Construction of a Zebrafish Model of Cardiac Hypertrophy Caused by ATIC Gene Deletion and Preliminary Exploration of Aerobic Exercise Improvement
Abstract
1. Introduction
2. Results
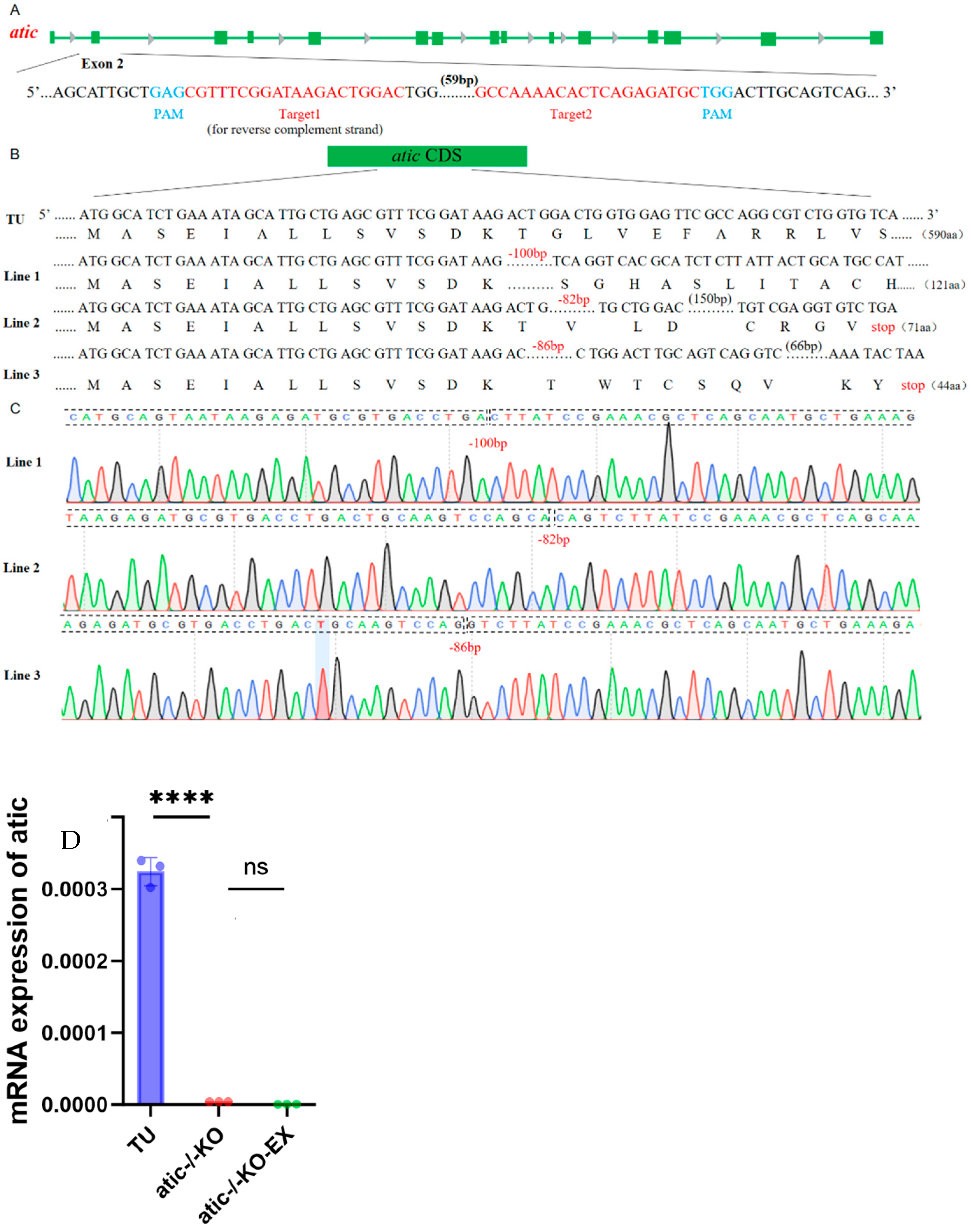
2.1. Construction of Zebrafish Lines with Atic Knockout
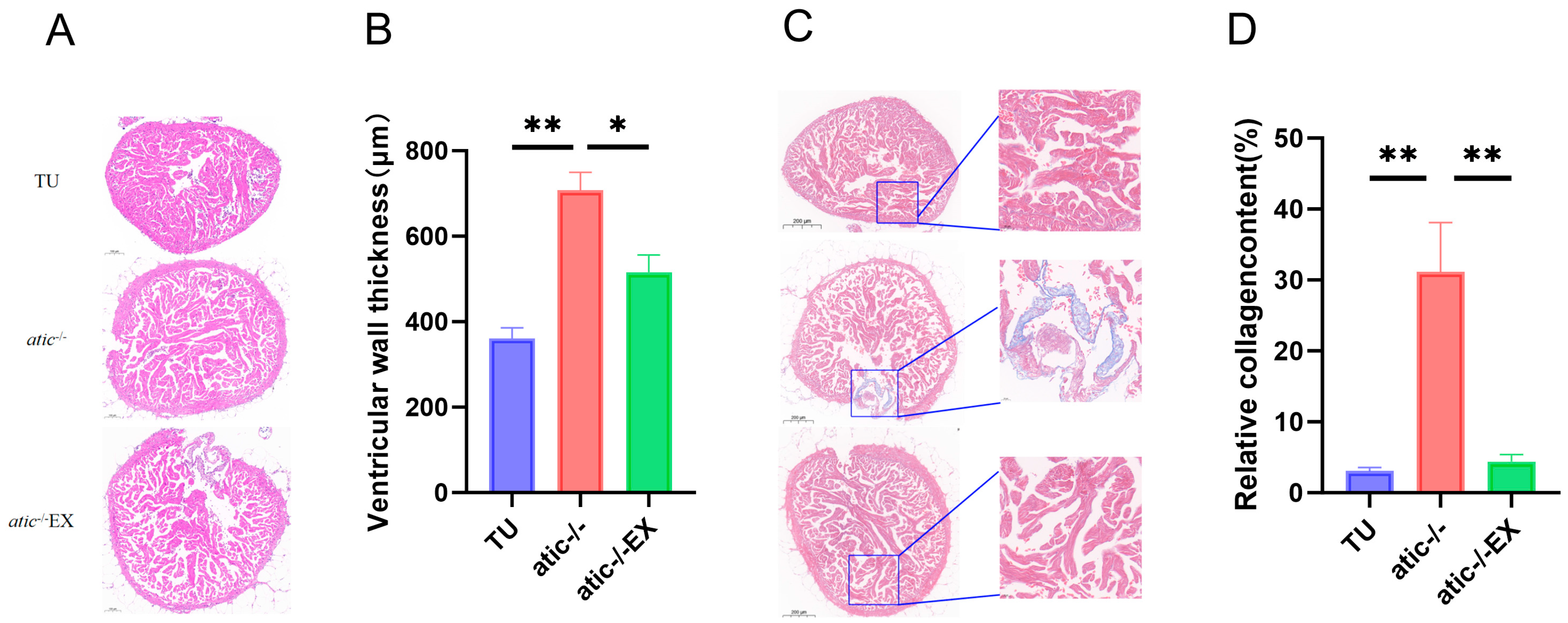
2.2. Aerobic Exercise Improves Cardiac Hypertrophy Phenotype of Atic−/− Zebrafish
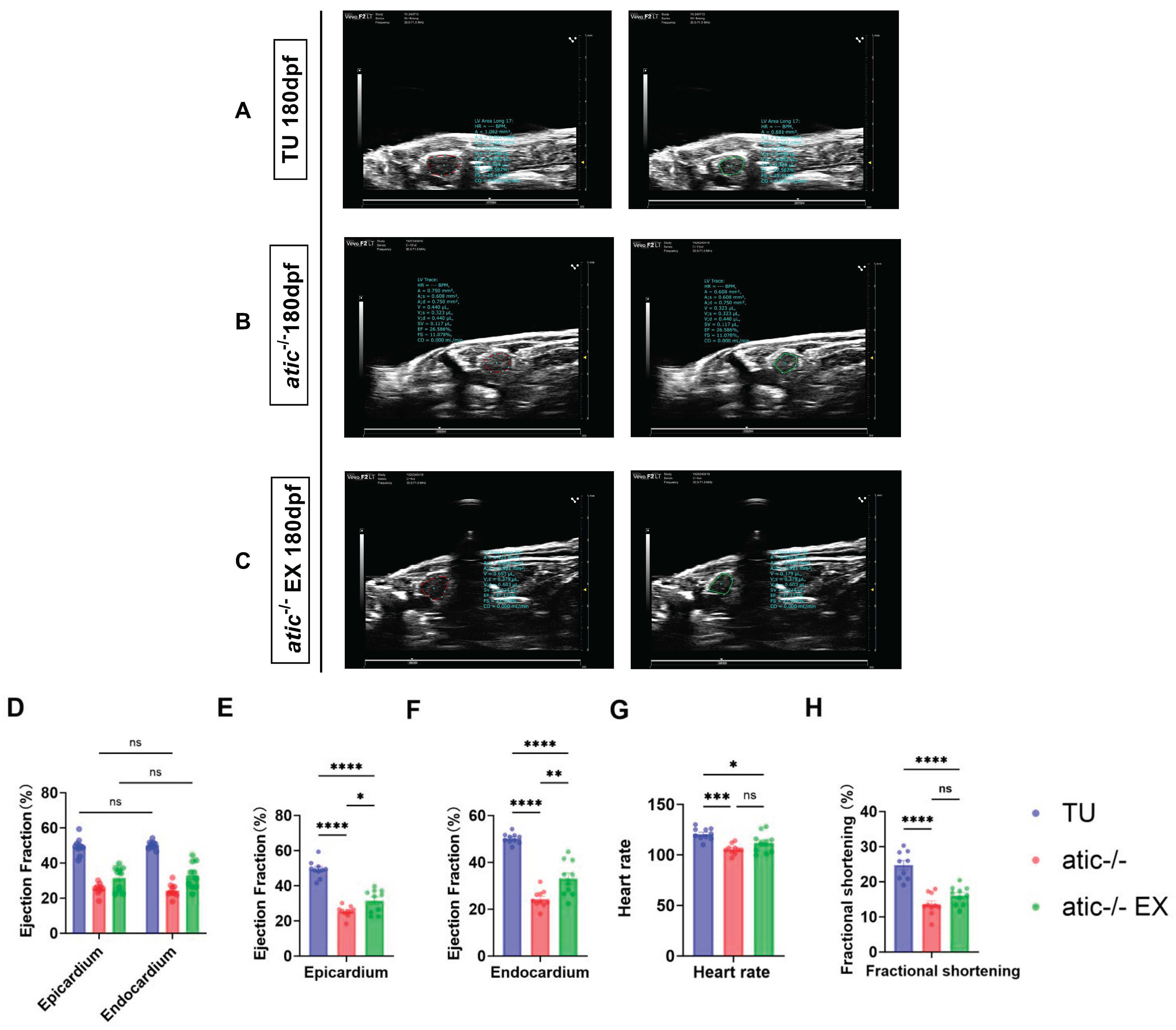
2.3. Aerobic Exercise Improves Cardiac Function in Atic−/− Zebrafish
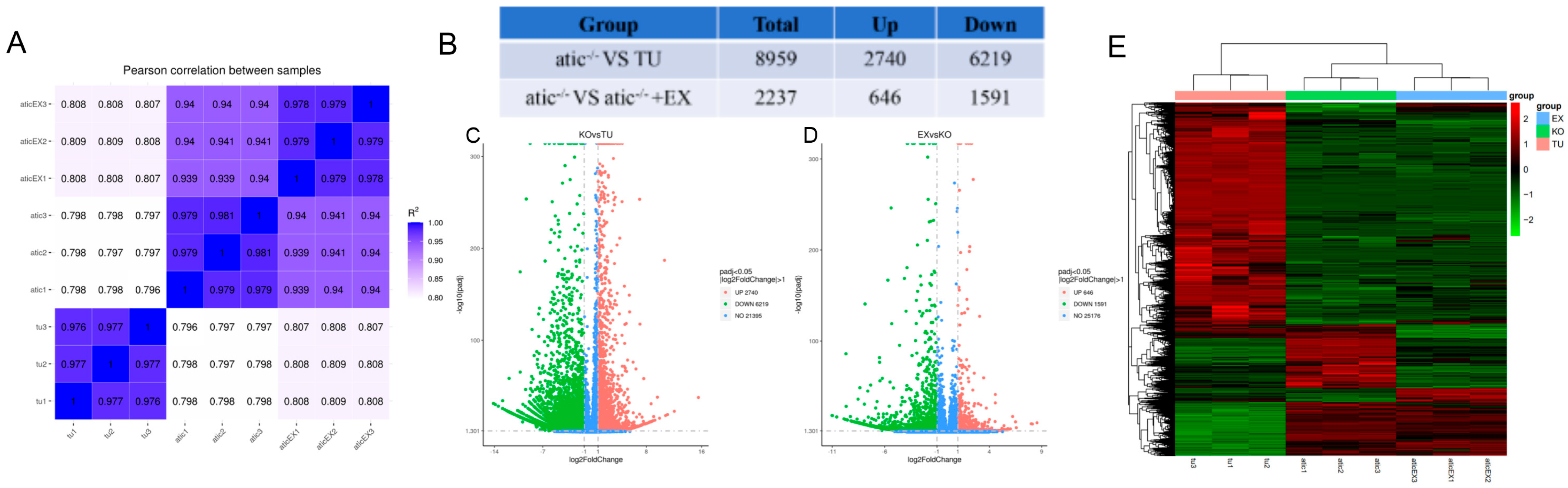
2.4. Atic−/− Zebrafish Heart Transcriptome Overview
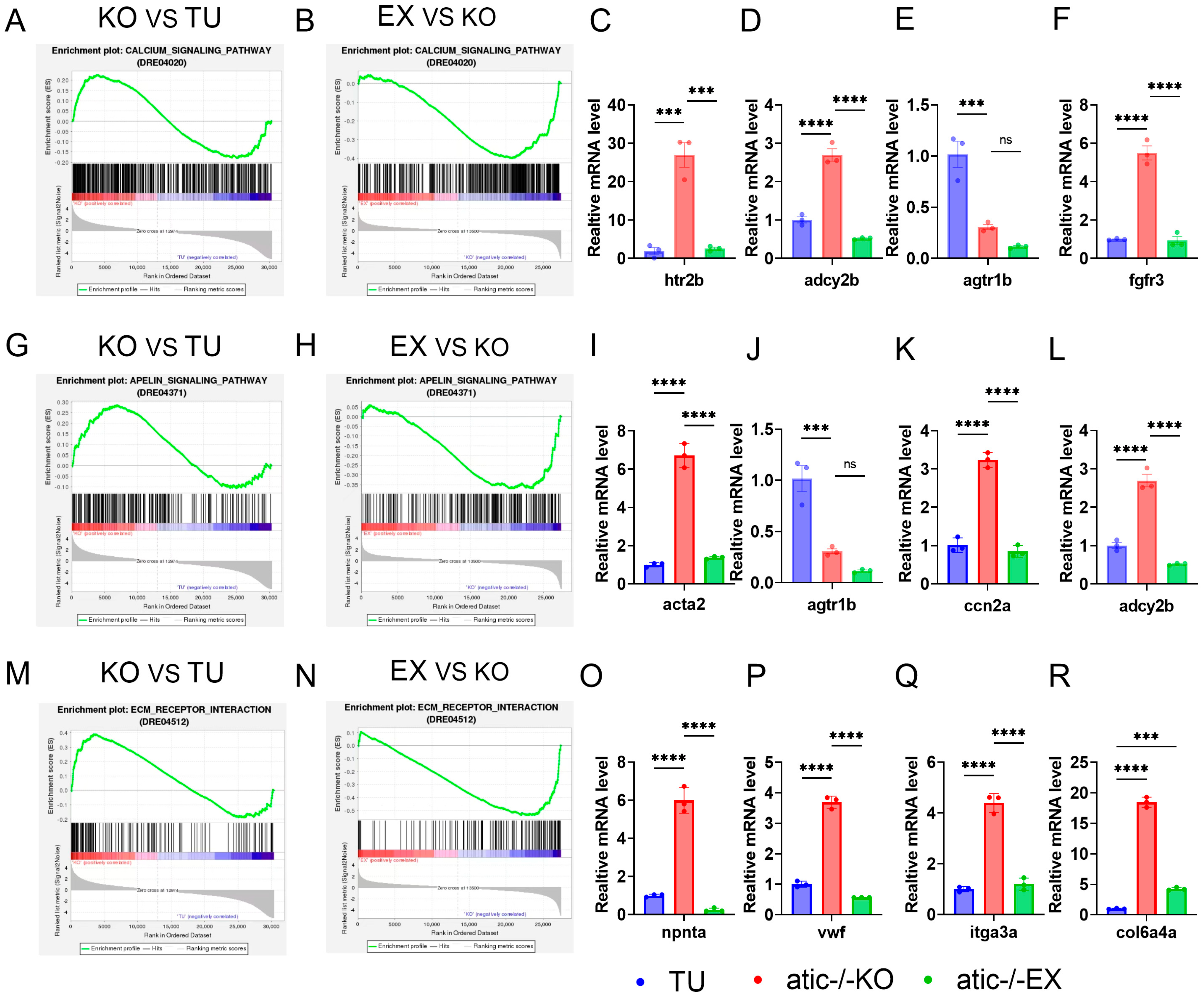
2.5. Biological Information Analysis of Aerobic Exercise Improving Cardiac Function of Atic−/− Zebrafish
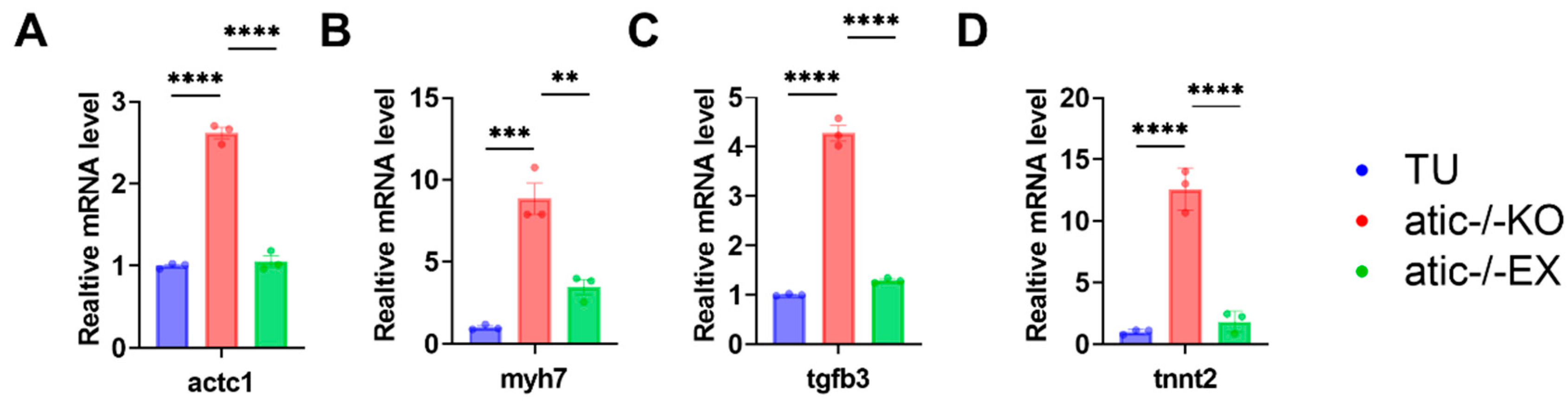
2.6. To Verify the Expression of Genes Related to Cardiac Hypertrophy in Atic−/− Zebrafish
2.7. To Verify the Molecular Mechanism of Exercise Improving Cardiac Function in Atic−/− Zebrafish
3. Discussion
3.1. Effects of Exercise on Cardiac Function of Zebrafish with Atic Gene Knockout
3.2. Transcriptome Characteristics of Motor-Regulated Atic−/−
3.3. Enlightenment for HCM Treatment
4. Materials and Methods
4.1. Experimental Animals and Groups
4.2. Aerobic Exercise Regimen
4.3. CRISPR/Cas9-Mediated Knockout of the Atic Gene
4.4. Hematoxylin–Eosin Staining
4.5. Masson Trichrome Staining
4.6. Echocardiographic Analysis
4.7. Transcriptome Analysis
4.8. qPCR Detection
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pareek, V.; Pedley, A.M.; Benkovic, S.J. Human de novo purine biosynthesis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 1–16. [Google Scholar] [CrossRef]
- Lane, A.N.; Fan, T.W. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, K.; Xie, Q.; Wu, Q.; Mack, S.C.; Shi, Y.; Kim, L.J.Y.; Prager, B.C.; Flavahan, W.A.; Liu, X.; et al. Purine synthesis promotes maintenance of brain tumor initiating cells in glioma. Nat. Neurosci. 2017, 20, 661–673. [Google Scholar] [CrossRef]
- Barfeld, S.J.; Fazli, L.; Persson, M.; Marjavaara, L.; Urbanucci, A.; Kaukoniemi, K.M.; Rennie, P.S.; Ceder, Y.; Chabes, A.; Visakorpi, T.; et al. Myc-dependent purine biosynthesis affects nucleolar stress and therapy response in prostate cancer. Oncotarget 2015, 6, 12587–12602. [Google Scholar] [CrossRef]
- Zhou, Q.; Lin, M.; Feng, X.; Ma, F.; Zhu, Y.; Liu, X.; Qu, C.; Sui, H.; Sun, B.; Zhu, A.; et al. Targeting CLK3 inhibits the progression of cholangiocarcinoma by reprogramming nucleotide metabolism. J. Exp. Med. 2020, 217, e20191779. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J.; Levinson, G.E. An index of the contractile state of the myocardium in man. J. Clin. Investig. 1968, 47, 1615–1626. [Google Scholar] [CrossRef]
- Shi, X.; Ma, Q.; Huo, Y.; Su, Y. 5-Aminoimidazole-4-carboxamide ribonucleotide formyltransferase/inosine monophosphate cyclohydrolase promotes pulmonary arterial smooth muscle cell proliferation via the Ras signaling pathway. Am. J. Physiol. Cell Physiol. 2024, 327, C901–C912. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, Q.; Xu, J.; Zhang, X.; Kim, D.; Liu, Z.; Da, Q.; Mao, X.; Zhou, Y.; Cai, Y.; et al. ATIC-Associated De Novo Purine Synthesis Is Critically Involved in Proliferative Arterial Disease. Circulation 2022, 146, 1444–1460. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, J.P.; Marie, S.; Nassogne, M.C. Disorders of purine biosynthesis metabolism. Mol. Genet. Metab. 2022, 136, 190–198. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, P.; Liu, J.; Chen, Z.; Ma, W.; Yuan, Y. ATIC inhibits autophagy in hepatocellular cancer through the AKT/FOXO3 pathway and serves as a prognostic signature for modeling patient survival. Int. J. Biol. Sci. 2021, 17, 4442–4458. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, D.; Li, N.; Chen, S.; Hu, L.; Yu, J.; Xiong, Y. Regulation of LRRK2 mRNA stability by ATIC and its substrate AICAR through ARE-mediated mRNA decay in Parkinson’s disease. EMBO J. 2023, 42, e113410. [Google Scholar] [CrossRef]
- Visser, S.; Koolen, S.; van Donk, N.; van Walree, N.; van der Leest, C.; Cornelissen, R.; van Schaik, R.; Mathijssen, R.; Aerts, J.; Stricker, B.H. Genetic polymorphism in ATIC is associated with effectiveness and toxicity of pemetrexed in non-small-cell lung cancer. Thorax 2021, 76, 1150–1153. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. A meta-analysis of the association between the ATIC 347 C/G polymorphism and methotrexate responsiveness and toxicity in rheumatoid arthritis. Semin. Arthritis Rheum. 2024, 64, 152337. [Google Scholar] [CrossRef]
- Li, B.; Liu, F.; Chen, X.; Chen, T.; Zhang, J.; Liu, Y.; Yao, Y.; Hu, W.; Zhang, M.; Wang, B.; et al. FARS2 Deficiency Causes Cardiomyopathy by Disrupting Mitochondrial Homeostasis and the Mitochondrial Quality Control System. Circulation 2024, 149, 1268–1284. [Google Scholar] [CrossRef]
- Zhang, Q.; He, X.; Yao, S.; Lin, T.; Zhang, L.; Chen, D.; Chen, C.; Yang, Q.; Li, F.; Zhu, Y.M.; et al. Ablation of Mto1 in zebrafish exhibited hypertrophic cardiomyopathy manifested by mitochondrion RNA maturation deficiency. Nucleic Acids Res. 2021, 49, 4689–4704. [Google Scholar] [CrossRef] [PubMed]
- Kawata, H.; Bao, H.; Curtis, J.P.; Minges, K.E.; Mitiku, T.; Birgersdotter-Green, U.; Feld, G.K.; Hsu, J.C. Cardiac Resynchronization Defibrillator Therapy for Nonspecific Intraventricular Conduction Delay Versus Right Bundle Branch Block. J. Am. Coll. Cardiol. 2019, 73, 3082–3099. [Google Scholar] [CrossRef]
- Teekakirikul, P.; Zhu, W.; Huang, H.C.; Fung, E. Hypertrophic Cardiomyopathy: An Overview of Genetics and Management. Biomolecules 2019, 9, 878. [Google Scholar] [CrossRef]
- Zhang, Y.; Adamo, M.; Zou, C.; Porcari, A.; Tomasoni, D.; Rossi, M.; Merlo, M.; Liu, H.; Wang, J.; Zhou, P.; et al. Management of hypertrophic cardiomyopathy. J. Cardiovasc. Med. Hagerst. 2024, 25, 399–419. [Google Scholar] [CrossRef] [PubMed]
- Marian, A.J.; Braunwald, E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef] [PubMed]
- Marian, A.J.; Roberts, R. The molecular genetic basis for hypertrophic cardiomyopathy. J. Mol. Cell Cardiol. 2001, 33, 655–670. [Google Scholar] [CrossRef]
- Sequeira, C.M.; Martins, M.A.; Alves, R.; Nascimento, A.L.R.; Botti, G.; Rocha, V.N.; Matsuura, C. Aerobic exercise training attenuates doxorubicin-induced ultrastructural changes in rat ventricular myocytes. Life Sci. 2021, 264, 118698. [Google Scholar] [CrossRef]
- Ma, M.; Chen, W.; Hua, Y.; Jia, H.; Song, Y.; Wang, Y. Aerobic exercise ameliorates cardiac hypertrophy by regulating mitochondrial quality control and endoplasmic reticulum stress through M(2) AChR. J. Cell Physiol. 2021, 236, 6581–6596. [Google Scholar] [CrossRef]
- Makarewich, C.A.; Thum, T. Exercise-Induced Long Noncoding RNAs As New Players in Cardiac Hypertrophy. Circulation 2022, 145, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Stolen, T.O.; Hoydal, M.A.; Ahmed, M.S.; Jorgensen, K.; Garten, K.; Hortigon-Vinagre, M.P.; Zamora, V.; Scrimgeour, N.R.; Berre, A.M.O.; Nes, B.M.; et al. Exercise training reveals micro-RNAs associated with improved cardiac function and electrophysiology in rats with heart failure after myocardial infarction. J. Mol. Cell Cardiol. 2020, 148, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.S.; Cunha, T.F.; Paixao, N.A.; Dourado, P.M.; Carrascoza, L.S.; Bacurau, A.V.N.; Brum, P.C. Exercise intolerance establishment in pulmonary hypertension: Preventive effect of aerobic exercise training. Life Sci. 2020, 261, 118298. [Google Scholar] [CrossRef]
- Ritterhoff, J.; Tian, R. Metabolic mechanisms in physiological and pathological cardiac hypertrophy: New paradigms and challenges. Nat. Rev. Cardiol. 2023, 20, 812–829. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, L.; Li, X.; Wang, J.; Zhang, Q.; Li, Z.; Peng, D.; Yang, P.; Ma, W.; Wang, F.; et al. Effect of Mavacamten on Chinese Patients With Symptomatic Obstructive Hypertrophic Cardiomyopathy: The EXPLORER-CN Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 957–965. [Google Scholar] [CrossRef]
- Luo, F.; Liu, W.; Bu, H. MicroRNAs in hypertrophic cardiomyopathy: Pathogenesis, diagnosis, treatment potential and roles as clinical biomarkers. Heart Fail. Rev. 2022, 27, 2211–2221. [Google Scholar] [CrossRef]
- Liu, T.Y.; Xiong, X.Q.; Ren, X.S.; Zhao, M.X.; Shi, C.X.; Wang, J.J.; Zhou, Y.B.; Zhang, F.; Han, Y.; Gao, X.Y.; et al. FNDC5 Alleviates Hepatosteatosis by Restoring AMPK/mTOR-Mediated Autophagy, Fatty Acid Oxidation, and Lipogenesis in Mice. Diabetes 2016, 65, 3262–3275. [Google Scholar] [CrossRef]
- Zhao, Y.; Hong, Z.; Lin, Y.; Shen, W.; Yang, Y.; Zuo, Z.; Hu, X. Exercise pretreatment alleviates neuroinflammation and oxidative stress by TFEB-mediated autophagic flux in mice with ischemic stroke. Exp. Neurol. 2023, 364, 114380. [Google Scholar] [CrossRef]
- Li, N.; Lan, J.; Yang, J.; Ding, H. Whole milk protein powder separated by low-temperature nanofiltration membrane administration alleviates sepsis-induced myopathy. Nutr. Metab. Lond 2024, 21, 85. [Google Scholar] [CrossRef]
- Qi, J.; Rittershaus, A.; Priya, R.; Mansingh, S.; Stainier, D.Y.R.; Helker, C.S.M. Apelin signaling dependent endocardial protrusions promote cardiac trabeculation in zebrafish. eLife 2022, 11, e73231. [Google Scholar] [CrossRef]
- Kim, J. Apelin-APJ signaling: A potential therapeutic target for pulmonary arterial hypertension. Mol. Cells 2014, 37, 196–201. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, Z.; Sun, C.; Yang, D.; Zhou, Z.; Peng, X.; Zheng, L.; Tang, C. Exercise Intervention Mitigates Pathological Liver Changes in NAFLD Zebrafish by Activating SIRT1/AMPK/NRF2 Signaling. Int. J. Mol. Sci. 2021, 22, 10940. [Google Scholar] [CrossRef] [PubMed]
- Palstra, A.P.; Tudorache, C.; Rovira, M.; Brittijn, S.A.; Burgerhout, E.; van den Thillart, G.E.; Spaink, H.P.; Planas, J.V. Establishing zebrafish as a novel exercise model: Swimming economy, swimming-enhanced growth and muscle growth marker gene expression. PLoS ONE 2010, 5, e14483. [Google Scholar] [CrossRef]
- Rovira, M.; Borras, D.M.; Marques, I.J.; Puig, C.; Planas, J.V. Physiological Responses to Swimming-Induced Exercise in the Adult Zebrafish Regenerating Heart. Front. Physiol. 2018, 9, 1362. [Google Scholar] [CrossRef]
- Alarcon, M.M.L.; Trentin-Sonoda, M.; Panico, K.; Schleier, Y.; Duque, T.; Moreno-Loaiza, O.; de Yurre, A.R.; Ferreira, F.; Caio-Silva, W.; Coury, P.R.; et al. Cardiac arrhythmias after renal I/R depend on IL-1beta. J. Mol. Cell Cardiol. 2019, 131, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Zheng, C.; Luo, R.; Cao, X.; Sun, H.; Ma, H.; Huang, J.; Yang, X.; Wu, X.; Li, X. Identification and functional characterization of BICD2 as a candidate disease gene in an consanguineous family with dilated cardiomyopathy. BMC Med. Genom. 2022, 15, 189. [Google Scholar] [CrossRef]
- Ding, Y.; Lang, D.; Yan, J.; Bu, H.; Li, H.; Jiao, K.; Yang, J.; Ni, H.; Morotti, S.; Le, T.; et al. A phenotype-based forward genetic screen identifies Dnajb6 as a sick sinus syndrome gene. eLife 2022, 11, e77327. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Zhang, Z.; Huang, S.; Cui, M.; Liu, S.; Ding, M.; Gu, W.; Yang, B.; Zheng, L. Construction of a Zebrafish Model of Cardiac Hypertrophy Caused by ATIC Gene Deletion and Preliminary Exploration of Aerobic Exercise Improvement. Int. J. Mol. Sci. 2025, 26, 10249. https://doi.org/10.3390/ijms262110249
Yang T, Zhang Z, Huang S, Cui M, Liu S, Ding M, Gu W, Yang B, Zheng L. Construction of a Zebrafish Model of Cardiac Hypertrophy Caused by ATIC Gene Deletion and Preliminary Exploration of Aerobic Exercise Improvement. International Journal of Molecular Sciences. 2025; 26(21):10249. https://doi.org/10.3390/ijms262110249
Chicago/Turabian StyleYang, Tianle, Zhilong Zhang, Shuaiwang Huang, Mengchao Cui, Siyuan Liu, Meng Ding, Wenzhi Gu, Boyu Yang, and Lan Zheng. 2025. "Construction of a Zebrafish Model of Cardiac Hypertrophy Caused by ATIC Gene Deletion and Preliminary Exploration of Aerobic Exercise Improvement" International Journal of Molecular Sciences 26, no. 21: 10249. https://doi.org/10.3390/ijms262110249
APA StyleYang, T., Zhang, Z., Huang, S., Cui, M., Liu, S., Ding, M., Gu, W., Yang, B., & Zheng, L. (2025). Construction of a Zebrafish Model of Cardiac Hypertrophy Caused by ATIC Gene Deletion and Preliminary Exploration of Aerobic Exercise Improvement. International Journal of Molecular Sciences, 26(21), 10249. https://doi.org/10.3390/ijms262110249




