Polysorbate-Based Carriers Encapsulating Oxygen-Deficient Nanoparticles for Targeted and Effective Chemo-Sonodynamic Therapy of Glioblastoma
Abstract
1. Introduction
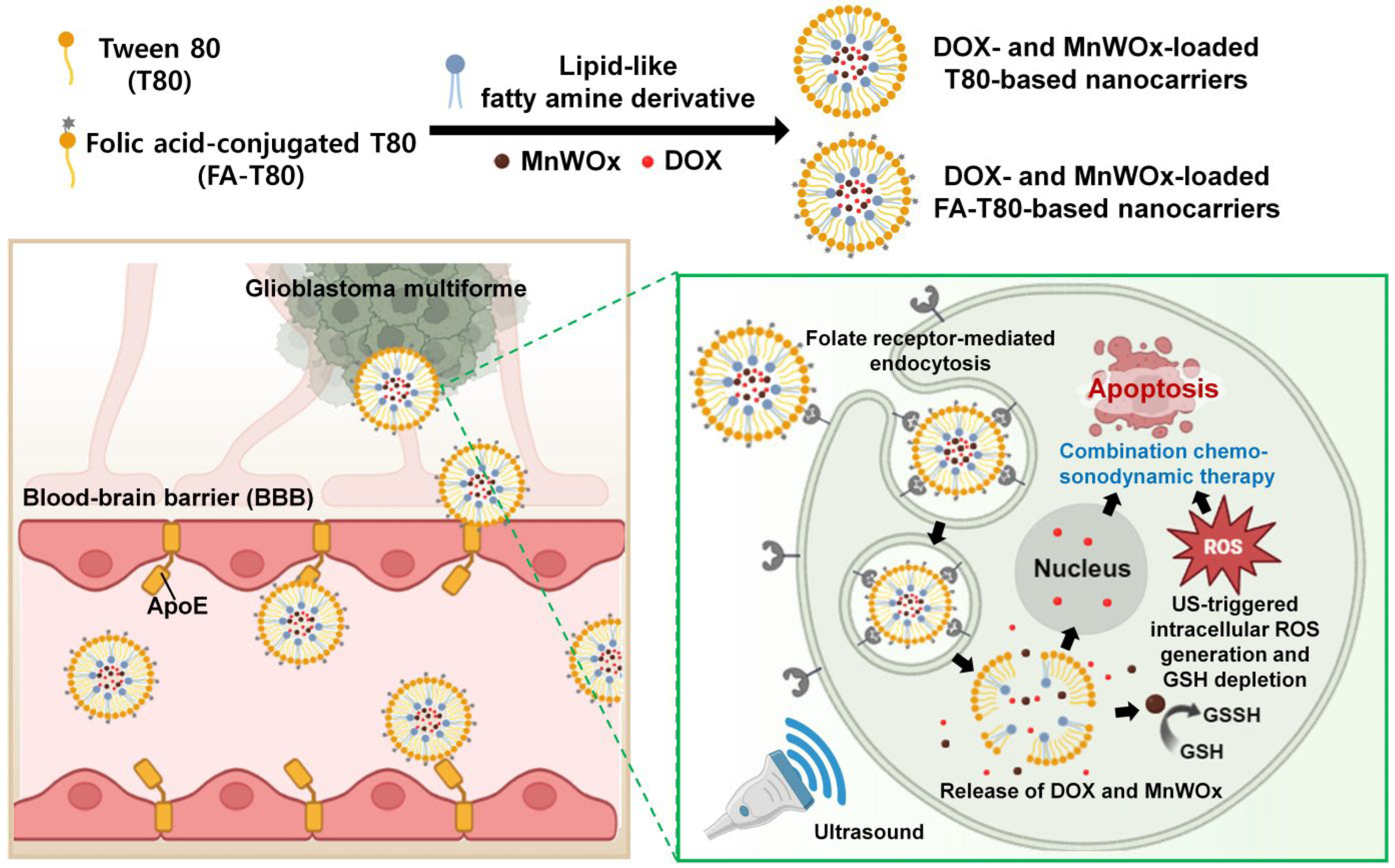
2. Results and Discussions
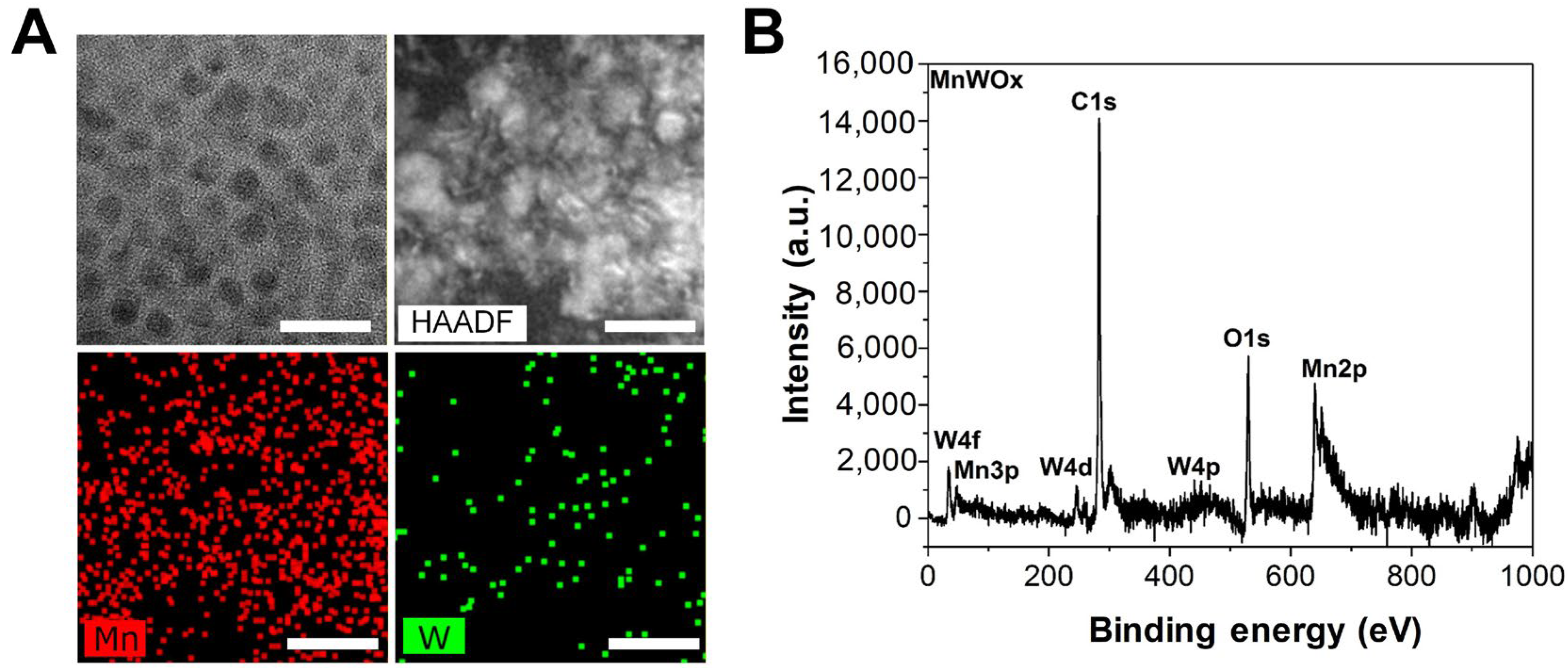
2.1. Fabrication and Characterization of MnWOx NPs
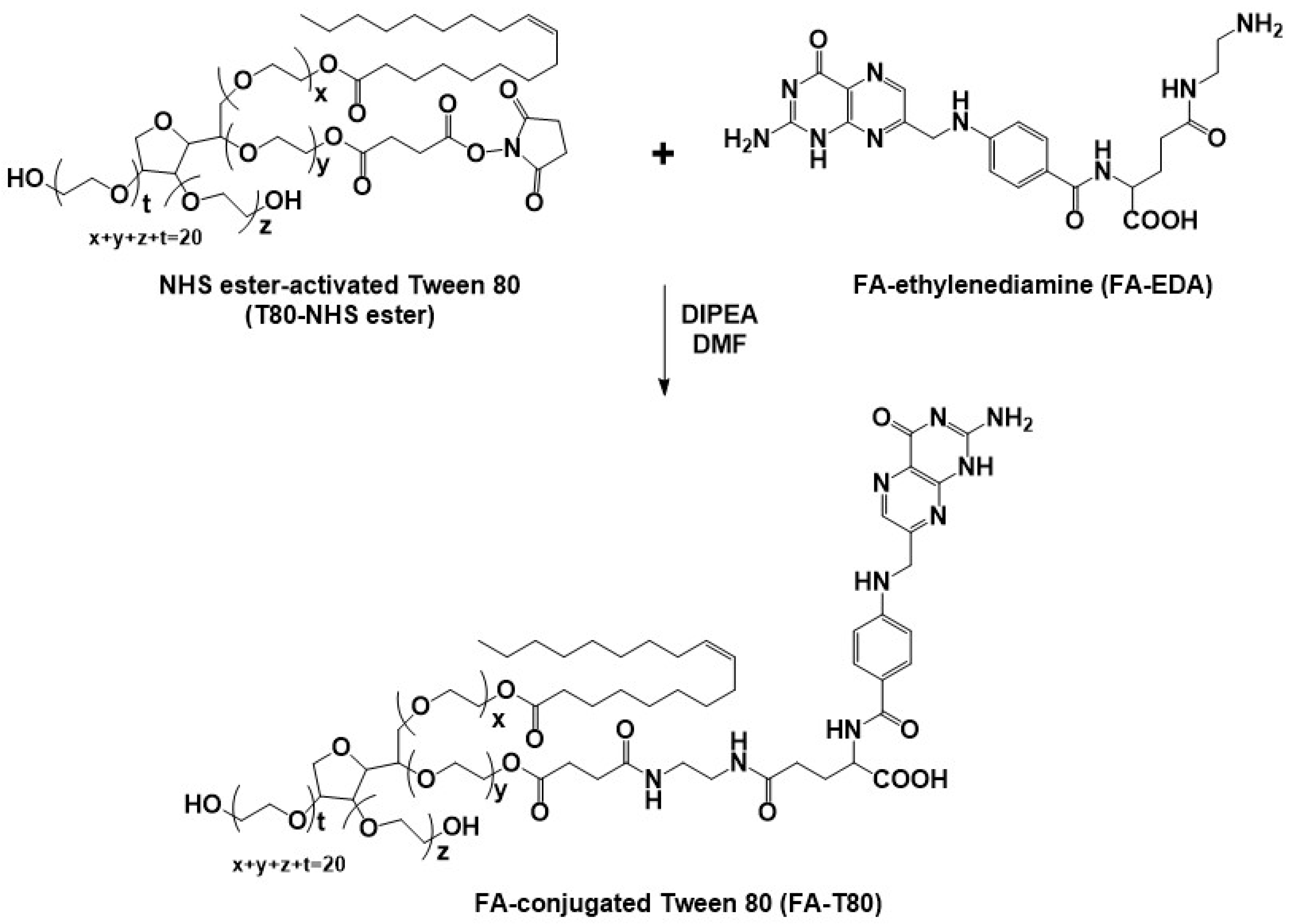
2.2. Synthesis and Characterization of FA-Conjugated Tween 80
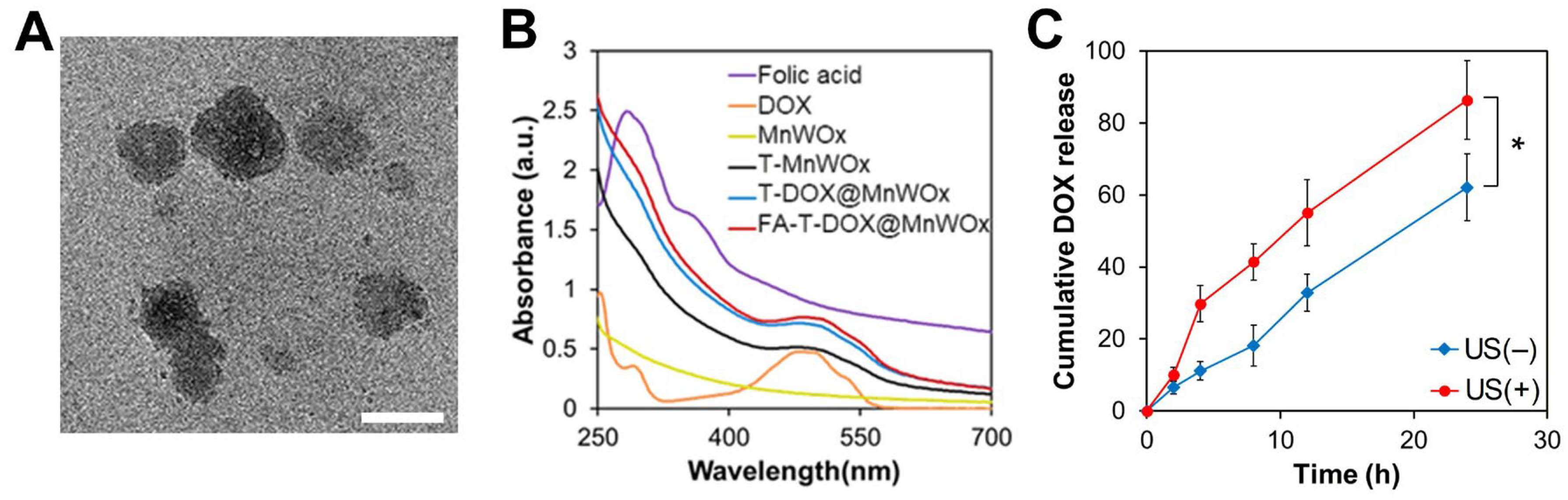
2.3. Fabrication and Characterization of MnWOx NP-Loaded T80 Nanocarriers
2.4. Enhanced DOX Release Profile from FA-T-DOX@MnWOx
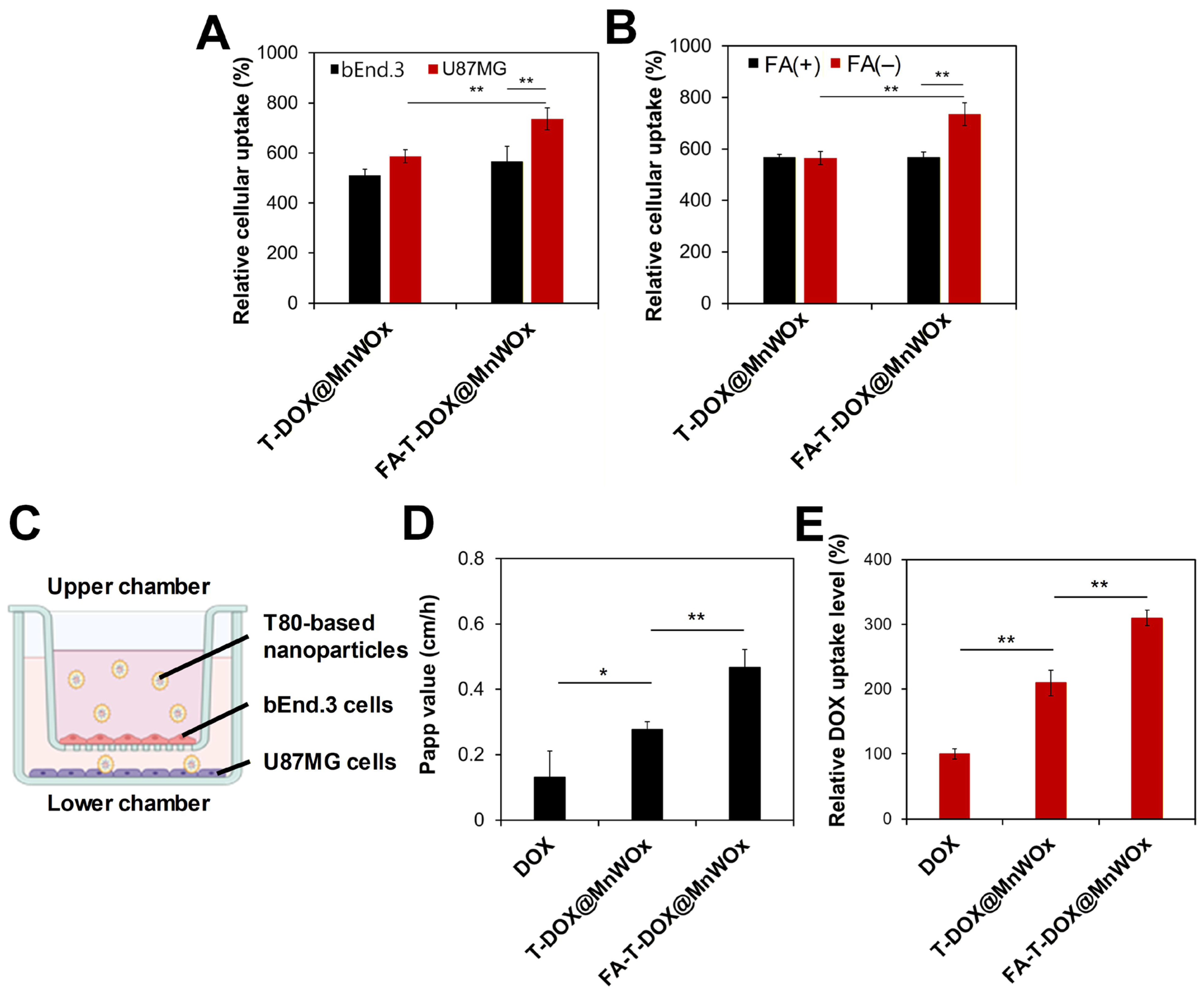
2.5. Cancer-Specific Cellular Uptake Analysis of FA-T-DOX@MnWOx
2.6. Efficient BBB Transcytosis of MnWOx-Loaded T80-Based Carriers
2.7. US-Activated Intracellular ROS Generation by T80 Nanocarriers Encapsulating MnWOx
2.8. Efficient GSH Depletion by MnWOx-Loaded T80 Nanocarriers
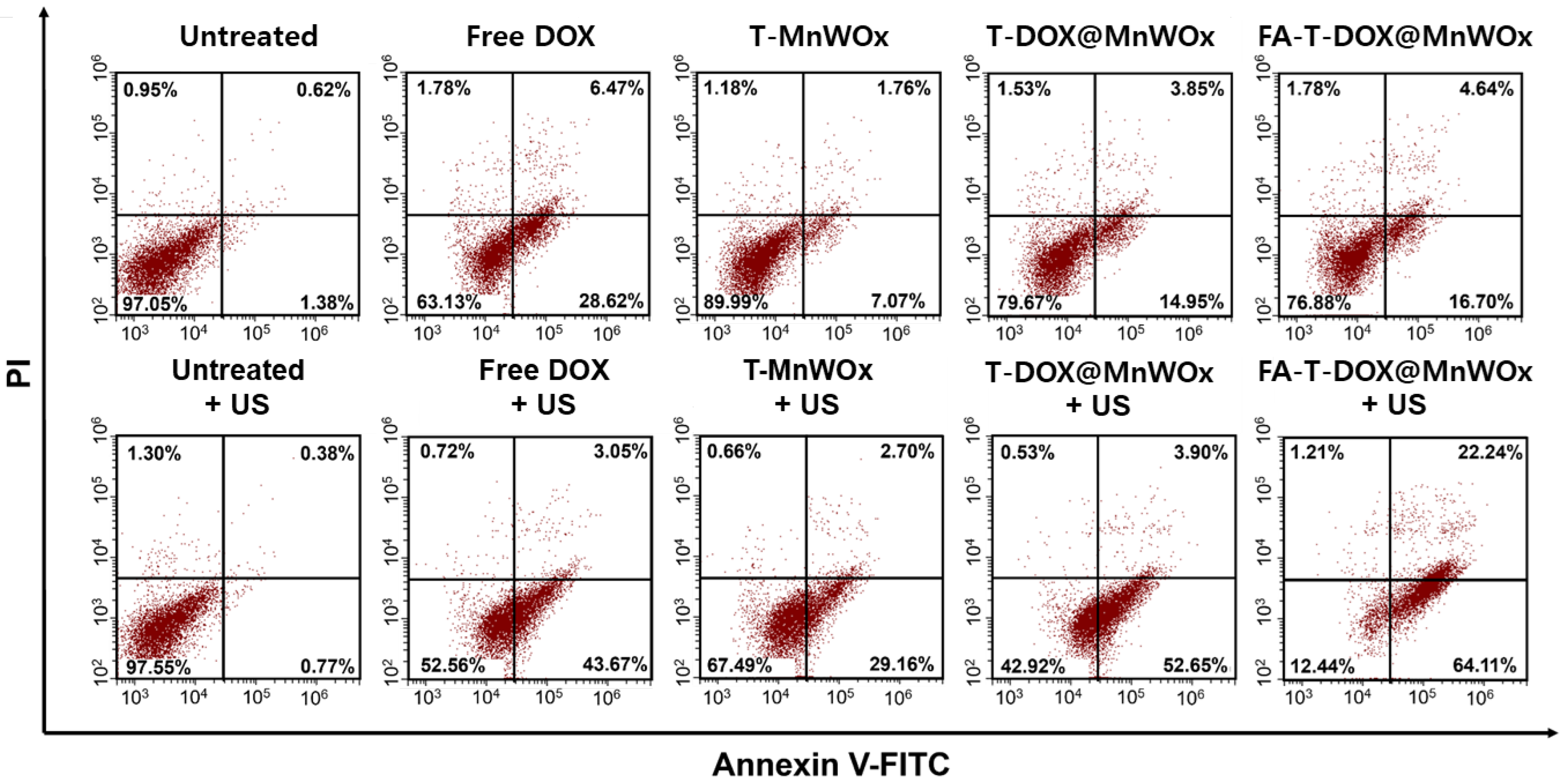
2.9. Cancer-Selective Combined Chemo-SDT Using FA-T-DOX@MnWOx
3. Materials and Methods
3.1. Materials
3.2. Preparation of MnWOx- and DOX-Loaded T80 Nanocarriers
3.3. Characterization of MnWOx NPs and MnWOx-Loaded T80-Based Carriers
3.4. DOX Release Profiles from FA-T-DOX@MnWOx
3.5. In Vitro Study
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in glioblastoma therapy: An update on current approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef] [PubMed]
- Chiariello, M.; Inzalaco, G.; Barone, V.; Gherardini, L. Overcoming challenges in glioblastoma treatment: Targeting infiltrating cancer cells and harnessing the tumor microenvironment. Front. Cell. Neurosci. 2023, 17, 1327621. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, J.; Jiang, S.; Tian, Y.; Zhang, Y.; Xu, L.; Hu, B.; Shi, H.; Li, Z.; Ran, G.; et al. Targeted reprogramming of tumor-associated macrophages for overcoming glioblastoma resistance to chemotherapy and immunotherapy. Biomaterials 2024, 311, 122708. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Antonio, M.; Daniel-da-Silva, A.L.; Sereno, J.; Oliveira, R.; Arnaut, L.G.; Gomes, C.; Ramos, M.L.; Castelo-Branco, M.; Sousa, J.; et al. A switch-on chemo-photothermal nanotherapy impairs glioblastoma. Mater. Horiz. 2025, 12, 4771–4787. [Google Scholar] [CrossRef]
- Xu, H.Z.; Li, T.F.; Ma, Y.; Li, K.; Zhang, Q.; Xu, Y.H.; Zhang, Y.C.; Zhao, L.; Chen, X. Targeted photodynamic therapy of glioblastoma mediated by platelets with photo-controlled release property. Biomaterials 2022, 290, 121833. [Google Scholar] [CrossRef]
- Ghosh, D.; Nandi, S.; Bhattacharjee, S. Combination therapy to checkmate glioblastoma: Clinical challenges and advances. Clin. Transl. Med. 2018, 7, 33. [Google Scholar] [CrossRef]
- Zhao, M.; van Straten, D.; Broekman, M.L.D.; Preat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef]
- Xing, X.; Zhao, S.; Xu, T.; Huang, L.; Zhang, Y.; Lan, M.; Lin, C.; Zheng, X.; Wang, P. Advances and perspectives in organic sonosensitizers for sonodynamic therapy. Coord. Chem. Rev. 2021, 445, 214087. [Google Scholar] [CrossRef]
- Shono, K.; Mizobuchi, Y.; Yamaguchi, I.; Nakajima, K.; Fujiwara, Y.; Fujihara, T.; Kitazato, K.; Matsuzaki, K.; Uto, Y.; Sampetrean, O.; et al. Elevated cellular PpIX potentiates sonodynamic therapy in a mouse glioma stem cell-bearing glioma model by downregulating the Akt/NF-kappaB/MDR1 pathway. Sci. Rep. 2021, 11, 15105. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Li, D.; Pan, J.; Chu, C.; Liu, G. Organic sonosensitizers for sonodynamic therapy: From small molecules and nanoparticles toward clinical development. Small 2021, 17, e2101976. [Google Scholar] [CrossRef]
- Jiang, Z.; Xiao, W.; Fu, Q. Stimuli responsive nanosonosensitizers for sonodynamic therapy. J. Control. Release 2023, 361, 547–567. [Google Scholar] [CrossRef] [PubMed]
- Hutton, D.L.; Burns, T.C.; Hossain-Ibrahim, K. A review of sonodynamic therapy for brain tumors. Neurosurg. Focus 2024, 57, E7. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, P.; Zhang, J.; Sun, Y.; Sun, S.; Xu, M.; Zhang, L.; Wang, S.; Liang, X.; Cui, L. Design and application of inorganic nanoparticles for sonodynamic cancer therapy. Biomater. Sci. 2021, 9, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Kim, J.H.; Wang, X.; Zhang, C.; Yoon, S.A.; Shin, J.; Sharma, A.; Lee, M.H.; Cheng, L.; Wu, J.; et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020, 49, 3244–3261. [Google Scholar] [CrossRef]
- Gong, F.; Cheng, L.; Yang, N.; Betzer, O.; Feng, L.; Zhou, Q.; Li, Y.; Chen, R.; Popovtzer, R.; Liu, Z. Ultrasmall oxygen-deficient bimetallic Oxide MnWO(X) nanoparticles for depletion of endogenous GSH and enhanced sonodynamic cancer therapy. Adv. Mater. 2019, 31, 1900730. [Google Scholar] [CrossRef]
- Dong, Y.; Dong, S.; Liu, B.; Yu, C.; Liu, J.; Yang, D.; Yang, P.; Lin, J. 2D piezoelectric Bi2MoO6 nanoribbons for GSH-enhanced sonodynamic therapy. Adv. Mater. 2021, 33, 2106838. [Google Scholar] [CrossRef]
- Ravichandran, V.; Hoang, Q.T.; Cao, T.G.N.; Shim, M.S. Glutathione-depleted and cancer-targeted nanocapsules encapsulating bimetallic oxide nanoparticles for enhanced chemo-sonodynamic therapy. J. Ind. Eng. Chem. 2022, 114, 171–180. [Google Scholar] [CrossRef]
- Ganipineni, L.P.; Danhier, F.; Preat, V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J. Control. Release 2018, 281, 42–57. [Google Scholar] [CrossRef]
- Guo, Q.L.; Dai, X.L.; Yin, M.Y.; Cheng, H.W.; Qian, H.S.; Wang, H.; Zhu, D.M.; Wang, X.W. Nanosensitizers for sonodynamic therapy for glioblastoma multiforme: Current progress and future perspectives. Mil. Med. Res. 2022, 9, 26. [Google Scholar] [CrossRef]
- Huang, Y.; Ouyang, W.; Lai, Z.; Qiu, G.; Bu, Z.; Zhu, X.; Wang, Q.; Yu, Y.; Liu, J. Nanotechnology-enabled sonodynamic therapy against malignant tumors. Nanoscale Adv. 2024, 6, 1974–1991. [Google Scholar] [CrossRef]
- Yang, N.; Li, J.; Yu, S.; Xia, G.; Li, D.; Yuan, L.; Wang, Q.; Ding, L.; Fan, Z.; Li, J. Application of Nanomaterial-Based Sonodynamic Therapy in Tumor Therapy. Pharmaceutics 2024, 16, 603. [Google Scholar] [CrossRef]
- Tao, X.; Li, Y.; Hu, Q.; Zhu, L.; Huang, Z.; Yi, J.; Yang, X.; Hu, J.; Feng, X. Preparation and drug release study of novel nanopharmaceuticals with polysorbate 80 surface adsorption. J. Nanomater. 2018, 2018, 4718045. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, M.; Alrobaian, M.M.; Alghamdi, S.A.; Warsi, M.H.; Sultana, S.; Khan, R.A. Brain targeted Polysorbate-80 coated PLGA thymoquinone nanoparticles for the treatment of Alzheimer’s disease, with biomechanistic insights. J. Drug Deliv. Sci. Technol. 2021, 61, 102214. [Google Scholar] [CrossRef]
- Ravichandran, V.; Lee, M.; Nguyen Cao, T.G.; Shim, M.S. Polysorbate-based drug formulations for brain-targeted drug delivery and anticancer therapy. Appl. Sci. 2021, 11, 9336. [Google Scholar] [CrossRef]
- Kreuter, J.; Shamenkov, D.; Petrov, V.; Ramge, P.; Cychutek, K.; Koch-Brandt, C.; Alyautdin, R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J. Drug Target. 2002, 10, 317–325. [Google Scholar] [CrossRef]
- McCord, E.; Pawar, S.; Koneru, T.; Tatiparti, K.; Sau, S.; Iyer, A.K. Folate receptors’ expression in gliomas may possess potential nanoparticle-based drug delivery opportunities. ACS Omega 2021, 6, 4111–4118. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ritter, C.A.; von Woedtke, T.; Bekeschus, S.; Wende, K. Package delivered: Folate receptor-mediated transporters in cancer therapy and diagnosis. Chem. Sci. 2024, 15, 1966–2006. [Google Scholar] [CrossRef]
- Allen, M.; Bjerke, M.; Edlund, H.; Nelander, S.; Westermark, B. Origin of the U87MG glioma cell line: Good news and bad news. Sci. Transl. Med. 2016, 8, 354re3. [Google Scholar] [CrossRef] [PubMed]
- Mazrad, Z.A.; Refaat, A.; Morrow, J.P.; Voelcker, N.H.; Nicolazzo, J.A.; Leiske, M.N.; Kempe, K. Folic acid-conjugated brush polymers show enhanced blood–brain barrier crossing in static and dynamic in vitro models toward brain cancer targeting therapy. ACS Biomater. Sci. Eng. 2024, 10, 2894–2910. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zeng, H.; Zheng, Y. Low-intensity ultrasound enhances the antitumor effects of doxorubicin on hepatocellular carcinoma cells through the ROS-miR-21-PTEN axis. Mol. Med. Rep. 2020, 21, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Feng, L.; Hao, Y.; Li, Q.; Chen, M.; Yang, Z.; Zhao, H.; Liu, Z. Synthesis of CaCO3-based nanomedicine for enhanced sonodynamic therapy via amplification of tumor oxidative stress. Chem 2020, 6, 1391–1407. [Google Scholar] [CrossRef]
- Lin, L.S.; Song, J.; Song, L.; Ke, K.; Liu, Y.; Zhou, Z.; Shen, Z.; Li, J.; Yang, Z.; Tang, W. Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Chem. Int. Ed. 2018, 130, 4996–5000. [Google Scholar]
- Schwartzberg, L.S.; Navari, R.M. Safety of polysorbate 80 in the oncology setting. Adv. Ther. 2018, 35, 754–767. [Google Scholar] [CrossRef]
- Kang, H.J.; Min, J.; Ravichandran, V.; Truong Hoang, Q.; Tram, L.T.H.; Kim, B.C.; Shim, M.S. Bioreducible polysorbate-based micelles encapsulating mitochondria-targeting sonosensitizers for efficient chemo-sonodynamic cancer therapy. Biotechnol. Bioprocess. Eng. 2025, 30, 642–651. [Google Scholar] [CrossRef]
- Nguyen Cao, T.G.; Kang, J.H.; Kang, S.J.; Truong Hoang, Q.; Kang, H.C.; Rhee, W.J.; Zhang, Y.S.; Ko, Y.T.; Shim, M.S. Brain endothelial cell-derived extracellular vesicles with a mitochondria-targeting photosensitizer effectively treat glioblastoma by hijacking the blood–brain barrier. Acta Pharm. Sin. B 2023, 13, 3834–3848. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, S.; Kang, H.C.; Shim, M.S. ROS-responsive thioether-based nanocarriers for efficient pro-oxidant cancer therapy. J. Ind. Eng. Chem. 2019, 75, 238–245. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.J.; Truong Hoang, Q.; Nguyen, N.C.; Pham, B.T.T.; Nguyen Cao, T.G.; Ravichandran, V.; Shim, M.S. Polysorbate-Based Carriers Encapsulating Oxygen-Deficient Nanoparticles for Targeted and Effective Chemo-Sonodynamic Therapy of Glioblastoma. Int. J. Mol. Sci. 2025, 26, 10235. https://doi.org/10.3390/ijms262010235
Kang HJ, Truong Hoang Q, Nguyen NC, Pham BTT, Nguyen Cao TG, Ravichandran V, Shim MS. Polysorbate-Based Carriers Encapsulating Oxygen-Deficient Nanoparticles for Targeted and Effective Chemo-Sonodynamic Therapy of Glioblastoma. International Journal of Molecular Sciences. 2025; 26(20):10235. https://doi.org/10.3390/ijms262010235
Chicago/Turabian StyleKang, Hyeon Ju, Quan Truong Hoang, Nguyen Cao Nguyen, Binh Thi Thanh Pham, Thuy Giang Nguyen Cao, Vasanthan Ravichandran, and Min Suk Shim. 2025. "Polysorbate-Based Carriers Encapsulating Oxygen-Deficient Nanoparticles for Targeted and Effective Chemo-Sonodynamic Therapy of Glioblastoma" International Journal of Molecular Sciences 26, no. 20: 10235. https://doi.org/10.3390/ijms262010235
APA StyleKang, H. J., Truong Hoang, Q., Nguyen, N. C., Pham, B. T. T., Nguyen Cao, T. G., Ravichandran, V., & Shim, M. S. (2025). Polysorbate-Based Carriers Encapsulating Oxygen-Deficient Nanoparticles for Targeted and Effective Chemo-Sonodynamic Therapy of Glioblastoma. International Journal of Molecular Sciences, 26(20), 10235. https://doi.org/10.3390/ijms262010235





