Palmitoylation Code and Endosomal Sorting Regulate ABHD17A Plasma Membrane Targeting and Activity
Abstract
1. Introduction
2. Results
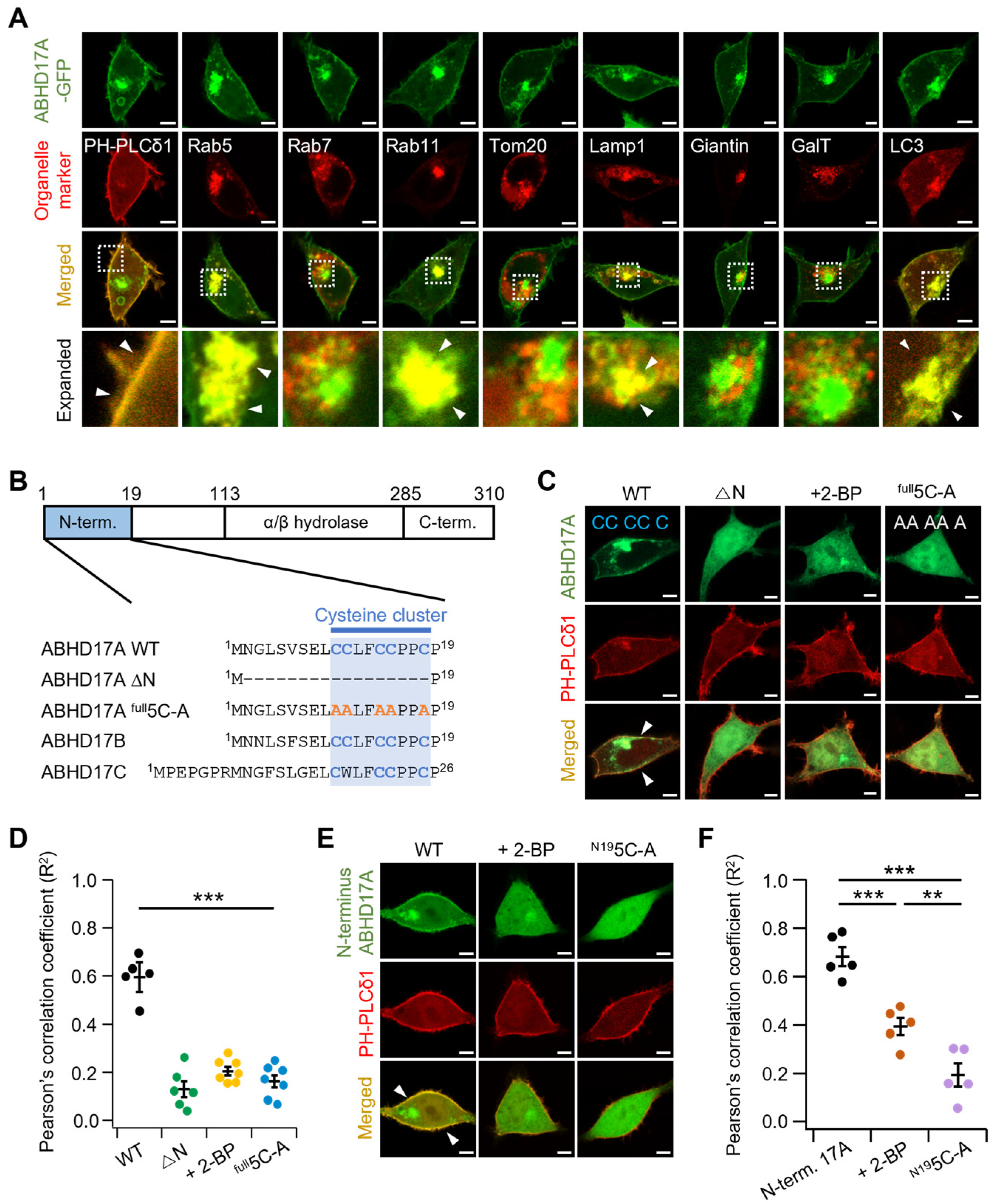
2.1. N-Terminal Palmitoylation of the ABHD17 Family Is Essential for PM Localization
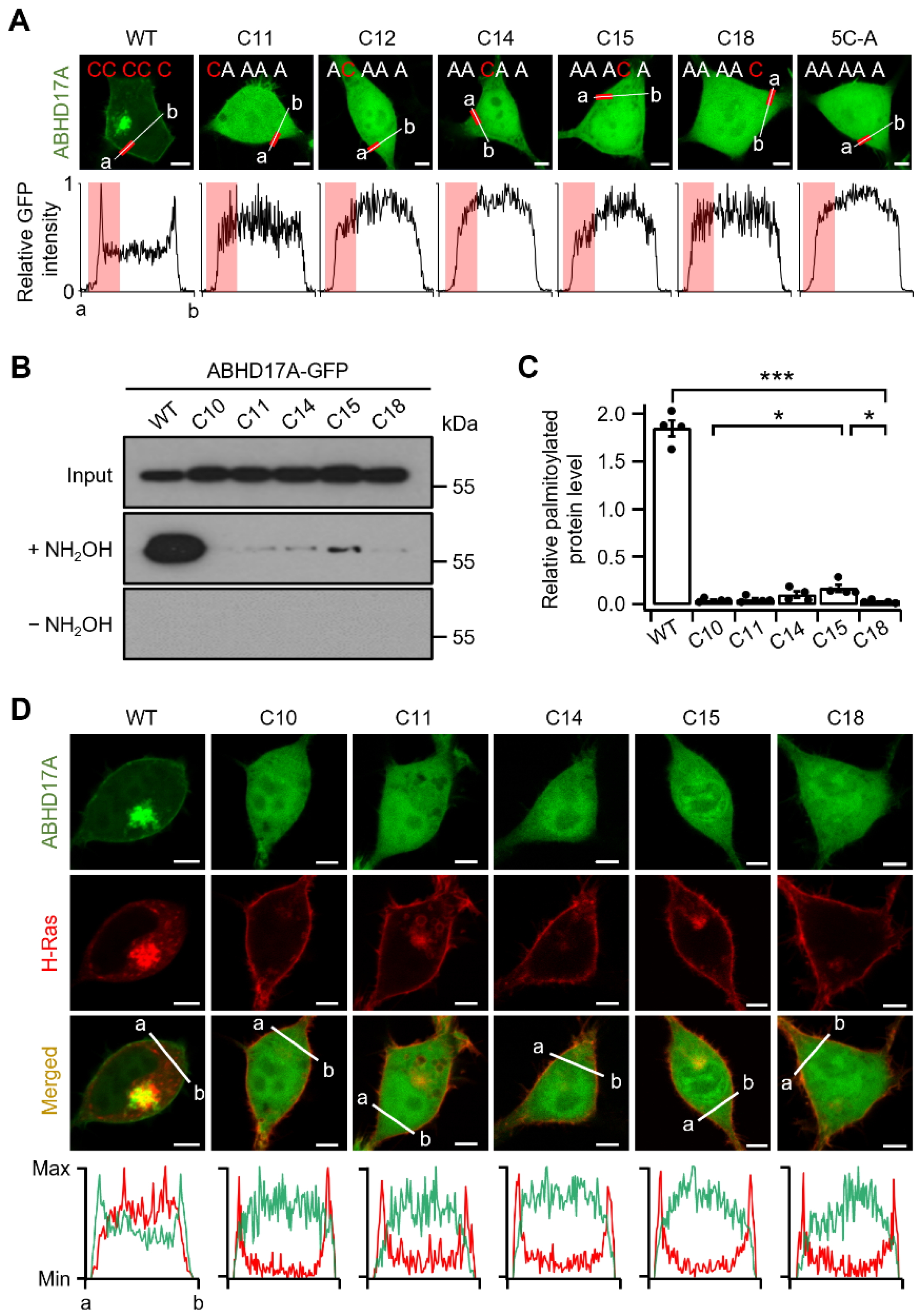
2.2. Single N-Terminal Cysteine Codes Are Insufficient for ABHD17A Palmitoylation and Membrane Association
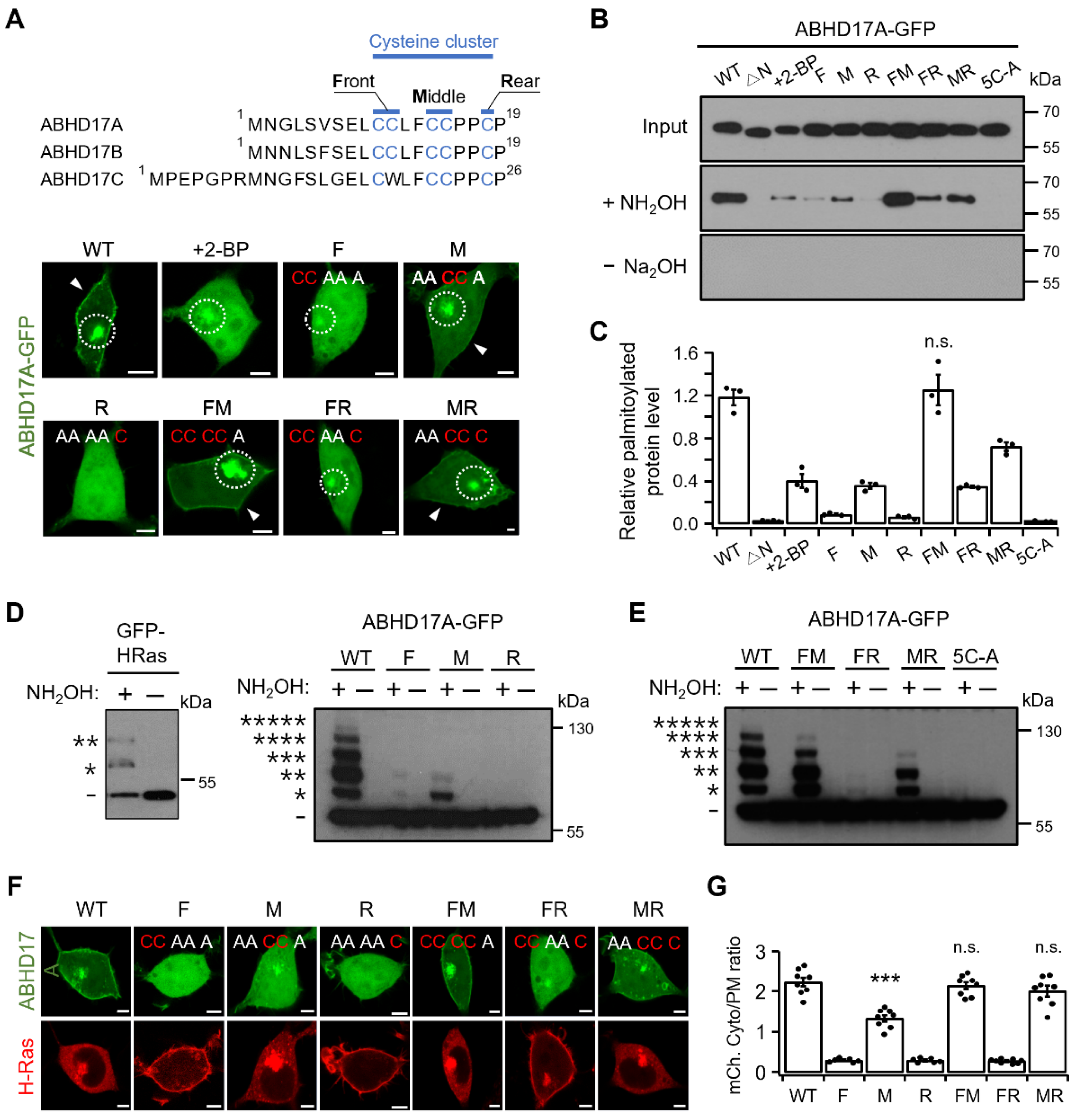
2.3. Middle Region Palmitoylation in ABHD17A Is Necessary for PM Localization and Catalytic Activity
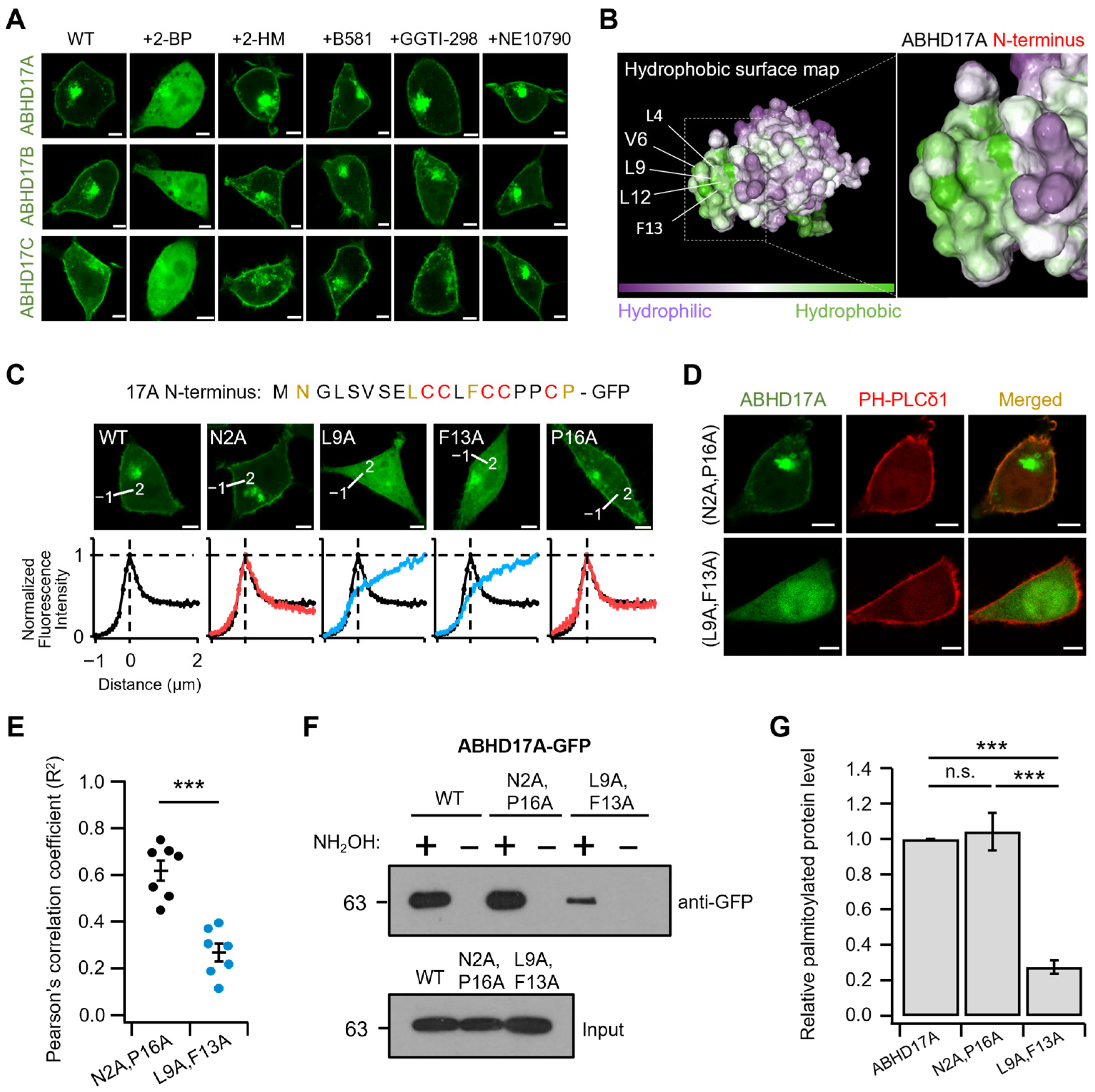
2.4. Hydrophobic Residues in the N-Terminus Regulate Initial Endomembrane Engagement Prior to Palmitoylation
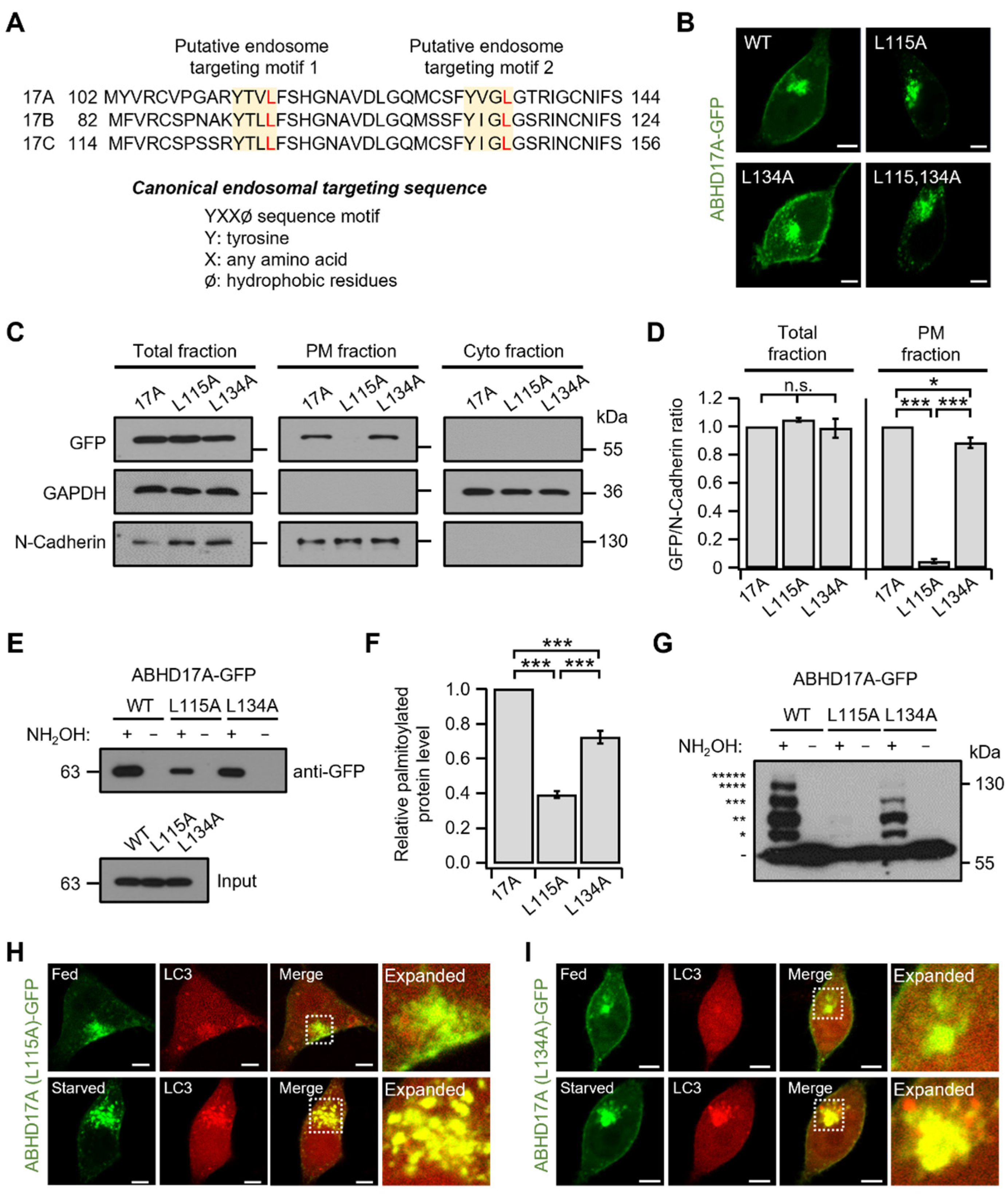
2.5. Endosomal Targeting Motif in ABHD17A Contributes to PM Targeting
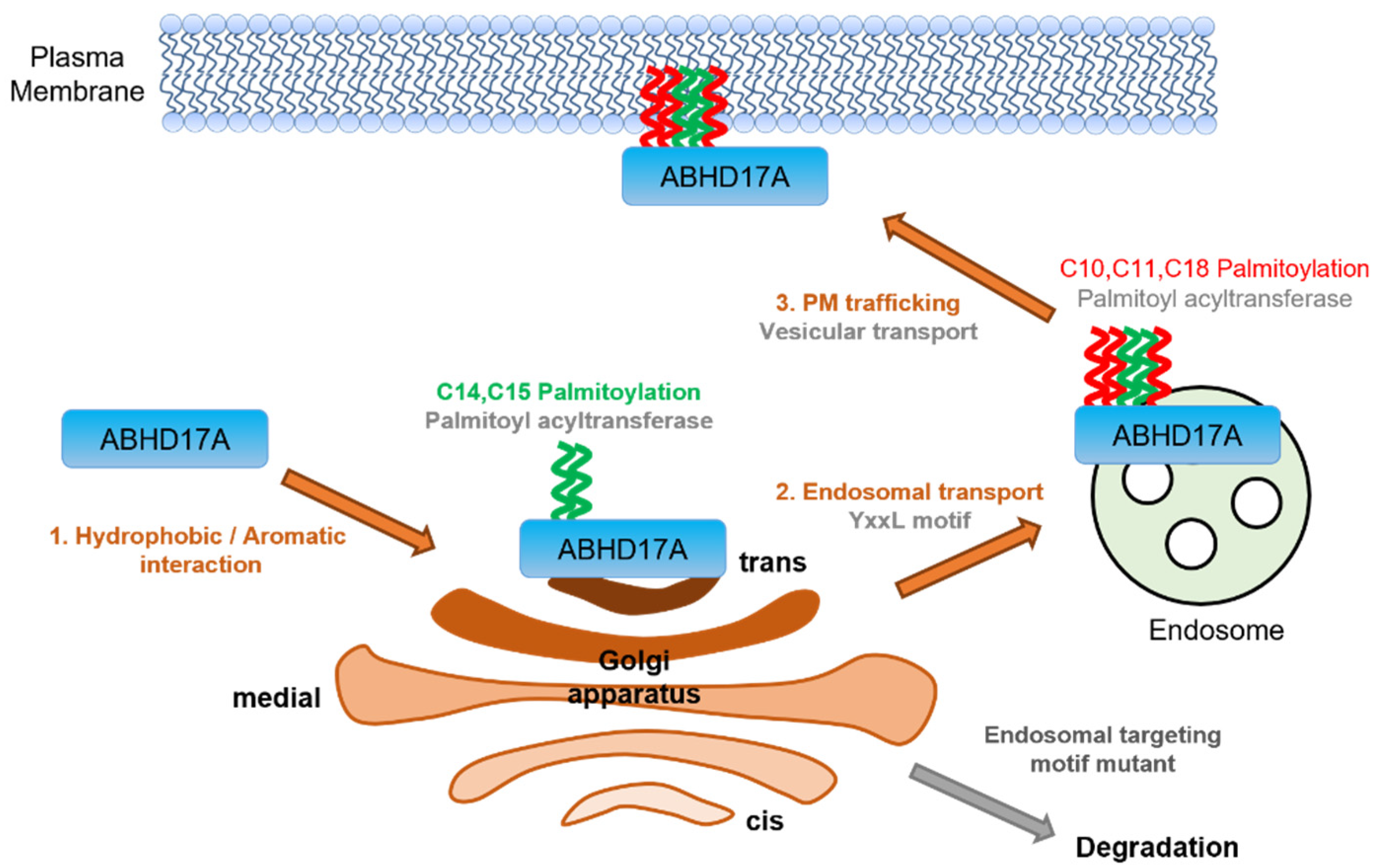
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Transfection
4.2. Western Blotting
4.3. PM–Cytosol Separation
4.4. In Silico Analyses
4.5. Plasmids and Molecular Cloning
4.6. Acyl-RAC Capture and Acyl-PEG Exchange Assays
4.7. Confocal Imaging and Quantitation
4.8. Pharmacological Treatments
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABHD17 | α/β hydrolase domain-containing protein 17 |
| 2-BP | 2-bromopalmitate |
| Acyl-RAC | acyl resin assisted capture |
| Acyl-PEG | acyl-polyethylene glycol |
| APT | acyl protein thioesterase |
| PPT1 | palmitoyl protein thioesterase 1 |
| PM | plasma membrane |
| zDHHC-PAT | zinc finger and DHHC motif containing palmitoyl acyltransferase |
| PI4KIIIα | phosphatidylinositol 4-kinase IIIα |
| PH-PLCδ1 | pleckstrin homology domain of phospholipase C-δ1 |
| SNAP25 | synaptosome associated protein 25 |
References
- Conibear, E.; Davis, N.G. Palmitoylation and depalmitoylation dynamics at a glance. J. Cell Sci. 2010, 123, 4007–4010. [Google Scholar] [CrossRef]
- Salaun, C.; Greaves, J.; Chamberlain, L.H. The intracellular dynamic of protein palmitoylation. J. Cell Biol. 2010, 191, 1229–1238. [Google Scholar] [CrossRef]
- Levental, I.; Lingwood, D.; Grzybek, M.; Coskun, Ü.; Simons, K. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc. Natl. Acad. Sci. USA 2010, 107, 22050–22054. [Google Scholar] [CrossRef]
- Kaur, I.; Yarov-Yarovoy, V.; Kirk, L.M.; Plambeck, K.E.; Barragan, E.V.; Ontiveros, E.S.; Díaz, E. Activity-dependent palmitoylation controls SynDIG1 stability, localization, and function. J. Neurosci. 2016, 36, 7562–7568. [Google Scholar] [CrossRef]
- Choy, E.; Chiu, V.K.; Silletti, J.; Feoktistov, M.; Morimoto, T.; Michaelson, D.; Ivanov, I.E.; Philips, M.R. Endomembrane trafficking of ras: The CAAX motif targets proteins to the ER and Golgi. Cell 1999, 98, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Blanc, M.; David, F.P.A.; van der Goot, F.G. SwissPalm 2: Protein S-palmitoylation database. In Protein Lipidation: Methods in Molecular Biology; Springer: New York, NY, USA, 2019; pp. 203–214. [Google Scholar]
- Sanders, S.S.; Martin, D.D.O.; Butland, S.L.; Lavallée-Adam, M.; Calzolari, D.; Kay, C.; Iii, J.R.Y.; Hayden, M.R. Curation of the mammalian palmitoylome indicates a pivotal role for palmitoylation in diseases and disorders of the nervous system and cancers. PLoS Comput. Biol. 2015, 11, e1004405. [Google Scholar] [CrossRef] [PubMed]
- Yeste-Velasco, M.; Linder, M.E.; Lu, Y.-J. Protein S-palmitoylation and cancer. Biochim. Biophys. Acta Rev. Cancer 2015, 1856, 107–120. [Google Scholar] [CrossRef]
- Ko, P.; Dixon, S.J. Protein palmitoylation and cancer. EMBO Rep. 2018, 19, e46666. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Xiao, Y.; Meng, J.; Hudmon, A.; Cummins, T.R. Cardiac sodium channel palmitoylation regulates channel availability and myocyte excitability with implications for arrhythmia generation. Nat. Commun. 2016, 7, 12035. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, B.; Yin, C.; Liu, W.; Han, C.; Chen, B.; Liu, T.; Li, X.; Chen, X.; Li, C.; et al. Palmitoylation-dependent activation of MC1R prevents melanomagenesis. Nature 2017, 549, 399–403. [Google Scholar] [CrossRef]
- Fukata, Y.; Fukata, M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat. Rev. Neurosci. 2010, 11, 161–175. [Google Scholar] [CrossRef]
- Fraser, N.J.; Howie, J.; Wypijewski, K.J.; Fuller, W. Therapeutic targeting of protein S-acylation for the treatment of disease. Biochem. Soc. Trans. 2019, 48, 281–290. [Google Scholar] [CrossRef]
- Cho, E.; Park, M. Palmitoylation in Alzheimer’s disease and other neurodegenerative diseases. Pharmacol. Res. 2016, 111, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.T.S.; Conibear, E. ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. eLife 2015, 4, e11306. [Google Scholar] [CrossRef]
- Greaves, J.; Chamberlain, L.H. DHHC palmitoyl transferases: Substrate interactions and (patho)physiology. Trends Biochem. Sci. 2011, 36, 245–253. [Google Scholar] [CrossRef]
- Greaves, J.; Chamberlain, L.H. Differential palmitoylation regulates intracellular patterning of SNAP25. J. Cell Sci. 2011, 124, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Batrouni, A.G.; Bag, N.; Phan, H.T.; Baird, B.A.; Baskin, J.M. A palmitoylation code controls PI4KIIIα complex formation and PI(4,5)P2 homeostasis at the plasma membrane. J. Cell Sci. 2022, 135, jcs259365. [Google Scholar] [CrossRef]
- Chumpen Ramirez, S.; Astrada, M.R.; Daniotti, J.L. PtdIns4P-mediated electrostatic forces influence S-acylation of peripheral proteins at the Golgi complex. Bios. Rep. 2020, 40, BSR20192911. [Google Scholar] [CrossRef] [PubMed]
- Abrami, L.; Audagnotto, M.; Ho, S.; Marcaida, M.J.; Mesquita, F.S.; Anwar, M.U.; Sandoz, P.A.; Fonti, G.; Pojer, F.; Dal Peraro, M.; et al. Palmitoylated acyl protein thioesterase APT2 deforms membranes to extract substrate acyl chains. Nat. Chem. Biol. 2021, 17, 438–447. [Google Scholar] [CrossRef]
- Remsberg, J.R.; Suciu, R.M.; Zambetti, N.A.; Hanigan, T.W.; Firestone, A.J.; Inguva, A.; Long, A.; Ngo, N.; Lum, K.M.; Henry, C.L.; et al. ABHD17 regulation of plasma membrane palmitoylation and N-Ras-dependent cancer growth. Nat. Chem. Biol. 2021, 17, 856–864. [Google Scholar] [CrossRef]
- Yokoi, N.; Fukata, Y.; Sekiya, A.; Murakami, T.; Kobayashi, K.; Fukata, M. Identification of PSD-95 depalmitoylating enzymes. J. Neurosci. 2016, 36, 6431–6444. [Google Scholar] [CrossRef]
- Martin, B.R.; Cravatt, B.F. Large-scale profiling of protein palmitoylation in mammalian Cells. Nat. Methods 2009, 6, 135–138. [Google Scholar] [CrossRef]
- Forrester, M.T.; Hess, D.T.; Thompson, J.W.; Hultman, R.; Moseley, M.A.; Stamler, J.S.; Casey, P.J. Site-specific analysis of protein S-acylation by resin-assisted capture. J. Lipid Res. 2011, 52, 393–398. [Google Scholar] [CrossRef]
- Percher, A.; Ramakrishnan, S.; Thinon, E.; Yuan, X.; Yount, J.S.; Hang, H.C. Mass-tag labeling reveals site-specific and endogenous levels of protein S-fatty acylation. Proc. Natl. Acad. Sci. USA 2016, 113, 4302–4307. [Google Scholar] [CrossRef]
- Devedjiev, Y.; Dauter, Z.; Kuznetsov, S.R.; Jones, T.L.Z.; Derewenda, Z.S. Crystal structure of the human acyl protein thioesterase I from a Single X-Ray Data Set to 1.5 Å. Structure 2000, 8, 1137–1146. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef]
- Papanikou, E.; Glick, B.S. Golgi compartmentation and identity. Curr. Opin. Cell Biol. 2014, 29, 74–81. [Google Scholar] [CrossRef]
- Sorkin, A.; von Zastrow, M. Endocytosis and signalling: Intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 2009, 10, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Chass, G.A.; Lovas, S.; Murphy, R.F.; Csizmadia, I.G. The role of enhanced aromatic π-electron donating aptitude of the tyrosyl sidechain with respect to that of phenylalanyl in intramolecular interactions. Eur. Phys. J. D 2002, 20, 481–497. [Google Scholar] [CrossRef]
- Ohno, H.; Stewart, J.; Fournier, M.-C.; Bosshart, H.; Rhee, I.; Miyatake, S.; Saito, T.; Gallusser, A.; Kirchhausen, T.; Bonifacino, J.S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 1995, 269, 1872–1875. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Traub, L.M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003, 72, 395–447. [Google Scholar] [CrossRef]
- Shahinian, S.; Silvius, J.R. Doubly-lipid-modified protein sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. Biochemistry 1995, 34, 3813–3822. [Google Scholar] [CrossRef]
- Goodwin, J.S.; Drake, K.R.; Rogers, C.; Wright, L.; Lippincott-Schwartz, J.; Philips, M.R.; Kenworthy, A.K. Depalmitoylated ras traffics to and from the Golgi complex via a nonvesicular pathway. J. Cell Biol. 2005, 170, 261–272. [Google Scholar] [CrossRef]
- Rocks, O.; Peyker, A.; Kahms, M.; Verveer, P.J.; Koerner, C.; Lumbierres, M.; Kuhlmann, J.; Waldmann, H.; Wittinghofer, A.; Bastiaens, P.I.H. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 2005, 307, 1746–1752. [Google Scholar] [CrossRef]
- Fukata, M.; Fukata, Y.; Adesnik, H.; Nicoll, R.A.; Bredt, D.S. Identification of PSD-95 palmitoylating enzymes. Neuron 2004, 44, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Greaves, J.; Gorleku, O.A.; Salaun, C.; Chamberlain, L.H. Palmitoylation of the SNAP25 protein family: Specificity and regulation by DHHC palmitoyl transferases. J. Biol. Chem. 2010, 285, 24629–24638. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Fukata, Y.; Noritake, J.; Iwanaga, T.; Perez, F.; Fukata, M. Identification of G protein α subunit-palmitoylating enzyme. Mol. Cell. Biol. 2009, 29, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.F.; Paterson, H.; Marshall, C.J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize P21Ras to the plasma membrane. Cell 1990, 63, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Dell’Angelica, E.C. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 1999, 145, 923–926. [Google Scholar] [CrossRef]
- Verkruyse, L.A.; Hofmann, S.L. Lysosomal targeting of palmitoyl-protein thioesterase. J. Biol. Chem. 1996, 271, 15831–15836. [Google Scholar] [CrossRef]
- Koster, K.P.; Yoshii, A. Depalmitoylation by palmitoyl-protein thioesterase 1 in Neuronal Health and Degeneration. Front. Synaptic Neurosci. 2019, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.L.; Martin, N.R.; Niphakis, M.J.; Cravatt, B.F.; Fairn, G.D. Attenuating ABHD17 isoforms augments the S-acylation and function of NOD2 and a subset of Crohn’s disease-associated NOD2 variants. Cell. Mol. Gastroenterol. Hepatol. 2025, 19, 101491. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold protein structure database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Hebditch, M.; Warwicker, J. Web-based display of protein surface and pH-dependent properties for assessing the developability of Biotherapeutics. Sci. Rep. 2019, 9, 1969. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-I.; Yeon, J.-H.; Suh, B.-C. Palmitoylation Code and Endosomal Sorting Regulate ABHD17A Plasma Membrane Targeting and Activity. Int. J. Mol. Sci. 2025, 26, 10190. https://doi.org/10.3390/ijms262010190
Kim B-I, Yeon J-H, Suh B-C. Palmitoylation Code and Endosomal Sorting Regulate ABHD17A Plasma Membrane Targeting and Activity. International Journal of Molecular Sciences. 2025; 26(20):10190. https://doi.org/10.3390/ijms262010190
Chicago/Turabian StyleKim, Byeol-I, Jun-Hee Yeon, and Byung-Chang Suh. 2025. "Palmitoylation Code and Endosomal Sorting Regulate ABHD17A Plasma Membrane Targeting and Activity" International Journal of Molecular Sciences 26, no. 20: 10190. https://doi.org/10.3390/ijms262010190
APA StyleKim, B.-I., Yeon, J.-H., & Suh, B.-C. (2025). Palmitoylation Code and Endosomal Sorting Regulate ABHD17A Plasma Membrane Targeting and Activity. International Journal of Molecular Sciences, 26(20), 10190. https://doi.org/10.3390/ijms262010190






