1. Introduction
Ichthyoses are a heterogeneous group of dermatological diseases, including both hereditary (autosomal dominant, autosomal recessive and X-linked forms) and acquired conditions, characterized by a common pathogenetic mechanism—a disruption of keratinization processes [
1]. Clinically, these diseases are manifested by generalized hyperkeratosis with the formation of large-plate scales, marked xerosis, and impaired skin barrier function. According to modern data, the pathogenesis of ichthyosis may be associated with a wide range of pathological conditions, including malignant neoplasms, autoimmune diseases, metabolic and endocrine disorders, and infectious processes [
2]. A special group consists of hereditary forms of ichthyosis caused by monogenic defects that lead to impaired formation of the stratum corneum of the epidermis.
Among hereditary ichthyoses, lamellar ichthyosis (LI) plays a special role as one of the most severe forms of nonsyndromal keratinization disorders characterized by high perinatal lethality (up to 20% of cases). The molecular basis of the disease in most cases involves mutations in the
TGM1 gene encoding the enzyme transglutaminase 1, which plays a key role in the processes of cross-linking of stratum corneum proteins [
3]. Deficiency of this enzyme leads to pronounced proliferative hyperkeratosis and impaired formation of corneocytes. Modern studies have revealed that the key pathogenetic mechanism of LI is a complex disorder of lipid metabolism in differentiated keratinocytes, which causes defects in the formation of the extracellular matrix of the stratum corneum and significantly impairs the barrier function of the skin [
4].
The clinical picture of LI is characterized by significant polymorphism. The most critical is the neonatal period, when 90% of patients develop the so-called collodion baby phenotype, a specific parchment-like membrane covering the entire body surface [
5]. This condition is accompanied by gross disorders of thermoregulation, significant fluid, and electrolyte losses, as well as a high risk of septic complications. Subsequently, the disease manifests by the formation of large lamellar scales of dark brown color with characteristic superficial cracks between them. Associated manifestations include dystrophic changes in the nail plates, alopecia areata, ocular involvement of varying severity (ectropion, keratitis), and possible eversion of mucous membranes [
6].
Current therapeutic approaches for LI are exclusively symptomatic and include the use of systemic retinoids, intensive emollient therapy, and prevention of secondary infections [
7]. Supportive therapy is of particular importance in the first months of life of patients, when the risk of lethal complications reaches maximum values. However, existing therapies are unable to address the underlying cause of the disease—TGM1 deficiency, which necessitates the development of fundamentally new etiotropic approaches [
8].
Gene therapy using viral vectors to deliver functional copies of the TGM1 gene appears to be a promising direction in the treatment of LI. Among various vector systems, adeno-associated viruses (AAVs) demonstrate a number of unique advantages, including low immunogenicity, non-integration into the host genome, and the ability to ensure long-term (up to several years) transgene expression [
9]. Of particular interest is the AAV2 serotype, which has shown high efficiency in transducing keratinocytes in experimental models. Studies demonstrating the efficacy of correcting an enzyme deficiency in Sjogren-Larsson syndrome by delivering the fatty aldehyde dehydrogenase (FALDH) gene using AAV vectors have become an important proof of concept [
10].
However, existing vector systems require substantial optimization, particularly in terms of improving the efficiency of epidermal stem cell transduction; ensuring stable long-term transgene expression; minimizing potential immune reactions; and developing efficient cutaneous delivery methods [
11].
In the present study, a comprehensive evaluation of the efficacy and safety of recombinant AAV2 vector carrying the TGM1 gene following intradermal injection in two experimental models, rats and pigs, was performed. Special attention was paid to the level of transgene expression in different epidermal layers, the duration of the therapeutic effect, and histological changes in the injection area, as well as potential toxic effects.
3. Discussion
Currently, gene therapy for skin diseases is primarily being investigated in preclinical studies. In fact, out of 1052 clinical trials involving gene therapy, only 23 focus on skin diseases [
11]. The skin is an attractive target for gene therapy for several reasons. The epidermis is frequently affected by monogenic skin diseases and can be readily modeled in vitro or manipulated ex vivo. Furthermore, the effects of therapy can be easily monitored. Additionally, current advances in cell culture techniques have made it possible to expand keratinocytes, including keratinocyte stem cells (KSCs), in vitro [
12].
Direct delivery of therapeutic vectors to the skin via topical application or intravenous/intradermal injection is an attractive strategy for treating genodermatoses [
13,
14]. Several local gene therapies have been developed using modified herpes simplex virus 1 (HSV-1) vector carrying wild-type genes, such as
COL7A1 for recessive dystrophic epidermolysis bullosa or
TGM1 for autosomal recessive congenital ichthyosis [
15]. The results of a phase 1 gene therapy with local delivery were promising, although transgene expression in the skin was transient due to the non-integrating nature of HSV-1, necessitating repeated vector injections [
12].
In our study, we performed a comprehensive evaluation of the functionality and safety of recombinant AAV2-TGM1. Initially, we developed and characterized the plasmid vector pAAV-TGM1 and demonstrated its functionality in human cells. Transfection of various cell lines with pAAV-TGM1 resulted in successful upregulation of TGM1 mRNA and protein expression and increased enzymatic activity. However, a 67% higher level of enzymatic activity was observed in HEK293 cells compared to HaCaT keratinocytes, likely due to the lower transfection efficiency of the latter. Previous studies have reported that transduction of primary keratinocytes with AAV2 is less than 5% efficient. It has been shown that while primary keratinocytes express AAV2 internalization receptors αvβ5 and α5β1 integrins, they lack heparan sulfate proteoglycan (HSPG), which serves as the primary attachment receptor [
16].
After confirming the functionality of pAAV-TGM1, recombinant AAV2-TGM1 was produced based on this plasmid, and its functionality and safety were analyzed both in vitro and in both small and large animal models.
We evaluated the functionality of AAV2-TGM1 in vitro by immunofluorescence analysis of TGM1 expression in epidermal and control cell lines. Endogenous TGM1 expression was observed in HaCaT cells, consistent with data from the Protein Atlas (
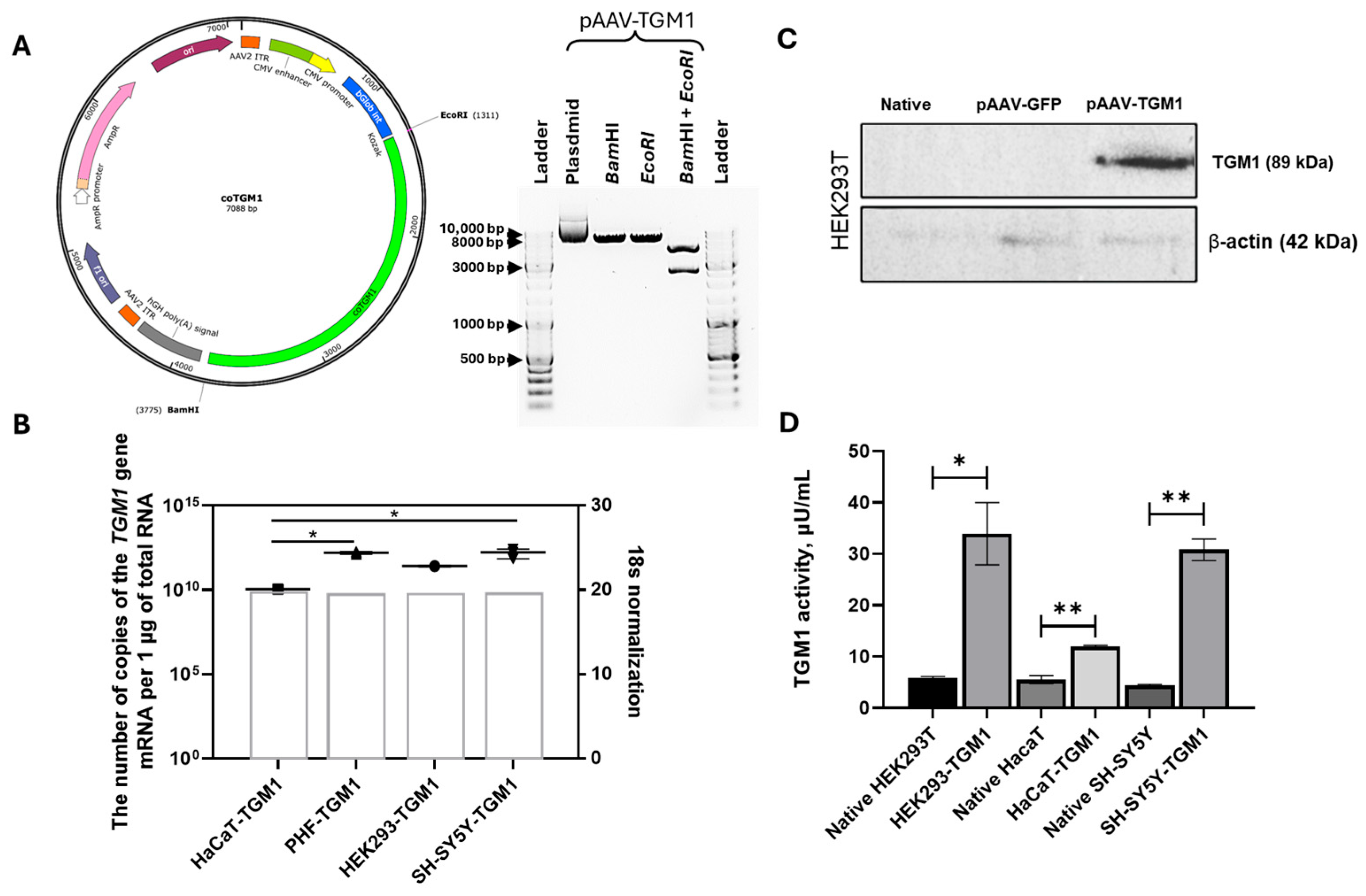
https://www.proteinatlas.org) (accessed on 12 October 2025). Western blot analysis confirmed TGM1 expression in AAV2-TGM1–modified HEK293 cells, which was also confirmed at the mRNA level by RT-PCR. The use of antibodies allowed to estimate the protein level only relative to the control group, taking into account the level of endogenous expression. In contrast, the use of primers specific to the codon-optimized sequence enabled the specific detection and quantification of recombinant mRNA.
In HEK293 cells after transfection with pAAV-TGM1 plasmid, TGM1 enzymatic activity increased 5.7-fold, and in HaCaT cells, it increased 2.15-fold compared to native cells. Similar results were obtained following viral transduction: TGM1 activity increased 16.3-fold in HEK293 cells and 2.19-fold in HaCaT cells compared to the control. Importantly, the increase in enzyme activity was accompanied by an increase in TGM1 mRNA and protein expression, as confirmed by RT-PCR and Western blot analysis. These findings suggest that even a temporary restoration of TGM1 enzymatic activity could significantly improve skin barrier function, which is supported by replacement therapy studies in animal models of ichthyosis [
17,
18].
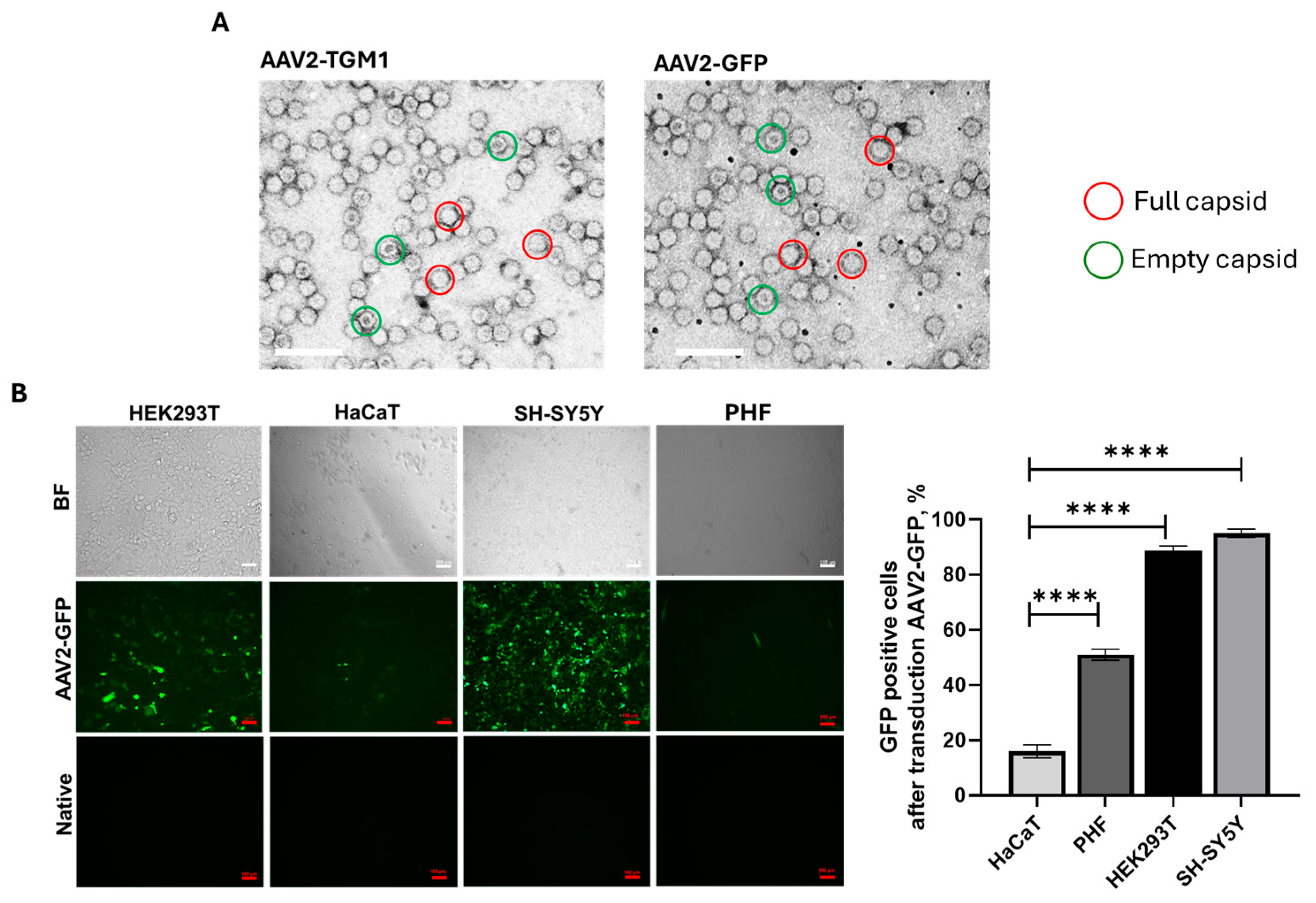
The proportion of full capsids was approximately 80% in both AAV2-TGM1 and AAV2-GFP samples, and the average size of the viral particles was 21.5 nm. Analysis of transduction efficiency in the tested cell lines (HEK293, HaCaT, PHF, and SH-SY5Y) revealed a low transduction level in HaCaT cells, which was confirmed both visually and by flow cytometry. This is likely attributable to the dense adhesion and differentiated state of these keratinocytes, accompanied by keratinization-related thickening of the cellular envelope.
Recombinant AAVs are the leading vectors for a wide range of gene therapy applications in humans due to their favorable safety profile in clinical trials, such as their ability to transduce both dividing and non-dividing cells, low cytotoxicity, and capacity for long-term transgene expression [
19,
20]. Multiple AAV serotypes from humans and non-human primates have been identified, each with distinct tissue tropism and transduction efficiency profiles. AAVs can efficiently transduce many tissues, including the liver, lung, eye, and skeletal muscle, as their tissue specificity depends on both capsid properties and the route of administration [
21].
It was shown that transduction with both AAV2-TGM1 and AAV2-GFP reduced the viability of HEK293 cells by 7–8% by 7 days. The effects of transfection with pAAV-TGM1 and transduction with AAV2-TGM1 on the cytokine profile of HaCaT and PHF cells were also evaluated. The plasmid vector pAAV-TGM1 showed moderate effects on pro-inflammatory (IL-1α, MCP-1) and regenerative markers (bFGF, HGF), whereas AAV2-TGM1 resulted in a stronger induction of these cytokines, especially in PHFs, suggesting a potential for more pronounced inflammation. The absence of changes in key markers (IP10, CTACK) associated with antiviral immunity and T-lymphocyte migration into the skin is a favorable factor for the therapeutic use of the vector in gene therapy [
22,
23]. Definitely, additional larger-scale studies are necessary for more specific conclusions.
Another research group has also explored the potential of gene therapy for TGM1 deficiency by developing a vector designed to deliver a functional human
TGM1 gene directly into the skin [
15]. This vector is based on HSV-1, which has a natural tropism for the epidermis and can more efficiently penetrate skin cells than other viral vectors. The vector was analyzed in vitro using patient-derived keratinocytes and in vivo in mice using a skin permeabilization model involving tape stripping or acetone treatment [
24]. Administration of HSV-1-TGM1 at a dose of 1.07 × 10
9 plaque-forming units (PFUs) was shown increase
TGM1 expression in mouse skin without adverse effects [
15].
Although AAV does not have natural mechanisms for penetrating the stratum corneum, it can still transduce skin cells. Physical damage or inflammation can compromise this barrier, facilitating access to keratinocytes in the basal layer and dermal fibroblasts [
25]. Furthermore, certain serotypes and engineered capsids (e.g., AAV5, AAV8, AAV-DJ) demonstrate enhanced transduction efficiency in skin cells [
16], and high local concentrations achieved through intradermal injection or scarification can further enhance this effect.
The homozygous deletion of the
TGM1 gene in mice results in neonatal lethality [
26], making a complete knockout model unsuitable for preclinical studies. We have performed functional studies and evaluated the acute toxicity of AAV2-TGM1 in small (rat) and large (pig) animal models. Chronic toxicity was not assessed due to the development of an immune response against the vector upon repeated administration. Neutralizing antibodies to the capsid and viral genome resulting from adaptive humoral immunity significantly reduce the efficacy of the viral vector without the use of immunosuppression [
27].
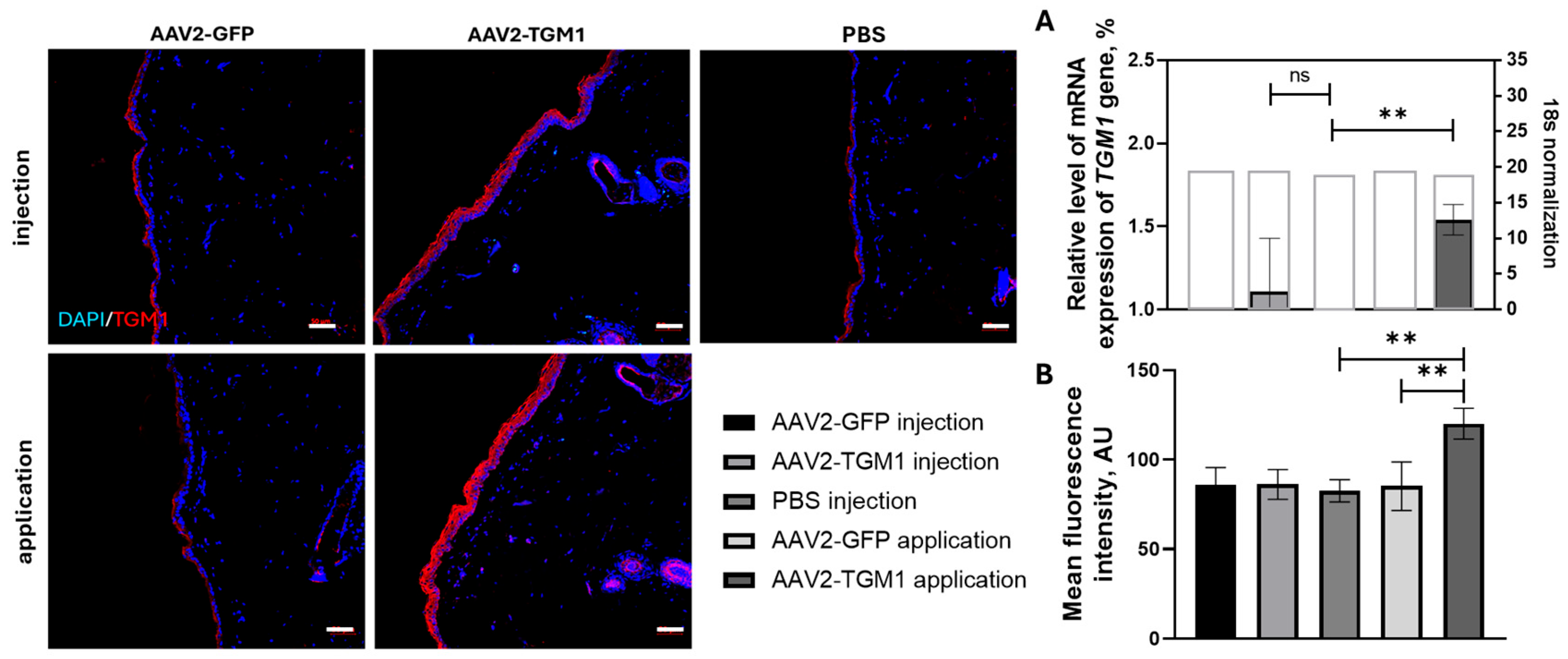
TGM1 gene expression in the skin was confirmed in both rats and pigs, indicating the functionality of recombinant AAV2-TGM1 in vivo. Following topical application in rats, a 1.41-fold increase in TGM1 fluorescence intensity was observed, while intradermal injection in pigs resulted in a 4.21-fold increase compared to controls. The observed
TGM1 expression levels were higher than those reported by Freedman et al. [
15], where local injection of HSV-1-TGM1 in mice caused a significant increase in protein levels in the absence of toxicity. However, this difference may be attributed to methodological variations and the use of different animal models (mice and pigs). In our study,
TGM1 expression in pigs decreased by 7 days, which is consistent with Jayarajan et al., who described transient expression upon non-integrating vector delivery to the skin [
12]. When evaluating the in vivo efficacy of the viral vector in this study, a number of limitations related to quantification have been identified. First, detection following both intradermal and topical application is challenging with standard histological methods. Second, there is cross-reactivity at the protein level with endogenous TGM1. Third, considerable intragroup variability was observed, likely due to factors such as skin heterogeneity, thickness, host immune responses, and even animal sex [
28].
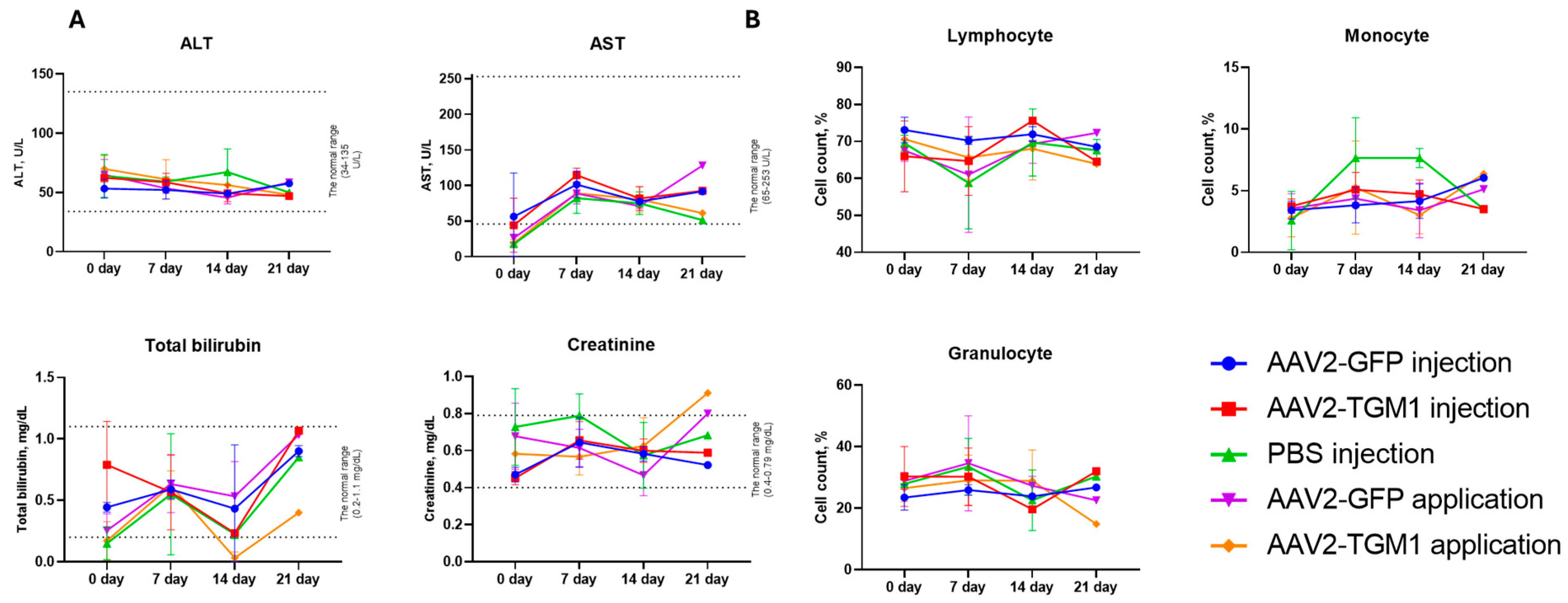
Biochemical blood analysis showed a moderate and reversible increase in bilirubin and creatinine in rats, mainly on day 21, which may indicate a transient burden on the liver and kidneys. Similar changes were observed in pigs on day 7, but the values returned to baseline by day 28. These fluctuations fall within the range of expected physiological variation, consistent with observations from other preclinical gene therapy studies [
29,
30]. Analysis of immune organ cellularity in rats showed a moderate decrease in thymic cell viability in the injection groups, aligning with reports of a localized stress response following AAV administration [
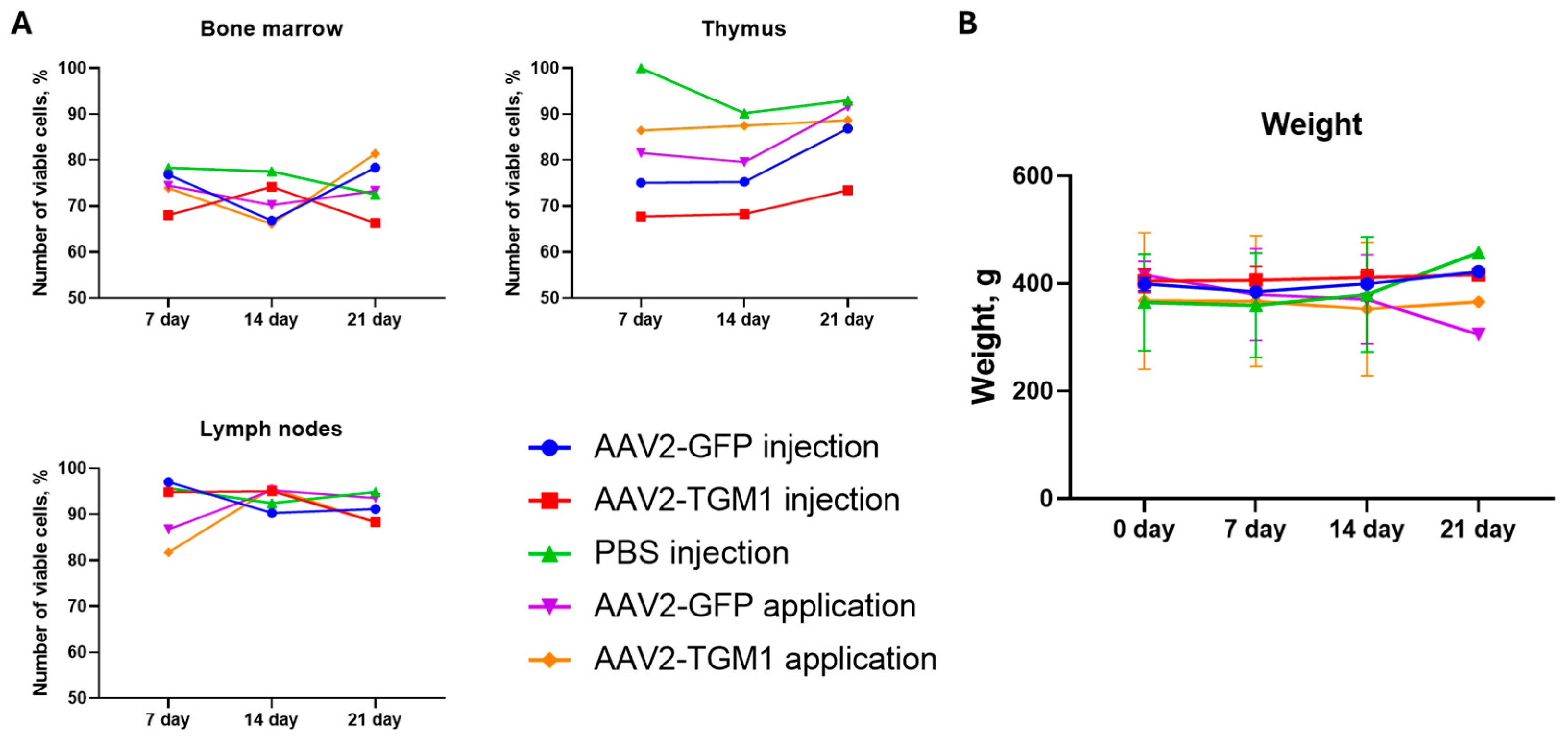
31]. The absence of significant changes in bone marrow and lymph nodes, however, suggests minimal systemic immunotoxicity.
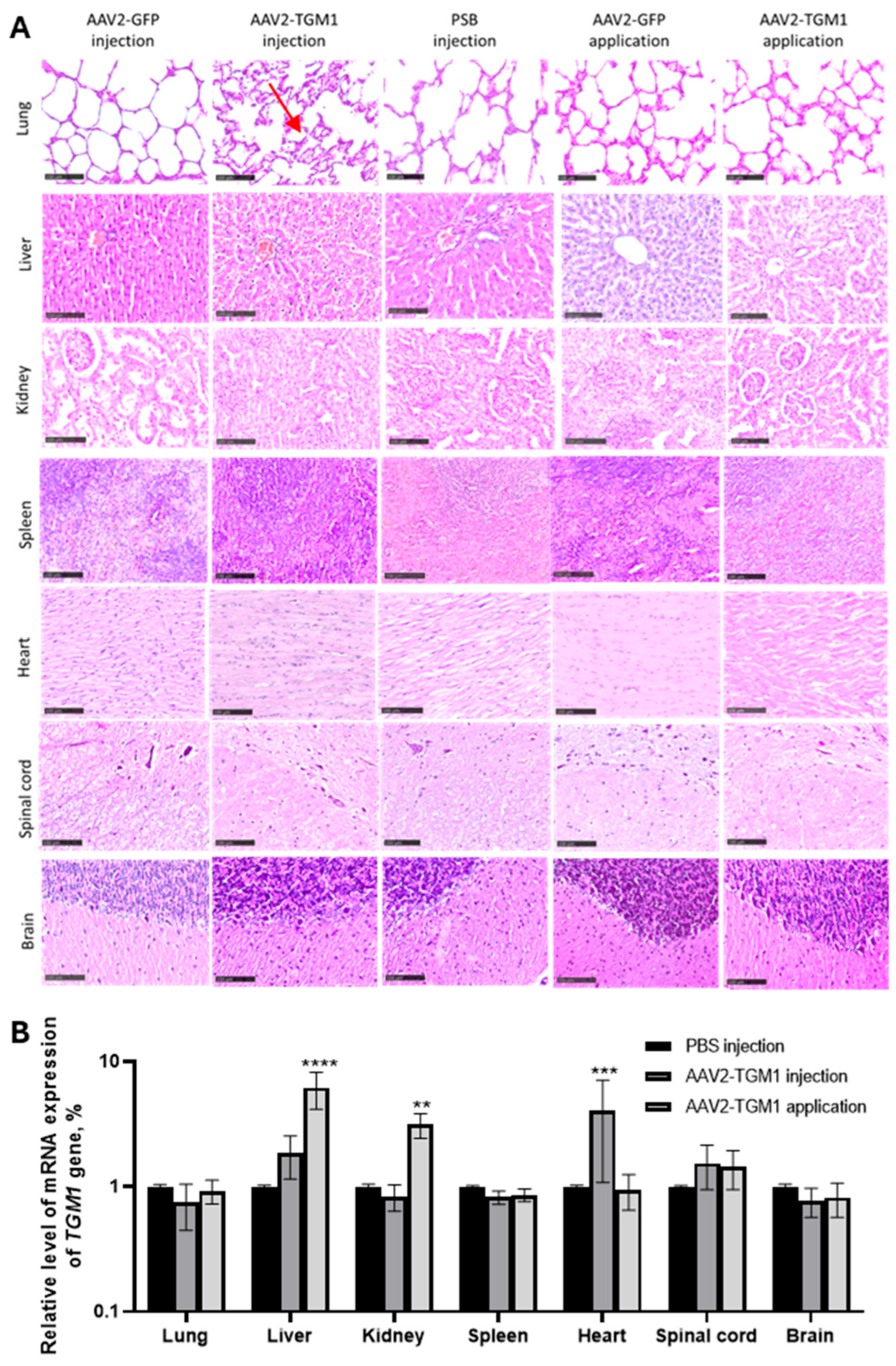
The literature describes isolated cases of cutaneous changes following AAV vector administration, including hyperkeratosis and activation of proliferative processes during wound healing, as well as dose-dependent perivascular inflammation and cellular infiltration at high viral titers [
17]. However, other studies using comparable AAV doses have reported no significant pathological changes in skin architecture. Consistent with the latter findings, our investigation in both rat and porcine models revealed no histological abnormalities or evidence of inflammatory reactions compared to untreated controls. This finding is consistent with the results of Aufenvenne et al. [
17], where topical application of TGM1 using liposomes in humanized mouse model also did not induce structural abnormalities in the skin. The preservation of normal dermal and epidermal architecture, along with the absence of pathological neovascularization, demonstrates the safety of both topical application and intradermal injection routes [
17].
Histopathological evaluation of internal organs and the CNS in both rats and pigs showed no significant differences between experimental and control animals. The absence of degenerative changes, inflammation, or neurotoxicity is consistent with observations indicating a favorable safety profile of AAV vectors. The findings add to the existing literature, confirming the promise of AAV2-TGM1 as a platform for gene therapy of cutaneous monogenic diseases with low risk of systemic toxicity, at appropriate doses [
32].
In conclusion, AAV2-TGM1 represents a promising candidate for restoring skin barrier function in lamellar ichthyosis. To fully realize its therapeutic potential, delivery to target cells needs to be optimized, for example, through the use of tissue-specific promoters or capsid engineering. Improving transduction efficiency would enable a lower multiplicity of infection (MOI), potentially reducing the cytotoxicity and immunogenicity. While the vector successfully upregulated TGM1 expression in vivo and was associated with only minor, reversible changes in blood biochemistry, further investigations are needed to refine the methods for assessing its efficacy accurately.
Due to the fact that wild-type animals were used in the study, an objective assessment of the therapeutic potential of the proposed gene strategy requires experiments on model animal systems that mimic autosomal recessive congenital ichthyosis. It is important to take into account that the use of AAVs excludes the probability of integration of their genetic material into the host genome of target cells. To ensure long-term stability of gene expression in regenerating epidermis, it is necessary to consider the use of alternative vector systems. Such systems, such as integrating vectors, have the ability to selectively target stem cells in the basal layer of the epidermis. Lentiviruses, non-integrating HSV serotypes, and other approaches could be explored as potential candidates.
4. Materials and Methods
4.1. TGM1 Gene Construct Design and Codon Optimization
The OptimumGene algorithm was used to optimize the codon composition of the nucleotide sequence of the CDS mRNA of the TGM1 gene, taking into account factors that could potentially affect gene expression levels. These factors included codon shift, GC composition, CpG dinucleotide content, mRNA secondary structure, tandem repeats, restriction sites interfering with cloning, premature polyadenylation sites, and additional minor ribosome binding sites. The nucleotide sequence of the CDS mRNA of the human TGM1 gene (GeneBank #NM_000359.3, 2454 bp) was used as a matrix for codon optimization (coTGM1). Codon optimization, de novo synthesis of the nucleotide sequence of the CDS mRNA of the TGM1 gene, and its cloning into the pAAV-MCS plasmid vector were performed by GenScript (Piscataway, NJ, USA). The correct assembly of the genetic construct was verified by restriction analysis and sequencing. After codon optimization, the CDS mRNA of coTGM1 gene was cloned into pAAV-MCS plasmid vector (Agilent Technologies, Santa Clara, CA, USA) using EcoRI/BamHI restriction site recombinase. The final construct contained the following elements: a CMV promoter/enhancer, β-globin intron, optimized TGM1 sequence, and a human growth hormone (hGH) polyadenylation signal. The construct was flanked by AAV2 inverted terminal repeats (ITRs). The reporter construct was plasmid pAAV-GFP carrying the gene of green fluorescent protein GFP.
4.2. Restriction Analysis of Plasmid Constructs
The restriction digestion of pAAV-TGM1 and pAAV-GFP was performed in 10 μL of reaction mixture using FastDigest EcoRI (#FD0274, Thermo Fisher Scientific Inc., Waltham, MA, USA) and BamHI (#FD0054, Thermo Fisher Scientific Inc., USA) enzymes according to the methodology recommended by the manufacturer. pDNA was used in the amount 300 ng for the reaction. The reaction mixture was incubated in the thermostat for 15 min at 37 °C and analyzed by electrophoresis in 1% agarose gel stained with ethidium bromide.
4.3. Preparation of Recombinant AAV and Characterization of Viral Particles
4.3.1. Obtaining and Concentrating Viral Particles
AAV Helper free system (Agilent Technologies, USA) was used to obtain recombinant vectors. AAV293 cells were transfected using polyethylenimine (#408727, Sigma-Aldrich, St. Louis, MO, USA). AAV293 cells were cultured at 37 °C in a humidified atmosphere with 5% CO
2, at a cell monolayer density of less than 50%, in DMEM/F-12 complete medium (#C470p, PanEco, Moscow, Russia) to which were added 10% fetal bovine serum (#S181B, BioSera, Cholet, France) 2 mM L-glutamine (#F032, PanEco, Moscow, Russia) and 1× penicillin and streptomycin antibiotic mixture (#A065p, PanEco, Moscow, Russia) (referred to as complete DMEM/F-12 medium). Immediately before transfection, the monolayer density was 70–80%; for this purpose, 2.2 × 10
6 cells were spread on a 10 cm Petri dish the day before transfection. The next day, co-transfection with three plasmids (pAAV-TGM1, pAAV-RC9, and pHelper) was performed as previously described [
33]. Seventy-two hours after transfection, 0.5% (
v/
v) Triton X-100 was added to the cell suspension. The lysate was centrifuged in 250 mL beakers at 3000×
g, 10 min, 4 °C. Purification was performed by centrifugation in an iodixanol density gradient 350,000×
g, 1 h, +18 °C on a Beckman Optima-XPN ultracentrifuge. Then, fractions of 60% + ½ 40% were collected.
The sample was dialyzed in dialysis bags with a 100 kDa pore size (Spectrum, #0867140, Stamford, CT, USA) against 1× phosphate-salt buffer (PBS)/213 mM NaCl/0.001% Pluronic F-68 buffer at +4 °C and sterilized by filtration using syringe filters with a pore diameter of 0.22 μm. The virus titer was determined by real-time polymerase chain reaction (PCR-RT) as described further in
Section 4.5.2.
4.3.2. Transmission Electron Microscopy
AAV samples were diluted in formulating buffer (2.27× PBS, 350 mM NaCl, 11.35% sorbitol, 0.0023% Pluronic F-68) depending on the initial concentration (1:10 dilution was optimal for 1–5 × 1014, 1:10 dilution was optimal for 1013, 1:5 dilution was optimal for 1012, and no dilution was required for 1012; if the concentration was unknown, the assay was performed without dilution followed by visual assessment), and the 1:5 dilution was performed with a pipette by mixing 5 µL of sample with 20 µL of water in an Eppendorf tube and vortexing for 30 s, after which the solution was stored for 14 days at 2–8 °C. Next, a contrast solution was prepared: 0.1 g of uranyl acetate was dissolved in 9.9 mL of water, filtered through a 0.2 μm membrane, and stored at 4 °C for up to 1 year.
For sample preparation, carbon film copper grids were treated with air plasma (5 s in a PELCO easiGlow™ unit), then 10 µL of the sample was applied to Parafilm, incubated for 2 min, removed excess with filter paper, and washed with water. Contrasting was performed with 10 µL of 1% uranyl acetate (1 min incubation), washed with water, dried at room temperature, and placed in a storage box. The results were analyzed on an HT7700 transmission electron microscope (Hitachi, Tokyo, Japan) at 100 kV, analyzing the distribution of particles after preparation. The diameter of viral particles was measured using the ImageJ program (version 1.54g) (National Institutes of Health, Bethesda, MD, USA).
4.4. Transfection and Transduction of Cell Cultures
The cells used in this work are as described below. HEK293 (Human Embryonic Kidney 293 cells) (ATCC: CRL-11268) is an immortalized primary human embryonic kidney cell line containing modifications for efficient assembly of adeno-associated virus. HaCaT (RRID: CVCL_0038), an immortalized keratinocyte line derived from human skin epidermis, is the target cell in this study. Primary human fibroblasts (PHF) were isolated from human dermis using standard techniques. Human neuroblastoma SH-SY5Y cells (ATCC: CRL-2266) were used as a line with low endogenous TGM1 expression. Cells were cultured in complete DMEM/F-12 medium in an incubator at 37 °C in a humidified atmosphere containing 5% CO2.
For transfection of cells with pAAV-TGM1 and pAAV-GFP, we used branched polyethylenimine 25 kDa (PEI, #408727, Sigma-Aldrich, St. Louis, MO, USA), a solution of positively charged polymer that forms compact, stable, positively charged complexes with nucleic acid molecules.
Cells were transduced with AAV2-TGM1 and AAV2-GFP with a multiplicity of infection (MOI) of 1 million genomic copies of AAV per cell. For this purpose, cells were seeded on a 6-well culture plate at 50,000 cells per well in complete DMEM/F-12 medium. AAV2, protamine sulfate (to a final concentration of 10 μg/mL), and DMEM/F-12 without the addition of serum (1 mL) were mixed. After cell attachment, the culture medium was changed to the prepared mixture with AAV2. Cells were incubated with AAV2 for 24 h, after which the medium was changed to fresh complete DMEM/F-12 medium. Transgene expression analysis and collection of conditioned medium for cytokine profile analysis (
Section 4.7) were performed 48 h after transfection and 96 h after transduction.
Genetic modification of HEK293, HaCaT, HPF, and SH-SY5Y cells using AAV-GFP is necessary to control the quality of cell transduction since the resulting transgene has a detectable fluorescence. Initial visual assessment of the genetic modification of cells was performed using an Axio Observer.Z1 microscope and Axio Vision Rel. 4.8 software (CarlZeiss, Oberkochen, Germany).
Transgene expression was also analyzed on a FACS Aria III flow cytometer (BD Biosciences, Milpitas, CA, USA). For this purpose, genetically modified HEK293 cells were stripped by trypsinization, resuspended in 500 μL of phosphate-salt buffer, and analyzed in the spectrum of FITC fluorophore (excitation wavelength = 495 nm, emission wavelength = 525 nm).
4.5. Analysis of TGM1 Expression and Enzymatic Activity
4.5.1. Western Blot Analysis
Cells (1 × 106 cells) were lysed in RIPA buffer containing a mixture of Halt™ Protease and Phosphatase Inhibitor Cocktail (#78440, Thermo Fisher Scientific Inc., Waltham, MA, USA). The concentration of total protein in the extracted samples was determined using Pierce BCA Protein Assay Kit (#23225, Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the protocol recommended by the manufacturer. Results were measured at a wavelength of 562 nm on a Tecan infinite 200 pro instrument (Tecan Trading AG, Zurich, Switzerland).
The isolated proteins (in equal amounts) were separated by gradient step electrophoresis in 4–12% polyacrylamide gel with sodium dodecyl sulfate (SDS-PAGE) and transferred to 0.22 μm PVDF membrane (#WGPVDF22, Servicebio, Huangshi, China) using paper blotting filters (#G6007-50, Servicebio, Wuhan, China) and a semi-dry transfer system (BioRad Laboratories, Hercules, CA, USA).
Before antibody staining, membranes were blocked in EveryBlot Blocking Buffer (#12010020, BioRad, Hercules, CA, USA) for 5 min at room temperature. Membranes were stained for one hour at room temperature in PBS with Tween-20 (0.1%) (PBST), supplemented with 5% bovine serum albumin (BSA) (#9048-46-8, Acros Organics, Waltham, MA, USA), with primary polyclonal antibodies against TGM1 (1 μg/mL, #PAC256Hu01, Cloud-Clone, Katy, TX, USA). Membranes were then washed in PBST buffer and stained with horseradish peroxidase (HRP)-conjugated secondary antibodies (#170-5046, BioRad, Hercules, CA, USA) for 2 h at room temperature in PBST with BSA.
To normalize the amount of protein, membranes were stained with antibodies against β-actin conjugated to HRP (#A00730, GenScript, Piscataway, NJ, USA) for 2 h at room temperature. Proteins were visualized with Clarity™ Western ECL substrate (#1705061, BioRad Laboratories, Hercules, CA, USA) using a ChemiDoc XRS+ instrument (BioRad Laboratories, Hercules, CA, USA). Relative signal intensity levels were determined using Image LabTM, version 6.0.1 (Bio-Rad Laboratories, Hercules, CA, USA).
4.5.2. Real-Time Polymerase Chain Reaction
Total RNA (tRNA) was isolated from cells using TRIzol reagent (#15596026, Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Organs were homogenized in 500–1000 μL of TRIzol reagent and sodium acetate buffer with the addition of glass beads in a FastPrep-24 homogenizer (MP Biomedicals, Irvine, CA, USA). The homogenizer was exposed for 20 s at a frequency of 5 Hz. After that, the homogenate was centrifuged for 5 min at 9500×
g, and the supernatant was used for RNA isolation and determination of enzymatic activity according to the instructions recommended by the manufacturer (
Section 4.5.4).
The concentration of total RNA samples was measured using a DS-11 FX+ spectrophotometer (DeNovix, Wilmington, DE, USA). cDNA synthesis was performed using the MMLV RT kit (#SK021, Eurogen, Moscow, Russia) according to the manufacturer’s instructions. PCR-RT was performed using specific primers and a probe to the nucleotide sequence of the TGM1 gene; primers and a fluorescent probe specific to the inverted terminal repeat (ITR) sequence of the AAV were used to estimate the virus titer. The primer sequences for TGM1 and ITR were designed using GenScript Online RT-PCR (TaqMan) Primer Design Tool software (GenScript, Piscataway, NJ, USA) and synthesized by Eurogen (Russia) (
Table 1).
PCR amplification was performed on a CFX96 Touch™ RT-PCR Detection System (Bio-Rad, Hercules, CA, USA) with the following cycling mode: 95 °C—5 min, 35 cycles of denaturation at 95 °C—10 s, annealing at 57 °C for coTGM1 sequence—30 s, elongation at 72 °C—30 s, 35 cycles. For the 18S sequence, preheating at 95 °C—5 min, 35 cycles of denaturation at 95 °C—10 s, annealing at 50 °C—30 s, elongation at 72 °C—30 s 35 cycles. For ITR sequence—preheating at 95 °C—15 min, 39 cycles of denaturation at 95 °C—30 s, annealing at 61 °C.
The mRNA levels of the tested genes were normalized relative to the mRNA level of 18S ribosomal RNA. The relative level of mRNA transcription was calculated by comparative quantification of the threshold cycle value (ΔΔCT). For the in vitro samples, the absolute mRNA copy numbers were additionally normalized using the 18S normalization coefficient.
4.5.3. Immunocytochemical Analysis
Cells were cultured in a 24-well plate on glass. The next day after seeding, transfection or transduction was performed. The cells were further cultured for another 4–6 days. The medium was removed, and the cells were fixed with 10% formalin (300 µL per well) for 25 min. The cells were washed three times with DPBS for 5 min. For intracellular staining, 300 μL per 1 well of 0.1% Triton X-100 was added. Incubation was performed for 20 min. Primary polyclonal antibody against TGM1 (#PAC256Hu01, Cloud-Clone, Houston, TX, USA) was diluted 0.5 μL per 500 μL PBS and incubated for 1.5 hr. Sample washed three times with DPBS for 5 min. Diluted secondary antibodies conjugated to Alexa Fluor™ 555 (#A-214281, Invitrogen, Carlsbad, CA, USA) 1 μL per 500 μL PBS and incubated for 1 h in the dark. Washed three times with DPBS for 5 min. The samples were encapsulated in Mounting Medium and visualized on an LSM 700 confocal microscope (Carl Zeiss, Oberkochen, Germany) using Zen black 2012 software (Carl Zeiss, Oberkochen, Germany). All samples were visualized using the same confocal settings (laser intensity, gain and offset). Semi-quantitative assessment of the arithmetic mean fluorescence intensity was performed as described further in
Section 4.8.4).
4.5.4. Evaluation of TGM1 Enzymatic Activity
TGM1 enzymatic activity was determined in lysates collected from native and genetically modified HEK293, HaCaT, and SH-SY5Y cells. Total protein concentration was determined using the Pierce BCA Protein Assay Kit (#23225, Thermo Fisher Scientific Inc., Waltham, MA, USA). Samples were normalized according to the concentration of total protein in the sample with the lowest concentration.
To determine the enzymatic activity of TGM1, a commercial TG1–CovTest kit (#opr0038, Covalab, France) was used according to the manufacturer’s instructions. Optical density was measured at 450 nm on a Tecan infinite 200 pro instrument (Tecan Trading AG, Zurich, Switzerland). To quantify TGM1 activity, a calibration curve was constructed using recombinant human TGM1 enzyme (50 μU/mL corresponded to an OD450 of 1.4 ± 0.06). All samples were analyzed in duplicate, monitoring nonspecific binding by negative control with ethylenediaminetetraacetic acid (EDTA).
4.6. Cell Viability Assessment
The viability of HEK293 cells at 3 and 7 days after AAV2-GFP viral transduction was determined using Alexa Fluor®488 Annexin V/Dead Cell Apoptosis Kit (#V13241, Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the manufacturer’s instructions on a FACSAria instrument (Bioscience, Conshohocken, PA, USA) using BD FACSDivaTM software version 7.0.
4.7. Analysis of Cytokine Profile of Conditioned Cell Culture Medium
The Bio-Plex Pro Human Cytokine Screening Panel, 48-Plex kit (#12007283, Bio-Rad, USA) was used to evaluate the cytokine profile of conditioned medium of HaCaT and PHF cells collected 48 h after transfection and 96 h after transduction. The assay was performed according to the manufacturer’s instructions. The obtained data were analyzed using the MAGPIX instrument (Luminex, Austin, TX, USA) and xPONENT software (version 4.2.1324.0) (Luminex, Austin, TX, USA).
4.8. Working with Laboratory Animals
4.8.1. Laboratory Animals, Groups, and Content
Ninety 12-week-old female and male Wistar line rats (weighing 230–250 g) were used in the study. The animals were randomly divided into three groups and three time points: control (5 animals per time point injected with 500 μL PBS), reference (5 animals per time point injected with AAV2-GFP), and experimental (5 animals per time point injected with AAV2-TGM1) at 7, 14, 21, days, respectively. Animals were kept in clear plastic cages, with a light:dark cycle of 12:12 h, at a temperature of 21–25 °C, and relative humidity of 40% to 70%, with unrestricted access to food and water. Scarification was performed using a scalpel; shallow wounds of 0.5 × 0.5 cm were made on the back of the rats. All experiments were conducted in accordance with ethical standards and current legislation, and the protocol was approved by the Animal Ethics Committee of Kazan Federal University (№ 50, approved 26 September 2024).
Recombinant AAV2-TGM1 was injected intradermally and applicatively at a concentration of 1 × 1010 per 1 kg in 300 μL PBS. Animals in the control group were injected with 100 μL of PBS. For application, the excipient gel was mixed with the viral vector in PBS at a ratio of 1.5:1. Before injection, whole blood samples were collected from rats in tubes containing the anticoagulant sodium citrate.
Laboratory pigs were also used to evaluate the functionality and safety of AAV2-TGM1. Animals were divided into 2 groups: control (5 individuals injected with PBS) and experimental (5 individuals injected with AAV2-TGM1). One week before sampling, pigs were injected intradermally with AAV2-TGM1 into a pre-prepared skin area, approximately 1.5 × 1.5 cm in size. The efficacy of the construct was assessed on days 1, 3, and 7. The obtained skin samples were further used to analyze TGM1 gene expression and secretion by PCR-RT (
Section 4.5.2) and immunofluorescence analysis (
Section 4.8.4). The scheme of experiments is presented in
Appendix A.
4.8.2. Determination of Leukocyte Formula and Biochemical Parameters of Blood
Blood was collected from rats and pigs to assess biochemical parameters, and leukocyte formula was additionally analyzed in rats. Blood was collected from rats at 7, 14, and 21 days after AAV2-TGM1 and AAV2-GFP administration. For evaluation of leukocyte formula and biochemical parameters, blood was collected from the tail vein in a volume of 1 mL. For leukocyte formula analysis, blood was collected in 1.5 mL microtubes with anticoagulant added, and whole blood was used. Lymphocytes, monocytes, and granulocytes were counted using an Abacus Junior Vet 5 hematology analyzer (Diatron, Budapest, Hungary). For biochemical analysis, blood was collected in tubes without anticoagulant, incubated for 40 min at room temperature, and then centrifuged at 4 °C, 2000× g for 20 min. The obtained serum was used to determine the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, and creatinine on a ChemWell 2900 biochemical analyzer (Awareness Technology, Palm City, FL, USA).
Blood was collected from the ear vein of pigs on days 7, 14, and 21 after AAV2-TGM1 injection. Blood in a volume of 1 mL was collected into tubes without anticoagulant, incubated at room temperature for 40 min, and then centrifuged at 4 °C, 2000× g for 20 min. Levels of AST, ALT, total bilirubin, and creatinine in serum were determined using a ChemWell 2900 biochemical analyzer (Awareness Technology, Palm City, FL, USA).
4.8.3. Determination of Immune System Organ Cellularity in Rats
Determination of immune system organ cellularity was performed to investigate the immunotoxicity of AAV2-TGM1. For this purpose, at 7, 14, and 21 days after administration of AAV2-TGM1, AAV2-GFP, or PBS, rats were subjected to CO2 euthanasia; the thymus, lymph nodes, and tubular bones were extracted. Lymphoid organs were homogenized and resuspended in cooled Hanks’ solution (pH = 7.2–7.4) (#P020p, PanEco, Moscow, Russia) in a volume of 5 mL for thymus and in a volume of 1 mL for hamstring lymph nodes and bone marrow. The resulting suspension was filtered through two layers of capron and washed twice by centrifugation at 200× g for 5 min. Then, 10 μL of the suspension was added to 0.4 mL of 3% acetic acid solution with trypan blue; the concentration of nucleated cells in the organ was counted, and the ratio of living and dead cells was determined using Goryaev’s chamber.
4.8.4. Immunofluorescence
Transversal paraffin skin sections were used to analyze TGM1 secretion in tissues. Sections were deparaffinized with solutions of xylene, alcohol, and distilled water and stained first with primary polyclonal antibodies against TGM1 (#PAC256Hu01, Cloud-Clone, USA) and then with secondary antibodies conjugated to Alexa Fluor™ 647 (#A-21244, Abcam, Waltham, MA, USA). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (#D8417, 10 μg/mL, Sigma-Aldrich, St. Louis, MO, USA). Coverslips were mounted on slides using ImmunoHistoMount medium (#ab104131, Abcam, Waltham, MA, USA). Sections incubated with secondary antibodies only (without primary antibodies) were used as reaction controls. The slides were examined and photographed with a confocal scanning microscope LSM 700 (Carl Zeiss, Oberkochen, Germany) using Zen black 2012 software (Carl Zeiss, Oberkochen, Germany).
Five sections in rats or pigs in the area of injection/application were analyzed in intact control (n = 5) and experimental group (n = 5) rats. The mean of fluorescence intensity (MFI units, semiquantitative analysis) was measured on confocal images of this area. Five areas in each region were examined, and for each channel, the lowest intensity signals were removed to minimize background. For semiquantitative analysis, all slices were visualized using identical confocal settings (laser intensity, gain, offset).
4.8.5. Histopathological Analysis
On the 7th day after vector injection, experimental animals were euthanized. Fragments of the following organs were collected from each animal: skin, lungs, kidneys, heart, spleen, and liver. Each of the above fragments was placed in 10% formalin solution. After 48 h from the beginning of fixation, each fragment was embedded in paraffin; the obtained samples of internal organs were sliced on Minus S700A microtome (RWD, Thermo Fisher Scientific, Waltham, MA, USA) with a thickness of 5–7 μm, then the slices were horizontally dried on glass in a thermostat of 60 °C for 1 h, followed by a dewaxing step to remove paraffin before staining. The sections were stained with hematoxylin (BioVitrum, Saint Petersburg, Russia) and eosin (BioVitrum, Saint Petersburg, Russia) or Mallory’s trichrome (BioVitrum, Saint Petersburg, Russia). The stained sections were encapsulated in Consul-Mount mounting medium (#9990440, Thermo Fisher Scientific, Waltham, MA, USA) and examined using an APERIOCS2 light scanning microscope (Leica, Wetzlar, Germany).
Epidermal thickness was measured in control and experimental groups of animals using an electronic ruler in NPD.view software. For each sample, multiple measurements were performed in standardized areas of histological sections. The obtained data were used for statistical analysis of morphological changes in the epidermis.
4.9. Statistical Analysis
The data were analyzed using GraphPad Prism 8 software (GraphPad Software, Boston, MA, USA). The normality of data distribution was determined by the Anderson–Darling, D’Agostino–Pearson, Shapiro–Wilk, and Kolmogorov–Smirnov methods. Depending on the type of data distribution, analysis of variance (ANOVA) followed by Tukey’s post hoc test or the Kruskal–Wallis method was used for analyses with 3 or more comparison groups. Student’s t-test or the Mann–Whitney method was used for 2 comparison groups for analyses, respectively. Quantitative variables are presented through their mean and standard deviation. Statistically significant differences are labeled as *—p < 0.05, **—p < 0.01 and ***—p < 0.001, and ****—p < 0.0001.