Deacidification of the Endolysosomal System by the Vesicular Proton Pump V-ATPase Inhibitor Bafilomycin A1 Affects EGF Receptor Endocytosis Differently in Endometrial MSC and HeLa Cells
Abstract
1. Introduction
2. Results
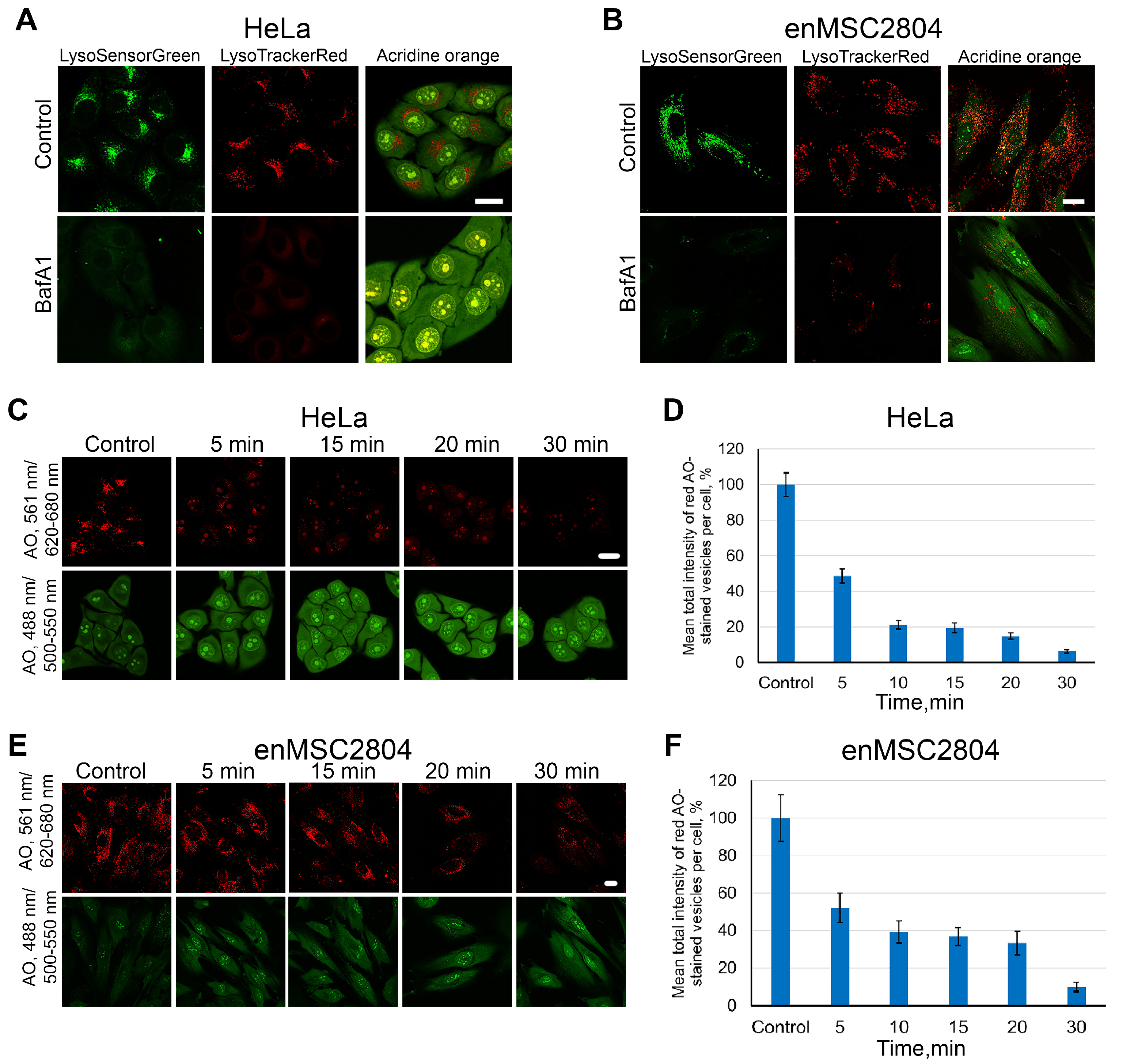
2.1. Dynamics of Endolysosome Deacidification During Pretreatment of Cells with BafA1
2.2. EGF Receptor Endocytosis Under BafA1 Pretreatment in Tumor-Derived and MSC Cells
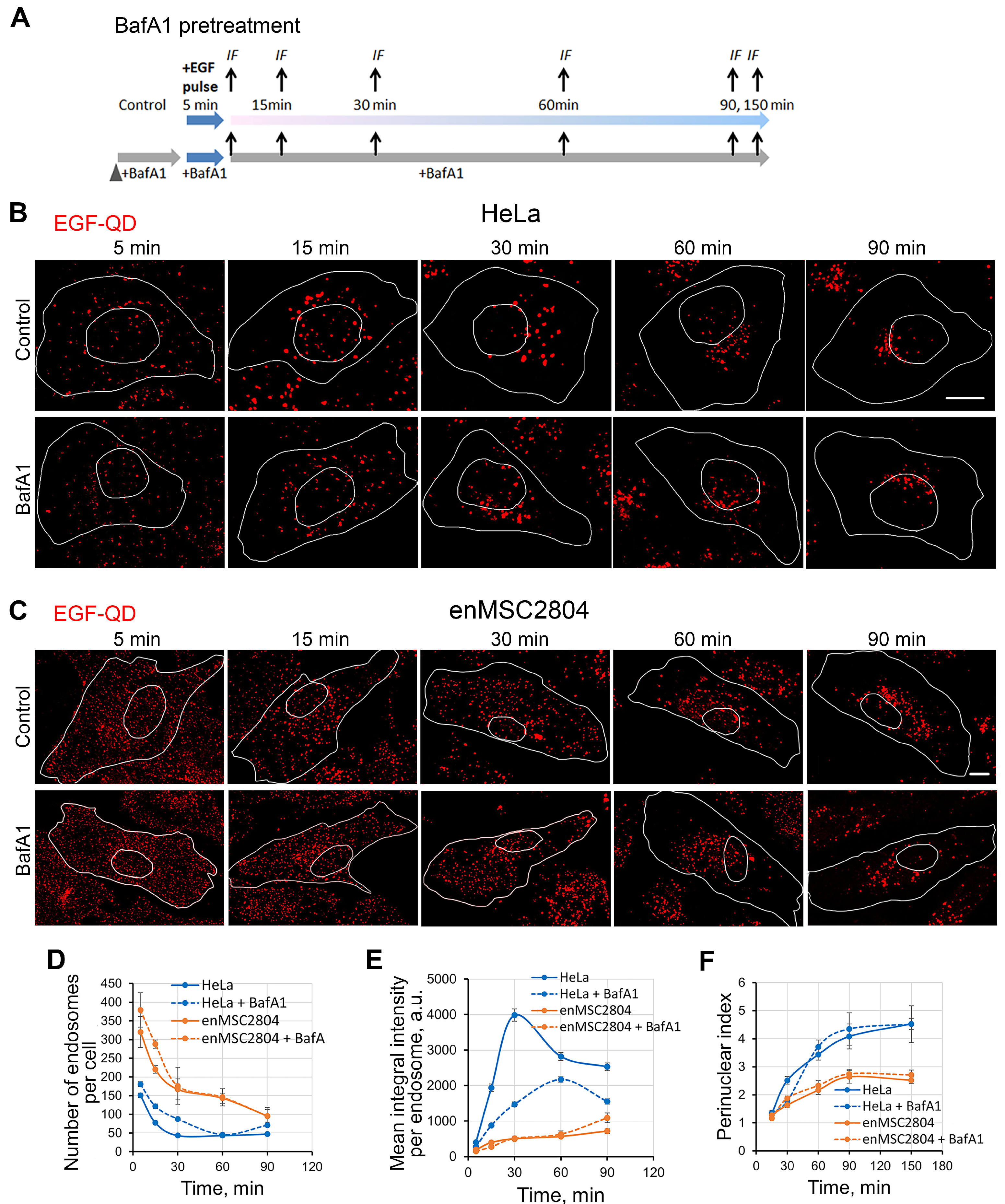
2.3. Effect of BafA1 Pretreatment on the Dynamics of the Main Stages of EGF Receptor Endocytosis Stimulated by EGF-QDs in Tumor and Mesenchymal Stromal Cells
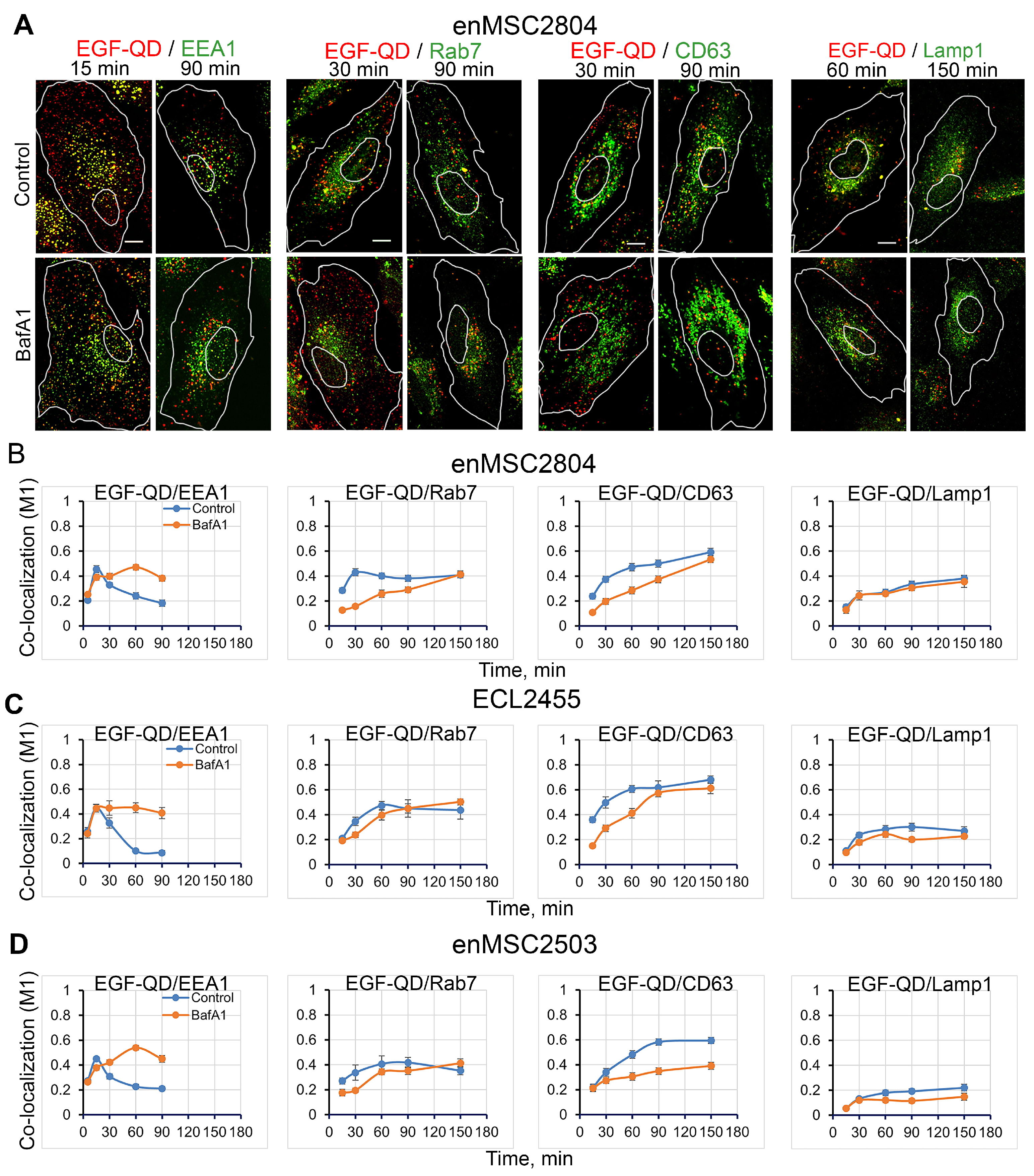
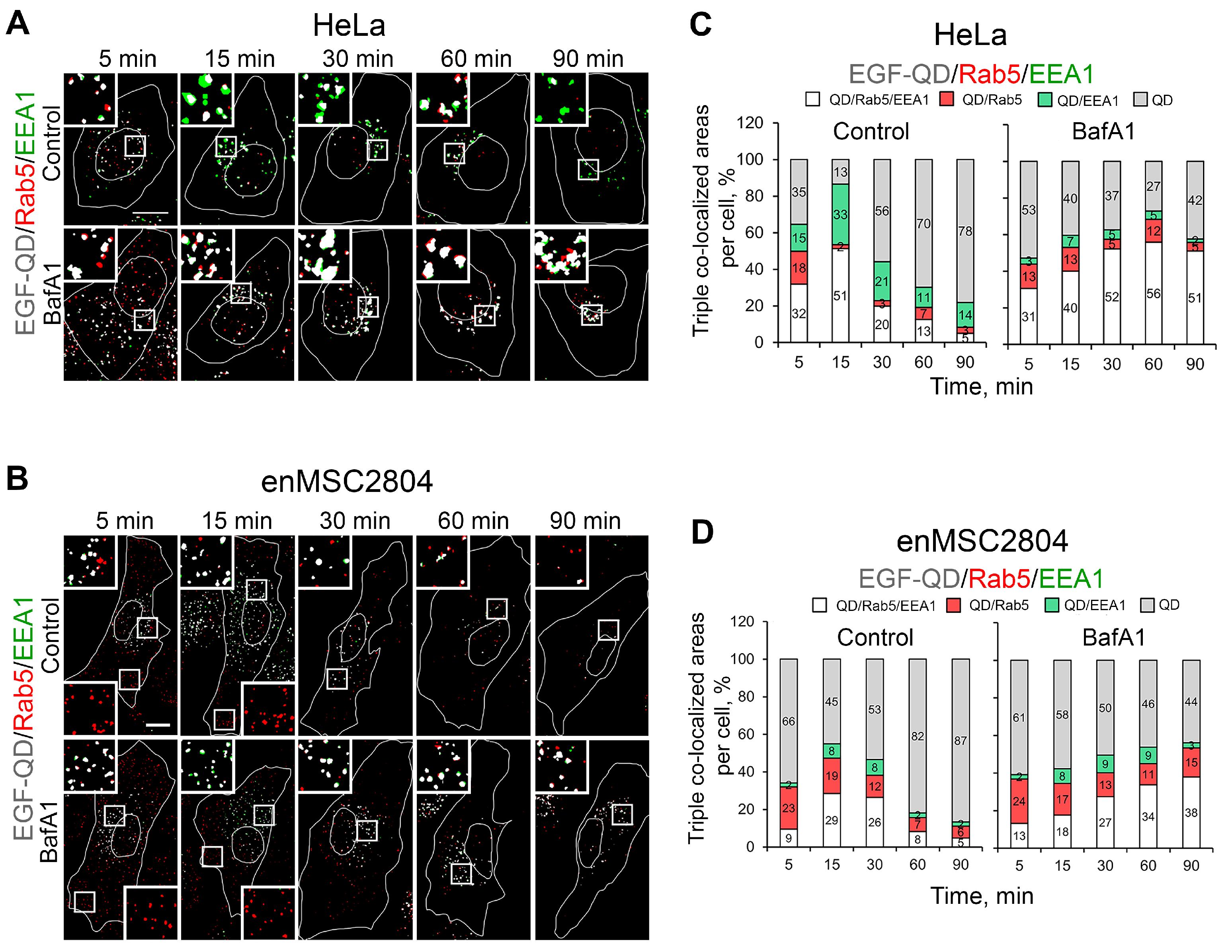
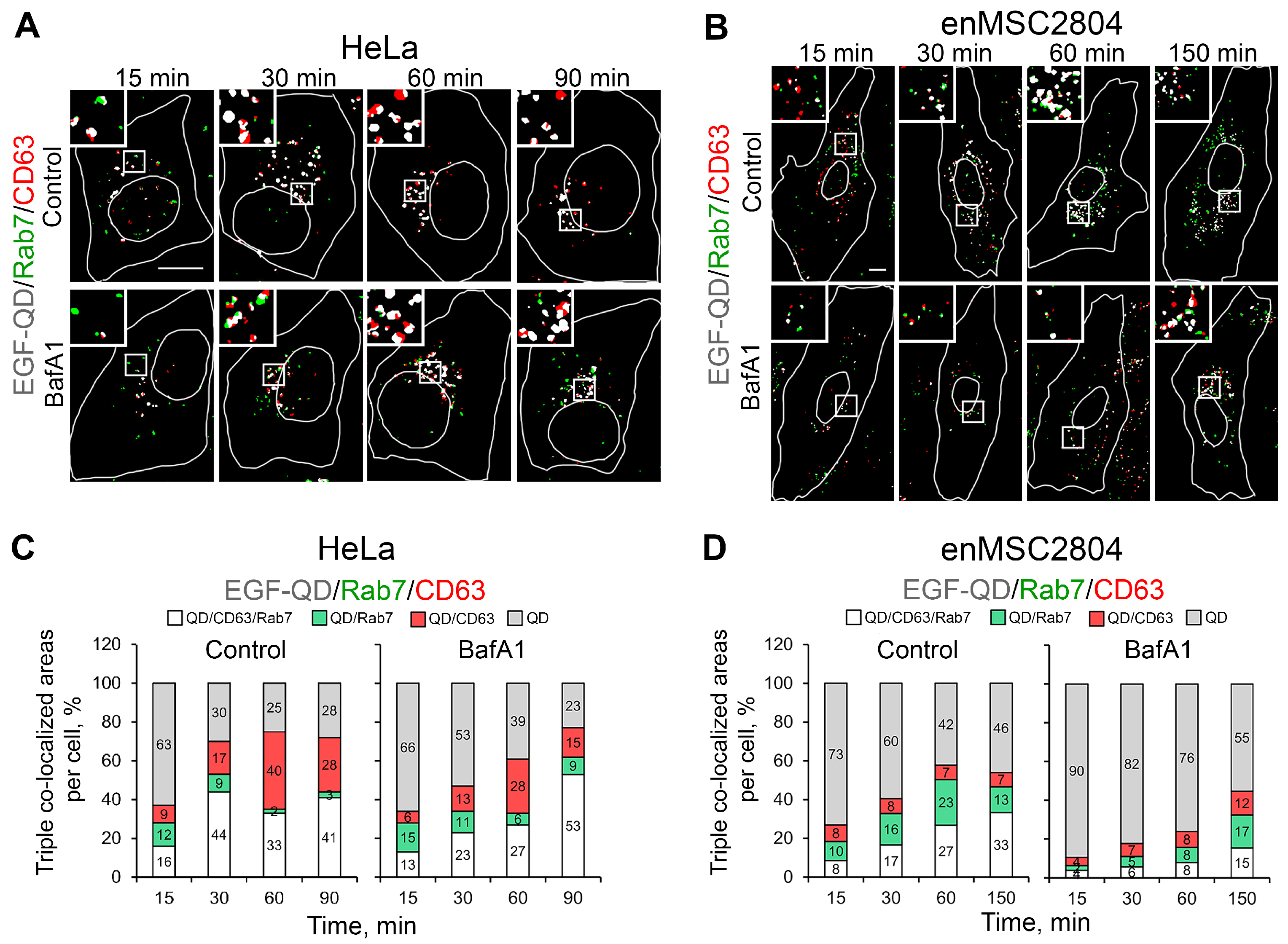
2.4. The Effect of Suppression of V-ATPase Activity on the Sorting Process of EGF-Receptor Complexes Within Endocytic Pathway Compartments
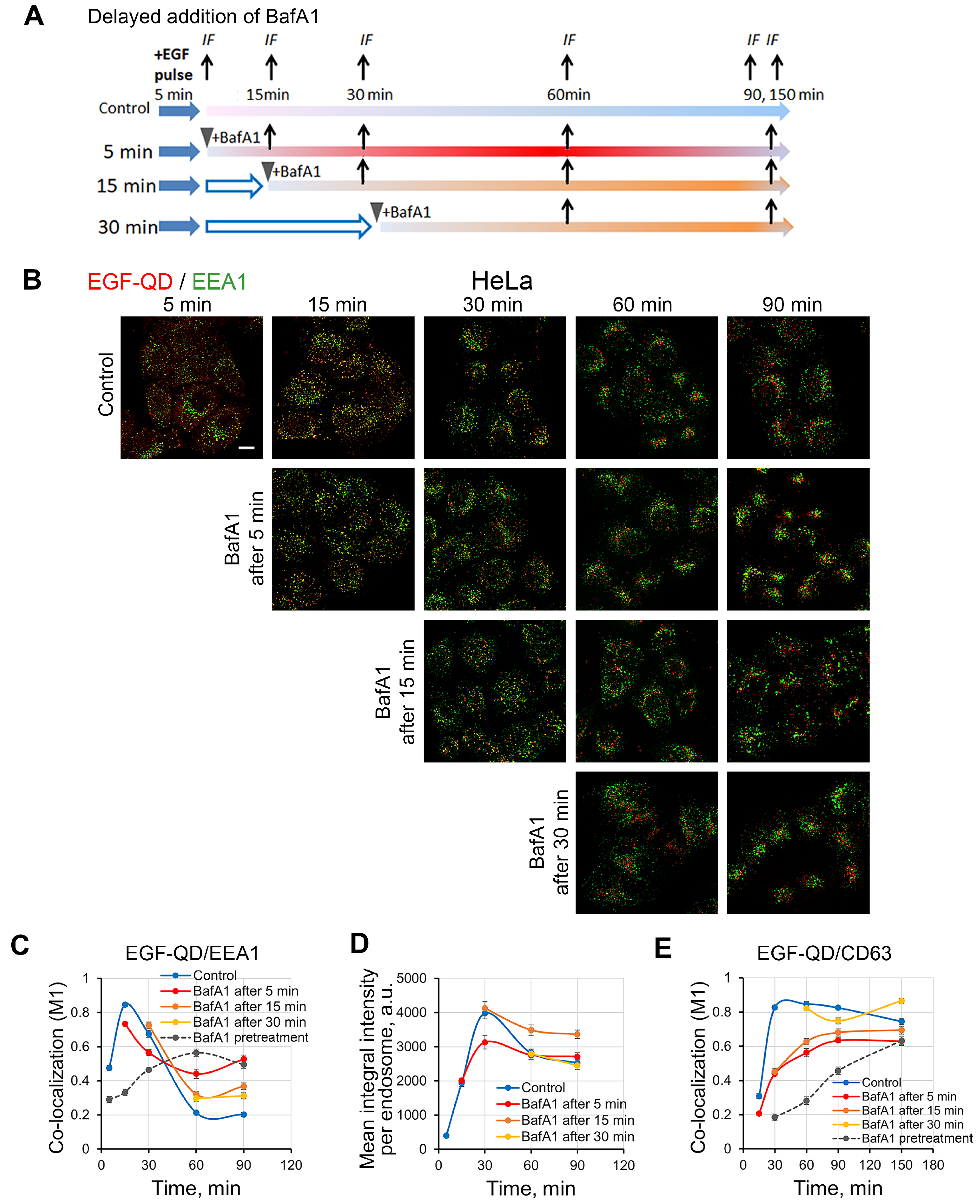
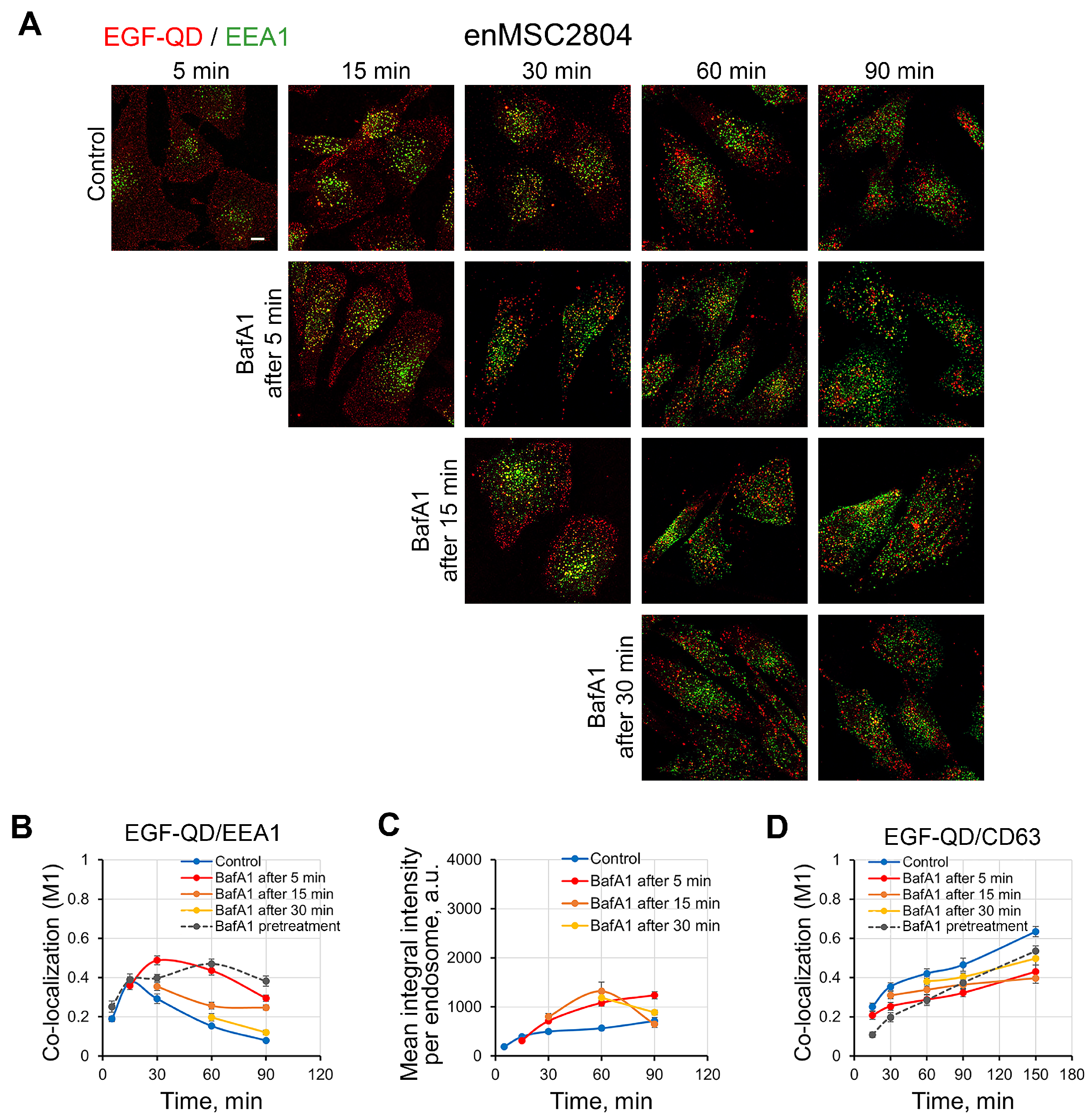
2.5. Effect of Delayed Addition of BafA1 on the Dynamics of EGF-QD Compartmentalization in HeLa Cells and enMSCs
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Stimulation of Endocytosis
4.3. Cell Treatments
4.4. Acidified Compartment Staining
4.5. Immunofluorescent Staining
4.6. Confocal Microscopy
4.7. Image and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BafA1 | Bafilomycin A1 |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| enMSCs | Endometrial Mesenchymal stem/stromal cells |
| MSCs | Mesenchymal stem/stromal cells |
| MVBs | Multivesicular bodies |
| QDs | Quantum dots |
References
- Skarpen, E.; Johannessen, L.E.; Bjerk, K.; Fasteng, H.; Guren, T.K.; Lindeman, B.; Thoresen, G.H.; Christoffersen, T.; Stang, E.; Huitfeldt, H.S.; et al. Endocytosed epidermal growth factor (EGF) receptors contribute to the EGF-mediated growth arrest in A431 cells by inducing a sustained increase in p21/CIP1. Exp. Cell Res. 1998, 243, 161–172. [Google Scholar] [CrossRef]
- Teis, D.; Wunderlich, W.; Huber, L.A. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell 2002, 3, 803–814. [Google Scholar] [CrossRef]
- Hammond, D.E.; Carter, S.; Clague, M.J. Met Receptor Dynamics and Signalling. In Signalling from Internalised Growth Factor Receptors; Madshus, I.H., Ed.; Springer: Heidelberg, Germany, 2004; pp. 21–46. [Google Scholar]
- Sigismund, S.; Confalonieri, S.; Ciliberto, A.; Polo, S.; Scita, G.; Di Fiore, P.P. Endocytosis and Signaling: Cell Logistics Shape the Eukaryotic Cell Plan. Physiol. Rev. 2012, 92, 273–366. [Google Scholar] [CrossRef] [PubMed]
- Kindberg, G.M.; Tolleshaug, H.; Gjøen, T.; Berg, T. Lysosomal and endosomal heterogeneity in the liver: A comparison of the intracellular pathways of endocytosis in rat liver cells. Hepatology 1991, 13, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, N.; Urukova, Y.; Cardelli, M.; Aubin, J.E.; Harrison, R.E. Lysosome dispersion in osteoblasts accommodates enhanced collagen production during differentiation. J. Biol. Chem. 2008, 283, 19678–19690. [Google Scholar] [CrossRef]
- Omari, S.; Makareeva, E.; Roberts-Pilgrim, A.; Mirigian, L.; Jarnik, M.; Ott, C.; Lippincott-Schwartz, J.; Leikin, S. Noncanonical autophagy at ER exit sites regulates procollagen turnover. Proc. Natl. Acad. Sci. USA 2018, 115, E10099–E10108. [Google Scholar] [CrossRef] [PubMed]
- Kamentseva, R.S.; Kharchenko, M.V.; Gabdrahmanova, G.V.; Kotov, M.A.; Kosheverova, V.V.; Kornilova, E.S. EGF, TGF-α and amphiregulin differently regulate endometrium-derived mesenchymal stromal/stem cells. Int. J. Mol. Sci. 2023, 24, 13408. [Google Scholar] [CrossRef]
- Kosheverova, V.; Kharchenko, M.; Kamentseva, R.; Kotov, M.; Schwarz, A.; Kuneev, I.; Kotova, A.; Enukashvily, N.; Kornilova, E. Human Mesenchymal Stromal Cells Derived from Different Tissues Show Similar Profiles of c-ErbB Receptor Family Expression at the mRNA and Protein Levels. Int. J. Mol. Sci. 2025, 26, 7201. [Google Scholar] [CrossRef]
- Sorkin, A.; Goh, L.K. Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 2008, 314, 3093–3106. [Google Scholar] [CrossRef]
- Decker, S.J. Epidermal growth factor and transforming growth factor-alpha induce differential processing of the epidermal growth factor receptor. Biochem. Biophys. Res. Commun. 1990, 166, 615–621. [Google Scholar] [CrossRef]
- Schultz, D.F.; Billadeau, D.D.; Jois, S.D. EGFR trafficking: Effect of dimerization, dynamics, and mutation. Front. Oncol. 2023, 13, 1258371. [Google Scholar] [CrossRef]
- Damare, R.; Engle, K.; Kumar, G. Targeting epidermal growth factor receptor and its downstream signaling pathways by natural products: A mechanistic insight. Phytother. Res. 2024, 38, 2406–2447. [Google Scholar] [CrossRef]
- Maxfield, F.R.; Yamashiro, D.J. Endosome acidification and the pathways of receptor-mediated endocytosis. Adv. Exp. Med. Biol. 1987, 225, 189–198. [Google Scholar] [PubMed]
- Mellman, I. The importance of being acid: The role of acidification in intracellular membrane traffic. J. Exp. Biol. 1992, 172, 39–45. [Google Scholar] [CrossRef]
- Clague, M.J.; Urbé, S. Multivesicular bodies. Curr. Biol. 2008, 18, R402–R404. [Google Scholar] [CrossRef][Green Version]
- Casey, J.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef]
- Murphy, R.F.; Powers, S.; Cantor, C.R. Endosome pH measured in single cells by dual fluorescence flow cytometry: Rapid acidification of insulin to pH 6. J. Cell Biol. 1984, 98, 1757–1762. [Google Scholar] [CrossRef]
- Bond, J.; Varley, J. Use of flow cytometry and SNARF to calibrate and measure intracellular pH in NS0 cells. Cytom. A 2005, 64, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Aloisio, F.M.; Charafeddine, R.A.; Cook, J.; Wittmann, T.; Barber, D.L. pHLARE: A new biosensor reveals decreased lysosome pH in cancer cells. Mol. Biol. Cell 2021, 32, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, D.J.; Fluss, S.R.; Maxfield, F.R. Acidification of endocytic vesicles by an ATP-dependent proton pump. J. Cell Biol. 1983, 97, 929–934. [Google Scholar] [CrossRef]
- Sun-Wada, G.-H.; Toyomura, T.; Murata, Y.; Yamamoto, A.; Futai, M.; Wada, Y. The a3 isoform of V-ATPase regulates insulin secretion from pancreatic β-cells. J. Cell Sci. 2006, 119, 4531–4540. [Google Scholar] [CrossRef] [PubMed]
- Marshansky, V.; Futai, M. The V-type H+-ATPase in vesicular trafficking: Targeting, regulation and function. Curr. Opin. Cell Biol. 2008, 20, 415–426. [Google Scholar] [CrossRef]
- Capecci, J.; Forgac, M. The Function of Vacuolar ATPase (V-ATPase) a Subunit Isoforms in Invasiveness of MCF10a and MCF10CA1a Human Breast Cancer Cells. J. Biol. Chem. 2013, 288, 32731–32741. [Google Scholar] [CrossRef]
- Marshansky, V.; Rubinstein, J.L.; Grüber, G. Eukaryotic V-ATPase: Novel structural findings and functional insights. Biochim. Biophys. Acta-(BBA) Bioenerg. 2014, 1837, 857–879. [Google Scholar] [CrossRef]
- Collins, M.P.; Forgac, M. Regulation and function of V-ATPases in physiology and disease. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183341. [Google Scholar] [CrossRef]
- van Weert, A.W.; Dunn, K.W.; Geuze, H.J.; Maxfield, F.R.; Stoorvogel, W. Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J. Cell Biol. 1995, 130, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, A.; Gaullier, J.M.; D’Arrigo, A.; Stenmark, H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J. Biol. Chem. 1999, 274, 28857–28860. [Google Scholar] [CrossRef]
- Lawe, D.C.; Sitouah, N.; Hayes, S.; Chawla, A.; Virbasius, J.V.; Tuft, R.; Fogarty, K.; Lifshitz, L.; Lambright, D.; Corvera, S. Essential role of Ca2+/calmodulin in Early Endosome Antigen-1 localization. Mol. Biol. Cell 2003, 14, 2935–2945. [Google Scholar] [CrossRef]
- Hurtado-Lorenzo, A.; Skinner, M.; El Annan, J.; Futai, M.; Sun-Wada, G.H.; Bourgoin, S.; Casanova, J.; Wildeman, A.; Bechoua, S.; Ausiello, D.A.; et al. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat. Cell Biol. 2006, 8, 124–136. [Google Scholar] [CrossRef]
- Xu, Y.; Parmar, A.; Roux, E.; Balbis, A.; Dumas, V.; Chevalier, S.; Posner, B.I. Epidermal growth factor-induced vacuolar (H+)-atpase assembly: A role in signaling via mTORC1 activation. J. Biol. Chem. 2012, 287, 26409–26422. [Google Scholar] [CrossRef] [PubMed]
- Astaburuaga, R.; Quintanar Haro, O.D.; Stauber, T.; Relógio, A. A Mathematical Model of Lysosomal Ion Homeostasis Points to Differential Effects of Cl- Transport in Ca2+ Dynamics. Cells 2019, 8, 1263. [Google Scholar] [CrossRef]
- Chen, F.; Kang, R.; Liu, J.; Tang, D. The V-ATPases in cancer and cell death. Cancer Gene Ther. 2022, 29, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Bowman, E.J.; Siebers, A.; Altendorf, K. Bafilomycins: A class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA 1988, 85, 7972–7976. [Google Scholar] [CrossRef]
- Baravalle, G.; Schober, D.; Huber, M.; Bayer, N.; Murphy, R.F.; Fuchs, R. Transferrin recycling and dextran transport to lysosomes is differentially affected by bafilomycin, nocodazole, and low temperature. Cell Tissue Res. 2005, 320, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, M.; Janjetovic, K.; Paunovic, V.; Ciric, D.; Kravic-Stevovic, T.; Trajkovic, V. Comparative analysis of cell death mechanisms induced by lysosomal autophagy inhibitors. Eur. J. Pharmacol. 2019, 859, 172540. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F.; Chang, A.C.; Chen, P.C.; Ho, C.Y.; Chen, H.E.; Chou, K.Y.; Hwang, T.I. Autophagy blockade potentiates cancer-associated immunosuppression through programmed death ligand-1 upregulation in bladder cancer. J. Cell Physiol. 2022, 237, 3587–3597. [Google Scholar] [CrossRef] [PubMed]
- Salova, A.V.; Belyaeva, T.N.; Leontieva, E.A.; Kornilova, E.S. EGF Receptor Lysosomal Degradation is Delayed in the Cells Stimulated with EGF-Quantum dot Bioconjugate but Earlier Key Events of Endocytic Degradative Pathway are Similar to that of Native EGF. Oncotarget 2017, 8, 44335–44350. [Google Scholar] [CrossRef][Green Version]
- Bayer, N.; Schober, D.; Prchla, E.; Murphy, R.F.; Blaas, D.; Fuchs, R. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: Implications for viral uncoating and infection. J. Virol. 1998, 72, 645–655. [Google Scholar] [CrossRef]
- Zelenin, A.V. Acridine orange as a probe for cell and molecular biology. In Fluorescent and Luminescent Probes for Biological Activity; Academic Press: London, UK, 1999; pp. 117–135. [Google Scholar]
- Belyaeva, T.N.; Krolenko, S.A.; Leontieva, E.A.; Mozhenok, T.P.; Salova, A.V.; Faddeeva, M.D. Acridine orange distribution and fluorescence spectra in myoblasts and single muscle fibers. Cell Tissue Biol. 2009, 3, 173–180. [Google Scholar] [CrossRef]
- Aniento, F.; Emans, N.; Griffiths, G.; Gruenberg, J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J. Cell Biol. 1993, 123 Pt 1, 1373–1387. [Google Scholar] [CrossRef]
- Yoshimori, T.; Yamamoto, A.; Moriyama, Y.; Futai, M.; Tashiro, Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem. 1991, 266, 17707–17712. [Google Scholar] [CrossRef]
- Melikova, M.S.; Blagoveshchenskaia, A.D.; Nikol’skiĭ, N.N.; Kornilova, E.S. Effect of an vacuolar proton pump inhibitor bafilomycin A1 on the intracellular processing of markers for receptor-mediated and liquid phase endocytosis. Tsitologiia 2002, 44, 807–816. (In Russian) [Google Scholar]
- Christoforidis, S.; McBride, H.M.; Burgoyne, R.D.; Zerial, M. The Rab5 effector EEA1 is a core component of endosome docking. Nature 1999, 397, 621–625. [Google Scholar] [CrossRef]
- Callaghan, J.; Nixon, S.; Bucci, C.; Toh, B.H.; Stenmark, H. Direct interaction of EEA1 with Rab5b. Eur. J. Biochem. 1999, 265, 361–366. [Google Scholar] [CrossRef]
- Kamentseva, R.; Kosheverova, V.; Kharchenko, M.; Zlobina, M.; Salova, A.; Belyaeva, T.; Nikolsky, N.; Kornilova, E. Functional cycle of EEA1-positive early endosome: Direct evidence for pre-existing compartment of degradative pathway. PLoS ONE 2020, 15, e0232532. [Google Scholar] [CrossRef]
- Metzelaar, M.J.; Wijngaard, P.L.; Peters, P.J.; Sixma, J.J.; Nieuwenhuis, H.K.; Clevers, H.C. CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J. Biol. Chem. 1991, 266, 3239–3245. [Google Scholar] [CrossRef]
- Thery, C.; Regnault, A.; Garin, J.; Wolfers, J.; Zitvogel, L.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 1999, 147, 599–610. [Google Scholar] [CrossRef]
- Pols, M.S.; Klumperman, J. Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 2009, 315, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Uder, C.; Brückner, S.; Winkler, S.; Tautenhahn, H.-M.; Christ, B. Mammalian MSC from selected species: Features and applications. Cytom. Part A 2017, 93, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K.; Kawasaki, H.; Wells, A. Epidermal Growth Factor (EGF) Treatment on Multipotential Stromal Cells (MSCs). Possible Enhancement of Therapeutic Potential of MSC. J. Biomed. Biotechnol. 2010, 2010, 795385. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Mills, S.J.; Cowin, A.J. Mesenchymal Stem Cell Secretome as an Emerging Cell-Free Alternative for Improving Wound Repair. Int. J. Mol. Sci. 2020, 21, 7038. [Google Scholar] [CrossRef]
- Gornostaeva, A.N.; Buravkova, L.B. Changes Induced by Inflammatory-Activated Immune Cell Microenvironment in the Paracrine Profile of MSC. Bull. Exp. Biol. Med. 2023, 174, 544–548. [Google Scholar] [CrossRef]
- Lee, J.H.; Chellasamy, G.; Yun, K.; Nam, M.J. EGF-expressed human mesenchymal stem cells inhibit collagenase1 expression in keratinocytes. Cell. Signal. 2023, 110, 110827. [Google Scholar] [CrossRef] [PubMed]
- Civenni, G.; Holbro, T.; Hynes, N.E. Wnt1 and Wnt5a induce cyclin D1 expression through erbB1 transactivation in HC11 mammary epithelial cells. EMBO Rep. 2003, 4, 166–171, Correction in EMBO Rep. 2003, 4, 326. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Li, Y.; Lin, T.; Siclari, V.A.; Chandra, A.; Candela, E.M.; Koyama, E.; Enomoto-Iwamoto, M.; Qin, L. Epidermal growth factor receptor (EGFR) signaling regulates epiphyseal cartilage development through β-catenin-dependent and -independent pathways. J. Biol. Chem. 2013, 288, 32229–32240. [Google Scholar] [CrossRef] [PubMed]
- Schuldiner, M.; Yanuka, O.; Itskovitz-Eldor, J.; Melton, D.A.; Benvenisty, N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11307–11312. [Google Scholar] [CrossRef]
- Aghajanova, L.; Bjuresten, K.; Altmäe, S.; Landgren, B.M.; Stavreus-Evers, A. HB-EGF but not amphiregulin or their receptors HER1 and HER4 is altered in endometrium of women with unexplained infertility. Reprod. Sci. 2008, 15, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Diaz, M.R.; Yee, D. Fulvestrant regulates epidermal growth factor (EGF) family ligands to activate EGF receptor (EGFR) signaling in breast cancer cells. Breast Cancer Res. Treat. 2013, 139, 351–360. [Google Scholar] [CrossRef]
- Trofimenko, E.; Homma, Y.; Fukuda, M.; Widmann, C. The endocytic pathway taken by cationic substances requires Rab14 but not Rab5 and Rab7. Cell Rep. 2021, 37, 109945. [Google Scholar] [CrossRef]
- Escola, J.M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef]
- Kobayashi, T.; Vischer, U.M.; Rosnoblet, C.; Lebrand, C.; Lindsay, M.; Parton, R.G.; Kruithof, E.K.; Gruenberg, J. The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol. Biol. Cell 2000, 11, 1829–1843. [Google Scholar] [CrossRef]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Edgar, J.R.; Eden, E.R.; Futter, C.E. Hrs- and CD63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic 2014, 15, 197–211. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, K.; Love, J.C.; Ploegh, H.L.; Vyas, J.M. Recruitment of CD63 to Cryptococcus neoformans phagosomes requires acidification. Proc. Natl. Acad. Sci. USA 2006, 103, 15945–15950. [Google Scholar] [CrossRef]
- Maxson, M.E.; Abbas, Y.M.; Wu, J.Z.; Plumb, J.D.; Grinstein, S.; Rubinstein, J.L. Detection and quantification of the vacuolar H+ATPase using the Legionella effector protein SidK. J. Cell Biol. 2022, 221, e202107174. [Google Scholar] [CrossRef]
- Smith, G.A.; Howell, G.J.; Phillips, C.; Muench, S.P.; Ponnambalam, S.; Harrison, M.A. Extracellular and Luminal pH Regulation by Vacuolar H+-ATPase Isoform Expression and Targeting to the Plasma Membrane and Endosomes. J. Biol. Chem. 2016, 291, 8500–8515. [Google Scholar] [CrossRef]
- Gerasimenko, J.V.; Tepikin, A.V.; Petersen, O.H.; Gerasimenko, O.V. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr. Biol. 1998, 8, 1335–1338. [Google Scholar] [CrossRef]
- Zhu, M.X.; Ma, J.; Parrington, J.; Calcraft, P.J.; Galione, A.; Evans, A.M. Calcium signaling via two-pore channels: Local or global, that is the question. Am. J. Physiol. Cell Physiol. 2010, 298, 430–441. [Google Scholar] [CrossRef]
- Saito, M.; Hanson, P.I.; Schlesinger, P. Luminal chloride-dependent activation of endosome calcium channels. Patch clamp study of enlarged endosomes. J. Biol. Chem. 2007, 282, 27327–27333. [Google Scholar] [CrossRef]
- Abe, K.; Puertollano, R. Role of TRP channels in the regulation of the endosomal pathway. Physiology 2011, 26, 14–22. [Google Scholar] [CrossRef]
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef]
- Burgstaller, S.; Bischof, H.; Gensch, T.; Stryeck, S.; Gottschalk, B.; Ramadani-Muja, J.; Eroglu, E.; Rost, R.; Balfanz, S.; Baumann, A.; et al. pH-Lemon, a Fluorescent Protein-Based pH Reporter for Acidic Compartments. ACS Sens. 2019, 4, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, P.; Khan, N.; Datta, G.; Ohm, J.E.; Chen, X.; Geiger, J.D. Importance of measuring endolysosome, cytosolic, and extracellular pH in understanding the pathogenesis of and possible treatments for glioblastoma multiforme. Cancer Rep. 2019, 2, e1193. [Google Scholar] [CrossRef] [PubMed]
- Rennick, J.J.; Nowell, C.J.; Pouton, C.W.; Johnston, A.P.R. Resolving subcellular pH with a quantitative fluorescent lifetime biosensor. Nat. Commun. 2022, 13, 6023. [Google Scholar] [CrossRef] [PubMed]
- Zemel’ko, V.I.; Grinchuk, T.M.; Domnina, A.P.; Artsybasheva, I.V.; Zenin, V.V.; Kirsanov, A.A.; Bichevaia, N.K.; Korsak, V.S.; Nikol’skii, N.N. Multipotent Mesenchymal Stem Cells of Desquamated Endometrium: Isolation, Characterization, and Application as a Feeder Layer for Maintenance of Human Embryonic Stem Cells. Cell Tissue Biol. 2012, 6, 1–11. [Google Scholar] [CrossRef]
- Petrosyan, M.A.; Melezhnikova, N.O.; Domnina, A.P.; Malysheva, O.V.; Shved, N.Y.; Petrova, L.I.; Polyanskikh, L.S.; Baziyan, E.V.; Molotkov, A.S. Decidual Differentiation of Endometrial Cell Lines in the Norm and Pathological Conditions. Cell Tissue Biol. 2020, 14, 113–123. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salova, A.V.; Belyaeva, T.N.; Litvinov, I.K.; Kharchenko, M.V.; Kornilova, E.S. Deacidification of the Endolysosomal System by the Vesicular Proton Pump V-ATPase Inhibitor Bafilomycin A1 Affects EGF Receptor Endocytosis Differently in Endometrial MSC and HeLa Cells. Int. J. Mol. Sci. 2025, 26, 10226. https://doi.org/10.3390/ijms262010226
Salova AV, Belyaeva TN, Litvinov IK, Kharchenko MV, Kornilova ES. Deacidification of the Endolysosomal System by the Vesicular Proton Pump V-ATPase Inhibitor Bafilomycin A1 Affects EGF Receptor Endocytosis Differently in Endometrial MSC and HeLa Cells. International Journal of Molecular Sciences. 2025; 26(20):10226. https://doi.org/10.3390/ijms262010226
Chicago/Turabian StyleSalova, Anna V., Tatiana N. Belyaeva, Ilia K. Litvinov, Marianna V. Kharchenko, and Elena S. Kornilova. 2025. "Deacidification of the Endolysosomal System by the Vesicular Proton Pump V-ATPase Inhibitor Bafilomycin A1 Affects EGF Receptor Endocytosis Differently in Endometrial MSC and HeLa Cells" International Journal of Molecular Sciences 26, no. 20: 10226. https://doi.org/10.3390/ijms262010226
APA StyleSalova, A. V., Belyaeva, T. N., Litvinov, I. K., Kharchenko, M. V., & Kornilova, E. S. (2025). Deacidification of the Endolysosomal System by the Vesicular Proton Pump V-ATPase Inhibitor Bafilomycin A1 Affects EGF Receptor Endocytosis Differently in Endometrial MSC and HeLa Cells. International Journal of Molecular Sciences, 26(20), 10226. https://doi.org/10.3390/ijms262010226





