Pharmacological Therapies for Consequences of Perinatal Hypoxic-Ischemic Brain Injury: Where Are We Now?
Abstract
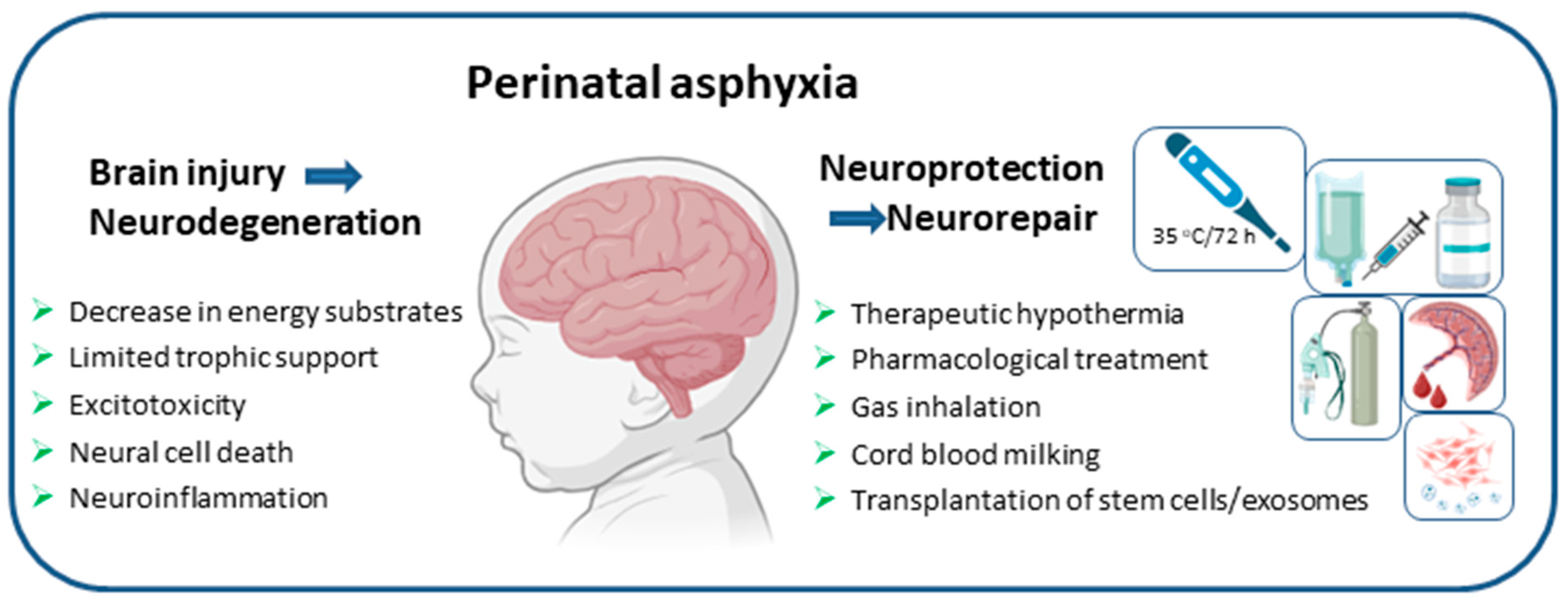
1. Introduction
2. Ethical and Practical Concerns of Clinical Trials in Neonates
3. Erythropoietin
4. Melatonin
5. Allopurinol
6. Sildenafil
7. Metformin
8. Glucocorticoids
9. Topiramate
10. Magnesium Sulphate
11. Sovateltide
12. Cerebrolysin
13. N-Acetylcysteine and Calcitriol
14. Vitamins and Ibuprofen
15. Caffeine Citrate
16. 2-Iminobiotin
17. Citicoline
18. RLS-0071
19. Xenon
20. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pawale, D.; Fursule, A.; Tan, J.; Wagh, D.; Patole, S.; Rao, S. Prevalence of Hearing Impairment in Neonatal Encephalopathy Due to Hypoxia-Ischemia: A Systematic Review and Meta-Analysis. Pediatr. Res. 2024, 97, 953–971. [Google Scholar] [CrossRef]
- Allen, K.A.; Brandon, D.H. Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs. Rev. 2011, 11, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Bruschettini, M.; Romantsik, O.; Moreira, A.; Ley, D.; Thébaud, B. Stem Cell-based Interventions for the Prevention of Morbidity and Mortality Following Hypoxic-ischaemic Encephalopathy in Newborn Infants. Cochrane Database Syst. Rev. 2020, 2020, CD013202. [Google Scholar] [CrossRef]
- Vries, L.; Cowan, F. Evolving Understanding of Hypoxic-Ischemic Encephalopathy in the Term Infant. Semin. Pediatr. Neurol. 2009, 16, 216–225. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Executive Summary: Neonatal Encephalopathy and Neurologic Outcome, Second Edition. Obstet. Gynecol. 2014, 123, 896–901. [Google Scholar] [CrossRef]
- Cotten, C.M.; Shankaran, S. Hypothermia for Hypoxic–Ischemic Encephalopathy. Expert Rev. Obstet. Gynecol. 2010, 5, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Gersh, B.J.; Stone, G.W.; White, H.D.; Holmes, D.R. Pharmacological Facilitation of Primary Percutaneous Coronary Intervention for Acute Myocardial InfarctionIs the Slope of the Curve the Shape of the Future? JAMA 2005, 293, 979–986. [Google Scholar] [CrossRef]
- Musiolik, J.; van Caster, P.; Skyschally, A.; Boengler, K.; Gres, P.; Schulz, R.; Heusch, G. Reduction of Infarct Size by Gentle Reperfusion without Activation of Reperfusion Injury Salvage Kinases in Pigs. Cardiovasc. Res. 2010, 85, 110–117. [Google Scholar] [CrossRef]
- Calcagno, D.M.; Zhang, C.; Toomu, A.; Huang, K.; Ninh, V.K.; Miyamoto, S.; Aguirre, A.D.; Fu, Z.; Heller Brown, J.; King, K.R. SiglecF(HI) Marks Late-Stage Neutrophils of the Infarcted Heart: A Single-Cell Transcriptomic Analysis of Neutrophil Diversification. J. Am. Heart Assoc. 2021, 10, e019019. [Google Scholar] [CrossRef]
- Li, J.; Conrad, C.; Mills, T.W.; Berg, N.K.; Kim, B.; Ruan, W.; Lee, J.W.; Zhang, X.; Yuan, X.; Eltzschig, H.K. PMN-Derived Netrin-1 Attenuates Cardiac Ischemia-Reperfusion Injury via Myeloid ADORA2B Signaling. J. Exp. Med. 2021, 218, e20210008. [Google Scholar] [CrossRef]
- Fraccarollo, D.; Neuser, J.; Möller, J.; Riehle, C.; Galuppo, P.; Bauersachs, J. Expansion of CD10neg Neutrophils and CD14+HLA-DRneg/Low Monocytes Driving Proinflammatory Responses in Patients with Acute Myocardial Infarction. eLife 2021, 10, e66808. [Google Scholar] [CrossRef] [PubMed]
- Noc, M.; Laanmets, P.; Neskovic, A.; Petrović, M.; Stanetic, B.; Aradi, D.; Kiss, R.; Ungi, I.; Merkely, B.; Hudec, M.; et al. A Multicentre, Prospective, Randomised Controlled Trial to Assess the Safety and Effectiveness of Cooling as an Adjunctive Therapy to Percutaneous Intervention in Patients with Acute Myocardial Infarction: The COOL AMI EU Pivotal Trial. Available online: https://eurointervention.pcronline.com/article/a-multicentre-prospective-randomised-controlled-trial-to-assess-the-safety-and-effectiveness-ofcooling-as-an-adjunctive-therapy-to-percutaneous-intervention-in-patients-with-acute-myocardial-infarction-thecoolamieu-pivotal-trial (accessed on 11 October 2025).
- Tissier, R.; Ghaleh, B.; Cohen, M.V.; Downey, J.M.; Berdeaux, A. Myocardial Protection with Mild Hypothermia. Cardiovasc. Res. 2012, 94, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Myocardial Ischemia/Reperfusion: Translational Pathophysiology of Ischemic Heart Disease. Med 2024, 5, 10–31. [Google Scholar] [CrossRef] [PubMed]
- Ginet, V.; Pittet, M.P.; Rummel, C.; Osterheld, M.C.; Meuli, R.; Clarke, P.G.H.; Puyal, J.; Truttmann, A.C. Dying Neurons in Thalamus of Asphyxiated Term Newborns and Rats Are Autophagic. Ann. Neurol. 2014, 76, 695–711. [Google Scholar] [CrossRef]
- Ziemka-Nalecz, M.; Jaworska, J.; Sypecka, J.; Zalewska, T. OGD Induced Modification of FAK- and PYK2-Coupled Pathways in Organotypic Hippocampal Slice Cultures. Brain Res. 2015, 1606, 21–33. [Google Scholar] [CrossRef]
- Ziemka-Nalecz, M.; Janowska, J.; Strojek, L.; Jaworska, J.; Zalewska, T.; Frontczak-Baniewicz, M.; Sypecka, J. Impact of Neonatal Hypoxia-Ischaemia on Oligodendrocyte Survival, Maturation and Myelinating Potential. J. Cell. Mol. Med. 2018, 22, 207–222. [Google Scholar] [CrossRef]
- Wootton, S.H.; Rysavy, M.; Davis, P.; Thio, M.; Romero-Lopez, M.; Holzapfel, L.F.; Thrasher, T.; Wade, J.D.; Owen, L.S. Practical Approaches for Supporting Informed Consent in Neonatal Clinical Trials. Acta Paediatr. 2024, 113, 923–930. [Google Scholar] [CrossRef]
- Janowska, J.; Gargas, J.; Zajdel, K.; Wieteska, M.; Lipinski, K.; Ziemka-Nalecz, M.; Frontczak-Baniewicz, M.; Sypecka, J. Oligodendrocyte Progenitor Cells’ Fate after Neonatal Asphyxia—Puzzling Implications for the Development of Hypoxic–Ischemic Encephalopathy. Brain Pathol. 2024, 34, e13255. [Google Scholar] [CrossRef]
- Gargas, J.; Janowska, J.; Gebala, P.; Maksymiuk, W.; Sypecka, J. Reactive Gliosis in Neonatal Disorders: Friend or Foe for Neuroregeneration? Cells 2024, 13, 131. [Google Scholar] [CrossRef]
- Nagai, A.; Nakagawa, E.; Choi, H.B.; Hatori, K.; Kobayashi, S.; Kim, S.U. Erythropoietin and Erythropoietin Receptors in Human CNS Neurons, Astrocytes, Microglia, and Oligodendrocytes Grown in Culture. J. Neuropathol. Exp. Neurol. 2001, 60, 386–392. [Google Scholar] [CrossRef]
- Yamaji, R.; Okada, T.; Moriya, M.; Naito, M.; Tsuruo, T.; Miyatake, K.; Nakano, Y. Brain Capillary Endothelial Cells Express Two Forms of Erythropoietin Receptor mRNA. Eur. J. Biochem. 1996, 239, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shacka, J.J.; Eells, J.B.; Suarez-Quian, C.; Przygodzki, R.M.; Beleslin-Cokic, B.; Lin, C.-S.; Nikodem, V.M.; Hempstead, B.; Flanders, K.C.; et al. Erythropoietin Receptor Signalling Is Required for Normal Brain Development. Dev. Camb. Engl. 2002, 129, 505–516. [Google Scholar] [CrossRef]
- Juul, S.E.; Yachnis, A.T.; Rojiani, A.M.; Christensen, R.D. Immunohistochemical Localization of Erythropoietin and Its Receptor in the Developing Human Brain. Pediatr. Dev. Pathol. 1999, 2, 148–158. [Google Scholar] [CrossRef]
- Gonzalez, F.F.; Larpthaveesarp, A.; McQuillen, P.; Derugin, N.; Wendland, M.; Spadafora, R.; Ferriero, D.M. Erythropoietin Increases Neurogenesis and Oligodendrogliosis of SVZ Precursor Cells after Neonatal Stroke. Stroke J. Cereb. Circ. 2013, 44, 753–758. [Google Scholar] [CrossRef]
- Kaneko, N.; Kako, E.; Sawamoto, K. Enhancement of Ventricular-Subventricular Zone-Derived Neurogenesis and Oligodendrogenesis by Erythropoietin and Its Derivatives. Front. Cell. Neurosci. 2013, 7, 235. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Meng, Y.; Zhang, Y.; Qu, C.; Schallert, T.; Chopp, M. Delayed Administration of Erythropoietin Reduces Hippocampal Cell Loss, Enhances Angiogenesis and Neurogenesis, and Improves Functional Outcome Following Traumatic Brain Injury in Rats: Comparison of Treatment with Single Dose and Triple Dose. J. Neurosurg. 2010, 113, 598–608. [Google Scholar] [CrossRef]
- Ikeda, E. Cellular Response to Tissue Hypoxia and Its Involvement in Disease Progression. Pathol. Int. 2005, 55, 603–610. [Google Scholar] [CrossRef]
- Villa, P.; van Beek, J.; Larsen, A.K.; Gerwien, J.; Christensen, S.; Cerami, A.; Brines, M.; Leist, M.; Ghezzi, P.; Torup, L. Reduced Functional Deficits, Neuroinflammation, and Secondary Tissue Damage after Treatment of Stroke by Nonerythropoietic Erythropoietin Derivatives. J. Cereb. Blood Flow Metab. 2007, 27, 552–563. [Google Scholar] [CrossRef]
- Juul, S.E.; Beyer, R.P.; Bammler, T.K.; McPherson, R.J.; Wilkerson, J.; Farin, F.M. Microarray Analysis of High-Dose Recombinant Erythropoietin Treatment of Unilateral Brain Injury in Neonatal Mouse Hippocampus. Pediatr. Res. 2009, 65, 485–492. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.G.; Zhang, R.L.; Gregg, S.R.; Hozeska-Solgot, A.; LeTourneau, Y.; Wang, Y.; Chopp, M. Matrix Metalloproteinase 2 (MMP2) and MMP9 Secreted by Erythropoietin-Activated Endothelial Cells Promote Neural Progenitor Cell Migration. J. Neurosci. 2006, 26, 5996–6003. [Google Scholar] [CrossRef]
- Wenger, R.H. Mammalian Oxygen Sensing, Signalling and Gene Regulation. J. Exp. Biol. 2000, 203, 1253–1263. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J. Inducible Operation of the Erythropoietin 3′ Enhancer in Multiple Cell Lines: Evidence for a Widespread Oxygen-Sensing Mechanism. Proc. Natl. Acad. Sci. USA 1993, 90, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W. Molecular Biology of Erythropoietin. Intern. Med. Tokyo Jpn. 2004, 43, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Biju, M.P.; Liu, Q.; Unger, T.L.; Rha, J.; Johnson, R.S.; Simon, M.C.; Keith, B.; Haase, V.H. Hypoxia-Inducible Factor–2 (HIF-2) Regulates Hepatic Erythropoietin in Vivo. J. Clin. Investig. 2007, 117, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, P.J. HIF-1 and HIF-2: Working Alone or Together in Hypoxia? J. Clin. Investig. 2007, 117, 862–865. [Google Scholar] [CrossRef]
- Jelkmann, W. Erythropoietin. In Frontiers of Hormone Research; Lanfranco, F., Strasburger, C.J., Eds.; S. Karger AG: Basel, Switzerland, 2016; Volume 47, pp. 115–127. ISBN 978-3-318-05868-0. [Google Scholar]
- Rogers, E.E.; Bonifacio, S.L.; Glass, H.C.; Juul, S.E.; Chang, T.; Mayock, D.E.; Durand, D.J.; Song, D.; Barkovich, A.J.; Ballard, R.A.; et al. Erythropoietin and Hypothermia for Hypoxic-Ischemic Encephalopathy. Pediatr. Neurol. 2014, 51, 657–662. [Google Scholar] [CrossRef]
- Wu, Y.W.; Bauer, L.A.; Ballard, R.A.; Ferriero, D.M.; Glidden, D.V.; Mayock, D.E.; Chang, T.; Durand, D.J.; Song, D.; Bonifacio, S.L.; et al. Erythropoietin for Neuroprotection in Neonatal Encephalopathy: Safety and Pharmacokinetics. Pediatrics 2012, 130, 683–691. [Google Scholar] [CrossRef]
- Massaro, A.N.; Wu, Y.W.; Bammler, T.K.; MacDonald, J.W.; Mathur, A.; Chang, T.; Mayock, D.; Mulkey, S.B.; van Meurs, K.; Afsharinejad, Z.; et al. Dried Blood Spot Compared to Plasma Measurements of Blood-Based Biomarkers of Brain Injury in Neonatal Encephalopathy. Pediatr. Res. 2019, 85, 655–661. [Google Scholar] [CrossRef]
- Wu, Y.W.; Goodman, A.M.; Chang, T.; Mulkey, S.B.; Gonzalez, F.F.; Mayock, D.E.; Juul, S.E.; Mathur, A.M.; Van Meurs, K.; McKinstry, R.C.; et al. Placental Pathology and Neonatal Brain MRI in a Randomized Trial of Erythropoietin for Hypoxic–Ischemic Encephalopathy. Pediatr. Res. 2020, 87, 879–884. [Google Scholar] [CrossRef]
- Mulkey, S.B.; Ramakrishnaiah, R.H.; McKinstry, R.C.; Chang, T.; Mathur, A.M.; Mayock, D.E.; Meurs, K.P.V.; Schaefer, G.B.; Luo, C.; Bai, S.; et al. Erythropoietin and Brain Magnetic Resonance Imaging Findings in Hypoxic-Ischemic Encephalopathy: Volume of Acute Brain Injury and 1-Year Neurodevelopmental Outcome. J. Pediatr. 2017, 186, 196–199. [Google Scholar] [CrossRef]
- Elmahdy, H.; El-Mashad, A.-R.; El-Bahrawy, H.; El-Gohary, T.; El-Barbary, A.; Aly, H. Human Recombinant Erythropoietin in Asphyxia Neonatorum: Pilot Trial. Pediatrics 2010, 125, e1135–e1142. [Google Scholar] [CrossRef]
- Baserga, M.C.; Beachy, J.C.; Roberts, J.K.; Ward, R.M.; DiGeronimo, R.J.; Walsh, W.F.; Ohls, R.K.; Anderson, J.; Mayock, D.E.; Juul, S.E.; et al. Darbepoetin Administration to Neonates Undergoing Cooling for Encephalopathy: A Safety and Pharmacokinetic Trial. Pediatr. Res. 2015, 78, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Comstock, B.A.; Gonzalez, F.F.; Mayock, D.E.; Goodman, A.M.; Maitre, N.L.; Chang, T.; Van Meurs, K.P.; Lampland, A.L.; Bendel-Stenzel, E.; et al. Trial of Erythropoietin for Hypoxic–Ischemic Encephalopathy in Newborns. N. Engl. J. Med. 2022, 387, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding Expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J. Measuring Melatonin by Immunoassay. J. Pineal Res. 2020, 69, e12657. [Google Scholar] [CrossRef]
- Cecon, E.; Boutin, J.A.; Jockers, R. Molecular Characterization and Pharmacology of Melatonin Receptors in Animals. Receptors 2023, 2, 127–147. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a Mitochondria-Targeted Antioxidant: One of Evolution’s Best Ideas. Cell. Mol. Life Sci. CMLS 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin and Brain Inflammaging. Prog. Neurobiol. 2015, 127–128, 46–63. [Google Scholar] [CrossRef]
- Aly, H.; Elmahdy, H.; El-Dib, M.; Rowisha, M.; Awny, M.; El-Gohary, T.; Elbatch, M.; Hamisa, M.; El-Mashad, A.-R. Melatonin Use for Neuroprotection in Perinatal Asphyxia: A Randomized Controlled Pilot Study. J. Perinatol. 2015, 35, 186–191. [Google Scholar] [CrossRef]
- Maiwald, C.A.; Annink, K.V.; Rüdiger, M.; Benders, M.J.N.L.; van Bel, F.; Allegaert, K.; Naulaers, G.; Bassler, D.; Klebermaß-Schrehof, K.; Vento, M.; et al. Effect of Allopurinol in Addition to Hypothermia Treatment in Neonates for Hypoxic-Ischemic Brain Injury on Neurocognitive Outcome (ALBINO): Study Protocol of a Blinded Randomized Placebo-Controlled Parallel Group Multicenter Trial for Superiority (Phase III). BMC Pediatr. 2019, 19, 210. [Google Scholar] [CrossRef]
- Deferm, N.; Annink, K.V.; Faelens, R.; Schroth, M.; Maiwald, C.A.; el Bakkali, L.; van Bel, F.; Benders, M.J.N.L.; van Weissenbruch, M.M.; Hagen, A.; et al. Glomerular Filtration Rate in Asphyxiated Neonates Under Therapeutic Whole-Body Hypothermia, Quantified by Mannitol Clearance. Clin. Pharmacokinet. 2021, 60, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.; Rüdiger, M.; Benders, M.J.N.L.; van Bel, F.; Allegaert, K.; Naulaers, G.; Bassler, D.; Klebermaß-Schrehof, K.; Vento, M.; Vilan, A.; et al. Detailed Statistical Analysis Plan for ALBINO: Effect of Allopurinol in Addition to Hypothermia for Hypoxic-Ischemic Brain Injury on Neurocognitive Outcome—A Blinded Randomized Placebo-Controlled Parallel Group Multicenter Trial for Superiority (Phase III). Trials 2024, 25, 81, Erratum in Trials 2024, 25, 192. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.Y.; Annink, K.V.; Nijstad, A.L.; Maiwald, C.A.; Schroth, M.; el Bakkali, L.; van Bel, F.; Benders, M.J.N.L.; van Weissenbruch, M.M.; Hagen, A.; et al. Pharmacokinetic/Pharmacodynamic Modelling of Allopurinol, Its Active Metabolite Oxypurinol, and Biomarkers Hypoxanthine, Xanthine and Uric Acid in Hypoxic-Ischemic Encephalopathy Neonates. Clin. Pharmacokinet. 2022, 61, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Klumper, J.; Kaandorp, J.J.; Schuit, E.; Groenendaal, F.; Koopman-Esseboom, C.; Mulder, E.J.H.; Van Bel, F.; Benders, M.J.N.L.; Mol, B.W.J.; van Elburg, R.M.; et al. Behavioral and Neurodevelopmental Outcome of Children after Maternal Allopurinol Administration during Suspected Fetal Hypoxia: 5-Year Follow up of the ALLO-Trial. PLoS ONE 2018, 13, e0201063. [Google Scholar] [CrossRef]
- Kaandorp, J.J.; Benders, M.J.N.L.; Schuit, E.; Rademaker, C.M.A.; Oudijk, M.A.; Porath, M.M.; Oetomo, S.B.; Wouters, M.G.A.J.; van Elburg, R.M.; Franssen, M.T.M.; et al. Maternal Allopurinol Administration during Suspected Fetal Hypoxia: A Novel Neuroprotective Intervention? A Multicentre Randomised Placebo Controlled Trial. Arch. Dis. Child.-Fetal Neonatal Ed. 2015, 100, F216–F223. [Google Scholar] [CrossRef]
- Kaandorp, J.J.; van den Broek, M.P.H.; Benders, M.J.N.L.; Oudijk, M.A.; Porath, M.M.; Oetomo, S.B.; Wouters, M.G.a.J.; van Elburg, R.; Franssen, M.T.M.; Bos, A.F.; et al. Rapid Target Allopurinol Concentrations in the Hypoxic Fetus after Maternal Administration during Labour. Arch. Dis. Child.-Fetal Neonatal Ed. 2014, 99, F144–F148. [Google Scholar] [CrossRef]
- Torrance, H.L.; Benders, M.J.; Derks, J.B.; Rademaker, C.M.A.; Bos, A.F.; Van Den Berg, P.; Longini, M.; Buonocore, G.; Venegas, M.; Baquero, H.; et al. Maternal Allopurinol During Fetal Hypoxia Lowers Cord Blood Levels of the Brain Injury Marker S-100B. Pediatrics 2009, 124, 350–357. [Google Scholar] [CrossRef]
- Yamamoto, T.; Moriwaki, Y.; Suda, M.; Nasako, Y.; Takahashi, S.; Hiroishi, K.; Nakano, T.; Hada, T.; Higashino, K. Effect of BOF-4272 on the Oxidation of Allopurinol and Pyrazinamide in Vivo. Biochem. Pharmacol. 1993, 46, 2277–2284. [Google Scholar] [CrossRef]
- McCord, J.M. Oxygen-Derived Free Radicals in Postischemic Tissue Injury. N. Engl. J. Med. 1985, 312, 159–163. [Google Scholar] [CrossRef]
- van Bel, F.; Groenendaal, F. Drugs for Neuroprotection after Birth Asphyxia: Pharmacologic Adjuncts to Hypothermia. Semin. Perinatol. 2016, 40, 152–159. [Google Scholar] [CrossRef]
- Annink, K.V.; Franz, A.R.; Derks, J.B.; Rudiger, M.; van Bel, F.; Benders, M.J.N.L. Allopurinol: Old Drug, New Indication in Neonates? Curr. Pharm. Des. 2017, 23, 5935–5942. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Huang, J.M.; Hampl, V.; Nelson, D.P.; Shultz, P.J.; Weir, E.K. Nitric Oxide and cGMP Cause Vasorelaxation by Activation of a Charybdotoxin-Sensitive K Channel by cGMP-Dependent Protein Kinase. Proc. Natl. Acad. Sci. USA 1994, 91, 7583–7587. [Google Scholar] [CrossRef] [PubMed]
- Perez, K.M.; Laughon, M. Sildenafil in Term and Premature Infants: A Systematic Review. Clin. Ther. 2015, 37, 2598–2607.e1. [Google Scholar] [CrossRef] [PubMed]
- Ölmestig, J.N.E.; Marlet, I.R.; Hainsworth, A.H.; Kruuse, C. Phosphodiesterase 5 Inhibition as a Therapeutic Target for Ischemic Stroke: A Systematic Review of Preclinical Studies. Cell. Signal. 2017, 38, 39–48. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Zhang, L.; Zhang, Z.; Tsang, W.; Lu, M.; Zhang, L.; Chopp, M. Sildenafil (Viagra) Induces Neurogenesis and Promotes Functional Recovery After Stroke in Rats. Stroke 2002, 33, 2675–2680. [Google Scholar] [CrossRef]
- Charriaut-Marlangue, C.; Nguyen, T.; Bonnin, P.; Duy, A.P.; Leger, P.-L.; Csaba, Z.; Pansiot, J.; Bourgeois, T.; Renolleau, S.; Baud, O. Sildenafil Mediates Blood-Flow Redistribution and Neuroprotection After Neonatal Hypoxia-Ischemia. Stroke 2014, 45, 850–856. [Google Scholar] [CrossRef]
- Moretti, R.; Leger, P.-L.; Besson, V.C.; Csaba, Z.; Pansiot, J.; Di Criscio, L.; Gentili, A.; Titomanlio, L.; Bonnin, P.; Baud, O.; et al. Sildenafil, a Cyclic GMP Phosphodiesterase Inhibitor, Induces Microglial Modulation after Focal Ischemia in the Neonatal Mouse Brain. J. Neuroinflamm. 2016, 13, 95. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Q.; Zhang, L.; Ding, G.; Zhang, Z.G.; Li, Q.; Ewing, J.R.; Lu, M.; Panda, S.; Ledbetter, K.A.; et al. Angiogenesis and Improved Cerebral Blood Flow in the Ischemic Boundary Area Detected by MRI after Administration of Sildenafil to Rats with Embolic Stroke. Brain Res. 2007, 1132, 185–192. [Google Scholar] [CrossRef]
- Zinni, M.; Pansiot, J.; Léger, P.-L.; El Kamouh, M.; Baud, O. Sildenafil-Mediated Neuroprotection from Adult to Neonatal Brain Injury: Evidence, Mechanisms, and Future Translation. Cells 2021, 10, 2766. [Google Scholar] [CrossRef]
- Julien, P.; Zinni, M.; Bonnel, N.; El Kamouh, M.; Odorcyk, F.; Peters, L.; Gautier, E.-F.; Leduc, M.; Broussard, C.; Baud, O. Synergistic Effect of Sildenafil Combined with Controlled Hypothermia to Alleviate Microglial Activation after Neonatal Hypoxia–Ischemia in Rats. J. Neuroinflamm. 2024, 21, 31. [Google Scholar] [CrossRef]
- Wintermark, P.; Lapointe, A.; Steinhorn, R.; Rampakakis, E.; Burhenne, J.; Meid, A.D.; Bajraktari-Sylejmani, G.; Khairy, M.; Altit, G.; Adamo, M.-T.; et al. Feasibility and Safety of Sildenafil to Repair Brain Injury Secondary to Birth Asphyxia (SANE-01): A Randomized, Double-Blind, Placebo-Controlled Phase Ib Clinical Trial. J. Pediatr. 2024, 266, 113879. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef] [PubMed]
- Venna, V.R.; Li, J.; Hammond, M.D.; Mancini, N.S.; McCullough, L.D. Chronic Metformin Treatment Improves Post-Stroke Angiogenesis and Recovery after Experimental Stroke. Eur. J. Neurosci. 2014, 39, 2129–2138. [Google Scholar] [CrossRef]
- Wang, Y.-W.; He, S.-J.; Feng, X.; Cheng, J.; Luo, Y.-T.; Tian, L.; Huang, Q. Metformin: A Review of Its Potential Indications. Drug Des. Devel. Ther. 2017, 11, 2421–2429. [Google Scholar] [CrossRef]
- Ruan, C.; Guo, H.; Gao, J.; Wang, Y.; Liu, Z.; Yan, J.; Li, X.; Lv, H. Neuroprotective Effects of Metformin on Cerebral Ischemia-reperfusion Injury by Regulating PI3K/Akt Pathway. Brain Behav. 2021, 11, e2335. [Google Scholar] [CrossRef]
- Sharma, S.; Nozohouri, S.; Vaidya, B.; Abbruscato, T. Repurposing Metformin to Treat Age-Related Neurodegenerative Disorders and Ischemic Stroke. Life Sci. 2021, 274, 119343. [Google Scholar] [CrossRef]
- Fang, M.; Jiang, H.; Ye, L.; Cai, C.; Hu, Y.; Pan, S.; Li, P.; Xiao, J.; Lin, Z. Metformin Treatment after the Hypoxia-Ischemia Attenuates Brain Injury in Newborn Rats. Oncotarget 2017, 8, 75308–75325. [Google Scholar] [CrossRef]
- Study Details|Metformin Treatment in Infants After Perinatal Brain Injury|ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/study/NCT05590676?cond=neonatal%20hypoxia-ischemia&rank=81 (accessed on 28 July 2024).
- Kovacs, K.; Szakmar, E.; Meder, U.; Szakacs, L.; Cseko, A.; Vatai, B.; Szabo, A.J.; McNamara, P.J.; Szabo, M.; Jermendy, A. A Randomized Controlled Study of Low-Dose Hydrocortisone Versus Placebo in Dopamine-Treated Hypotensive Neonates Undergoing Hypothermia Treatment for Hypoxic−Ischemic Encephalopathy. J. Pediatr. 2019, 211, 13–19.e3. [Google Scholar] [CrossRef]
- Filippi, L.; Fiorini, P.; Daniotti, M.; Catarzi, S.; Savelli, S.; Fonda, C.; Bartalena, L.; Boldrini, A.; Giampietri, M.; Scaramuzzo, R.; et al. Safety and Efficacy of Topiramate in Neonates with Hypoxic Ischemic Encephalopathy Treated with Hypothermia (NeoNATI). BMC Pediatr. 2012, 12, 144. [Google Scholar] [CrossRef]
- Filippi, L.; Fiorini, P.; Catarzi, S.; Berti, E.; Padrini, L.; Landucci, E.; Donzelli, G.; Bartalena, L.; Fiorentini, E.; Boldrini, A.; et al. Safety and Efficacy of Topiramate in Neonates with Hypoxic Ischemic Encephalopathy Treated with Hypothermia (NeoNATI): A Feasibility Study. J. Matern. Fetal Neonatal Med. 2018, 31, 973–980. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Butt, T.K. Role of Intravenous Magnesium Sulphate in Term Neonates with Hypoxic Ischemic Encephalopathy (HIE) in a Low-Income Country: A Randomised Clinical Trial. J. Coll. Physicians Surg. Pak. JCPSP 2021, 31, 817–820. [Google Scholar] [CrossRef]
- Hassanein, S.M.A.; Deifalla, S.M.; El-Houssinie, M.; Mokbel, S.A. Safety and Efficacy of Cerebrolysin in Infants with Communication Defects Due to Severe Perinatal Brain Insult: A Randomized Controlled Clinical Trial. J. Clin. Neurol. 2016, 12, 79–84. [Google Scholar] [CrossRef]
- Moss, H.G.; Brown, T.R.; Wiest, D.B.; Jenkins, D.D. N-Acetylcysteine Rapidly Replenishes Central Nervous System Glutathione Measured via Magnetic Resonance Spectroscopy in Human Neonates with Hypoxic-Ischemic Encephalopathy. J. Cereb. Blood Flow Metab. 2018, 38, 950–958. [Google Scholar] [CrossRef]
- Sánchez-Illana, Á.; Thayyil, S.; Montaldo, P.; Jenkins, D.; Quintás, G.; Oger, C.; Galano, J.-M.; Vigor, C.; Durand, T.; Vento, M.; et al. Novel Free-Radical Mediated Lipid Peroxidation Biomarkers in Newborn Plasma. Anal. Chim. Acta 2017, 996, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.; Abd-Rabboh, L.; El-Dib, M.; Nawwar, F.; Hassan, H.; Aaref, M.; Abdelrahman, S.; Elsayed, A. Ascorbic Acid Combined with Ibuprofen in Hypoxic Ischemic Encephalopathy: A Randomized Controlled Trial. J. Perinatol. 2009, 29, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.; Gonzalez, D.; Greenberg, R.G.; Lee, Y.Z.; Laughon, M.M. A Phase I Trial of Caffeine to Evaluate Safety in Infants with Hypoxic-Ischemic Encephalopathy. J. Perinatol. 2024, 44, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, D.; Robertson, N.J.; Bainbridge, A.; Cady, E.; Charles-Edwards, G.; Deierl, A.; Fagiolo, G.; Franks, N.P.; Griffiths, J.; Hajnal, J.; et al. Moderate Hypothermia within 6 h of Birth plus Inhaled Xenon versus Moderate Hypothermia Alone after Birth Asphyxia (TOBY-Xe): A Proof-of-Concept, Open-Label, Randomised Controlled Trial. Lancet Neurol. 2016, 15, 145–153. [Google Scholar] [CrossRef]
- Dingley, J.; Liu, X.; Gill, H.; Smit, E.; Sabir, H.; Tooley, J.; Chakkarapani, E.; Windsor, D.; Thoresen, M. The Feasibility of Using a Portable Xenon Delivery Device to Permit Earlier Xenon Ventilation with Therapeutic Cooling of Neonates during Ambulance Retrieval. Anesth. Analg. 2015, 120, 1331–1336. [Google Scholar] [CrossRef]
- Patra, A.; Huang, H.; Bauer, J.A.; Giannone, P.J. Neurological Consequences of Systemic Inflammation in the Premature Neonate. Neural Regen. Res. 2017, 12, 890–896. [Google Scholar] [CrossRef]
- Gallini, F.; Coppola, M.; De Rose, D.U.; Maggio, L.; Arena, R.; Romano, V.; Cota, F.; Ricci, D.; Romeo, D.M.; Mercuri, E.M.; et al. Neurodevelopmental Outcomes in Very Preterm Infants: The Role of Severity of Bronchopulmonary Dysplasia. Early Hum. Dev. 2021, 152, 105275. [Google Scholar] [CrossRef]
- Harding, B.; Conception, K.; Li, Y.; Zhang, L. Glucocorticoids Protect Neonatal Rat Brain in Model of Hypoxic-Ischemic Encephalopathy (HIE). Int. J. Mol. Sci. 2016, 18, 17. [Google Scholar] [CrossRef]
- Guerrini, R.; Parmeggiani, L. Topiramate and Its Clinical Applications in Epilepsy. Expert Opin. Pharmacother. 2006, 7, 811–823. [Google Scholar] [CrossRef]
- Shank, R.P.; Gardocki, J.F.; Streeter, A.J.; Maryanoff, B.E. An Overview of the Preclinical Aspects of Topiramate: Pharmacology, Pharmacokinetics, and Mechanism of Action. Epilepsia 2000, 41, 3–9. [Google Scholar] [CrossRef]
- Filippi, L.; Poggi, C.; la Marca, G.; Furlanetto, S.; Fiorini, P.; Cavallaro, G.; Plantulli, A.; Donzelli, G.; Guerrini, R. Oral Topiramate in Neonates with Hypoxic Ischemic Encephalopathy Treated with Hypothermia: A Safety Study. J. Pediatr. 2010, 157, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Glier, C.; Dzietko, M.; Bittigau, P.; Jarosz, B.; Korobowicz, E.; Ikonomidou, C. Therapeutic Doses of Topiramate Are Not Toxic to the Developing Rat Brain. Exp. Neurol. 2004, 187, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.-R.; Kim, S.K.; Sun, W.; Park, S.K.; Choi, H.C.; Lim, J.H.; Kim, I.H.; Kim, H.-J.; Kim, H.; Eun, B.-L. Neuroprotective Effect of Topiramate on Hypoxic Ischemic Brain Injury in Neonatal Rats. Exp. Neurol. 2006, 201, 470–478. [Google Scholar] [CrossRef]
- Albrecht, E.; Kirkham, K.R.; Liu, S.S.; Brull, R. Peri-Operative Intravenous Administration of Magnesium Sulphate and Postoperative Pain: A Meta-Analysis. Anaesthesia 2013, 68, 79–90. [Google Scholar] [CrossRef]
- Do, S.-H. Magnesium: A Versatile Drug for Anesthesiologists. Korean J. Anesthesiol. 2013, 65, 4–8. [Google Scholar] [CrossRef]
- Sohn, H.-M.; Jheon, S.-H.; Nam, S.; Do, S.-H. Magnesium Sulphate Improves Pulmonary Function after Video-Assisted Thoracoscopic Surgery: A Randomised Double-Blind Placebo-Controlled Study. Eur. J. Anaesthesiol. EJA 2017, 34, 508–514. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Farber, N.B.; Olney, J.W. NMDA Receptor Function, Memory, and Brain Aging. Dialogues Clin. Neurosci. 2000, 2, 219–232. [Google Scholar] [CrossRef]
- Kemp, P.A.; Gardiner, S.M.; March, J.E.; Rubin, P.C.; Bennett, T. Assessment of the Effects of Endothelin-1 and Magnesium Sulphate on Regional Blood Flows in Conscious Rats, by the Coloured Microsphere Reference Technique. Br. J. Pharmacol. 1999, 126, 621–626. [Google Scholar] [CrossRef]
- Chang, J.J.; Mack, W.J.; Saver, J.L.; Sanossian, N. Magnesium: Potential Roles in Neurovascular Disease. Front. Neurol. 2014, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.L.; Palanisamy, A. What Can You Do to Protect the Newborn Brain? Curr. Opin. Anesthesiol. 2015, 28, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A Novel Potent Vasoconstrictor Peptide Produced by Vascular Endothelial Cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef]
- Ramos, M.D.; Briyal, S.; Prazad, P.; Gulati, A. Neuroprotective Effect of Sovateltide (IRL 1620, PMZ 1620) in a Neonatal Rat Model of Hypoxic-Ischemic Encephalopathy. Neuroscience 2022, 480, 194–202. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Gulati, A. Advances in Therapies to Treat Neonatal Hypoxic-Ischemic Encephalopathy. J. Clin. Med. 2023, 12, 6653. [Google Scholar] [CrossRef]
- Teng, H.; Li, C.; Zhang, Y.; Lu, M.; Chopp, M.; Zhang, Z.G.; Melcher-Mourgas, M.; Fleckenstein, B. Therapeutic Effect of Cerebrolysin on Reducing Impaired Cerebral Endothelial Cell Permeability. NeuroReport 2021, 32, 359–366. [Google Scholar] [CrossRef]
- Muresanu, D.F.; Strilciuc, S.; Stan, A. Current Drug Treatment of Acute Ischemic Stroke: Challenges and Opportunities. CNS Drugs 2019, 33, 841–847. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The Chemistry and Biological Activities of N-Acetylcysteine. Biochim. Biophys. Acta BBA-Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Frye, R.E.; Andrus, J.P.; Lemley, K.V.; De Rosa, S.C.; Ghezzi, P.; Holmgren, A.; Jones, D.; Jahoor, F.; Kopke, R.; Cotgreave, I.; et al. Pharmacology, Formulations, and Adverse Effects. In The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine; Frye, R.E., Berk, M., Eds.; Springer: Singapore, 2019; pp. 387–394. ISBN 978-981-10-5311-5. [Google Scholar]
- Arakawa, M.; Ito, Y. N-Acetylcysteine and Neurodegenerative Diseases: Basic and Clinical Pharmacology. Cerebellum Lond. Engl. 2007, 6, 308–314. [Google Scholar] [CrossRef]
- Jenkins, D.D.; Moss, H.G.; Brown, T.R.; Yazdani, M.; Thayyil, S.; Montaldo, P.; Vento, M.; Kuligowski, J.; Wagner, C.; Hollis, B.W.; et al. NAC and Vitamin D Improve CNS and Plasma Oxidative Stress in Neonatal HIE and Are Associated with Favorable Long-Term Outcomes. Antioxidants 2021, 10, 1344. [Google Scholar] [CrossRef]
- Cui, X.; McGrath, J.J.; Burne, T.H.J.; Mackay-Sim, A.; Eyles, D.W. Maternal Vitamin D Depletion Alters Neurogenesis in the Developing Rat Brain. Int. J. Dev. Neurosci. 2007, 25, 227–232. [Google Scholar] [CrossRef]
- Eyles, D.; Almeras, L.; Benech, P.; Patatian, A.; Mackay-Sim, A.; McGrath, J.; Féron, F. Developmental Vitamin D Deficiency Alters the Expression of Genes Encoding Mitochondrial, Cytoskeletal and Synaptic Proteins in the Adult Rat Brain. J. Steroid Biochem. Mol. Biol. 2007, 103, 538–545. [Google Scholar] [CrossRef]
- Gomez-Pinedo, U.; Cuevas, J.A.; Benito-Martín, M.S.; Moreno-Jiménez, L.; Esteban-Garcia, N.; Torre-Fuentes, L.; Matías-Guiu, J.A.; Pytel, V.; Montero, P.; Matías-Guiu, J. Vitamin D Increases Remyelination by Promoting Oligodendrocyte Lineage Differentiation. Brain Behav. 2019, 10, e01498. [Google Scholar] [CrossRef] [PubMed]
- Al-Niaimi, F.; Chiang, N.Y.Z. Topical Vitamin C and the Skin: Mechanisms of Action and Clinical Applications. J. Clin. Aesthetic Dermatol. 2017, 10, 14–17. [Google Scholar]
- Mohd Zaffarin, A.S.; Ng, S.-F.; Ng, M.H.; Hassan, H.; Alias, E. Pharmacology and Pharmacokinetics of Vitamin E: Nanoformulations to Enhance Bioavailability. Int. J. Nanomed. 2020, 15, 9961–9974. [Google Scholar] [CrossRef]
- La Torre, M.E.; Villano, I.; Monda, M.; Messina, A.; Cibelli, G.; Valenzano, A.; Pisanelli, D.; Panaro, M.A.; Tartaglia, N.; Ambrosi, A.; et al. Role of Vitamin E and the Orexin System in Neuroprotection. Brain Sci. 2021, 11, 1098. [Google Scholar] [CrossRef]
- Singhi, S.; Johnston, M. Recent Advances in Perinatal Neuroprotection. F1000Research 2019, 8, 2031. [Google Scholar] [CrossRef]
- Traber, M.G. Vitamin E: Necessary Nutrient for Neural Development and Cognitive Function. Proc. Nutr. Soc. 2021, 80, 319–326. [Google Scholar] [CrossRef]
- Brion, L.P.; Bell, E.F.; Raghuveer, T.S. Vitamin E Supplementation for Prevention of Morbidity and Mortality in Preterm Infants. Cochrane Database Syst. Rev. 2003, 2010, CD003665. [Google Scholar] [CrossRef]
- Bell, E.F.; Hansen, N.I.; Brion, L.P.; Ehrenkranz, R.A.; Kennedy, K.A.; Walsh, M.C.; Shankaran, S.; Acarregui, M.J.; Johnson, K.J.; Hale, E.C.; et al. Serum Tocopherol Levels in Very Preterm Infants After a Single Dose of Vitamin E at Birth. Pediatrics 2013, 132, e1626–e1633. [Google Scholar] [CrossRef]
- Paul, I.M.; Walson, P.D. Acetaminophen and Ibuprofen in the Treatment of Pediatric Fever: A Narrative Review. Curr. Med. Res. Opin. 2021, 37, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Janitschke, D.; Lauer, A.A.; Bachmann, C.M.; Seyfried, M.; Grimm, H.S.; Hartmann, T.; Grimm, M.O.W. Unique Role of Caffeine Compared to Other Methylxanthines (Theobromine, Theophylline, Pentoxifylline, Propentofylline) in Regulation of AD Relevant Genes in Neuroblastoma SH-SY5Y Wild Type Cells. Int. J. Mol. Sci. 2020, 21, 9015. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of Caffeine in the Brain with Special Reference to Factors That Contribute to Its Widespread Use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Chen, J.-F.; Cunha, R.A.; Svenningsson, P.; Vaugeois, J.-M. Adenosine and Brain Function. In International Review of Neurobiology; Academic Press: Cambridge, MA, USA, 2005; Volume 63, pp. 191–270. [Google Scholar]
- Lopes, J.P.; Pliássova, A.; Cunha, R.A. The Physiological Effects of Caffeine on Synaptic Transmission and Plasticity in the Mouse Hippocampus Selectively Depend on Adenosine A1 and A2A Receptors. Biochem. Pharmacol. 2019, 166, 313–321. [Google Scholar] [CrossRef]
- Cunha, R.A. How Does Adenosine Control Neuronal Dysfunction and Neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Yang, L.; Yu, X.; Zhang, Y.; Liu, N.; Xue, X.; Fu, J. Caffeine Treatment Started before Injury Reduces Hypoxic–Ischemic White-Matter Damage in Neonatal Rats by Regulating Phenotypic Microglia Polarization. Pediatr. Res. 2022, 92, 1543–1554. [Google Scholar] [CrossRef]
- Dobson, N.R.; Hunt, C.E. Pharmacology Review: Caffeine Use in Neonates: Indications, Pharmacokinetics, Clinical Effects, Outcomes. NeoReviews 2013, 14, e540–e550. [Google Scholar] [CrossRef]
- Sup, S.J.; Green, B.G.; Grant, S.K. 2-Iminobiotin Is an Inhibitor of Nitric Oxide Synthases. Biochem. Biophys. Res. Commun. 1994, 204, 962–968. [Google Scholar] [CrossRef]
- Fan, X.; van Bel, F. Pharmacological Neuroprotection after Perinatal Asphyxia. J. Matern. Fetal Neonatal Med. 2010, 23, 17–19. [Google Scholar] [CrossRef]
- Favié, L.M.A.; Cox, A.R.; van den Hoogen, A.; Nijboer, C.H.A.; Peeters-Scholte, C.M.P.C.D.; van Bel, F.; Egberts, T.C.G.; Rademaker, C.M.A.; Groenendaal, F. Nitric Oxide Synthase Inhibition as a Neuroprotective Strategy Following Hypoxic–Ischemic Encephalopathy: Evidence From Animal Studies. Front. Neurol. 2018, 9, 258. [Google Scholar] [CrossRef]
- van Hoogdalem, E.-J.; Peeters-Scholte, C.M.P.C.D.; Leufkens, P.W.T.J.; Hartstra, J.; van Lier, J.J.; de Leede, L.G.J. First-in-Human Study of the Safety, Tolerability, Pharmacokinetics and -Preliminary Dynamics of Neuroprotectant 2-Iminobiotin in Healthy Subjects. Curr. Clin. Pharmacol. 2020, 15, 152–163. [Google Scholar] [CrossRef]
- Iulia, C.; Ruxandra, T.; Costin, L.-B.; Liliana-Mary, V. Citicoline—A Neuroprotector with Proven Effects on Glaucomatous Disease. Romanian J. Ophthalmol. 2017, 61, 152–158. [Google Scholar]
- Salamah, A.; Mehrez, M.; Faheem, A.; El Amrousy, D. Efficacy of Citicoline as a Neuroprotector in Children with Post Cardiac Arrest: A Randomized Controlled Clinical Trial. Eur. J. Pediatr. 2021, 180, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Hobson, A.; Baines, J.; Weiss, M.D. Beyond Hypothermia: Alternative Therapies for Hypoxic Ischemic Encephalopathy. Open Pharmacol. J. 2013, 7, 26–40. [Google Scholar] [CrossRef]
- Álvarez-Sabín, J.; Román, G.C. The Role of Citicoline in Neuroprotection and Neurorepair in Ischemic Stroke. Brain Sci. 2013, 3, 1395–1414. [Google Scholar] [CrossRef]
- Hair, P.S.; Enos, A.I.; Krishna, N.K.; Cunnion, K.M. Inhibition of Immune Complex Complement Activation and Neutrophil Extracellular Trap Formation by Peptide Inhibitor of Complement C1. Front. Immunol. 2018, 9, 558. [Google Scholar] [CrossRef]
- Goss, J.; Hair, P.; Kumar, P.; Iacono, G.; Redden, L.; Morelli, G.; Krishna, N.; Thienel, U.; Cunnion, K. RLS-0071, a Dual-Targeting Anti-Inflammatory Peptide–Biomarker Findings from a First in Human Clinical Trial. Transl. Med. Commun. 2023, 8, 1. [Google Scholar] [CrossRef]
- Rüegger, C.M.; Davis, P.G.; Cheong, J.L. Xenon as an Adjuvant to Therapeutic Hypothermia in Near-term and Term Newborns with Hypoxic-ischaemic Encephalopathy. Cochrane Database Syst. Rev. 2018, 2018, CD012753. [Google Scholar] [CrossRef]
- Chakkarapani, E.; Dingley, J.; Liu, X.; Hoque, N.; Aquilina, K.; Porter, H.; Thoresen, M. Xenon Enhances Hypothermic Neuroprotection in Asphyxiated Newborn Pigs. Ann. Neurol. 2010, 68, 330–341. [Google Scholar] [CrossRef]
- Dingley, J.; Hobbs, C.; Ferguson, J.; Stone, J.; Thoresen, M. Xenon/Hypothermia Neuroprotection Regimes in Spontaneously Breathing Neonatal Rats After Hypoxic-Ischemic Insult: The Respiratory and Sedative Effects. Anesth. Analg. 2008, 106, 916–923. [Google Scholar] [CrossRef]
- Thoresen, M.; Hobbs, C.E.; Wood, T.; Chakkarapani, E.; Dingley, J. Cooling Combined with Immediate or Delayed Xenon Inhalation Provides Equivalent Long-Term Neuroprotection after Neonatal Hypoxia—Ischemia. J. Cereb. Blood Flow Metab. 2009, 29, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Franks, N.P.; Dickinson, R.; de Sousa, S.L.M.; Hall, A.C.; Lieb, W.R. How Does Xenon Produce Anaesthesia? Nature 1998, 396, 324. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.P.; Banks, P.J.; McKitrick, T.J.W.; Geldart, C.H.; Edge, C.J.; Babla, R.; Simillis, C.; Franks, N.P.; Dickinson, R. Identification of Two Mutations (F758W and F758Y) in the N -Methyl-D-Aspartate Receptor Glycine-Binding Site That Selectively Prevent Competitive Inhibition by Xenon without Affecting Glycine Binding. Anesthesiology 2012, 117, 38–47. [Google Scholar] [CrossRef]
- Dickinson, R.; Peterson, B.K.; Banks, P.; Simillis, C.; Martin, J.C.S.; Valenzuela, C.A.; Maze, M.; Franks, N.P. Competitive Inhibition at the Glycine Site of the N -Methyl-d-Aspartate Receptor by the Anesthetics Xenon and Isoflurane: Evidence from Molecular Modeling and Electrophysiology. Anesthesiology 2007, 107, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Lim, T.; Xu, J.; Tang, H.; Wan, Y.; Zhao, H.; Hossain, M.; Maxwell, P.H.; Maze, M. Xenon Preconditioning Protects against Renal Ischemic-Reperfusion Injury via HIF-1α Activation. J. Am. Soc. Nephrol. JASN 2009, 20, 713–720. [Google Scholar] [CrossRef]
- Bauchner, H.; Rivara, F.P. Improving Child Health Research: The Role of Randomized Clinical Trials. J. Pediatr. 2023, 262, 113641. [Google Scholar] [CrossRef]
- Marques, K.L.; Rodrigues, V.; Balduci, C.T.N.; Montes, G.C.; Barradas, P.C.; Cunha-Rodrigues, M.C. Emerging Therapeutic Strategies in Hypoxic-Ischemic Encephalopathy: A Focus on Cognitive Outcomes. Front. Pharmacol. 2024, 15, 1347529. [Google Scholar] [CrossRef]
- Gulati, A.; Agrawal, N.; Vibha, D.; Misra, U.K.; Paul, B.; Jain, D.; Pandian, J.; Borgohain, R. Safety and Efficacy of Sovateltide (IRL-1620) in a Multicenter Randomized Controlled Clinical Trial in Patients with Acute Cerebral Ischemic Stroke. CNS Drugs 2021, 35, 85–104. [Google Scholar] [CrossRef]
- Rüegger, C.M.; Dawson, J.A.; Donath, S.M.; Owen, L.S.; Davis, P.G. Nonpublication and Discontinuation of Randomised Controlled Trials in Newborns. Acta Paediatr. 2017, 106, 1940–1944. [Google Scholar] [CrossRef]
| NCT Number/ Country | Recruitment Status | Pharmacological Substance | Administration | Schedule of Administration | Apgar Score | Age | Number of Participants | Reported Outcomes | References |
|---|---|---|---|---|---|---|---|---|---|
| NCT00719407 United States | Completed (study completion—November 2012) | Erythropoietin α (Procrit) | Intravenously | Six doses (250, 500, 1000, 2500 U/kg per dose) first dose < 24 h, subsequent at 48-h intervals | ≤5 at 10 min | Up to 24 h | 24 | No deaths reported, moderate to severe disabilities occurred, cases of brain injury on neonatal MRI | [38,39] |
| NCT03079167 Australia New Zealand Singapore | Completed (study completion—April 2024) | Erythropoietin α (Procrit, Epogen) | Intravenously | 1000 IU/kg (capped up to 4000 IU/kg per day) on 1, 2, 3, 5 and 7 days after birth | ≤5 at 10 min | Up to 23 h | 313 | Not reported | - |
| NCT00808704 China | Completed (study completion—July 2008) | Recombinant human erythropoietin (r-hu-EPO) | Subcutaneously and intravenously | Either 300 U/kg and 500 U/kg first subcutaneously then intravenously for every day for 2 weeks | ≤5 at 5 min | 1 h to 48 h | 167 | Not reported | - |
| NCT01913340 United States | Completed (study completion—September 2016) | Erythropoietin α (Procrit) | Intravenously | 1000 U/kg per dose; five doses | ≤5 at 10 min | 30 min to 24 h | 50 | Low rate of deaths, not reported adverse events and moderate or severe neurodevelopmental impairments | [40,41,42] |
| NCT00945789 Egypt | Completed (study completion—June 2009) | Recombinant human erythropoietin | Subcutaneously | 2500 IU/kg per dose daily for 5 days | ≤3 at 5 min | Up to 24 h | 45 | No seizures reported, MRI did not differ between groups, lower neurologic and developmental abnormalities | [43] |
| NCT01471015 United States | Completed (study completion—January 2014) | Darbepoetin α | Intravenously or subcutaneously | High dose: 10 mcg/kg/dose first dose within 12 h of delivery, second dose given at 7 days old; low dose: 2 mcg/kg/dose first dose within 12 h of delivery, second dose given at 7 days old | ≤5 at 10 min | Up to 12 h | 30 | Adverse events occurred | [44] |
| NCT01732146 France | Completed (study completion—February 2017) | Erythropoietin β | Intravenously | 1000–1500 U/kg/dose given three times every 24 h, first dose within 12 h of delivery | ≤5 at 10 min | Up to 12 h | 120 | Not reported | - |
| NCT02811263 United States | Completed (study completion—April 2022) | Erythropoietin α (Epogen) | Intravenously | 1000 U/kg per dose before 26 h of age and at 2, 3, 4, and 7 days of age | ≤5 at 10 min | Up to 24 h | 500 | Higher number of serious adverse events, no changes in deaths/neurodevelopmental impairments | [45] |
| NCT02002039 India | Completed (study completion—June 2016) | Erythropoietin β | Intravenously | 500 U/kg per dose every day for five doses starting at first 6 h of life | ≤5 at 10 min | Up to 6 h | 100 | Not reported | - |
| NCT05395195 Bangladesh India Sri Lanka UK-sponsor | Recruiting (study start—December 2022) | Erythropoietin | Intravenously or subcutaneously | 500 U/kg per dose. First dose within 6 h of birth; second between 12–24 h from the first dose; subsequent seven doses every 24 h from the second dose; total nine doses | <6 at 5 min | 1 h to 6 h | 504 | Not reported | - |
| NCT03163589 Egypt | Unknown (study completion—June 2022) | Erythropoietin | Intravenously | 1000 U/kg within 4–6 h after birth on days 1, 2, 3, 5, 7, and 9 (nine doses) | <5 at 10 min | Up to 24 h | 40 | Not reported | - |
| NCT Number/ Country | Recruitment Status | Pharmacological Substance | Administration | Schedule of Administration | Apgar Score | Age | Number of Participants | Reported Outcomes | References |
|---|---|---|---|---|---|---|---|---|---|
| NCT02071160 Egypt | Completed (study completion—December 2013) | Melatonin (Puritan’s Pride) | Intraesophageally | 10 mg/kg daily, five doses started at enrollment | ≤3 at 5 min | Up to 6 h | 45 | Fewer seizures, less white matter abnormalities on MRI, at 6 months improved survival without neurological or developmental abnormalities | [51] |
| NCT02621944 United States | Recruiting (study start—November 2016) | Melatonin | Enterally | Dose escalation study—0.5 mg/kg; 3 mg/kg; 5 mg/kg enteral route within 12 h with a target of 6 h of life | <5 at 10 min | Up to 6 h | 70 | Not reported | - |
| NCT01904786 United States | Withdrawn (lack of enrollment for suitable candidates) (study completion—November 2018) | Melatonin (PureBulk) | Orally | 40 mg/kg every 8 h for a total of six doses given over 48 h | No Apgar | 1 h to 8 h | 0 | Not reported | - |
| NCT03806816 Italy | Unknown (study completion—December 2022) | Melatonin (Buona Circadiem) | Enterally | 10 mg/kg/daily five doses | <5 at 10 min | 1 h to 6 h | 100 | Not reported | - |
| NCT Number/ Country | Recruitment Status | Pharmacological Substance | Administration | Schedule of Administration | Apgar Score | Age | Number of Participants | Reported Outcomes | References |
|---|---|---|---|---|---|---|---|---|---|
| NCT03162653 Austria Belgium Estonia Finland German Italy Netherlands Norway Poland-withdrawn Portugal-withdrawn Spain Switzerland | Recruiting (study start—March 2018) | Allopurinol (powder for injection) | Intravenously | First dose—20 mg/kg in 2 mL/kg sterile water no later than 30 min postnatally; second dose—10 mg/kg in 2 mL/kg sterile water 12 h thereafter | ≤5 at 10 min | Up to 45 min | 760 | Not reported | [52,53,54,55] |
| NCT00189007 Netherlands | Terminated (study completion—December 2016) | Allopurinol (Acepurin) | Intravenously | One dose 500 mg/50 mL intravenously to women before delivery | Not reported | Child, adult, older adult | 222 | Not reported long-term developmental and behavioral outcomes at 5 years of age | [56,57,58,59] |
| NCT Number/ Country | Recruitment Status | Pharmacological Substance | Administration | Schedule of Administration | Apgar Score | Age | Number of Participants | Reported Outcomes | References |
|---|---|---|---|---|---|---|---|---|---|
| NCT02812433 Canada | Completed (study completion—January 2022) | Sildenafil | Orally | 2 mg/kg/dose twice a day for 7 days (from day 2 of life to day 9 of life) | Apgar ≤ 5 at 10 min | 0 min to 48 h | 28 | Few severe neurodevelopmental impairments occurred | [69] |
| NCT05275725 Uganda | Recruiting (study start—July 2022) | Sildenafil | Not reported | Five groups: first group—4 mg/kg; second group—5 mg/kg; third group—6 mg/kg; fourth group—6 mg/kg; fifth group—6 mg/kg | Apgar ≤ 5 at 5 min | 0 days to 18 months | 30 | Not reported | - |
| NCT06098833 Canada | Recruiting (study start—July 2024) | Sildenafil (Viagra) | Orally | Three doses twice a day: 2 mg/kg, 2.5 mg/kg and 3 mg/kg (day 2 to day 9) | Apgar ≤ 5 at 10 min | 0 min to 48 h | 60 | Not reported | - |
| NCT04169191 Canada | Active, not recruiting (study start—September 2019) | Sildenafil citrate | Not reported | Dose escalation study (3 + 3) with maximum dose 6 mg/kg/dose every 12 h | Apgar ≤ 5 at 10 min | 0 days to 2 days | 20 | Not reported | - |
| NCT Number/ Country | Recruitment Status | Pharmacological Substance | Administration | Schedule of Administration | Apgar Score | Age | Number of Participants | Reported Outcomes | References |
|---|---|---|---|---|---|---|---|---|---|
| Metformin | |||||||||
| NCT05590676 Canada | Terminated (low recruitment) (study completion—February 2024) | Metformin | Intravenously | 4 group (15 mg/kg, 20 mg/kg, 25 mg/kg) | Not reported | Up to 3 months | 1 | Not reported | [80] |
| Glucocorticoids | |||||||||
| NCT02700828 Hungary | Completed (study completion—December 2022) | Hydrocortisone (Solu-Cortef) | Intravenously | 4 × 0.5 mg/kg for 24 h (in every 6 h) | ≤5 at 10 min | Up to 72 h | 32 | Effectiveness in raising blood pressure, decreasing inotrope requirement | [81] |
| NCT05836610 Hungary | Recruiting (study start—September 2021) | Hydrocortisone (Solu-Cortef) | Intravenously | 0.5 mg/kg (in every 6 h) | Not reported | Up to 72 h | 50 | Not reported | - |
| Topiramate | |||||||||
| NCT01241019 Italy | Completed (study completion—December 2013) | Topiramate (Topamax, Janssen-Cilag, Cologno Monzese, Milan, Italy) | Orogastrically | 10 mg/kg once a day, total of three doses per patient started on the third day of life | <5 at 10 min | 36 weeks and older | 64 | No statistically or clinically significant differences between both groups, reduction in the prevalence of epilepsy | [82,83] |
| NCT01765218 United States | Terminated (study completion—January 2022) | Topiramate (Topimax, Topiragen) | Enterally | 5 mg/kg in five doses as soon as possible | <5 at 10 min | Up to 6 h | 34 | Low rate of seizures, no mortality or adverse events reported | - |
| Magnesium sulphate | |||||||||
| NCT04705142 Pakistan | Completed (study completion—December 2020) | Magnesium sulphate | Intravenously | 250 mg/kg, total of three doses, first within 6 h of life, second after 24 h, third after 48 h | Not reported | Up to 6 h | 200 | Low rate of seizures and neurological problems, better ability to suck feed | [84] |
| NCT06342362 Pakistan | Not yet recruiting (study start—April 2024) | Magnesium sulphate | Intravenously | Not reported | Not reported | 1 day to 30 days | 102 | Not reported | - |
| NCT05707962 Pakistan | Not yet recruiting (study start—March 2023) | Magnesium sulphate | Intravenously | 250 mg/kg at 3 doses given 24 h apart, not later than 24 h of life | Not reported | 1 h to 24 h | 178 | Not reported | - |
| Sovateltide | |||||||||
| NCT05514340 India | Recruiting (study start—September 2023) | Sovateltide (IRL-1620) | Intravenously | 0.3 µg/kg in 3 doses, every 3 h on day 1, day 3, and day 6 post randomization | <5 at 10 min | Child, adult, older adult | 40 | Not reported | - |
| Cerebrolysine | |||||||||
| NCT01059461 Egypt | Completed (study completion—September 2013) | Cerebrolysin | Intramuscularly | 0.1 mL/kg twice weekly, ten injections | <5 at 10 min | 3 months to 6 months | 40 | Improved social and speech composite, minimal side effects, no changes in seizures frequency or in duration | [85] |
| N-acetylcysteine and calcitriol | |||||||||
| NCT04643821 United States | Completed (study completion—March 2020) | N-acetylcystein (NAC), Vitamin D (calcitriol) | Intravenously | First group: NAC 25 mg/kg every 12 h, calcitriol 0.05 mcg/kg every 12 h for 10 days; second group: NAC 25 mg/kg every 12 h, calcitriol 0.03 mcg/kg every 24 h for 10 days; third group: NAC 40 mg/kg every 12 h, calcitriol 0.03 mcg/kg every 24 h for 10 days; starting within 6 h after birth | <5 at 5 min | Up to 6 h | 30 | NAC increased GSH levels in brains after HIE | [86,87] |
| Vitamins and ibuprofen | |||||||||
| NCT01743742 India | Completed (study completion—October 2013) | Vitamin E (Evion), vitamin C (Limcee) | Orally | 200 IU single dose of vit E, 250 mg vit C (two doses at 24 h interval) | <6 at 5 min | 1 min to 6 h | 95 | Not reported | - |
| NCT00624871 Egypt | Completed (study completion—April 2009) | Vitamin C, ibuprofen | Intravenously/orally | Vitamin C: 100 mg/kg/dose every day for 3 days, ibuprofen: 10 mg/kg on first day, 5 mg/kg on second and third day for 3 days | <6 at 5 min | Up to 2 h | 60 | No severity in HIE, no difference in the incidence of neurological abnormalities, no developmental delay | [88] |
| Caffeine citrate | |||||||||
| NCT03913221 United States | Active, not recruiting (study start—August 2019) | Caffeine citrate (Cafcit) | Intravenously | Low-dose: caffeine citrate 5 mg/kg twice a day; high-dose: 10 mg/kg twice a day within 24 h of delivery (loading dose 20 mg/kg) | Not reported | Up to 24 h | 17 | Reported adverse events | [89] |
| NCT06448780 United States | Not yet recruiting (study start—July 2024) | Caffeine citrate (Cafcit) | Intravenously | Low-dose: caffeine citrate 10 mg/kg twice a day (loading dose 20 mg/kg); high-dose: 10 mg/kg twice a day (loading dose 30 mg/kg) within 24 h of delivery | Not reported | Up to 24 h | 16 | Not reported | - |
| NCT05295784 United States | Withdrawn (data no longer support this study) (study completion—May 2024) | Caffeine citrate | Intravenously | Low dose: 5 mg/kg; medium dose: 15 mg/kg; high dose: 25 mg/kg once in the first 24 h of life | Not reported | 0 h to 24 h | 0 | Not reported | - |
| 2-iminobiotin | |||||||||
| NCT01626924 Turkey | Terminated (study completion—October 2014) | 2-iminobiotin | Intravenously | 0.2 mg/kg/dose/6 doses given in 20 h | ≤5 at 10 min | Up to 6 h | 6 | Not reported | - |
| Citicoline | |||||||||
| NCT03181646 Pakistan | Unknown status (study completion—December 2017) | Citicoline | Intravenously | 15 mg/kg/dose | Not reported | 1 h to 14 days | 50 | Not reported | - |
| RLS-0071 | |||||||||
| NCT05778188 United States | Recruiting (study start—July 2023) | RLS-0071 | Intravenously | 3 mg/kg, 10 mg/kg, 20 mg/kg every 8 h, total 10 doses for 72 h | ≤5 at 10 min | Up to 10 h | 42 | Not reported | - |
| Xenon | |||||||||
| NCT00934700 United Kingdom | Completed (study completion—September 2014) | Xenon gas (LENOXe) | Endotracheally | 30% xenon gas for 24 h | <5 at 10 min | 1 h to 12 h | 92 | Low rate of adverse events, cases of deaths occurred | [90] |
| NCT02071394 United Kingdom | Completed (study completion—April 2020) | Xenon gas (LENOXe) | Endotracheally | 50% xenon gas for 18 h within 5 h after birth | ≤5 at 10 min | Child, adult, older adult | 50 | No reduction of mortality, no substantial differences between groups after analysis of brain damage biomarkers, no seizures occurred during primary hospitalization | [91] |
| Drug | Advantages | Pitfalls |
|---|---|---|
| EPO | Neuroprotective agent; enhances neuronal and glial migration around the injured site; increases neurodevelopment in children affected by HIE; prevents brain injury in newborns; low rate of deaths; fewer neurologic and developmental abnormalities; no significant side effects | Disabilities and brain injuries, ranging from moderate to severe, are sometimes experienced, and a high number of adverse events were reported. |
| Melatonin | Antioxidant and anti-inflammatory agent; ability to easily cross the BBB; fewer seizures; improves survival without neurological and/or developmental abnormalities; less white matter abnormalities | - |
| Allopurinol | Decreases the release of oxygen radicals; not reported long-term developmental and behavioral outcomes | - |
| Sildenafil | Neuroprotective agent; enhances hemodynamic redistribution and increases vascular density; improves microcirculation | The combined rate of death or survival with severe neurodevelopmental impairment was 57% at 18 months. |
| Metformin | A common treatment for diabetes type 2; antioxidant and anti-inflammatory agent; enhances neurogenesis; supports remyelination in neonatal white matter after injury | - |
| GCs | Effective for reducing inflammation; impacts brain development through intracellular glucocorticoid and mineralocorticoid receptors; administration to the neonatal brain confer neuroprotection and mitigate brain damage; increases blood pressure and reduces need for inotropes in neonates | Administration of GCs in preterm infants is a controversial topic. |
| Topiramate | Anticonvulsant drug; effective absorption, high bioavailability, good tolerability; considered as a neuroprotective drug; short-term use has minimal neurotoxic effects; low rate of seizures and no mortality events; reduction in the prevalence of epilepsy | The rate of death or severe neurological disability remains unchanged, but the percentage of adverse events has increased. |
| Magnesium sulphate | Ability to reduce pain and decrease the need for anesthetics; involves in vasodilatation, hemostasis and maintenance of the BBB; neuroprotective agent; supportive drug for newborns with HI injury; low rate of seizures and neurological problems | - |
| Sovateltide | Neuroprotective agent; potential drug for enhancing neuronal recovery after HIE; decreases oxidative stress and cell death in the neonatal HI; “first in class” drug for HIE | - |
| Cerebrolysin | Enhances the therapeutic efficacy and safety of thrombolytic agents; has complex mechanism of action, proven multimodal and pleiotropic effects; neuroprotection; long-term regeneration; improves social and speech composite; occurs minimal side effects | There are no changes either in the frequency or duration of the seizures. |
| NAC and calcitriol | Antioxidant and free radical scavenger (NAC); has a role in neuroplasticity, myelination and normal brain development (calcitriol); mitigates oxidative stress and contributes to neuroprotection of the injured neonatal brains; NAC increases GSH levels in brains after HIE | - |
| Vitamins and ibuprofen | Antioxidant agent (vitamin C); antioxidant and anti-inflammatory agent with neuroprotective properties (vitamin E); crucial for the development of the nervous system during embryonic development (vitamin E); anti-inflammatory drug that alleviates pain and reduces inflammation (ibuprofen) | The neuroprotective efficacy of vitamin E remains controversial due to its varying effectiveness. Observed incidence of mortality, neurological abnormalities, and developmental delay at 6 months of age. |
| Caffeine citrate | Has a neuroprotective effect; influences on neuronal functions | In animal models, it mitigates white matter brain damage. There is no data on the efficacy and safety of the therapy in newborns after hypoxic-ischemic (HI) injury. Some adverse events occur. |
| 2-IB | Provides neuroprotection by inhibiting the neuronal and inducible forms of NOS; the aim of using 2-IB was to modulate the pathophysiological pathways activated by oxygen deprivation after HI | - |
| Citicoline | Aids in neurorepair; protects damaged tissue from mechanism of brain injury; enhances brain plasticity | - |
| RLS-0071 | Anti-inflammatory agent, inhibits cellular and humoral inflammation; established as the therapy for HIE and neurotrophic pulmonary diseases | - |
| Xenon | Odorless gas use in inhalations; in neonates, it has a quick onset and provides anticonvulsant effects; provides most of neuroprotective benefits; reduces apoptotic cell death during phases of reperfusion injury; low rate of adverse events | There were a few deaths reported; TH with xenon was not associated with a reduction in mortality; there were no substantial differences between the groups in terms of the analyses of the selected biomarkers of brain damage or the occurrence of seizures during the initial hospitalization. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebala, P.; Janowska, J.; Sypecka, J. Pharmacological Therapies for Consequences of Perinatal Hypoxic-Ischemic Brain Injury: Where Are We Now? Int. J. Mol. Sci. 2025, 26, 10200. https://doi.org/10.3390/ijms262010200
Gebala P, Janowska J, Sypecka J. Pharmacological Therapies for Consequences of Perinatal Hypoxic-Ischemic Brain Injury: Where Are We Now? International Journal of Molecular Sciences. 2025; 26(20):10200. https://doi.org/10.3390/ijms262010200
Chicago/Turabian StyleGebala, Paulina, Justyna Janowska, and Joanna Sypecka. 2025. "Pharmacological Therapies for Consequences of Perinatal Hypoxic-Ischemic Brain Injury: Where Are We Now?" International Journal of Molecular Sciences 26, no. 20: 10200. https://doi.org/10.3390/ijms262010200
APA StyleGebala, P., Janowska, J., & Sypecka, J. (2025). Pharmacological Therapies for Consequences of Perinatal Hypoxic-Ischemic Brain Injury: Where Are We Now? International Journal of Molecular Sciences, 26(20), 10200. https://doi.org/10.3390/ijms262010200









