Characterization of Recombinant Human Type II Collagen from CHO Cells, Functional Assessment of Chondrocytes and Alleviation of Cartilage Degeneration
Abstract
1. Introduction
2. Results
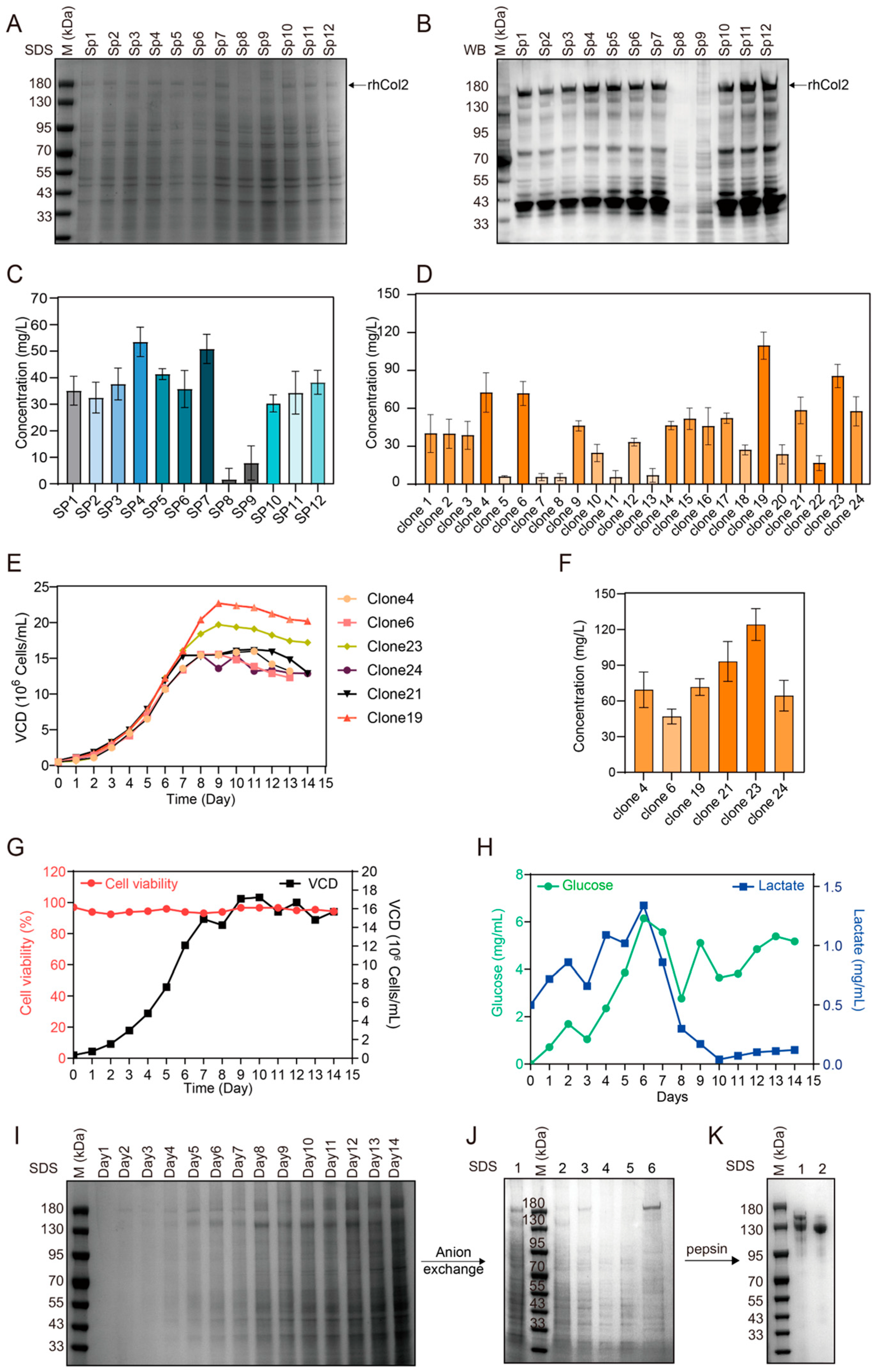
2.1. Evaluation of Various Signal Peptides
2.2. High-Level Expression and Purification of rhCol2
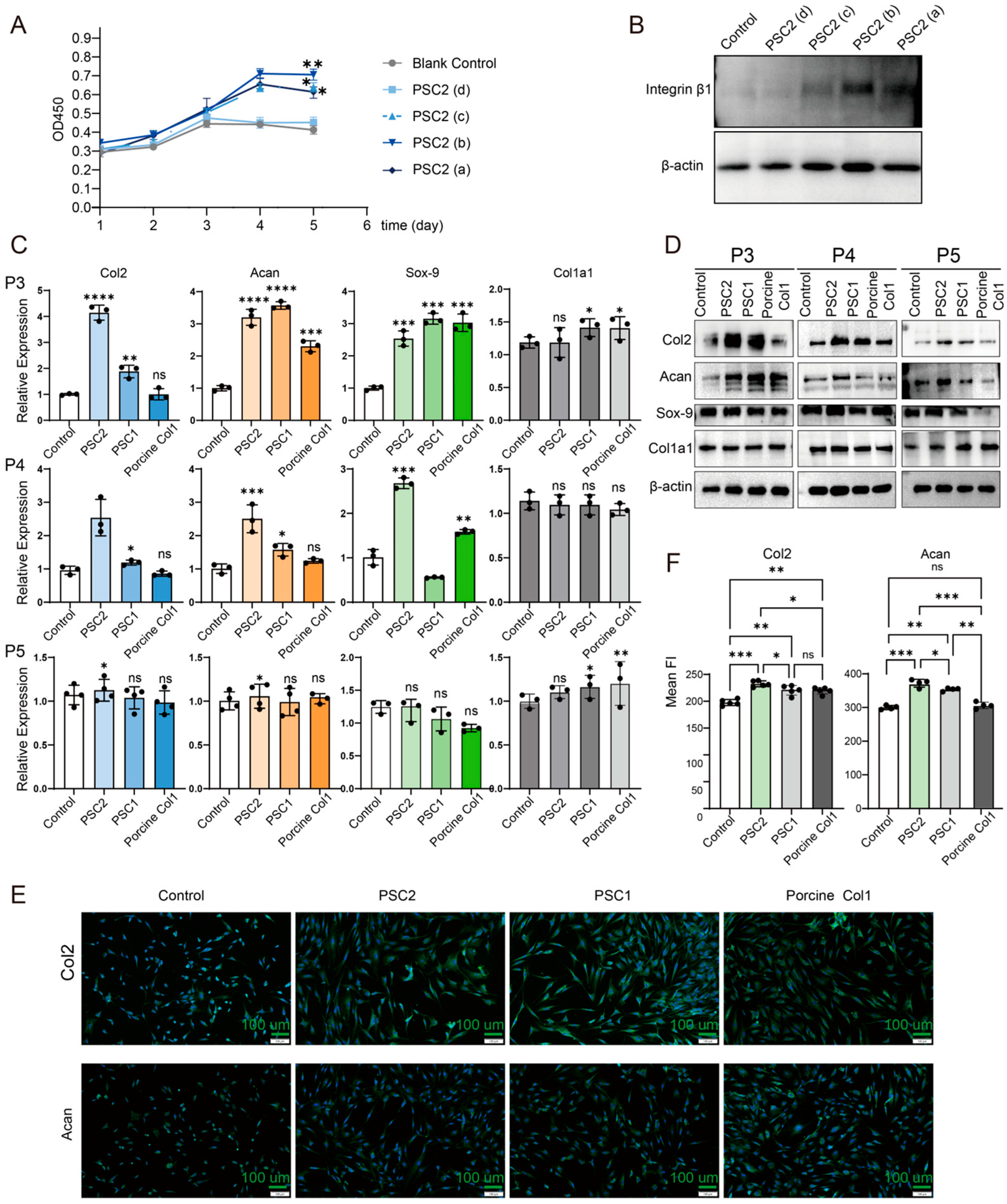
2.3. Characterization of PSC2
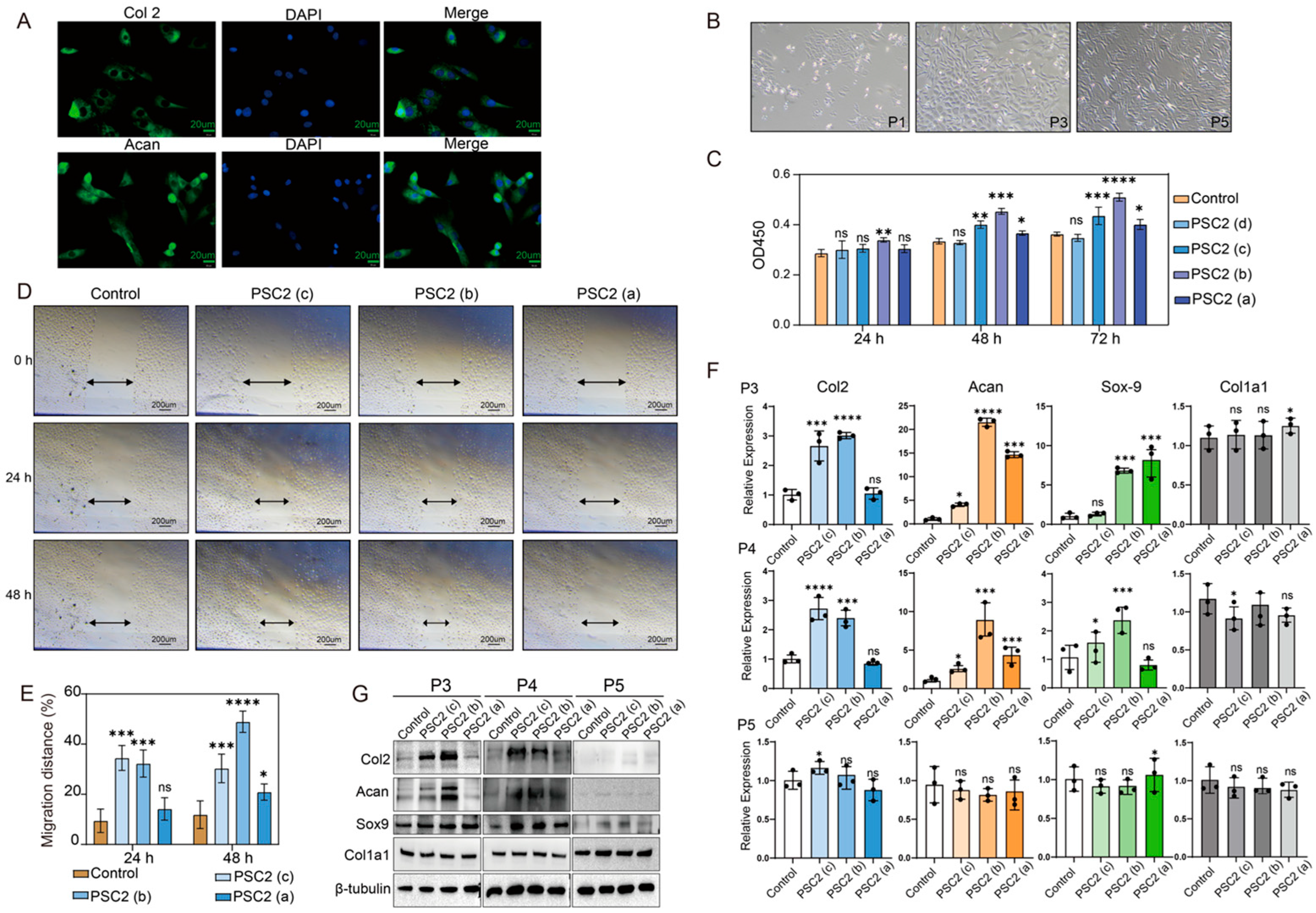
2.4. Effects of PSC2 on Rat Chondrocytes
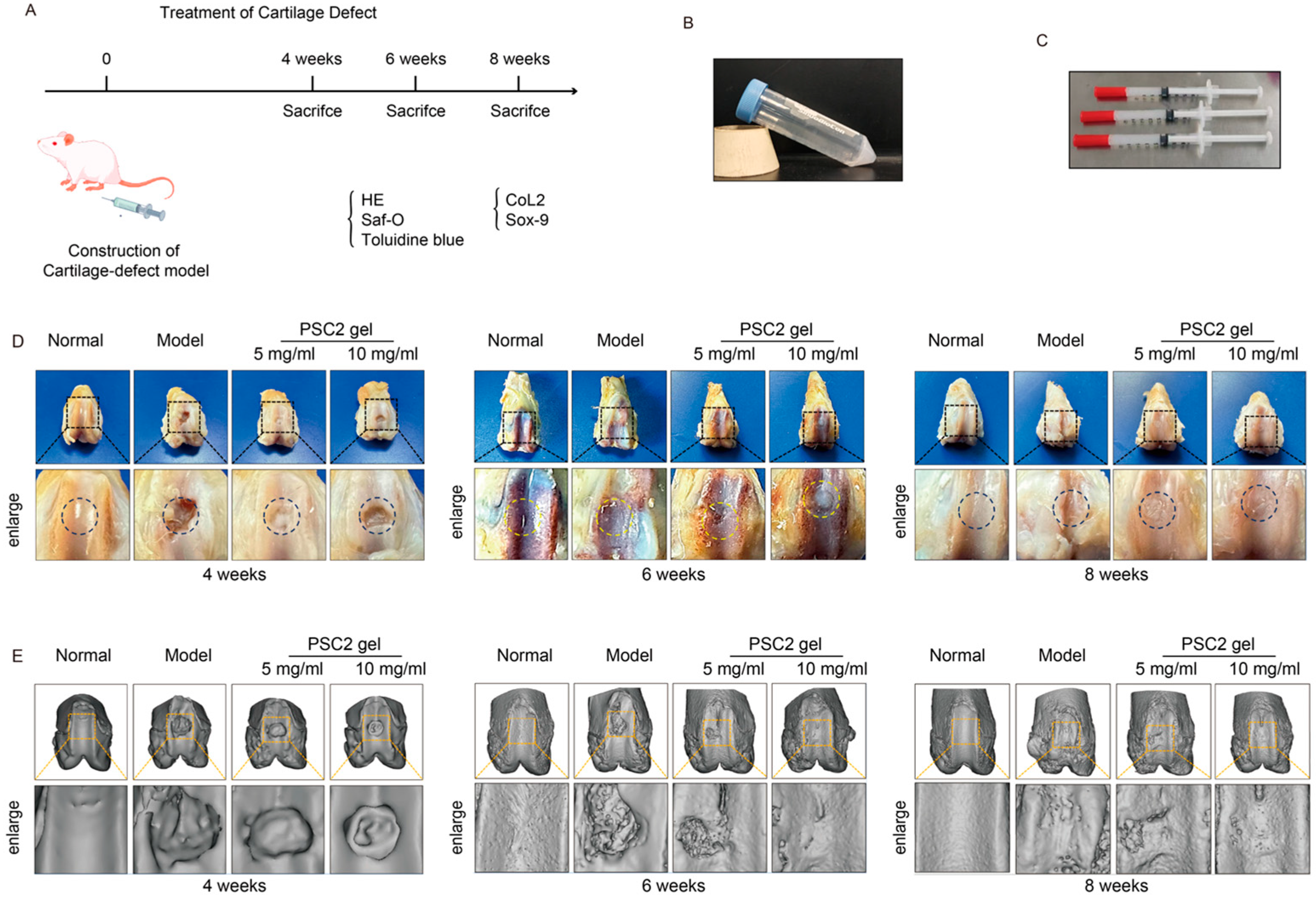
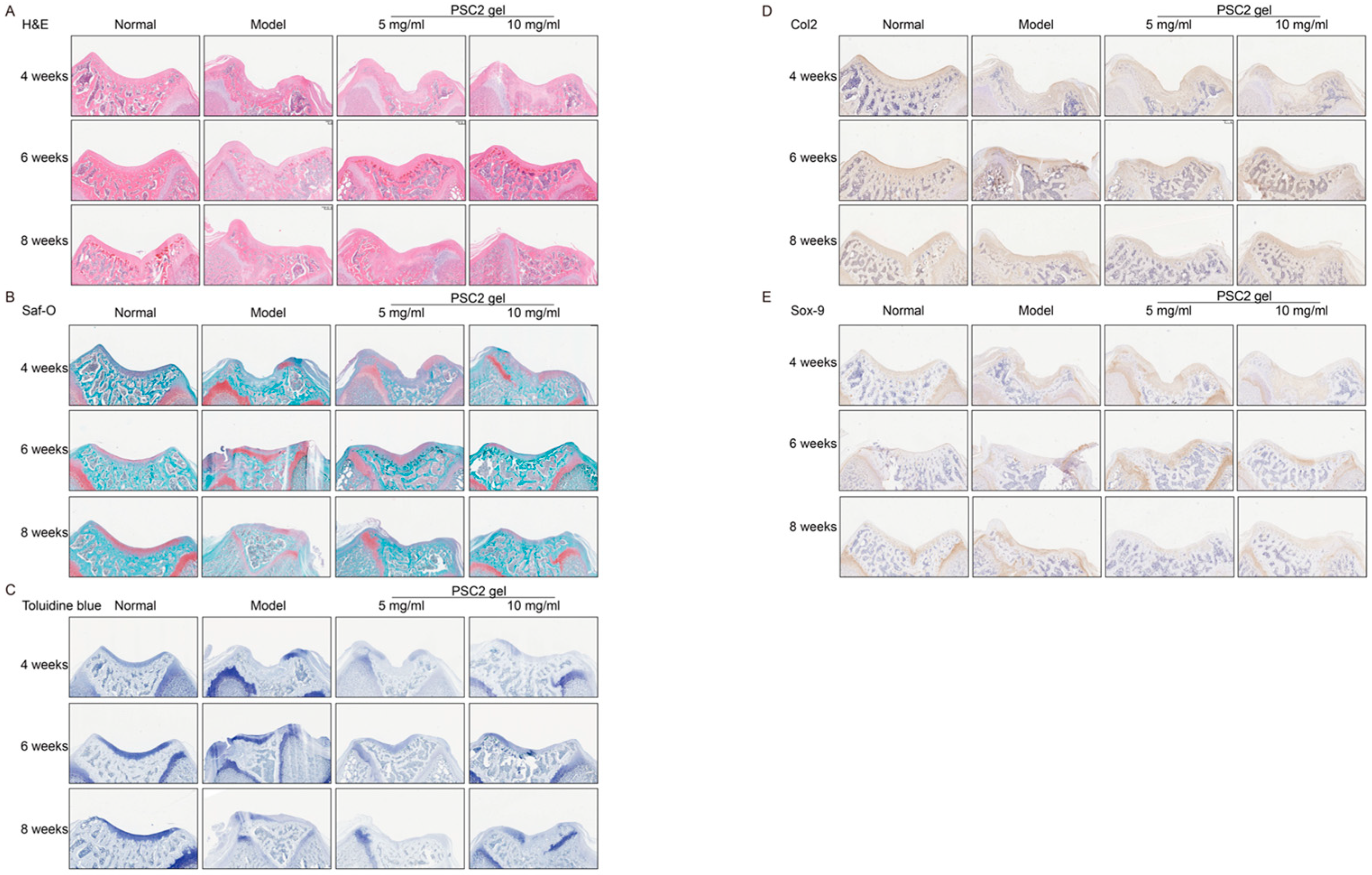
2.5. PSC2 Promotes In Vivo Cartilage Regeneration
2.6. Effects of Different Types of Collagen on Human Chondrocytes
3. Discussion
4. Materials and Methods
4.1. CHO Cell Culture
4.2. Expression of rhCol2
4.2.1. Vector Construct
4.2.2. Transfection and Stable Expression
4.2.3. Screening of the Optimal Signal Peptide
4.2.4. Screening for High-Yield Cloning
4.2.5. Production of rhCol2 in a Bioreactor
4.3. Purification of rhCol2
4.4. SDS–PAGE and Western Blot Analysis
4.5. Peptide Mapping Analysis
4.6. Amino Acid Analysis
4.7. SEC-MALS Experiments
4.8. UV–VIS Absorption Spectra
4.9. Fourier Transform Infrared Spectroscopy (FTIR)
4.10. Circular Dichroism (CD) Spectroscopy
4.11. Zeta Potential Analysis
4.12. Differential Scanning Calorimetry (DSC)
4.13. Transmission Electron Microscopy (TEM)
4.14. Scanning Electron Microscopy (SEM)
4.15. Chondrocyte Isolation and Culture
4.16. Immunofluorescence Assays
4.17. Analysis of Chondrocyte Activity
4.18. Cell Migration
4.19. Gene Expression Analysis
4.20. Regeneration of Articular Cartilage Defects In Vivo
4.21. Micro-CT Scan
4.22. Histological and Immunohistochemical Staining
4.23. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Korntner, S.H.; Mullen, A.M.; Zeugolis, D.I. Collagen type II: From biosynthesis to advanced biomaterials for cartilage engineering. Biomater. Biosyst. 2021, 4, 100030. [Google Scholar] [CrossRef]
- Intini, C.; Hodgkinson, T.; Casey, S.M.; Gleeson, J.P.; O’Brien, F.J. Highly Porous Type II Collagen-Containing Scaffolds for Enhanced Cartilage Repair with Reduced Hypertrophic Cartilage Formation. Bioengineering 2022, 9, 232. [Google Scholar] [CrossRef]
- Ko, C.S.; Huang, J.P.; Huang, C.W.; Chu, I.M. Type II collagen-chondroitin sulfate-hyaluronan scaffold cross-linked by genipin for cartilage tissue engineering. J. Biosci. Bioeng. 2009, 107, 177–182. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2010, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Sadigursky, D.; Magnavita, V.F.S.; Sa, C.K.C.; Monteiro, H.S.; Braghiroli, O.F.M.; Matos, M.A.A. Undenatured Collagen Type Ii for the Treatment of Osteoarthritis of the Knee. Acta Ortop. Bras. 2022, 30, e240572. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Dai, H.; Zhang, Y.; Fu, Y. The potential of undenatured type II collagen against arthritis: A review. Collagen Leather 2024, 6, 1–19, Correction in Collagen Leather 2025, 7, 1. [Google Scholar] [CrossRef]
- Dai, M.; Sui, B.; Xue, Y.; Liu, X.; Sun, J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials 2018, 180, 91–103. [Google Scholar] [CrossRef]
- Choi, B.; Kim, S.; Lin, B.; Wu, B.M.; Lee, M. Cartilaginous extracellular matrix-modified chitosan hydrogels for cartilage tissue engineering. ACS Appl. Mater. Interfaces 2014, 6, 20110–20121. [Google Scholar] [CrossRef]
- Rutgers, M.; Saris, D.B.; Vonk, L.A.; van Rijen, M.H.; Akrum, V.; Langeveld, D.; van Boxtel, A.; Dhert, W.J.; Creemers, L.B. Effect of collagen type I or type II on chondrogenesis by cultured human articular chondrocytes. Tissue Eng. Part A 2013, 19, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.B.; Su, D.H.; Liu, P.; Ma, Y.Q.; Shao, Z.Z.; Dong, J. Shape-memory collagen scaffold for enhanced cartilage regeneration: Native collagen versus denatured collagen. Osteoarthr. Cartil. 2018, 26, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Pulkkinen, H.J.; Tiitu, V.; Valonen, P.; Jurvelin, J.S.; Lammi, M.J.; Kiviranta, I. Engineering of cartilage in recombinant human type II collagen gel in nude mouse model in vivo. Osteoarthr. Cartil. 2010, 18, 1077–1087. [Google Scholar] [CrossRef]
- Muhonen, V.; Narcisi, R.; Nystedt, J.; Korhonen, M.; van Osch, G.J.; Kiviranta, I. Recombinant human type II collagen hydrogel provides a xeno-free 3D micro-environment for chondrogenesis of human bone marrow-derived mesenchymal stromal cells. J. Tissue Eng. Regen. Med. 2017, 11, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Korntner, S.H.; Mullen, A.M.; Skoufos, I.; Zeugolis, D.I. In the quest of the optimal tissue source (porcine male and female articular, tracheal and auricular cartilage) for the development of collagen sponges for articular cartilage. Biomed. Eng. Adv. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Xiao, S.; Huang, X.; He, X.; Chen, Z.; Li, X.; Wei, X.; Liu, Q.; Dong, H.; Zeng, X.; Bai, W. Interactions between curcumin and fish/bovine-derived (type I and II) collagens: Preparation of nanoparticle and their application in Pickering emulsions. Food Chem. 2025, 487, 144781. [Google Scholar] [CrossRef]
- Akram, A.N.; Zhang, C. Extraction of collagen-II with pepsin and ultrasound treatment from chicken sternal cartilage; physicochemical and functional properties. Ultrason. Sonochem 2020, 64, 105053. [Google Scholar] [CrossRef]
- Cao, H.; Xu, S.Y. Purification and characterization of type II collagen from chick sternal cartilage. Food Chem. 2008, 108, 439–445. [Google Scholar] [CrossRef]
- Dai, M.; Liu, X.; Wang, N.; Sun, J. Squid type II collagen as a novel biomaterial: Isolation, characterization, immunogenicity and relieving effect on degenerative osteoarthritis via inhibiting STAT1 signaling in pro-inflammatory macrophages. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Sewing, J.; Klinger, M.; Notbohm, H. Jellyfish collagen matrices conserve the chondrogenic phenotype in two- and three-dimensional collagen matrices. J. Tissue Eng. Regen. Med. 2017, 11, 916–925. [Google Scholar] [CrossRef]
- Zhang, X.; Adachi, S.; Ura, K.; Takagi, Y. Properties of collagen extracted from Amur sturgeon Acipenser schrenckii and assessment of collagen fibrils in vitro. Int. J. Biol. Macromol. 2019, 137, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2017, 17, 20–26. [Google Scholar] [CrossRef]
- Chen, C.-X.; Zhang, Y.-Y.; Yang, J.; Yan, M.-H.; Jia, Y.; Jiang, S. An overview of progress in the application of recombinant collagen in cosmetics. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100059. [Google Scholar] [CrossRef]
- Xi, C.; Liu, N.; Liang, F.; Zhao, X.; Long, J.; Yuan, F.; Yun, S.; Sun, Y.; Xi, Y. Molecular assembly of recombinant chicken type II collagen in the yeast Pichia pastoris. Sci. China Life Sci. 2018, 61, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yu, S.; Sun, R.; Xu, K.; Zhao, X.; Zhou, J.; Rao, Y.; Wang, X. Biosynthesis of a Functional Fragment of Human Collagen II in Pichia pastoris. ACS Synth. Biol. 2024, 13, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Lioi, M.; Tengattini, S.; Bagatin, F.; Galliani, S.; Daly, S.; Massolini, G.; Temporini, C. Development of a rapid, efficient, and reusable magnetic bead-based immunocapture system for recombinant human procollagen type II isolation from yeast fermentation broth. Anal. Bioanal. Chem. 2023, 415, 3155–3166. [Google Scholar] [CrossRef]
- Qi, Q.; Yao, L.; Liang, Z.; Yan, D.; Li, Z.; Huang, Y.; Sun, J. Production of human type II collagen using an efficient baculovirus-silkworm multigene expression system. Mol. Genet. Genom. 2016, 291, 2189–2198. [Google Scholar] [CrossRef]
- Ala-Kokko, L.; Hyland, J.; Smith, C.; Kivirikko, K.; Jimenez, S.; Prockop, D. Expression of a human cartilage procollagen gene (COL2A1) in mouse 3T3 cells. J. Biol. Chem. 1991, 266, 14175. [Google Scholar] [CrossRef]
- Zhou, H.; Mu, Y.; Ma, C.; Zhang, Z.; Tao, C.; Wang, D.A. Rejuvenating Hyaline Cartilaginous Phenotype of Dedifferentiated Chondrocytes in Collagen II Scaffolds: A Mechanism Study Using Chondrocyte Membrane Nanoaggregates as Antagonists. ACS Nano 2024, 18, 2077–2090. [Google Scholar] [CrossRef]
- Rottmar, M.; Mhanna, R.; Guimond-Lischer, S.; Vogel, V.; Zenobi-Wong, M.; Maniura-Weber, K. Interference with the contractile machinery of the fibroblastic chondrocyte cytoskeleton induces re-expression of the cartilage phenotype through involvement of PI3K, PKC and MAPKs. Exp. Cell Res. 2014, 320, 175–187. [Google Scholar] [CrossRef]
- Xin, W.; Heilig, J.; Paulsson, M.; Zaucke, F. Collagen II regulates chondroycte integrin expression profile and differentiation. Connect. Tissue Res. 2015, 56, 307–314. [Google Scholar] [CrossRef]
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineering 2020, 7, 155. [Google Scholar] [CrossRef]
- Revell, C.K.; Jensen, O.E.; Shearer, T.; Lu, Y.; Holmes, D.F.; Kadler, K.E. Collagen fibril assembly: New approaches to unanswered questions. Matrix Biol. Plus 2021, 12, 100079. [Google Scholar] [CrossRef] [PubMed]
- Aravamudhan, A.; Ramos, D.M.; Jenkins, N.A.; Dyment, N.A.; Sanders, M.M.; Rowe, D.W.; Kumbar, S.G. Collagen nanofibril self-assembly on a natural polymeric material for the osteoinduction of stem cells in vitro and biocompatibility in vivo. RSC Adv. 2016, 6, 80851–80866. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.; Liang, Y.; Yuan, W.; Bian, L.; Duan, L.; Rong, Z.; Xiong, J.; Wang, D.; Xia, J. 3D printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomater. Sci. 2021, 9, 2620–2630. [Google Scholar] [CrossRef]
- Hu, X.; Wu, Z.; Zhang, Z.; Yao, H.; Wang, D.-A. Type II collagen scaffolds for tissue engineering. Commun. Mater. 2024, 5, 149. [Google Scholar] [CrossRef]
- Chen, R.; Pye, J.S.; Li, J.; Little, C.B.; Li, J.J. Multiphasic scaffolds for the repair of osteochondral defects: Outcomes of preclinical studies. Bioact. Mater. 2023, 27, 505–545. [Google Scholar] [CrossRef]
- Zhou, L.; Gjvm, V.O.; Malda, J.; Stoddart, M.J.; Lai, Y.; Richards, R.G.; Ki-Wai Ho, K.; Qin, L. Innovative Tissue-Engineered Strategies for Osteochondral Defect Repair and Regeneration: Current Progress and Challenges. Adv. Healthc. Mater. 2020, 9, e2001008. [Google Scholar] [CrossRef] [PubMed]
- Kober, L.; Zehe, C.; Bode, J. Optimized signal peptides for the development of high expressing CHO cell lines. Biotechnol. Bioeng. 2013, 110, 1164–1173. [Google Scholar] [CrossRef]
- Attallah, C.; Etcheverrigaray, M.; Kratje, R.; Oggero, M. A highly efficient modified human serum albumin signal peptide to secrete proteins in cells derived from different mammalian species. Protein Expr. Purif. 2017, 132, 27–33. [Google Scholar] [CrossRef]
- Bender, M.F.; Li, Y.; Ivleva, V.B.; Gowetski, D.B.; Paula Lei, Q. Protein and glycan molecular weight determination of highly glycosylated HIV-1 envelope trimers by HPSEC-MALS. Vaccine 2021, 39, 3650–3654. [Google Scholar] [CrossRef]
- Lebendiker, M.; Tsadok, A.; Amartely, H.; Some, D. Characterization of Proteins by Size-Exclusion Chromatography Coupled to Multi-Angle Light Scattering (SEC-MALS). J. Vis. Exp. 2019, 148, e59615. [Google Scholar]
- Anand, S.; Kamath, S.; Chuang, L.; Kasapis, S.; Lopata, A.L. Biochemical and thermo-mechanical analysis of collagen from the skin of Asian Sea bass (Lates calcarifer) and Australasian Snapper (Pagrus auratus), an alternative for mammalian collagen. Eur. Food Res. Technol. 2013, 236, 873–882. [Google Scholar] [CrossRef]
- Li, C.; Tian, H.; Duan, L.; Tian, Z.; Li, G. Characterization of acylated pepsin-solubilized collagen with better surface activity. Int. J. Biol. Macromol. 2013, 57, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yuan, Q.; Yin, T.; You, J.; Gu, Z.; Xiong, S.; Hu, Y. Self-assembly of collagen-based biomaterials: Preparation, characterizations and biomedical applications. J. Mater. Chem. B 2018, 6, 2650–2676. [Google Scholar] [CrossRef]
- Sharma, V.; Sakhalkar, U.; Nadkarni, P.; Mishal, R.; Parandhaman, D.; Vichare, K.; Francis, A.; Khanna, M.; Kukreja, M.; Sharma, A. Cytoprotective Effect of Growth Factors Derived from Platelets on Corticosteroid-Treated Primary Anterior Cruciate Ligament-Derived Stromal Cells and Chondrocytes. Cureus 2024, 16, e65566. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Q.; Zhang, Y.; Xu, L.; Chen, X.; He, F.; Zhang, M.; Yang, H.; Yu, S.; Liu, X.; et al. Stress causes lipid droplet accumulation in chondrocytes by impairing microtubules. Osteoarthr. Cartil. 2025, 33, 351–363. [Google Scholar] [CrossRef]
- Xuan, H.; Hu, H.; Geng, C.; Song, J.; Shen, Y.; Lei, D.; Guan, Q.; Zhao, S.; You, Z. Biofunctionalized chondrogenic shape-memory ternary scaffolds for efficient cell-free cartilage regeneration. Acta Biomater. 2020, 105, 97–110. [Google Scholar] [CrossRef]
- Wang, C.; Guo, X.; Fan, M.; Yue, L.; Wang, H.; Wang, J.; Zha, Z.; Yin, H. Production of recombinant human type I collagen homotrimers in CHO cells and their physicochemical and functional properties. J. Biotechnol. 2024, 395, 149–160. [Google Scholar] [CrossRef]
- Li, J.; Ke, H.; Lei, X.; Zhang, J.; Wen, Z.; Xiao, Z.; Chen, H.; Yao, J.; Wang, X.; Wei, Z.; et al. Controlled-release hydrogel loaded with magnesium-based nanoflowers synergize immunomodulation and cartilage regeneration in tendon-bone healing. Bioact. Mater. 2024, 36, 62–82. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Sun, M.; Yang, P.; Hu, X.; Ao, Y.; Cheng, J. The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater. 2021, 125, 253–266. [Google Scholar] [CrossRef]
- Klatt, A.R.; Paul-Klausch, B.; Klinger, G.; Kuhn, G.; Renno, J.H.; Banerjee, M.; Malchau, G.; Wielckens, K. A critical role for collagen II in cartilage matrix degradation: Collagen II induces pro-inflammatory cytokines and MMPs in primary human chondrocytes. J. Orthop. Res. 2009, 27, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.F.; Doulabi, B.Z.; Huang, C.L.; Bank, R.A.; Helder, M.N. Collagen type II enhances chondrogenesis in adipose tissue-derived stem cells by affecting cell shape. Tissue Eng. Part A 2010, 16, 81–90. [Google Scholar] [PubMed]
- Wang, K.Y.; Jin, X.Y.; Ma, Y.H.; Cai, W.J.; Xiao, W.Y.; Li, Z.W.; Qi, X.; Ding, J. Injectable stress relaxation gelatin-based hydrogels with positive surface charge for adsorption of aggrecan and facile cartilage tissue regeneration. J. Nanobiotechnol. 2021, 19, 214. [Google Scholar] [CrossRef]
- Zhou, Z.; Lv, C.; Wang, Y.; Zhang, B.; Liu, L.; Yang, J.; Leng, X.; Zhao, D.; Yao, B.; Wang, J.; et al. BuShen JianGu Fang alleviates cartilage degeneration via regulating multiple genes and signaling pathways to activate NF-kappaB/Sox9 axis. Phytomedicine 2023, 113, 154742. [Google Scholar]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1−SMAD1 interaction. Bone Res. 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, S.; Yuan, G.; Jing, D.; Qin, L.; Zhao, H.; Yang, S. Type II collagen-positive progenitors are important stem cells in controlling skeletal development and vascular formation. Bone Res. 2022, 10, 46. [Google Scholar] [CrossRef] [PubMed]


| Number | Amino Acid | PSC2 | Triple Helix Region of the α1 Chain of Human Type II Collagen |
|---|---|---|---|
| 1 | Asp | 4.85% | 3.06% |
| 2 | Glu | 5.53% | 4.64% |
| 3 | Ser | 3.40% | 3.35% |
| 4 | His | 0.25% | 0.20% |
| 5 | Gly | 33.10% | 33.63% |
| 6 | Thr | 0.55% | 1.58% |
| 7 | Arg | 5.1% | 5.03% |
| 8 | Ala | 15.0% | 11.64% |
| 9 | Tyr | 0.18% | 0 |
| 10 | Cys | 0.36% | 0 |
| 11 | Val | 1.70% | 1.97% |
| 12 | Met | 0.26% | 0.69% |
| 13 | Trp | 0 | 0 |
| 14 | Phe | 1.56% | 0 |
| 15 | Ile | 1.06% | 0.59% |
| 16 | Leu | 2.87% | 1.87% |
| 17 | Lys | 2.19% | 3.55% |
| 18 | Gln | 1.26% | 2.66% |
| 19 | Asn | 0.74% | 1.08% |
| 20 | Hyp | 9.10% | 10.95% |
| 21 | Pro | 11.50% | 12.33% |
| Signal Peptide | Number | Amino Acid Sequence |
|---|---|---|
| Human Col2a1 (WT) | 1 | MIRLGAPQTLVLLTLLVAAVLRCQG |
| Human IgG | 2 | MGSAALLLWVLLLWVPGSNG |
| Modified IgG | 3 | MGSAALLLWVLLLWVPSSRA |
| Human Col3a1 | 4 | MMSFVQKGSWLLLALLHPTI ILA |
| Mouse Col1a1 | 5 | MFSFVDLRLLLLLGATALLTHG |
| Mouse Col2a1 | 6 | MIRLGAPQSLVLLTLLIAAVLRCQG |
| Mouse Col3a1 | 7 | MMSFVQSGTWFLLTLLHPTLILA |
| SA | 8 | MKWVTFISLLFLFSSAYS |
| modify SA | 9 | MKWVTFISLLFLFSSSSRA |
| AZ | 10 | MTRLTVLALLAGLLASSRA |
| Immunoglobulin heavy chain | 11 | MAWSPLFLTLITHCAGSWA |
| Immunoglobulin light chain | 12 | MDWTWRVFCLLAVTPGAHP |
| Gene | Primer Sequence (5′ to 3′) | Size (bp) | NCBI Reference |
|---|---|---|---|
| Col2a1 | F: ACACCGCTAACGTCCAGATG R: TCGGTACTCGATGATGGTCT | 254 | NM_001414896.1 |
| Acan | F: TTGGTAGGGTCTGCTTCTGG R: GATGGGCCACTTCCAATGTC | 143 | NM_022190.2 |
| Sox-9 | F: AAATTCCCAGTGTGCATCCG R: TGACGTGTGGCTTGTTCTTG | 115 | NM_080403.3 |
| Gapdh | F: CAAGGCTGAGAATGGGAAGC R: GAAGACGCCAGTAGACTCCA | 127 | NM_017008 |
| Col1a2 | F: CTGAGGGCAACAGCAGATTC R: CAGGCGAGATGGCTTATTCG | 113 | NM_053356.2 |
| Gene | Primer Sequence (5′ to 3′) | Size (bp) | NCBI Reference |
|---|---|---|---|
| Col2a1 | F: TGGACGATCAGGCGAAACC R: GCTGCGGATGCTCTCAATCT | 244 | NM_001844.5 |
| Acan | F: GTGCCTATCAGGACAAGGTCT R: GATGCCTTTCACCACGACTTC | 167 | NM_001135.4 |
| Sox-9 | F: AGCGAACGCACATCAAGAC R: CTGTAGGCGATCTGTTGGGG | 86 | NM_000346.4 |
| Gapdh | F: GGAGCGAGATCCCTCCAAAAT R: GGCTGTTGTCATACTTCTCATG | 197 | NM_001256799.3 |
| Col1a2 | F: GAGGGCCAAGACGAAGACATC R: CAGATCACGTCATCGCACAAC | 140 | NM_000089.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhang, Z.; Zha, Z.; Lu, C.; Wang, H.; Yue, L.; Yin, H. Characterization of Recombinant Human Type II Collagen from CHO Cells, Functional Assessment of Chondrocytes and Alleviation of Cartilage Degeneration. Int. J. Mol. Sci. 2025, 26, 10232. https://doi.org/10.3390/ijms262010232
Wang C, Zhang Z, Zha Z, Lu C, Wang H, Yue L, Yin H. Characterization of Recombinant Human Type II Collagen from CHO Cells, Functional Assessment of Chondrocytes and Alleviation of Cartilage Degeneration. International Journal of Molecular Sciences. 2025; 26(20):10232. https://doi.org/10.3390/ijms262010232
Chicago/Turabian StyleWang, Chuan, Zhijie Zhang, Zhengqi Zha, Chunyang Lu, Hang Wang, Long Yue, and Hongping Yin. 2025. "Characterization of Recombinant Human Type II Collagen from CHO Cells, Functional Assessment of Chondrocytes and Alleviation of Cartilage Degeneration" International Journal of Molecular Sciences 26, no. 20: 10232. https://doi.org/10.3390/ijms262010232
APA StyleWang, C., Zhang, Z., Zha, Z., Lu, C., Wang, H., Yue, L., & Yin, H. (2025). Characterization of Recombinant Human Type II Collagen from CHO Cells, Functional Assessment of Chondrocytes and Alleviation of Cartilage Degeneration. International Journal of Molecular Sciences, 26(20), 10232. https://doi.org/10.3390/ijms262010232







