Reactive Oxygen Species Across Death Pathways: Gatekeepers of Apoptosis, Ferroptosis, Pyroptosis, Paraptosis, and Beyond
Abstract
1. Introduction
2. An Evolutionary Perspective on ROS as Master Regulators of Cell Fate
3. Reactive Oxygen Species: Types and Cellular Sources
3.1. Types of Reactive Oxygen Species
3.2. Key Features of O2•−, H2O2, and •OH
3.3. Primary Sources of ROS
3.4. Cellular Mechanisms Maintaining the Redox Homeostasis
4. ROS-Dependent Pathways of Regulated Cell Death
4.1. ROS as Modulators of Apoptosis
4.1.1. ROS and the Regulation of Death Ligands in Extrinsic Apoptosis
4.1.2. ROS and Bcl-2 Family-Dependent Control of Intrinsic Apoptosis
4.1.3. ROS and the Regulation of Caspase Activity
4.2. ROS as Modulators of Anoikis
4.3. ROS as Modulators of Necroptosis
4.4. ROS as Modulators of Ferroptosis
4.5. ROS as Modulators of Pyroptosis
4.6. ROS as Modulators of Paraptosis
4.7. ROS as Modulators of Parthanatos
4.8. ROS as Modulators of Lysosome-Dependent Cell Death
4.9. ROS as Modulators of Oxeiptosis
4.10. ROS as Modulators of NETosis
4.11. ROS as Modulators of Autophagy
4.12. ROS and Other Forms of Cell Death
4.12.1. ROS and Mitochondrial Permeability Transition-Driven Necrosis
4.12.2. ROS and Entotic Cell Death
4.12.3. ROS and Mitotic Catastrophe
4.12.4. ROS and Mitotic Death
4.12.5. ROS and Cuproptosis
4.12.6. ROS and Alkaliptosis
4.12.7. ROS and Methuosis
4.12.8. ROS and Disulfidptosis
5. ROS-Driven Crosstalk Between RCD Pathways
6. Emerging Technologies, Future Directions, and Challenges in Redox–RCD Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gülow, K.; Tümen, D.; Heumann, P.; Schmid, S.; Kandulski, A.; Müller, M.; Kunst, C. Unraveling the Role of Reactive Oxygen Species in T Lymphocyte Signaling. Int. J. Mol. Sci. 2024, 25, 6114. [Google Scholar] [CrossRef]
- Seitz, R.; Tümen, D.; Kunst, C.; Heumann, P.; Schmid, S.; Kandulski, A.; Müller, M.; Gülow, K. Exploring the Thioredoxin System as a Therapeutic Target in Cancer: Mechanisms and Implications. Antioxidants 2024, 13, 1078. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Reth, M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 2002, 3, 1129–1134. [Google Scholar] [CrossRef]
- Krammer, P.H.; Kamiński, M.; Kiessling, M.; Gülow, K. No life without death. Adv. Cancer Res. 2007, 97, 111–138. [Google Scholar] [CrossRef] [PubMed]
- Kunst, C.; Tümen, D.; Ernst, M.; Tews, H.C.; Müller, M.; Gülow, K. Paraptosis—A Distinct Pathway to Cell Death. Int. J. Mol. Sci. 2024, 25, 11478. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Dixon, S.J.; Olzmann, J.A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- von Krusenstiern, A.N.; Robson, R.N.; Qian, N.; Qiu, B.; Hu, F.; Reznik, E.; Smith, N.; Zandkarimi, F.; Estes, V.M.; Dupont, M.; et al. Identification of essential sites of lipid peroxidation in ferroptosis. Nat. Chem. Biol. 2023, 19, 719–730. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.-L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Ye, X.-J.; He, X.-H.; Ouyang, D.-Y. The Signaling Pathways Regulating NLRP3 Inflammasome Activation. Inflammation 2021, 44, 1229–1245. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, J.-Y.; Liu, X.-S.; Chen, H.-Z.; Ai, Y.-L.; Cheng, K.; Sun, R.-Y.; Zhou, D.; Han, J.; Wu, Q. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018, 28, 1171–1185. [Google Scholar] [CrossRef]
- Grootjans, S.; Vanden Berghe, T.; Vandenabeele, P. Initiation and execution mechanisms of necroptosis: An overview. Cell Death Differ. 2017, 24, 1184–1195. [Google Scholar] [CrossRef]
- Gupta, G.; Afzal, M.; Moglad, E.; Goyal, A.; Almalki, W.H.; Goyal, K.; Rana, M.; Ali, H.; Rekha, A.; Kazmi, I.; et al. Parthanatos and apoptosis: Unraveling their roles in cancer cell death and therapy resistance. EXCLI J. 2025, 24, 351–380. [Google Scholar] [CrossRef]
- Greenwald, S.H.; Pierce, E.A. Parthanatos as a Cell Death Pathway Underlying Retinal Disease. In Retinal Degenerative Diseases: Mechanisms and Experimental Therapy; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; Volume 1185, pp. 323–327. [Google Scholar] [CrossRef]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef]
- Scaturro, P.; Pichlmair, A. Oxeiptosis: A discreet way to respond to radicals. Curr. Opin. Immunol. 2019, 56, 37–43. [Google Scholar] [CrossRef]
- Holze, C.; Michaudel, C.; Mackowiak, C.; Haas, D.A.; Benda, C.; Hubel, P.; Pennemann, F.L.; Schnepf, D.; Wettmarshausen, J.; Braun, M.; et al. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 2018, 19, 130–140. [Google Scholar] [CrossRef]
- Frisch, S.M.; Screaton, R.A. Anoikis mechanisms. Curr. Opin. Cell Biol. 2001, 13, 555–562. [Google Scholar] [CrossRef]
- Hamann, J.C.; Surcel, A.; Chen, R.; Teragawa, C.; Albeck, J.G.; Robinson, D.N.; Overholtzer, M. Entosis Is Induced by Glucose Starvation. Cell Rep. 2017, 20, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Mc Gee, M.M. Targeting the Mitotic Catastrophe Signaling Pathway in Cancer. Mediat. Inflamm. 2015, 2015, 146282. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewska, E.; Florek, K.; Zagórski, K.; Gachowska, M.; Wietrzyk, A.; Hutny, A.; Nowakowska-Toporowska, A.; Kulbacka, J. Methuosis, Alkaliptosis, and Oxeiptosis and Their Significance in Anticancer Therapy. Cells 2024, 13, 2095. [Google Scholar] [CrossRef]
- Cong, Y.; Li, N.; Zhang, Z.; Shang, Y.; Zhao, H. Cuproptosis: Molecular mechanisms, cancer prognosis, and therapeutic applications. J. Transl. Med. 2025, 23, 104. [Google Scholar] [CrossRef]
- Lyons, T.W.; Reinhard, C.T.; Planavsky, N.J. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 2014, 506, 307–315. [Google Scholar] [CrossRef]
- Fischer, W.W.; Hemp, J.; Valentine, J.S. How did life survive Earth’s great oxygenation? Curr. Opin. Chem. Biol. 2016, 31, 166–178. [Google Scholar] [CrossRef]
- Mills, D.B.; Boyle, R.A.; Daines, S.J.; Sperling, E.A.; Pisani, D.; Donoghue, P.C.J.; Lenton, T.M. Eukaryogenesis and oxygen in Earth history. Nat. Ecol. Evol. 2022, 6, 520–532. [Google Scholar] [CrossRef]
- Craig, J.M.; Kumar, S.; Hedges, S.B. The origin of eukaryotes and rise in complexity were synchronous with the rise in oxygen. Front. Bioinform. 2023, 3, 1233281. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Conrad, M.; Kagan, V.E.; Bayir, H.; Pagnussat, G.C.; Head, B.; Traber, M.G.; Stockwell, B.R. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 2018, 32, 602–619. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Vanlangenakker, N.; Vanden Berghe, T.; Krysko, D.V.; Festjens, N.; Vandenabeele, P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr. Mol. Med. 2008, 8, 207–220. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Winterbourn, C.C. The biological chemistry of hydrogen peroxide. Methods Enzymol. 2013, 528, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; de la Lastra, J.M.P.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry-Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Kruszewski, M. Labile iron pool: The main determinant of cellular response to oxidative stress. Mutat. Res. 2003, 531, 81–92. [Google Scholar] [CrossRef]
- Kiessling, M.K.; Klemke, C.D.; Kaminski, M.M.; Galani, I.E.; Krammer, P.H.; Gülow, K. Inhibition of constitutively activated nuclear factor-kappaB induces reactive oxygen species- and iron-dependent cell death in cutaneous T-cell lymphoma. Cancer Res. 2009, 69, 2365–2374. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Starkov, A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, M.M.; Sauer, S.W.; Kamiński, M.; Opp, S.; Ruppert, T.; Grigaravičius, P.; Grudnik, P.; Gröne, H.-J.; Krammer, P.H.; Gülow, K. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep. 2012, 2, 1300–1315. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, M.M.; Röth, D.; Krammer, P.H.; Gülow, K. Mitochondria as oxidative signaling organelles in T-cell activation: Physiological role and pathological implications. Arch. Immunol. Ther. Exp. 2013, 61, 367–384. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Dröse, S.; Brandt, U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. In Mitochondrial Oxidative Phosphorylation: Nuclear-Encoded Genes, Enzyme Regulation, and Pathophysiology; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2012; Volume 748, pp. 145–169. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef]
- Kamiński, M.M.; Röth, D.; Sass, S.; Sauer, S.W.; Krammer, P.H.; Gülow, K. Manganese superoxide dismutase: A regulator of T cell activation-induced oxidative signaling and cell death. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 1041–1052. [Google Scholar] [CrossRef]
- Okoye, C.N.; Koren, S.A.; Wojtovich, A.P. Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. 2023, 67, 102926. [Google Scholar] [CrossRef]
- Mikhed, Y.; Daiber, A.; Steven, S. Mitochondrial Oxidative Stress, Mitochondrial DNA Damage and Their Role in Age-Related Vascular Dysfunction. Int. J. Mol. Sci. 2015, 16, 15918–15953. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, A.; Viviano, M.; Feoli, A.; Milite, C.; Sarno, G.; Castellano, S.; Sbardella, G. NADPH Oxidases: From Molecular Mechanisms to Current Inhibitors. J. Med. Chem. 2023, 66, 11632–11655. [Google Scholar] [CrossRef] [PubMed]
- Geiszt, M.; Witta, J.; Baffi, J.; Lekstrom, K.; Leto, T.L. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003, 17, 1502–1504. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.; Kiessling, M.; Süss, D.; Krammer, P.H.; Gülow, K. Novel role for mitochondria: Protein kinase Ctheta-dependent oxidative signaling organelles in activation-induced T-cell death. Mol. Cell. Biol. 2007, 27, 3625–3639. [Google Scholar] [CrossRef]
- Qi, X.; Miao, L.; Cai, Y.; Gong, L.; Ren, J. ROS generated by CYP450, especially CYP2E1, mediate mitochondrial dysfunction induced by tetrandrine in rat hepatocytes. Acta Pharmacol. Sin. 2013, 34, 1229–1236. [Google Scholar] [CrossRef]
- Nieto, N.; Friedman, S.L.; Cederbaum, A.I. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J. Biol. Chem. 2002, 277, 9853–9864. [Google Scholar] [CrossRef]
- Xu, J.; Ma, H.-Y.; Liang, S.; Sun, M.; Karin, G.; Koyama, Y.; Hu, R.; Quehenberger, O.; Davidson, N.O.; Dennis, E.A.; et al. The role of human cytochrome P450 2E1 in liver inflammation and fibrosis. Hepatol. Commun. 2017, 1, 1043–1057. [Google Scholar] [CrossRef]
- Fruehauf, J.P.; Meyskens, F.L. Reactive oxygen species: A breath of life or death? Clin. Cancer Res. 2007, 13, 789–794. [Google Scholar] [CrossRef]
- Kim, C.; Kim, J.-Y.; Kim, J.-H. Cytosolic phospholipase A(2), lipoxygenase metabolites, and reactive oxygen species. BMB Rep. 2008, 41, 555–559. [Google Scholar] [CrossRef]
- Chandrasekharan, N.V.; Simmons, D.L. The cyclooxygenases. Genome Biol. 2004, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Dufrusine, B.; Di Francesco, A.; Oddi, S.; Scipioni, L.; Angelucci, C.B.; D’Addario, C.; Serafini, M.; Häfner, A.-K.; Steinhilber, D.; Maccarrone, M.; et al. Iron-Dependent Trafficking of 5-Lipoxygenase and Impact on Human Macrophage Activation. Front. Immunol. 2019, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Walton, P.A.; Pizzitelli, M. Effects of peroxisomal catalase inhibition on mitochondrial function. Front. Physiol. 2012, 3, 108. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J. Glutathione synthesis and its role in redox signaling. Semin. Cell Dev. Biol. 2012, 23, 722–728. [Google Scholar] [CrossRef]
- Veszelyi, K.; Czegle, I.; Varga, V.; Németh, C.E.; Besztercei, B.; Margittai, É. Subcellular Localization of Thioredoxin/Thioredoxin Reductase System-A Missing Link in Endoplasmic Reticulum Redox Balance. Int. J. Mol. Sci. 2024, 25, 6647. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Gaetani, G.F. Mammalian catalase: A venerable enzyme with new mysteries. Trends Biochem. Sci. 2007, 32, 44–50. [Google Scholar] [CrossRef]
- Conrad, M.; Pratt, D.A. The chemical basis of ferroptosis. Nat. Chem. Biol. 2019, 15, 1137–1147, Correction to Nat. Chem. Biol. 2020, 16, 223–224. https://doi.org/10.1038/s41589-019-0434-z. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Gregolin, C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1985, 839, 62–70. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.-S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol.-Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; He, L.; Lemasters, J.J. Mitochondrial permeability transition: A common pathway to necrosis and apoptosis. Biochem. Biophys. Res. Commun. 2003, 304, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes and oxidative stress in aging and apoptosis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 1291–1303. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225, Erratum in Nature 2011, 475, 122. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Brinkmann, V.; Zychlinsky, A. Beneficial suicide: Why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007, 5, 577–582. [Google Scholar] [CrossRef]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Vitale, I.; Pietrocola, F.; Guilbaud, E.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostini, M.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; et al. Apoptotic cell death in disease-Current understanding of the NCCD 2023. Cell Death Differ. 2023, 30, 1097–1154. [Google Scholar] [CrossRef] [PubMed]
- Bode, K.; Bujupi, F.; Link, C.; Hein, T.; Zimmermann, S.; Peiris, D.; Jaquet, V.; Lepenies, B.; Weyd, H.; Krammer, P.H. Dectin-1 Binding to Annexins on Apoptotic Cells Induces Peripheral Immune Tolerance via NADPH Oxidase-2. Cell Rep. 2019, 29, 4435–4446.e9. [Google Scholar] [CrossRef] [PubMed]
- Krammer, P.H.; Weyd, H. Life, death and tolerance. Biochem. Biophys. Res. Commun. 2017, 482, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Guilbaud, E.; Schmidt, D.; Kroemer, G.; Marincola, F.M. Targeting immunogenic cell stress and death for cancer therapy. Nat. Rev. Drug Discov. 2024, 23, 445–460. [Google Scholar] [CrossRef]
- Krammer, P.H. CD95’s deadly mission in the immune system. Nature 2000, 407, 789–795. [Google Scholar] [CrossRef]
- Krammer, P.H.; Arnold, R.; Lavrik, I.N. Life and death in peripheral T cells. Nat. Rev. Immunol. 2007, 7, 532–542. [Google Scholar] [CrossRef]
- Lavrik, I.N. Systems biology of death receptor networks: Live and let die. Cell Death Dis. 2014, 5, e1259. [Google Scholar] [CrossRef]
- Lavrik, I.; Golks, A.; Krammer, P.H. Death receptor signaling. J. Cell Sci. 2005, 118, 265–267. [Google Scholar] [CrossRef]
- Li-Weber, M.; Laur, O.; Hekele, A.; Coy, J.; Walczak, H.; Krammer, P.H. A regulatory element in the CD95 (APO-1/Fas) ligand promoter is essential for responsiveness to TCR-mediated activation. Eur. J. Immunol. 1998, 28, 2373–2383. [Google Scholar] [CrossRef]
- Li-Weber, M.; Laur, O.; Dern, K.; Krammer, P.H. T cell activation-induced and HIV Tat-enhanced CD95(APO-1/Fas) ligand transcription involves NF-κB. Eur. J. Immunol. 2000, 30, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M.; Krammer, P.H. Function and regulation of the CD95 (APO-1/Fas) ligand in the immune system. Semin. Immunol. 2003, 15, 145–157. [Google Scholar] [CrossRef]
- Müller, M.; Wilder, S.; Bannasch, D.; Israeli, D.; Lehlbach, K.; Li-Weber, M.; Friedman, S.L.; Galle, P.R.; Stremmel, W.; Oren, M.; et al. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J. Exp. Med. 1998, 188, 2033–2045. [Google Scholar] [CrossRef]
- Müller, M.; Strand, S.; Hug, H.; Heinemann, E.M.; Walczak, H.; Hofmann, W.J.; Stremmel, W.; Krammer, P.H.; Galle, P.R. Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J. Clin. Investig. 1997, 99, 403–413. [Google Scholar] [CrossRef]
- Gülow, K.; Kaminski, M.; Darvas, K.; Süss, D.; Li-Weber, M.; Krammer, P.H. HIV-1 trans-activator of transcription substitutes for oxidative signaling in activation-induced T cell death. J. Immunol. 2005, 174, 5249–5260. [Google Scholar] [CrossRef]
- Beiqing, L.; Chen, M.; Whisler, R.L. Sublethal levels of oxidative stress stimulate transcriptional activation of c-jun and suppress IL-2 promoter activation in Jurkat T cells. J. Immunol. 1996, 157, 160–169. [Google Scholar] [CrossRef]
- Los, M.; Dröge, W.; Stricker, K.; Baeuerle, P.A.; Schulze-Osthoff, K. Hydrogen peroxide as a potent activator of T lymphocyte functions. Eur. J. Immunol. 1995, 25, 159–165. [Google Scholar] [CrossRef]
- Galter, D.; Mihm, S.; Dröge, W. Distinct effects of glutathione disulphide on the nuclear transcription factors κB and the activator protein-1. Eur. J. Biochem. 1994, 221, 639–648. [Google Scholar] [CrossRef]
- Griffith, C.E.; Zhang, W.; Wange, R.L. ZAP-70-dependent and -independent activation of Erk in Jurkat T cells. Differences in signaling induced by H2O2 and Cd3 cross-linking. J. Biol. Chem. 1998, 273, 10771–10776. [Google Scholar] [CrossRef]
- Hehner, S.P.; Breitkreutz, R.; Shubinsky, G.; Unsoeld, H.; Schulze-Osthoff, K.; Schmitz, M.L.; Dröge, W. Enhancement of T cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J. Immunol. 2000, 165, 4319–4328. [Google Scholar] [CrossRef]
- Saitoh, M.; Nishitoh, H.; Fujii, M.; Takeda, K.; Tobiume, K.; Sawada, Y.; Kawabata, M.; Miyazono, K.; Ichijo, H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998, 17, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Kamata, H.; Honda, S.-I.; Maeda, S.; Chang, L.; Hirata, H.; Karin, M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005, 120, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, M.K.; Linke, B.; Brechmann, M.; Süss, D.; Krammer, P.H.; Gülow, K. Inhibition of NF-κB induces a switch from CD95L-dependent to CD95L-independent and JNK-mediated apoptosis in T cells. FEBS Lett. 2010, 584, 4679–4688. [Google Scholar] [CrossRef] [PubMed]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Israël, N.; Gougerot-Pocidalo, M.A.; Aillet, F.; Virelizier, J.L. Redox status of cells influences constitutive or induced NF-kappa B translocation and HIV long terminal repeat activity in human T and monocytic cell lines. J. Immunol. 1992, 149, 3386–3393. [Google Scholar] [CrossRef]
- Kretz-Remy, C.; Mehlen, P.; Mirault, M.E.; Arrigo, A.P. Inhibition of I kappa B-alpha phosphorylation and degradation and subsequent NF-kappa B activation by glutathione peroxidase overexpression. J. Cell Biol. 1996, 133, 1083–1093. [Google Scholar] [CrossRef]
- Schoonbroodt, S.; Ferreira, V.; Best-Belpomme, M.; Boelaert, J.R.; Legrand-Poels, S.; Korner, M.; Piette, J. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of I kappa B alpha in NF-kappa B activation by an oxidative stress. J. Immunol. 2000, 164, 4292–4300. [Google Scholar] [CrossRef]
- Traenckner, E.B.; Wilk, S.; Baeuerle, P.A. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. EMBO J. 1994, 13, 5433–5441. [Google Scholar] [CrossRef]
- Schroeder, A.; Warnken, U.; Röth, D.; Klika, K.D.; Vobis, D.; Barnert, A.; Bujupi, F.; Oberacker, T.; Schnölzer, M.; Nicolay, J.P.; et al. Targeting Thioredoxin-1 by dimethyl fumarate induces ripoptosome-mediated cell death. Sci. Rep. 2017, 7, 43168. [Google Scholar] [CrossRef]
- Devadas, S.; Zaritskaya, L.; Rhee, S.G.; Oberley, L.; Williams, M.S. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: Selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 2002, 195, 59–70. [Google Scholar] [CrossRef]
- Röth, D.; Krammer, P.H.; Gülow, K. Dynamin related protein 1-dependent mitochondrial fission regulates oxidative signalling in T cells. FEBS Lett. 2014, 588, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ueta, E.; Kimura, T.; Yamamoto, T.; Osaki, T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004, 95, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.J.F.; Marchi, S.; Petroni, G.; Kroemer, G.; Galluzzi, L.; Pervaiz, S. Noncanonical Cell Fate Regulation by Bcl-2 Proteins. Trends Cell Biol. 2020, 30, 537–555. [Google Scholar] [CrossRef]
- Chen, Z.X.; Pervaiz, S. Bcl-2 induces pro-oxidant state by engaging mitochondrial respiration in tumor cells. Cell Death Differ. 2007, 14, 1617–1627, Erratum in Cell Death Differ. 2012, 19, 551. [Google Scholar] [CrossRef]
- Um, H.-D. Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: A review focusing on mitochondrial respiration and reactive oxygen species. Oncotarget 2016, 7, 5193–5203. [Google Scholar] [CrossRef]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxidative Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Lee, H.H.; Dadgostar, H.; Cheng, Q.; Shu, J.; Cheng, G. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc. Natl. Acad. Sci. USA 1999, 96, 9136–9141. [Google Scholar] [CrossRef]
- Chen, C.; Edelstein, L.C.; Gélinas, C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol. Cell. Biol. 2000, 20, 2687–2695. [Google Scholar] [CrossRef]
- Nie, C.; Tian, C.; Zhao, L.; Petit, P.X.; Mehrpour, M.; Chen, Q. Cysteine 62 of Bax is critical for its conformational activation and its proapoptotic activity in response to H2O2-induced apoptosis. J. Biol. Chem. 2008, 283, 15359–15369. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Davis, R.J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Oda, E.; Ohki, R.; Murasawa, H.; Nemoto, J.; Shibue, T.; Yamashita, T.; Tokino, T.; Taniguchi, T.; Tanaka, N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 2000, 288, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Villunger, A.; Michalak, E.M.; Coultas, L.; Müllauer, F.; Böck, G.; Ausserlechner, M.J.; Adams, J.M.; Strasser, A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003, 302, 1036–1038. [Google Scholar] [CrossRef]
- Schilling, T.; Kairat, A.; Melino, G.; Krammer, P.H.; Stremmel, W.; Oren, M.; Müller, M. Interference with the p53 family network contributes to the gain of oncogenic function of mutant p53 in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2010, 394, 817–823. [Google Scholar] [CrossRef]
- Müller, M.; Schleithoff, E.S.; Stremmel, W.; Melino, G.; Krammer, P.H.; Schilling, T. One, two, three—p53, p63, p73 and chemosensitivity. Drug Resist. Updat. 2006, 9, 288–306. [Google Scholar] [CrossRef]
- Kagan, V.E.; Tyurin, V.A.; Jiang, J.; Tyurina, Y.Y.; Ritov, V.B.; Amoscato, A.A.; Osipov, A.N.; Belikova, N.A.; Kapralov, A.A.; Kini, V.; et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005, 1, 223–232. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656, Erratum in Cold Spring Harb. Perspect. Biol. 2015, 7, a026716. https://doi.org/10.1101/cshperspect.a026716. [Google Scholar] [CrossRef]
- Bao, Q.; Shi, Y. Apoptosome: A platform for the activation of initiator caspases. Cell Death Differ. 2007, 14, 56–65. [Google Scholar] [CrossRef]
- Huang, Z.; Pinto, J.T.; Deng, H.; Richie, J.P. Inhibition of caspase-3 activity and activation by protein glutathionylation. Biochem. Pharmacol. 2008, 75, 2234–2244. [Google Scholar] [CrossRef]
- Nobel, C.S.; Burgess, D.H.; Zhivotovsky, B.; Burkitt, M.J.; Orrenius, S.; Slater, A.F. Mechanism of dithiocarbamate inhibition of apoptosis: Thiol oxidation by dithiocarbamate disulfides directly inhibits processing of the caspase-3 proenzyme. Chem. Res. Toxicol. 1997, 10, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, T.H.; Chung, H.T.; Talanian, R.V.; Yin, X.M.; Billiar, T.R. Nitric oxide prevents tumor necrosis factor alpha-induced rat hepatocyte apoptosis by the interruption of mitochondrial apoptotic signaling through S-nitrosylation of caspase-8. Hepatology 2000, 32, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, D.J.; Cheung, H.H.; Mrad, R.L.; Plenchette, S.; Simard, C.; Enwere, E.; Arora, V.; Mak, T.W.; Lacasse, E.C.; Waring, J.; et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc. Natl. Acad. Sci. USA 2008, 105, 11778–11783. [Google Scholar] [CrossRef] [PubMed]
- Graber, T.E.; Holcik, M. Distinct roles for the cellular inhibitors of apoptosis proteins 1 and 2. Cell Death Dis. 2011, 2, e135. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Vousden, K.H.; Lane, D.P. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 275–283. [Google Scholar] [CrossRef]
- Del Gaizo Moore, V.; Brown, J.R.; Certo, M.; Love, T.M.; Novina, C.D.; Letai, A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J. Clin. Investig. 2007, 117, 112–121. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Barbouti, A.; Amorgianiotis, C.; Kolettas, E.; Kanavaros, P.; Galaris, D. Hydrogen peroxide inhibits caspase-dependent apoptosis by inactivating procaspase-9 in an iron-dependent manner. Free Radic. Biol. Med. 2007, 43, 1377–1387. [Google Scholar] [CrossRef]
- Ueda, S.; Nakamura, H.; Masutani, H.; Sasada, T.; Yonehara, S.; Takabayashi, A.; Yamaoka, Y.; Yodoi, J. Redox regulation of caspase-3(-like) protease activity: Regulatory roles of thioredoxin and cytochrome c. J. Immunol. 1998, 161, 6689–6695. [Google Scholar] [CrossRef]
- Curtin, J.F.; Donovan, M.; Cotter, T.G. Regulation and measurement of oxidative stress in apoptosis. J. Immunol. Methods 2002, 265, 49–72. [Google Scholar] [CrossRef]
- Hampton, M.B.; Orrenius, S. Dual regulation of caspase activity by hydrogen peroxide: Implications for apoptosis. FEBS Lett. 1997, 414, 552–556. [Google Scholar] [CrossRef]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Frisch, S.M.; Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994, 124, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Taddei, M.L.; Giannoni, E.; Fiaschi, T.; Chiarugi, P. Anoikis: An emerging hallmark in health and diseases. J. Pathol. 2012, 226, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Buricchi, F.; Grimaldi, G.; Parri, M.; Cialdai, F.; Taddei, M.L.; Raugei, G.; Ramponi, G.; Chiarugi, P. Redox regulation of anoikis: Reactive oxygen species as essential mediators of cell survival. Cell Death Differ. 2008, 15, 867–878. [Google Scholar] [CrossRef]
- Chiarugi, P.; Giannoni, E. Anoikis: A necessary death program for anchorage-dependent cells. Biochem. Pharmacol. 2008, 76, 1352–1364. [Google Scholar] [CrossRef]
- Li, A.E.; Ito, H.; Rovira, I.I.; Kim, K.S.; Takeda, K.; Yu, Z.Y.; Ferrans, V.J.; Finkel, T. A role for reactive oxygen species in endothelial cell anoikis. Circ. Res. 1999, 85, 304–310. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.; Kroemer, G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef]
- Preedy, M.K.; White, M.R.H.; Tergaonkar, V. Cellular heterogeneity in TNF/TNFR1 signalling: Live cell imaging of cell fate decisions in single cells. Cell Death Dis. 2024, 15, 202. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Kellert, B.; Dimitrova, D.P.; Langlais, C.; Hupe, M.; Cain, K.; MacFarlane, M.; Häcker, G.; Leverkus, M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 2011, 43, 449–463. [Google Scholar] [CrossRef]
- Schilling, R.; Geserick, P.; Leverkus, M. Characterization of the ripoptosome and its components: Implications for anti-inflammatory and cancer therapy. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 545, pp. 83–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, S.S.; Zhao, S.; Yang, Z.; Zhong, C.-Q.; Chen, X.; Cai, Q.; Yang, Z.-H.; Huang, D.; Wu, R.; et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 2017, 8, 14329. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Zhang, Y.; He, X.; Zhong, C.-Q.; Ni, H.; Chen, X.; Liang, Y.; Wu, J.; Zhao, S.; et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat. Cell Biol. 2018, 20, 186–197, Erratum in Nat. Cell Biol. 2024, 26, 1225. https://doi.org/10.1038/s41556-024-01455-2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.-J.; Lin, S.-C.; Dong, M.-Q.; Han, J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Bozonet, S.M.; Magon, N.J.; Schwartfeger, A.J.; Konigstorfer, A.; Heath, S.G.; Vissers, M.C.M.; Morris, V.K.; Göbl, C.; Murphy, J.M.; Salvesen, G.S.; et al. Oxidation of caspase-8 by hypothiocyanous acid enables TNF-mediated necroptosis. J. Biol. Chem. 2023, 299, 104792. [Google Scholar] [CrossRef]
- Borutaite, V.; Brown, G.C. Caspases are reversibly inactivated by hydrogen peroxide. FEBS Lett. 2001, 500, 114–118. [Google Scholar] [CrossRef]
- Mannick, J.B.; Schonhoff, C.; Papeta, N.; Ghafourifar, P.; Szibor, M.; Fang, K.; Gaston, B. S-Nitrosylation of mitochondrial caspases. J. Cell Biol. 2001, 154, 1111–1116. [Google Scholar] [CrossRef]
- Choi, M.E.; Price, D.R.; Ryter, S.W.; Choi, A.M.K. Necroptosis: A crucial pathogenic mediator of human disease. JCI Insight 2019, 4, e128834. [Google Scholar] [CrossRef]
- Chen, J.; Kos, R.; Garssen, J.; Redegeld, F. Molecular Insights into the Mechanism of Necroptosis: The Necrosome As a Potential Therapeutic Target. Cells 2019, 8, 1486. [Google Scholar] [CrossRef]
- Liu, H.; Sidiropoulos, P.; Song, G.; Pagliari, L.J.; Birrer, M.J.; Stein, B.; Anrather, J.; Pope, R.M. TNF-alpha gene expression in macrophages: Regulation by NF-kappa B is independent of c-Jun or C/EBP beta. J. Immunol. 2000, 164, 4277–4285. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.B.; Leonard, W.J. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc. Natl. Acad. Sci. USA 1991, 88, 4328–4332. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.R.; Botting, C.H.; Panico, M.; Morris, H.R.; Hay, R.T. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996, 24, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Nicolay, J.P.; Müller-Decker, K.; Schroeder, A.; Brechmann, M.; Möbs, M.; Géraud, C.; Assaf, C.; Goerdt, S.; Krammer, P.H.; Gülow, K. Dimethyl fumarate restores apoptosis sensitivity and inhibits tumor growth and metastasis in CTCL by targeting NF-κB. Blood 2016, 128, 805–815. [Google Scholar] [CrossRef]
- Nicolay, J.P.; Melchers, S.; Albrecht, J.D.; Assaf, C.; Dippel, E.; Stadler, R.; Wehkamp, U.; Wobser, M.; Zhao, J.; Burghaus, I.; et al. Dimethyl fumarate treatment in relapsed and refractory cutaneous T-cell lymphoma: A multicenter phase 2 study. Blood 2023, 142, 794–805. [Google Scholar] [CrossRef]
- Micheau, O.; Lens, S.; Gaide, O.; Alevizopoulos, K.; Tschopp, J. NF-kappaB signals induce the expression of c-FLIP. Mol. Cell. Biol. 2001, 21, 5299–5305. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Wenzel, S.E.; Tyurina, Y.Y.; Zhao, J.; St Croix, C.M.; Dar, H.H.; Mao, G.; Tyurin, V.A.; Anthonymuthu, T.S.; Kapralov, A.A.; Amoscato, A.A.; et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 2017, 171, 628–641.e26. [Google Scholar] [CrossRef]
- Kakhlon, O.; Cabantchik, Z.I. The labile iron pool: Characterization, measurement, and participation in cellular processes(1). Free Radic. Biol. Med. 2002, 33, 1037–1046. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X.L. Glutathione: A key component of the cytoplasmic labile iron pool. Biometals 2011, 24, 1179–1187. [Google Scholar] [CrossRef]
- Cabantchik, Z.I. Labile iron in cells and body fluids: Physiology, pathology, and pharmacology. Front. Pharmacol. 2014, 5, 45. [Google Scholar] [CrossRef]
- Stoyanovsky, D.A.; Tyurina, Y.Y.; Shrivastava, I.; Bahar, I.; Tyurin, V.A.; Protchenko, O.; Jadhav, S.; Bolevich, S.B.; Kozlov, A.V.; Vladimirov, Y.A.; et al. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Radic. Biol. Med. 2019, 133, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.G.; Bubici, C.; Zazzeroni, F.; Papa, S.; Jones, J.; Alvarez, K.; Jayawardena, S.; de Smaele, E.; Cong, R.; Beaumont, C.; et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell 2004, 119, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Bridges, R.J.; Natale, N.R.; Patel, S.A. System xc- cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol. 2012, 165, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Hewett, S.J.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The cystine/glutamate antiporter system xc- in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013, 18, 522–555. [Google Scholar] [CrossRef]
- Koppula, P.; Zhang, Y.; Zhuang, L.; Gan, B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018, 38, 12. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.-J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Weaver, K.; Skouta, R. The Selenoprotein Glutathione Peroxidase 4: From Molecular Mechanisms to Novel Therapeutic Opportunities. Biomedicines 2022, 10, 891. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Yant, L.J.; Ran, Q.; Rao, L.; van Remmen, H.; Shibatani, T.; Belter, J.G.; Motta, L.; Richardson, A.; Prolla, T.A. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 2003, 34, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.-H.; Zhong, C.-Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.-D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef]
- Xia, X.; Wang, X.; Cheng, Z.; Qin, W.; Lei, L.; Jiang, J.; Hu, J. The role of pyroptosis in cancer: Pro-cancer or pro-“host”? Cell Death Dis. 2019, 10, 650. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Oberacker, T.; Bajorat, J.; Ziola, S.; Schroeder, A.; Röth, D.; Kastl, L.; Edgar, B.A.; Wagner, W.; Gülow, K.; Krammer, P.H. Enhanced expression of thioredoxin-interacting-protein regulates oxidative DNA damage and aging. FEBS Lett. 2018, 592, 2297–2307. [Google Scholar] [CrossRef]
- Meissner, F.; Seger, R.A.; Moshous, D.; Fischer, A.; Reichenbach, J.; Zychlinsky, A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood 2010, 116, 1570–1573. [Google Scholar] [CrossRef]
- Mohamed, I.N.; Li, L.; Ismael, S.; Ishrat, T.; El-Remessy, A.B. Thioredoxin interacting protein, a key molecular switch between oxidative stress and sterile inflammation in cellular response. World J. Diabetes 2021, 12, 1979–1999. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ismael, S.; Nasoohi, S.; Sakata, K.; Liao, F.-F.; McDonald, M.P.; Ishrat, T. Thioredoxin-Interacting Protein (TXNIP) Associated NLRP3 Inflammasome Activation in Human Alzheimer’s Disease Brain. J. Alzheimer’s Dis. 2019, 68, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-H.; Park, S.-J. TXNIP: A key protein in the cellular stress response pathway and a potential therapeutic target. Exp. Mol. Med. 2023, 55, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, S.; de Belle, I.; Bredesen, D.E. An alternative, nonapoptotic form of programmed cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 14376–14381. [Google Scholar] [CrossRef]
- Sperandio, S.; Poksay, K.; de Belle, I.; Lafuente, M.J.; Liu, B.; Nasir, J.; Bredesen, D.E. Paraptosis: Mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ. 2004, 11, 1066–1075. [Google Scholar] [CrossRef]
- Yoon, M.J.; Kim, E.H.; Lim, J.H.; Kwon, T.K.; Choi, K.S. Superoxide anion and proteasomal dysfunction contribute to curcumin-induced paraptosis of malignant breast cancer cells. Free Radic. Biol. Med. 2010, 48, 713–726. [Google Scholar] [CrossRef]
- Raimondi, M.; Fontana, F.; Marzagalli, M.; Audano, M.; Beretta, G.; Procacci, P.; Sartori, P.; Mitro, N.; Limonta, P. Ca2+ overload- and ROS-associated mitochondrial dysfunction contributes to δ-tocotrienol-mediated paraptosis in melanoma cells. Apoptosis 2021, 26, 277–292. [Google Scholar] [CrossRef]
- Chang, L.C.; Chiang, S.K.; Chen, S.E.; Hung, M.C. Exploring paraptosis as a therapeutic approach in cancer treatment. J. Biomed. Sci. 2024, 31, 101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sang, J.; Li, W.; Diao, H.-J.; Fan, R.-Z.; Huang, J.-L.; Gan, L.; Zou, M.-F.; Tang, G.-H.; Yin, S. Jolkinolide B targets thioredoxin and glutathione systems to induce ROS-mediated paraptosis and apoptosis in bladder cancer cells. Cancer Lett. 2021, 509, 13–25. [Google Scholar] [CrossRef]
- Yu, S.-W.; Wang, H.; Poitras, M.F.; Coombs, C.; Bowers, W.J.; Federoff, H.J.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 2002, 297, 259–263. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Kim, N.S.; Yu, S.-W.; Wang, H.; Koh, D.W.; Sasaki, M.; Klaus, J.A.; Otsuka, T.; Zhang, Z.; Koehler, R.C.; et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc. Natl. Acad. Sci. USA 2006, 103, 18308–18313. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, N.S.; Haince, J.-F.; Kang, H.C.; David, K.K.; Andrabi, S.A.; Poirier, G.G.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos). Sci. Signal. 2011, 4, ra20. [Google Scholar] [CrossRef] [PubMed]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Artus, C.; Boujrad, H.; Bouharrour, A.; Brunelle, M.-N.; Hoos, S.; Yuste, V.J.; Lenormand, P.; Rousselle, J.-C.; Namane, A.; England, P.; et al. AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX. EMBO J. 2010, 29, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, R.; Umanah, G.K.; Park, H.; Nambiar, K.; Eacker, S.M.; Kim, B.; Bao, L.; Harraz, M.M.; Chang, C.; et al. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science 2016, 354, 6308. [Google Scholar] [CrossRef]
- Fatokun, A.A.; Dawson, V.L.; Dawson, T.M. Parthanatos: Mitochondrial-linked mechanisms and therapeutic opportunities. Br. J. Pharmacol. 2014, 171, 2000–2016. [Google Scholar] [CrossRef]
- Huang, P.; Chen, G.; Jin, W.; Mao, K.; Wan, H.; He, Y. Molecular Mechanisms of Parthanatos and Its Role in Diverse Diseases. Int. J. Mol. Sci. 2022, 23, 7292. [Google Scholar] [CrossRef]
- Wang, R.; Li, C.; Qiao, P.; Xue, Y.; Zheng, X.; Chen, H.; Zeng, X.; Liu, W.; Boldogh, I.; Ba, X. OGG1-initiated base excision repair exacerbates oxidative stress-induced parthanatos. Cell Death Dis. 2018, 9, 628. [Google Scholar] [CrossRef]
- Cheong, K.J.H.; Huang, D.-Y.; Sekar, P.; Chen, R.J.; Cheng, I.H.-J.; Chan, C.-M.; Chen, Y.-S.; Lin, W.-W. CASK Mediates Oxidative Stress-Induced Microglial Apoptosis-Inducing Factor-Independent Parthanatos Cell Death via Promoting PARP-1 Hyperactivation and Mitochondrial Dysfunction. Antioxidants 2024, 13, 343. [Google Scholar] [CrossRef]
- Aits, S.; Jäättelä, M. Lysosomal cell death at a glance. J. Cell Sci. 2013, 126, 1905–1912. [Google Scholar] [CrossRef]
- Radulovic, M.; Schink, K.O.; Wenzel, E.M.; Nähse, V.; Bongiovanni, A.; Lafont, F.; Stenmark, H. ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J. 2018, 37, e99753. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tan, J.X. Lysosomal quality control: Molecular mechanisms and therapeutic implications. Trends Cell Biol. 2023, 33, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, M.J.; Swarup, S.; Harper, J.W. Mechanisms Controlling Selective Elimination of Damaged Lysosomes. Curr. Opin. Physiol. 2022, 29, 100590. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-Y.; Yao, R.-Q.; Li, Y.-X.; Zhao, P.-Y.; Ren, C.; Du, X.-H.; Yao, Y.-M. Lysosomal quality control of cell fate: A novel therapeutic target for human diseases. Cell Death Dis. 2020, 11, 817. [Google Scholar] [CrossRef]
- Johansson, A.-C.; Appelqvist, H.; Nilsson, C.; Kågedal, K.; Roberg, K.; Ollinger, K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis 2010, 15, 527–540. [Google Scholar] [CrossRef]
- Kurz, T.; Eaton, J.W.; Brunk, U.T. The role of lysosomes in iron metabolism and recycling. Int. J. Biochem. Cell Biol. 2011, 43, 1686–1697. [Google Scholar] [CrossRef]
- Lin, Y.; Epstein, D.L.; Liton, P.B. Intralysosomal iron induces lysosomal membrane permeabilization and cathepsin D-mediated cell death in trabecular meshwork cells exposed to oxidative stress. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6483–6495. [Google Scholar] [CrossRef]
- Wang, F.; Gómez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; von Bernuth, H.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 2017, 6, e24437. [Google Scholar] [CrossRef]
- Tatsiy, O.; McDonald, P.P. Physiological Stimuli Induce PAD4-Dependent, ROS-Independent NETosis, with Early and Late Events Controlled by Discrete Signaling Pathways. Front. Immunol. 2018, 9, 2036. [Google Scholar] [CrossRef]
- Tatsiy, O.; Mayer, T.Z.; de Carvalho Oliveira, V.; Sylvain-Prévost, S.; Isabel, M.; Dubois, C.M.; McDonald, P.P. Cytokine Production and NET Formation by Monosodium Urate-Activated Human Neutrophils Involves Early and Late Events, and Requires Upstream TAK1 and Syk. Front. Immunol. 2019, 10, 2996. [Google Scholar] [CrossRef]
- Khan, M.A.; Philip, L.M.; Cheung, G.; Vadakepeedika, S.; Grasemann, H.; Sweezey, N.; Palaniyar, N. Regulating NETosis: Increasing pH Promotes NADPH Oxidase-Dependent NETosis. Front. Med. 2018, 5, 19. [Google Scholar] [CrossRef]
- Ohsumi, Y. Yoshinori Ohsumi: Autophagy from beginning to end. Interview by Caitlin Sedwick. J. Cell Biol. 2012, 197, 164–165. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Anding, A.L.; Baehrecke, E.H. Cleaning House: Selective Autophagy of Organelles. Dev. Cell 2017, 41, 10–22. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Teng, X.; Laddha, S.V.; Ma, S.; van Nostrand, S.C.; Yang, Y.; Khor, S.; Chan, C.S.; Rabinowitz, J.D.; White, E. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes Dev. 2016, 30, 1704–1717. [Google Scholar] [CrossRef] [PubMed]
- Marquez, R.T.; Xu, L. Bcl-2:Beclin 1 complex: Multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am. J. Cancer Res. 2012, 2, 214–221. [Google Scholar]
- Palabiyik, A.A. The role of Bcl-2 in controlling the transition between autophagy and apoptosis (Review). Mol. Med. Rep. 2025, 32, 172. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, T.; Wang, X. The Interplay Between Autophagy and Regulated Necrosis. Antioxid. Redox Signal. 2023, 38, 550–580. [Google Scholar] [CrossRef]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Hu, Y.; Shi, Y.; Chen, H.; Tao, M.; Zhou, X.; Li, J.; Ma, X.; Wang, Y.; Liu, N. Blockade of Autophagy Prevents the Progression of Hyperuricemic Nephropathy Through Inhibiting NLRP3 Inflammasome-Mediated Pyroptosis. Front. Immunol. 2022, 13, 858494. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Pan, J.; Cao, J.; Sun, X.; Li, X.; Zhang, L.; Qin, C. Degradation of NLRP3 by p62-dependent-autophagy improves cognitive function in Alzheimer’s disease by maintaining the phagocytic function of microglia. CNS Neurosci. Ther. 2023, 29, 2826–2842. [Google Scholar] [CrossRef]
- Wible, D.J.; Bratton, S.B. Reciprocity in ROS and autophagic signaling. Curr. Opin. Toxicol. 2018, 7, 28–36. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Interactions between reactive oxygen species and autophagy: Special issue: Death mechanisms in cellular homeostasis. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 119041. [Google Scholar] [CrossRef]
- Chen, Y.; McMillan-Ward, E.; Kong, J.; Israels, S.J.; Gibson, S.B. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008, 15, 171–182. [Google Scholar] [CrossRef]
- Ornatowski, W.; Lu, Q.; Yegambaram, M.; Garcia, A.E.; Zemskov, E.A.; Maltepe, E.; Fineman, J.R.; Wang, T.; Black, S.M. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020, 36, 101679. [Google Scholar] [CrossRef]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- Huang, X.; Yan, H.; Xu, Z.; Yang, B.; Luo, P.; He, Q. The inducible role of autophagy in cell death: Emerging evidence and future perspectives. Cell Commun. Signal. 2025, 23, 151. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007, 26, 1749–1760, Correction in EMBO J. 2019, 38, e101812. https://doi.org/10.15252/embj.2019101812. [Google Scholar] [CrossRef]
- Liu, Z.; Lenardo, M.J. Reactive oxygen species regulate autophagy through redox-sensitive proteases. Dev. Cell 2007, 12, 484–485. [Google Scholar] [CrossRef]
- Sarkar, S.; Ravikumar, B.; Floto, R.A.; Rubinsztein, D.C. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009, 16, 46–56. [Google Scholar] [CrossRef]
- Qi, Z.; He, Q.; Ji, L.; Ding, S. Antioxidant supplement inhibits skeletal muscle constitutive autophagy rather than fasting-induced autophagy in mice. Oxidative Med. Cell. Longev. 2014, 2014, 315896. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011, 36, 30–38. [Google Scholar] [CrossRef]
- Filomeni, G.; de Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Hasan, A.; Rizvi, S.F.; Parveen, S.; Pathak, N.; Nazir, A.; Mir, S.S. Crosstalk Between ROS and Autophagy in Tumorigenesis: Understanding the Multifaceted Paradox. Front. Oncol. 2022, 12, 852424. [Google Scholar] [CrossRef]
- Robichaux, D.J.; Harata, M.; Murphy, E.; Karch, J. Mitochondrial permeability transition pore-dependent necrosis. J. Mol. Cell. Cardiol. 2023, 174, 47–55. [Google Scholar] [CrossRef]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.-S.; Soukas, A.A. Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- Marchi, S.; Giorgi, C.; Suski, J.M.; Agnoletto, C.; Bononi, A.; Bonora, M.; de Marchi, E.; Missiroli, S.; Patergnani, S.; Poletti, F.; et al. Mitochondria-ros crosstalk in the control of cell death and aging. J. Signal Transduct. 2012, 2012, 329635. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Kianfar, M.; Balcerak, A.; Chmielarczyk, M.; Tarnowski, L.; Grzybowska, E.A. Cell Death by Entosis: Triggers, Molecular Mechanisms and Clinical Significance. Int. J. Mol. Sci. 2022, 23, 4985. [Google Scholar] [CrossRef]
- Lystad, A.H.; Simonsen, A. Mechanisms and Pathophysiological Roles of the ATG8 Conjugation Machinery. Cells 2019, 8, 973. [Google Scholar] [CrossRef]
- Huang, J.; Canadien, V.; Lam, G.Y.; Steinberg, B.E.; Dinauer, M.C.; Magalhaes, M.A.O.; Glogauer, M.; Grinstein, S.; Brumell, J.H. Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl. Acad. Sci. USA 2009, 106, 6226–6231. [Google Scholar] [CrossRef]
- Vitale, I.; Galluzzi, L.; Castedo, M.; Kroemer, G. Mitotic catastrophe: A mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell Biol. 2011, 12, 385–392. [Google Scholar] [CrossRef]
- Sazonova, E.V.; Petrichuk, S.V.; Kopeina, G.S.; Zhivotovsky, B. A link between mitotic defects and mitotic catastrophe: Detection and cell fate. Biol. Direct 2021, 16, 25. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K.-I. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Zhao, H.; Traganos, F.; Albino, A.P.; Darzynkiewicz, Z. Oxidative stress induces cell cycle-dependent Mre11 recruitment, ATM and Chk2 activation and histone H2AX phosphorylation. Cell Cycle 2008, 7, 1490–1495. [Google Scholar] [CrossRef]
- Guo, Z.; Kozlov, S.; Lavin, M.F.; Person, M.D.; Paull, T.T. ATM activation by oxidative stress. Science 2010, 330, 517–521. [Google Scholar] [CrossRef]
- Das, K.C.; Dashnamoorthy, R. Hyperoxia activates the ATR-Chk1 pathway and phosphorylates p53 at multiple sites. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 286, L87–L97. [Google Scholar] [CrossRef]
- Willis, J.; Patel, Y.; Lentz, B.L.; Yan, S. APE2 is required for ATR-Chk1 checkpoint activation in response to oxidative stress. Proc. Natl. Acad. Sci. USA 2013, 110, 10592–10597. [Google Scholar] [CrossRef]
- Peng, C.Y.; Graves, P.R.; Thoma, R.S.; Wu, Z.; Shaw, A.S.; Piwnica-Worms, H. Mitotic and G2 checkpoint control: Regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 1997, 277, 1501–1505. [Google Scholar] [CrossRef]
- Savitsky, P.A.; Finkel, T. Redox regulation of Cdc25C. J. Biol. Chem. 2002, 277, 20535–20540. [Google Scholar] [CrossRef]
- Sohn, J.; Rudolph, J. Catalytic and chemical competence of regulation of cdc25 phosphatase by oxidation/reduction. Biochemistry 2003, 42, 10060–10070. [Google Scholar] [CrossRef]
- Patterson, J.C.; Joughin, B.A.; van de Kooij, B.; Lim, D.C.; Lauffenburger, D.A.; Yaffe, M.B. ROS and Oxidative Stress Are Elevated in Mitosis during Asynchronous Cell Cycle Progression and Are Exacerbated by Mitotic Arrest. Cell Syst. 2019, 8, 163–167.e2. [Google Scholar] [CrossRef]
- Di Ghelli Luserna Rorà, A.; Martinelli, G.; Simonetti, G. The balance between mitotic death and mitotic slippage in acute leukemia: A new therapeutic window? J. Hematol. Oncol. 2019, 12, 123. [Google Scholar] [CrossRef]
- Ruan, W.; Lim, H.H.; Surana, U. Mapping Mitotic Death: Functional Integration of Mitochondria, Spindle Assembly Checkpoint and Apoptosis. Front. Cell Dev. Biol. 2018, 6, 177. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Boaru, D.L.; de Leon-Oliva, D.; de Castro-Martinez, P.; Garcia-Montero, C.; Fraile-Martinez, O.; García-González, B.; Pérez-González, I.; Michael Alhaddadin, M.N.; Barrena-Blázquez, S.; Lopez-Gonzalez, L.; et al. Cuproptosis: Current insights into its multifaceted role in disease, cancer, and translational/therapeutic opportunities. Biomed. Pharmacother. 2025, 190, 118422. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261, Erratum in Science 2022, 376, eabq4855. https://doi.org/10.1126/science.abq4855. [Google Scholar] [CrossRef]
- Vo, T.T.T.; Peng, T.-Y.; Nguyen, T.H.; Bui, T.N.H.; Wang, C.-S.; Lee, W.-J.; Chen, Y.-L.; Wu, Y.-C.; Lee, I.-T. The crosstalk between copper-induced oxidative stress and cuproptosis: A novel potential anticancer paradigm. Cell Commun. Signal. 2024, 22, 353. [Google Scholar] [CrossRef]
- Chen, F.; Kang, R.; Liu, J.; Tang, D. Mechanisms of alkaliptosis. Front. Cell Dev. Biol. 2023, 11, 1213995. [Google Scholar] [CrossRef]
- Song, X.; Zhu, S.; Xie, Y.; Liu, J.; Sun, L.; Zeng, D.; Wang, P.; Ma, X.; Kroemer, G.; Bartlett, D.L.; et al. JTC801 Induces pH-dependent Death Specifically in Cancer Cells and Slows Growth of Tumors in Mice. Gastroenterology 2018, 154, 1480–1493. [Google Scholar] [CrossRef]
- Overmeyer, J.H.; Kaul, A.; Johnson, E.E.; Maltese, W.A. Active ras triggers death in glioblastoma cells through hyperstimulation of macropinocytosis. Mol. Cancer Res. MCR 2008, 6, 965–977. [Google Scholar] [CrossRef]
- Maltese, W.A.; Overmeyer, J.H. Methuosis: Nonapoptotic cell death associated with vacuolization of macropinosome and endosome compartments. Am. J. Pathol. 2014, 184, 1630–1642. [Google Scholar] [CrossRef]
- Bhanot, H.; Young, A.M.; Overmeyer, J.H.; Maltese, W.A. Induction of nonapoptotic cell death by activated Ras requires inverse regulation of Rac1 and Arf6. Mol. Cancer Res. MCR 2010, 8, 1358–1374. [Google Scholar] [CrossRef]
- Ritter, M.; Bresgen, N.; Kerschbaum, H.H. From Pinocytosis to Methuosis-Fluid Consumption as a Risk Factor for Cell Death. Front. Cell Dev. Biol. 2021, 9, 651982. [Google Scholar] [CrossRef]
- Cho, H.; Geno, E.; Patoor, M.; Reid, A.; McDonald, R.; Hild, M.; Jenkins, J.L. Indolyl-Pyridinyl-Propenone-Induced Methuosis through the Inhibition of PIKFYVE. ACS Omega 2018, 3, 6097–6103, Erratum in ACS Omega 2018, 3, 9034. https://doi.org/10.1021/acsomega.8b01656. [Google Scholar] [CrossRef]
- Overmeyer, J.H.; Young, A.M.; Bhanot, H.; Maltese, W.A. A chalcone-related small molecule that induces methuosis, a novel form of non-apoptotic cell death, in glioblastoma cells. Mol. Cancer 2011, 10, 69. [Google Scholar] [CrossRef]
- Li, Z.; Mbah, N.E.; Overmeyer, J.H.; Sarver, J.G.; George, S.; Trabbic, C.J.; Erhardt, P.W.; Maltese, W.A. The JNK signaling pathway plays a key role in methuosis (non-apoptotic cell death) induced by MOMIPP in glioblastoma. BMC Cancer 2019, 19, 77. [Google Scholar] [CrossRef]
- Liu, X.; Nie, L.; Zhang, Y.; Yan, Y.; Wang, C.; Colic, M.; Olszewski, K.; Horbath, A.; Chen, X.; Lei, G.; et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat. Cell Biol. 2023, 25, 404–414. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, X.; Deng, G. Disulfidptosis: A new form of regulated cell death for cancer treatment. Mol. Biomed. 2023, 4, 18. [Google Scholar] [CrossRef]
- Liu, X.; Zhuang, L.; Gan, B. Disulfidptosis: Disulfide stress-induced cell death. Trends Cell Biol. 2024, 34, 327–337. [Google Scholar] [CrossRef]
- Fritsch, M.; Günther, S.D.; Schwarzer, R.; Albert, M.-C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, J.; Kang, R.; Zhou, B.; Tang, D. The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 2019, 510, 278–283. [Google Scholar] [CrossRef]
- Frank, D.; Vince, J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef]
- Los, M.; Mozoluk, M.; Ferrari, D.; Stepczynska, A.; Stroh, C.; Renz, A.; Herceg, Z.; Wang, Z.-Q.; Schulze-Osthoff, K. Activation and caspase-mediated inhibition of PARP: A molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 2002, 13, 978–988. [Google Scholar] [CrossRef]
- Vanden Berghe, T.; Kaiser, W.J.; Bertrand, M.J.; Vandenabeele, P. Molecular crosstalk between apoptosis, necroptosis, and survival signaling. Mol. Cell. Oncol. 2015, 2, e975093. [Google Scholar] [CrossRef]
- Kinnally, K.W.; Peixoto, P.M.; Ryu, S.-Y.; Dejean, L.M. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 616–622. [Google Scholar] [CrossRef]
- Pak, V.V.; Ezeriņa, D.; Lyublinskaya, O.G.; Pedre, B.; Tyurin-Kuzmin, P.A.; Mishina, N.M.; Thauvin, M.; Young, D.; Wahni, K.; Martínez Gache, S.A.; et al. Ultrasensitive Genetically Encoded Indicator for Hydrogen Peroxide Identifies Roles for the Oxidant in Cell Migration and Mitochondrial Function. Cell Metab. 2020, 31, 642–653.e6. [Google Scholar] [CrossRef]
- Gutscher, M.; Pauleau, A.-L.; Marty, L.; Brach, T.; Wabnitz, G.H.; Samstag, Y.; Meyer, A.J.; Dick, T.P. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 2008, 5, 553–559. [Google Scholar] [CrossRef]
- Morgan, B.; van Laer, K.; Owusu, T.N.E.; Ezeriņa, D.; Pastor-Flores, D.; Amponsah, P.S.; Tursch, A.; Dick, T.P. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat. Chem. Biol. 2016, 12, 437–443. [Google Scholar] [CrossRef]
- Takemoto, K.; Matsuda, T.; Sakai, N.; Fu, D.; Noda, M.; Uchiyama, S.; Kotera, I.; Arai, Y.; Horiuchi, M.; Fukui, K.; et al. SuperNova, a monomeric photosensitizing fluorescent protein for chromophore-assisted light inactivation. Sci. Rep. 2013, 3, 2629. [Google Scholar] [CrossRef]
- Steinhorn, B.; Sorrentino, A.; Badole, S.; Bogdanova, Y.; Belousov, V.; Michel, T. Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nat. Commun. 2018, 9, 4044, Erratum in Nat. Commun. 2021, 12, 357. https://doi.org/10.1038/s41467-020-20668-1. [Google Scholar] [CrossRef]
- Viswanathan, V.S.; Ryan, M.J.; Dhruv, H.D.; Gill, S.; Eichhoff, O.M.; Seashore-Ludlow, B.; Kaffenberger, S.D.; Eaton, J.K.; Shimada, K.; Aguirre, A.J.; et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 2017, 547, 453–457. [Google Scholar] [CrossRef]
- Bhat, K.P.; Vijay, J.; Vilas, C.K.; Asundi, J.; Zou, J.; Lau, T.; Cai, X.; Ahmed, M.; Kabza, M.; Weng, J.; et al. CRISPR activation screens identify the SWI/SNF ATPases as suppressors of ferroptosis. Cell Rep. 2024, 43, 114345. [Google Scholar] [CrossRef]
- Brandes, N.; Reichmann, D.; Tienson, H.; Leichert, L.I.; Jakob, U. Using quantitative redox proteomics to dissect the yeast redoxome. J. Biol. Chem. 2011, 286, 41893–41903. [Google Scholar] [CrossRef]
- Yang, J.; Gupta, V.; Tallman, K.A.; Porter, N.A.; Carroll, K.S.; Liebler, D.C. Global, in situ, site-specific analysis of protein S-sulfenylation. Nat. Protoc. 2015, 10, 1022–1037. [Google Scholar] [CrossRef]
- Drummen, G.P.C.; van Liebergen, L.C.M.; Op den Kamp, J.A.F.; Post, J.A. C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 2002, 33, 473–490. [Google Scholar] [CrossRef]
- Wang, F.; Naowarojna, N.; Zou, Y. Stratifying ferroptosis sensitivity in cells and mouse tissues by photochemical activation of lipid peroxidation and fluorescent imaging. STAR Protoc. 2022, 3, 101189. [Google Scholar] [CrossRef]
- Lamontagne, F.; Paz-Trejo, C.; Zamorano Cuervo, N.; Grandvaux, N. Redox signaling in cell fate: Beyond damage. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119722. [Google Scholar] [CrossRef]
- Dho, S.H.; Cho, M.; Woo, W.; Jeong, S.; Kim, L.K. Caspases as master regulators of programmed cell death: Apoptosis, pyroptosis and beyond. Exp. Mol. Med. 2025, 57, 1121–1132. [Google Scholar] [CrossRef]
- Chen, H.; Lin, Y.; Chen, J.; Luo, X.; Kan, Y.; He, Y.; Zhu, R.; Jin, J.; Li, D.; Wang, Y.; et al. Targeting caspase-8: A new strategy for combating hepatocellular carcinoma. Front. Immunol. 2024, 15, 1501659. [Google Scholar] [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef]
- Ziehr, B.K.; MacDonald, J.A. Regulation of NLRPs by reactive oxygen species: A story of crosstalk. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119823. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Wang, Z.; Jin, Y.; Gu, W. Ferroptosis as a new tool for tumor suppression through lipid peroxidation. Commun. Biol. 2024, 7, 1475. [Google Scholar] [CrossRef]
- Cao, X.; Tan, J.; Zheng, R.; Wang, F.; Zhou, L.; Yi, J.; Yuan, R.; Dai, Q.; Song, L.; Dai, A. Targeting necroptosis: A promising avenue for respiratory disease treatment. Cell Commun. Signal. 2024, 22, 418. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Meng, Y.; Yan, B.; Zhou, Q.; Wang, X. The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol. Cell 2024, 84, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, Y.; Chen, D.; Sun, Y.; Li, D.; Meng, Y.; Zhou, Q.; Zeng, F.; Deng, G.; Chen, X. Targeting regulated cell death: Apoptosis, necroptosis, pyroptosis, ferroptosis, and cuproptosis in anticancer immunity. J. Transl. Int. Med. 2025, 13, 10–32. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’en, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar] [CrossRef]
- Weisel, K.; Scott, N.E.; Tompson, D.J.; Votta, B.J.; Madhavan, S.; Povey, K.; Wolstenholme, A.; Simeoni, M.; Rudo, T.; Richards-Peterson, L.; et al. Randomized clinical study of safety, pharmacokinetics, and pharmacodynamics of RIPK1 inhibitor GSK2982772 in healthy volunteers. Pharmacol. Res. Perspect. 2017, 5, e00365. [Google Scholar] [CrossRef]
- Ozaki, E.; Campbell, M.; Doyle, S.L. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J. Inflamm. Res. 2015, 8, 15–27. [Google Scholar] [CrossRef]
- Cabral, J.E.; Wu, A.; Zhou, H.; Pham, M.A.; Lin, S.; McNulty, R. Targeting the NLRP3 inflammasome for inflammatory disease therapy. Trends Pharmacol. Sci. 2025, 46, 503–519. [Google Scholar] [CrossRef]
- Pan, W.; Jia, Z.; Du, J.; Chang, K.; Liu, Y.; Liu, W.; Zhao, X.; Tan, W. NLRP3 Inflammasome Upregulates PD-L1 in Ovarian Cancer and Contributes to an Immunosuppressive Microenvironment. Immunotargets Ther. 2024, 13, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Pratz, K.W.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Döhner, H.; Récher, C.; Fiedler, W.; Yamamoto, K.; Wang, J.; et al. Long-term follow-up of VIALE-A: Venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia. Am. J. Hematol. 2024, 99, 615–624. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Maiti, A.; Rausch, C.R.; Pemmaraju, N.; Naqvi, K.; Daver, N.G.; Kadia, T.M.; Borthakur, G.; Ohanian, M.; Alvarado, Y.; et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: A single-centre, phase 2 trial. Lancet Haematol. 2020, 7, e724–e736. [Google Scholar] [CrossRef] [PubMed]
- Michalski, M.; Bauer, M.; Walz, F.; Tümen, D.; Heumann, P.; Stöckert, P.; Gunckel, M.; Kunst, C.; Kandulski, A.; Schmid, S.; et al. Simultaneous Inhibition of Mcl-1 and Bcl-2 Induces Synergistic Cell Death in Hepatocellular Carcinoma. Biomedicines 2023, 11, 1666. [Google Scholar] [CrossRef]
- Pelosi, E.; Castelli, G.; Testa, U. The Growing Role of the BH3 Mimetic Drug Venetoclax in the Therapy of Acute Myeloid Leukemia. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022080. [Google Scholar] [CrossRef]
- Roca-Portoles, A.; Rodriguez-Blanco, G.; Sumpton, D.; Cloix, C.; Mullin, M.; Mackay, G.M.; O’Neill, K.; Lemgruber, L.; Luo, X.; Tait, S.W.G. Venetoclax causes metabolic reprogramming independent of BCL-2 inhibition. Cell Death Dis. 2020, 11, 616. [Google Scholar] [CrossRef]
- Chen, J.; Pan, Q.; Bai, Y.; Chen, X.; Zhou, Y. Hydroxychloroquine Induces Apoptosis in Cholangiocarcinoma via Reactive Oxygen Species Accumulation Induced by Autophagy Inhibition. Front. Mol. Biosci. 2021, 8, 720370. [Google Scholar] [CrossRef]
- Buccarelli, M.; Marconi, M.; Pacioni, S.; de Pascalis, I.; D’Alessandris, Q.G.; Martini, M.; Ascione, B.; Malorni, W.; Larocca, L.M.; Pallini, R.; et al. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 2018, 9, 841. [Google Scholar] [CrossRef]
- Iglesias-Corral, D.; García-Valles, P.; Arroyo-Garrapucho, N.; Bueno-Martínez, E.; Ruiz-Robles, J.M.; Ovejero-Sánchez, M.; González-Sarmiento, R.; Herrero, A.B. Chloroquine-induced DNA damage synergizes with DNA repair inhibitors causing cancer cell death. Front. Oncol. 2024, 14, 1390518. [Google Scholar] [CrossRef]
- Rojas-Sanchez, G.; García-Miranda, A.; Montes-Alvarado, J.B.; Cotzomi-Ortega, I.; Sarmiento-Salinas, F.L.; Jimenez-Ignacio, E.E.; Ramírez-Ramírez, D.; Romo-Rodríguez, R.E.; Reyes-Leyva, J.; Vallejo-Ruiz, V.; et al. Chloroquine Induces ROS-mediated Macrophage Migration Inhibitory Factor Secretion and Epithelial to Mesenchymal Transition in ER-positive Breast Cancer Cell Lines. J. Mammary Gland Biol. Neoplasia 2021, 26, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; An, L.-L.; Chaerkady, R.; Mittereder, N.; Clarke, L.; Cohen, T.S.; Chen, B.; Hess, S.; Sims, G.P.; Mustelin, T. Evidence for a direct link between PAD4-mediated citrullination and the oxidative burst in human neutrophils. Sci. Rep. 2018, 8, 15228. [Google Scholar] [CrossRef] [PubMed]
- Baranov, M.V.; Bianchi, F.; Schirmacher, A.; van Aart, M.A.C.; Maassen, S.; Muntjewerff, E.M.; Dingjan, I.; ter Beest, M.; Verdoes, M.; Keyser, S.G.L.; et al. The Phosphoinositide Kinase PIKfyve Promotes Cathepsin-S-Mediated Major Histocompatibility Complex Class II Antigen Presentation. iScience 2019, 11, 160–177. [Google Scholar] [CrossRef] [PubMed]
- Saffi, G.T.; Tang, E.; Mamand, S.; Inpanathan, S.; Fountain, A.; Salmena, L.; Botelho, R.J. Reactive oxygen species prevent lysosome coalescence during PIKfyve inhibition. PLoS ONE 2021, 16, e0259313. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, M.; Yan, C.; Kong, W.; Lan, F.; Narengaowa; Zhao, S.; Yang, Q.; Bai, Z.; Qing, H.; et al. Cathepsin B in programmed cell death machinery: Mechanisms of execution and regulatory pathways. Cell Death Dis. 2023, 14, 255. [Google Scholar] [CrossRef]
- Liu, B.; Yang, C.; Liu, J.; Peng, M.; Mao, J.; Tang, S.; Huang, W. Targeting of Lysosomes as a Therapeutic Target in Cancer. Curr. Mol. Pharmacol. 2024, 17, e18761429354659. [Google Scholar] [CrossRef]
- Wojtovich, A.P.; Berry, B.J.; Galkin, A. Redox Signaling Through Compartmentalization of Reactive Oxygen Species: Implications for Health and Disease. Antioxid. Redox Signal. 2019, 31, 591–593. [Google Scholar] [CrossRef]
- Brandl, N.; Seitz, R.; Sendtner, N.; Müller, M.; Gülow, K. Living on the Edge: ROS Homeostasis in Cancer Cells and Its Potential as a Therapeutic Target. Antioxidants 2025, 14, 1002. [Google Scholar] [CrossRef]
- Glorieux, C.; Liu, S.; Trachootham, D.; Huang, P. Targeting ROS in cancer: Rationale and strategies. Nat. Rev. Drug Discov. 2024, 23, 583–606. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Shah, M.A.; Rogoff, H.A. Implications of reactive oxygen species on cancer formation and its treatment. Semin. Oncol. 2021, 48, 238–245. [Google Scholar] [CrossRef]


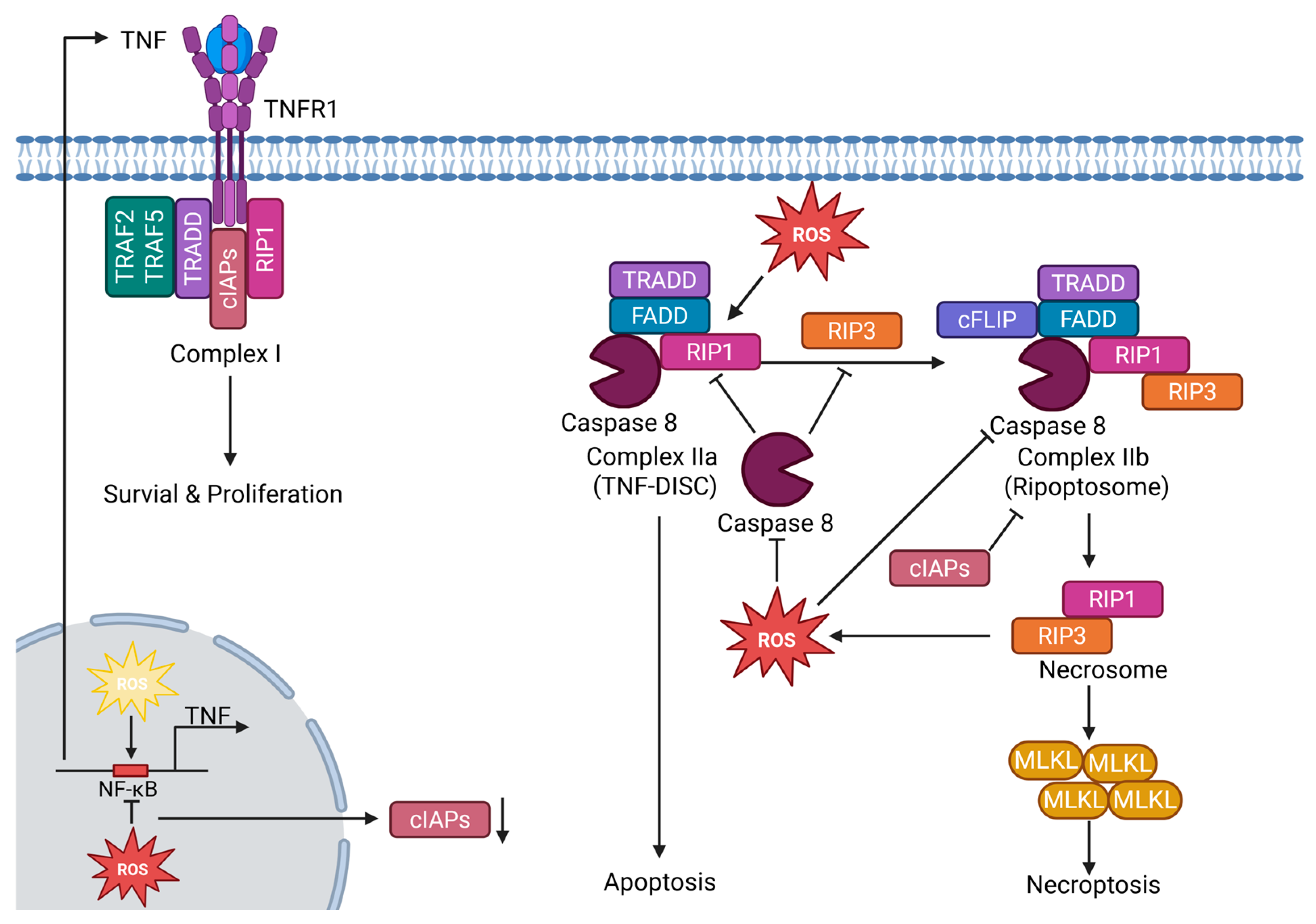
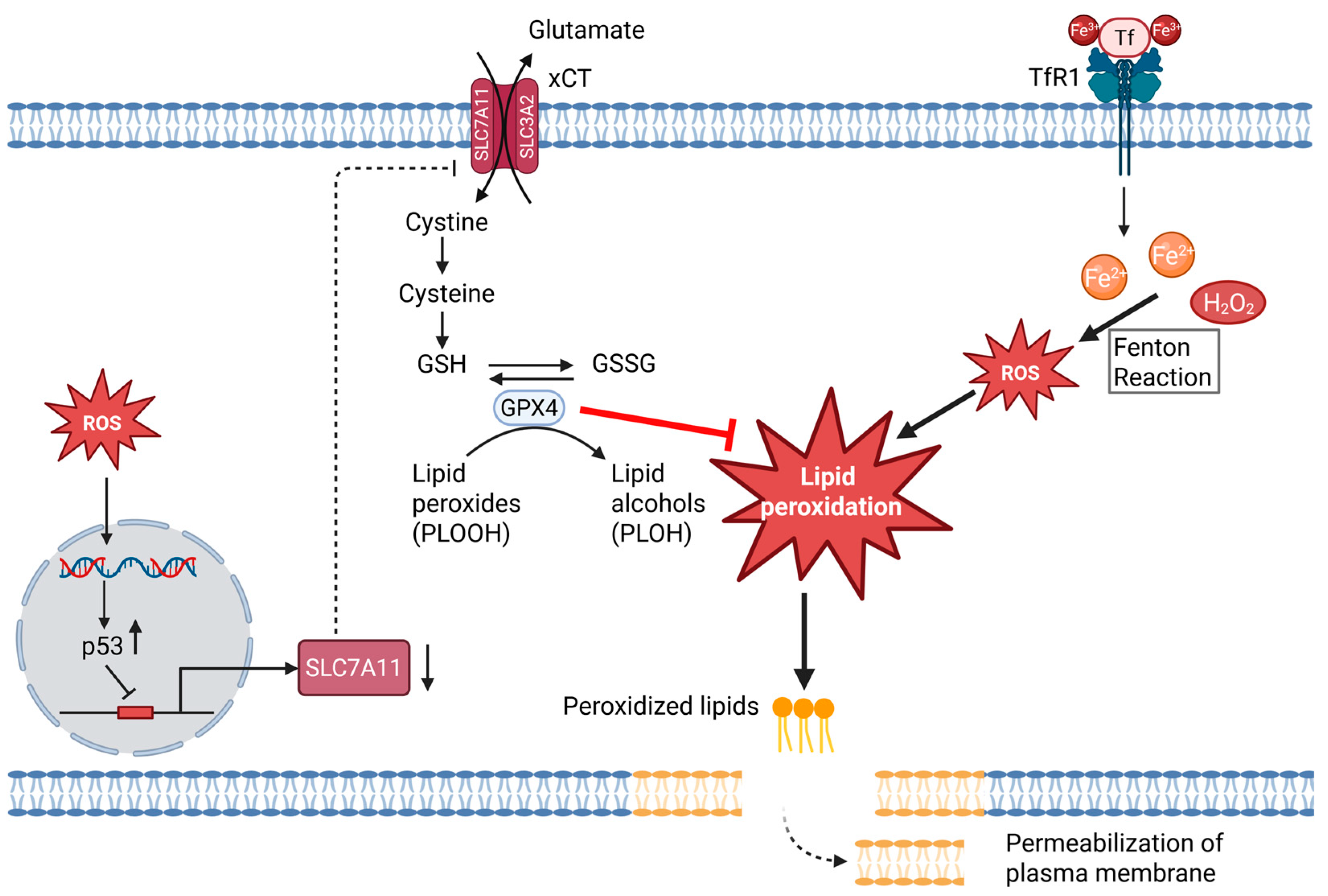
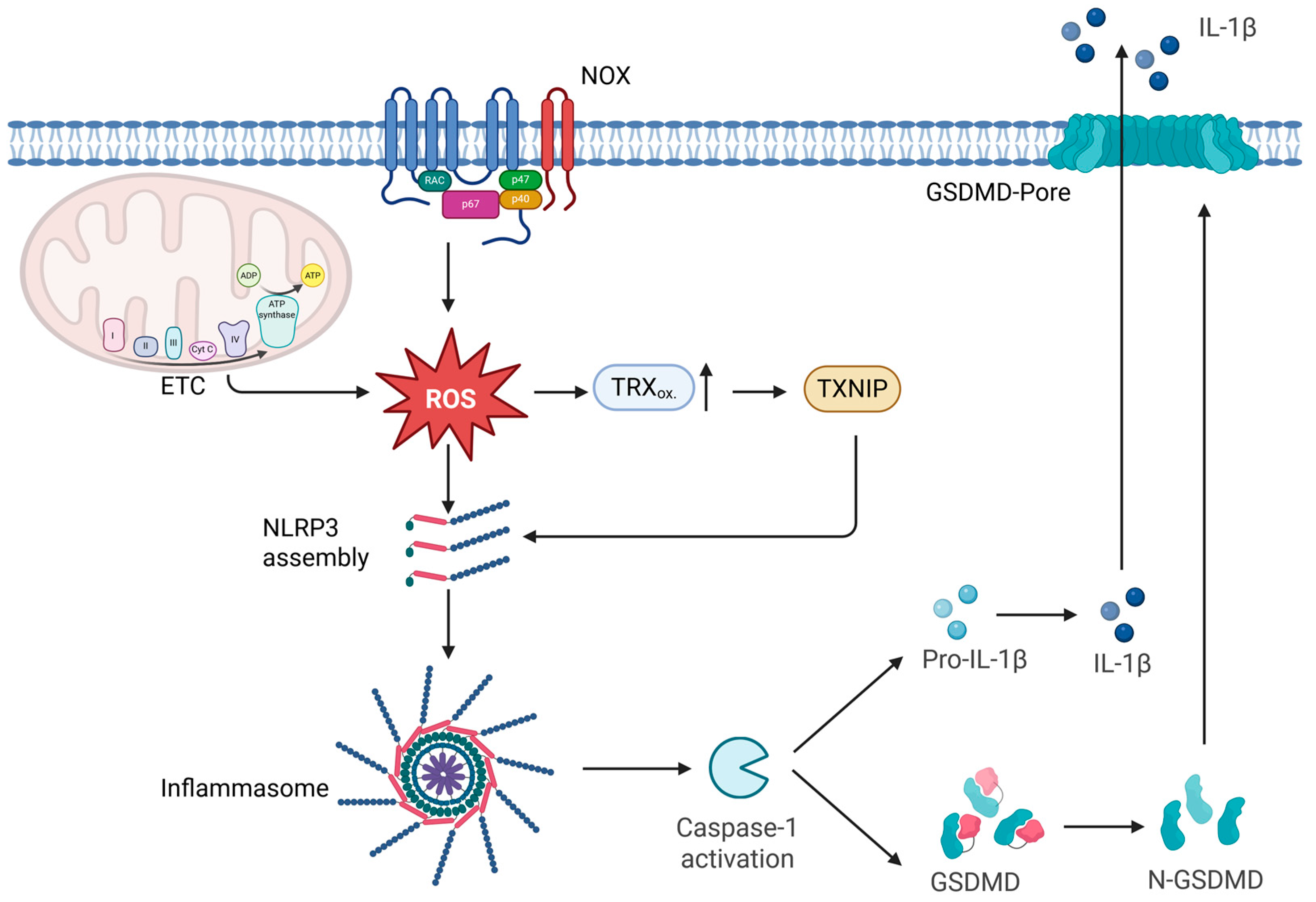
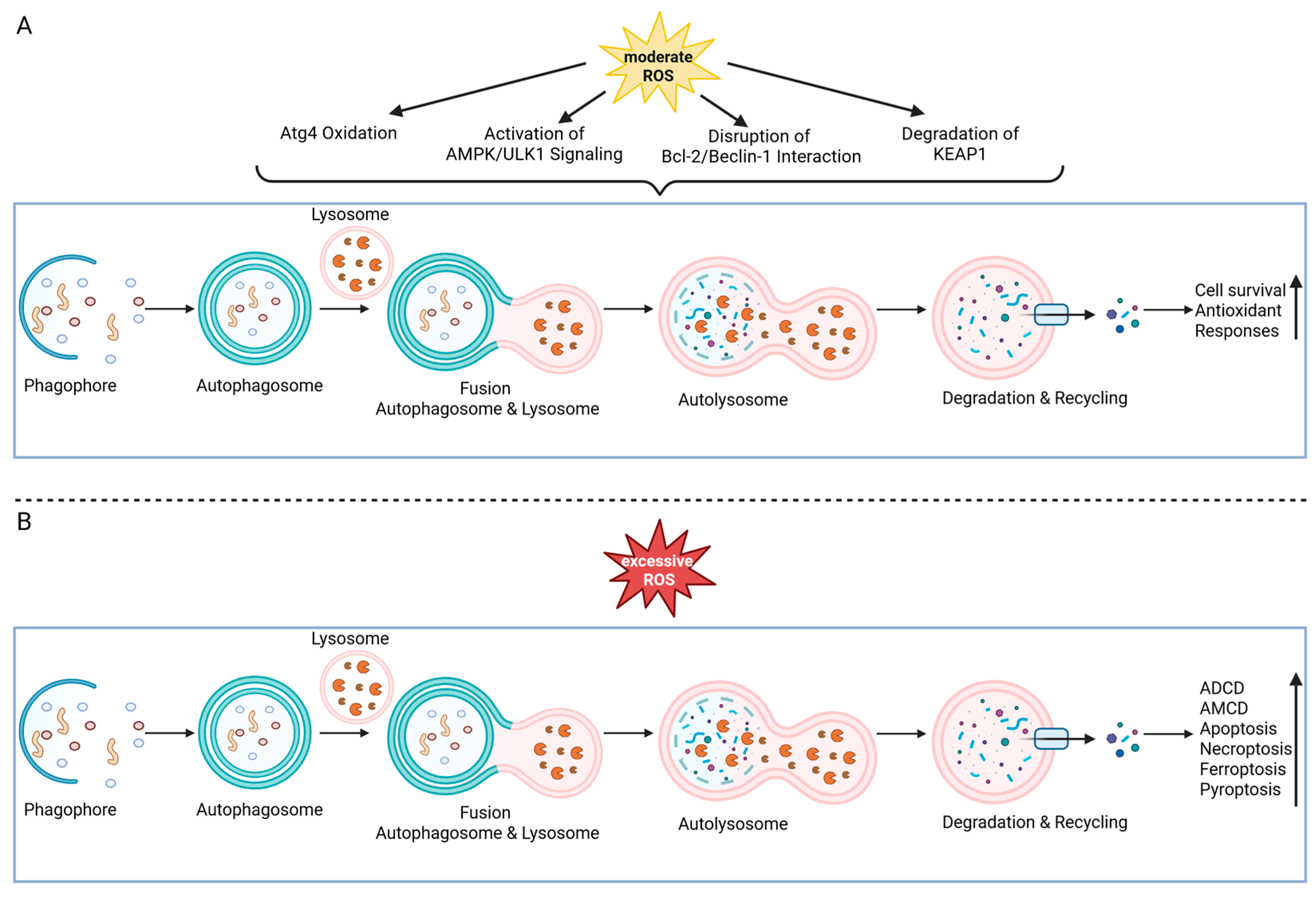

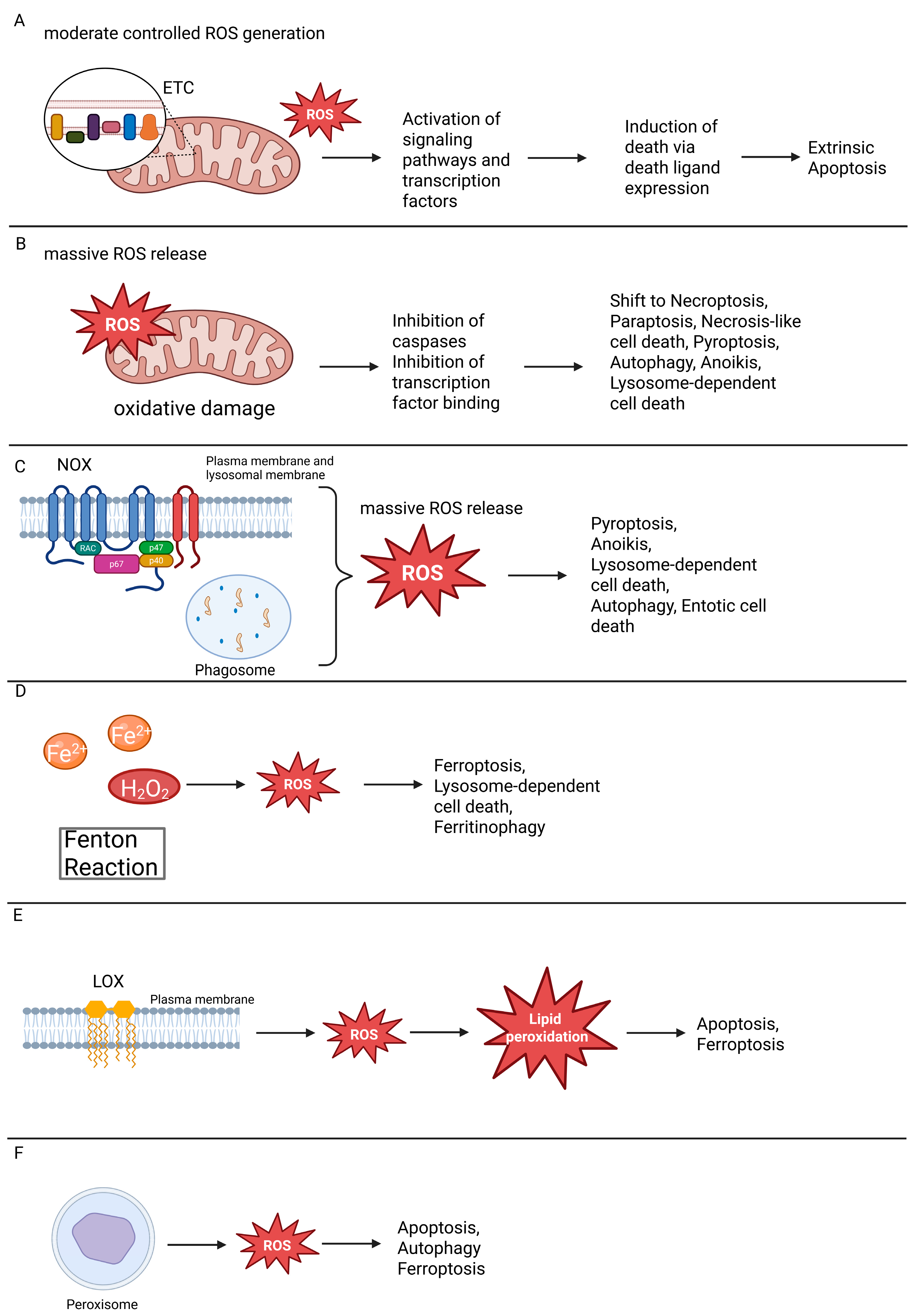
| Pathway | Condition/Stimulus | ROS Requirement | Mechanism | Key References |
|---|---|---|---|---|
| Extrinsic | ROS-dependent regulation of CD95L transcription (via NF-κB, AP-1) | Required | ROS sustain AP-1 (ASK1–JNK, c-Jun) and promote NF-κB nuclear translocation, enabling CD95L expression and receptor clustering | [108,115,119,120,121,122,123] |
| Extrinsic | Mitochondrial ROS (Complex I/III) driving CD95L induction | Required | Complex I/III-derived ROS enhance CD95L transcription, linking mitochondrial dysfunction with death receptor signaling | [46,68] |
| Intrinsic | ROS-mediated modulation of BCL-2 family and mitochondria | Required | mtROS drive cardiolipin oxidation, tBid docking, Bax/Bak activation, and support p53-dependent PUMA/NOXA induction → MOMP | [133,134,135,136,148] |
| Intrinsic | BH3 mimetics (venetoclax/navitoclax) | Optional | Direct antagonism of BCL-2 triggers MOMP; ROS act only as amplifiers | [149,150,151,152] |
| Intrinsic | DNA damage (p53-competent) | Optional | p53 induces PUMA/NOXA; apoptosis proceeds under ROS scavenging, with ROS accelerating responses | [136,148] |
| Extrinsic | Strong ligand stimulation (efficient DISC formation) | Optional | Suprathreshold receptor signaling drives caspase activation; ROS accelerate but are not essential | [39,153] |
| Execution | Excess ROS (supraphysiological) | Inhibitory | Oxidation of catalytic cysteines in caspases blocks execution; cells may deviate to necrosis/necroptosis | [143,144,154,155] |
| Regulatory Level | Condition/Stimulus | ROS Requirement | Mechanism | Key References |
|---|---|---|---|---|
| RIPK1 activation | Mitochondrial ROS oxidize RIPK1 (Cys → pS161 autophosphorylation) | Required | mtROS drive RIPK1 activation and RIPK1–RIPK3 necrosome assembly; positive feedback loop | [165] |
| Metabolic reprogramming | RIPK3 phosphorylates pyruvate dehydrogenase → ↑ mitochondrial respiration/ROS | Required | RIPK3–MLKL axis enhances aerobic metabolism, boosting ROS to amplify necroptosis | [166,167] |
| Checkpoint regulation | Redox-dependent inhibition of caspase-8 | Required | Caspase-8 oxidation disables apoptotic brake, permitting RIPK1–RIPK3 necrosome formation | [168,169,170] |
| Transcriptional control | NF-κB-dependent TNF, cIAPs, c-FLIP expression | Optional | Moderate ROS support NF-κB activity (pro-survival); excessive ROS block NF-κB DNA binding, impair IAPs, tilting balance to necroptosis | [117,122,147,173,174,175,176] |
| Pharmacological modulation | Trx-1 inhibition by dimethyl fumarate | Optional | Loss of redox buffering promotes Ripoptosome assembly; mixed apoptosis/necroptosis phenotype | [122,177,178] |
| Execution phase | Excess ROS beyond physiological range | Inhibitory/Shift | Overoxidation of NF-κB or metabolic collapse suppresses survival and apoptotic checkpoints, biasing outcome toward necroptosis | [122,175,176,179,180] |
| Regulatory Level | Condition/Stimulus | ROS Requirement | Mechanism | Key References |
|---|---|---|---|---|
| Initiation | Labile iron pool (Fe2+) + H2O2 → Fenton chemistry | Required | Fe2+/H2O2 generate •OH radicals that indiscriminately oxidize lipids, initiating ferroptosis | [41,185,186,187,188] |
| Enzymatic amplification | 15-LOX–PEBP1 complex drives phosphatidylethanolamine peroxidation | Required | Enzymatic lipid peroxidation amplifies lipid ROS beyond detoxification capacity | [182,184] |
| Transcriptional control | Oncogenic/stress pathways p53 represses SLC7A11 | Optional | ROS-induced stress and p53 signaling determine sensitivity to ferroptosis by regulating cystine uptake | [192,193] |
| Execution phase | Genetic or pharmacologic loss of GPX4 | Required | Loss of GPX4 peroxidase activity causes lethal accumulation of lipid peroxides | [84,181,183,194,195,196] |
| Context-specific modulation | NF-κB inhibition (e.g., in CTCL) | Shift to ferroptosis | Disturbed iron/ROS homeostasis under NF-κB loss leads to ROS- and iron-dependent cell death | [53,189] |
| Regulatory Level | Condition/Stimulus | ROS Requirement | Mechanism | Key References |
|---|---|---|---|---|
| Inflammasome activation | Mitochondrial ROS promote NLRP3 assembly | Required | mtROS and mtDNA release amplify NLRP3 inflammasome formation and caspase-1 activation | [15,88,89] |
| Inflammasome activation | NOX-derived ROS drive priming and activation in phagocytes | Required/Context-dependent | Oxidative burst from NOX couples microbial sensing to inflammasome activation | [15,89,203] |
| Inflammasome activation | TXNIP released upon TRX oxidation binds NLRP3 | Required | TXNIP acts as a redox-sensitive switch linking oxidative imbalance to caspase-1 activation and pyroptosis | [201,204,205,206] |
| Additional ROS sources | Peroxisomal H2O2 | Optional | Peroxisomes contribute H2O2 as a cofactor in inflammasome signaling | [15] |
| Regulatory Level | Condition/Stimulus | ROS Requirement | Mechanistic Handle (One-Liner) | Key References |
|---|---|---|---|---|
| Lysosomal ROS generation | Intra-lysosomal Fenton chemistry (H2O2 + Fe2+ → •OH) | Required | Hydroxyl radicals attack lysosomal membranes, destabilizing integrity and triggering LMP | [228,229] |
| Protective modulation | Lysosomotropic iron chelators | Optional | Chelation prevents ROS-driven lipid peroxidation and cathepsin release | [230] |
| Apoptotic outcome (limited LMP) | Partial cathepsin release → BID cleavage → MOMP → caspase activation | Shift to apoptosis | Moderate ROS promote controlled LMP, linking lysosomes to mitochondrial apoptosis | [21,223,231] |
| Necrotic outcome (extensive LMP) | Massive cathepsin/hydrolase leakage → direct proteolysis | Required | Excessive ROS cause catastrophic lysosomal rupture and caspase-independent necrotic cell death | [21,223] |
| Repair/clearance balance | ESCRT machinery and lysophagy counteract LMP | Shift | When repair or lysophagy fails, ROS-induced LMP tilts balance toward LDCD | [224,225,226,227] |
| Regulatory Level | Condition/Stimulus | ROS Requirement | Mechanism | Key References |
|---|---|---|---|---|
| Canonical (Suicidal NETosis) | NOX2-derived oxidative burst | Required | NOX2-dependent ROS drive NE and MPO translocation to the nucleus, promoting chromatin decondensation and NET release; absent in chronic granulomatous disease | [90,235,236,237] |
| Mitochondrial ROS (vital NETosis) | Mitochondrial ROS and Ca2+ influx | Required/Context-dependent | Mitochondrial ROS trigger rapid NET release while preserving neutrophil viability (vital NETosis) | [234] |
| ROS-independent NETosis | Physiological agonists (Ca2+ ionophores, monosodium urate crystals) | Absent/Alternative pathway | Certain stimuli induce NET release without detectable ROS burst, indicating stimulus-specific, ROS-independent NETosis | [238,239,240] |
| Regulatory Level | Condition/Stimulus | ROS Requirement | Mechanism | Key References |
|---|---|---|---|---|
| Initiation (nutrient stress) | Starvation-induced oxidation of Atg4 | Required | ROS oxidize Atg4 to enable LC3 processing and autophagosome formation | [259,260] |
| Energy-sensing pathway | AMPK/ULK1 activation under oxidative stress | Required/Context-dependent | ROS activate AMPK and ULK1, promoting autophagy initiation and metabolic adaptation | [253,254] |
| Regulation via Bcl-2/Beclin-1 and KEAP1 degradation | Moderate oxidative stress | Required/Context-dependent | ROS disrupt Bcl-2–Beclin-1 complexes and trigger KEAP1 degradation, enhancing Nrf2-dependent antioxidant responses | [253,254] |
| Negative feedback control (mitophagy, pexophagy) | Selective removal of ROS-producing organelles | Optional/Feedback | Autophagy reduces ROS burden via degradation of damaged mitochondria or peroxisomes | [253] |
| Autophagy-dependent cell death (ADCD/AMCD) | Sustained or excessive ROS accumulation | Required | Persistent ROS drive destructive, non-protective autophagy contributing directly to cell death | [255,256,257,258] |
| mTOR inhibition (rapamycin-induced autophagy) | Pharmacological mTOR blockade | ROS-independent | Rapamycin triggers autophagy even under antioxidant conditions, indicating ROS-independent induction | [261,262] |
| RCD Modality | Plasma Membrane Integrity | Inflammatory Potential |
|---|---|---|
| Apoptosis | Preserved | Non-inflammatory |
| Anoikis | Preserved | Non-inflammatory |
| Necroptosis | Ruptured | Inflammatory |
| Ferroptosis | Ruptured | Inflammatory |
| Pyroptosis | Ruptured | Inflammatory |
| Paraptosis | Ruptured | Inflammatory |
| Parthanatos | Ruptured | Inflammatory |
| LDCD | Ruptured | Inflammatory |
| Oxeiptosis | Preserved | Non-inflammatory |
| suicidal NETosis | Ruptured | Inflammatory |
| Vital NETosis | Preserved | Inflammatory (controlled release of DAMPs) |
| Autophagy | Preserved | Non-inflammatory |
| MPT-driven necrosis | Ruptured | Inflammatory |
| Entotic cell death | Preserved | Non-inflammatory |
| Mitotic catastrophe | Variable (often progresses to apoptosis → preserved) | Non-inflammatory |
| Mitotic death | Preserved | Non-inflammatory |
| Cuproptosis | Ruptured | Inflammatory |
| Alkaliptosis | Ruptured | Inflammatory |
| Methuosis | Ruptured (late vacuole collapse) | Inflammatory |
| Disulfidptosis | Likely ruptured | Likely inflammatory |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sendtner, N.; Seitz, R.; Brandl, N.; Müller, M.; Gülow, K. Reactive Oxygen Species Across Death Pathways: Gatekeepers of Apoptosis, Ferroptosis, Pyroptosis, Paraptosis, and Beyond. Int. J. Mol. Sci. 2025, 26, 10240. https://doi.org/10.3390/ijms262010240
Sendtner N, Seitz R, Brandl N, Müller M, Gülow K. Reactive Oxygen Species Across Death Pathways: Gatekeepers of Apoptosis, Ferroptosis, Pyroptosis, Paraptosis, and Beyond. International Journal of Molecular Sciences. 2025; 26(20):10240. https://doi.org/10.3390/ijms262010240
Chicago/Turabian StyleSendtner, Noah, Rebecca Seitz, Noah Brandl, Martina Müller, and Karsten Gülow. 2025. "Reactive Oxygen Species Across Death Pathways: Gatekeepers of Apoptosis, Ferroptosis, Pyroptosis, Paraptosis, and Beyond" International Journal of Molecular Sciences 26, no. 20: 10240. https://doi.org/10.3390/ijms262010240
APA StyleSendtner, N., Seitz, R., Brandl, N., Müller, M., & Gülow, K. (2025). Reactive Oxygen Species Across Death Pathways: Gatekeepers of Apoptosis, Ferroptosis, Pyroptosis, Paraptosis, and Beyond. International Journal of Molecular Sciences, 26(20), 10240. https://doi.org/10.3390/ijms262010240






