Rep. 2025, 8, e70044. [Google Scholar] [CrossRef]
- Parvez, A.; Choudhary, F.; Mudgal, P.; Khan, R.; Qureshi, K.A.; Farooqi, H.; Aspatwar, A. PD-1 and PD-L1: Architects of Immune Symphony and Immunotherapy Breakthroughs in Cancer Treatment. Front. Immunol. 2023, 14, 1296341. [Google Scholar] [CrossRef]
- Sui, X.; Ma, J.; Han, W.; Wang, X.; Fang, Y.; Li, D.; Pan, H.; Zhang, L. The Anticancer Immune Response of Anti-PD-1/PD-L1 and the Genetic Determinants of Response to Anti-PD-1/PD-L1 Antibodies in Cancer Patients. Oncotarget 2015, 6, 19393–19404. [Google Scholar] [CrossRef]
- Cha, J.-H.; Chan, L.-C.; Li, C.-W.; Hsu, J.L.; Hung, M.-C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in Cancer: Significance and Impact on Clinical Outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Zito, E.; Guarrera, L.; Janssen-Heininger, Y.M.W. Fingerprint of the Oxido-Reductase ERO1: A Protein Disulfide Bond Producer and Supporter of Cancer. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189027. [Google Scholar] [CrossRef] [PubMed]
- Paule, S.; Aljofan, M.; Simon, C.; Rombauts, L.J.F.; Nie, G. Cleavage of Endometrial -Integrins into Their Functional Forms Is Mediated by Proprotein Convertase 5/6. Human Reprod. 2012, 27, 2766–2774. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, X.; Wang, N.; Su, Z.; Bi, Y.; Sun, L.; Liu, L.; Piao, Y.; Piao, J.; Lin, Z.; et al. 2′,4′-Dihydroxychalcone Induces Ferroptosis through ERO1A/GPX4 Regulatory Axis in Cholangiocarcinoma. Phytomedicine 2025, 147, 157192. [Google Scholar] [CrossRef]
- Li, X.; Zhong, J.; Deng, X.; Guo, X.; Lu, Y.; Lin, J.; Huang, X.; Wang, C. Targeting Myeloid-Derived Suppressor Cells to Enhance the Antitumor Efficacy of Immune Checkpoint Blockade Therapy. Front. Immunol. 2021, 12, 754196. [Google Scholar] [CrossRef]
- Cabibbo, A.; Pagani, M.; Fabbri, M.; Rocchi, M.; Farmery, M.R.; Bulleid, N.J.; Sitia, R. ERO1-L, a Human Protein That Favors Disulfide Bond Formation in the Endoplasmic Reticulum. J. Biol. Chem. 2000, 275, 4827–4833. [Google Scholar] [CrossRef]
| # | Year | Authors | Name | Species | Cell Lines | Key Findings |
|---|---|---|---|---|---|---|
| 1 | 2013 | Kutomi et al. [24] | Human endoplasmic reticulum oxidoreductin 1-α is a novel predictor for poor prognosis of breast cancer | Human, mice | 4T1 | ERO1α is overexpressed in breast cancer and promotes tumor progression and metastasis. Its mRNA is detected in breast cancer tissues but not in normal tissues, and its overexpression is confirmed in MCF7 cells. Knockdown of ERO1α in 4T1 cells leads to reduced tumor growth, fewer lung metastases, and decreased VEGF-A production. |
| 2 | 2015 | Tanaka et al. [25] | Cancer-associated oxidoreductase ERO1-α drives the production of tumor-promoting myeloid-derived suppressor cells via oxidative protein folding | Human, mice | 4T1 MCF7 BT-474 UACC-893 SK-BR-3 MDA-MB-15 MDA-MB-231 and MDA-MB-468 | ERO1α promotes tumor growth and immune evasion in breast cancer by enhancing the secretion of immunosuppressive cytokines. Knockdown of ERO1α in 4T1 cells reduced tumor growth and improved survival in immunocompetent mice, effects that were lost when CD4+ and CD8+ T cells were depleted, highlighting its role in suppressing T cell-mediated immunity. ERO1α-overexpressing tumors had higher levels of G-CSF, CXCL1, and CXCL2 proteins, leading to PMN-MDSC accumulation and immune suppression, despite unchanged mRNA levels. |
| 3 | 2016 | Tanaka et al. [26] | Cancer-associated oxidoreductase ERO1-α drives the production of VEGF via oxidative protein folding and regulating the mRNA level | human, mice | MDA-MB-157 MDA-MB-231 MDA-MB-468 MCF7 | ERO1α is significantly upregulated in TNBC cell lines and tissues, correlating with poorer overall survival in patients. Knockdown of ERO1α in MDA-MB-231 cells slowed tumor growth and reduced tumor angiogenesis, as shown by fewer CD31+ blood vessels, while overexpression led to more aggressive tumor growth in NOD/SCID mice. ERO1α regulates VEGF at the protein level without altering its mRNA expression—knockdown decreased the mature, oxidized form of VEGF, and inhibition with EN460 reduced VEGF protein secretion. Additionally, ERO1α overexpression increased HIF-1α and reactive oxygen species. |
| 4 | 2017 | Tanaka et al. [27] | Cancer-associated oxidoreductase ERO1-α promotes immune escape through up-regulation of PD-L1 in human breast cancer | human, mice | MDA-MB-231 MDA-MB-468 | ERO1α enhances PD-L1 expression and maturation in MDA-MB-231 cancer cells. OE of ERO1-α increased both PD-L1 surface protein and mRNA levels, while KD reduced them. OE cells showed higher HIF-1α protein and ROS levels, and silencing HIF-1α lowered PD-L1 mRNA in some cells. ERO1α promoted the oxidized (mature) form of PD-L1, with KD cells showing a significantly lower oxidized-to-reduced PD-L1 ratio. |
| 5 | 2019 | Takei et al. [28] | ERO1α is a novel endogenous marker of hypoxia in human cancer cell lines | human, mice | MDA-MB-231 MCF7 | Under normoxic conditions, ERO1α is expressed across all tested cell lines, with notably higher levels in cancer cell lines. Additionally, the hypoxia marker CA9 is significantly elevated in the aggressive MDA-MB-231 breast cancer cells compared to both normal cells and MCF7 cells. |
| 6 | 2021 | Varone et al. [13] | The ER Stress Response Mediator ERO1 Triggers Cancer Metastasis by Favoring the Angiogenic Switch in Hypoxic Conditions | Human, mice | MDAMB231 4T1 E0771 | ERO1α is highly expressed in several breast cancer cells, particularly elevated in aggressive basal/TNBC types. Under hypoxia, ERO1α levels increase in most cells except luminal CAMA1. Loss of ERO1α impairs cell migration and leads to an accumulation of proteins with free thiols and reduced disulfide-bonded secreted factors. VEGFA secretion is significantly decreased in ERO1α KO cells, especially under hypoxia, while VEGFR2 is upregulated, possibly as compensation. Key ER stress markers ATF4 and CHOP are downregulated in ERO1α KO cells during hypoxia, unlike in wild-type cells where they increase, indicating impaired unfolded protein response activation. |
| 7 | 2022 | Varone et al. [29] | Endoplasmic reticulum oxidoreductin 1-alpha deficiency and activation of protein translation synergistically impair breast tumour resilience | Human, mice | MDAMB231 MCF7 | Under hypoxia, ERO1α KO cells showed increased accumulation of VEGF121 and chaperone BIP in the detergent-insoluble fraction, along with higher phosphorylated eIF2α, indicating suppressed protein translation. While wild-type MDA-MB-231 cells maintained protein synthesis under hypoxia, ERO1α KO cells exhibited reduced translation. ISRIB modestly decreased ATF4 and CHOP transcripts without affecting ERO1α expression. VEGFA expression was reduced in ERO1α KO cells under hypoxia, whereas VEGFB remained unchanged. Additionally, ERO1α KO breast tumors upregulated the PERK pathway of the unfolded protein response. |
| 8 | 2022 | Varone et al. [30] | ERO1 alpha deficiency impairs angiogenesis by increasing N-glycosylation of a proangiogenic VEGFA | mice | ERO1 KO-MDAMB231 | ERO1α KO TNBC xenografts exhibited significantly increased protein N-hyperglycosylation compared to wild-type tumors, with a five-fold increase in cluster volume observed in ERO1α KO tumors. |
| 9 | 2024 | Wang et al. [31] | Augmented ERO1α upon mTORC1 activation induces ferroptosis resistance and tumor progression via upregulation of SLC7A11 | Human, mice | MDA-MB-231 | ERO1α acts as a downstream effector of mTORC1, promoting ferroptosis resistance and tumor progression by upregulating SLC7A11 through activation of the IL-6/STAT3 pathway. Combining ERO1α inhibition with the ferroptosis inducer imidazole ketone erastin (IKE) produced a synergistic antitumor effect in mTORC1-driven tumor models, including cell line xenografts, LSCC organoids, and patient-derived xenografts. |
| 10 | 2024 | Hermawan et al. [32] | Transcriptomics analysis reveals distinct mechanism of breast cancer stem cells regulation in mammospheres from MCF-7 and T47D cells | human | MCF-7 T47D cells | ERO1α ranked among the top 10 upregulated genes in breast cancer. Additionally, DNA methylation analysis showed significant differences in ERO1L gene expression between low-risk and high-risk breast cancer patient groups. |
| 11 | 2025 | Varone et al. [33] | Small molecule-mediated inhibition of the oxidoreductase ERO1A restrains aggressive breast cancer by impairing VEGF and PD-L1 in the tumor microenvironment | Human, mice | MDA-MB-231 | ERO1A is overexpressed in MDA-MB-231 cell line and drives breast cancer aggressiveness. EN460 and I2 downregulated proliferative pathways (E2F, G2M, MYC), consistent with suppressed tumor growth. ERO1A inhibition limits tumor fitness by impairing proliferation, suppressing angiogenesis, and modulating the immune microenvironment. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khojayeva, K.; Moldasheva, A.; Aljofan, M. ERO1α as a Potential Drug Target for Breast Cancer: A Systematic Review of Current Evidence. Int. J. Mol. Sci. 2025, 26, 10276. https://doi.org/10.3390/ijms262110276
Khojayeva K, Moldasheva A, Aljofan M. ERO1α as a Potential Drug Target for Breast Cancer: A Systematic Review of Current Evidence. International Journal of Molecular Sciences. 2025; 26(21):10276. https://doi.org/10.3390/ijms262110276
Chicago/Turabian StyleKhojayeva, Kamilla, Aiman Moldasheva, and Mohamad Aljofan. 2025. "ERO1α as a Potential Drug Target for Breast Cancer: A Systematic Review of Current Evidence" International Journal of Molecular Sciences 26, no. 21: 10276. https://doi.org/10.3390/ijms262110276
APA StyleKhojayeva, K., Moldasheva, A., & Aljofan, M. (2025). ERO1α as a Potential Drug Target for Breast Cancer: A Systematic Review of Current Evidence. International Journal of Molecular Sciences, 26(21), 10276. https://doi.org/10.3390/ijms262110276







