Preparation and Characterization of New pH-Sensitive Polyurethane Hydrogels as Anti-Cancer Drug Delivery Systems for 5-Fluorouracyl and Fluorodeoxyuridine
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of CL, LA, and PEG Copolymers
- Copolymers of CL, LA, and PEG-1500 (molar ratio CL:LA—1:1).For process yield:y1 = 93.2936 + 2.0825x1 − 1.3025x2 − 6.5625x3For CL contained in the copolymer chain:y2 = 0.6882 − 0.0213x1 − 0.0813x2 + 0.0038x3
- Copolymers of CL, LA, and PEG-1500 (molar ratio CL:LA—0.6:0.4).For process yield:y1 = 95.2255 + 2.3813x1 − 1.5288x2 − 5.5663x3For CL contained in the copolymer chain:y2 = 0.7518 − 0.0213x1 − 0.0863x2 + 0.0038x3
- Copolymers of CL, LA, and PEG-1500 (molar ratio CL:LA—0.7:0.3).For process yield:y1 = 94.0409 + 2.2525x1 − 1.415x2 − 6.3475x3For CL contained in the copolymer chain:y2 = 0.7891 − 0.0225x1 − 0.09x2 + 0.005x3
2.2. Hydrogel DDSs Preparation
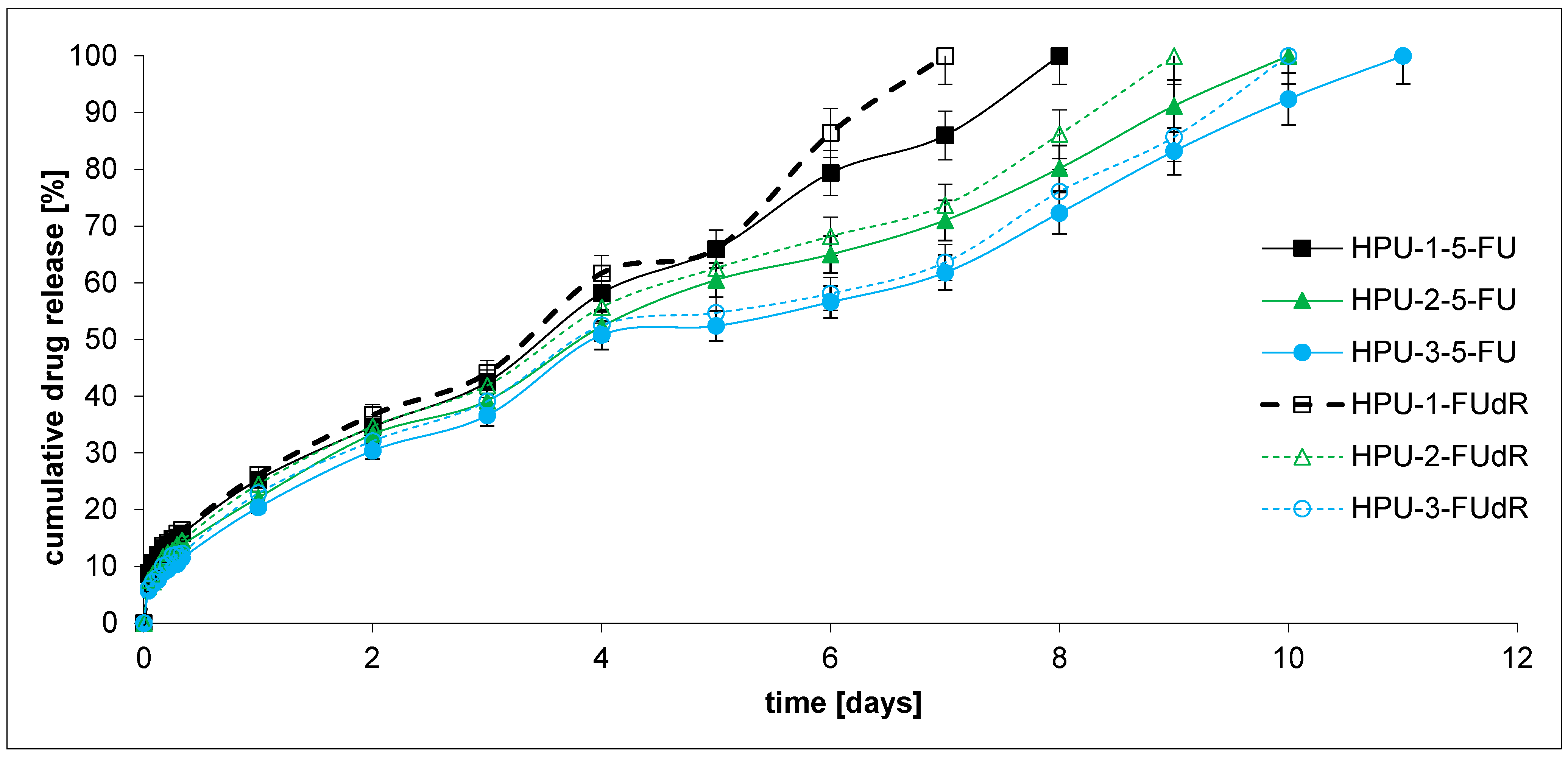
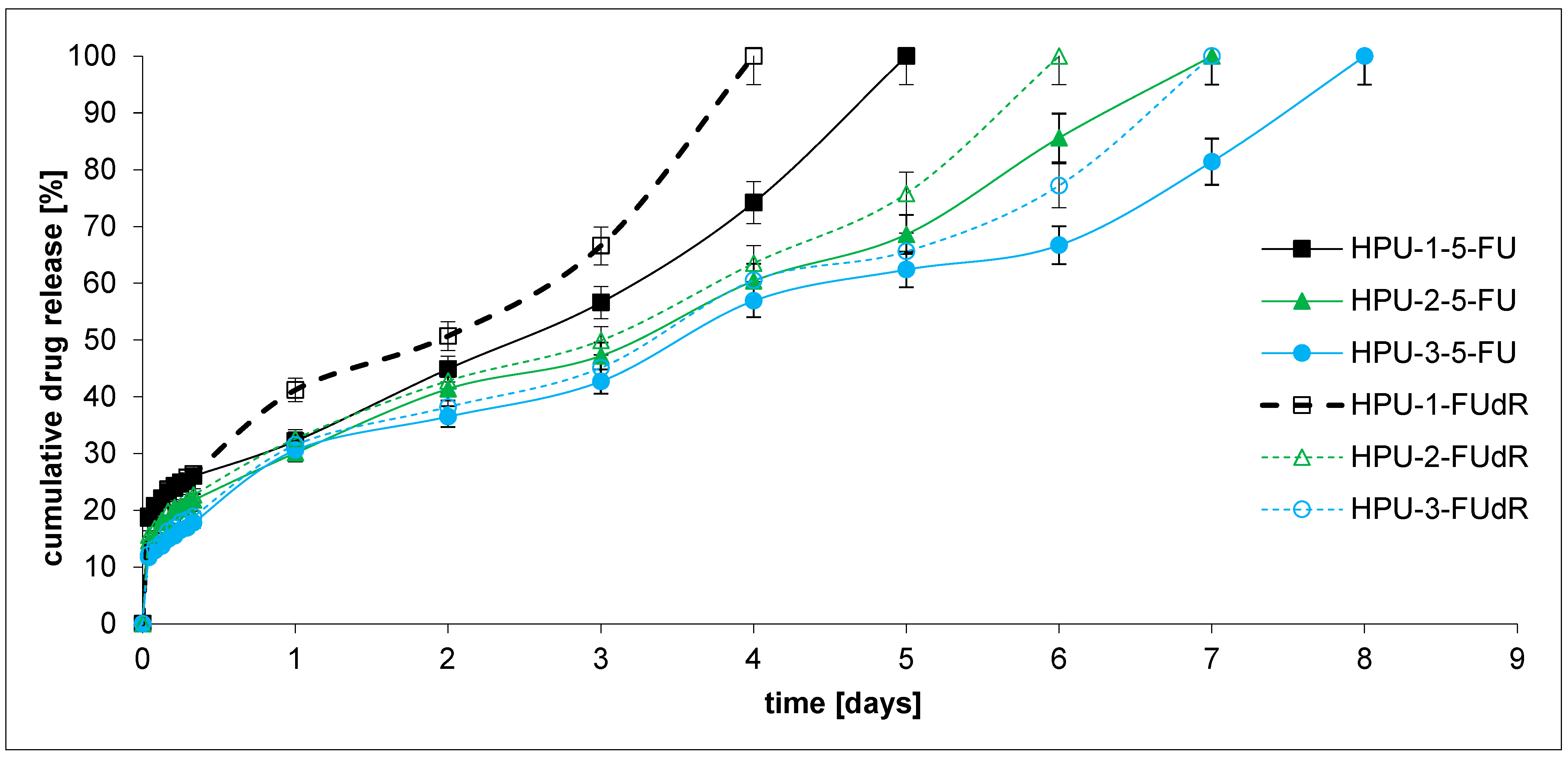
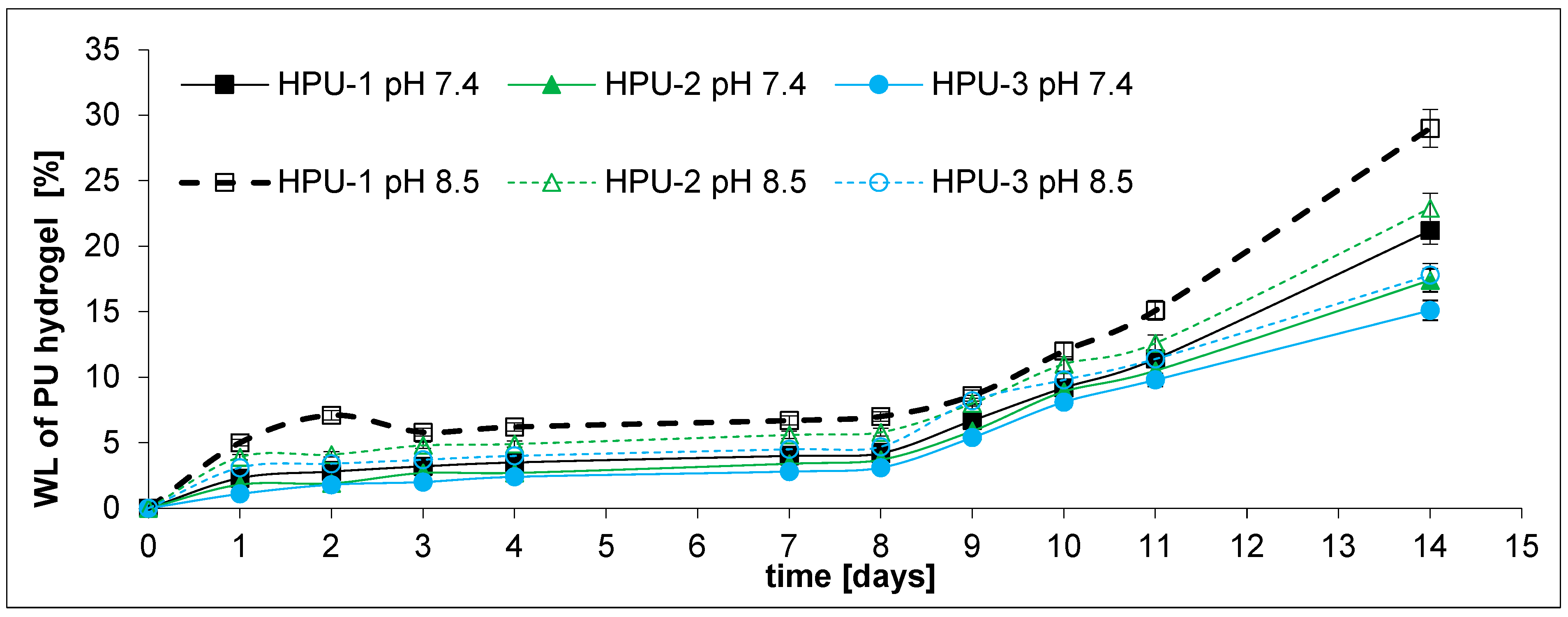
2.3. Drug Release and DDS Degradation Studies
3. Materials and Methods
3.1. Materials
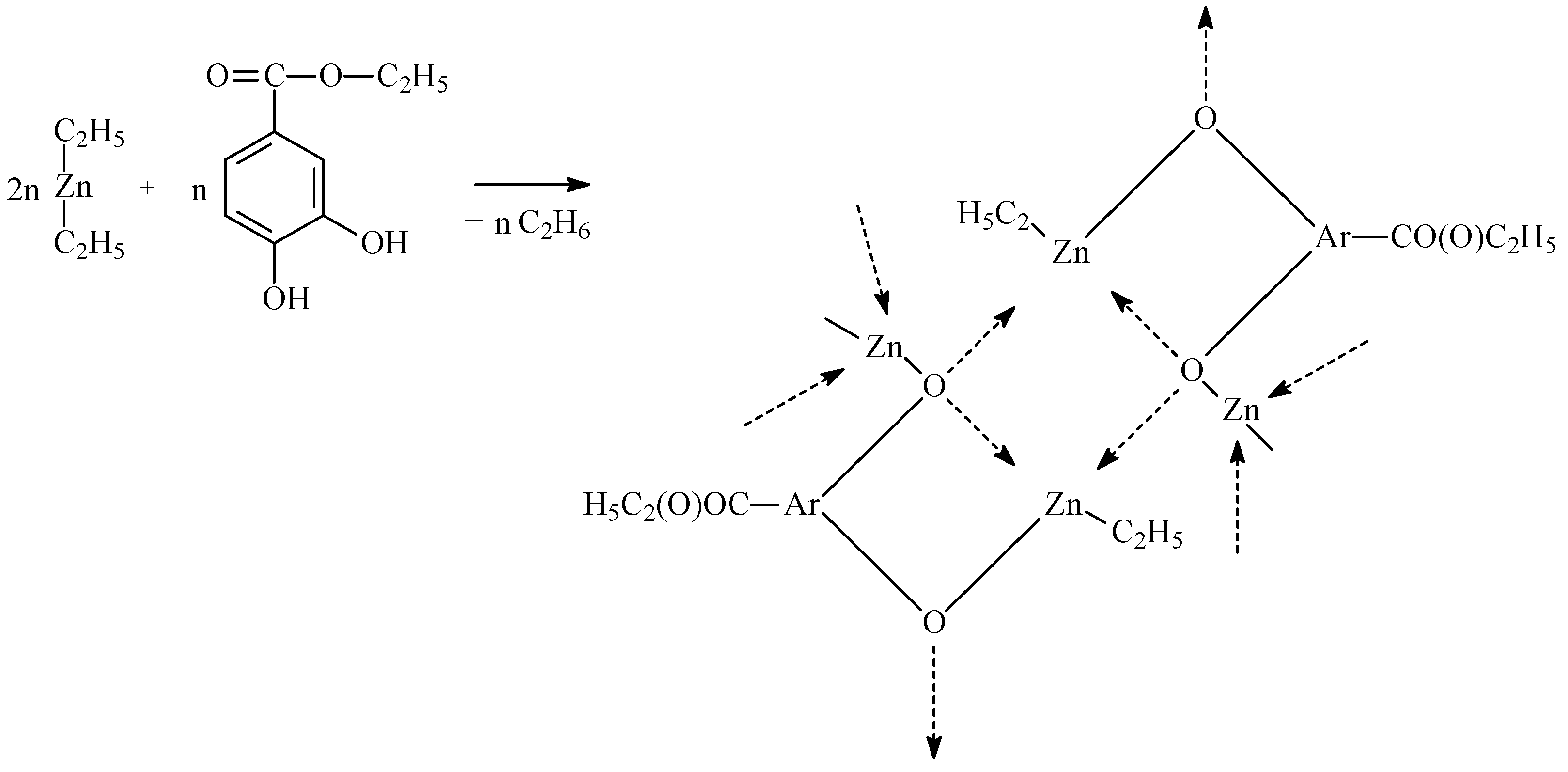
3.2. Synthesis of Catalytic Systems
3.3. Copolymerization of CL, rac-LA, and PEG Procedure
Design of Experiments
- z1—polyreaction time (h).
- z2—polyreaction temperature (°C).
- z3—CL (LA): PEG molar ratio.
- y1—process yield (%).
- y2—CL contained in the copolymer chain (mol %).
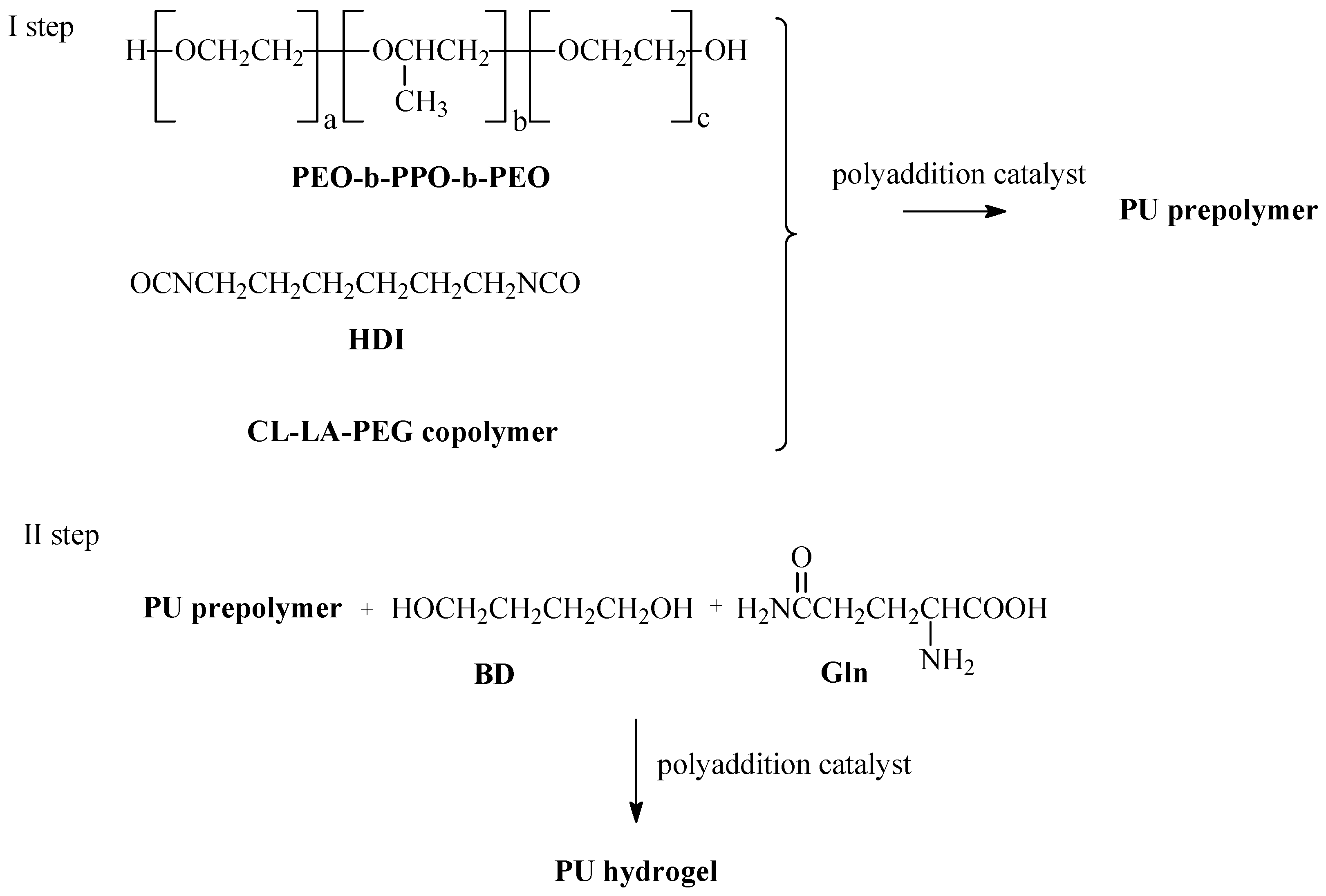
3.4. Preparation of PU Hydrogels
3.5. Preparation of Hydrogel DDSs Containing 5-FU or FUdR
3.6. Drug Release Studies
- F is the fraction of drug released from the matrix after time t;
- F0 is the initial amount of drug;
- k is a model constant and n is the drug release exponent in the Korsmeyer–Peppas model [60].
3.7. NMR Measurements
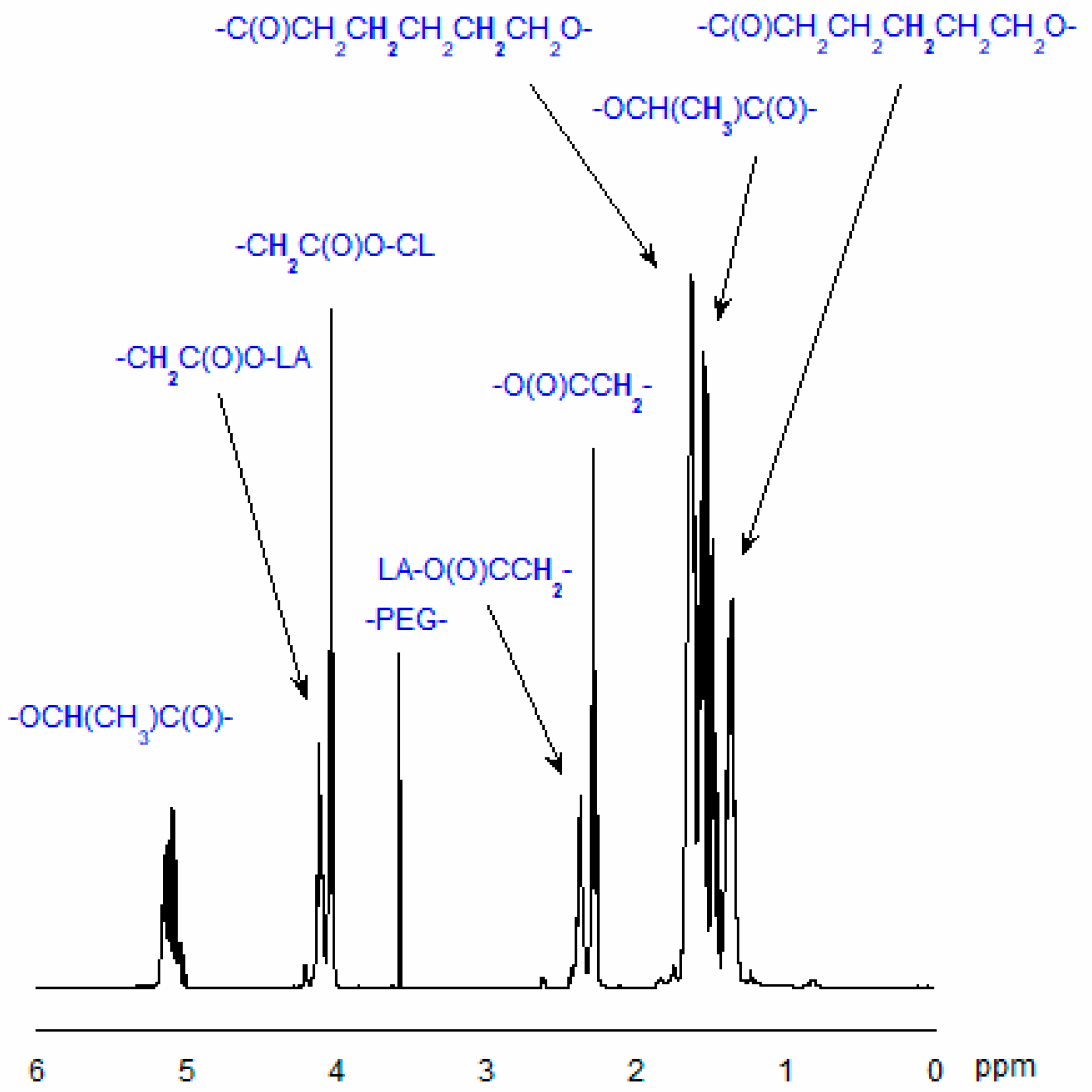
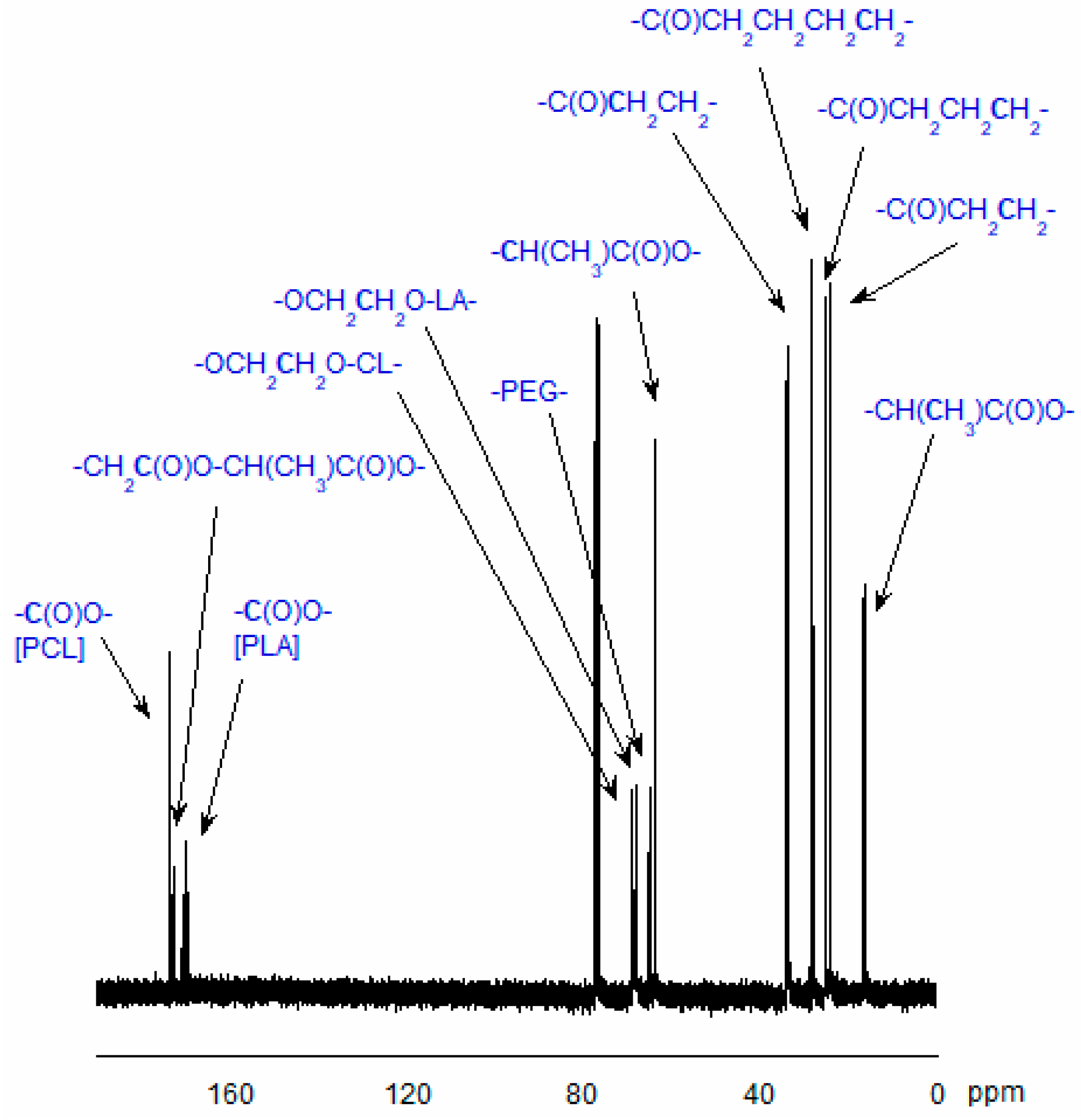
3.7.1. Analysis of Copolymer Structure
- ICL—the integral intensity of the protons adjacent to the carbon atom of CL units (-CO-CH2-CH2-CH2-CH2-CH2-O-) in the copolymeric chain;
- ILA—the integral intensity of the protons of LA units (-CO-CH(CH3)-O-);
- IEG—the integral intensity of methylene protons of PEG (-CH2-CH2-O-), and nEG is the average number of ethylene glycol mers in the PEG molecule.
3.7.2. NMR Data
3.8. GPC Measurements
3.9. HPLC Measurements
3.10. Toxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BD | 1,4-butanediol |
| CHIT | chitosan |
| CL | є-caprolactone |
| CL-LA-PEG | copolymer of є-caprolactone, lactide, and poly(ethylene glycol) |
| DBTDL | dibutyltin dilaurate |
| DDSs | drug delivery systems |
| EDHB | ethyl-3,4-dihydroxybenzoate |
| 5-FU | 5-fluorouracil |
| FUdR | 5-fluoro-2′-deoxyuridine |
| Gln | L-glutamine |
| HDI | hexamethylene diisocyanate |
| LA | rac-lactide |
| PCL | poly(є-caprolactone) |
| PDAC | pancreatic ductal adenocarcinoma |
| PEG | poly(ethylene glycol) |
| PEO-bPPO-b-PEO | poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) |
| PLA | polylactide |
| PU | polyurethane |
| PUs | polyurethanes |
| ZnEt2 | diethylzinc |
References
- Santucci, C.; Mignozzi, S.; Levi, F.; Malvezzi, M.; Boffetta, P.; Negri, E.; La Vecchia, C. European Cancer Mortality Predictions for the Year 2025 with Focus on Breast Cancer. Ann. Oncol. 2025, 36, 460–468. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kwaśniewska, D.; Fudalej, M.; Nurzyński, P.; Badowska-Kozakiewicz, A.; Czerw, A.; Cipora, E.; Sygit, K.; Bandurska, E.; Deptała, A. How A Patient with Resectable or Borderline Resectable Pancreatic Cancer Should Be Treated—A Comprehensive Review. Cancers 2023, 15, 4275. [Google Scholar] [CrossRef]
- Leiphrakpam, P.D.; Chowdhury, S.; Zhang, M.; Bajaj, V.; Dhir, M.; Are, C. Trends in the Global Incidence of Pancreatic Cancer and a Brief Review of Its Histologic and Molecular Subtypes. J. Gastrointest. Cancer 2025, 56, 71. [Google Scholar] [CrossRef] [PubMed]
- Entezar-Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadian, F. Recent Advances in Designing 5-Fluorouracil Delivery Systems: A Stepping Stone in the Safe Treatment of Colorectal Cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Okechukwu, C.C. Review of 5-FU Resistance Mechanisms in Colorectal Cancer: Clinical Significance of Attenuated on-Target Effects. Cancer Drug Resist. 2023, 6, 257–272. [Google Scholar] [CrossRef]
- Gu, C.; Le, V.; Lang, M.; Liu, J. Preparation of Polysaccharide Derivates Chitosan-Graft-Poly(ε-Caprolactone) Amphiphilic Copolymer Micelles for 5-Fluorouracil Drug Delivery. Colloids Surf. B Biointerfaces 2014, 116, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Horo, H.; Das, S.; Mandal, B.; Kundu, L.M. Development of a Photoresponsive Chitosan Conjugated Prodrug Nano-Carrier for Controlled Delivery of Antitumor Drug 5-Fluorouracil. Int. J. Biol. Macromol. 2019, 121, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, D.; Bhadra, S.; Jain, S.; Jain, N.K. A PEGylated Dendritic Nanoparticulate Carrier of Fluorouracil. Int. J. Pharm. 2003, 257, 111–124. [Google Scholar] [CrossRef]
- Ullah, A.; Lim, S.I. Bioinspired Tunable Hydrogels: An Update on Methods of Preparation, Classification, and Biomedical and Therapeutic Applications. Int. J. Pharm. 2022, 612, 121368. [Google Scholar] [CrossRef]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart Hydrogels—Synthetic Stimuli-Responsive Antitumor Drug Release Systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef]
- Ono, K.; Hashimoto, H.; Katayama, T.; Ueda, N.; Nagahama, K. Injectable Biocatalytic Nanocomposite Hydrogel Factories for Focal Enzyme-Prodrug Cancer Therapy. Biomacromolecules 2021, 22, 4217–4227. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical Applications of Hydrogels in Drug Delivery System: An Update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Chronopoulou, L.; Haghighi, F.H.; Fratoddi, I.; Palocci, C. Peptide-Based Hydrogels: New Materials for Biosensing and Biomedical Applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef] [PubMed]
- Hasallari, F.; Gallo, E.; Rizzuti, S.; Diaferia, C.; Salvatore, M.; Accardo, A.; Gianolio, E.; Aime, S. A Biocompatible, Highly Sensitive Hydrogel-Based T1 Thermometer for in Vivo MRI Applications. Mater. Today Chem. 2025, 49, 103040. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, J.; Ren, J. Recent Advances in Glycopeptide Hydrogels: Design, Biological Functions, and Biomedical Applications. Front. Bioeng. Biotechnol. 2025, 13, 1577192. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.Y.H.; Lee, C.S.; Pichika, M.R.; Cheng, S.F.; Lam, K.Y. PH Responsive Polyurethane for the Advancement of Biomedical and Drug Delivery. Polymers 2022, 14, 1672. [Google Scholar] [CrossRef]
- Boffito, M.; Torchio, A.; Tonda-Turo, C.; Laurano, R.; Gisbert-Garzarán, M.; Berkmann, J.C.; Cassino, C.; Manzano, M.; Duda, G.N.; Vallet-Regí, M.; et al. Hybrid Injectable Sol-Gel Systems Based on Thermo-Sensitive Polyurethane Hydrogels Carrying pH-Sensitive Mesoporous Silica Nanoparticles for the Controlled and Triggered Release of Therapeutic Agents. Front. Bioeng. Biotechnol. 2020, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Nguyen, T.; Padanilam, D.; Xu, C.; Saha, D.; Nguyen, K.T.; Hong, Y. Glutathione-Responsive Biodegradable Polyurethane Nanoparticles for Lung Cancer Treatment. J. Control. Release 2020, 321, 363–371. [Google Scholar] [CrossRef]
- Yu, C.; Tan, X.; Xu, Z.; Zhu, G.; Teng, W.; Zhao, Q.; Liang, Z.; Wu, Z.; Xiong, D. Smart Drug Carrier Based on Polyurethane Material for Enhanced and Controlled DOX Release Triggered by Redox Stimulus. React. Funct. Polym. 2020, 148, 104507. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, C.; Qiao, Z.; Yao, Y.; Luo, J. Reduction Responsive and Surface Charge Switchable Polyurethane Micelles with Acid Cleavable Crosslinks for Intracellular Drug Delivery. RSC Adv. 2018, 8, 17888–17897. [Google Scholar] [CrossRef]
- Sun, J.; Rust, T.; Kuckling, D. Light—Responsive Serinol—Based Polyurethane Nanocarrier for Controlled Drug Release. Macromol. Rapid Commun. 2019, 40, 1900348. [Google Scholar] [CrossRef]
- Aluri, R.; Saxena, S.; Joshi, D.C.; Jayakannan, M. Multistimuli-Responsive Amphiphilic Poly(Ester-Urethane) Nanoassemblies Based on L-Tyrosine for Intracellular Drug Delivery to Cancer Cells. Biomacromolecules 2018, 19, 2166–2181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, L.; Ding, M.; Li, J.; Tan, H.; Wang, Z.; Fu, Q. Synthesis and Characterization of pH-Sensitive Biodegradable Polyurethane for Potential Drug Delivery Applications. Macromolecules 2011, 44, 857–864. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, D.; He, X.; Li, J.; Tan, H.; Li, J.; Fu, Q.; Gu, Q. The Degradation and Biocompatibility of pH-Sensitive Biodegradable Polyurethanes for Intracellular Multifunctional Antitumor Drug Delivery. Biomaterials 2012, 33, 2734–2745. [Google Scholar] [CrossRef]
- Song, N.; Zhou, L.; Li, J.; Pan, Z.; He, X.; Tan, H.; Wan, X.; Li, J.; Ran, R.; Fu, Q. Inspired by Nonenveloped Viruses Escaping from Endo-Lysosomes: A pH-Sensitive Polyurethane Micelle for Effective Intracellular Trafficking. Nanoscale 2016, 8, 7711–7722. [Google Scholar] [CrossRef]
- Pardini, F.M.; Amalvy, J.I. Synthesis and Swelling Behavior of pH-Responsive Polyurethane/Poly[2-(Diethylamino)Ethyl Methacrylate] Hybrid Materials. J. Appl. Polym. Sci. 2014, 131, 39799. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, D.; Liu, C.; Guan, Y.; Zhang, J.; Su, Y.; Zhao, L.; Meng, F.; Luo, J. Biodegradable pH-Sensitive Polyurethane Micelles with Different Polyethylene Glycol (PEG) Locations for Anti-Cancer Drug Carrier Applications. RSC Adv. 2016, 6, 97684–97693. [Google Scholar] [CrossRef]
- Kim, S.; Traore, Y.L.; Ho, E.A.; Shafiq, M.; Kim, S.H.; Liu, S. Design and Development of pH-Responsive Polyurethane Membranes for Intravaginal Release of Nanomedicines. Acta Biomater. 2018, 82, 12–23. [Google Scholar] [CrossRef]
- He, W.; Zheng, X.; Zhao, Q.; Duan, L.; Lv, Q.; Gao, G.H.; Yu, S. pH—Triggered Charge—Reversal Polyurethane Micelles for Controlled Release of Doxorubicin. Macromol. Biosci. 2016, 16, 925–935. [Google Scholar] [CrossRef]
- Kim, S.; Traore, Y.L.; Chen, Y.; Ho, E.A.; Liu, S. Switchable On-Demand Release of a Nanocarrier from a Segmented Reservoir Type Intravaginal Ring Filled with a pH-Responsive Supramolecular Polyurethane Hydrogel. ACS Appl. Bio Mater. 2018, 1, 652–662. [Google Scholar] [CrossRef]
- Duru Kamacı, U.; Kamacı, M. Preparation of Polyvinyl Alcohol, Chitosan and Polyurethane-Based pH-Sensitive and Biodegradable Hydrogels for Controlled Drug Release Applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1167–1177. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mukhopadhyay, P.; Kundu, P.P. Synthesis of a Novel pH-sensitive Polyurethane–Alginate Blend with Poly(Ethylene Terephthalate) Waste for the Oral Delivery of Protein. J. Appl. Polym. Sci. 2014, 131, 40650. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mukhopadhyay, P.; Pramanik, N.; Kundu, P.P. Effect of Polyethylene Glycol on Bis(2-hydroxyethyl) terephthalate—Based Polyurethane/Alginate pH-Sensitive Blend for Oral Protein Delivery. Adv. Polym. Technol. 2016, 35, 21525. [Google Scholar] [CrossRef]
- Solanki, A.; Thakore, S. Cellulose Crosslinked pH-Responsive Polyurethanes for Drug Delivery: α-Hydroxy Acids as Drug Release Modifiers. Int. J. Biol. Macromol. 2015, 80, 683–691. [Google Scholar] [CrossRef]
- Nabid, M.R.; Omrani, I. Facile Preparation of pH-Responsive Polyurethane Nanocarrier for Oral Delivery. Mater. Sci. Eng. C 2016, 69, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Liu, Z.; Wang, F.; Gao, B.; Chen, F.; Zhang, Q.; Xiong, R.; Han, J.; Samal, S.K.; De Smedt, S.C.; et al. pH Responsive Polyurethane (Core) and Cellulose Acetate Phthalate (Shell) Electrospun Fibers for Intravaginal Drug Delivery. Carbohydr. Polym. 2016, 151, 1240–1244. [Google Scholar] [CrossRef]
- Shoaib, M.; Bahadur, A.; Saeed, A.; Rahman, M.S.U.; Naseer, M.M. Biocompatible, pH-Responsive, and Biodegradable Polyurethanes as Smart Anti-Cancer Drug Delivery Carriers. React. Funct. Polym. 2018, 127, 153–160. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zhang, X.M.; Duan, L.J.; Zhang, M.Y.; Gao, G.H.; Zhang, H.X. Environmental pH-responsive Fluorescent PEG-polyurethane for Potential Optical Imaging. J. Appl. Polym. Sci. 2013, 129, 846–852. [Google Scholar] [CrossRef]
- Zhou, H.; Xun, R.; Liu, Q.; Fan, H.; Liu, Y. Preparation of Thermal and pH Dually Sensitive Polyurethane Membranes and Their Properties. J. Macromol. Sci. Part B 2014, 53, 398–411. [Google Scholar] [CrossRef]
- Wang, A.; Gao, H.; Sun, Y.; Sun, Y.; Yang, Y.-W.; Wu, G.; Wang, Y.; Fan, Y.; Ma, J. Temperature- and pH-Responsive Nanoparticles of Biocompatible Polyurethanes for Doxorubicin Delivery. Int. J. Pharm. 2013, 441, 30–39. [Google Scholar] [CrossRef]
- Pardini, F.M.; Faccia, P.A.; Pardini, O.R.; Amalvy, J.I. Thermal and pH Dual Responsive Polyurethane/2-(Diisopropylamino)Ethyl Methacrylate Hybrids: Synthesis, Characterization, and Swelling Behavior. Int. J. Polym. Anal. Charact. 2018, 23, 207–225. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Liu, D.; Wang, W.; Liu, Y.; Zhou, S. pH-Responsive Shape Memory Poly(Ethylene Glycol)–Poly(ε-Caprolactone)-Based Polyurethane/Cellulose Nanocrystals Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 12988–12999. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Gooch, J.; Sun, W.; Wang, H.; Wang, K. Photo- and pH-Sensitive Azo-Containing Cationic Waterborne Polyurethane. Polym. Bull. 2015, 72, 881–895. [Google Scholar] [CrossRef]
- Guan, Y.; Su, Y.; Zhao, L.; Meng, F.; Wang, Q.; Yao, Y.; Luo, J. Biodegradable Polyurethane Micelles with pH and Reduction Responsive Properties for Intracellular Drug Delivery. Mater. Sci. Eng. C 2017, 75, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Zhang, H.; Xu, K.; Du, B.; Zhu, C.; Li, Y. pH and Reduction Dual-Responsive Micelles Based on Novel Polyurethanes with Detachable Poly(2-Ethyl-2-Oxazoline) Shell for Controlled Release of Doxorubicin. Drug Deliv. 2019, 26, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; He, C.; Ding, J.; Cheng, Y.; Song, W.; Zhuang, X.; Chen, X. pH and Reduction Dual Responsive Polyurethane Triblock Copolymers for Efficient Intracellular Drug Delivery. Soft Matter 2013, 9, 2637. [Google Scholar] [CrossRef]
- Yu, S.; He, C.; Lv, Q.; Sun, H.; Chen, X. pH and Reduction Dual Responsive Cross-Linked Polyurethane Micelles as an Intracellular Drug Delivery System. RSC Adv. 2014, 4, 63070–63078. [Google Scholar] [CrossRef]
- Song, Q.; Chen, H.; Zhou, S.; Zhao, K.; Wang, B.; Hu, P. Thermo- and pH-Sensitive Shape Memory Polyurethane Containing Carboxyl Groups. Polym. Chem. 2016, 7, 1739–1746. [Google Scholar] [CrossRef]
- Kasiński, A.; Zielińska-Pisklak, M.; Kowalczyk, S.; Plichta, A.; Zgadzaj, A.; Oledzka, E.; Sobczak, M. Synthesis and Characterization of New Biodegradable Injectable Thermosensitive Smart Hydrogels for 5-Fluorouracil Delivery. Int. J. Mol. Sci. 2021, 22, 8330. [Google Scholar] [CrossRef]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Nałęcz-Jawecki, G.; Drobniewska, A.; Sobczak, M. Hydrogels Based on Poly(Ether-Ester)s as Highly Controlled 5-Fluorouracil Delivery Systems—Synthesis and Characterization. Materials 2020, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Kasiński, A.; Świerczek, A.; Zielińska-Pisklak, M.; Kowalczyk, S.; Plichta, A.; Zgadzaj, A.; Oledzka, E.; Sobczak, M. Dual-Stimuli-Sensitive Smart Hydrogels Containing Magnetic Nanoparticles as Antitumor Local Drug Delivery Systems—Synthesis and Characterization. Int. J. Mol. Sci. 2023, 24, 6906. [Google Scholar] [CrossRef] [PubMed]
- Zagórska-Dziok, M.; Kleczkowska, P.; Olędzka, E.; Figat, R.; Sobczak, M. Poly(Chitosan-Ester-Ether-Urethane) Hydrogels as Highly Controlled Genistein Release Systems. Int. J. Mol. Sci. 2021, 22, 3339. [Google Scholar] [CrossRef]
- Sobczak, M.; Oledzka, E.; Kwietniewska, M.; Nałęcz-Jawecki, G.; Kołodziejski, W. Promising Macromolecular Conjugates of Camptothecin—The Synthesis, Characterization and in Vitro Studies. J. Macromol. Sci. Part A 2014, 51, 254–262. [Google Scholar] [CrossRef]
- Tabet, A.; Mommer, S.; Vigil, J.A.; Hallou, C.; Bulstrode, H.; Scherman, O.A. Mechanical Characterization of Human Brain Tissue and Soft Dynamic Gels Exhibiting Electromechanical Neuro-Mimicry. Adv. Healthc. Mater. 2019, 8, 1900068. [Google Scholar] [CrossRef]
- Speidel, A.T.; Chivers, P.R.A.; Wood, C.S.; Roberts, D.A.; Correia, I.P.; Caravaca, A.S.; Chan, Y.K.V.; Hansel, C.S.; Heimgärtner, J.; Müller, E.; et al. Tailored Biocompatible Polyurethane-Poly(Ethylene Glycol) Hydrogels as a Versatile Nonfouling Biomaterial. Adv. Healthc. Mater. 2022, 11, 2201378. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) Resistance and the New Strategy to Enhance the Sensitivity against Cancer: Implication of DNA Repair Inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Marín, R.; Muñoz-Guerra, S. Carbohydrate-Based Poly(Ester-Urethane)s: A Comparative Study Regarding Cyclic Alditols Extenders and Polymerization Procedures. J. Appl. Polym. Sci. 2009, 114, 3723–3736. [Google Scholar] [CrossRef]
- Asefnejad, A.; Khorasani, M.T.; Behnamghader, A.; Farsadzadeh, B.; Bonakdar, S. Manufacturing of Biodegradable Polyurethane Scaffolds Based on Polycaprolactone Using a Phase Separation Method: Physical Properties and in vitro Assay. Int. J. Nanomed. 2011, 6, 2375–2384. [Google Scholar] [CrossRef]
- Kamaci, M. Polyurethane-Based Hydrogels for Controlled Drug Delivery Applications. Eur. Polym. J. 2020, 123, 109444. [Google Scholar] [CrossRef]
- Kamaci, M.; Kaya, I. Chitosan Based Hybrid Hydrogels for Drug Delivery: Preparation, Biodegradation, Thermal, and Mechanical Properties. Polym. Adv. Technol. 2023, 34, 779–788. [Google Scholar] [CrossRef]
- Domańska, I.M.; Zalewska, A.; Cieśla, K.; Plichta, A.; Głuszewski, W.; Łyczko, M.; Kowalczyk, S.; Oledzka, E.; Sobczak, M. The Influence of Electron Beam and Gamma Irradiation on Paclitaxel-Loaded Nanoparticles of Fully Randomized Copolymers in Relation to Potential Sterilization. J. Drug Deliv. Sci. Technol. 2023, 90, 105115. [Google Scholar] [CrossRef]
- Jiang, Z.; Deng, X.; Hao, J. Thermogelling Hydrogels of Poly(ε-caprolactone-co-D,L-lactide)–Poly(Ethylene Glycol)–Poly(ε-caprolactone-co-D,L-lactide) and Poly(ε-caprolactone-co-L-lactide)–Poly(Ethylene Glycol)–Poly(ε-caprolactone-co-L-lactide) Aqueous Solutions. J. Polym. Sci. A Polym. Chem. 2007, 45, 4091–4099. [Google Scholar] [CrossRef]
- Maring, J.G.; Schouten, L.; Greijdanus, B.; De Vries, E.G.E.; Uges, D.R.A. A Simple and Sensitive Fully Validated HPLC-UV Method for the Determination of 5-Fluorouracil and Its Metabolite 5,6-Dihydrofluorouracil in Plasma. Ther. Drug Monit. 2005, 27, 25–30. [Google Scholar] [CrossRef]
- Wang, J.-X.; Sun, X.; Zhang, Z.-R. Enhanced Brain Targeting by Synthesis of 3′,5′-Dioctanoyl-5-Fluoro-2′-Deoxyuridine and Incorporation into Solid Lipid Nanoparticles. Eur. J. Pharm. Biopharm. 2002, 54, 285–290. [Google Scholar] [CrossRef] [PubMed]

| Sample | CL/LA/PEG 1 | Temp. [°C] | Time [h] | Yield [%] | Yieldcal. 2 [%] | CL in Polym. 3 | CL in Polym.cal. 2 | Mn 4 [g/mol] |
|---|---|---|---|---|---|---|---|---|
| CLLAPEG-1 | 10:10:1 | 120 | 12 | 99.7 | 99.0 | 0.81 | 0.79 | 4100 |
| CLLAPEG-2 | 10:10:1 | 120 | 36 | 98.3 | 100 | 0.75 | 0.74 | 4200 |
| CLLAPEG-3 | 10:10:1 | 150 | 12 | 100 | 96.4 | 0.59 | 0.62 | 4500 |
| CLLAPEG-4 | 10:10:1 | 150 | 36 | 99.5 | 100 | 0.62 | 0.58 | 4600 |
| CLLAPEG-5 | 20:20:1 | 120 | 12 | 87.2 | 85.9 | 0.79 | 0.79 | 4700 |
| CLLAPEG-6 | 20:20:1 | 120 | 36 | 91.2 | 90.1 | 0.76 | 0.75 | 4900 |
| CLLAPEG-7 | 20:20:1 | 150 | 12 | 76.0 | 83.3 | 0.68 | 0.63 | 5100 |
| CLLAPEG-8 | 20:20:1 | 150 | 36 | 90.5 | 87.5 | 0.57 | 0.59 | 5200 |
| CLLAPEG-9 | 15:15:1 | 135 | 24 | 92.6 | 93.2 | 0.67 | 0.69 | 5400 |
| CLLAPEG-10 | 15:15:1 | 135 | 24 | 94.0 | 93.2 | 0.68 | 0.69 | 5500 |
| CLLAPEG-11 | 15:15:1 | 135 | 24 | 96.8 | 93.2 | 0.65 | 0.69 | 5400 |
| Sample | CL/LA/PEG 1 | Temp. [°C] | Time [h] | Yield [%] | Yieldcal. 2 [%] | CL in Polym. 3 | CL in Polym.cal. 2 | Mn 4 [g/mol] |
|---|---|---|---|---|---|---|---|---|
| CLLAPEG-1A | 12:8:1 | 120 | 12 | 100 | 99.9 | 0.88 | 0.86 | 4000 |
| CLLAPEG-2A | 12:8:1 | 120 | 36 | 100 | 100 | 0.82 | 0.81 | 4200 |
| CLLAPEG-3A | 12:8:1 | 150 | 12 | 100 | 96.8 | 0.65 | 0.68 | 4400 |
| CLLAPEG-4A | 12:8:1 | 150 | 36 | 100 | 100 | 0.68 | 0.64 | 4700 |
| CLLAPEG-5A | 24:16:1 | 120 | 12 | 89.8 | 88.8 | 0.86 | 0.86 | 5700 |
| CLLAPEG-6A | 24:16:1 | 120 | 36 | 93.9 | 93.5 | 0.83 | 0.82 | 5800 |
| CLLAPEG-7A | 24:16:1 | 150 | 12 | 78.3 | 85.7 | 0.74 | 0.69 | 6000 |
| CLLAPEG-8A | 24:16:1 | 150 | 36 | 93.2 | 90.5 | 0.63 | 0.65 | 6100 |
| CLLAPEG-9A | 18:12:1 | 135 | 24 | 95.4 | 95.2 | 0.73 | 0.75 | 5000 |
| CLLAPEG-10A | 18:12:1 | 135 | 24 | 96.8 | 95.2 | 0.74 | 0.75 | 5100 |
| CLLAPEG-11A | 18:12:1 | 135 | 24 | 99.7 | 95.2 | 0.71 | 0.75 | 5000 |
| Sample | CL/LA/PEG 1 | Temp. [°C] | Time [h] | Yield [%] | Yieldcal. 2 [%] | CL in Polym. 3 | CL in Polym.cal. 2 | Mn 4 [g/mol] |
|---|---|---|---|---|---|---|---|---|
| CLLAPEG-1B | 14:6:1 | 120 | 12 | 100 | 99.5 | 0.92 | 0.90 | 4100 |
| CLLAPEG-2B | 14:6:1 | 120 | 36 | 99.3 | 100 | 0.86 | 0.85 | 4300 |
| CLLAPEG-3B | 14:6:1 | 150 | 12 | 100 | 96.7 | 0.68 | 0.72 | 4500 |
| CLLAPEG-4B | 14:6:1 | 150 | 36 | 100 | 101.2 | 0.71 | 0.67 | 4800 |
| CLLAPEG-5B | 28:12:1 | 120 | 12 | 88.1 | 86.8 | 0.90 | 0.91 | 5600 |
| CLLAPEG-6B | 28:12:1 | 120 | 36 | 92.1 | 91.3 | 0.87 | 0.86 | 5700 |
| CLLAPEG-7B | 28:12:1 | 150 | 12 | 76.8 | 84.0 | 0.78 | 0.73 | 5900 |
| CLLAPEG-8B | 28:12:1 | 150 | 36 | 91.4 | 88.5 | 0.66 | 0.68 | 6200 |
| CLLAPEG-9B | 21:9:1 | 135 | 24 | 97.0 | 94.0 | 0.77 | 0.79 | 5100 |
| CLLAPEG-10B | 21:9:1 | 135 | 24 | 94.5 | 94.0 | 0.78 | 0.79 | 5000 |
| CLLAPEG-11B | 21:9:1 | 135 | 24 | 94.9 | 94.0 | 0.75 | 0.79 | 5200 |
| Sample | CL/LA/PEG 1 | Temp. [°C] | Time [h] | Yield [%] | CL in Polym. 2 | Mn 3 [g/mol] | Mn 4 [g/mol] | Đ 4 |
|---|---|---|---|---|---|---|---|---|
| CLLAPEG-4-OC | 10:10:1 | 150 | 36 | 100 | 0.59 | 4300 | 4600 | 1.45 |
| CLLAPEG-4A-OC | 12:8:1 | 150 | 36 | 100 | 0.66 | 4200 | 4300 | 1.39 |
| CLLAPEG-3B-OC | 14:6:1 | 150 | 12 | 100 | 0.72 | 4200 | 4500 | 1.51 |
| Sample | CL Cont. 1 | MSR 2,a [%] | MSR 2,b [%] | MSR 2,c [%] | MSR 2,d [%] |
|---|---|---|---|---|---|
| HPU-1 | 0.59 | 197 ± 9 | 236 ± 10 | 241 ± 11 | 243 ± 9 |
| HPU-2 | 0.66 | 182 ± 9 | 224 ± 9 | 228 ± 9 | 229 ± 10 |
| HPU-3 | 0.72 | 154 ± 7 | 182 ± 10 | 185 ± 9 | 187 ± 11 |
| No. | Zero-Order Model | First-Order Model | Higuchi Model | Korsmeyer–Peppas Model | Transport Mechanism | |
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | n | ||
| HPU-1-5-FU a | 0.9897 | 0.9602 | 0.9715 | 0.9740 | 0.421 | Fickian transport |
| HPU-2-5-FU a | 0.9872 | 0.9254 | 0.9817 | 0.9861 | 0.477 | non-Fickian transport |
| HPU-3-5-FU a | 0.9868 | 0.8678 | 0.9737 | 0.9855 | 0.499 | non-Fickian transport |
| HPU-1-FUdR a | 0.9851 | 0.9116 | 0.9587 | 0.9764 | 0.424 | Fickian transport |
| HPU-2-FUdR a | 0.9845 | 09635 | 0.9801 | 0.9833 | 0.462 | non-Fickian transport |
| HPU-3-FUdR a | 0.9806 | 0.9359 | 0.9727 | 0.9903 | 0.486 | non-Fickian transport |
| HPU-1-5-FU b | 0.9454 | 0.9394 | 0.9252 | 0.9339 | 0.248 | Fickian transport |
| HPU-2-5-FU b | 0.9737 | 0.9275 | 0.9580 | 0.9617 | 0.329 | Fickian transport |
| HPU-3-5-FU b | 0.9738 | 0.9621 | 0.9625 | 0.9679 | 0.377 | Fickian transport |
| HPU-1-FUdR b | 0.9360 | 0.9466 | 0.9344 | 0.9401 | 0.295 | Fickian transport |
| HPU-2-FUdR b | 0.9618 | 0.9640 | 0.9463 | 0.9572 | 0.308 | Fickian transport |
| HPU-3-FUdR b | 0.9715 | 0.9748 | 0.9575 | 0.9685 | 0.367 | Fickian transport |
| Natural Variable, zi | Center of the Plan | Variable Step |
|---|---|---|
| z1 | 24 | 12 |
| z2 | 135 | 15 |
| z3 | 15 or 18 or 21 | 5 or 6 or 7 |
| Experiment No. | Random Variables | ||
|---|---|---|---|
| x1 | x2 | x3 | |
| 1 | −1 | −1 | −1 |
| 2 | +1 | −1 | −1 |
| 3 | −1 | +1 | −1 |
| 4 | +1 | +1 | −1 |
| 5 | −1 | −1 | +1 |
| 6 | +1 | −1 | +1 |
| 7 | −1 | +1 | +1 |
| 8 | +1 | +1 | +1 |
| 9 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 |
| 11 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczak, M.; Kasiński, A.; Kędra, K.; Frankowski, J.; Kurzątkowska, M.; Watrakiewicz, K.; Mulas, K.; Strzelecka, K.; Chodkowski, M.; Krzyżowska, M.; et al. Preparation and Characterization of New pH-Sensitive Polyurethane Hydrogels as Anti-Cancer Drug Delivery Systems for 5-Fluorouracyl and Fluorodeoxyuridine. Int. J. Mol. Sci. 2025, 26, 10258. https://doi.org/10.3390/ijms262110258
Sobczak M, Kasiński A, Kędra K, Frankowski J, Kurzątkowska M, Watrakiewicz K, Mulas K, Strzelecka K, Chodkowski M, Krzyżowska M, et al. Preparation and Characterization of New pH-Sensitive Polyurethane Hydrogels as Anti-Cancer Drug Delivery Systems for 5-Fluorouracyl and Fluorodeoxyuridine. International Journal of Molecular Sciences. 2025; 26(21):10258. https://doi.org/10.3390/ijms262110258
Chicago/Turabian StyleSobczak, Marcin, Adam Kasiński, Karolina Kędra, Joachim Frankowski, Matylda Kurzątkowska, Karolina Watrakiewicz, Karolina Mulas, Katarzyna Strzelecka, Marcin Chodkowski, Małgorzata Krzyżowska, and et al. 2025. "Preparation and Characterization of New pH-Sensitive Polyurethane Hydrogels as Anti-Cancer Drug Delivery Systems for 5-Fluorouracyl and Fluorodeoxyuridine" International Journal of Molecular Sciences 26, no. 21: 10258. https://doi.org/10.3390/ijms262110258
APA StyleSobczak, M., Kasiński, A., Kędra, K., Frankowski, J., Kurzątkowska, M., Watrakiewicz, K., Mulas, K., Strzelecka, K., Chodkowski, M., Krzyżowska, M., Deptała, A., & Oledzka, E. (2025). Preparation and Characterization of New pH-Sensitive Polyurethane Hydrogels as Anti-Cancer Drug Delivery Systems for 5-Fluorouracyl and Fluorodeoxyuridine. International Journal of Molecular Sciences, 26(21), 10258. https://doi.org/10.3390/ijms262110258











