Gel-Phase Microextraction Using Microfluidic-Directed Ultrashort Peptide Assemblies for the Determination of Drugs in Oral Fluids
Abstract
1. Introduction
2. Results and Discussion
2.1. Materials Choice and Objective
2.2. Materials (Microfluidic Device) Preparation and Fiber Characterization
Co-Assembly of Peptide Fibers with Small Molecules Under Microfluidic Conditions
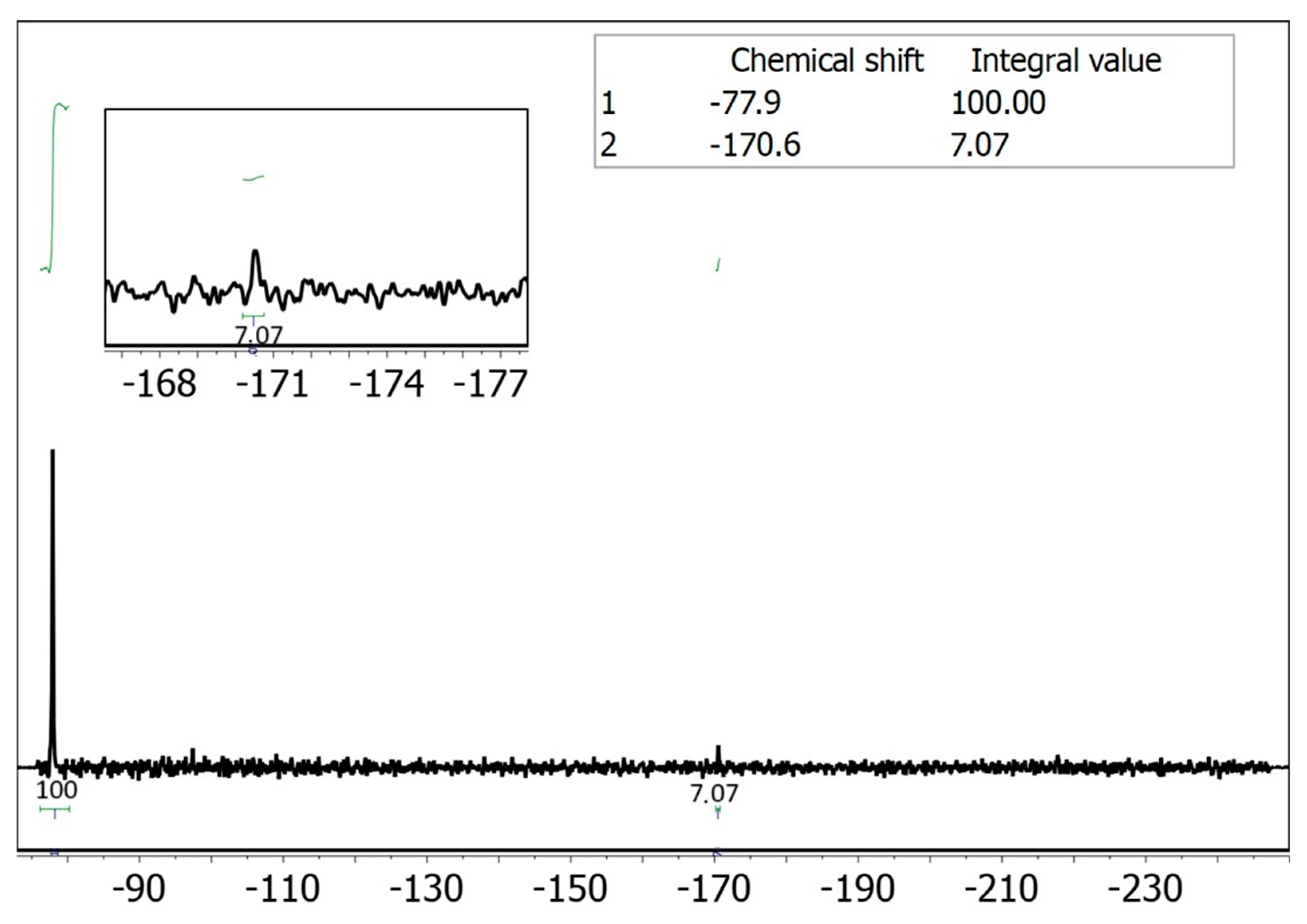
2.3. Drug Extraction and Analysis
3. Materials and Methods
3.1. Reagents and Instrumentation
3.2. In-Flow Gelation of the Tripeptide in Microfluidic Devices
3.2.1. Peptide Fiber Formation
3.2.2. Peptide Fiber Formation in the Presence of Drugs (5-FU or Naproxen)
3.3. Microfluidic Device Preparation
3.4. Extraction Procedure and Analysis
3.4.1. Absorption Experiments
Adsorption Using Solutions of Drugs in Buffer
Adsorption Using Solutions of Drugs in Saliva and 19F NMR Detection
3.4.2. Elution Experiments
3.5. Calibration Curves from UV Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittman, T.W.; Decsi, D.B.; Punyadeera, C.; Henry, C.S. Saliva-Based Microfluidic Point-of-Care Diagnostic. Theranostics 2023, 13, 1091–1108. [Google Scholar] [CrossRef]
- Ashraf, Z.; Farhat, S.; Rather, M.Y. Use of Saliva as an Alternative Matrix to Serum/Plasma for Therapeutic Drug Monitoring Using Reverse-Phase HPLC. Clin. Ther. 2021, 43, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Pandit, G.; Chetia, M.; Sarkar, A.K.; Chowdhuri, S.; Bidkar, A.P.; Chatterjee, S. Peptide Hydrogels as Platforms for Sustained Release of Antimicrobial and Antitumor Drugs and Proteins. ACS Appl. Bio Mater. 2020, 3, 6251–6262. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Kennedy, S.R.; Steed, J.W.; Valcárcel, M. Fluorescent Carbon Quantum Dot Hydrogels for Direct Determination of Silver Ions. Talanta 2016, 151, 100–105. [Google Scholar] [CrossRef]
- Cayuela, A.; Kennedy, S.R.; Soriano, M.L.; Jones, C.D.; Valcárcel, M.; Steed, J.W. Fluorescent Carbon Dot-Molecular Salt Hydrogels. Chem. Sci. 2015, 6, 6139–6146. [Google Scholar] [CrossRef]
- Alampanos, V.D.; Lambropoulou, D.A. Hydrogel Sorbent-Based Sample Preparation Processes as Green Alternatives for the Extraction of Organic Contaminants Followed by Chromatographic Analysis. TrAC-Trends Anal. Chem. 2024, 174, 117687. [Google Scholar] [CrossRef]
- Liao, P.H.; Urban, P.L. Agarose-Based Gel-Phase Microextraction Technique for Quick Sampling of Polar Analytes Adsorbed on Surfaces. ACS Omega 2019, 4, 19063–19070. [Google Scholar] [CrossRef] [PubMed]
- Guzella, C.S.; Souto, D.E.P.; Silva, B.J.G. Alginate-Based Hydrogel Fiber as a Restricted Access Material for Microextraction of Drugs in Biological Samples. Carbohydr. Polym. 2022, 294, 119810. [Google Scholar] [CrossRef]
- Levin, A.; Hakala, T.A.; Schnaider, L.; Bernardes, G.J.L.; Gazit, E.; Knowles, T.P.J. Biomimetic Peptide Self-Assembly for Functional Materials. Nat. Rev. Chem. 2020, 4, 615–634. [Google Scholar] [CrossRef]
- Marchesan, S.; Qu, Y.; Waddington, L.J.; Easton, C.D.; Glattauer, V.; Lithgow, T.J.; McLean, K.M.; Forsythe, J.S.; Hartley, P.G. Self-Assembly of Ciprofloxacin and a Tripeptide into an Antimicrobial Nanostructured Hydrogel. Biomaterials 2013, 34, 3678–3687. [Google Scholar] [CrossRef]
- Marchesan, S.; Vargiu, A.V.; Styan, K.E. The Phe-Phe Motif for Peptide Self-Assembly in Nanomedicine. Molecules 2015, 20, 19775–19788. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Wu, H.; Adler-Abramovich, L.; Zhang, J.; Fan, X.; Wang, Y.; Zhang, Y.; Tofail, S.A.M.; Mei, D.; Li, J.; et al. Aromatic Short Peptide Architectonics: Assembly and Engineering. Prog. Mater. Sci. 2024, 142, 101240. [Google Scholar] [CrossRef]
- Garcia, A.M.; Iglesias, D.; Parisi, E.; Styan, K.E.; Waddington, L.J.; Deganutti, C.; De Zorzi, R.; Grassi, M.; Melchionna, M.; Vargiu, A.V.; et al. Chirality Effects on Peptide Self-Assembly Unraveled from Molecules to Materials. Chem 2018, 4, 1862–1876. [Google Scholar] [CrossRef]
- Marchesan, S.; Easton, C.D.; Kushkaki, F.; Waddington, L.; Hartley, P.G. Tripeptide Self-Assembled Hydrogels: Unexpected Twists of Chirality. Chem. Commun. 2012, 48, 2195–2197. [Google Scholar] [CrossRef]
- Parisi, E.; Garcia, A.M.; Marson, D.; Posocco, P.; Marchesan, S. Supramolecular Tripeptide Hydrogel Assembly with 5-Fluorouracil. Gels 2019, 5, 5. [Google Scholar] [CrossRef]
- Kurbasic, M.; Romano, C.D.; Garcia, A.M.; Kralj, S.; Marchesan, S. Assembly of a Tripeptide and Anti-Inflammatory Drugs into Supramolecular Hydrogels for Sustained Release. Gels 2017, 3, 29. [Google Scholar] [CrossRef]
- Garcia, A.M.; Garcia-Romero, J.A.; Mejias, S.H.; Prieto, P.; Saggiomo, V.; Velders, A.H.; Soriano, M.L.; Ruiz-Díez, V.; Cabanillas-González, J.; Gomez, M.V. Microfluidic-Driven Short Peptide Hydrogels with Optical Waveguiding Properties. J. Mater. Chem. C 2024, 12, 6027–6034. [Google Scholar] [CrossRef]
- Willems, S.B.J.; Schijven, L.M.I.; Bunschoten, A.; Van Leeuwen, F.W.B.; Velders, A.H.; Saggiomo, V. Covalently Bound Monolayer Patterns Obtained by Plasma Etching on Glass Surfaces. Chem. Commun. 2019, 55, 7667–7670. [Google Scholar] [CrossRef]
- Willems, S.B.J.; Zegers, J.; Bunschoten, A.; Wagterveld, R.M.; Van Leeuwen, F.W.B.; Velders, A.H.; Saggiomo, V. COvalent Monolayer Patterns in Microfluidics by PLasma Etching Open Technology-COMPLOT. Analyst 2020, 145, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, X.; Li, X.; Wei, Y.; Wang, T.; Liu, S.; Yang, H.; Sun, X. Saliva Analysis Based on Microfluidics: Focusing the Wide Spectrum of Target Analyte. Crit. Rev. Anal. Chem. 2025, 55, 330–352. [Google Scholar] [CrossRef] [PubMed]
- Mompeán, M.; Sánchez-donoso, R.M.; de la Hoz, A.; Saggiomo, V.; Velders, A.H.; Gomez, M.V. Pushing Nuclear Magnetic Resonance Sensitivity Limits with Microfluidics and Photo-Chemically Induced Dynamic Nuclear Polarization. Nat. Commun. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Andreou, C.; Hoonejani, M.R.; Barmi, M.R.; Moskovits, M.; Meinhart, C.D. Rapid Detection of Drugs of Abuse in Saliva Using Surface Enhanced Raman Spectroscopy and Microfluidics. ACS Nano 2013, 7, 7157–7164. [Google Scholar] [CrossRef]
- Buchholz, C.R.; Pomerantz, W.C.K. 19F NMR Viewed through Two Different Lenses: Ligand-Observed and Protein-Observed 19F NMR Applications for Fragment-Based Drug Discovery. RSC Chem. Biol. 2021, 2, 1312–1330. [Google Scholar] [CrossRef]
- Li, Q.; Kang, C.B. Perspectives on Applications of 19F-NMR in Fragment-Based Drug Discovery. Molecules 2024, 29, 5748. [Google Scholar] [CrossRef]
- Gomez, M.V.; Velders, A.H. NMR Microcoils for On-Line Reaction Monitoring. In Flow Chemistry; The Royal Society of Chemistry: London, UK, 2019; pp. 340–365. [Google Scholar]
- Gomez, M.V.; Baas, S.; Velders, A.H. Multinuclear 1D and 2D NMR with 19F-Photo-CIDNP Hyperpolarization in a Microfluidic Chip with Untuned Microcoil. Nat. Commun. 2023, 14, 3885. [Google Scholar] [CrossRef]
- Luis, S.V.; Garcia-Verdugo, E. Flow Chemistry: Integrated Approaches for Practical Applications; Royal Society of Chemistry: London, UK, 2019; ISBN 978-1-78801-498-4/978-1-78801-609-4/978-1-78801-893-7. [Google Scholar]
- Gomez, M.V.; Rodriguez, A.M.; De La Hoz, A.; Jimenez-Marquez, F.; Fratila, R.M.; Barneveld, P.A.; Velders, A.H. Determination of Kinetic Parameters within a Single Nonisothermal On-Flow Experiment by Nanoliter NMR Spectroscopy. Anal. Chem. 2015, 87, 10547–10555. [Google Scholar] [CrossRef]
- Gomez, M.V.; Verputten, H.H.J.; Díaz-Ortíz, A.; Moreno, A.; De La Hoz, A.; Velders, A.H. On-Line Monitoring of a Microwave-Assisted Chemical Reaction by Nanolitre NMR-Spectroscopy. Chem. Commun. 2010, 46, 4514–4516. [Google Scholar] [CrossRef] [PubMed]
- Fratila, R.M.; Gomez, M.V.; Sýkora, S.; Velders, A.H. Multinuclear Nanoliter One-Dimensional and Two-Dimensional NMR Spectroscopy with a Single Non-Resonant Microcoil. Nat. Commun. 2014, 5, 3025. [Google Scholar] [CrossRef]
- Rho, H.S.; Hanke, A.T.; Ottens, M.; Gardeniers, H. A Microfluidic Device for the Batch Adsorption of a Protein on Adsorbent Particles. Analyst 2017, 142, 3656–3665. [Google Scholar] [CrossRef]
- Kleeberg, U.R. Plasma and Salivary Pharmacokinetics of 5-Fluorouracil. Strahlenther. Onkol. 2000, 176, 240–241. [Google Scholar] [PubMed]
- Sivilotti, M.L.A. Initial Management of the Critically Ill Adult with an Unknown Overdose. Available online: https://www.uptodate.com/contents/initial-management-of-the-critically-ill-adult-with-an-unknown-overdose (accessed on 17 July 2025).
- Masiliūnienė, G.; Stankevičius, E.; Kaduševičius, E. Trends in Adverse Drug Reaction Reporting in Eight Selected Countries after the Implementation of New Pharmacovigilance Regulation in 2012: A Joinpoint Regression Analysis. Eur. J. Clin. Pharmacol. 2025, 81, 1287–1299. [Google Scholar] [CrossRef]
- Gomez, M.V.; Juan, A.; Jiménez-Márquez, F.; De La Hoz, A.; Velders, A.H. Illumination of Nanoliter-NMR Spectroscopy Chips for Real-Time Photochemical Reaction Monitoring. Anal. Chem. 2018, 90, 1542–1546. [Google Scholar] [CrossRef]
- Anders, J.; Velders, A.H. Microcoils for Broadband Multinuclei Detection. In Microcoils for Broadband Multinuclei Detection; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 265–296. [Google Scholar]
- Sierra, S.; Gomez, M.V.; Jiménez, A.I.; Pop, A.; Silvestru, C.; Marín, M.L.; Boscá, F.; Sastre, G.; Gómez-Bengoa, E.; Urriolabeitia, E.P. Stereoselective, Ruthenium-Photocatalyzed Synthesis of 1,2-Diaminotruxinic Bis-Amino Acids from 4-Arylidene-5(4 H)-Oxazolones. J. Org. Chem. 2022, 87, 3529–3545. [Google Scholar] [CrossRef] [PubMed]
- Hines, E.P.; Calafat, A.M.; Silva, M.J.; Mendola, P.; Fenton, S.E. Concentrations of Phthalate Metabolites in Milk, Urine, Saliva, and Serum of Lactating North Carolina Women. Environ. Health Perspect. 2009, 117, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Spencer, E.P.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs: A 2013 Update. Ther. Drug Monit. 2013, 35, 1–47. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriano, M.L.; Garcia, A.M.; Garcia-Romero, J.A.; Prieto, P.; Velders, A.H.; Gomez, M.V. Gel-Phase Microextraction Using Microfluidic-Directed Ultrashort Peptide Assemblies for the Determination of Drugs in Oral Fluids. Int. J. Mol. Sci. 2025, 26, 9982. https://doi.org/10.3390/ijms26209982
Soriano ML, Garcia AM, Garcia-Romero JA, Prieto P, Velders AH, Gomez MV. Gel-Phase Microextraction Using Microfluidic-Directed Ultrashort Peptide Assemblies for the Determination of Drugs in Oral Fluids. International Journal of Molecular Sciences. 2025; 26(20):9982. https://doi.org/10.3390/ijms26209982
Chicago/Turabian StyleSoriano, M. Laura, Ana M. Garcia, Juan A. Garcia-Romero, Pilar Prieto, Aldrik H. Velders, and M. Victoria Gomez. 2025. "Gel-Phase Microextraction Using Microfluidic-Directed Ultrashort Peptide Assemblies for the Determination of Drugs in Oral Fluids" International Journal of Molecular Sciences 26, no. 20: 9982. https://doi.org/10.3390/ijms26209982
APA StyleSoriano, M. L., Garcia, A. M., Garcia-Romero, J. A., Prieto, P., Velders, A. H., & Gomez, M. V. (2025). Gel-Phase Microextraction Using Microfluidic-Directed Ultrashort Peptide Assemblies for the Determination of Drugs in Oral Fluids. International Journal of Molecular Sciences, 26(20), 9982. https://doi.org/10.3390/ijms26209982







