Proteomic Profiling of Cardiomyocytes Revealed Potential Radioprotective Effects of Different Resveratrol Pretreatment Regimens
Abstract
1. Introduction
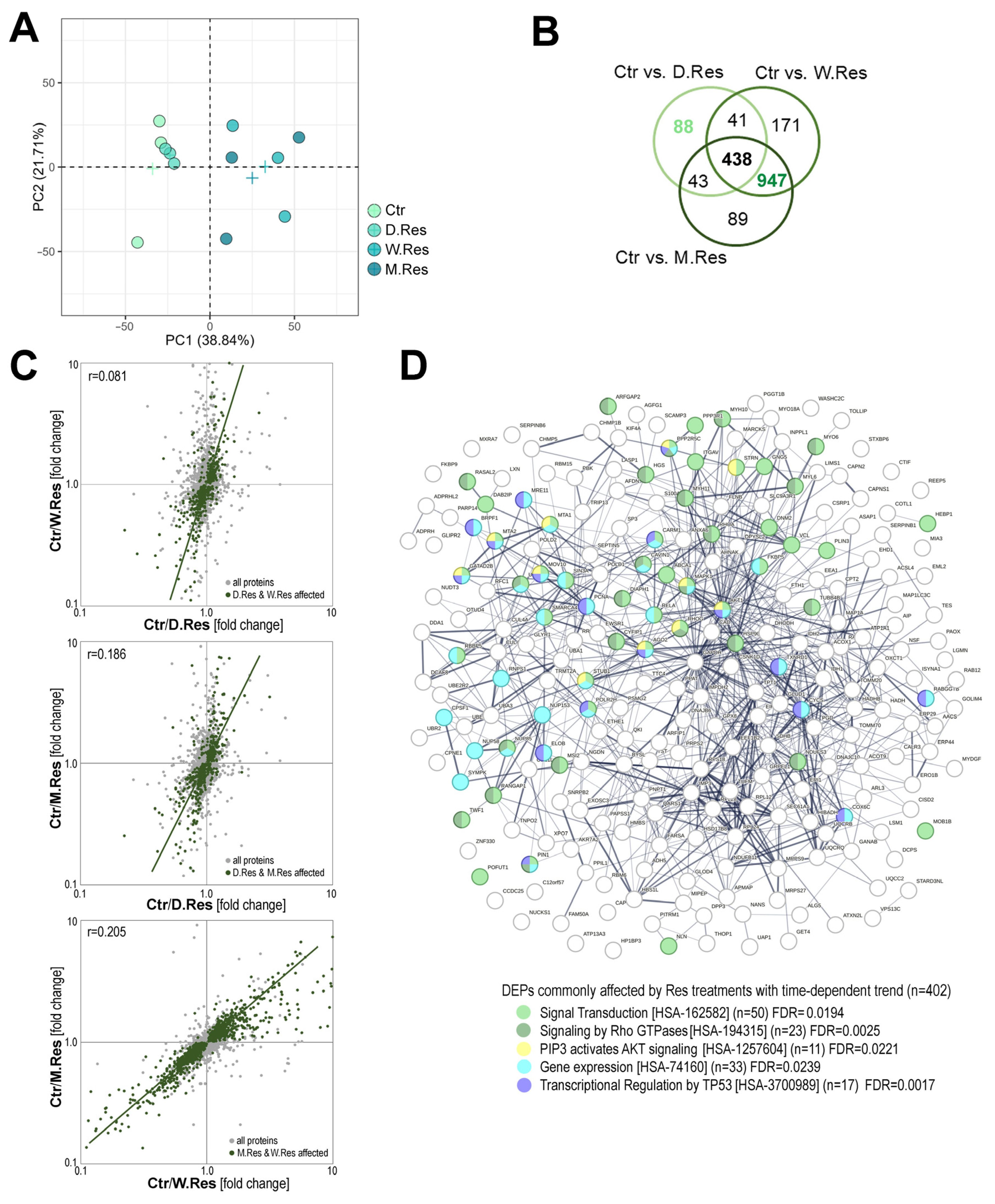
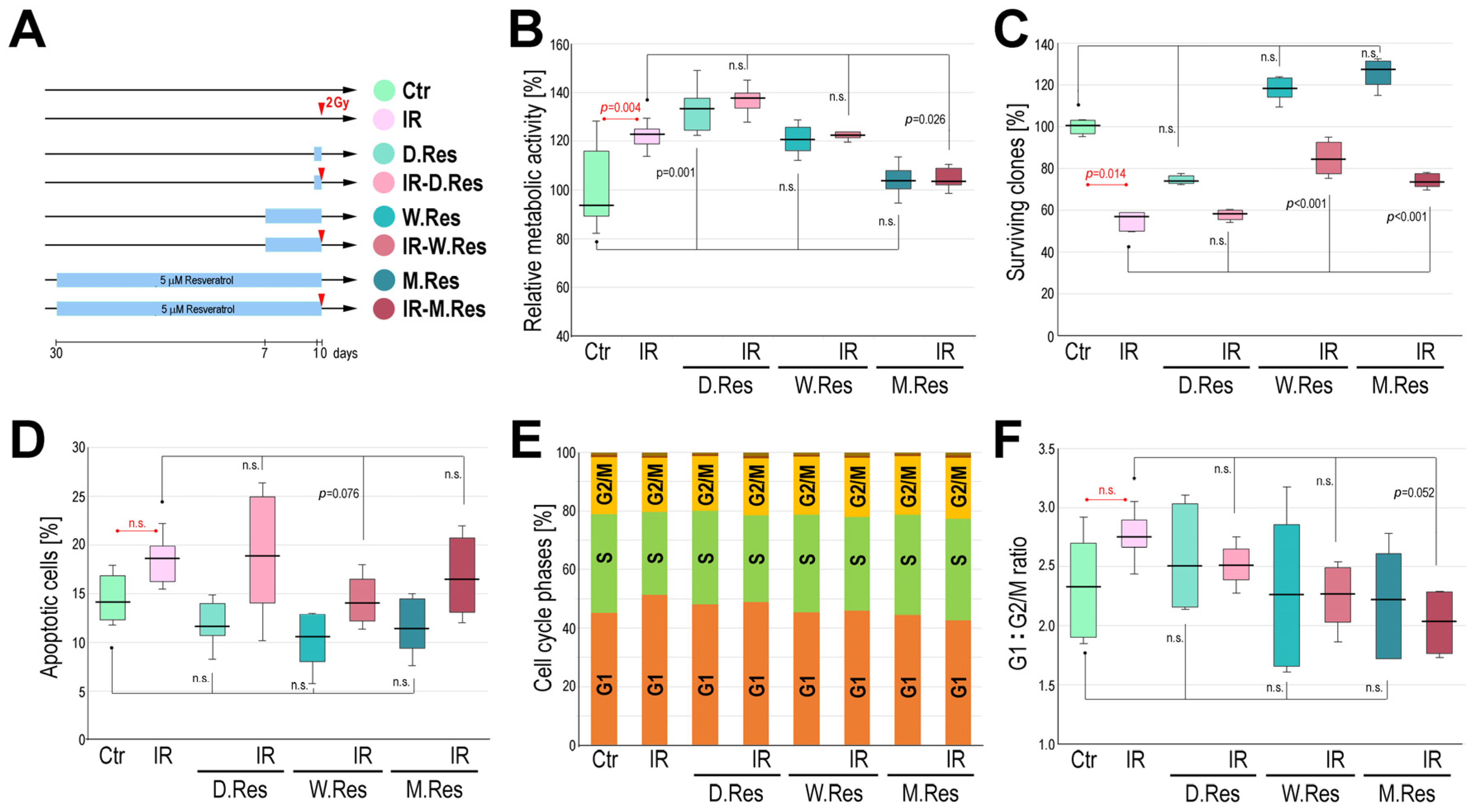
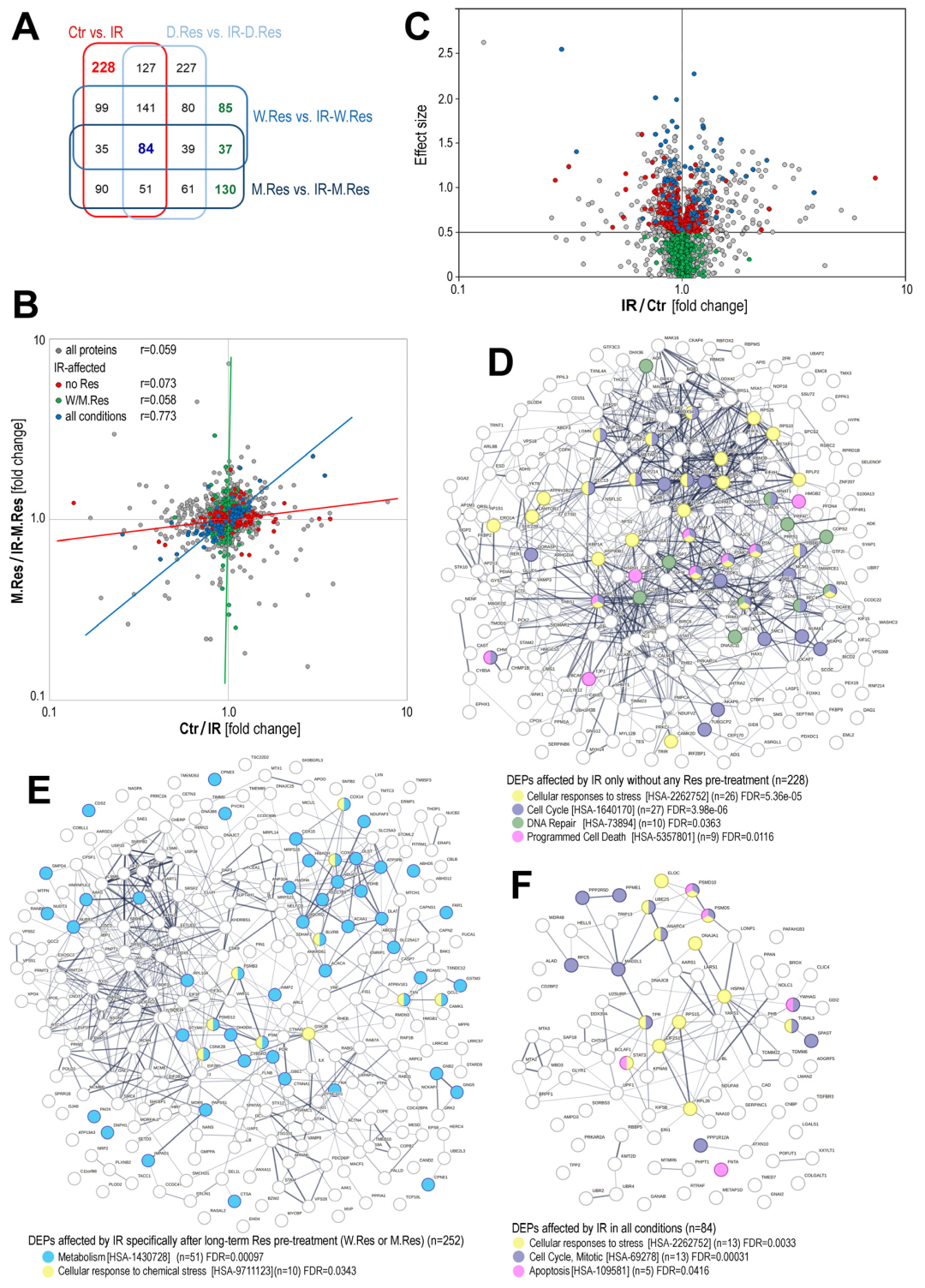
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| cAMP | cyclic form of adenosine monophosphate |
| DEPs | differentially expressed proteins |
| DMSO | dimethyl sulfoxide |
| IR | ionizing radiation |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor-kappa B |
| PCA | principal component analysis |
| PI | propidium iodide |
| PI3K | phosphatidylinositol 3-kinase |
| Rho-GTPases | Rho family guanosine triphosphatase |
| Sirt1 | sirtuin 1 |
References
- Powathil, G.G.; Munro, A.J.; Chaplain, M.A.; Swat, M. Bystander Effects and Their Implications for Clinical Radiation Therapy: Insights from Multiscale in Silico Experiments. J. Theor. Biol. 2016, 401, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, Z.; Wu, H.; Zou, B.; Xu, Y. Exploring Natural Products as Radioprotective Agents for Cancer Therapy: Mechanisms, Challenges, and Opportunities. Cancers 2023, 15, 3585. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, K.; Hu, L. Advancements in Delivery Systems for Dietary Polyphenols in Enhancing Radioprotection Effects: Challenges and Opportunities. npj Sci. Food 2025, 9, 51. [Google Scholar] [CrossRef]
- Arenas, M.; Sabater, S.; Hernández, V.; Rovirosa, A.; Lara, P.C.; Biete, A.; Panés, J. Anti-Inflammatory Effects of Low-Dose Radiotherapy: Indications, Dose, and Radiobiological Mechanisms Involved. Strahlenther. Onkol. 2012, 188, 975–981. [Google Scholar] [CrossRef]
- Hess, C.B.; Buchwald, Z.S.; Stokes, W.; Nasti, T.H.; Switchenko, J.M.; Weinberg, B.D.; Steinberg, J.P.; Godette, K.D.; Murphy, D.; Ahmed, R.; et al. Low-dose Whole-lung Radiation for COVID-19 Pneumonia: Planned Day 7 Interim Analysis of a Registered Clinical Trial. Cancer 2020, 126, 5109–5113. [Google Scholar] [CrossRef]
- Boerma, M.; Sridharan, V.; Mao, X.-W.; Nelson, G.A.; Cheema, A.K.; Koturbash, I.; Singh, S.P.; Tackett, A.J.; Hauer-Jensen, M. Effects of Ionizing Radiation on the Heart. Mutat. Res./Rev. Mutat. Res. 2016, 770, 319–327. [Google Scholar] [CrossRef]
- Barjaktarovic, Z.; Schmaltz, D.; Shyla, A.; Azimzadeh, O.; Schulz, S.; Haagen, J.; Dörr, W.; Sarioglu, H.; Schäfer, A.; Atkinson, M.J.; et al. Radiation–Induced Signaling Results in Mitochondrial Impairment in Mouse Heart at 4 Weeks after Exposure to X-Rays. PLoS ONE 2011, 6, e27811. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.K.; Pathak, R.; Zandkarimi, F.; Kaur, P.; Alkhalil, L.; Singh, R.; Zhong, X.; Ghosh, S.; Aykin-Burns, N.; Hauer-Jensen, M. Liver Metabolomics Reveals Increased Oxidative Stress and Fibrogenic Potential in Gfrp Transgenic Mice in Response to Ionizing Radiation. J. Proteome Res. 2014, 13, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, O.; Azizova, T.; Merl-Pham, J.; Subramanian, V.; Bakshi, M.V.; Moseeva, M.; Zubkova, O.; Hauck, S.M.; Anastasov, N.; Atkinson, M.J.; et al. A Dose-Dependent Perturbation in Cardiac Energy Metabolism Is Linked to Radiation-Induced Ischemic Heart Disease in Mayak Nuclear Workers. Oncotarget 2017, 8, 9067. [Google Scholar] [CrossRef]
- Sebastià, N.; Montoro, A.; Hervás, D.; Pantelias, G.; Hatzi, V.I.; Soriano, J.M.; Villaescusa, J.I.; Terzoudi, G.I. Curcumin and Trans-Resveratrol Exert Cell Cycle-Dependent Radioprotective or Radiosensitizing Effects as Elucidated by the PCC and G2-Assay. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2014, 766–767, 49–55. [Google Scholar] [CrossRef]
- Fischer, N.; Seo, E.-J.; Efferth, T. Prevention from Radiation Damage by Natural Products. Phytomedicine 2018, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-Y. Cardioprotection by Phytochemicals via Antiplatelet Effects and Metabolism Modulations. Cell Biochem. Biophys. 2015, 73, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Ji, C.-B.; Lu, X.-W.; Ni, Y.-H.; Gao, C.-L.; Chen, X.-H.; Zhao, Y.-P.; Gu, G.-X.; Guo, X.-R. Resveratrol Attenuates Radiation Damage in Caenorhabditis Elegans by Preventing Oxidative Stress. J. Radiat. Res. 2010, 51, 473–479. [Google Scholar] [CrossRef]
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.A.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, D.; Gottfried, C.; Lin, H.-Y.; et al. What Is New for an Old Molecule? Systematic Review and Recommendations on the Use of Resveratrol. PLoS ONE 2011, 6, e19881. [Google Scholar] [CrossRef]
- Movahed, A.; Yu, L.; Thandapilly, S.J.; Louis, X.L.; Netticadan, T. Resveratrol Protects Adult Cardiomyocytes against Oxidative Stress Mediated Cell Injury. Arch. Biochem. Biophys. 2012, 527, 74–80. [Google Scholar] [CrossRef]
- Fu, D.-G. Regulation of Redox Signalling and Autophagy during Cardiovascular Diseases-Role of Resveratrol. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1530–1536. [Google Scholar]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, H.; Zhou, X.; Wang, H.; Yang, Y.; Zhang, J.; Wang, H. The Protective Effects of Resveratrol against Radiation-Induced Intestinal Injury. BMC Complement. Altern. Med. 2017, 17, 410. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Fan, Y.; Zhang, H.; Pan, J.; Yu, J.; Zhang, J.; Wang, C. Resveratrol Suppresses Doxorubicin-Induced Cardiotoxicity by Disrupting E2F1 Mediated Autophagy Inhibition and Apoptosis Promotion. Biochem. Pharmacol. 2018, 150, 202–213. [Google Scholar] [CrossRef]
- Carsten, R.E.; Bachand, A.M.; Bailey, S.M.; Ullrich, R.L. Resveratrol Reduces Radiation-Induced Chromosome Aberration Frequencies in Mouse Bone Marrow Cells. Radiat. Res. 2008, 169, 633–638. [Google Scholar] [CrossRef]
- Chekalina, N.I. Resveratrol Has a Positive Effect on Parameters of Central Hemodynamics and Myocardial Ischemia in Patients with Stable Coronary Heart Disease. Wiad. Lek. 2017, 70, 286–291. [Google Scholar]
- Gal, R.; Praksch, D.; Kenyeres, P.; Rabai, M.; Toth, K.; Halmosi, R.; Habon, T. Hemorheological Alterations in Patients with Heart Failure with Reduced Ejection Fraction Treated by Resveratrol. Cardiovasc. Ther. 2020, 2020, 7262474. [Google Scholar] [CrossRef]
- Gal, R.; Deres, L.; Horvath, O.; Eros, K.; Sandor, B.; Urban, P.; Soos, S.; Marton, Z.; Sumegi, B.; Toth, K.; et al. Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure. Antioxidants 2020, 9, 1108. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, H.; Zheng, X. The Clinical Effects of Resveratrol on Atherosclerosis Treatment and Its Effect on the Expression of NADPH Oxidase Complex Genes in Vascular Smooth Muscle Cell Line. Cell. Mol. Biol. 2021, 67, 148–152. [Google Scholar] [CrossRef]
- Yan, R.; Shan, H.; Lin, L.; Zhang, M.; Diao, J.-Y.; Li, Q.; Liu, X.; Wei, J. Chronic Resveratrol Treatment Improves Cardiac Function in a Rat Model of Diabetic Cardiomyopathy via Attenuation of Mitochondrial Injury and Myocardial Apoptosis. Int. J. Clin. Exp. Med. 2016, 9, 21156–21167. [Google Scholar]
- Gramatyka, M. Time Does Matter: The Cellular Response to Resveratrol Varies Depending on the Exposure Duration. Int. J. Mol. Sci. 2025, 26, 5542. [Google Scholar] [CrossRef]
- Prager, I.; Patties, I.; Himmelbach, K.; Kendzia, E.; Merz, F.; Müller, K.; Kortmann, R.-D.; Glasow, A. Dose-Dependent Short- and Long-Term Effects of Ionizing Irradiation on Neural Stem Cells in Murine Hippocampal Tissue Cultures: Neuroprotective Potential of Resveratrol. Brain Behav. 2016, 6, e00548. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Monceau, V.; Meziani, L.; Strup-Perrot, C.; Morel, E.; Schmidt, M.; Haagen, J.; Escoubet, B.; Dörr, W.; Vozenin, M.-C. Enhanced Sensitivity to Low Dose Irradiation of ApoE−/− Mice Mediated by Early Pro-Inflammatory Profile and Delayed Activation of the TGFβ1 Cascade Involved in Fibrogenesis. PLoS ONE 2013, 8, e57052. [Google Scholar] [CrossRef] [PubMed]
- Torres Santiago, G.; Serrano Contreras, J.I.; Meléndez Camargo, M.E.; Zepeda Vallejo, L.G. NMR-Based Metabonomic Approach Reveals Changes in the Urinary and Fecal Metabolome Caused by Resveratrol. J. Pharm. Biomed. Anal. 2019, 162, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Pignet, A.-L.; Schellnegger, M.; Hecker, A.; Kohlhauser, M.; Kotzbeck, P.; Kamolz, L.-P. Resveratrol-Induced Signal Transduction in Wound Healing. Int. J. Mol. Sci. 2021, 22, 12614. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK Signalling Pathway Coordinates Cell Growth, Autophagy and Metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Tao, J.; Xin, P.; Liu, M.; Li, J.; Wei, M. Resveratrol Protects Cardiomyocytes from Oxidative Stress through SIRT1 and Mitochondrial Biogenesis Signaling Pathways. Biochem. Biophys. Res. Commun. 2013, 438, 270–276. [Google Scholar] [CrossRef]
- Fodor, K.; Tit, D.M.; Pasca, B.; Bustea, C.; Uivarosan, D.; Endres, L.; Iovan, C.; Abdel-Daim, M.M.; Bungau, S. Long-Term Resveratrol Supplementation as a Secondary Prophylaxis for Stroke. Oxidative Med. Cell. Longev. 2018, 2018, 4147320. [Google Scholar] [CrossRef]
- Aronica, S.M.; Kraus, W.L.; Katzenellenbogen, B.S. Estrogen Action via the cAMP Signaling Pathway: Stimulation of Adenylate Cyclase and cAMP-Regulated Gene Transcription. Proc. Natl. Acad. Sci. USA 1994, 91, 8517–8521. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A. Anti-Inflammatory Effects of Resveratrol: Possible Role in Prevention of Age-Related Cardiovascular Disease: Anti-Inflammatory Effects of Resveratrol in Aging. Ann. N. Y. Acad. Sci. 2011, 1215, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Thaung Zaw, J.J.; Howe, P.R.; Wong, R.H. Long-Term Effects of Resveratrol on Cognition, Cerebrovascular Function and Cardio-Metabolic Markers in Postmenopausal Women: A 24-Month Randomised, Double-Blind, Placebo-Controlled, Crossover Study. Clin. Nutr. 2021, 40, 820–829. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen Receptor Signaling Mechanisms. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 116, pp. 135–170. ISBN 978-0-12-815561-5. [Google Scholar]
- Gramatyka, M. The Radioprotective Activity of Resveratrol—Metabolomic Point of View. Metabolites 2022, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Simsek, Y.; Gurocak, S.; Turkoz, Y.; Akpolat, N.; Celik, O.; Ozer, A.; Yılmaz, E.; Turhan, U.; Ozyalin, F. Ameliorative Effects of Resveratrol on Acute Ovarian Toxicity Induced by Total Body Irradiation in Young Adult Rats. J. Pediatr. Adolesc. Gynecol. 2012, 25, 262–266. [Google Scholar] [CrossRef]
- Koohian, F.; Shanei, A.; Shahbazi-Gahrouei, D.; Hejazi, S.H.; Moradi, M.-T. The Radioprotective Effect of Resveratrol Against Genotoxicity Induced by γ-Irradiation in Mice Blood Lymphocytes. Dose-Response 2017, 15, 155932581770569. [Google Scholar] [CrossRef]
- Gao, P.; Li, N.; Ji, K.; Wang, Y.; Xu, C.; Liu, Y.; Wang, Q.; Wang, J.; He, N.; Sun, Z.; et al. Resveratrol Targets TyrRS Acetylation to Protect against Radiation-induced Damage. FASEB J. 2019, 33, 8083–8093. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, X.; Liang, X.; Liu, J.; Liu, J.; Han, Z.; Lu, Q.; Wang, K.; Meng, B.; Zhang, C.; et al. Resveratrol Rescues Cutaneous Radiation-Induced DNA Damage via a Novel AMPK/SIRT7/HMGB1 Regulatory Axis. Cell Death Dis. 2023, 13, 847. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-R.; Tsai, Y.-F.; Lau, Y.-T.; Yu, H.-P. Plasma Metabolite Profiles Following Trauma-Hemorrhage: Effect of Posttreatment With Resveratrol. Shock 2015, 43, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Massimi, M.; Tomassini, A.; Sciubba, F.; Sobolev, A.P.; Devirgiliis, L.C.; Miccheli, A. Effects of Resveratrol on HepG2 Cells as Revealed by 1H-NMR Based Metabolic Profiling. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2012, 1820, 1–8. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakashima, Y. Repeated and Long-Term Treatment with Physiological Concentrations of Resveratrol Promotes NO Production in Vascular Endothelial Cells. Br. J. Nutr. 2012, 107, 774–780. [Google Scholar] [CrossRef]
- Yin, E.; Nelson, D.O.; Coleman, M.A.; Peterson, L.E.; Wyrobek, A.J. Gene Expression Changes in Mouse Brain after Exposure to Low-dose Ionizing Radiation. Int. J. Radiat. Biol. 2003, 79, 759–775. [Google Scholar] [CrossRef]
- Snyder, A.R.; Morgan, W.F. Gene Expression Profiling after Irradiation: Clues to Understanding Acute and Persistent Responses? Cancer Metastasis Rev. 2004, 23, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Templin, T.; Paul, S.; Amundson, S.A.; Young, E.F.; Barker, C.A.; Wolden, S.L.; Smilenov, L.B. Radiation-Induced Micro-RNA Expression Changes in Peripheral Blood Cells of Radiotherapy Patients. Int. J. Radiat. Oncol. * Biol. * Phys. 2011, 80, 549–557. [Google Scholar] [CrossRef]
- Azimzadeh, O.; Sievert, W.; Sarioglu, H.; Merl-Pham, J.; Yentrapalli, R.; Bakshi, M.V.; Janik, D.; Ueffing, M.; Atkinson, M.J.; Multhoff, G.; et al. Integrative Proteomics and Targeted Transcriptomics Analyses in Cardiac Endothelial Cells Unravel Mechanisms of Long-Term Radiation-Induced Vascular Dysfunction. J. Proteome Res. 2015, 14, 1203–1219. [Google Scholar] [CrossRef]
- Chen, C.; Brenner, D.J.; Brown, T.R. Identification of Urinary Biomarkers from X-Irradiated Mice Using NMR Spectroscopy. Radiat. Res. 2011, 175, 622–630. [Google Scholar] [CrossRef]
- Pannkuk, E.; Laiakis, E.; Girgis, M.; Dowd, S.; Dhungana, S.; Nishita, D.; Bujold, K.; Bakke, J.; Gahagen, J.; Authier, S.; et al. Temporal Effects on Radiation Responses in Nonhuman Primates: Identification of Biofluid Small Molecule Signatures by Gas Chromatography–Mass Spectrometry Metabolomics. Metabolites 2019, 9, 98. [Google Scholar] [CrossRef]
- Gramatyka, M.; Boguszewicz, ᴌ.; Ciszek, M.; Gabryś, D.; Kulik, R.; Sokół, M. Metabolic Changes in Mice Cardiac Tissue after Low-Dose Irradiation Revealed by 1H NMR Spectroscopy. J. Radiat. Res. 2020, 61, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, L.; Yu, Z.; Gao, Y.; Wang, R.; Du, B.; Cui, X.; Yuan, J. Multi-Omics Integrated Analysis to Generate Transcriptome and Protome Profiles of Resveratrol Improving Paraquat-Induced Acute Lung Injury. Ecotoxicol. Environ. Saf. 2025, 300, 118459. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, M.K.; Kroager, T.P.; Sanggaard, K.W.; Knudsen, A.D.; Stensballe, A.; Enghild, J.J.; Ølholm, J.; Richelsen, B.; Pedersen, S.B. SILAC-MS Based Characterization of LPS and Resveratrol Induced Changes in Adipocyte Proteomics—Resveratrol as Ameliorating Factor on LPS Induced Changes. PLoS ONE 2016, 11, e0159747. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal Sample Preparation Method for Proteome Analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Conover, W.J.; Conover, W.J. Practical Nonparametric Statistics. In Wiley Series in Probability and Statistics Applied Probability and Statistics Section, 3rd ed.; Wiley: New York, NY, USA, 1999; ISBN 978-0-471-16068-7. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 2013; ISBN 978-1-134-74270-7. [Google Scholar]
- Pallant, J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS, 7th ed.; Routledge: London, UK, 2020; ISBN 978-1-003-11745-2. [Google Scholar]
- Jonckheere, A.R. A Distribution-Free k-Sample Test Against Ordered Alternatives. Biometrika 1954, 41, 133–145. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein–Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bandla, C.; Kundu, D.J.; Kamatchinathan, S.; Bai, J.; Hewapathirana, S.; John, N.S.; Prakash, A.; Walzer, M.; Wang, S.; et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2025, 53, D543–D553. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gramatyka, M.; Gawin, M.; Kurczyk, A.; Gądek, A.; Pietrowska, M.; Widłak, P. Proteomic Profiling of Cardiomyocytes Revealed Potential Radioprotective Effects of Different Resveratrol Pretreatment Regimens. Int. J. Mol. Sci. 2025, 26, 10223. https://doi.org/10.3390/ijms262010223
Gramatyka M, Gawin M, Kurczyk A, Gądek A, Pietrowska M, Widłak P. Proteomic Profiling of Cardiomyocytes Revealed Potential Radioprotective Effects of Different Resveratrol Pretreatment Regimens. International Journal of Molecular Sciences. 2025; 26(20):10223. https://doi.org/10.3390/ijms262010223
Chicago/Turabian StyleGramatyka, Michalina, Marta Gawin, Agata Kurczyk, Adam Gądek, Monika Pietrowska, and Piotr Widłak. 2025. "Proteomic Profiling of Cardiomyocytes Revealed Potential Radioprotective Effects of Different Resveratrol Pretreatment Regimens" International Journal of Molecular Sciences 26, no. 20: 10223. https://doi.org/10.3390/ijms262010223
APA StyleGramatyka, M., Gawin, M., Kurczyk, A., Gądek, A., Pietrowska, M., & Widłak, P. (2025). Proteomic Profiling of Cardiomyocytes Revealed Potential Radioprotective Effects of Different Resveratrol Pretreatment Regimens. International Journal of Molecular Sciences, 26(20), 10223. https://doi.org/10.3390/ijms262010223






