Validating TDP1 as an Inhibition Target for Lipophilic Nucleoside Derivative in Human Cells
Abstract
1. Introduction
2. Results and Discussion
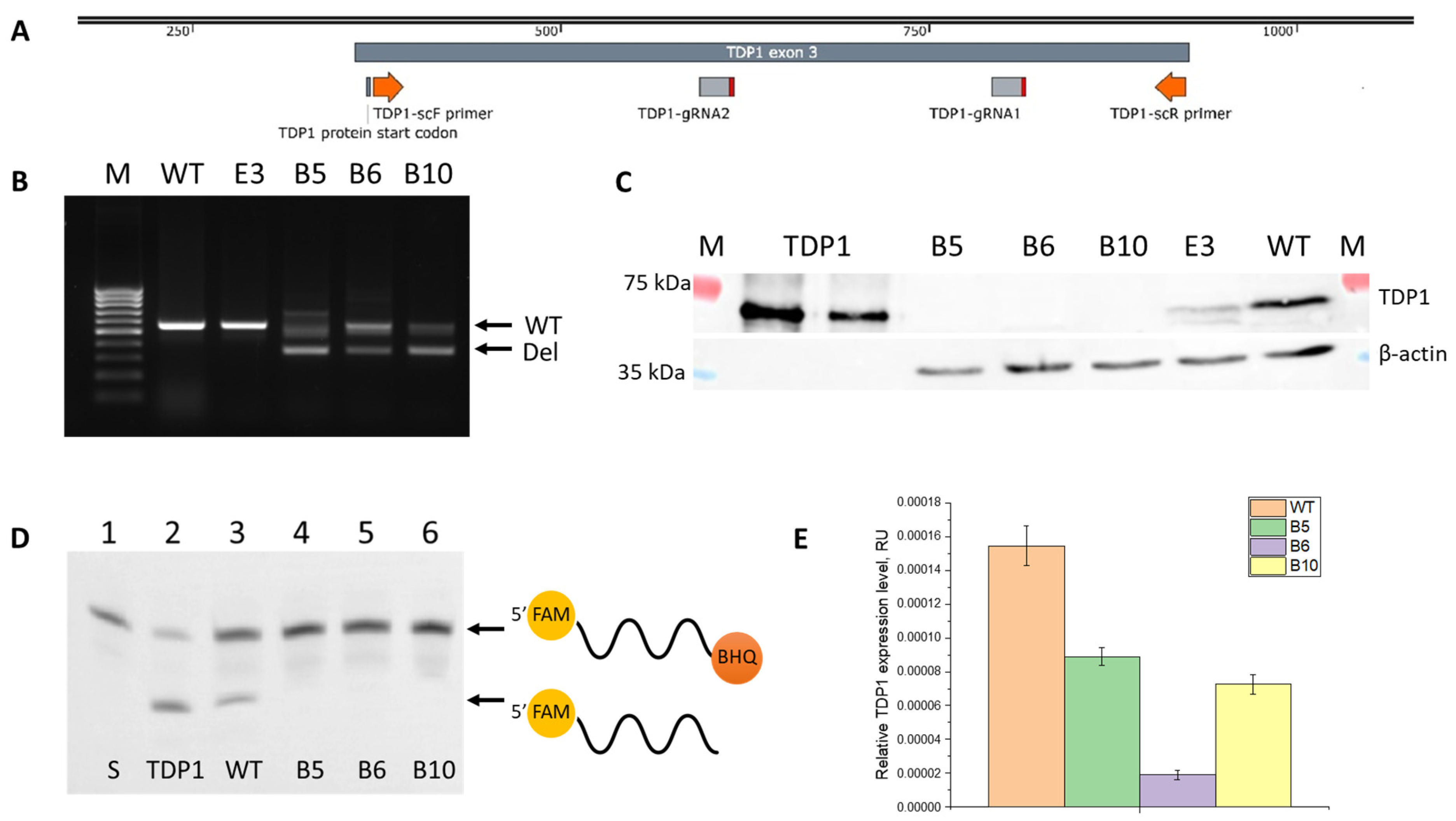
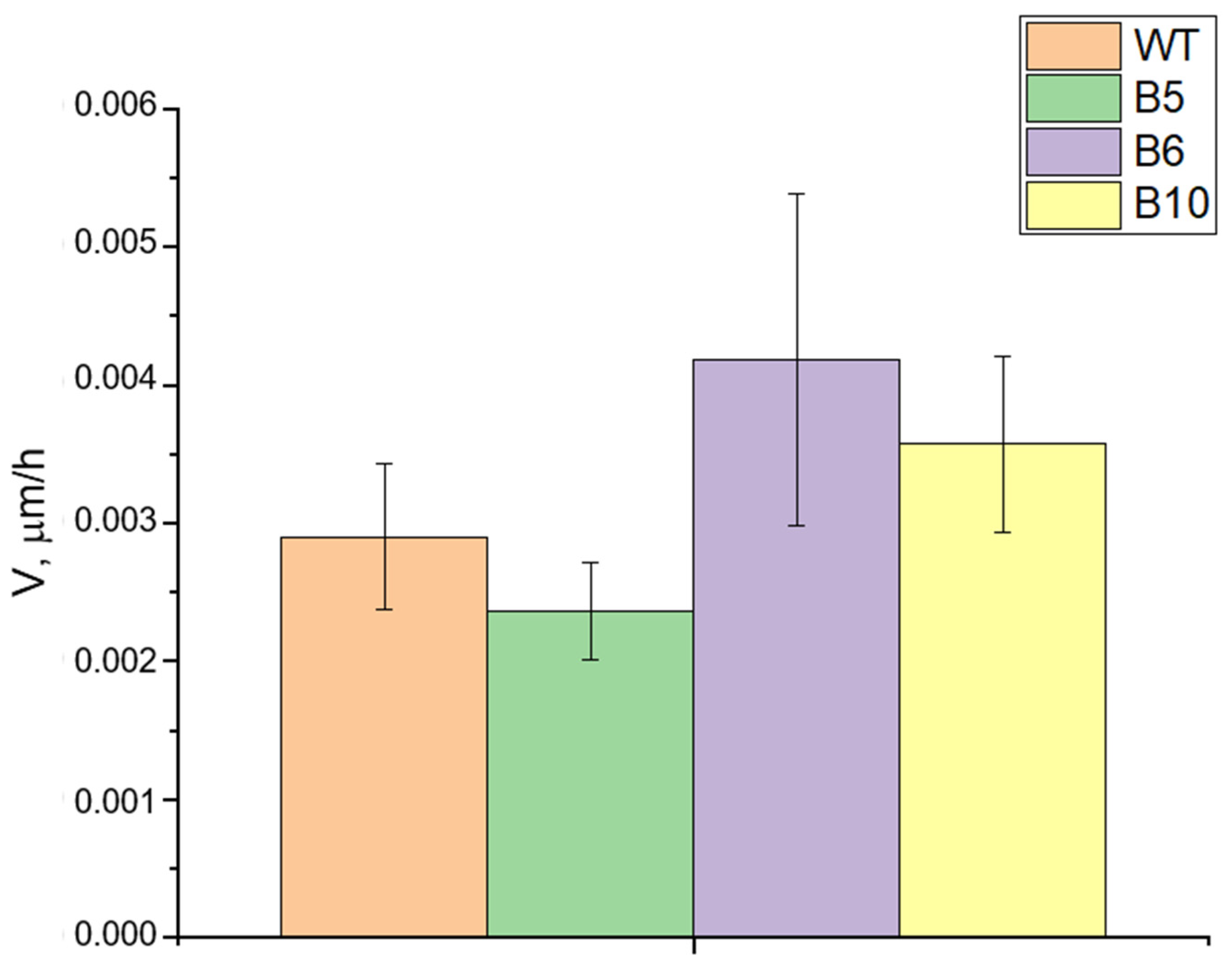
2.1. Generation of TDP1 Knockout Cell Lines
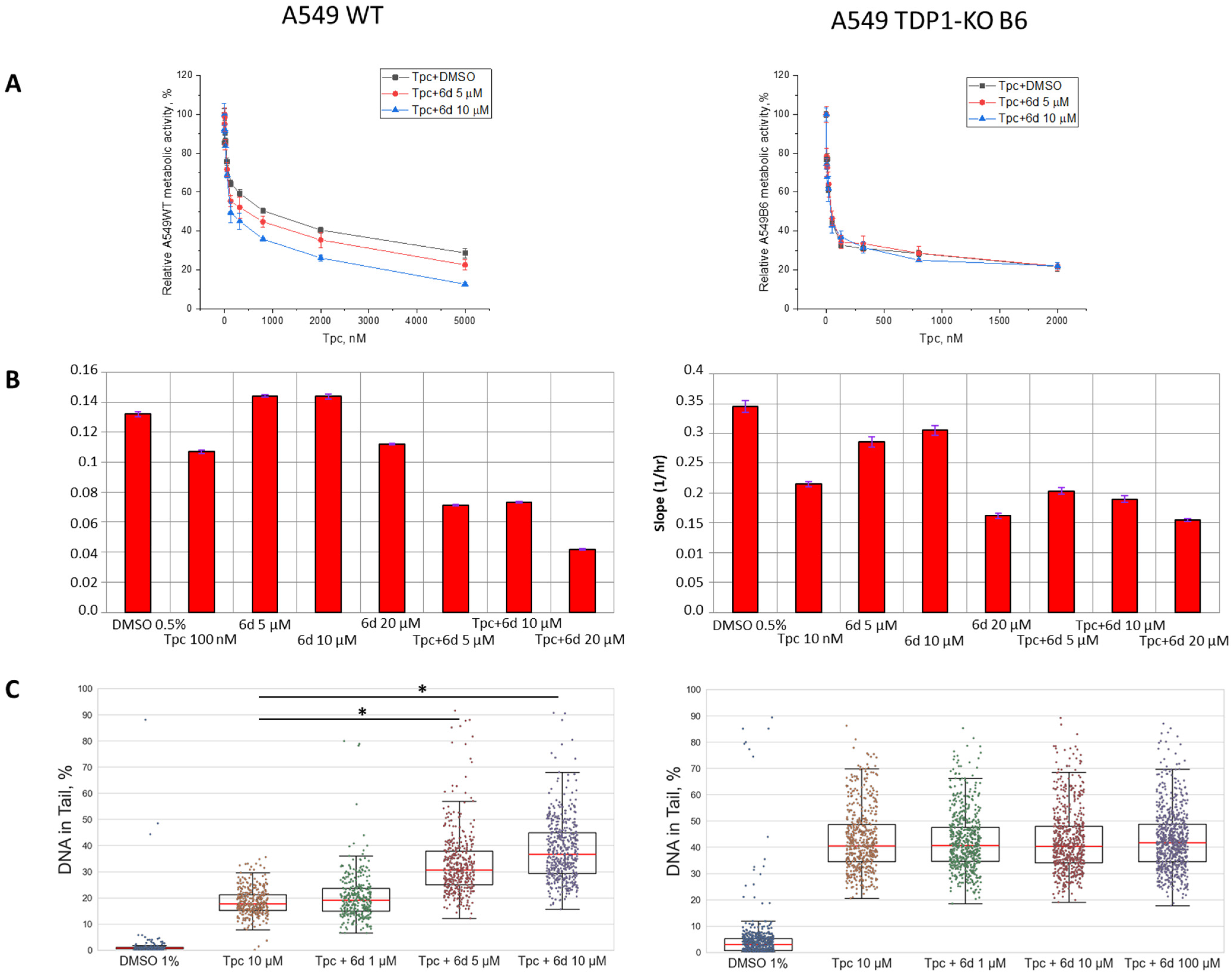
2.2. The Effect of Compound 6d on Topotecan Action in Cell Lines with TDP1 Deficiency
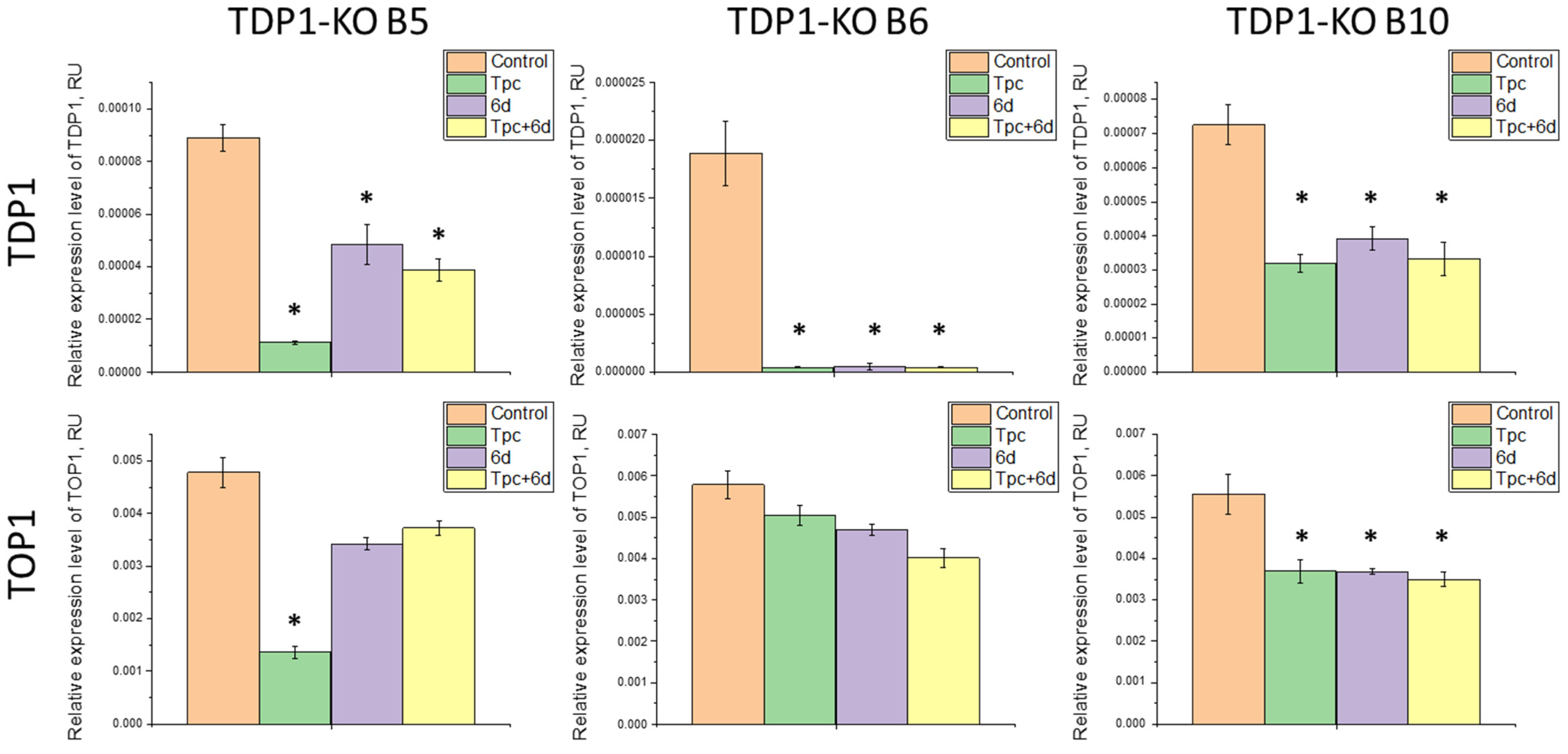
2.3. Study of the Effect of Compound 6d and Its Combination with Tpc on the Expression Levels of TDP1 and TOP1 Genes
3. Materials and Methods
3.1. Cell Lines and Growth Conditions
3.2. TDP1 Knockout A549 Clones
3.3. Western Blotting
3.4. TDP1 Gel-Based Enzyme Activity Assay
3.5. MTT Assay
3.6. xCELLigence Real-Time Cell Analysis (RTCA)
3.7. Alkaline Comet Assay
3.8. Wound Healing Assay
3.9. RT-qPCR
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pommier, Y.; Huang, S.N.; Gao, R.; Das, B.B.; Murai, J.; Marchand, C. Tyrosyl-DNA-Phosphodiesterases (TDP1 and TDP2). DNA Repair 2014, 19, 114–129. [Google Scholar] [CrossRef]
- Murai, J.; Huang, S.N.; Das, B.B.; Dexheimer, T.S.; Takeda, S.; Pommier, Y. Tyrosyl-DNA Phosphodiesterase 1 (TDP1) Repairs DNA Damage Induced by Topoisomerases I and II and Base Alkylation in Vertebrate Cells. J. Biol. Chem. 2012, 287, 12848–12857. [Google Scholar] [CrossRef]
- Nitiss, K.C.; Malik, M.; He, X.; White, S.W.; Nitiss, J.L. Tyrosyl-DNA Phosphodiesterase (Tdp1) Participates in the Repair of Top2-Mediated DNA Damage. Proc. Natl. Acad. Sci. USA 2006, 103, 8953–8958. [Google Scholar] [CrossRef]
- Lorusso, D.; Pietragalla, A.; Mainenti, S.; Masciullo, V.; Di Vagno, G.; Scambia, G. Review Role of Topotecan in Gynaecological Cancers: Current Indications and Perspectives. Crit. Rev. Oncol. Hematol. 2010, 74, 163–174. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Eckardt, J.; Ramlau, R. Recent Advances with Topotecan in the Treatment of Lung Cancer. Oncologist 2007, 12, 1194–1204. [Google Scholar] [CrossRef]
- Delgado, J.L.; Hsieh, C.-M.; Chan, N.-L.; Hiasa, H. Topoisomerases as Anticancer Targets. Biochem. J. 2018, 475, 373–398. [Google Scholar] [CrossRef]
- Pourquier, P.; Pilon, A.A.; Kohlhagen, G.; Mazumder, A.; Sharma, A.; Pommier, Y. Trapping of Mammalian Topoisomerase I and Recombinations Induced by Damaged DNA Containing Nicks or Gaps. Importance of DNA End Phosphorylation and Camptothecin Effects. J. Biol. Chem. 1997, 272, 26441–26447. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, L.F. Processing of Topoisomerase I Cleavable Complexes into DNA Damage by Transcription. Nucleic Acids Res. 1997, 25, 4181–4186. [Google Scholar] [CrossRef]
- Pommier, Y. Topoisomerase I Inhibitors: Camptothecins and Beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W.; Burgin, A.B.; Huizenga, B.N.; Robertson, C.A.; Yao, K.C.; Nash, H.A. A Eukaryotic Enzyme That Can Disjoin Dead-End Covalent Complexes between DNA and Type I Topoisomerases. Proc. Natl. Acad. Sci. USA 1996, 93, 11534–11539. [Google Scholar] [CrossRef]
- Interthal, H.; Pouliot, J.J.; Champoux, J.J. The Tyrosyl-DNA Phosphodiesterase Tdp1 Is a Member of the Phospholipase D Superfamily. Proc. Natl. Acad. Sci. USA 2001, 98, 12009–12014. [Google Scholar] [CrossRef]
- Li, J.; Summerlin, M.; Nitiss, K.C.; Nitiss, J.L.; Hanakahi, L.A. TDP1 Is Required for Efficient Non-Homologous End Joining in Human Cells. DNA Repair 2017, 60, 40–49. [Google Scholar] [CrossRef]
- Brettrager, E.J.; Segura, I.A.; van Waardenburg, R.C.A.M. Tyrosyl-DNA Phosphodiesterase I N-Terminal Domain Modifications and Interactions Regulate Cellular Function. Genes 2019, 10, 897. [Google Scholar] [CrossRef]
- Takashima, H.; Boerkoel, C.F.; John, J.; Saifi, G.M.; Salih, M.A.M.; Armstrong, D.; Mao, Y.; Quiocho, F.A.; Roa, B.B.; Nakagawa, M.; et al. Mutation of TDP1, Encoding a Topoisomerase I-Dependent DNA Damage Repair Enzyme, in Spinocerebellar Ataxia with Axonal Neuropathy. Nat. Genet. 2002, 32, 267–272. [Google Scholar] [CrossRef]
- El-Khamisy, S.F.; Saifi, G.M.; Weinfeld, M.; Johansson, F.; Helleday, T.; Lupski, J.R.; Caldecott, K.W. Defective DNA Single-Strand Break Repair in Spinocerebellar Ataxia with Axonal Neuropathy-1. Nature 2005, 434, 108–113. [Google Scholar] [CrossRef]
- Jakobsen, A.-K.; Yuusufi, S.; Madsen, L.B.; Meldgaard, P.; Knudsen, B.R.; Stougaard, M. TDP1 and TOP1 as Targets in Anticancer Treatment of NSCLC: Activity and Protein Level in Normal and Tumor Tissue from 150 NSCLC Patients Correlated to Clinical Data. Lung Cancer 2022, 164, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Meisenberg, C.; Ward, S.E.; Schmid, P.; El-Khamisy, S.F. TDP1/TOP1 Ratio as a Promising Indicator for the Response of Small Cell Lung Cancer to Topotecan. J. Cancer Sci. Ther. 2014, 6, 258–267. [Google Scholar] [CrossRef]
- Kim, J.W.; Min, A.; Im, S.-A.; Jang, H.; Kim, Y.J.; Kim, H.-J.; Lee, K.-H.; Kim, T.-Y.; Lee, K.W.; Oh, D.-Y.; et al. TDP1 and TOP1 Modulation in Olaparib-Resistant Cancer Determines the Efficacy of Subsequent Chemotherapy. Cancers 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Perego, P.; Cossa, G.; Tinelli, S.; Corna, E.; Carenini, N.; Gatti, L.; De Cesare, M.; Ciusani, E.; Zunino, F.; Luison, E.; et al. Role of Tyrosyl-DNA Phosphodiesterase 1 and Inter-Players in Regulation of Tumor Cell Sensitivity to Topoisomerase I Inhibition. Biochem. Pharmacol. 2012, 83, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.L.; Luzina, O.A.; Chepanova, A.A.; Dyrkheeva, N.S.; Salakhutdinov, N.F.; Lavrik, O.I. Natural Products and Their Derivatives as Inhibitors of the DNA Repair Enzyme Tyrosyl-DNA Phosphodiesterase 1. Int. J. Mol. Sci. 2023, 24, 5781. [Google Scholar] [CrossRef]
- Antony, S.; Marchand, C.; Stephen, A.G.; Thibaut, L.; Agama, K.K.; Fisher, R.J.; Pommier, Y. Novel High-Throughput Electrochemiluminescent Assay for Identification of Human Tyrosyl-DNA Phosphodiesterase (Tdp1) Inhibitors and Characterization of Furamidine (NSC 305831) as an Inhibitor of Tdp1. Nucleic Acids Res. 2007, 35, 4474–4484. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, A.; Price, E.; Marchand, C.; Chergui, A.; Naumova, A.; Whitson, E.L.; Krumpe, L.R.H.; Goncharova, E.I.; Evans, J.R.; McKee, T.C.; et al. Identification of Natural Products That Inhibit the Catalytic Function of Human Tyrosyl-DNA Phosphodiesterase (TDP1). SLAS Discov. 2017, 22, 1093–1105. [Google Scholar] [CrossRef]
- Jun, J.H.; Kumar, V.; Dexheimer, T.S.; Wedlich, I.; Nicklaus, M.C.; Pommier, Y.; Malhotra, S.V. Synthesis, Anti-Cancer Screening and Tyrosyl-DNA Phosphodiesterase 1 (Tdp1) Inhibition Activity of Novel Piperidinyl Sulfamides. Eur. J. Pharm. Sci. 2018, 111, 337–348. [Google Scholar] [CrossRef]
- Liao, Z.; Thibaut, L.; Jobson, A.; Pommier, Y. Inhibition of Human Tyrosyl-DNA Phosphodiesterase by Aminoglycoside Antibiotics and Ribosome Inhibitors. Mol. Pharmacol. 2006, 70, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.-C.; Agama, K.; Marchand, C.; Pommier, Y.; Cushman, M. Design, Synthesis, and Biological Evaluation of O-2-Modified Indenoisoquinolines as Dual Topoisomerase I–Tyrosyl-DNA Phosphodiesterase I Inhibitors. J. Med. Chem. 2014, 57, 4324–4336. [Google Scholar] [CrossRef]
- Marchand, C.; Lea, W.A.; Jadhav, A.; Dexheimer, T.S.; Austin, C.P.; Inglese, J.; Pommier, Y.; Simeonov, A. Identification of Phosphotyrosine Mimetic Inhibitors of Human Tyrosyl-DNA Phosphodiesterase I by a Novel AlphaScreen High-Throughput Assay. Mol. Cancer Ther. 2009, 8, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Della Volpe, S.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A.; Sala, A.; Collina, S. The Long Story of Camptothecin: From Traditional Medicine to Drugs. Bioorg. Med. Chem. Lett. 2017, 27, 701–707. [Google Scholar] [CrossRef]
- Meisenberg, C.; Gilbert, D.C.; Chalmers, A.; Haley, V.; Gollins, S.; Ward, S.E.; El-Khamisy, S.F. Clinical and Cellular Roles for TDP1 and TOP1 in Modulating Colorectal Cancer Response to Irinotecan. Mol. Cancer Ther. 2015, 14, 575–585. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Abdelmalak, M.; Marchand, C.; Agama, K.; Pommier, Y.; Cushman, M. Synthesis and Biological Evaluation of Nitrated 7-, 8-, 9-, and 10-Hydroxyindenoisoquinolines as Potential Dual Topoisomerase I (Top1)–Tyrosyl-DNA Phosphodiesterase I (TDP1) Inhibitors. J. Med. Chem. 2015, 58, 3188–3208. [Google Scholar] [CrossRef]
- Sirivolu, V.R.; Vernekar, S.K.V.; Marchand, C.; Naumova, A.; Chergui, A.; Renaud, A.; Stephen, A.G.; Chen, F.; Sham, Y.Y.; Pommier, Y.; et al. 5-Arylidenethioxothiazolidinones as Inhibitors of Tyrosyl-DNA Phosphodiesterase I. J. Med. Chem. 2012, 55, 8671–8684. [Google Scholar] [CrossRef]
- Moshawih, S.; Goh, H.; Kifli, N.; Darwesh, M.; Ardianto, C.; Goh, K.W.; Ming, L.C. Identification and Optimization of TDP1 Inhibitors from Anthraquinone and Chalcone Derivatives: Consensus Scoring Virtual Screening and Molecular Simulations. J. Biomol. Struct. Dyn. 2023, 42, 10286–10310. [Google Scholar] [CrossRef]
- Conda-Sheridan, M.; Reddy, P.V.N.; Morrell, A.; Cobb, B.T.; Marchand, C.; Agama, K.; Chergui, A.; Renaud, A.; Stephen, A.G.; Bindu, L.K.; et al. Synthesis and Biological Evaluation of Indenoisoquinolines That Inhibit Both Tyrosyl-DNA Phosphodiesterase I (Tdp1) and Topoisomerase I (Top1). J. Med. Chem. 2013, 56, 182–200. [Google Scholar] [CrossRef]
- Wang, P.; Elsayed, M.S.A.; Plescia, C.B.; Ravji, A.; Redon, C.E.; Kiselev, E.; Marchand, C.; Zeleznik, O.; Agama, K.; Pommier, Y.; et al. Synthesis and Biological Evaluation of the First Triple Inhibitors of Human Topoisomerase 1, Tyrosyl–DNA Phosphodiesterase 1 (Tdp1), and Tyrosyl–DNA Phosphodiesterase 2 (Tdp2). J. Med. Chem. 2017, 60, 3275–3288. [Google Scholar] [CrossRef]
- Kornienko, T.E.; Chepanova, A.A.; Zakharenko, A.L.; Filimonov, A.S.; Luzina, O.A.; Dyrkheeva, N.S.; Nikolin, V.P.; Popova, N.A.; Salakhutdinov, N.F.; Lavrik, O.I. Enhancement of the Antitumor and Antimetastatic Effect of Topotecan and Normalization of Blood Counts in Mice with Lewis Carcinoma by Tdp1 Inhibitors-New Usnic Acid Derivatives. Int. J. Mol. Sci. 2024, 25, 1210. [Google Scholar] [CrossRef]
- Zakharenko, A.L.; Luzina, O.A.; Sokolov, D.N.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Patel, J.; Zakharova, O.D.; Chepanova, A.A.; Zafar, A.; et al. Novel Tyrosyl-DNA Phosphodiesterase 1 Inhibitors Enhance the Therapeutic Impact of Topotecan on in Vivo Tumor Models. Eur. J. Med. Chem. 2019, 161, 581–593. [Google Scholar] [CrossRef]
- Nikolin, V.P.; Popova, N.A.; Kaledin, V.I.; Luzina, O.A.; Zakharenko, A.L.; Salakhutdinov, N.F.; Lavrik, O.I. The Influence of an Enamine Usnic Acid Derivative (a Tyrosyl-DNA Phosphodiesterase 1 Inhibitor) on the Therapeutic Effect of Topotecan against Transplanted Tumors In Vivo. Clin. Exp. Metastasis 2021, 38, 431–440. [Google Scholar] [CrossRef]
- Zakharenko, A.L.; Drenichev, M.S.; Dyrkheeva, N.S.; Ivanov, G.A.; Oslovsky, V.E.; Ilina, E.S.; Chernyshova, I.A.; Lavrik, O.I.; Mikhailov, S.N. Inhibition of Tyrosyl-DNA Phosphodiesterase 1 by Lipophilic Pyrimidine Nucleosides. Molecules 2020, 25, 3694. [Google Scholar] [CrossRef]
- Chernyshova, I.A.; Zakharenko, A.L.; Kurochkin, N.N.; Dyrkheeva, N.S.; Kornienko, T.E.; Popova, N.A.; Nikolin, V.P.; Ilina, E.S.; Zharkov, T.D.; Kupryushkin, M.S.; et al. The Lipophilic Purine Nucleoside-Tdp1 Inhibitor-Enhances DNA Damage Induced by Topotecan In Vitro and Potentiates the Antitumor Effect of Topotecan In Vivo. Molecules 2022, 28, 323. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Hosoya, N.; Miyagawa, K. Targeting DNA Damage Response in Cancer Therapy. Cancer Sci. 2014, 105, 370–388. [Google Scholar] [CrossRef]
- Drew, Y.; Zenke, F.T.; Curtin, N.J. DNA Damage Response Inhibitors in Cancer Therapy: Lessons from the Past, Current Status and Future Implications. Nat. Rev. Drug Discov. 2025, 24, 19–39. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Ao, D. Targeting DNA Repair Pathway in Cancer: Mechanisms and Clinical Application. MedComm 2021, 2, 654–691. [Google Scholar] [CrossRef]
- Majd, N.K.; Yap, T.A.; Koul, D.; Balasubramaniyan, V.; Li, X.; Khan, S.; Gandy, K.S.; Yung, W.K.A.; De Groot, J.F. The Promise of DNA Damage Response Inhibitors for the Treatment of Glioblastoma. Neuro-Oncol. Adv. 2021, 3, vdab015. [Google Scholar] [CrossRef]
- Vennepureddy, A.; Atallah, J.-P.; Terjanian, T. Role of Topotecan in Non-Small Cell Lung Cancer: A Review of Literature. World J. Oncol. 2015, 6, 429–436. [Google Scholar] [CrossRef]
- Spigel, D.R.; Dowlati, A.; Chen, Y.; Navarro, A.; Yang, J.C.-H.; Stojanovic, G.; Jove, M.; Rich, P.; Andric, Z.G.; Wu, Y.-L.; et al. RESILIENT Part 2: A Randomized, Open-Label Phase III Study of Liposomal Irinotecan Versus Topotecan in Adults with Relapsed Small Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 2317–2326. [Google Scholar] [CrossRef]
- El-Khamisy, S.F.; Katyal, S.; Patel, P.; Ju, L.; McKinnon, P.J.; Caldecott, K.W. Synergistic Decrease of DNA Single-Strand Break Repair Rates in Mouse Neural Cells Lacking Both Tdp1 and Aprataxin. DNA Repair 2009, 8, 760–766. [Google Scholar] [CrossRef]
- Dyrkheeva, N.S.; Zakharenko, A.L.; Malakhova, A.A.; Okorokova, L.S.; Shtokalo, D.N.; Medvedev, S.P.; Tupikin, A.A.; Kabilov, M.R.; Lavrik, O.I. Transcriptomic Analysis of HEK293A Cells with a CRISPR/Cas9-Mediated TDP1 Knockout. Biochim. Biophys. Acta Gen. Subj. 2024, 1868, 130616. [Google Scholar] [CrossRef]
- Chou, T.-C.; Talalay, P. Quantitative Analysis of Dose-Effect Relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Chou, T.-C.; Martin, N. A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 Values. In CompuSyn for Drug Combinations User’s Guide; ComboSyn, Inc.: Paramus, NJ, USA, 2005. [Google Scholar]
- Limame, R.; Wouters, A.; Pauwels, B.; Fransen, E.; Peeters, M.; Lardon, F.; De Wever, O.; Pauwels, P. Comparative Analysis of Dynamic Cell Viability, Migration and Invasion Assessments by Novel Real-Time Technology and Classic Endpoint Assays. PLoS ONE 2012, 7, e46536. [Google Scholar] [CrossRef]
- Kustermann, S.; Boess, F.; Buness, A.; Schmitz, M.; Watzele, M.; Weiser, T.; Singer, T.; Suter, L.; Roth, A. A Label-Free, Impedance-Based Real Time Assay to Identify Drug-Induced Toxicities and Differentiate Cytostatic from Cytotoxic Effects. Toxicology 2013, 27, 1589–1595. [Google Scholar] [CrossRef]
- Il’ina, I.V.; Dyrkheeva, N.S.; Zakharenko, A.L.; Sidorenko, A.Y.; Li-Zhulanov, N.S.; Korchagina, D.V.; Chand, R.; Ayine-Tora, D.M.; Chepanova, A.A.; Zakharova, O.D.; et al. Design, Synthesis, and Biological Investigation of Novel Classes of 3-Carene-Derived Potent Inhibitors of TDP1. Molecules 2020, 25, 3496. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Regairaz, M.; Seiler, J.A.; Agama, K.K.; Doroshow, J.H.; Pommier, Y. Poly(ADP-Ribose) Polymerase and XPF–ERCC1 Participate in Distinct Pathways for the Repair of Topoisomerase I-Induced DNA Damage in Mammalian Cells. Nucleic Acids Res. 2011, 39, 3607–3620. [Google Scholar] [CrossRef] [PubMed]
- Sacho, E.J.; Maizels, N. DNA Repair Factor MRE11/RAD50 Cleaves 3′-Phosphotyrosyl Bonds and Resects DNA to Repair Damage Caused by Topoisomerase 1 Poisons*. J. Biol. Chem. 2011, 286, 44945–44951. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Spitz, G.S.; Veturi, U.; Lach, F.P.; Auerbach, A.D.; Smogorzewska, A. Regulation of Multiple DNA Repair Pathways by the Fanconi Anemia Protein SLX4. Blood 2013, 121, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]

| Gene | Primer Sequence | Amplification Efficiency for HEK293A Cells | Amplification Efficiency for A549 Cells |
|---|---|---|---|
| GAPDH | AGATCATCAGCAATGCCTCCT | 2.01 ± 0.16 | 1.9 ± 0.2 |
| TGGTCATGAGTCCTTCCACG | |||
| B2M | CGCTCCGTGGCCTTAGCTGT | 1.95 ± 0.09 | 1.87 ± 0.15 |
| AAAGACAAGTCTGAATGCTC | |||
| TOP1 | CCTCCTGGACTTTTCCGTGG | 2.0 ± 0.2 | 2.07 ± 0.13 |
| GGAACCTTGGCATCTTTGCTAC | |||
| TDP1 | AAGACATCTCTGCTCCCAATG | 2.2 ± 0.3 | 2.2 ± 0.15 |
| TTCCCTTTATCCAGCATGTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernyshova, I.A.; Kornienko, T.E.; Dyrkheeva, N.S.; Zakharenko, A.L.; Chepanova, A.A.; Orishchenko, K.E.; Kurochkin, N.N.; Drenichev, M.S.; Lavrik, O.I. Validating TDP1 as an Inhibition Target for Lipophilic Nucleoside Derivative in Human Cells. Int. J. Mol. Sci. 2025, 26, 10193. https://doi.org/10.3390/ijms262010193
Chernyshova IA, Kornienko TE, Dyrkheeva NS, Zakharenko AL, Chepanova AA, Orishchenko KE, Kurochkin NN, Drenichev MS, Lavrik OI. Validating TDP1 as an Inhibition Target for Lipophilic Nucleoside Derivative in Human Cells. International Journal of Molecular Sciences. 2025; 26(20):10193. https://doi.org/10.3390/ijms262010193
Chicago/Turabian StyleChernyshova, Irina A., Tatyana E. Kornienko, Nadezhda S. Dyrkheeva, Alexandra L. Zakharenko, Arina A. Chepanova, Konstantin E. Orishchenko, Nikolay N. Kurochkin, Mikhail S. Drenichev, and Olga I. Lavrik. 2025. "Validating TDP1 as an Inhibition Target for Lipophilic Nucleoside Derivative in Human Cells" International Journal of Molecular Sciences 26, no. 20: 10193. https://doi.org/10.3390/ijms262010193
APA StyleChernyshova, I. A., Kornienko, T. E., Dyrkheeva, N. S., Zakharenko, A. L., Chepanova, A. A., Orishchenko, K. E., Kurochkin, N. N., Drenichev, M. S., & Lavrik, O. I. (2025). Validating TDP1 as an Inhibition Target for Lipophilic Nucleoside Derivative in Human Cells. International Journal of Molecular Sciences, 26(20), 10193. https://doi.org/10.3390/ijms262010193





