Rhythmic Dynamics of Stress Granules in Wild-Type and Bmal1−/− Fibroblasts Lacking a Functional Canonical Circadian Clock
Abstract
1. Introduction
2. Results
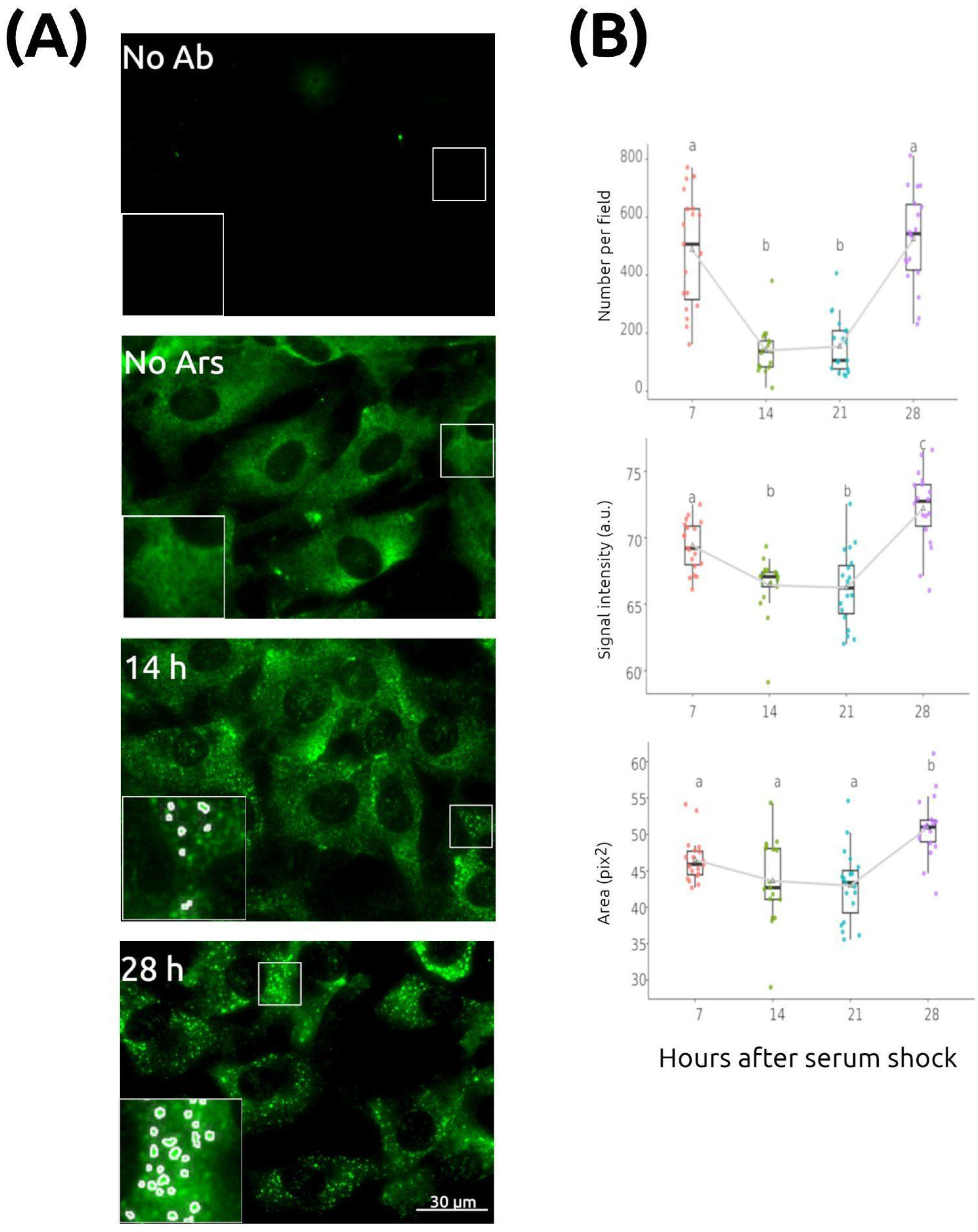
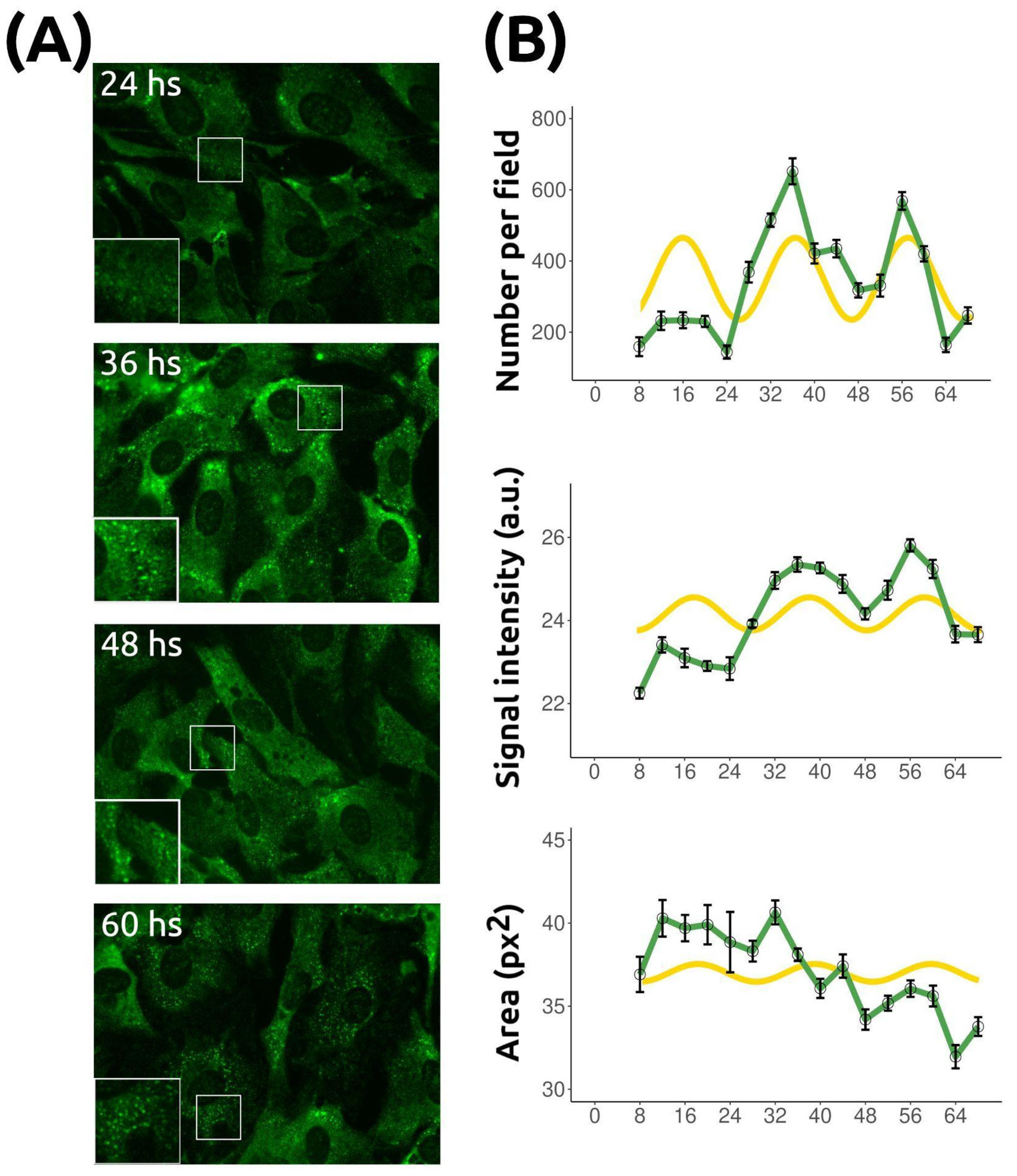
2.1. Stress Granule Number Varies over Time in Fibroblasts Synchronized by Serum Shock
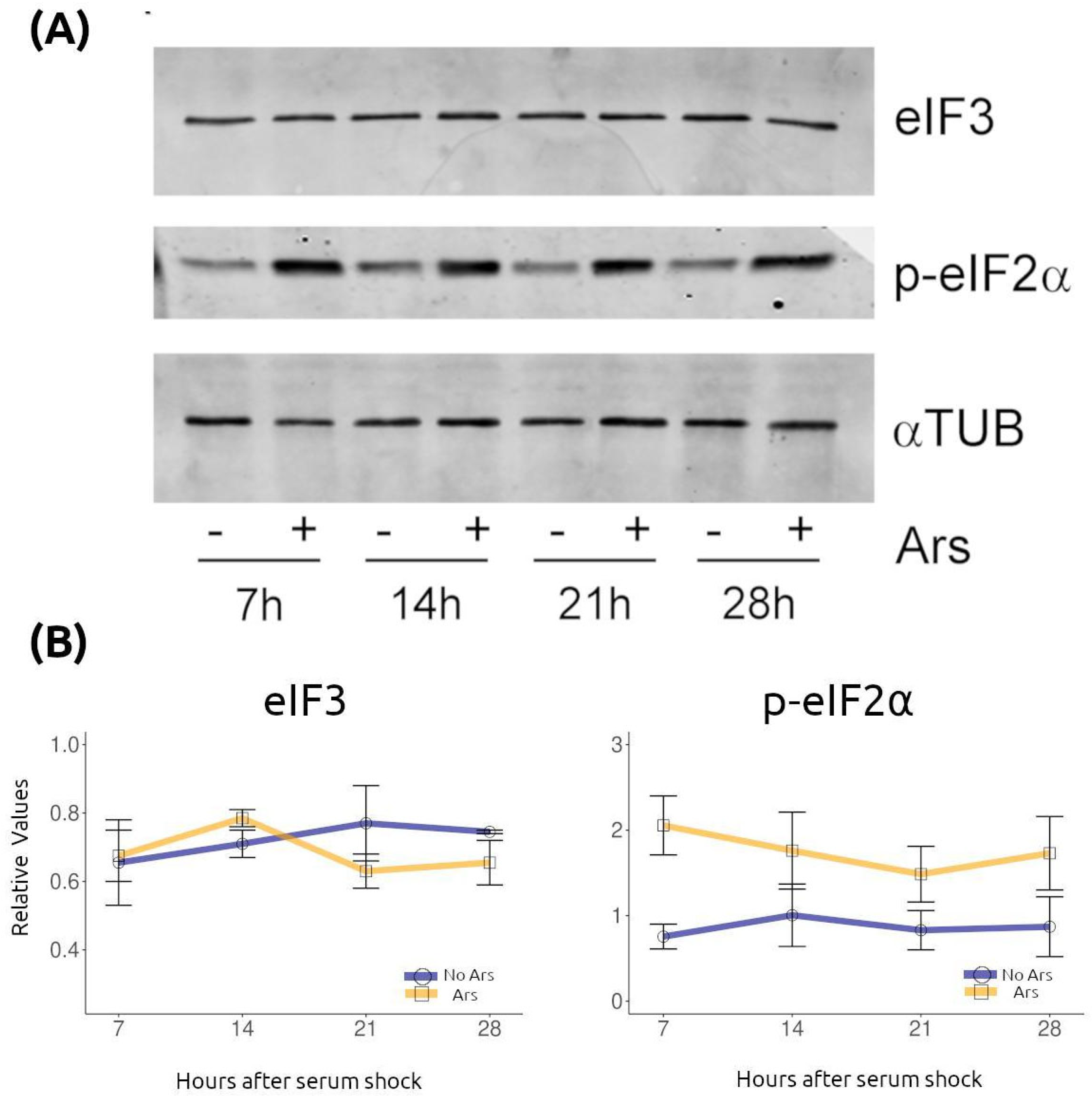
2.2. Temporal Changes in Stress Granules Are Not Attributable to eIF3 Expression or eIF2α Phosphorylation
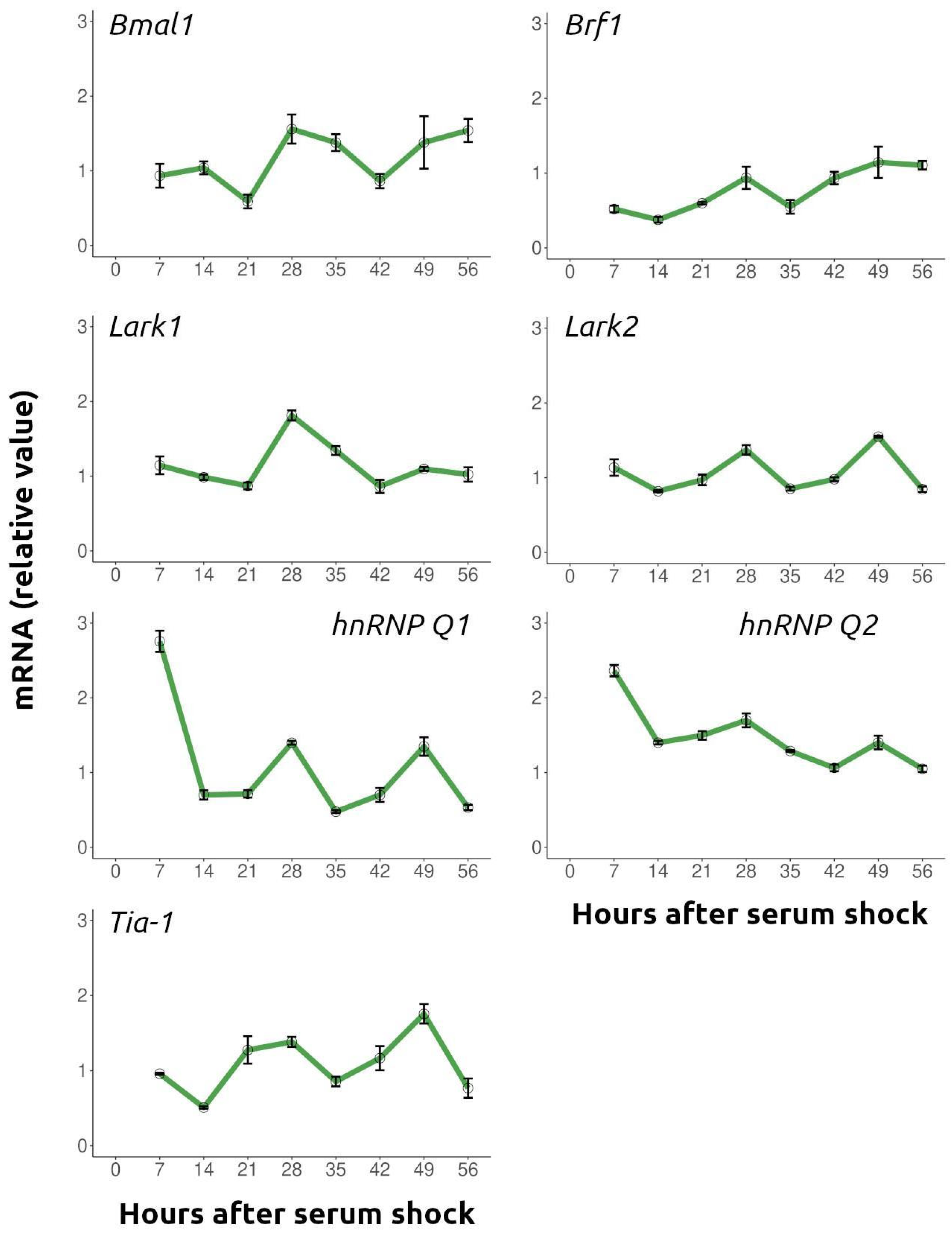
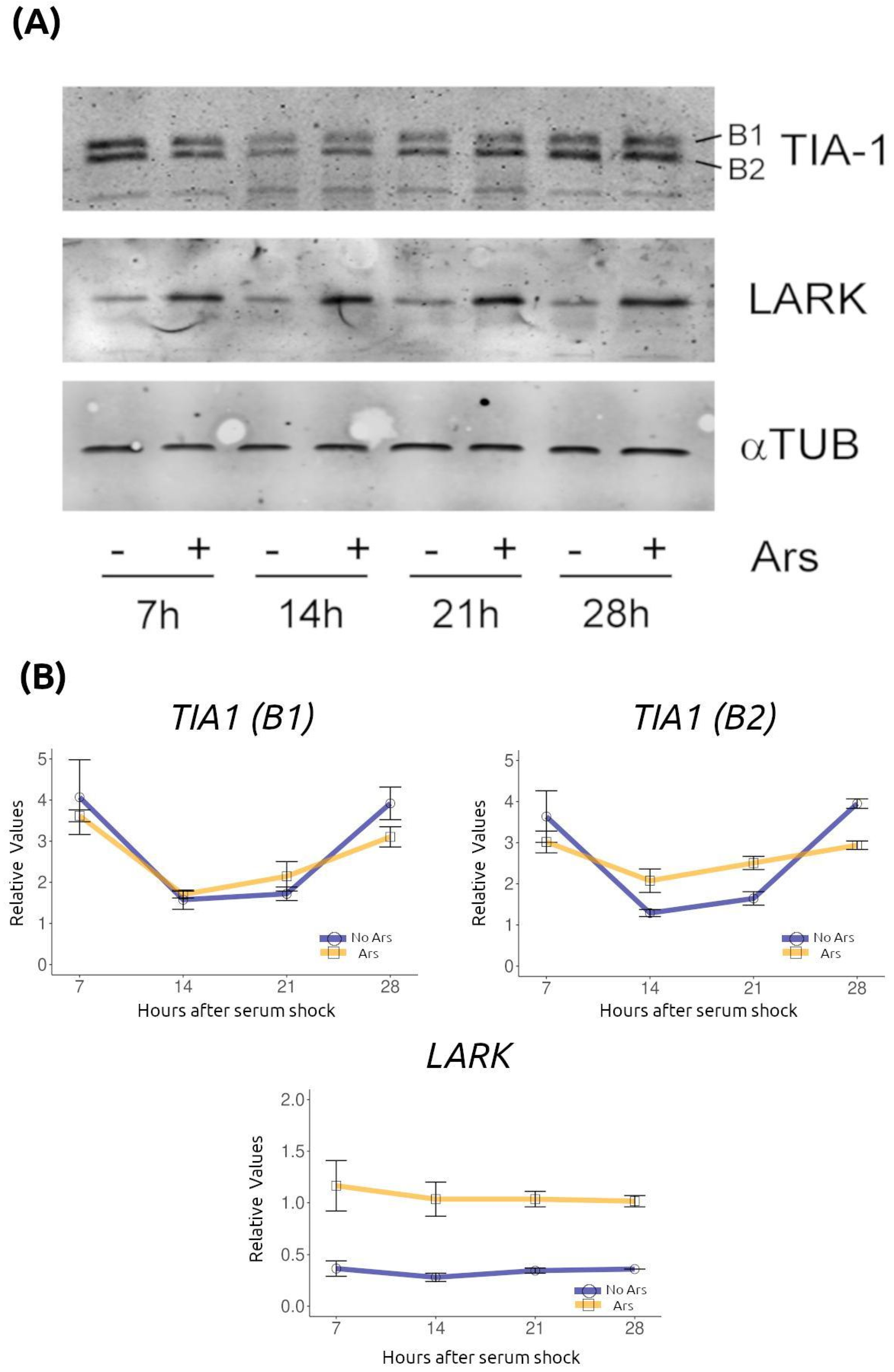
2.3. Several RNA-Binding Proteins That Are Components of RNA Granules Exhibit Temporal Regulation
2.4. Stress Granule Temporal Changes Are Rhythmic
2.5. Stress Granule Oscillations Persist in Cells with Impaired Canonical Molecular Circadian Clock
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Immunocytochemistry (ICC) and Image Acquisition
4.3. Stress Granule Quantification
- Process > “Smooth”;
- Process > “Subtract background” (rolling ball radius: 10 pixels);
- Plugins > Segmentation > “Otsu thresholding”;
- Analyze > “Analyze particles” (size: 17–1650 pixels2; circularity: 0.6–1.0).
4.4. Western Blotting
4.5. RNA Isolation, cDNA Synthesis and Real-Time Quantitative PCR
4.6. Bioluminescence
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SG | Stress granule |
| TTFL | Transcription–translation feedback loop |
| RBP | RNA-binding protein |
| PB | Processing body |
| p-eIF2α | phosphorylated eIF2α |
| ICC | Immunocytochemistry |
| SSA | Singular Spectrum Analysis |
| MEF | Mouse embryonic fibroblast |
| DMEM | Dulbecco’s Modified Eagle Medium |
| CS | Calf serum |
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
References
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of Circadian Rhythms in Health and Disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef]
- Bass, J.; Lazar, M.A. Circadian Time Signatures of Fitness and Disease. Science 2016, 354, 994–999. [Google Scholar] [CrossRef]
- Jabbur, M.L.; Dani, C.; Spoelstra, K.; Dodd, A.N.; Johnson, C.H. Evaluating the Adaptive Fitness of Circadian Clocks and Their Evolution. J. Biol. Rhythms 2024, 39, 115–134. [Google Scholar] [CrossRef]
- Pittendrigh, C.S. Temporal Organization: Reflections of a Darwinian Clock-Watcher. Annu. Rev. Physiol. 1993, 55, 17–54. [Google Scholar] [CrossRef]
- Putker, M.; O’Neill, J.S. Reciprocal Control of the Circadian Clock and Cellular Redox State—A Critical Appraisal. Mol. Cells 2016, 39, 6–19. [Google Scholar] [CrossRef]
- Oster, H. The Interplay between Stress, Circadian Clocks, and Energy Metabolism. J. Endocrinol. 2020, 247, R13–R25. [Google Scholar] [CrossRef]
- Levine, D.C.; Ramsey, K.M.; Bass, J. Circadian NAD(P)(H) Cycles in Cell Metabolism. Semin. Cell Dev. Biol. 2022, 126, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Protter, D.S.W.; Parker, R. Principles and Properties of Stress Granules. Trends Cell Biol. 2016, 26, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Riggs, C.L.; Kedersha, N.; Ivanov, P.; Anderson, P. Mammalian Stress Granules and P Bodies at a Glance. J. Cell Sci. 2020, 133, jcs242487. [Google Scholar] [CrossRef] [PubMed]
- Putnam, A.; Thomas, L.; Seydoux, G. RNA Granules: Functional Compartments or Incidental Condensates? Genes Dev. 2023, 37, 354–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jiang, X.; Bao, P.; Qin, M.; Xu, J. Circadian Control of Stress Granules by Oscillating EIF2α. Cell Death Dis. 2019, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Lahens, N.F.; Hogenesch, J.B.; Sehgal, A. Ribosome Profiling Reveals an Important Role for Translational Control in Circadian Gene Expression. Genome Res. 2015, 25, 1836–1847. [Google Scholar] [CrossRef]
- Malcolm, M.; Saad, L.; Penazzi, L.G.; Garbarino-Pico, E. Processing Bodies Oscillate in Neuro 2A Cells. Front. Cell. Neurosci. 2019, 13, 487. [Google Scholar] [CrossRef]
- Chawla, S.; O’Neill, J.; Knight, M.I.; He, Y.; Wang, L.; Maronde, E.; Rodríguez, S.G.; van Ooijen, G.; Garbarino-Pico, E.; Wolf, E.; et al. Timely Questions Emerging in Chronobiology: The Circadian Clock Keeps on Ticking. J. Circadian Rhythms 2024, 22, 2. [Google Scholar] [CrossRef]
- Balsalobre, A.; Damiola, F.; Schibler, U. A Serum Shock Induces Circadian Gene Expression in Mammalian Tissue Culture Cells. Cell 1998, 93, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, E.; Saini, C.; Bauer, C.; Laroche, T.; Naef, F.; Schibler, U. Circadian Gene Expression in Individual Fibroblasts: Cell-Autonomous and Self-Sustained Oscillators Pass Time to Daughter Cells. Cell 2004, 119, 693–705. [Google Scholar] [CrossRef]
- Marquez, S.; Crespo, P.; Carlini, V.; Garbarino-Pico, E.; Baler, R.; Caputto, B.L.; Guido, M.E. The Metabolism of Phospholipids Oscillates Rhythmically in Cultures of Fibroblasts and Is Regulated by the Clock Protein PERIOD 1. FASEB J. 2004, 18, 519–521. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, V.A.; Márquez, S.; Salvador, G.A.; Pasquaré, S.J.; Gorné, L.D.; Garbarino-Pico, E.; Giusto, N.M.; Guido, M.E. Daily Rhythms of Glycerophospholipid Synthesis in Fibroblast Cultures Involve Differential Enzyme Contributions. J. Lipid Res. 2013, 54, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Gilks, N.; Kedersha, N.; Ayodele, M.; Shen, L.; Stoecklin, G.; Dember, L.M.; Anderson, P. Stress Granule Assembly Is Mediated by Prion-like Aggregation of TIA-1. Mol. Biol. Cell 2004, 15, 5383–5398. [Google Scholar] [CrossRef]
- Kedersha, N.; Anderson, P. Mammalian Stress Granules and Processing Bodies. Methods Enzymol. 2007, 431, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 2016, 164, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.L.; Gupta, M.; Li, W.; Miller, I.; Anderson, P. RNA-Binding Proteins TIA-1 and TIAR Link the Phosphorylation of eIF-2 Alpha to the Assembly of Mammalian Stress Granules. J. Cell Biol. 1999, 147, 1431–1442. [Google Scholar] [CrossRef]
- Pizarro, A.; Hayer, K.; Lahens, N.F.; Hogenesch, J.B. CircaDB: A Database of Mammalian Circadian Gene Expression Profiles. Nucleic Acids Res. 2013, 41, D1009–D1013. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Anafi, R.C.; Hughes, M.E.; Kornacker, K.; Hogenesch, J.B. MetaCycle: An Integrated R Package to Evaluate Periodicity in Large Scale Data. Bioinformatics 2016, 32, 3351–3353. [Google Scholar] [CrossRef]
- Golyandina, N.; Korobeynikov, A.; Zhigljavsky, A. Singular Spectrum Analysis with R; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-662-57378-5. [Google Scholar]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 Is an Essential Component of the Master Circadian Pacemaker in Mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.-K.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2:LUCIFERASE Real-Time Reporting of Circadian Dynamics Reveals Persistent Circadian Oscillations in Mouse Peripheral Tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef]
- Kornmann, B.; Schaad, O.; Bujard, H.; Takahashi, J.S.; Schibler, U. System-Driven and Oscillator-Dependent Circadian Transcription in Mice with a Conditionally Active Liver Clock. PLoS Biol. 2007, 5, e34. [Google Scholar] [CrossRef]
- Koronowski, K.B.; Kinouchi, K.; Welz, P.-S.; Smith, J.G.; Zinna, V.M.; Shi, J.; Samad, M.; Chen, S.; Magnan, C.N.; Kinchen, J.M.; et al. Defining the Independence of the Liver Circadian Clock. Cell 2019, 177, 1448–1462.e14. [Google Scholar] [CrossRef]
- Pathak, S.S.; Liu, D.; Li, T.; de Zavalia, N.; Zhu, L.; Li, J.; Karthikeyan, R.; Alain, T.; Liu, A.C.; Storch, K.-F.; et al. The eIF2α Kinase GCN2 Modulates Period and Rhythmicity of the Circadian Clock by Translational Control of Atf4. Neuron 2019, 104, 724–735.e6. [Google Scholar] [CrossRef]
- Karki, S.; Castillo, K.; Ding, Z.; Kerr, O.; Lamb, T.M.; Wu, C.; Sachs, M.S.; Bell-Pedersen, D. Circadian Clock Control of eIF2α Phosphorylation Is Necessary for Rhythmic Translation Initiation. Proc. Natl. Acad. Sci. USA 2020, 117, 10935–10945. [Google Scholar] [CrossRef] [PubMed]
- Castillo, K.D.; Wu, C.; Ding, Z.; Lopez-Garcia, O.K.; Rowlinson, E.; Sachs, M.S.; Bell-Pedersen, D. A Circadian Clock Translational Control Mechanism Targets Specific mRNAs to Cytoplasmic Messenger Ribonucleoprotein Granules. Cell Rep. 2022, 41, 111879. [Google Scholar] [CrossRef]
- Preh, E.O.; Ramirez, M.A.; Mohan, S.; Guy, C.R.; Bell-Pedersen, D. Circadian Clock Control of Interactions between eIF2α Kinase CPC-3 and GCN1 with Ribosomes Regulates Rhythmic Translation Initiation. Proc. Natl. Acad. Sci. USA 2025, 122, e2411916122. [Google Scholar] [CrossRef]
- Izquierdo, J.M.; Valcárcel, J. Two Isoforms of the T-Cell Intracellular Antigen 1 (TIA-1) Splicing Factor Display Distinct Splicing Regulation Activities. Control of TIA-1 Isoform Ratio by TIA-1-Related Protein. J. Biol. Chem. 2007, 282, 19410–19417. [Google Scholar] [CrossRef] [PubMed]
- Stoecklin, G.; Colombi, M.; Raineri, I.; Leuenberger, S.; Mallaun, M.; Schmidlin, M.; Gross, B.; Lu, M.; Kitamura, T.; Moroni, C. Functional Cloning of BRF1, a Regulator of ARE-Dependent mRNA Turnover. EMBO J. 2002, 21, 4709–4718. [Google Scholar] [CrossRef] [PubMed]
- Franks, T.M.; Lykke-Andersen, J. TTP and BRF Proteins Nucleate Processing Body Formation to Silence mRNAs with AU-Rich Elements. Genes Dev. 2007, 21, 719–735. [Google Scholar] [CrossRef]
- Kedersha, N.; Stoecklin, G.; Ayodele, M.; Yacono, P.; Lykke-Andersen, J.; Fritzler, M.J.; Scheuner, D.; Kaufman, R.J.; Golan, D.E.; Anderson, P. Stress Granules and Processing Bodies Are Dynamically Linked Sites of mRNP Remodeling. J. Cell Biol. 2005, 169, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Woo, K.-C.; Lee, K.-H.; Kim, T.-D.; Kim, K.-T. HnRNP Q and PTB Modulate the Circadian Oscillation of Mouse Rev-Erb Alpha via IRES-Mediated Translation. Nucleic Acids Res. 2010, 38, 7068–7078. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Kwak, E.; Kim, S.-H.; Lee, K.-H.; Woo, K.-C.; Kim, K.-T. HnRNP Q Mediates a Phase-Dependent Translation-Coupled mRNA Decay of Mouse Period3. Nucleic Acids Res. 2011, 39, 8901–8914. [Google Scholar] [CrossRef]
- Lee, K.-H.; Woo, K.-C.; Kim, D.-Y.; Kim, T.-D.; Shin, J.; Park, S.M.; Jang, S.K.; Kim, K.-T. Rhythmic Interaction between Period1 mRNA and hnRNP Q Leads to Circadian Time-Dependent Translation. Mol. Cell. Biol. 2012, 32, 717–728. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kim, S.-H.; Kim, D.-Y.; Kim, S.; Kim, K.-T. Internal Ribosomal Entry Site-Mediated Translation Is Important for Rhythmic PERIOD1 Expression. PLoS ONE 2012, 7, e37936. [Google Scholar] [CrossRef][Green Version]
- Lim, I.; Jung, Y.; Kim, D.-Y.; Kim, K.-T. HnRNP Q Has a Suppressive Role in the Translation of Mouse Cryptochrome1. PLoS ONE 2016, 11, e0159018. [Google Scholar] [CrossRef]
- Jung, Y.; Ryu, H.G.; Kim, S.W.; Lee, K.-H.; Gu, S.; Yi, H.; Ku, H.-O.; Jang, S.K.; Kim, K.-T. The RNA-Binding Protein hnRNP Q Represses Translation of the Clock Gene Bmal1 in Murine Cells. J. Biol. Chem. 2019, 294, 7682–7691. [Google Scholar] [CrossRef]
- Kanai, Y.; Dohmae, N.; Hirokawa, N. Kinesin Transports RNA: Isolation and Characterization of an RNA-Transporting Granule. Neuron 2004, 43, 513–525. [Google Scholar] [CrossRef]
- Bannai, H.; Fukatsu, K.; Mizutani, A.; Natsume, T.; Iemura, S.-I.; Ikegami, T.; Inoue, T.; Mikoshiba, K. An RNA-Interacting Protein, SYNCRIP (Heterogeneous Nuclear Ribonuclear Protein Q1/NSAP1) Is a Component of mRNA Granule Transported with Inositol 1,4,5-Trisphosphate Receptor Type 1 mRNA in Neuronal Dendrites. J. Biol. Chem. 2004, 279, 53427–53434. [Google Scholar] [CrossRef]
- Quaresma, A.J.C.; Bressan, G.C.; Gava, L.M.; Lanza, D.C.F.; Ramos, C.H.I.; Kobarg, J. Human hnRNP Q Re-Localizes to Cytoplasmic Granules upon PMA, Thapsigargin, Arsenite and Heat-Shock Treatments. Exp. Cell Res. 2009, 315, 968–980. [Google Scholar] [CrossRef]
- Kojima, S.; Matsumoto, K.; Hirose, M.; Shimada, M.; Nagano, M.; Shigeyoshi, Y.; Hoshino, S.; Ui-Tei, K.; Saigo, K.; Green, C.B.; et al. LARK Activates Posttranscriptional Expression of an Essential Mammalian Clock Protein, PERIOD1. Proc. Natl. Acad. Sci. USA 2007, 104, 1859–1864. [Google Scholar] [CrossRef]
- Lin, J.-C.; Tarn, W.-Y. RNA-Binding Motif Protein 4 Translocates to Cytoplasmic Granules and Suppresses Translation via Argonaute2 during Muscle Cell Differentiation. J. Biol. Chem. 2009, 284, 34658–34665. [Google Scholar] [CrossRef]
- Milev, N.B.; Rhee, S.-G.; Reddy, A.B. Cellular Timekeeping: It’s Redox o’Clock. Cold Spring Harb. Perspect. Biol. 2018, 10, a027698. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.C.; O’Neill, J.S. Non-Transcriptional Processes in Circadian Rhythm Generation. Curr. Opin. Physiol. 2018, 5, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Lakin-Thomas, P. The Case for the Target of Rapamycin Pathway as a Candidate Circadian Oscillator. Int. J. Mol. Sci. 2023, 24, 13307. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Perfecting the Life Clock: The Journey from PTO to TTFL. Int. J. Mol. Sci. 2023, 24, 2402. [Google Scholar] [CrossRef]
- Lipton, J.O.; Yuan, E.D.; Boyle, L.M.; Ebrahimi-Fakhari, D.; Kwiatkowski, E.; Nathan, A.; Güttler, T.; Davis, F.; Asara, J.M.; Sahin, M. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell 2015, 161, 1138–1151. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.S.; Cox, J.; Mann, M. In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism. PLoS Genet. 2014, 10, e1004047. [Google Scholar] [CrossRef]
- Mauvoisin, D.; Wang, J.; Jouffe, C.; Martin, E.; Atger, F.; Waridel, P.; Quadroni, M.; Gachon, F.; Naef, F. Circadian Clock-Dependent and -Independent Rhythmic Proteomes Implement Distinct Diurnal Functions in Mouse Liver. Proc. Natl. Acad. Sci. USA 2014, 111, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Jouffe, C.; Cretenet, G.; Symul, L.; Martin, E.; Atger, F.; Naef, F.; Gachon, F. The Circadian Clock Coordinates Ribosome Biogenesis. PLOS Biol. 2013, 11, e1001455. [Google Scholar] [CrossRef]
- James, N.R.; O’Neill, J.S. Circadian Control of Protein Synthesis. BioEssays 2025, 47, e202300158. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.S.; Reddy, A.B. Circadian Clocks in Human Red Blood Cells. Nature 2011, 469, 498–503. [Google Scholar] [CrossRef]
- O’Neill, J.S.; van Ooijen, G.; Dixon, L.E.; Troein, C.; Corellou, F.; Bouget, F.-Y.; Reddy, A.B.; Millar, A.J. Circadian Rhythms Persist without Transcription in a Eukaryote. Nature 2011, 469, 554–558. [Google Scholar] [CrossRef]
- Garbarino-Pico, E.; Niu, S.; Rollag, M.D.; Strayer, C.A.; Besharse, J.C.; Green, C.B. Immediate Early Response of the Circadian polyA Ribonuclease Nocturnin to Two Extracellular Stimuli. RNA 2007, 13, 745–755. [Google Scholar] [CrossRef]
- Niu, S.; Shingle, D.L.; Garbarino-Pico, E.; Kojima, S.; Gilbert, M.; Green, C.B. The Circadian Deadenylase Nocturnin Is Necessary for Stabilization of the iNOS mRNA in Mice. PLoS ONE 2011, 6, e26954. [Google Scholar] [CrossRef]
- Nissan, T.; Parker, R. Analyzing P-Bodies in Saccharomyces Cerevisiae. Methods Enzymol. 2008, 448, 507–520. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakahata, Y.; Tanaka, M.; Yoshida, M.; Soma, H.; Shinohara, K.; Yasuda, A.; Mamine, T.; Takumi, T. Acute Physical Stress Elevates Mouse Period1 mRNA Expression in Mouse Peripheral Tissues via a Glucocorticoid-Responsive Element. J. Biol. Chem. 2005, 280, 42036–42043. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Akashi, M.; Trcka, D.; Yasuda, A.; Takumi, T. The in Vitro Real-Time Oscillation Monitoring System Identifies Potential Entrainment Factors for Circadian Clocks. BMC Mol. Biol. 2006, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Lin, R.; Ghosh, S.; Anderson, R.A.; Urban, J.F. Production and Characterization of ZFP36L1 Antiserum against Recombinant Protein from Escherichia Coli. Biotechnol. Prog. 2008, 24, 326–333. [Google Scholar] [CrossRef][Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Seed, B. A PCR Primer Bank for Quantitative Gene Expression Analysis. Nucleic Acids Res. 2003, 31, e154. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, RESEARCH0034-1. [Google Scholar] [CrossRef]
- Yang, R.; Su, Z. Analyzing Circadian Expression Data by Harmonic Regression Based on Autoregressive Spectral Estimation. Bioinformatics 2010, 26, i168–i174. [Google Scholar] [CrossRef]
- Hughes, M.E.; Hogenesch, J.B.; Kornacker, K. JTK_CYCLE: An Efficient Nonparametric Algorithm for Detecting Rhythmic Components in Genome-Scale Data Sets. J. Biol. Rhythms 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Glynn, E.F.; Chen, J.; Mushegian, A.R. Detecting Periodic Patterns in Unevenly Spaced Gene Expression Time Series Using Lomb-Scargle Periodograms. Bioinformatics 2006, 22, 310–316. [Google Scholar] [CrossRef]

| Cell Type | ANOVA | MetaCycle (meta2d) [24] | n 1 | |||||
|---|---|---|---|---|---|---|---|---|
| p | F | p | BH, Q | Period | Phase | Amp | ||
| 1. Stress granule number per field | ||||||||
| NIH/3T3 | <0.001 | 38.3 | <0.001 | <0.001 | 23.98 | 21.82 | 330.8 | 13 |
| wt | <0.001 | 14.3 | 3.5 × 10−14 | 3.5 × 10−14 | 34.48 | 0.67 | 83.91 | 55–64 |
| Bmal1−/− | <0.001 | 29.8 | 1.3 × 10−7 | 1.3 × 10−7 | 35.00 | 6.10 | 72.77 | 51–65 |
| 2. Stress granule signal intensity | ||||||||
| NIH/3T3 | <0.001 | 33.2 | <0.001 | <0.001 | 20.08 | 17.43 | 28.14 | 13 |
| wt | <0.001 | 48.4 | <0.001 | <0.001 | 35.00 | 34.84 | 1.69 | 55–64 |
| Bmal1−/− | <0.001 | 44.5 | <0.001 | <0.001 | 33.29 | 2.05 | 1.35 | 51–65 |
| 3. Stress granule area | ||||||||
| NIH/3T3 | <0.001 | 9.4 | 0.005 | 0.005 | 20.00 | 8.000 | 0.566 | 13 |
| wt | <0.001 | 22.5 | <0.001 | <0.001 | 35.00 | 32.83 | 17.06 | 55–64 |
| Bmal1−/− | <0.001 | 28.6 | 1.1 × 10−12 | 1.1 × 10−12 | 27.33 | 6.60 | 9.71 | 51–65 |
| 4. Bioluminescence | ||||||||
| wt | <0.001 | 8.80 | <0.001 | <0.001 | 28.13 | 4.21 | 32,207 | 3 |
| Bmal1−/− | 0.188 | 1.69 | 0.516 | 0.516 | - | - | - | 3 |
| Abbr. | Names | Accession Number | Forward Reverse | Ampl. Size |
|---|---|---|---|---|
| 18S rRNA | 18S ribosomal RNA | X00686 | CGCCGCTAGAGGTGAAATTC [60,63] CGAACCTCCGACTTTCGTTCT | 101 nt |
| B2m | β2-Microglobulin | NM_009735 | TTCTGGTGCTTGTCTCACTGA [60] CAGTATGTTCGGCTTCCCATTC | 104 nt |
| Bmal1 | Aryl hydrocarbon receptor nuclear translocator-like | NM_007489 | GCAGTGCCACTGACTACCAAGA [64] TCCTGGACATTGCATTGCAT | 201 nt |
| Brf1 | zinc finger protein 36, C3H type-like 1 (Zfp36l1) | NM_007564 | TGCGAACGCCCACGAT [65] CTTCGCTCAAGTCAAAAATGG | 60 nt |
| hnRNP Q1 | synaptotagmin binding, cytoplasmic RNA interacting protein (Syncrip) 1 | NM_019666 | GCCGCGGTGGAAATGTAGGAGG [66] TGATTATTGGTCTGGCGCCGCT | 82 nt |
| hnRNP Q2 | synaptotagmin binding, cytoplasmic RNA interacting protein (Syncrip), 2 | NM_019796 | CAACAACAAAGAGGCCGCGGG [66] ACCATTACTCCACTGCAAGCTTCTG | 116 nt |
| Lark1 | RNA binding motif protein 4 (Rbm4) | NM_009032 | GGTTACGGGCATGACAGTGAG [67] GGCCATGTCGTACAGGGAAT | 69 nt |
| Lark2 | RNA binding motif protein 4B (Rbm4b) | NM_025717 | CCAGTAGACCGTACAGGGC [67] GGACTCCCCATAACCCATAGT | 99 nt |
| Tbp | TATA box binding protein | NM_013684 | AGAACAATCCAGACTAGCAGCA [60] GGGAACTTCACATCACAGCTC | 120 nt |
| Tia-1 | cytotoxic granule-associated RNA binding protein 1 | NM_011585 | GGTGCCTCAAGGATTCCCTGTTGG [66] GCACGAGACTGATGCGGCGA | 149 nt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malcolm, M.; Pusterla, J.M.; Penazzi, L.G.; Trenchi, A.; Acosta-Rodríguez, V.A.; Ríos, M.N.; Villarreal, M.; Guido, M.E.; Garbarino-Pico, E. Rhythmic Dynamics of Stress Granules in Wild-Type and Bmal1−/− Fibroblasts Lacking a Functional Canonical Circadian Clock. Int. J. Mol. Sci. 2025, 26, 9943. https://doi.org/10.3390/ijms26209943
Malcolm M, Pusterla JM, Penazzi LG, Trenchi A, Acosta-Rodríguez VA, Ríos MN, Villarreal M, Guido ME, Garbarino-Pico E. Rhythmic Dynamics of Stress Granules in Wild-Type and Bmal1−/− Fibroblasts Lacking a Functional Canonical Circadian Clock. International Journal of Molecular Sciences. 2025; 26(20):9943. https://doi.org/10.3390/ijms26209943
Chicago/Turabian StyleMalcolm, Melisa, Julio M. Pusterla, Laura G. Penazzi, Alejandra Trenchi, Victoria A. Acosta-Rodríguez, Maximiliano N. Ríos, Marcos Villarreal, Mario E. Guido, and Eduardo Garbarino-Pico. 2025. "Rhythmic Dynamics of Stress Granules in Wild-Type and Bmal1−/− Fibroblasts Lacking a Functional Canonical Circadian Clock" International Journal of Molecular Sciences 26, no. 20: 9943. https://doi.org/10.3390/ijms26209943
APA StyleMalcolm, M., Pusterla, J. M., Penazzi, L. G., Trenchi, A., Acosta-Rodríguez, V. A., Ríos, M. N., Villarreal, M., Guido, M. E., & Garbarino-Pico, E. (2025). Rhythmic Dynamics of Stress Granules in Wild-Type and Bmal1−/− Fibroblasts Lacking a Functional Canonical Circadian Clock. International Journal of Molecular Sciences, 26(20), 9943. https://doi.org/10.3390/ijms26209943








