Kaposi’s Sarcoma: A Non-Communicable Outcome Mainly Prompted by Communicable Diseases in Sub-Saharan Africa
Abstract
1. Introduction
2. Crosstalk Between HIV-KSHV Co-Infection in SSA
2.1. Epidemiology and Risk Factors of KSHV in Africa
2.2. Description of the Prevalence of KSHV Infection in SSA Countries
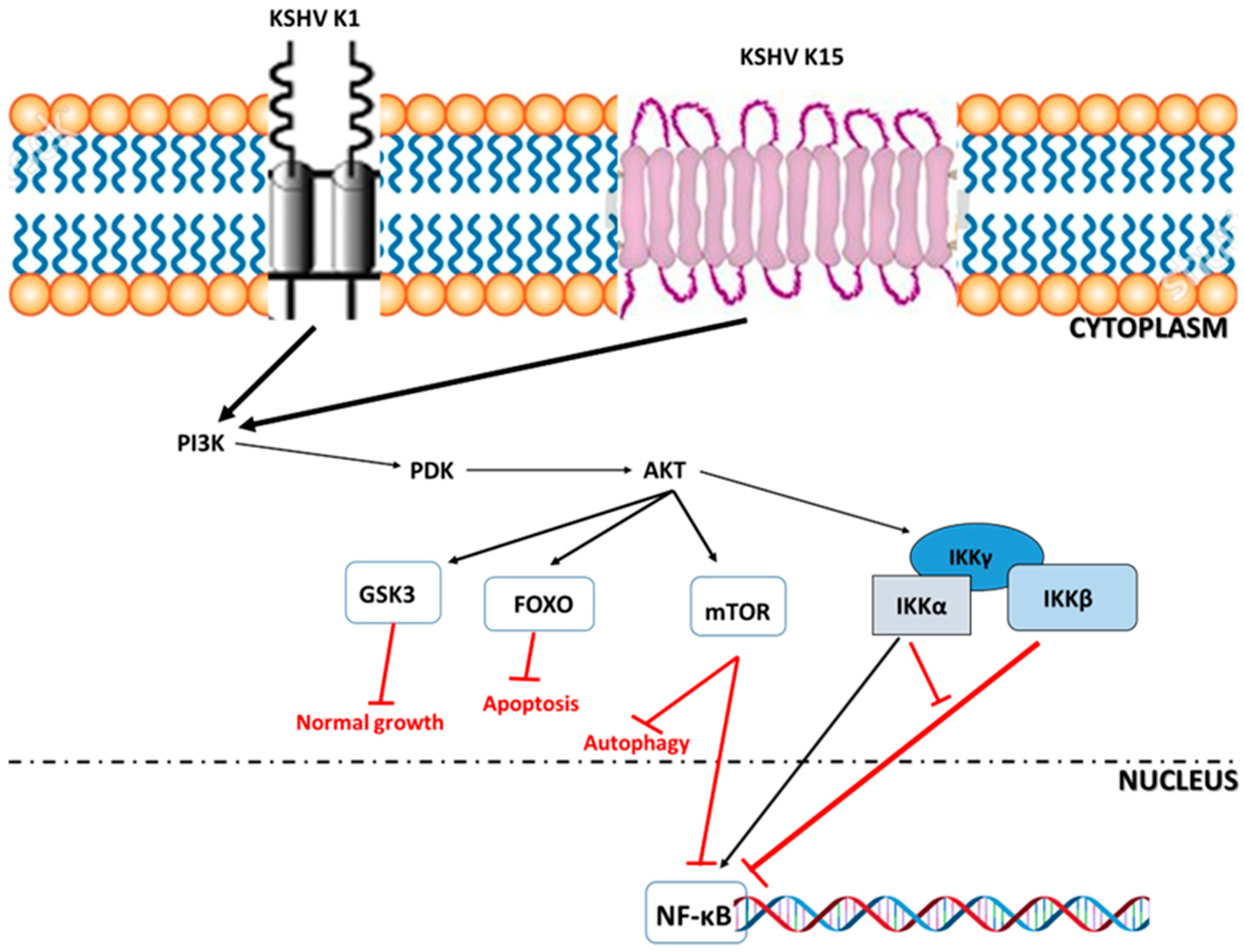
2.3. Transmission and Pathogenesis of KSHV
3. Kaposi’s Sarcoma Malignancy
3.1. Epidemiology and Carcinogenesis
3.2. Other KS-Facilitating Factors and Mitigating Measures in SSA
4. A Potential “All-in-One” Solution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Douglas, J.L.; Gustin, J.K.; Moses, A.V.; Dezube, B.J.; Pantanowitz, L. Kaposi Sarcoma Pathogenesis: A Triad of Viral Infection, Oncogenesis and Chronic Inflammation. Transl. Biomed. 2010, 1, 172. [Google Scholar]
- Ablashi, D.V.; Chatlynne, L.G.; Whitman, J.E., Jr.; Cesarman, E. Spectrum of Kaposi’s sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin. Microbiol. Rev. 2002, 15, 439–464. [Google Scholar] [CrossRef]
- Jary, A.; Veyri, M.; Gothland, A.; Leducq, V.; Calvez, V.; Marcelin, A.G. Kaposi’s Sarcoma-Associated Herpesvirus, the Etiological Agent of All Epidemiological Forms of Kaposi’s Sarcoma. Cancers 2021, 13, 6208. [Google Scholar] [CrossRef]
- Dittmer, D.P.; Damania, B. Kaposi’s Sarcoma-Associated Herpesvirus (KSHV)-Associated Disease in the AIDS Patient: An Update. Cancer Treat. Res. 2019, 177, 63–80. [Google Scholar] [CrossRef]
- Lifson, A.R.; Darrow, W.W.; Hessol, N.A.; O’Malley, P.M.; Barnhart, L.; Jaffe, H.W.; Rutherford, G.W. Kaposi’s sarcoma among homosexual and bisexual men enrolled in the San Francisco City Clinic Cohort Study. J. Acquir. Immune Defic. Synd. 1990, 3, S32–S37. [Google Scholar] [PubMed]
- Phillips, A.M.; Jones, A.G.; Osmond, D.H.; Pollack, L.M.; Catania, J.A.; Martin, J.N. Awareness of Kaposi’s sarcoma-associated herpesvirus among men who have sex with men. Sex. Transm. Dis. 2008, 35, 1011–1014. [Google Scholar] [CrossRef]
- Blumenthal, M.J.; Cornejo Castro, E.M.; Whitby, D.; Katz, A.A.; Schäfer, G. Evidence for altered host genetic factors in KSHV infection and KSHV-related disease development. Rev. Med. Virol. 2021, 31, e2160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shao, X.; Chen, Y.; Zhang, T.; Minhas, V.; Wood, C.; He, N. Human herpesvirus 8 seroprevalence, China. Emerg. Infect. Dis. 2012, 18, 150–152. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.A.; Sumita, L.M.; Nascimento, M.C.; Oliveira, J.; Mascheretti, M.; Quiroga, M.; Freire, W.S.; Tateno, A.; Boulos, M.; Mayaud, P.; et al. Human herpesvirus-8 infection and oral shedding in Amerindian and non-Amerindian populations in the Brazilian Amazon region. J. Infect. Dis. 2007, 196, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Kazanji, M.; Dussart, P.; Duprez, R.; Tortevoye, P.; Pouliquen, J.F.; Vandekerkhove, J.; Couppié, P.; Morvan, J.; Talarmin, A.; Gessain, A. Serological and molecular evidence that human herpesvirus 8 is endemic among Amerindians in French Guiana. J. Infect. Dis. 2005, 192, 1525–1529. [Google Scholar] [CrossRef]
- Angeletti, P.C.; Zhang, L.; Wood, C. The viral etiology of AIDS-associated malignancies. Adv. Pharmacol. 2008, 56, 509–557. [Google Scholar] [CrossRef]
- Dittmer, D.P.; Damania, B. Kaposi sarcoma associated herpesvirus pathogenesis (KSHV)—An update. Curr. Opin. Virol. 2013, 3, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.J.; Pantanowitz, L.; Casper, C.; Stebbing, J.; Dezube, B.J. HIV/AIDS: Epidemiology, pathophysiology, and treatment of Kaposi sarcoma-associated herpesvirus disease: Kaposi sarcoma, primary effusion lymphoma, and multicentric Castleman disease. Clin. Infect. Dis. 2008, 47, 1209–1215. [Google Scholar] [CrossRef]
- Gaglia, M.M. Kaposi’s sarcoma-associated herpesvirus at 27. Tumour Virus Res. 2021, 12, 200223. [Google Scholar] [CrossRef]
- Buh, A.; Deonandan, R.; Gomes, J.; Krentel, A.; Oladimeji, O.; Yaya, S. Prevalence and factors associated with HIV treatment non-adherence among people living with HIV in three regions of Cameroon: A cross-sectional study. PLoS ONE 2023, 18, e0283991. [Google Scholar] [CrossRef]
- Alexandrova, Y.; Costiniuk, C.T.; Jenabian, M.A. Pulmonary Immune Dysregulation and Viral Persistence During HIV Infection. Front. Immunol. 2022, 12, 808722. [Google Scholar] [CrossRef]
- UNAIDS 2024 Epidemiological Estimates. UNAIDS Financial Estimates. 2024. Available online: http://hivfinancial.unaids.org/hivfinancialdashboards.html (accessed on 23 September 2024).
- Lorenc, A.; Ananthavarathan, P.; Lorigan, J.; Jowata, M.; Brook, G.; Banarsee, R. The prevalence of comorbidities among people living with HIV in Brent: A diverse London Borough. Lond. J. Prim. Care 2014, 6, 84–90. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Reveals Leading Causes of Death and Disability Worldwide Between 2000 and 2019. 2020. Available online: https://www.paho.org/pt/noticias/9-12-2020-oms-revela-principais-causas-morte-e-incapacidade-em-todo-mundo-entre-2000-e (accessed on 17 October 2025).
- Deeks, S.G.; Walker, B.D. The immune response to AIDS virus infection: Good, bad, or both? J. Clin. Investig. 2004, 113, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, M.; Pandhare, J.; Dash, C. Immune Control of HIV. J. Life Sci. 2019, 1, 4–37. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Crane, M.; Zhou, J.; Mina, M.; Post, J.J.; Cameron, B.A.; Lloyd, A.R.; Jaworowski, A.; French, M.A.; Lewin, S.R. HIV and co-infections. Immunol. Rev. 2013, 254, 114–142. [Google Scholar] [CrossRef]
- Mulherkar, T.H.; Gómez, D.J.; Sandel, G.; Jain, P. Co-Infection and Cancer: Host-Pathogen Interaction between Dendritic Cells and HIV-1, HTLV-1, and Other Oncogenic Viruses. Viruses 2022, 14, 2037. [Google Scholar] [CrossRef]
- Skrzat-Klapaczyńska, A.; Matłosz, B.; Bednarska, A.; Paciorek, M.; Firląg-Burkacka, E.; Horban, A.; Kowalska, J.D. Factors associated with urinary tract infections among HIV-1 infected patients. PLoS ONE 2018, 13, e0190564. [Google Scholar] [CrossRef] [PubMed]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients Under Long-Term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Stolka, K.; Ndom, P.; Hemingway-Foday, J.; Iriondo-Perez, J.; Miley, W.; Labo, N.; Stella, J.; Abassora, M.; Woelk, G.; Ryder, R.; et al. Risk factors for Kaposi’s sarcoma among HIV-positive individuals in a case control study in Cameroon. Cancer Epidemiol. 2014, 38, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Zhang, Y.; Wei, M.; Tao, S.; Yang, Y. Seroprevalence and risk factors for Kaposi’s Sarcoma associated herpesvirus among men who have sex with men in Shanghai, China. BMC Infect. Dis. 2023, 23, 59. [Google Scholar] [CrossRef]
- He, M.; Cheng, F.; da Silva, S.R.; Tan, B.; Sorel, O.; Gruffaz, M.; Li, T.; Gao, S.J. Molecular Biology of KSHV in Relation to HIV/AIDS-Associated Oncogenesis. Cancer Treat. Res. 2019, 177, 23–62. [Google Scholar] [CrossRef]
- Sandfort, T.G.; Reddy, V. African same-sex sexualities and gender-diversity: An introduction. Cult. Health Sex. 2013, 15 (Suppl. S1), 1–6. [Google Scholar] [CrossRef]
- McLaren, P.J.; Porreca, I.; Iaconis, G.; Mok, H.P.; Mukhopadhyay, S.; Karakoc, E. Africa-specific human genetic variation near CHD1L associates with HIV-1 load. Nature 2023, 620, 1025–1030. [Google Scholar] [CrossRef]
- Shimelis, T.; Tebeje, M.; Tadesse, E.; Tegbaru, B.; Terefe, A. Sero-prevalence of latent Toxoplasma gondii infection among HIV-infected and HIV-uninfected people in Addis Ababa, Ethiopia: A comparative cross-sectional study. BMC Res. Notes 2009, 2, 213. [Google Scholar] [CrossRef]
- Broussard, G.; Damania, B. KSHV: Immune Modulation and Immunotherapy. Front. Immunol. 2020, 10, 3084. [Google Scholar] [CrossRef]
- Mayer, K.H.; Nelson, L.; Hightow-Weidman, L.; Mimiaga, M.J.; Mena, L.; Reisner, S.; Daskalakis, D.; Safren, S.A.; Beyrer, C.; Sullivan, P.S. The persistent and evolving HIV epidemic in American men who have sex with men. Lancet 2021, 397, 1116–1126. [Google Scholar] [CrossRef]
- Mamimandjiami, A.I.; Mouinga-Ondémé, A.; Ramassamy, J.L.; Djuicy, D.D.; Afonso, P.V.; Mahé, A.; Lekana-Douki, J.B. Epidemiology and Genetic Variability of HHV-8/KSHV among Rural Populations and Kaposi’s Sarcoma Patients in Gabon, Central Africa. Review of the Geographical Distribution of HHV-8 K1 Genotypes in Africa. Viruses 2021, 13, 175. [Google Scholar] [CrossRef]
- White, S.L.; Rawlinson, W.; Boan, P.; Sheppeard, V.; Wong, G.; Waller, K.; Opdam, H.; Kaldor, J.; Fink, M.; Verran, D.; et al. Infectious Disease Transmission in Solid Organ Transplantation: Donor Evaluation, Recipient Risk, and Outcomes of Transmission. Transplant. Direct 2018, 5, e416. [Google Scholar] [CrossRef]
- Diakite, M.; Shaw-Saliba, K.; Lau, C.Y. Malignancy and viral infections in Sub-Saharan Africa: A review. Front. Virol. 2023, 3, 1103737. [Google Scholar] [CrossRef]
- Motlhale, M.; Sitas, F.; Bradshaw, D.; Chen, W.C.; Singini, M.G.; de Villiers, C.B.; Lewis, C.M.; Muchengeti, M.; Waterboer, T.; Mathew, C.G.; et al. Epidemiology of Kaposi’s sarcoma in sub-Saharan Africa. Cancer Epidemiol. 2022, 78, 102167. [Google Scholar] [CrossRef]
- Butler, L.M.; Were, W.A.; Balinandi, S.; Downing, R.; Dollard, S.; Neilands, T.B.; Gupta, S.; Rutherford, G.W.; Mermin, J. Human herpesvirus 8 infection in children and adults in a population-based study in rural Uganda. J. Infect. Dis. 2011, 203, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Takaiwa, A.; Takeshima, S.N.; Okada, A.; Inoshima, Y. Genetic Variability of 3′-Proximal Region of Genomes of Orf Viruses Isolated From Sheep and Wild Japanese Serows (Capricornis crispus) in Japan. Front. Vet. Sci. 2020, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.C.; Ciufo, D.M.; Alcendor, D.J.; Wan, X.; Nicholas, J.; Browning, P.J.; Rady, P.L.; Tyring, S.K.; Orenstein, J.M.; Rabkin, C.S.; et al. High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi’s sarcoma-associated herpesvirus genome defines four major virus subtypes and multiple variants or clades in different human populations. J. Virol. 1999, 73, 4156–4170. [Google Scholar] [CrossRef]
- Zong, J.; Ciufo, D.M.; Viscidi, R.; Alagiozoglou, L.; Tyring, S.; Rady, P.; Orenstein, J.; Boto, W.; Kalumbuja, H.; Romano, N. Genotypic analysis at multiple loci across Kaposi’s sarcoma herpesvirus (KSHV) DNA molecules: Clustering patterns, novel variants and chimerism. J. Clin. Virol. 2002, 23, 119–148. [Google Scholar] [CrossRef]
- Hosseinipour, M.C.; Sweet, K.M.; Xiong, J.; Namarika, D.; Mwafongo, A.; Nyirenda, M.; Chiwoko, L.; Kamwendo, D.; Hoffman, I.; Lee, J.; et al. Viral profiling identifies multiple subtypes of Kaposi’s sarcoma. mBio 2014, 5, e01633-14. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Kanno, T.; Hasegawa, H.; Katano, H. Pathology of Kaposi’s Sarcoma-Associated Herpesvirus Infection. Front. Microbiol. 2011, 2, 175. [Google Scholar] [CrossRef]
- Casper, C.; Corey, L.; Cohen, J.I.; Damania, B.; Gershon, A.A.; Kaslow, D.C.; Krug, L.T.; Martin, J.; Mbulaiteye, S.M.; Mocarski, E.S.; et al. KSHV (HHV8) vaccine: Promises and potential pitfalls for a new anti-cancer vaccine. NPJ Vaccines 2022, 7, 108. [Google Scholar] [CrossRef]
- Ouyang, X.; Zeng, Y.; Fu, B.; Wang, X.; Chen, W.; Fang, Y.; Luo, M.; Wang, L. Genotypic analysis of Kaposi’s sarcoma-associated herpesvirus from patients with Kaposi’s sarcoma in Xinjiang, China. Viruses 2014, 6, 4800–4810. [Google Scholar] [CrossRef]
- Anders, P.M.; Zhang, Z.; Bhende, P.M.; Giffin, L.; Damania, B. The KSHV K1 Protein Modulates AMPK Function to Enhance Cell Survival. PLoS Pathog. 2016, 12, e1005985. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Damania, B. AKTivation of PI3K/AKT/mTOR signaling pathway by KSHV. Front. Immunol. 2013, 3, 401. [Google Scholar] [CrossRef]
- Broussard, G.; Damania, B. Regulation of KSHV Latency and Lytic Reactivation. Viruses 2020, 12, 1034. [Google Scholar] [CrossRef]
- Yan, L.; Majerciak, V.; Zheng, Z.M.; Lan, K. Towards Better Understanding of KSHV Life Cycle: From Transcription and Posttranscriptional Regulations to Pathogenesis. Virol. Sin. 2019, 34, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Cho, H.; Roh, S.W.; Kim, S.J.; Myoung, J. Cell Type-Specific Interferon-γ-mediated Antagonism of KSHV Lytic Replication. Sci. Rep. 2019, 9, 2372. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E.; Damania, B.; Krown, S.E.; Martin, J.; Bower, M.; Whitby, D. Kaposi sarcoma. Nat. Rev. Dis. Primers 2019, 5, 9. [Google Scholar] [CrossRef]
- Parker, E.; Judge, M.A.; Macete, E.; Nhampossa, T.; Dorward, J.; Langa, D.C.; Schacht, C. HIV infection in Eastern and Southern Africa: Highest burden, largest challenges, greatest potential. S. Afr. J. HIV Med. 2021, 22, 1237. [Google Scholar] [CrossRef] [PubMed]
- Nalwoga, A.; Webb, E.L.; Muserere, C.; Chihota, B.; Miley, W.; Labo, N.; Elliott, A.; Cose, S.; Whitby, D.; Newton, R. Variation in KSHV prevalence between geographically proximate locations in Uganda. Infect. Agent. Cancer 2020, 15, 49. [Google Scholar] [CrossRef]
- Whitby, D.; Marshall, V.A.; Bagni, R.K.; Miley, W.J.; McCloud, T.G.; Hines-Boykin, R.; Goedert, J.J.; Conde, B.A.; Nagashima, K.; Mikovits, J. Reactivation of Kaposi’s sarcoma-associated herpesvirus by natural products from Kaposi’s sarcoma endemic regions. Int. J. Cancer 2007, 120, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.; Polizzotto, M.N.; Uldrick, T.S.; Yarchoan, R. Kaposi sarcoma-associated herpesvirus-associated malignancies: Epidemiology, pathogenesis, and advances in treatment. Semin. Oncol. 2015, 42, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, Y. Identification of the Kaposi’s Sarcoma-associated Herpesvirus (KSHV) Surface Glycoprotein Targets of Human KSHV-Specific Neutralizing Antibody Responses. Master’s Thesis, University of Nebraska-Lincoln, Lincoln, NE, USA, 2019. [Google Scholar]
- Gaye-Diallo, A.; Touré, A.T.; Gessain, A.; Guèye-Ndiaye, A.; Ndour, A.N.; Touré-Kane, N.C.; Dia, M.C. Preliminary study of human Herpesvirus type 8 infection in pregnant women in Dakar (Senegal). Bull. Soc. Pathol. Exot. 2001, 94, 231–234. (In French) [Google Scholar] [PubMed]
- Collenberg, E.; Ouedraogo, T.; Ganamé, J.; Fickenscher, H.; Kynast-Wolf, G.; Becher, H.; Kouyaté, B.; Kräusslich, H.G.; Sangaré, L.; Tebit, D.M. Seroprevalence of six different viruses among pregnant women and blood donors in rural and urban Burkina Faso: A comparative analysis. J. Med. Virol. 2006, 78, 683–692. [Google Scholar] [CrossRef]
- Ogoina, D.; Onyemelukwe, G.; Musa, B.O.; Babadoko, A. Seroprevalence and determinants of human herpes virus 8 infection in adult Nigerians with and without HIV-1 infection. Afr. Health Sci. 2011, 11, 158–162. [Google Scholar] [PubMed]
- Ablashi, D.; Chatlynne, L.; Cooper, H.; Thomas, D.; Yadav, M.; Norhanom, A.W.; Chandana, A.K.; Churdboonchart, V.; Kulpradist, S.A.; Patnaik, M. Seroprevalence of human herpesvirus-8 (HHV-8) in countries of Southeast Asia compared to the USA, the Caribbean and Africa. Br. J. Cancer 1999, 81, 893–897. [Google Scholar] [CrossRef]
- Dedicoat, M.; Newton, R. Review of the distribution of Kaposi’s sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi’s sarcoma. Br. J. Cancer. 2003, 88, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Biryahwaho, B.; Dollard, S.C.; Pfeiffer, R.M.; Shebl, F.M.; Munuo, S.; Amin, M.M.; Hladik, W.; Parsons, R.; Mbulaiteye, S.M. Sex and geographic patterns of human herpesvirus 8 infection in a nationally representative population-based sample in Uganda. J. Infect. Dis. 2010, 2, 1347–1353. [Google Scholar] [CrossRef]
- Engels, E.A.; Sinclair, M.D.; Biggar, R.J.; Whitby, D.; Ebbesen, P.; Goedert, J.J.; Gastwirth, J.L. Latent class analysis of human herpesvirus 8 assay performance and infection prevalence in sub-saharan Africa and Malta. Int. J. Cancer 2000, 88, 1003–1008. [Google Scholar] [CrossRef]
- Newton, R.; Ziegler, J.; Bourboulia, D.; Casabonne, D.; Beral, V.; Mbidde, E.; Carpenter, L.; Reeves, G.; Parkin, D.M.; Wabinga, H.; et al. The sero-epidemiology of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in adults with cancer in Uganda. Int. J. Cancer 2003, 103, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.; Labo, N.; Wakeham, K.; Miley, W.; Asiki, G.; Johnston, W.T.; Whitby, D. Kaposi Sarcoma-Associated Herpesvirus in a Rural Ugandan Cohort, 1992–2008. J. Infect. Dis. 2018, 217, 263–269. [Google Scholar] [CrossRef] [PubMed]
- de Sanjose, S.; Mbisa, G.; Perez-Alvarez, S.; Benavente, Y.; Sukvirach, S.; Hieu, N.T.; Shin, H.R.; Anh, P.T.; Thomas, J.; Lazcano, E.; et al. Geographic variation in the prevalence of Kaposi sarcoma-associated herpesvirus and risk factors for transmission. J. Infect. Dis. 2009, 199, 1449–1456. [Google Scholar] [CrossRef]
- Meschi, S.; Schepisi, M.S.; Nicastri, E.; Bevilacqua, N.; Castilletti, C.; Sciarrone, M.R.; Paglia, M.G.; Fumakule, R.; Mohamed, J.; Kitwa, A. The prevalence of antibodies to human herpesvirus 8 and hepatitis B virus in patients in two hospitals in Tanzania. J. Med. Virol. 2010, 82, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Lemma, E.; Constantine, N.T.; Kassa, D.; Messele, T.; Mindaye, T.; Taye, G.; Abebe, A.; Tamene, W.; Tebje, M.; Gebremeskel, W.; et al. Human herpesvirus 8 infection in HIV-1-infected and uninfected pregnant women in Ethiopia. Ethiop. Med. J. 2009, 47, 205–211. [Google Scholar]
- Mbulaiteye, S.M.; Pfeiffer, R.M.; Whitby, D.; Brubaker, G.R.; Shao, J.; Biggar, R.J. Human herpesvirus 8 infection within families in rural Tanzania. J. Infect. Dis. 2003, 187, 1780–1785. [Google Scholar] [CrossRef]
- Phipps, W.; Saracino, M.; Selke, S.; Huang, M.L.; Jaoko, W.; Mandaliya, K.; Wald, A.; Casper, C.; McClelland, R.S. Oral HHV-8 replication among women in Mombasa, Kenya. J. Med. Virol. 2014, 86, 1759–1765. [Google Scholar] [CrossRef]
- Lavreys, L.; Chohan, B.; Ashley, R.; Richardson, B.A.; Corey, L.; Mandaliya, K.; Ndinya-Achola, J.O.; Kreiss, J.K. Human herpesvirus 8: Seroprevalence and correlates in prostitutes in Mombasa, Kenya. J. Infect. Dis. 2003, 187, 359–363. [Google Scholar] [CrossRef]
- Whitby, D.; Marshall, V.A.; Bagni, R.K.; Wang, C.D.; Gamache, C.J.; Guzman, J.R.; Kron, M.; Ebbesen, P.; Biggar, R.J. Genotypic characterization of Kaposi’s sarcoma-associated herpesvirus in asymptomatic infected subjects from isolated populations. J. Gen. Virol. 2004, 85 Pt 1, 155–163. [Google Scholar] [CrossRef]
- Etta, E.M.; Alayande, D.P.; Mavhandu-Ramarumo, L.G.; Gachara, G.; Bessong, P.O. HHV-8 Seroprevalence and Genotype Distribution in Africa, 1998–2017: A Systematic Review. Viruses 2018, 10, 458. [Google Scholar] [CrossRef]
- Maskew, M.; Macphail, A.P.; Whitby, D.; Egger, M.; Wallis, C.L.; Fox, M.P. Prevalence and predictors of kaposi sarcoma herpes virus seropositivity: A cross-sectional analysis of HIV-infected adults initiating ART in Johannesburg, South Africa. Infect. Agent. Cancer 2011, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.M.; Wheeler, W.A.; Mbisa, G.; Whitby, D.; Goedert, J.J.; de Thé, G.; Mbulaiteye, S.M. Geographic heterogeneity of prevalence of the human herpesvirus 8 in sub-Saharan Africa: Clues about etiology. Ann. Epidemiol. 2010, 20, 958–963. [Google Scholar] [CrossRef]
- Olsen, S.J.; Chang, Y.; Moore, P.S.; Biggar, R.J.; Melbye, M. Increasing Kaposi’s sarcoma-associated herpesvirus seroprevalence with age in a highly Kaposi’s sarcoma endemic region, Zambia in 1985. AIDS 1998, 12, 1921–1925. [Google Scholar] [CrossRef]
- Plancoulaine, S.; Gessain, A. Epidemiological aspects of human herpesvirus 8 infection and of Kaposi’s sarcoma. Med. Mal. Infect. 2005, 35, 314–321. [Google Scholar] [CrossRef]
- Serraino, D.; Toma, L.; Andreoni, M.; Buttò, S.; Tchangmena, O.; Sarmati, L.; Monini, P.; Franceschi, S.; Ensoli, B.; Rezza, G. A seroprevalence study of human herpesvirus type 8 (HHV8) in eastern and Central Africa and in the Mediterranean area. Eur. J. Epidemiol. 2001, 17, 871–876. [Google Scholar] [CrossRef]
- Betsem, E.; Cassar, O.; Afonso, P.V.; Fontanet, A.; Froment, A.; Gessain, A. Epidemiology and genetic variability of HHV-8/KSHV in Pygmy and Bantu populations in Cameroon. PLoS Negl. Trop. Dis. 2014, 8, e2851. [Google Scholar] [CrossRef] [PubMed]
- Kalengayi, M.M.; Kashala, L. Clinicopathological features of Kaposi’s sarcoma in Zaire. IARC Sci. Publ. 1984, 63, 559–582. [Google Scholar]
- Capan-Melser, M.; Mombo-Ngoma, G.; Akerey-Diop, D.; Basra, A.; Manego-Zoleko, R.; Würbel, H.; Lötsch, F.; Groger, M.; Skoll, M.; Schwing, J.; et al. Epidemiology of Human Herpes Virus 8 in Pregnant Women and their Newborns—A cross-sectional delivery survey in Central Gabon. Int. J. Infect. Dis. 2015, 39, 16–19. [Google Scholar] [CrossRef]
- Bélec, L.; Cancré, N.; Hallouin, M.C.; Morvan, J.; Si Mohamed, A.; Grésenguet, G. High prevalence in Central Africa of blood donors who are potentially infectious for human herpesvirus 8. Transfusion 1998, 38, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, V.; Judde, J.G.; Brière, J.; Tulliez, M.; Garin, B.; Kassa-Kelembho, E.; Morvan, J.; Couppié, P.; Clyti, E.; Forteza-Vila, J.; et al. Molecular epidemiology of human herpesvirus 8 in Africa: Both B and A5 K1 genotypes, as well as the M and P genotypes of K14.1/K15 loci, are frequent and widespread. Virology 2000, 278, 60–74. [Google Scholar] [CrossRef]
- Mbondji-Wonje, C.; Ragupathy, V.; Lee, S.; Wood, O.; Awazi, B.; Hewlett, I.K. Seroprevalence of human herpesvirus-8 in HIV-1 infected and uninfected individuals in Cameroon. Viruses 2013, 5, 2253–2259. [Google Scholar] [CrossRef]
- Voufo, R.A.; Kouotou, A.E.; Tatah, N.J.; TeTo, G.; Gueguim, C.; Ngondé, C.M.E.; Njiguet Tepa, A.G.; Gabin, A.; Amazia, F.; Yembeau, N.L.; et al. Relation between interleukin-6 concentrations and oxidative status of HIV infected patients with/or at risk of Kaposi disease in Yaounde. Virol. J. 2023, 20, 165. [Google Scholar] [CrossRef]
- Malonga, G.A.; Jary, A.; Leducq, V.; Moudiongui Mboungou Malanda, D.; Boumba, A.L.M.; Chicaud, E.; Malet, I.; Calvez, V.; Peko, J.F.; Marcelin, A.G. Seroprevalence and molecular diversity of Human Herpesvirus 8 among people living with HIV in Brazzaville, Congo. Sci. Rep. 2021, 11, 17442. [Google Scholar] [CrossRef]
- Iloukou, P.J.; Boumba, A.L.; Ngombe, D.F.; Massengo, N.R.; Malonga, G.A.; Moukassa, D.; Ennaji, M.M. Molecular detection and genotyping of human herpes virus 8 in blood donors in Congo. Vopr. Virusol. 2024, 69, 277–284. [Google Scholar] [CrossRef]
- Dedicoat, M.; Newton, R.; Alkharsah, K.R.; Sheldon, J.; Szabados, I.; Ndlovu, B.; Page, T.; Casabonne, D.; Gilks, C.F.; Cassol, S.A.; et al. Mother-to-child transmission of human herpesvirus-8 in South Africa. J. Infect. Dis. 2004, 190, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Minhas, V.; Crabtree, K.L.; Chao, A.; M’soka, T.J.; Kankasa, C.; Bulterys, M.; Mitchell, C.D.; Wood, C. Early childhood infection by human herpesvirus 8 in Zambia and the role of human immunodeficiency virus type 1 coinfection in a highly endemic area. Am. J. Epidemiol. 2008, 168, 311–320. [Google Scholar] [CrossRef]
- Lidenge, S.J.; Tran, T.; Tso, F.Y.; Ngowi, J.R.; Shea, D.M.; Mwaiselage, J.; Wood, C.; West, J.T. Prevalence of Kaposi’s sarcoma-associated herpesvirus and transfusion-transmissible infections in Tanzanian blood donors. Int. J. Infect. Dis. 2020, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Nzivo, M.M.; Lwembe, R.M.; Odari, E.O.; Kang’ethe, J.M.; Budambula, N.L.M. Prevalence and Risk Factors of Human Herpes Virus Type 8 (HHV-8), Human Immunodeficiency Virus-1 (HIV-1), and Syphilis among Female Sex Workers in Malindi, Kenya. Interdiscip. Perspect. Infect. Dis. 2019, 2019, 5345161. [Google Scholar] [CrossRef] [PubMed]
- Adjei, A.A.; Armah, H.B.; Gbagbo, F.; Boamah, I.; Adu-Gyamfi, C.; Asare, I. Seroprevalence of HHV-8, CMV, and EBV among the general population in Ghana, West Africa. BMC Infect. Dis. 2008, 8, 111. [Google Scholar] [CrossRef]
- Gobbini, F.; Owusu-Ofori, S.; Marcelin, A.G.; Candotti, D.; Allain, J.P. Human herpesvirus 8 transfusion transmission in Ghana, an endemic region of West Africa. Transfusion 2012, 52, 2294–2299. [Google Scholar] [CrossRef]
- Biatougou, N.M.; Ouedraogo, M.S.; Soubeiga, S.T.; Zohoncon, T.M.; Ouedraogo, P.; Obiri-Yeboah, D.; Tapsoba, A.S.A.; Kiendrebeogo, T.I.; Sagna, T.; Niamba, P. Molecular Epidemiology of Human Herpes Virus Type 8 Among Patients with Compromised Immune System in Ouagadougou, Burkina Faso. HIV AIDS 2022, 14, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Martró, E.; Esteve, A.; Schulz, T.F.; Sheldon, J.; Gambús, G.; Muñoz, R.; Whitby, D.; Casabona, J.; Euro-Shaks Study Group. Risk factors for human Herpesvirus 8 infection and AIDS-associated Kaposi’s sarcoma among men who have sex with men in a European multicentre study. Int. J. Cancer 2007, 120, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Simpore, J.; Granato, M.; Santarelli, R.; Nsme, R.A.; Coluzzi, M.; Pietra, V.; Pignatelli, S.; Bere, A.; Faggioni, A.; Angeloni, A. Prevalence of infection by HHV-8, HIV, HCV and HBV among pregnant women in Burkina Faso. J. Clin. Virol. 2004, 31, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Gras, J.; Helary, M.; Carette, D.; Minier, M.; Salmona, M.; Gabassi, A.; Saouzanet, M.; Charreau, I.; Meyer, L.; Molina, J.M.; et al. Prevalence, Risk factors, and Shedding of Human Herpes Virus-8 among Men Having Sex with Men Enrolled in a Pre-exposure Prophylaxis Study. Clin. Infect. Dis. 2023, ciad502. [Google Scholar] [CrossRef] [PubMed]
- Rohner, E.; Wyss, N.; Trelle, S.; Mbulaiteye, S.M.; Egger, M.; Novak, U.; Zwahlen, M.; Bohlius, J. HHV-8 seroprevalence: A global view. Syst. Rev. 2014, 3, 11. [Google Scholar] [CrossRef]
- Rohner, E.; Wyss, N.; Heg, Z.; Faralli, Z.; Mbulaiteye, S.M.; Novak, U.; Zwahlen, M.; Egger, M.; Bohlius, J. HIV and human herpesvirus 8 co-infection across the globe: Systematic review and meta-analysis. Int. J. Cancer 2016, 138, 45–54. [Google Scholar] [CrossRef]
- Plancoulaine, S.; Abel, L.; van Beveren, M.; Trégouët, D.A.; Joubert, M.; Tortevoye, P.; de Thé, G.; Gessain, A. Human herpesvirus 8 transmission from mother to child and between siblings in an endemic population. Lancet 2000, 356, 1062–1065. [Google Scholar] [CrossRef]
- Cattani, P.; Capuano, M.; Cerimele, F.; La Parola, I.L.; Santangelo, R.; Masini, C.; Cerimele, D.; Fadda, G. Human herpesvirus 8 seroprevalence and evaluation of nonsexual transmission routes by detection of DNA in clinical specimens from human immunodeficiency virus-seronegative patients from central and southern Italy, with and without Kaposi’s sarcoma. J. Clin. Microbiol. 1999, 37, 1150–1153. [Google Scholar] [CrossRef]
- Rezza, G.; Lennette, E.T.; Giuliani, M.; Pezzotti, P.; Caprilli, F.; Monini, P.; Buttò, S.; Lodi, G.; Di Carlo, A.; Levy, J.A.; et al. Prevalence and determinants of anti-lytic and anti-latent antibodies to human herpesvirus-8 among Italian individuals at risk of sexually and parenterally transmitted infections. Int. J. Cancer 1998, 77, 361–365. [Google Scholar] [CrossRef]
- Vangipuram, R.; Tyring, S.K. Epidemiology of Kaposi sarcoma: Review and description of the nonepidemic variant. Int. J. Dermatol. 2019, 58, 538–542. [Google Scholar] [CrossRef]
- Vamvakas, E.C. Is human herpesvirus-8 transmitted by transfusion? Transfus. Med. Rev. 2010, 24, 1–14. [Google Scholar] [CrossRef]
- Pearce, M.; Matsumura, S.; Wilson, A.C. Transcripts encoding K12, v-FLIP, v-cyclin, and the microRNA cluster of Kaposi’s sarcoma-associated herpesvirus originate from a common promoter. J. Virol. 2005, 79, 14457–14464. [Google Scholar] [CrossRef]
- Schulz, T.F.; Cesarman, E. Kaposi sarcoma-associated herpesvirus: Mechanisms of oncogenesis. Curr. Opin. Virol. 2015, 14, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, D.P.; Damania, B. Kaposi sarcoma-associated herpesvirus: Immunobiology, oncogenesis, and therapy. J. Clin. Investig. 2016, 126, 3165–3175. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liang, D.; Gao, Y.; Lan, K. Kaposi’s sarcoma-associated herpesvirus-encoded LANA interacts with host KAP1 to facilitate establishment of viral latency. J. Virol. 2014, 88, 7331–7344. [Google Scholar] [CrossRef]
- Aneja, K.K.; Yuan, Y. Reactivation and Lytic Replication of Kaposi’s Sarcoma-Associated Herpesvirus: An Update. Front. Microbiol. 2017, 8, 613. [Google Scholar] [CrossRef]
- Moore, P.S.; Kingsley, L.A.; Holmberg, S.D.; Spira, T.; Gupta, P.; Hoover, D.R. Kaposi’s sarcoma-associated herpesvirus infection prior to onset of Kaposi’s sarcoma. AIDS 1996, 10, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Wies, E.; Neipel, F. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor 3 inhibits gamma interferon and major histocompatibility complex class II expression. J. Virol. 2011, 85, 4530–4537. [Google Scholar] [CrossRef]
- Ishido, S.; Wang, C.; Lee, B.S.; Cohen, G.B.; Jung, J.U. Down regulation of major histocompatibility complex class I molecules by Kaposi’s sarcoma associated herpes virus K3 and K5 proteins. J. Virol. 2000, 74, 5300–5309. [Google Scholar] [CrossRef]
- Hunte, R.; Alonso, P.; Thomas, R.; Bazile, C.A.; Ramos, J.C.; van der Weyden, L.; Dominguez-Bendala, J.; Khan, W.N.; Shembade, N. CADM1 is essential for KSHV-encoded vGPCR-and vFLIP-mediated chronic NF-κB activation. PLoS Pathog. 2018, 14, e1006968. [Google Scholar] [CrossRef]
- Sakakibara, S.; Espigol-Frigole, G.; Gasperini, P.; Uldrick, T.S.; Yarchoan, R.; Tosato, G. A20/TNFAIP3 inhibits NF-κB activation induced by the Kaposi’s sarcoma-associated herpesvirus vFLIP oncoprotein. Oncogene 2013, 32, 1223–1232. [Google Scholar] [CrossRef]
- Aoki, Y.; Yarchoan, R.; Wyvill, K.; Okamoto, S.; Little, R.F.; Tosato, G. Detection of viral interleukin-6 in Kaposi sarcoma-associated herpesvirus-linked disorders. Blood 2001, 97, 2173–2176. [Google Scholar] [CrossRef]
- Cai, Q.; Verma, S.C.; Choi, J.Y.; Ma, M.; Robertson, E.S. Kaposi’s sarcoma-associated herpesvirus inhibits interleukin-4-mediated STAT6 phosphorylation to regulate apoptosis and maintain latency. J. Virol. 2010, 84, 11134–11144. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, S.; Tosato, G. Viral interleukin-6: Role in Kaposi’s sarcoma-associated herpesvirus: Associated malignancies. J. Interferon Cytokine Res. 2011, 31, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Guerin, L.N.; Chen, Z.; Rajendren, S.; Dunker, W.; Zhao, Y.; Zhang, R.; Hodges, E.; Karijolich, J. Enhancer-promoter activation by the Kaposi sarcoma-associated herpesvirus episome maintenance protein LANA. Cell Rep. 2024, 43, 113888. [Google Scholar] [CrossRef] [PubMed]
- Chinna, P.; Bratl, K.; Lambarey, H.; Blumenthal, M.J.; Schäfer, G. The Impact of Co-Infections for Human Gammaherpesvirus Infection and Associated Pathologies. Int. J. Mol. Sci. 2023, 24, 13066. [Google Scholar] [CrossRef]
- Gonçalves, P.H.; Uldrick, T.S.; Yarchoan, R. HIV-associated Kaposi sarcoma and related diseases. AIDS 2017, 31, 1903–1916. [Google Scholar] [CrossRef]
- Sirera, G.; Videla, S.; Saludes, V.; Castellà, E.; Sanz, C.; Ariza, A.; Clotet, B.; Martró, E. Prevalence of HPV-DNA and E6 mRNA in lung cancer of HIV-infected patients. Sci. Rep. 2022, 12, 13196. [Google Scholar] [CrossRef]
- Galati, D.; Zanotta, S. The Role of Cancer Biomarkers in HIV Infected Hosts. Curr. Med. Chem. 2016, 23, 2333–2349. [Google Scholar] [CrossRef]
- Grabar, S.; Costagliola, D. Epidemiology of Kaposi’s sarcoma. Cancers 2021, 13, 5692. [Google Scholar] [CrossRef]
- Moyo, E.; Moyo, P.; Murewanhema, G.; Mhango, M.; Chitungo, I.; Dzinamarira, T. Key populations and Sub-Saharan Africa’s HIV response. Front. Public Health 2023, 11, 1079990. [Google Scholar] [CrossRef]
- Thakker, S.; Verma, S.C. Co-infections and Pathogenesis of KSHV-Associated Malignancies. Front. Microbiol. 2016, 7, 151. [Google Scholar] [CrossRef]
- Grulich, A.E.; Vajdic, C.M. The epidemiology of cancers in human immunodeficiency virus infection and after organ transplantation. Semin. Oncol. 2015, 42, 247–257. [Google Scholar] [CrossRef]
- Kumar, A.; Abbas, W.; Herbein, G. HIV-1 latency in monocytes/macrophages. Viruses 2014, 6, 1837–1860. [Google Scholar] [CrossRef]
- Labo, N.; Miley, W.; Benson, C.A.; Campbell, T.B.; Whitby, D. Epidemiology of Kaposi’s sarcoma-associated herpesvirus in HIV-1-infected US persons in the era of combination antiretroviral therapy. AIDS 2015, 29, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, R.; Gonnella, R.; Di Giovenale, G.; Cuomo, L.; Capobianchi, A.; Granato, M.; Gentile, G.; Faggioni, A.; Cirone, M. STAT3 activation by KSHV correlates with IL-10, IL-6 and IL-23 release and an autophagic block in dendritic cells. Sci. Rep. 2014, 4, 4241. [Google Scholar] [CrossRef]
- Ensoli, B.; Moretti, S.; Borsetti, A.; Maggiorella, M.T.; Buttò, S.; Picconi, O.; Tripiciano, A.; Sgadari, C.; Monini, P.; Cafaro, A. New insights into pathogenesis point to HIV-1 Tat as a key vaccine target. Arch. Virol. 2021, 166, 2955–2974. [Google Scholar] [CrossRef] [PubMed]
- Ajasin, D.; Eugenin, E.A. HIV-1 Tat: Role in Bystander Toxicity. Front. Cell Infect. Microbiol. 2020, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, A.; Schietroma, I.; Sernicola, L.; Belli, R.; Campagna, M.; Mancini, F.; Farcomeni, S.; Pavone-Cossut, M.R.; Borsetti, A.; Monini, P.; et al. Role of HIV-1 Tat Protein Interactions with Host Receptors in HIV Infection and Pathogenesis. Int. J. Mol. Sci. 2024, 25, 1704. [Google Scholar] [CrossRef]
- Stănescu, L.; Foarfă, C.; Georgescu, A.C.; Georgescu, I. Kaposi’s sarcoma associated with AIDS. Rom. J. Morphol. Embryol. 2007, 48, 181–187. [Google Scholar]
- Iscovich, J.; Boffetta, P.; Franceschi, S.; Azizi, E.; Sarid, R. Classic kaposi sarcoma: Epidemiology and risk factors. Cancer 2000, 88, 500–517. [Google Scholar] [CrossRef]
- Fatahzadeh, M. Kaposi sarcoma: Review and medical management update. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 2–16, Erratum in Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 708. [Google Scholar] [CrossRef]
- Tounouga, D.N.; Kouotou, E.A.; Nansseu, J.R.; Zoung-Kanyi Bissek, A.C. Epidemiological and Clinical Patterns of Kaposi Sarcoma: A 16-Year Retrospective Cross-Sectional Study from Yaoundé, Cameroon. Dermatology 2018, 234, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, A.; Brockmeyer, N.; Stücker, M.; Wieland, U.; Kreuter, A.; Competence Network HIV AIDS. Kaposi sarcoma in a HIV uninfected man who has sex with men. Eur. J. Med. Res. 2010, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.C.; Paya, C.V. Kaposi’s sarcoma and Transplantation. Herpes 2000, 7, 18–23. [Google Scholar]
- Yarchoan, R.; Uldrick, T.S. HIV-Associated Cancers and Related Diseases. N. Engl. J. Med. 2018, 378, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Temelkova, I.; Tronnier, M.; Terziev, I.; Wollina, U.; Lozev, I.; Goldust, M.; Tchernev, G. A Series of Patients with Kaposi Sarcoma (Mediterranean/Classical Type): Case Presentations and Short Update on Pathogenesis and Treatment. Open Access Maced. J. Med. Sci. 2018, 6, 1688–1693. [Google Scholar] [CrossRef]
- Gbabe, O.F.; Okwundu, C.I.; Dedicoat, M.; Freeman, E.E. Treatment of severe or progressive Kaposi’s sarcoma in HIV-infected adults. Cochrane Database Syst. Rev. 2014, 9, CD003256. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, E.; Ruocco, V.; Tornesello, M.L.; Gambardella, A.; Wolf, R.; Buonaguro, F.M. Kaposi’s sarcoma: Etiology and pathogenesis, inducing factors, causal associations, and treatments: Facts and controversies. Clin. Dermatol. 2013, 31, 413–422. [Google Scholar] [CrossRef]
- Nakamura, H.; Li, M.; Zarycki, J.; Jung, J.U. Inhibition of p53 tumor suppressor by viral interferon regulatory factor. J. Virol. 2001, 75, 7572–7582. [Google Scholar] [CrossRef]
- Lee, H.R.; Toth, Z.; Shin, Y.C.; Lee, J.S.; Chang, H.; Gu, W.; Oh, T.K.; Kim, M.H.; Jung, J.U. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor 4 targets MDM2 to deregulate the p53 tumor suppressor pathway. J. Virol. 2009, 83, 6739–6747. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N. Papillomaviruses and cancer: Commonalities and differences in HPV carcinogenesis at different sites of the body. Int. J. Clin. Oncol. 2023, 28, 956–964. [Google Scholar] [CrossRef]
- Phillips, M.; Burrows, J.; Manyando, C.; van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria. Nat. Rev. Dis. Primers 2017, 3, 17050. [Google Scholar] [CrossRef] [PubMed]
- CDC—Malaria—About Malaria—Disease. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/malaria/about/index.html (accessed on 17 October 2025).
- Malaria. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/malaria/hcp/drug-malaria/index.html (accessed on 17 October 2025).
- McDonnell, M.R. World Malaria 2023: What You Need to Know. Available online: https://beatmalaria.org/blog/world-malaria-report-2023-what-you-need-to-know/ (accessed on 12 April 2024).
- Robbiani, D.F.; Deroubaix, S.; Feldhahn, N.; Oliveira, T.Y.; Callen, E.; Wang, Q.; Jankovic, M.; Silva, I.T.; Rommel, P.C.; Bosque, D.; et al. Plasmodium Infection Promotes Genomic Instability and AID-Dependent B Cell Lymphoma. Cell 2015, 62, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, K.U.; Kotepui, M. Malaria Infection and Risk for Endemic Burkitt Lymphoma: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 5886. [Google Scholar] [CrossRef]
- Barnes, K.I.; Mwenechanya, J.; Tembo, M.; McIlleron, H.; Folb, P.I.; Ribeiro, I.; Little, F.; Gomes, M.; Molyneux, M.E. Efficacy of rectal artesunate compared with parenteral quinine in initial treatment of moderately severe malaria in African children and adults: A randomised study. Lancet 2004, 363, 1598–1605. [Google Scholar] [CrossRef]
- Abdin, M.Z.; Israr, M.; Rehman, R.U.; Jain, S.K. Artemisinin, a novel antimalarial drug: Biochemical and molecular approaches for enhanced production. Planta Med. 2003, 69, 289–299. [Google Scholar] [CrossRef]
- Nevin, R.L.; Croft, A.M. Psychiatric effects of malaria and anti-malarial drugs: Historical and modern perspectives. Malar. J. 2016, 15, 332. [Google Scholar] [CrossRef]
- Franceschi, S.; Geddes, M. Epidemiology of classic Kaposi’s sarcoma, with special reference to mediterranean population. Tumori J. 1995, 81, 308–314. [Google Scholar] [CrossRef]
- Mertelsmann, A.M.; Mukerebe, C.; Miyaye, D.; Shigella, P.; Mhango, L.; Lutonja, P.; Corstjens, P.L.A.M.; de Dood, C.; van Dam, G.J.; Colombe, S.; et al. Clinical and Demographic Factors Associated With Kaposi Sarcoma–Associated Herpesvirus Shedding in Saliva or Cervical Secretions in a Cohort of Tanzanian Women. Open Forum Infect. Dis. 2024, 11, ofae161. [Google Scholar] [CrossRef]
- Crabtree, K.L.; Wojcicki, J.M.; Minhas, V.; Kankasa, C.; Mitchell, C.; Wood, C. Association of Household Food- and Drink-Sharing Practices with Human Herpesvirus 8 Seroconversion in a Cohort of Zambian Children. J. Infect. Dis. 2017, 216, 842–849. [Google Scholar] [CrossRef]
- Shin, J.E.; Han, K.; An, H.J.; Park, H.S.; Shim, B.Y.; Kim, H. Common Cancer-Related Factors and the Risk of Developing Kaposi Sarcoma in Individuals without AIDS: Korea National Health Insurance Services Claims Database. J. Clin. Med. 2024, 13, 5634. [Google Scholar] [CrossRef]
- Mbulaiteye, S.M.; Atkinson, J.O.; Whitby, D.; Wohl, D.A.; Gallant, J.E.; Royal, S.; Goedert, J.J.; Rabkin, C.S. Risk factors for human herpesvirus 8 seropositivity in the AIDS Cancer Cohort Study. J. Clin. Virol. 2006, 35, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Abigun, A. Top 10 Countries with Highest Alcohol Consumption in Africa. 2024. Available online: https://tribuneonlineng.com/top-10-countries-with-highest-alcohol-consumption-in-africa/ (accessed on 17 September 2024).
- Motlhale, M.; Sitas, F.; Bradshaw, D.; Chen, W.C.; Singini, M.G.; de Villiers, C.B. Lifestyle factors associated with sex differences in Kaposi sarcoma incidence among adult black South Africans: A case-control study. Cancer Epidemiol. 2022, 78, 102158. [Google Scholar] [CrossRef]
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef] [PubMed]
- Fares, A. Factors influencing the seasonal patterns of infectious diseases. Int. J. Prev. Med. 2013, 4, 128–132. [Google Scholar] [PubMed]
- Fu, L.; Tian, T.; Wang, B.; Lu, Z.; Gao, Y.; Sun, Y.; Lin, Y.F.; Zhang, W.; Li, Y.; Zou, H. Global patterns and trends in Kaposi sarcoma incidence: A population-based study. Lancet Glob. Health 2023, 11, e1566–e1575. [Google Scholar] [CrossRef]
- Shetty, K. Management of oral Kaposi’s sarcoma lesions on HIV-positive patient using highly active antiretroviral therapy: Case report and a review of the literature. Oral Oncol. Extra 2005, 4, 226–229. [Google Scholar]
- Sigala, P.A.; Crowley, J.R.; Henderson, J.P.; Goldberg, D.E. Deconvoluting heme biosynthesis to target blood-stage malaria parasites. eLife 2015, 4, e09143. [Google Scholar] [CrossRef]
- Graves, M.S.; Lloyd, A.A.; Ross, E.V. Defining the Absorption Spectrum of the Skin After Application of a Popular Sunless Tanner, Dihydroxyacetone, Using Re ectance Photospectrometry. J. Drugs Dermatol. 2016, 15, 1459–1460. [Google Scholar] [PubMed]
- Peter, S.; Jama, S.; Alven, S.; Aderibigbe, B.A. Artemisinin and Derivatives-Based Hybrid Compounds: Promising Therapeutics for the Treatment of Cancer and Malaria. Molecules 2021, 26, 7521. [Google Scholar] [CrossRef]
- Zeng, Z.W.; Chen, D.; Chen, L.; He, B.; Li, Y. A comprehensive overview of Artemisinin and its derivatives as anticancer agents. Eur. J. Med. Chem. 2023, 247, 115000. [Google Scholar] [CrossRef]
- Efferth, T. Cancer combination therapies with artemisinin-type drugs. Biochem. Pharmacol. 2017, 139, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.; Sevilla, A. Current Advances in Photodynamic Therapy (PDT) and the Future Potential of PDT-Combinatorial Cancer Therapies. Int. J. Mol. Sci. 2024, 25, 1023. [Google Scholar] [CrossRef]
- Aebisher, D.; Serafin, I.; Batóg-Szczęch, K.; Dynarowicz, K.; Chodurek, E.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Photodynamic Therapy in the Treatment of Cancer—The Selection of Synthetic Photosensitizers. Pharmaceuticals 2024, 17, 932. [Google Scholar] [CrossRef]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT) for the treatment of malaria, leishmaniasis and trypanosomiasis. Braz. J. Med. Biol. Res. 2011, 44, 1–10. [Google Scholar] [CrossRef]
- Jurak, I.; Cokarić Brdovčak, M.; Djaković, L.; Bertović, I.; Knežević, K.; Lončarić, M.; Jurak-Begonja, A.; Malatesti, N. Photodynamic Inhibition of Herpes Simplex Virus 1 Infection by Tricationic Amphiphilic Porphyrin with a Long Alkyl Chain. Pharmaceutics 2023, 15, 956. [Google Scholar] [CrossRef] [PubMed]
- Kubizna, M.; Dawiec, G.; Wiench, R. Efficacy of Curcumin-Mediated Antimicrobial Photodynamic Therapy on Candida spp.—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 8136. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Ramalho, K.M.; Cunha, S.R.; Gonçalves, F.; Escudeiro, G.S.; Steiner-Oliveira, C.; Horliana, A.C.R.T.; Eduardo, C.P. Photodynamic therapy and Acyclovir in the treatment of recurrent herpes labialis: A controlled randomized clinical trial. Photodiagnosis Photodyn. Ther. 2021, 33, 102093. [Google Scholar] [CrossRef]
- Suresh, N.; Joseph, B.; Sathyan, P.; Sweety, V.K.; Waltimo, T.; Anil, S. Photodynamic therapy: An emerging therapeutic modality in dentistry. Bioorg Med. Chem. 2024, 114, 117962. [Google Scholar] [CrossRef]
- Gholami, L.; Shahabi, S.; Jazaeri, M.; Hadilou, M.; Fekrazad, R. Clinical applications of antimicrobial photodynamic therapy in dentistry. Front. Microbiol. 2023, 13, 1020995. [Google Scholar] [CrossRef] [PubMed]
- Algorri, J.F.; López-Higuera, J.M.; Rodríguez-Cobo, L.; Cobo, A. Advanced Light Source Technologies for Photodynamic Therapy of Skin Cancer Lesions. Pharmaceutics 2023, 15, 2075. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ni, J.; Yuan, L.; Li, X. Hematoporphyrin derivative photodynamic therapy induces apoptosis and suppresses the migration of human esophageal squamous cell carcinoma cells by regulating the PI3K/AKT/mTOR signaling pathway. Oncol. Lett. 2023, 27, 17. [Google Scholar] [CrossRef] [PubMed]
- Vinita, N.M.; Devan, U.; Durgadevi, S.; Anitha, S.; Prabhu, D.; Rajamanikandan, S.; Govarthanan, M.; Yuvaraj, A.; Biruntha, M.; Velanganni, A.J.; et al. Triphenylphosphonium conjugated gold nanotriangles impact Pi3K/AKT pathway in breast cancer cells: A photodynamic therapy approach. Sci. Rep. 2023, 13, 2230. [Google Scholar] [CrossRef] [PubMed]
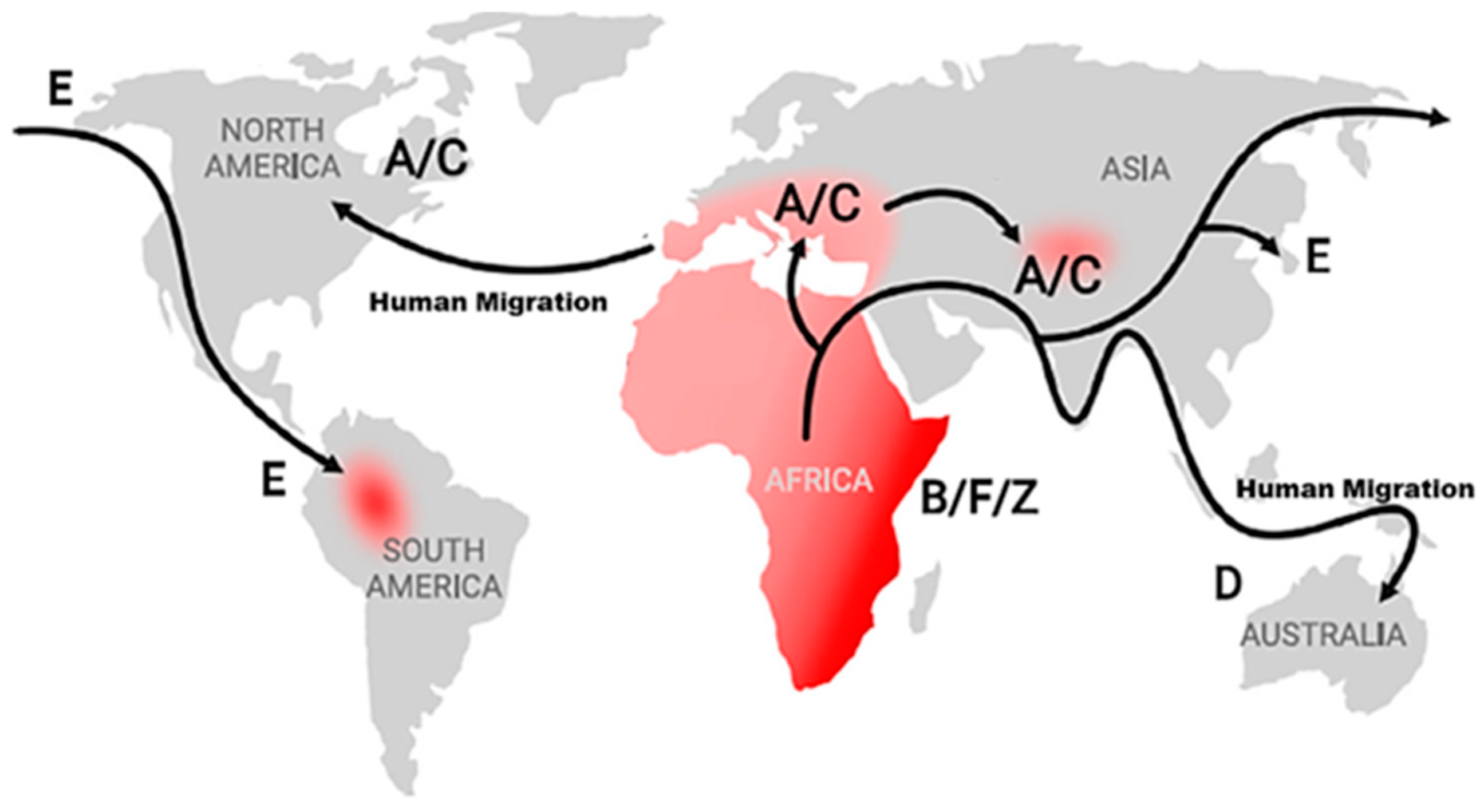

| SSA Regions | Countries | KSHV-Subtypes | Seroprevalence |
KS-Incidence (Per 1000) | Ref. |
|---|---|---|---|---|---|
| Central | Cameroon | A5, B, P | >50% | 4–8 | [25,72,75,76,77,78,79,83,84] |
| Gabon | A5 | 25–50% | 2–4 | [33,59,72,76,80] | |
| Congo Brazzaville | B, M | 25–50% | 0–0.5 | [85,86] | |
| Southern | South Africa | A5, B, N | >50% | 4–8 | [59,64,72,73,74,87] |
| Zambia | A5, B, C, Z, M, N, P | >50% | 1–8 | [59,72,75,88] | |
| Zimbabwe | A5 | >50% | 4–8 | [8,58,59,71,72] | |
| Eastern | Uganda | A5, C, F, P, M | >50% | 1–8 | [58,59,60,62,64,68,72,77] |
| Tanzania | A5, B, P | >50% | 2–4 | [59,65,66,72,89] | |
| Kenya | A5, B, C, F, P | >50% | 0.5–2 | [69,70,72,90] | |
| Western | Ghana | A5, B | >50% | 0–0.5 | [58,59,72,91,92] |
| Nigeria | A5 | >50% | 0.5–2 | [57,59,65,72,74] | |
| Burkina Faso | A5 | >25% | 0–0,5 | [56,59,93,94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamimandjiami, A.I.; Engone-Ondo, J.-D.; Moussavou-Boundzanga, P.; Mouinga-Ondeme, A.; Mfouo-Tynga, I.S. Kaposi’s Sarcoma: A Non-Communicable Outcome Mainly Prompted by Communicable Diseases in Sub-Saharan Africa. Int. J. Mol. Sci. 2025, 26, 10198. https://doi.org/10.3390/ijms262010198
Mamimandjiami AI, Engone-Ondo J-D, Moussavou-Boundzanga P, Mouinga-Ondeme A, Mfouo-Tynga IS. Kaposi’s Sarcoma: A Non-Communicable Outcome Mainly Prompted by Communicable Diseases in Sub-Saharan Africa. International Journal of Molecular Sciences. 2025; 26(20):10198. https://doi.org/10.3390/ijms262010198
Chicago/Turabian StyleMamimandjiami, Anthony Idam, Jéordy-Dimitri Engone-Ondo, Pamela Moussavou-Boundzanga, Augustin Mouinga-Ondeme, and Ivan S. Mfouo-Tynga. 2025. "Kaposi’s Sarcoma: A Non-Communicable Outcome Mainly Prompted by Communicable Diseases in Sub-Saharan Africa" International Journal of Molecular Sciences 26, no. 20: 10198. https://doi.org/10.3390/ijms262010198
APA StyleMamimandjiami, A. I., Engone-Ondo, J.-D., Moussavou-Boundzanga, P., Mouinga-Ondeme, A., & Mfouo-Tynga, I. S. (2025). Kaposi’s Sarcoma: A Non-Communicable Outcome Mainly Prompted by Communicable Diseases in Sub-Saharan Africa. International Journal of Molecular Sciences, 26(20), 10198. https://doi.org/10.3390/ijms262010198






