Chemical Characterization and In Vitro Antioxidant, Anti-Inflammatory, and Colon Cancer-Preventive Potential of a Polysaccharide Fraction from Macrolepiota procera
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of Crude Polysaccharides from M. procera
2.2. Biological Activity of Crude Polysaccharides from M. procera
2.2.1. Antioxidant Activity
2.2.2. Anti-Inflammatory Potential
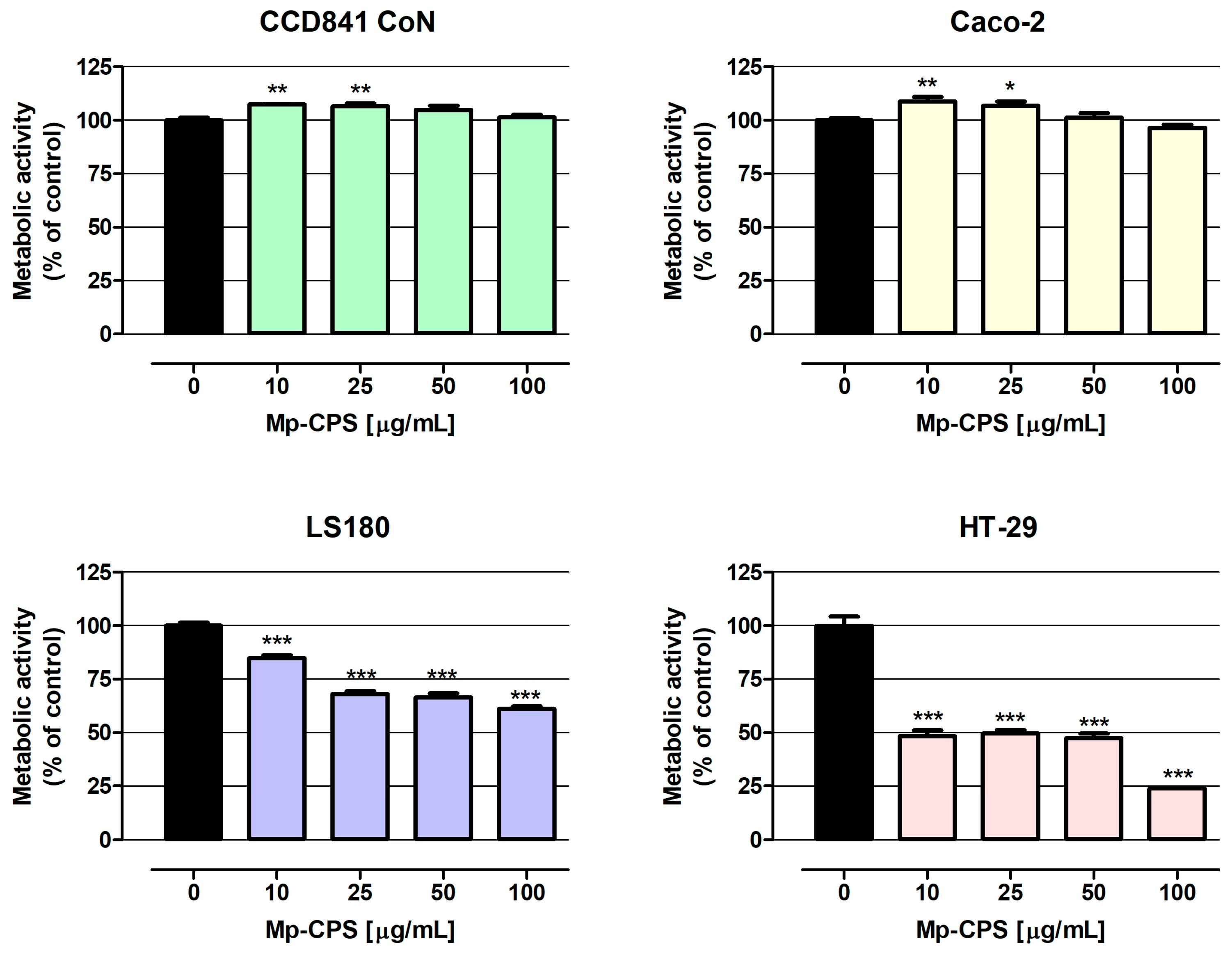
2.2.3. Anticancer Potential
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemicals
4.3. Preparation of Crude Polysaccharides from M. procera Mp-CPS
4.4. Determination of the Total Sugar Content
4.5. Determination of Uronic Acids
4.6. Determination of Proteins
4.7. Determination of the Total Content of Phenolic Compounds
4.8. Monosaccharide Composition of the Mp-CPS
4.9. Evaluation of the Total α- and β-Glucans Content
4.10. Determination of Biological Activity of the Mp-CPS
4.10.1. TEAC (Trolox Equivalent Antioxidant Capacity) Assay
4.10.2. ORAC (Oxygen Radical Absorbance Capacity) Assay
4.10.3. Inhibition of Cyclooxygenase (COX) Activity
4.10.4. Inhibition of Lipoxygenase (LOX) Activity
4.10.5. Anticancer Potential—In Vitro Studies
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, P.; Wang, S.; Liu, M.; Chen, F.; Yang, W.; Chang, X.; Liu, N.; Zhao, Y.; Wang, J.; Chen, X. Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: A mini-review. Carbohydr. Res. 2020, 494, 108037. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, Y.; Xue, J.; Chen, S.; Wang, H. Edible mushroom polysaccharides: Structural characteristics, chemical modification strategies, and structure-activity relationship: A review. Int. J. Biol. Macromol. 2025, 320, 145888. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Rodrigues, H.; Sousa, A.S.; Relvas, J.B.; Tavaria, F.K.; Pintado, M. An overview on mushroom polysaccharides: Health-promoting properties, prebiotic and gut microbiota modulation effects and structure-function correlation. Carbohydr. Polym. 2024, 333, 121978. [Google Scholar] [CrossRef]
- Yin, Z.; Liang, Z.; Li, C.; Wang, J.; Ma, C.; Kang, W. Immunomodulatory effects of polysaccharides from edible fungus: A review. Food Sci. Hum. Wellness 2021, 10, 393–400. [Google Scholar] [CrossRef]
- Deveci, E.; Tel-Çayan, G.; Çayan, F.; Yılmaz Altınok, B.; Aktaş, S. Characterization of polysaccharide extracts of four edible mushrooms and determination of in vitro antioxidant, enzyme inhibition and anticancer activities. ACS Omega 2024, 9, 25887–25901. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, G.; Li, F.; Fang, S.; Zhou, S.; Ishiwata, A.; Tonevitsky, A.G.; Shkurnikov, M.; Cai, H.; Ding, F. Immunomodulatory effect and biological significance of β-glucans. Pharmaceutics 2023, 15, 1615. [Google Scholar] [CrossRef]
- Park, H.J. Current uses of mushrooms in cancer treatment and their anticancer mechanisms. Int. J. Mol. Sci. 2022, 23, 10502. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, S.; Ding, W.; Zhang, C.; Rehman, M.U.; Tareen, M.F.; Wang, L.; Huang, S. From structure to function: A comprehensive overview of polysaccharide roles and applications. Food Front. 2025, 6, 15–39. [Google Scholar] [CrossRef]
- Guo, D.; Liu, C.; Zhu, H.; Cheng, Y.; Guo, Y.; Yao, W.; Jiang, J.; Qian, H. Advanced insights into mushroom polysaccharides: Extraction methods, structure-activity, prebiotic properties, and health-promoting effects. Int. J. Biol. Macromol. 2025, 308, 142319. [Google Scholar] [CrossRef]
- Adamska, I.; Tokarczyk, G. Possibilities of using Macrolepiota procera in the production of prohealth food and in medicine. Int. J. Food Sci. 2022, 2022, 5773275. [Google Scholar] [CrossRef]
- Shim, S.M.; Oh, Y.H.; Lee, K.R.; Kim, S.H.; Im, K.H.; Kim, J.W.; Lee, U.Y.; Shim, J.O.; Shim, M.J.; Lee, M.W.; et al. The characteristics of cultural conditions for the mycelial growth of Macrolepiota procera. Mycobiology 2005, 33, 15. [Google Scholar] [CrossRef]
- Aytar, E.C.; Akata, I.; Açik, L. Antioxidant and antimicrobial activities of Armillaria mellea and Macrolepiota procera extracts. J. Fungus 2020, 11, 121–128. [Google Scholar]
- Robaszkiewicz, A.; Bartosz, G.; Ławrynowicz, M.; Soszyński, M. The role of polyphenols, β-carotene, and lycopene in the antioxidative action of the extracts of dried, edible mushrooms. J. Nutr. Metab. 2010, 2010, 173274. [Google Scholar] [CrossRef]
- Georgiev, Y.N.; Vasicek, O.; Dzhambazov, B.; Batsalova, T.G.; Denev, P.N.; Dobreva, L.I.; Danova, S.T.; Simova, S.D.; Wold, C.W.; Ognyanov, M.H.; et al. Structural features and immunomodulatory effects of water-extractable polysaccharides from Macrolepiota procera (Scop.) Singer. J. Fungi 2022, 8, 848. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Q.; Wang, G.; Wu, J.Y. Contents and antioxidant activities of polysaccharides in 14 wild mushroom species from the forest of northeastern China. Int. J. Med. Mushrooms 2015, 17, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Nowacka-Jechalke, N.; Juda, M.; Malm, A. The preliminary study of prebiotic potential of polish wild mushroom polysaccharides: The stimulation effect on Lactobacillus strains growth. Eur. J. Nutr. 2018, 57, 1511–1521. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Zhang, Y.; Zhang, J.; Jia, L. Mycelium polysaccharides of Macrolepiota procera alleviate reproductive impairments induced by nonylphenol. Food Funct. 2022, 13, 5794–5806. [Google Scholar] [CrossRef]
- Zara, R.; Rasul, A.; Sultana, T.; Jabeen, F.; Selamoglu, Z. Identification of Macrolepiota procera extract as a novel G6PD inhibitor for the treatment of lung cancer. Saudi J. Biol. Sci. 2022, 29, 3372–3379. [Google Scholar] [CrossRef] [PubMed]
- Özgür, A.; Kaplan, Ö.; Gökşen Tosun, N.; Türkekul, İ.; Gökçe, İ. Green synthesis of silver nanoparticles using Macrolepiota procera extract and investigation of their HSP27, HSP70, and HSP90 inhibitory potentials in human cancer cells. Part. Sci. Technol. 2023, 41, 330–340. [Google Scholar] [CrossRef]
- Nowacka-Jechalke, N.; Nowak, R.; Lemieszek, M.K.; Rzeski, W.; Gawlik-Dziki, U.; Szpakowska, N.; Kaczyński, Z. Promising potential of crude polysaccharides from Sparassis crispa against colon cancer: An in vitro study. Nutrients 2021, 13, 161. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Ahmed, D.; Eide, P.W.; Eilertsen, I.A.; Danielsen, S.A.; Eknæs, M.; Hektoen, M.; Lind, G.E.; Lothe, R.A. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2013, 2, e71. [Google Scholar] [CrossRef]
- Shabahang, M.; Buras, R.R.; Davoodi, F.; Schumaker, L.M.; Nauta, R.J.; Evans, S.R.T. 1,25-dihydroxyvitamin D3 receptor as a marker of human colon carcinoma cell line differentiation and growth inhibition. Cancer Res. 1993, 53, 3712–3718. [Google Scholar]
- Mirończuk-Chodakowska, I.; Witkowska, A.M. Evaluation of Polish wild mushrooms as beta-glucan sources. Int. J. Environ. Res. Public Health 2020, 17, 7299. [Google Scholar] [CrossRef]
- Pekşen, A.; Kibar, B. Determination of optimum culture conditions for mycelial growth of Macrolepiota procera Mushroom. Acta Sci. Pol. Hortorum Cultus 2020, 19, 11–20. [Google Scholar] [CrossRef]
- Bucurica, I.A.; Dulama, I.D.; Radulescu, C.; Banica, A.L.; Stanescu, S.G. Heavy metals and associated risks of wild edible mushrooms consumption: Transfer factor, carcinogenic risk, and health risk index. J. Fungi 2024, 10, 844. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Sapkota, A.; Dryżałowska, A.; Mędyk, M.; Feng, X. Analysis of some metallic elements and metalloids composition and relationships in parasol mushroom Macrolepiota procera. Environ. Sci. Pollut. Res. Int. 2017, 24, 15528. [Google Scholar] [CrossRef]
- Chun, S.; Gopal, J.; Muthu, M.; Pablos, P.A.; Omaye, S. Antioxidant activity of mushroom extracts/polysaccharides—Their antiviral properties and plausible antiCOVID-19 properties. Antioxidants 2021, 10, 1899. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Mizuno, M.; Minato, K.I. Anti-inflammatory and immunomodulatory properties of polysaccharides in mushrooms. Curr. Opin. Biotechnol. 2024, 86, 103076. [Google Scholar] [CrossRef]
- Rao, C.V.; Janakiram, N.B.; Mohammed, A. Lipoxygenase and cyclooxygenase pathways and colorectal cancer prevention. Curr. Color. Cancer Rep. 2012, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.S.; Kim, E.H.; Kim, D.H.; Song, N.Y.; Kim, W.; Na, H.K.; Surh, Y.J. Targeting cyclooxygenase-2 for chemoprevention of inflammation-associated intestinal carcinogenesis: An update. Biochem. Pharmacol. 2024, 228, 116259. [Google Scholar] [CrossRef]
- Li, C.; Wu, G.; Zhao, H.; Dong, N.; Wu, B.; Chen, Y.; Lu, Q. Natural-derived polysaccharides from plants, mushrooms, and seaweeds for the treatment of inflammatory bowel disease. Front. Pharmacol. 2021, 12, 651813. [Google Scholar] [CrossRef] [PubMed]
- Nowacka-Jechalke, N.; Kanak, S.; Moczulski, M.; Martyna, A.; Kubiński, K.; Masłyk, M.; Szpakowska, N.; Kaczyński, Z.; Nowak, R.; Olech, M. Crude polysaccharides from wild-growing Armillaria mellea—Chemical composition and antidiabetic, anti-inflammatory, antioxidant, and antiproliferative potential. Appl. Sci. 2023, 13, 3853. [Google Scholar] [CrossRef]
- Khan, T.; Date, A.; Chawda, H.; Patel, K. Polysaccharides as potential anticancer agents—A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef]
- Pandya, U.; Dhuldhaj, U.; Sahay, N.S. Bioactive mushroom polysaccharides as antitumor: An overview. Nat. Prod. Res. 2019, 33, 2668–2680. [Google Scholar] [CrossRef]
- Ren, L.; Perera, C.; Hemar, Y. Antitumor activity of mushroom polysaccharides: A review. Food Funct. 2012, 3, 1118–1130. [Google Scholar] [CrossRef]
- Bentharavithana, J.; Islam, T.; Xu, B. medicinal mushrooms in colon cancer therapy: Mechanisms of action of bioactive compounds and therapeutic potential. Int. J. Mol. Sci. 2025, 26, 5304. [Google Scholar] [CrossRef]
- Arora, S.; Goyal, S.; Balani, J.; Tandon, S. Enhanced antiproliferative effects of aqueous extracts of some medicinal mushrooms on colon cancer cells. Int. J. Med. Mushrooms 2013, 15, 301–314. [Google Scholar] [CrossRef]
- Seçme, M.; Kaygusuz, O.; Eroğlu, C.; Dodurga, Y.; Çolak, Ö.F.; Atmaca, P. Potential anticancer activity of the parasol mushroom, Macrolepiota procera (Agaricomycetes), against the A549 human lung cancer cell line. Int. J. Med. Mushrooms 2018, 20, 1075–1086. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Rančić, A.; Stanojković, T. Evaluation of metal concentration and antioxidant, antimicrobial, and anticancer potentials of two edible mushrooms Lactarius deliciosus and Macrolepiota procera. J. Food Drug Anal. 2016, 24, 477–484. [Google Scholar] [CrossRef]
- Ćirić, A.; Kruljević, I.; Stojković, D.; Fernandes, Â.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R.; Soković, M.; Glamočlija, J. Comparative investigation on edible mushrooms Macrolepiota mastoidea, M. rhacodes and M. procera: Functional foods with diverse biological activities. Food Funct. 2019, 10, 7678–7686. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- He, C.; Zhang, R.; Jia, X.; Dong, L.; Ma, Q.; Zhao, D.; Sun, Z.; Zhang, M.; Huang, F. Variation in characterization and probiotic activities of polysaccharides from litchi pulp fermented for different times. Front. Nutr. 2022, 9, 993828. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R. Influence of different extraction procedures on the antiradical activity and phenolic profile of Rosa rugosa petals. Acta Pol. Pharm. 2012, 69, 501–507. [Google Scholar]
- Rovio, S.; Yli-Kauhaluoma, J.; Sirén, H. Determination of neutral carbohydrates by CZE with direct uv detection. Electrophoresis 2007, 28, 3129–3135. [Google Scholar] [CrossRef]
- Łubek-Nguyen, A.; Olech, M.; Nowacka-Jechalke, N.; Martyna, A.; Kubiński, K.; Masłyk, M.; Moczulski, M.; Kanak, S. Crude polysaccharide fraction from Rosa rugosa Thunb. root—Chemical characterisation, enzyme inhibitory, antioxidant and antiproliferative activity. Appl. Sci. 2022, 12, 10126. [Google Scholar] [CrossRef]
- Dienaitė, L.; Pukalskas, A.; Pukalskienė, M.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Phytochemical composition, antioxidant and antiproliferative activities of defatted sea buckthorn (Hippophaë rhamnoides L.) berry pomace fractions consecutively recovered by pressurized ethanol and water. Antioxidants 2020, 9, 274. [Google Scholar] [CrossRef]
- Olech, M.; Łyko, L.; Nowak, R. Influence of accelerated solvent extraction conditions on the LC-ESI-MS/MS polyphenolic profile, triterpenoid content, and antioxidant and anti-lipoxygenase activity of Rhododendron luteum sweet leaves. Antioxidants 2020, 9, 822. [Google Scholar] [CrossRef]
- Maiga, A.; Malterud, K.E.; Diallo, D.; Paulsen, B.S. Antioxidant and 15-lipoxygenase inhibitory activities of the malian medicinal plants Diospyros abyssinica (Hiern) F. White (Ebenaceae), Lannea velutina A. Rich (Anacardiaceae) and Crossopteryx febrifuga (Afzel) Benth. (Rubiaceae). J. Ethnopharmacol. 2006, 104, 132–137. [Google Scholar] [CrossRef] [PubMed]
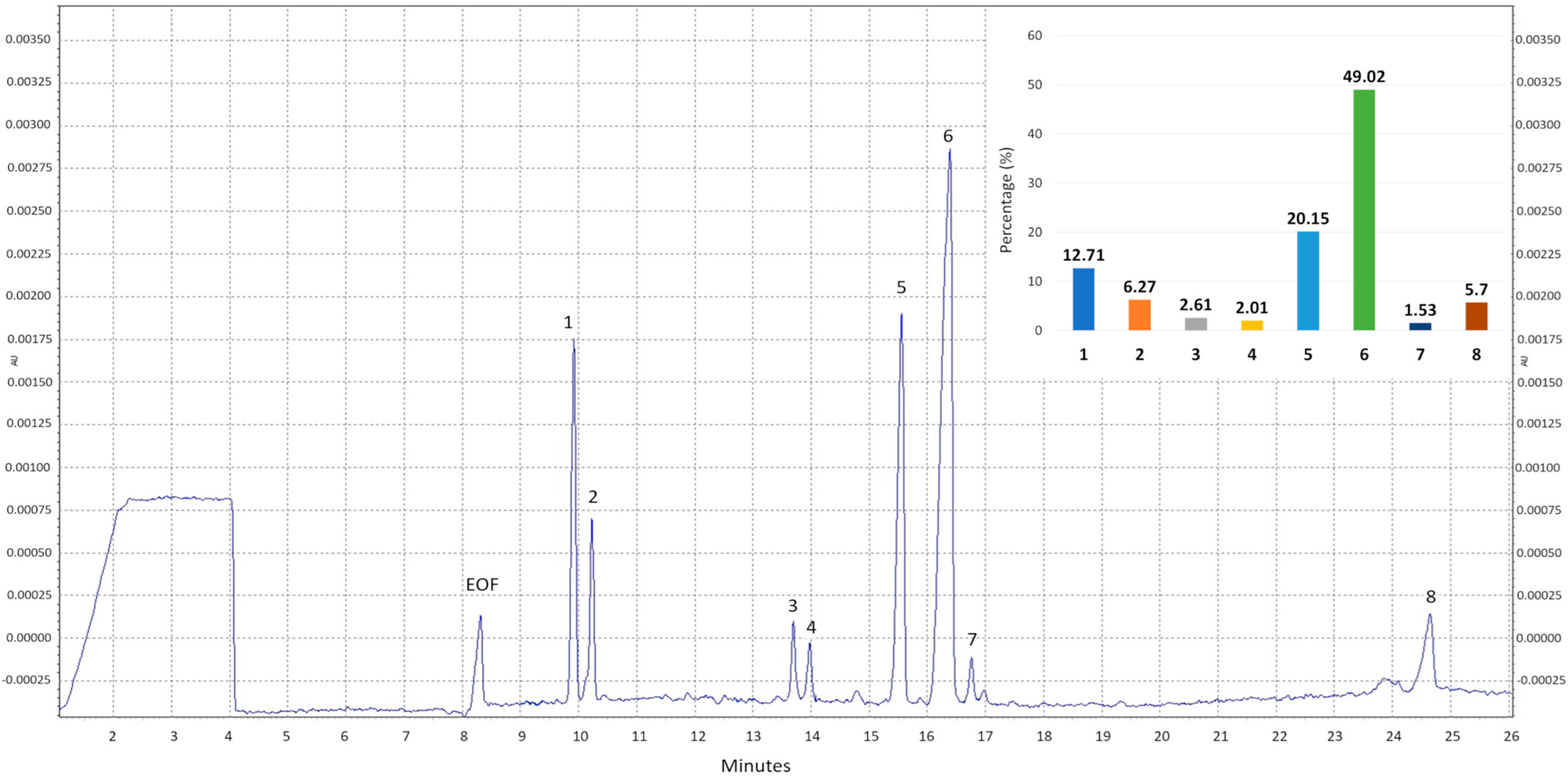

| Group of Compounds | Result (Mean Value ± SD) |
|---|---|
| Sugars (% of Mp-CPS) | 63.86 ± 0.92 |
| Uronic acids (% of Mp-CPS) | 6.71 ± 0.21 |
| Proteins (% of Mp-CPS) | 4.01 ± 0.18 |
| Phenolic compounds (% of Mp-CPS) | 2.19 ± 0.09 |
| Total glucans (g/100 g of d.w.) | 13.51 ± 0.41 |
| α-glucans (g/100 g of d.w.) | 2.75 ± 0.13 |
| β-glucans (g/100 g of d.w.) | 10.76 ± 0.53 |
| Yield (%) | 15.7 ± 0.38 |
| Antioxidant Test | Result (Mean Value ± SD) |
|---|---|
| TEAC (µM Trolox/g of Mp-CPS) | 102.00 ± 2.09 |
| ORAC (µM Trolox/g of Mp-CPS) | 358.56 ± 13.18 |
| COX-1 | COX-2 | LOX | |
|---|---|---|---|
| (% of Inhibition) | |||
| Mp-CPS | 74.23 ± 1.26 | 39.09 ± 0.42 | 43.69 ± 0.94 |
| ASA | 40.21 ± 0.78 | 96.84 ± 0.93 | 92.86 ± 0.51 |
| Assay | LDH Assay | MTT Assay | |||
|---|---|---|---|---|---|
| Cell Line | EC50 [µg/mL] | Trust Range [µg/mL] | IC50 [µg/mL] | Trust Range [µg/mL] | |
| CCD841 CoN | 2.67 × 1021 | no data | no data | no data | |
| Caco-2 | 524 | 141–1949 | no data | no data | |
| LS180 | 371 | 166–828 | 232 | 137–394 | |
| HT-29 | 174 | 48–631 | 15 | 8–29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka-Jechalke, N.; Lemieszek, M.K. Chemical Characterization and In Vitro Antioxidant, Anti-Inflammatory, and Colon Cancer-Preventive Potential of a Polysaccharide Fraction from Macrolepiota procera. Int. J. Mol. Sci. 2025, 26, 9978. https://doi.org/10.3390/ijms26209978
Nowacka-Jechalke N, Lemieszek MK. Chemical Characterization and In Vitro Antioxidant, Anti-Inflammatory, and Colon Cancer-Preventive Potential of a Polysaccharide Fraction from Macrolepiota procera. International Journal of Molecular Sciences. 2025; 26(20):9978. https://doi.org/10.3390/ijms26209978
Chicago/Turabian StyleNowacka-Jechalke, Natalia, and Marta Kinga Lemieszek. 2025. "Chemical Characterization and In Vitro Antioxidant, Anti-Inflammatory, and Colon Cancer-Preventive Potential of a Polysaccharide Fraction from Macrolepiota procera" International Journal of Molecular Sciences 26, no. 20: 9978. https://doi.org/10.3390/ijms26209978
APA StyleNowacka-Jechalke, N., & Lemieszek, M. K. (2025). Chemical Characterization and In Vitro Antioxidant, Anti-Inflammatory, and Colon Cancer-Preventive Potential of a Polysaccharide Fraction from Macrolepiota procera. International Journal of Molecular Sciences, 26(20), 9978. https://doi.org/10.3390/ijms26209978








