Inhibition of Casein Kinase 1δ as a Novel Therapeutic Strategy for Amyotrophic Lateral Sclerosis: A Theoretical Study
Abstract
1. Introduction
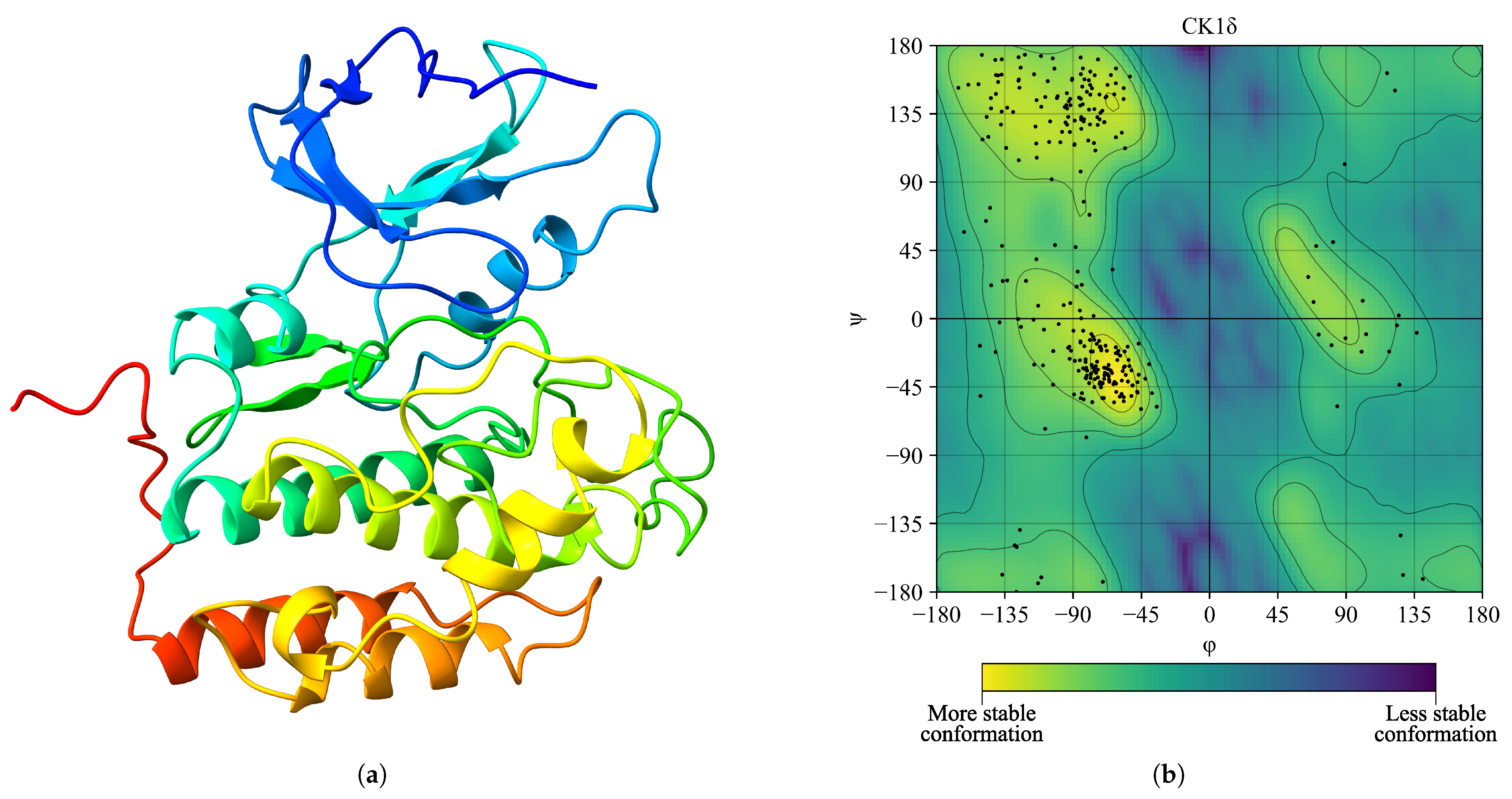
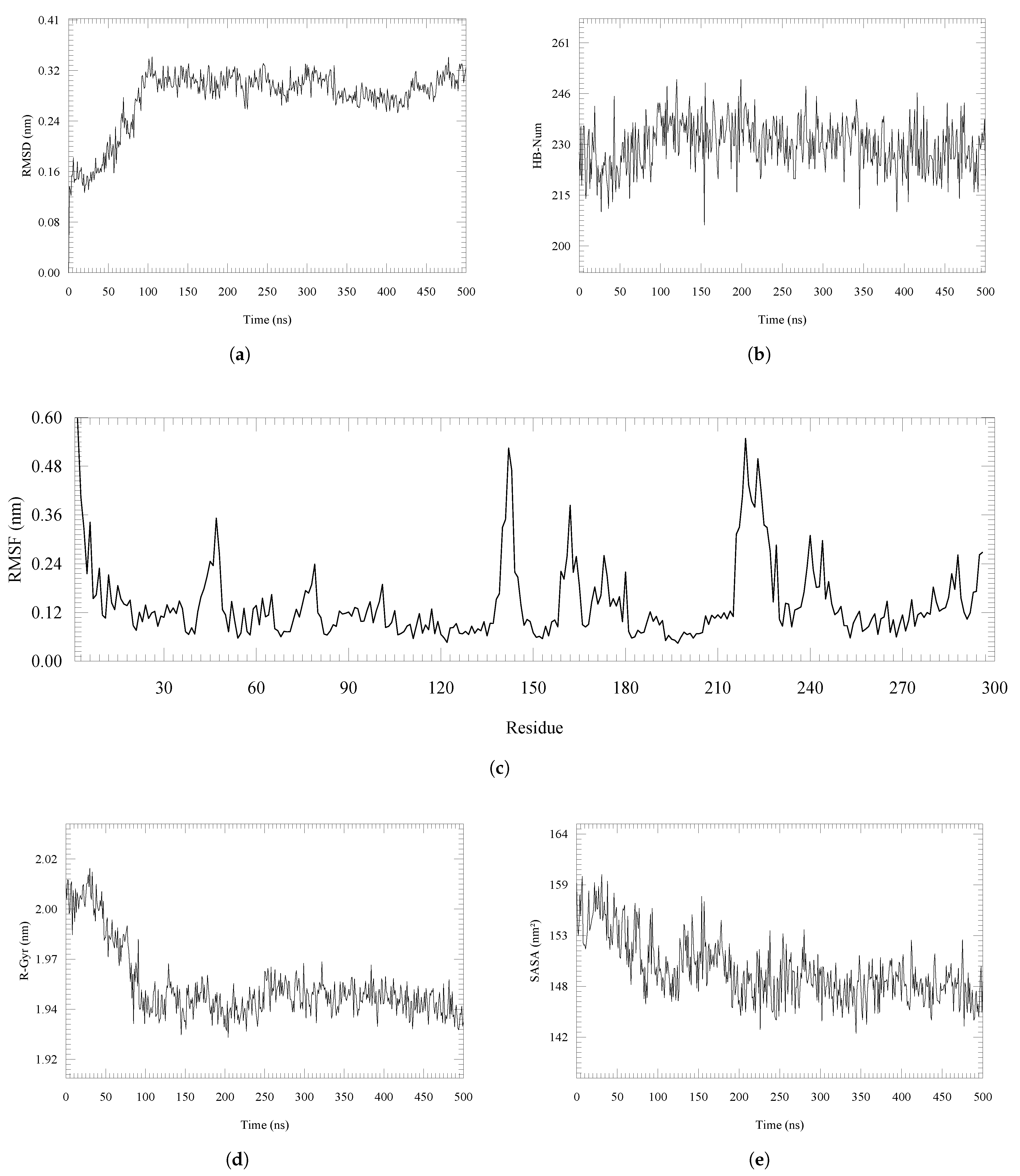
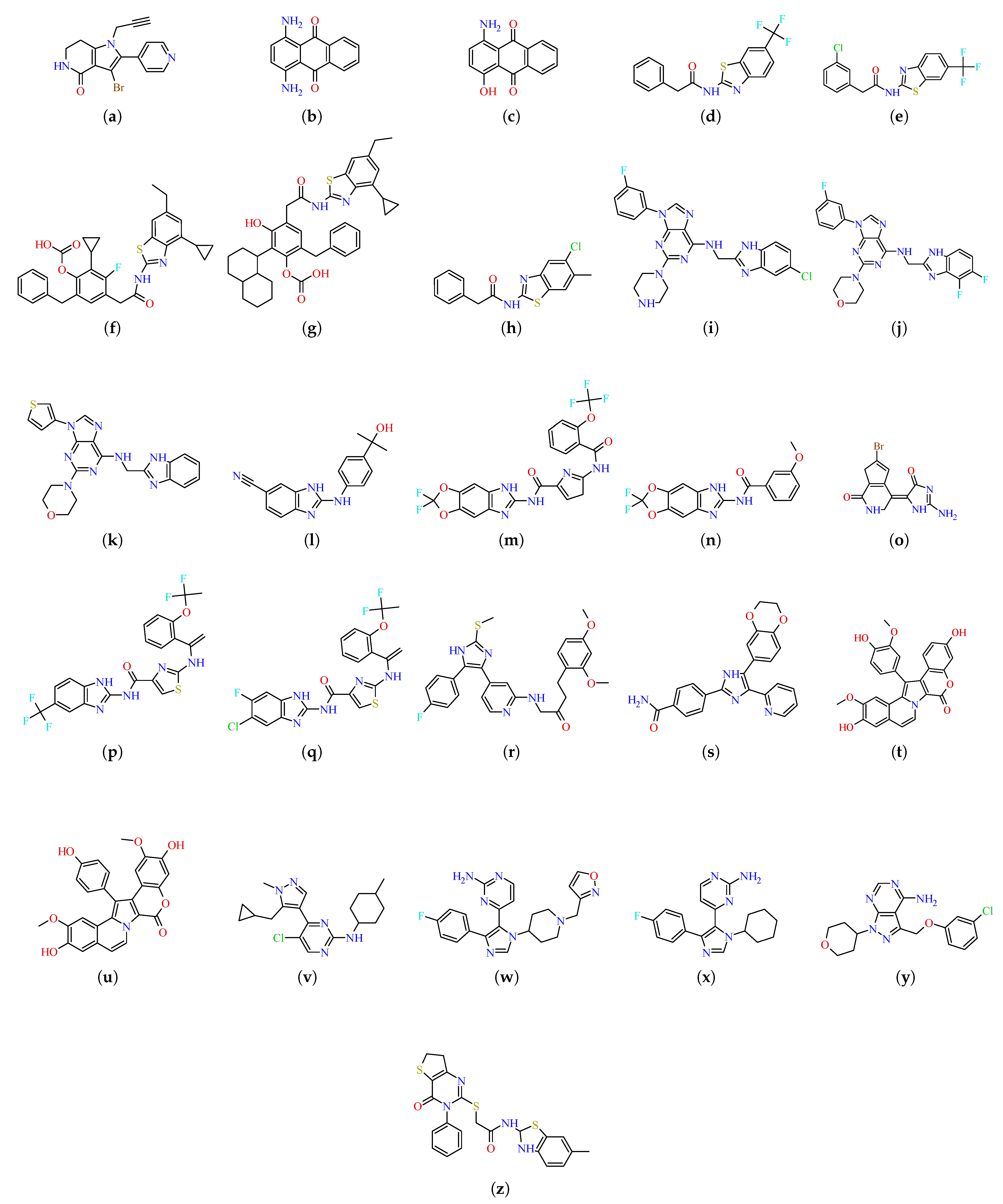
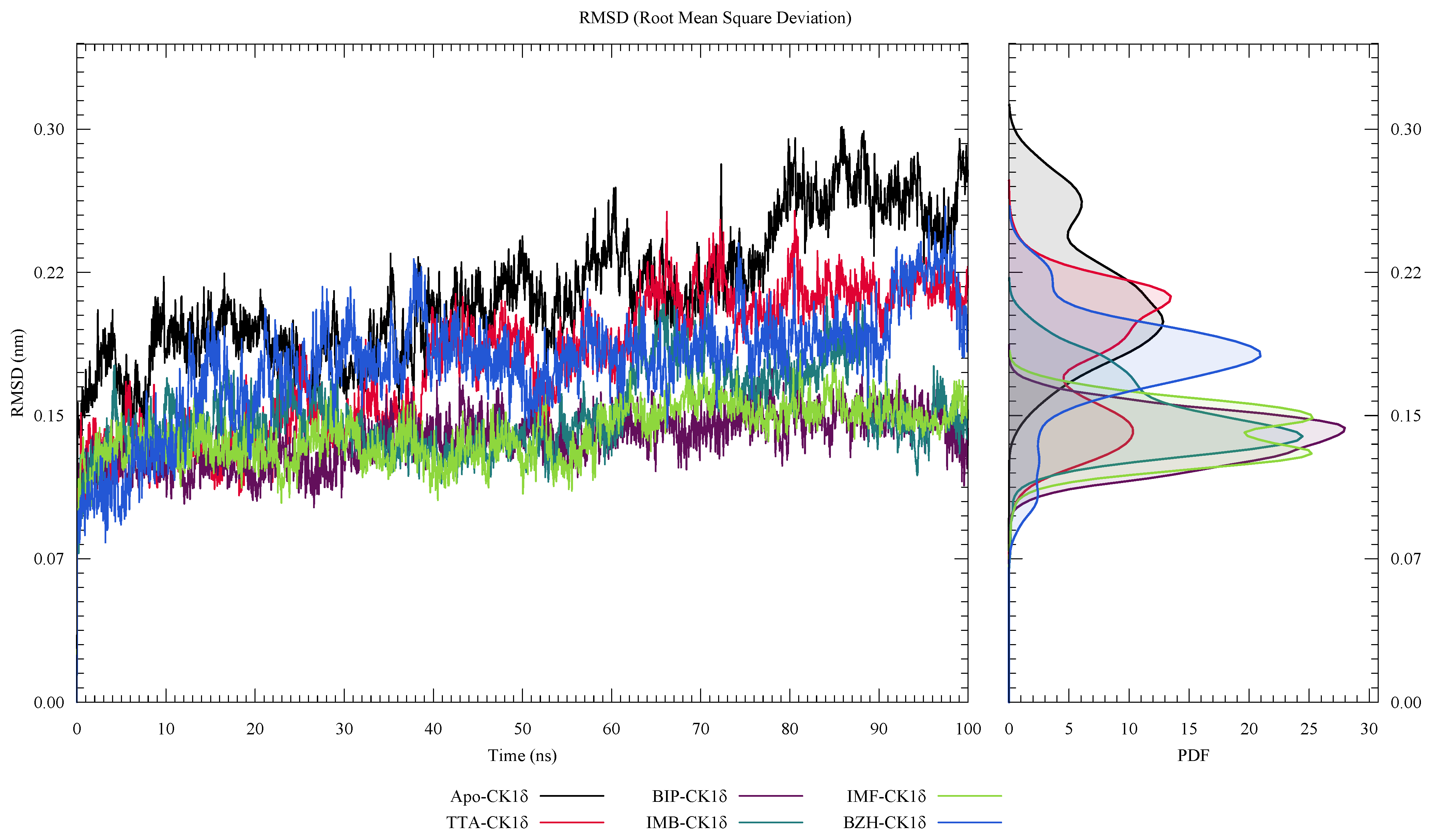
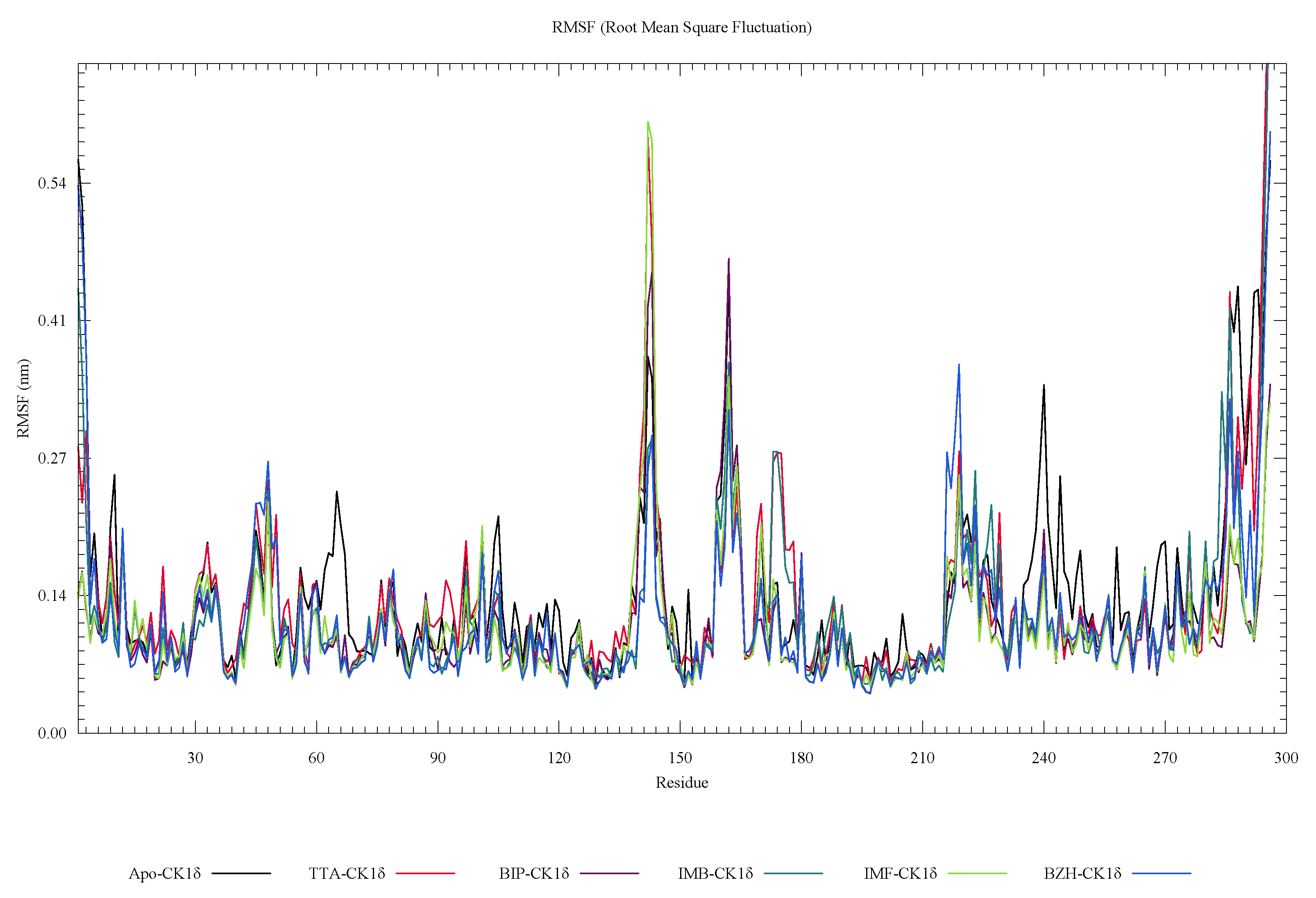
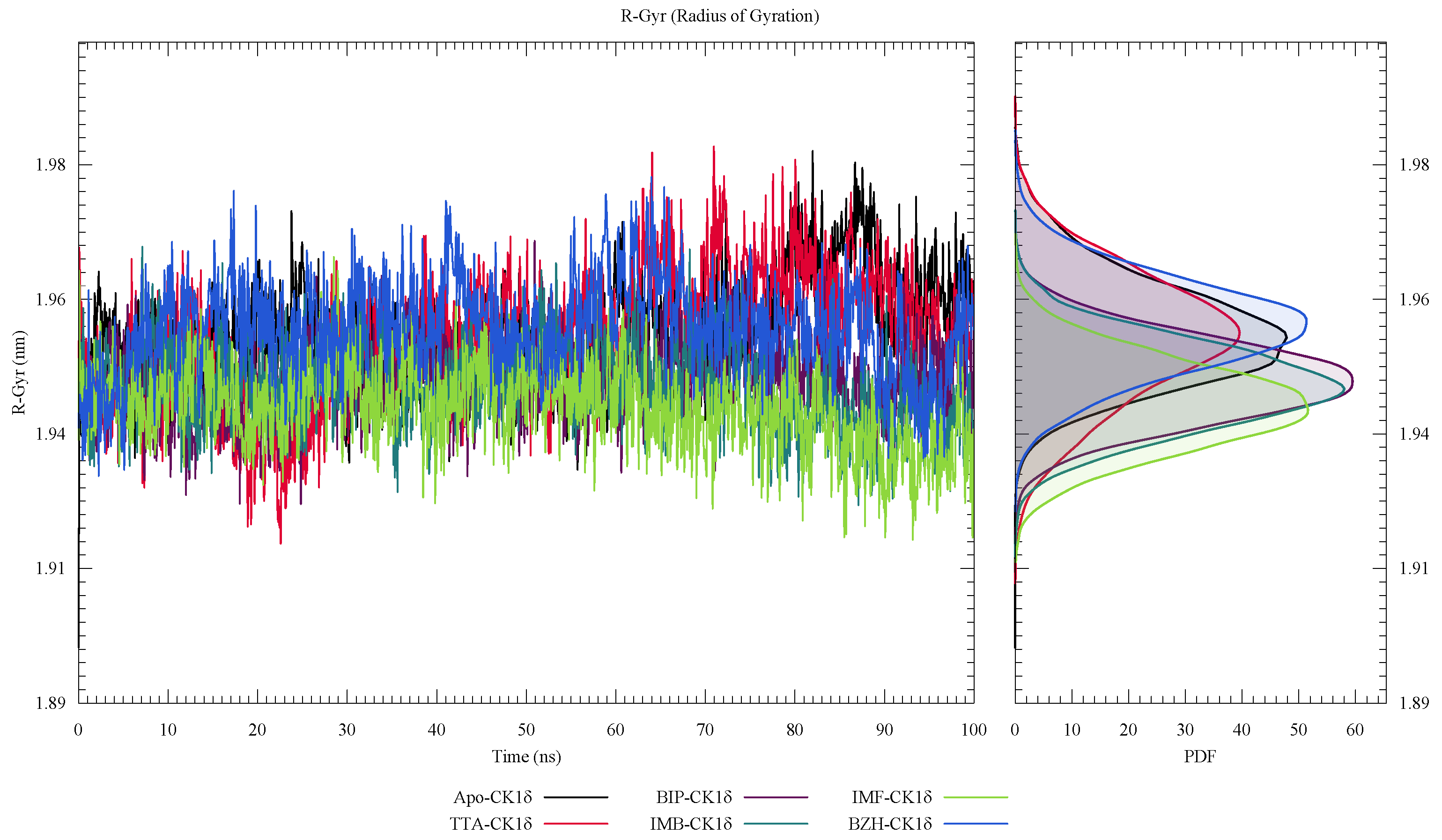
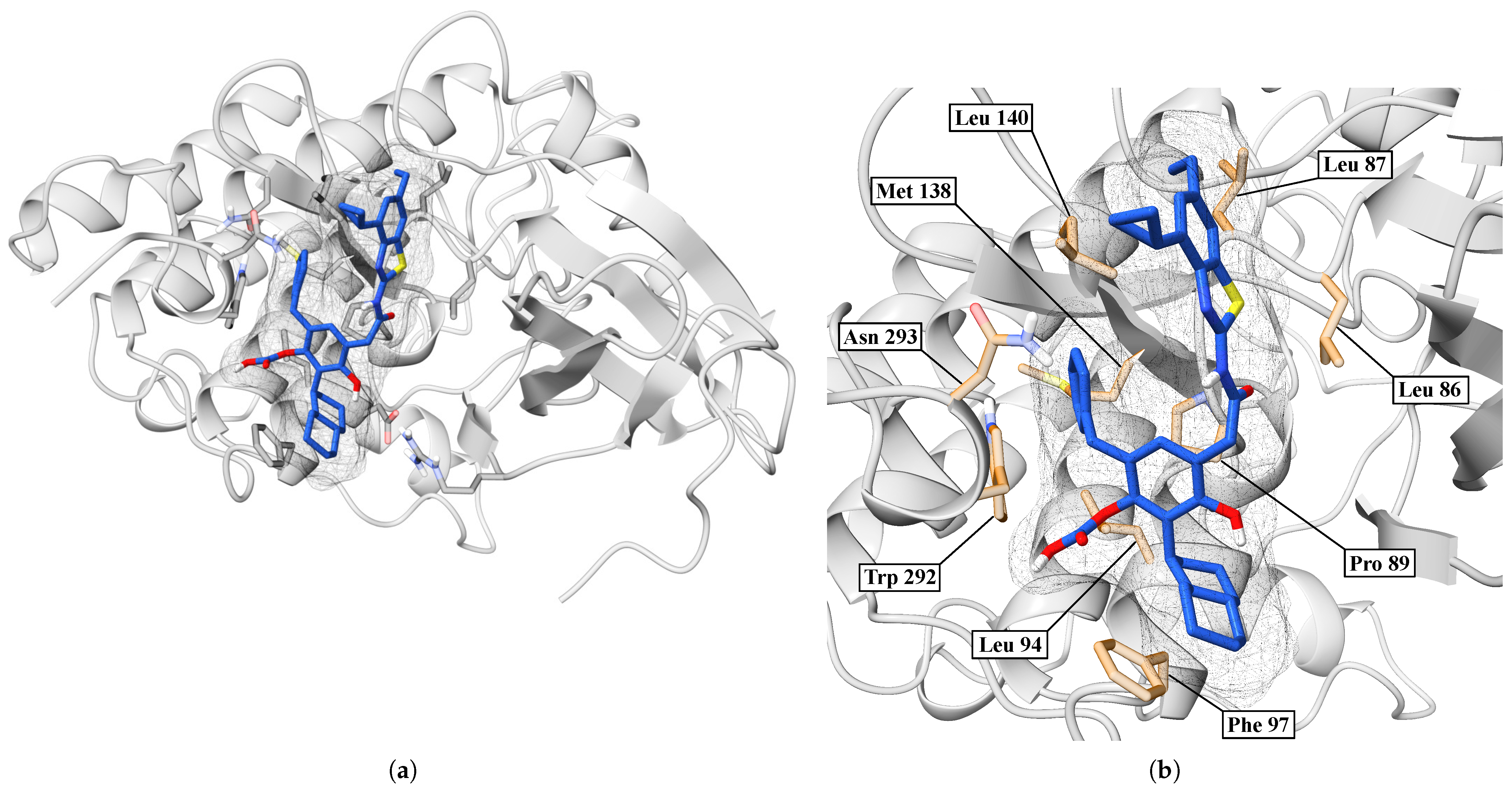
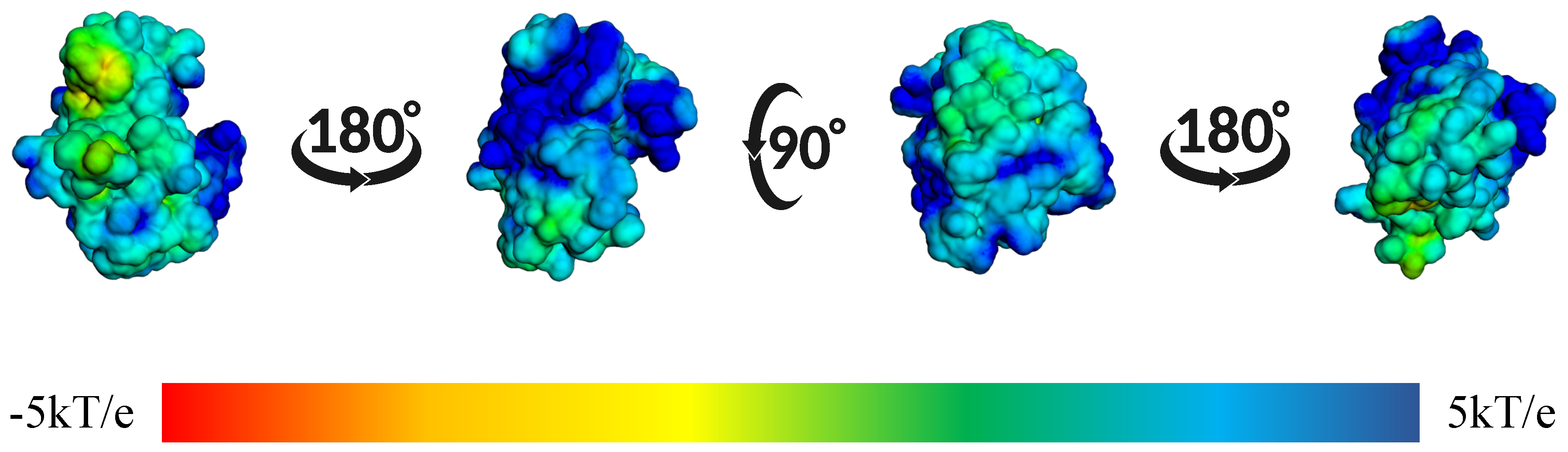
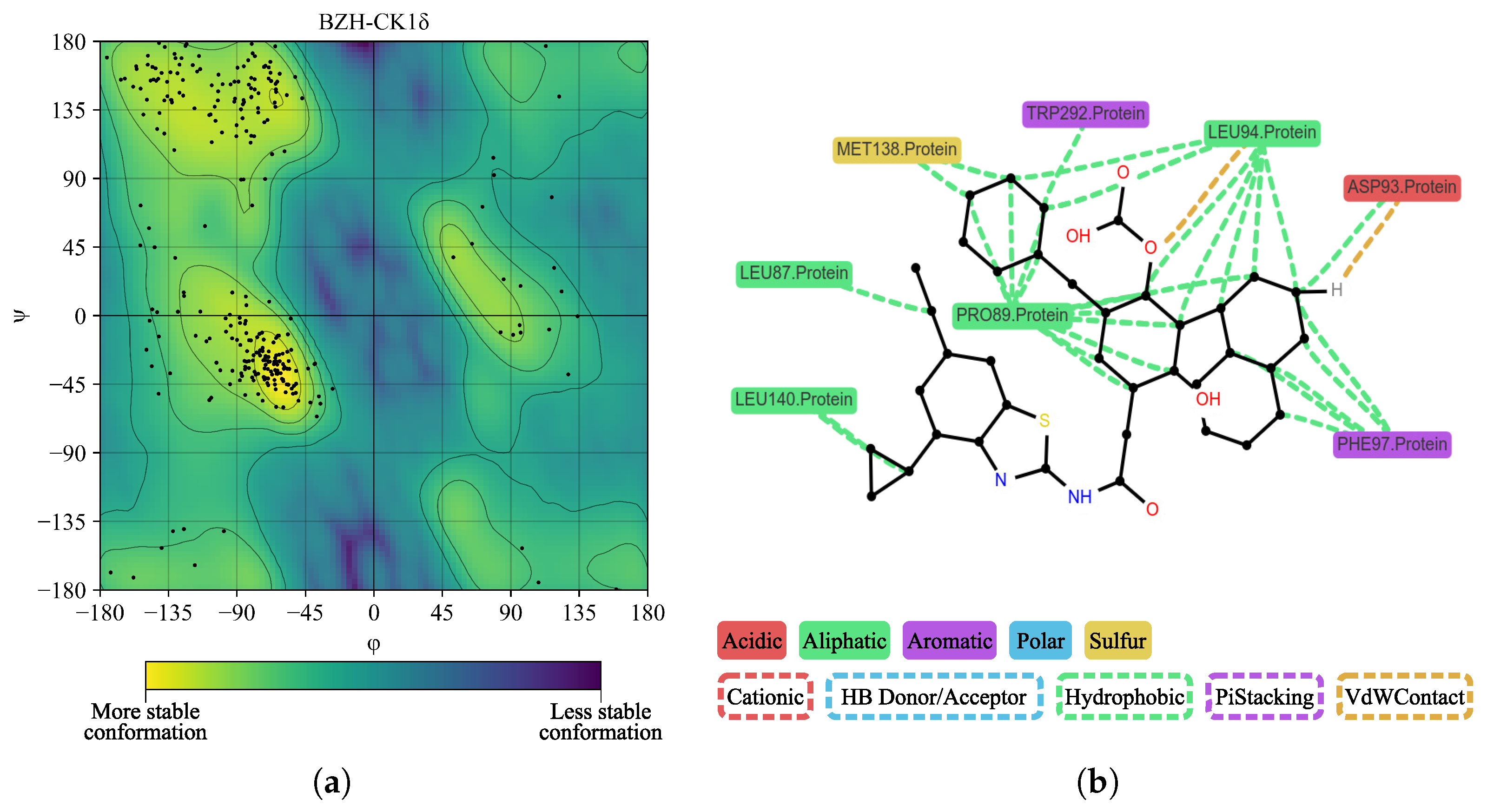
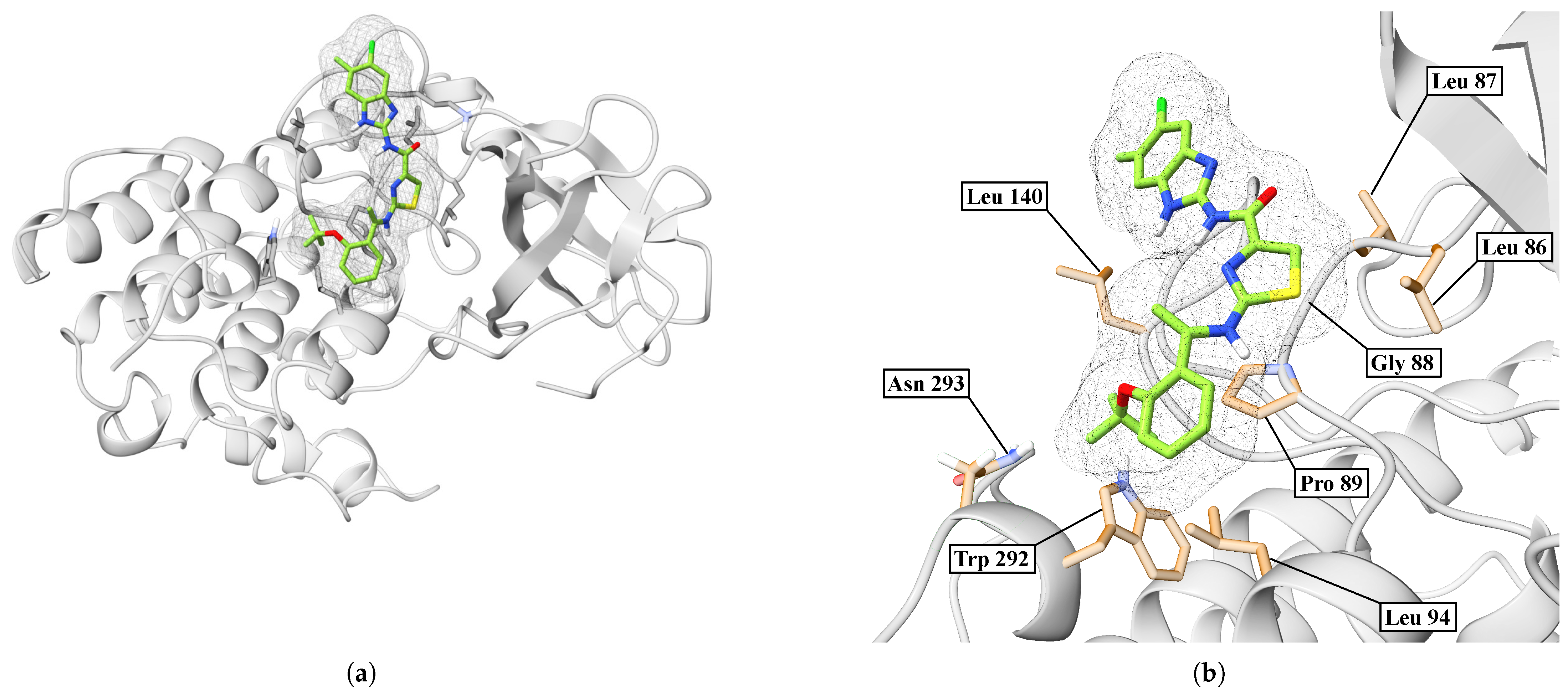
2. Results
3. Computational Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Schependom, J.; D’haeseleer, M. Advances in Neurodegenerative Diseases. J. Clin. Med. 2023, 12, 1709. [Google Scholar] [CrossRef]
- Ryan, M.; Heverin, M.; McLaughlin, R.L.; Hardiman, O. Lifetime Risk and Heritability of Amyotrophic Lateral Sclerosis. JAMA Neurol. 2019, 76, 1367. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; van den Berg, L.H.; Veldink, J. Gene discovery in amyotrophic lateral sclerosis: Implications for clinical management. Nat. Rev. Neurol. 2016, 13, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shen, D.; Gao, Y.; Zhou, Q.; Ni, Y.; Meng, H.; Shi, H.; Le, W.; Chen, S.; Chen, S. A perspective on therapies for amyotrophic lateral sclerosis: Can disease progression be curbed? Transl. Neurodegener. 2021, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Kaye, W.; Bryan, L.; Larson, T.; Copeland, T.; Wu, J.; Muravov, O.; Horton, K. Prevalence of Amyotrophic Lateral Sclerosis - United States, 2012–2013. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2016, 65, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sayana, P.; Zhang, X.; Le, W. Genetics of amyotrophic lateral sclerosis: An update. Mol. Neurodegener. 2013, 8, 28. [Google Scholar] [CrossRef]
- Ravits, J.M.; La Spada, A.R. ALS motor phenotype heterogeneity, focality, and spread: Deconstructing motor neuron degeneration. Neurology 2009, 73, 805–811. [Google Scholar] [CrossRef]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Marin, B.; Boumédiene, F.; Logroscino, G.; Couratier, P.; Babron, M.C.; Leutenegger, A.L.; Copetti, M.; Preux, P.M.; Beghi, E. Variation in worldwide incidence of amyotrophic lateral sclerosis: A meta-analysis. Int. J. Epidemiol. 2016, 46, 57–74. [Google Scholar] [CrossRef]
- Hoxhaj, P.; Hastings, N.; Kachhadia, M.P.; Gupta, R.; Sindhu, U.; Durve, S.A.; Azam, A.; Auz Vinueza, M.J.; Bhuvan; Win, S.H.; et al. Exploring Advancements in the Treatment of Amyotrophic Lateral Sclerosis: A Comprehensive Review of Current Modalities and Future Prospects. Cureus 2023, 15, e45489. [Google Scholar] [CrossRef]
- Czaplinski, A.; Yen, A.A.; Simpson, E.P.; Appel, S.H. Slower Disease Progression and Prolonged Survival in Contemporary Patients With Amyotrophic Lateral Sclerosis: Is the Natural History of Amyotrophic Lateral Sclerosis Changing? Arch. Neurol. 2006, 63, 1139. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.G.; Moreno-Escobar, M.C.; Joseph, J.; Munakomi, S.; Pawar, G. Amyotrophic Lateral Sclerosis; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Garcia-Montojo, M.; Fathi, S.; Rastegar, C.; Simula, E.R.; Doucet-O’Hare, T.; Cheng, Y.H.H.; Abrams, R.P.M.; Pasternack, N.; Malik, N.; Bachani, M.; et al. TDP-43 proteinopathy in ALS is triggered by loss of ASRGL1 and associated with HML-2 expression. Nat. Commun. 2024, 15, 4163. [Google Scholar] [CrossRef] [PubMed]
- Droppelmann, C.A.; Campos-Melo, D.; Noches, V.; McLellan, C.; Szabla, R.; Lyons, T.A.; Amzil, H.; Withers, B.; Kaplanis, B.; Sonkar, K.S.; et al. Mitigation of TDP-43 toxic phenotype by an RGNEF fragment in amyotrophic lateral sclerosis models. Brain 2024, 147, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Oiwa, K.; Watanabe, S.; Onodera, K.; Iguchi, Y.; Kinoshita, Y.; Komine, O.; Sobue, A.; Okada, Y.; Katsuno, M.; Yamanaka, K. Monomerization of TDP-43 is a key determinant for inducing TDP-43 pathology in amyotrophic lateral sclerosis. Sci. Adv. 2023, 9, eadf6895. [Google Scholar] [CrossRef]
- Jo, M.; Lee, S.; Jeon, Y.M.; Kim, S.; Kwon, Y.; Kim, H.J. The role of TDP-43 propagation in neurodegenerative diseases: Integrating insights from clinical and experimental studies. Exp. Mol. Med. 2020, 52, 1652–1662. [Google Scholar] [CrossRef]
- Wu, C.C.; Jin, L.W.; Wang, I.F.; Wei, W.Y.; Ho, P.C.; Liu, Y.C.; Tsai, K.J. HDAC1 dysregulation induces aberrant cell cycle and DNA damage in progress of TDP-43 proteinopathies. EMBO Mol. Med. 2020, 12, e10622. [Google Scholar] [CrossRef]
- Ko, V.I.; Ong, K.; Cleveland, D.W.; Yu, H.; Ravits, J.M. CK1δ/ε kinases regulate TDP-43 phosphorylation and are therapeutic targets for ALS-related TDP-43 hyperphosphorylation. Neurobiol. Dis. 2024, 196, 106516. [Google Scholar] [CrossRef]
- Choksi, D.K.; Roy, B.; Chatterjee, S.; Yusuff, T.; Bakhoum, M.F.; Sengupta, U.; Ambegaokar, S.; Kayed, R.; Jackson, G.R. TDP-43 Phosphorylation by casein kinase Iε promotes oligomerization and enhances toxicity in vivo. Hum. Mol. Genet. 2013, 23, 1025–1035. [Google Scholar] [CrossRef]
- Hicks, D.A.; Cross, L.L.; Williamson, R.; Rattray, M. Endoplasmic Reticulum Stress Signalling Induces Casein Kinase 1-Dependent Formation of Cytosolic TDP-43 Inclusions in Motor Neuron-Like Cells. Neurochem. Res. 2019, 45, 1354–1364. [Google Scholar] [CrossRef]
- Liachko, N.F.; Guthrie, C.R.; Kraemer, B.C. Phosphorylation Promotes Neurotoxicity in aCaenorhabditis elegansModel of TDP-43 Proteinopathy. J. Neurosci. 2010, 30, 16208–16219. [Google Scholar] [CrossRef]
- Martínez-González, L.; Rodríguez-Cueto, C.; Cabezudo, D.; Bartolomé, F.; Andrés-Benito, P.; Ferrer, I.; Gil, C.; Martín-Requero, Á.; Fernández-Ruiz, J.; Martínez, A.; et al. Motor neuron preservation and decrease of in vivo TDP-43 phosphorylation by protein CK-1δ kinase inhibitor treatment. Sci. Rep. 2020, 10, 4449. [Google Scholar] [CrossRef] [PubMed]
- Akharume, F.; Adedeji, A. Molecular dynamic (in silico) modeling of structure-function of glutelin type-B 5-like from proso millet storage protein: Effects of temperature and pressure. J. Food Sci. Technol. 2022, 60, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Chaikuad, A.; Arrowsmith, C.; Edwards, A.; Bountra, C.; Roush, W.; Knapp, S. Crystal Structure of Casein Kinase 1 Delta (CK1 Delta) Complexed with SR3029 Inhibitor: 6rcg. 2019. Available online: https://www.rcsb.org/structure/6RCG (accessed on 15 October 2025).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Hu, Q.; Gao, S.; Ma, X.; Zhang, W.; Shen, Y.; Chen, F.; Lai, L.; Pei, J. CavityPlus: A web server for protein cavity detection with pharmacophore modelling, allosteric site identification and covalent ligand binding ability prediction. Nucleic Acids Res. 2018, 46, W374–W379. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, C.; Wang, J.; Bo, Z.; Zhu, Z.; Zheng, H. Uni-Dock: GPU-Accelerated Docking Enables Ultralarge Virtual Screening. J. Chem. Theory Comput. 2023, 19, 3336–3345. [Google Scholar] [CrossRef]
- Gohlke, H.; Klebe, G. Approaches to the description and prediction of the binding affinity of small-molecule ligands to macromolecular receptors. Angew. Chem. (Int. Ed. Engl.) 2002, 41, 2644–2676. [Google Scholar] [CrossRef]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the Performance of the MM/PBSA and MM/GBSA Methods. 1. The Accuracy of Binding Free Energy Calculations Based on Molecular Dynamics Simulations. J. Chem. Inf. Model. 2010, 51, 69–82. [Google Scholar] [CrossRef]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating Structures and Free Energies of Complex Molecules: Combining Molecular Mechanics and Continuum Models. Accounts Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef]
- Knippschild, U.; Krüger, M.; Richter, J.; Xu, P.; Garcà a-Reyes, B.; Peifer, C.; Halekotte, J.; Bakulev, V.; Bischof, J. The CK1 Family: Contribution to Cellular Stress Response and Its Role in Carcinogenesis. Front. Oncol. 2014, 4, 96. [Google Scholar] [CrossRef]
- Long, A.; Zhao, H.; Huang, X. Structural Basis for the Interaction between Casein Kinase 1 Delta and a Potent and Selective Inhibitor. J. Med. Chem. 2012, 55, 956–960. [Google Scholar] [CrossRef]
- Córdova-Bahena, L.; Sánchez-Álvarez, A.A.; Ruiz-Moreno, A.J.; Velasco-Velázquez, M.A. Repositioning of Etravirine as a Potential CK1ε Inhibitor by Virtual Screening. Pharmaceuticals 2021, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Sunkari, Y.K.; Meijer, L.; Flajolet, M. The protein kinase CK1: Inhibition, activation, and possible allosteric modulation. Front. Mol. Biosci. 2022, 9, 916232. [Google Scholar] [CrossRef]
- Fu, L.; Shi, S.; Yi, J.; Wang, N.; He, Y.; Wu, Z.; Peng, J.; Deng, Y.; Wang, W.; Wu, C.; et al. ADMETlab 3.0: An updated comprehensive online ADMET prediction platform enhanced with broader coverage, improved performance, API functionality and decision support. Nucleic Acids Res. 2024, 52, W422–W431. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Walton, K.M.; Fisher, K.; Rubitski, D.; Marconi, M.; Meng, Q.J.; Sládek, M.; Adams, J.; Bass, M.; Chandrasekaran, R.; Butler, T.; et al. Selective Inhibition of Casein Kinase 1ε Minimally Alters Circadian Clock Period. J. Pharmacol. Exp. Ther. 2009, 330, 430–439. [Google Scholar] [CrossRef]
- Joshi, K.; Goyal, S.; Grover, S.; Jamal, S.; Singh, A.; Dhar, P.; Grover, A. Novel group-based QSAR and combinatorial design of CK-1δ inhibitors as neuroprotective agents. BMC Bioinform. 2016, 17, 515. [Google Scholar] [CrossRef]
- Bischof, J.; Leban, J.; Zaja, M.; Grothey, A.; Radunsky, B.; Othersen, O.; Strobl, S.; Vitt, D.; Knippschild, U. 2-Benzamido-N-(1H-benzo[d]imidazol-2-yl)thiazole-4-carboxamide derivatives as potent inhibitors of CK1δ/ε. Amino Acids 2012, 43, 1577–1591. [Google Scholar] [CrossRef]
- Halekotte, J.; Witt, L.; Ianes, C.; Krüger, M.; Bührmann, M.; Rauh, D.; Pichlo, C.; Brunstein, E.; Luxenburger, A.; Baumann, U.; et al. Optimized 4,5-Diarylimidazoles as Potent/Selective Inhibitors of Protein Kinase CK1δ and Their Structural Relation to p38α MAPK. Molecules 2017, 22, 522. [Google Scholar] [CrossRef]
- García-Reyes, B.; Witt, L.; Jansen, B.; Karasu, E.; Gehring, T.; Leban, J.; Henne-Bruns, D.; Pichlo, C.; Brunstein, E.; Baumann, U.; et al. Discovery of Inhibitor of Wnt Production 2 (IWP-2) and Related Compounds As Selective ATP-Competitive Inhibitors of Casein Kinase 1 (CK1) δ/ε. J. Med. Chem. 2018, 61, 4087–4102. [Google Scholar] [CrossRef]
- Bibian, M.; Rahaim, R.J.; Choi, J.Y.; Noguchi, Y.; Schürer, S.; Chen, W.; Nakanishi, S.; Licht, K.; Rosenberg, L.H.; Li, L.; et al. Development of highly selective casein kinase 1δ/1ε (CK1δ/ε) inhibitors with potent antiproliferative properties. Bioorg. Med. Chem. Lett. 2013, 23, 4374–4380. [Google Scholar] [CrossRef]
- Rena, G.; Bain, J.; Elliott, M.; Cohen, P. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 2004, 5, 60–65. [Google Scholar] [CrossRef]
- Liu, G.; Li, H.; Zhang, W.; Yu, J.; Zhang, X.; Wu, R.; Niu, M.; Liu, X.; Yu, R. Csnk1a1 inhibition modulates the inflammatory secretome and enhances response to radiotherapy in glioma. J. Cell. Mol. Med. 2021, 25, 7395–7406, Erratum in J. Cell. Mol. Med. 2022, 26, 2754–2755. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Song, J.; Wang, Z.; Zhou, L.; Xia, Y.; Yu, S.; Sun, Q.; Liu, S.S.; Zhao, L.; Li, S.; et al. Tumor promoter TPA activates Wnt/β-catenin signaling in a casein kinase 1-dependent manner. Proc. Natl. Acad. Sci. USA 2018, 115, E7522–E7531. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.J.; Stein, A.S.; Borthakur, G.; Murray, C.; Kook, K.; Chan, K.W.; Stein, E.M. Trial in Progress: A Phase I Trial of BTX-A51 in Patients with Relapsed or Refractory AML or High-Risk MDS. Blood 2020, 136, 18–19. [Google Scholar] [CrossRef]
- Cunningham, P.S.; Ahern, S.A.; Smith, L.C.; da Silva Santos, C.S.; Wager, T.T.; Bechtold, D.A. Targeting of the circadian clock via CK1δ/ε to improve glucose homeostasis in obesity. Sci. Rep. 2016, 6, 29983. [Google Scholar] [CrossRef]
- Wager, T.T.; Chandrasekaran, R.Y.; Bradley, J.; Rubitski, D.; Berke, H.; Mente, S.; Butler, T.; Doran, A.; Chang, C.; Fisher, K.; et al. Casein Kinase 1δ/ε Inhibitor PF-5006739 Attenuates Opioid Drug-Seeking Behavior. ACS Chem. Neurosci. 2014, 5, 1253–1265. [Google Scholar] [CrossRef]
- Baunbæk, D.; Trinkler, N.; Ferandin, Y.; Lozach, O.; Ploypradith, P.; Rucirawat, S.; Ishibashi, F.; Iwao, M.; Meijer, L. Anticancer Alkaloid Lamellarins Inhibit Protein Kinases. Mar. Drugs 2008, 6, 514–527. [Google Scholar] [CrossRef]
- Monastyrskyi, A.; Nilchan, N.; Quereda, V.; Noguchi, Y.; Ruiz, C.; Grant, W.; Cameron, M.; Duckett, D.; Roush, W. Development of dual casein kinase 1δ/1ε (CK1δ/ε) inhibitors for treatment of breast cancer. Bioorg. Med. Chem. 2018, 26, 590–602. [Google Scholar] [CrossRef]
- Hua, Z.; Huang, X.; Bregman, H.; Chakka, N.; DiMauro, E.F.; Doherty, E.M.; Goldstein, J.; Gunaydin, H.; Huang, H.; Mercede, S.; et al. 2-Phenylamino-6-cyano-1H-benzimidazole-based isoform selective casein kinase 1 gamma (CK1γ) inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 5392–5395. [Google Scholar] [CrossRef]
- Meng, Q.J.; Maywood, E.S.; Bechtold, D.A.; Lu, W.Q.; Li, J.; Gibbs, J.E.; Dupré, S.M.; Chesham, J.E.; Rajamohan, F.; Knafels, J.; et al. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc. Natl. Acad. Sci. USA 2010, 107, 15240–15245. [Google Scholar] [CrossRef]
- Adler, P.; Mayne, J.; Walker, K.; Ning, Z.; Figeys, D. Therapeutic Targeting of Casein Kinase 1δ/ε in an Alzheimer’s Disease Mouse Model. J. Proteome Res. 2019, 18, 3383–3393. [Google Scholar] [CrossRef] [PubMed]
- Badura, L.; Swanson, T.; Adamowicz, W.; Adams, J.; Cianfrogna, J.; Fisher, K.; Holland, J.; Kleiman, R.; Nelson, F.; Reynolds, L.; et al. An Inhibitor of Casein Kinase Iε Induces Phase Delays in Circadian Rhythms under Free-Running and Entrained Conditions. J. Pharmacol. Exp. Ther. 2007, 322, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.K.; Hung, N.T.; Wang, H.; Tan, P.; Voorhoeve, P.M.; Lee, S.H.; Virshup, D.M. IC261 induces cell cycle arrest and apoptosis of human cancer cells via CK1δ/ε and Wnt/β-catenin independent inhibition of mitotic spindle formation. Oncogene 2011, 30, 2558–2569. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Nagaraj, S.; Mahoney, H.; Portugues, A.; Li, W.; Millsaps, K.; Faulkner, J.; Yunus, A.; Burns, C.; Bloom, C.; et al. Inhibition of casein kinase 1δ/εimproves cognitive-affective behavior and reduces amyloid load in the APP-PS1 mouse model of Alzheimer’s disease. Sci. Rep. 2019, 9, 13743. [Google Scholar] [CrossRef]
- Saito, A.N.; Matsuo, H.; Kuwata, K.; Ono, A.; Kinoshita, T.; Yamaguchi, J.; Nakamichi, N. Structure-function study of a novel inhibitor of the casein kinase 1 family inArabidopsis thaliana. Plant Direct 2019, 3, e00172. [Google Scholar] [CrossRef]
- Alquezar, C.; Salado, I.G.; de la Encarnación, A.; Pérez, D.I.; Moreno, F.; Gil, C.; de Munain, A.L.; Martínez, A.; Martín-Requero, Á. Targeting TDP-43 phosphorylation by Casein Kinase-1δ inhibitors: A novel strategy for the treatment of frontotemporal dementia. Mol. Neurodegener. 2016, 11, 36. [Google Scholar] [CrossRef]
- Morales-Garcia, J.A.; Salado, I.G.; Sanz-San Cristobal, M.; Gil, C.; Pérez-Castillo, A.; Martínez, A.; Pérez, D.I. Biological and Pharmacological Characterization of Benzothiazole-Based CK-1δ Inhibitors in Models of Parkinson’s Disease. ACS Omega 2017, 2, 5215–5220. [Google Scholar] [CrossRef]
- Posa, D.; Martínez-González, L.; Bartolomé, F.; Nagaraj, S.; Porras, G.; Martínez, A.; Martín-Requero, Á. Recapitulation of Pathological TDP-43 Features in Immortalized Lymphocytes from Sporadic ALS Patients. Mol. Neurobiol. 2018, 56, 2424–2432. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, L.; Cuevas, E.P.; Tosat-Bitrián, C.; Nozal, V.; Gil, C.; Palomo, V.; Martín-Requero, Á.; Martinez, A. TTBK1 and CK1 inhibitors restore TDP-43 pathology and avoid disease propagation in lymphoblast from Alzheimer’s disease patients. Front. Mol. Neurosci. 2023, 16, 1243277. [Google Scholar] [CrossRef]
- Lee, J.W.; Hirota, T.; Peters, E.C.; Garcia, M.; Gonzalez, R.; Cho, C.Y.; Wu, X.; Schultz, P.G.; Kay, S.A. A Small Molecule Modulates Circadian Rhythms through Phosphorylation of the Period Protein. Angew. Chem. Int. Ed. 2011, 50, 10608–10611. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Bischof, J.; Zaja, M.; Kohlhof, H.; Othersen, O.; Vitt, D.; Alscher, V.; Pospiech, I.; García-Reyes, B.; Berg, S.; et al. Difluoro-dioxolo-benzoimidazol-benzamides As Potent Inhibitors of CK1δ and ε with Nanomolar Inhibitory Activity on Cancer Cell Proliferation. J. Med. Chem. 2014, 57, 7933–7946. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.; Thunnissen, A.M.; White, A.; Garnier, M.; Nikolic, M.; Tsai, L.H.; Walter, J.; Cleverley, K.; Salinas, P.; Wu, Y.Z.; et al. Inhibition of cyclin-dependent kinases, GSK-3β and CK1 by hymenialdisine, a marine sponge constituent. Chem. Biol. 2000, 7, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Plisson, F.; Prasad, P.; Xiao, X.; Piggott, A.M.; Huang, X.c.; Khalil, Z.; Capon, R.J. Callyspongisines A-D: Bromopyrrole alkaloids from an Australian marine sponge, Callyspongia sp. Org. Biomol. Chem. 2014, 12, 1579–1584. [Google Scholar] [CrossRef]
- Wan, Y.; Hur, W.; Cho, C.Y.; Liu, Y.; Adrian, F.J.; Lozach, O.; Bach, S.; Mayer, T.; Fabbro, D.; Meijer, L.; et al. Synthesis and Target Identification of Hymenialdisine Analogs. Chem. Biol. 2004, 11, 247–259. [Google Scholar] [CrossRef]
- Zhang, H.; Khalil, Z.; Conte, M.M.; Plisson, F.; Capon, R.J. A search for kinase inhibitors and antibacterial agents: Bromopyrrolo-2-aminoimidazoles from a deep-water Great Australian Bight sponge, Axinella sp. Tetrahedron Lett. 2012, 53, 3784–3787. [Google Scholar] [CrossRef]
- Cozza, G.; Gianoncelli, A.; Montopoli, M.; Caparrotta, L.; Venerando, A.; Meggio, F.; Pinna, L.A.; Zagotto, G.; Moro, S. Identification of novel protein kinase CK1 delta (CK1δ) inhibitors through structure-based virtual screening. Bioorg. Med. Chem. Lett. 2008, 18, 5672–5675. [Google Scholar] [CrossRef]
- Aliev, A.E.; Kulke, M.; Khaneja, H.S.; Chudasama, V.; Sheppard, T.D.; Lanigan, R.M. Motional timescale predictions by molecular dynamics simulations: Case study using proline and hydroxyproline sidechain dynamics: Proline Force Field Parameters. Proteins Struct. Funct. Bioinform. 2013, 82, 195–215. [Google Scholar] [CrossRef]
- Bakan, A.; Meireles, L.M.; Bahar, I. ProDy: Protein Dynamics Inferred from Theory and Experiments. Bioinformatics 2011, 27, 1575–1577. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Costanzo, L.D.; Christie, C.; Duarte, J.M.; Dutta, S.; Feng, Z.; et al. Protein Data Bank: The single global archive for 3D macromolecular structure data. Nucleic Acids Res. 2018, 47, D520–D528. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDB): The Single Global Macromolecular Structure Archive. In Protein Crystallography; Springer: New York, NY, USA, 2017; pp. 627–641. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2022, 51, D1373–D1380. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.; Zielesny, A.; Steinbeck, C. DECIMER: Towards deep learning for chemical image recognition. J. Cheminform. 2020, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Landrum, G.; Tosco, P.; Kelley, B.; Rodriguez, R.; Cosgrove, D.; Vianello, R.; Sriniker; Gedeck, P.; Jones, G.; Kawashima, E.; et al. rdkit/rdkit: 2025_03_4 (Q1 2025) Release. 2025. Available online: https://zenodo.org/records/15773589 (accessed on 15 October 2025).
- Van Rossum, G.; Drake, F. Python 3 Reference Manual: (Python Documentation Manual Part 2); Documentation for Python; CreateSpace Independent Publishing Platform: North Charleston, SC, USA, 2009. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Cerutti, D.S.; Cisneros, G.A.; Cruzeiro, V.W.D.; Forouzesh, N.; Giese, T.J.; Götz, A.W.; Gohlke, H.; et al. AmberTools. J. Chem. Inf. Model. 2023, 63, 6183–6191. [Google Scholar] [CrossRef]
- Dodda, L.S.; Cabeza de Vaca, I.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen web server: An automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian~16 Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Abraham, M.; Alekseenko, A.; Basov, V.; Bergh, C.; Briand, E.; Brown, A.; Doijade, M.; Fiorin, G.; Fleischmann, S.; Gorelov, S.; et al. GROMACS 2024.5 Manual. 2025. Available online: https://manual.gromacs.org/documentation/2024.5/install-guide/index.html (accessed on 15 October 2025).
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Rooijers, K.; Kolmeder, C.; Juste, C.; Doré, J.; de Been, M.; Boeren, S.; Galan, P.; Beauvallet, C.; de Vos, W.M.; Schaap, P.J. An iterative workflow for mining the human intestinal metaproteome. BMC Genom. 2011, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Sang, P.; Yang, Q.; Du, X.; Yang, N.; Yang, L.Q.; Ji, X.L.; Fu, Y.X.; Meng, Z.H.; Liu, S.Q. Effect of the Solvent Temperatures on Dynamics of Serine Protease Proteinase K. Int. J. Mol. Sci. 2016, 17, 254. [Google Scholar] [CrossRef] [PubMed]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2017, 27, 112–128. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Lynn, A. g_mmpbsa-A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Hermans, J. Interaction Models for Water in Relation to Protein Hydration. In Intermolecular Forces; Springer: Dordrecht, The Netherlands, 1981; pp. 331–342. [Google Scholar] [CrossRef]
- Hooft, R.W.; Sander, C.; Vriend, G. Objectively judging the quality of a protein structure from a Ramachandran plot. Bioinformatics 1997, 13, 425–430. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
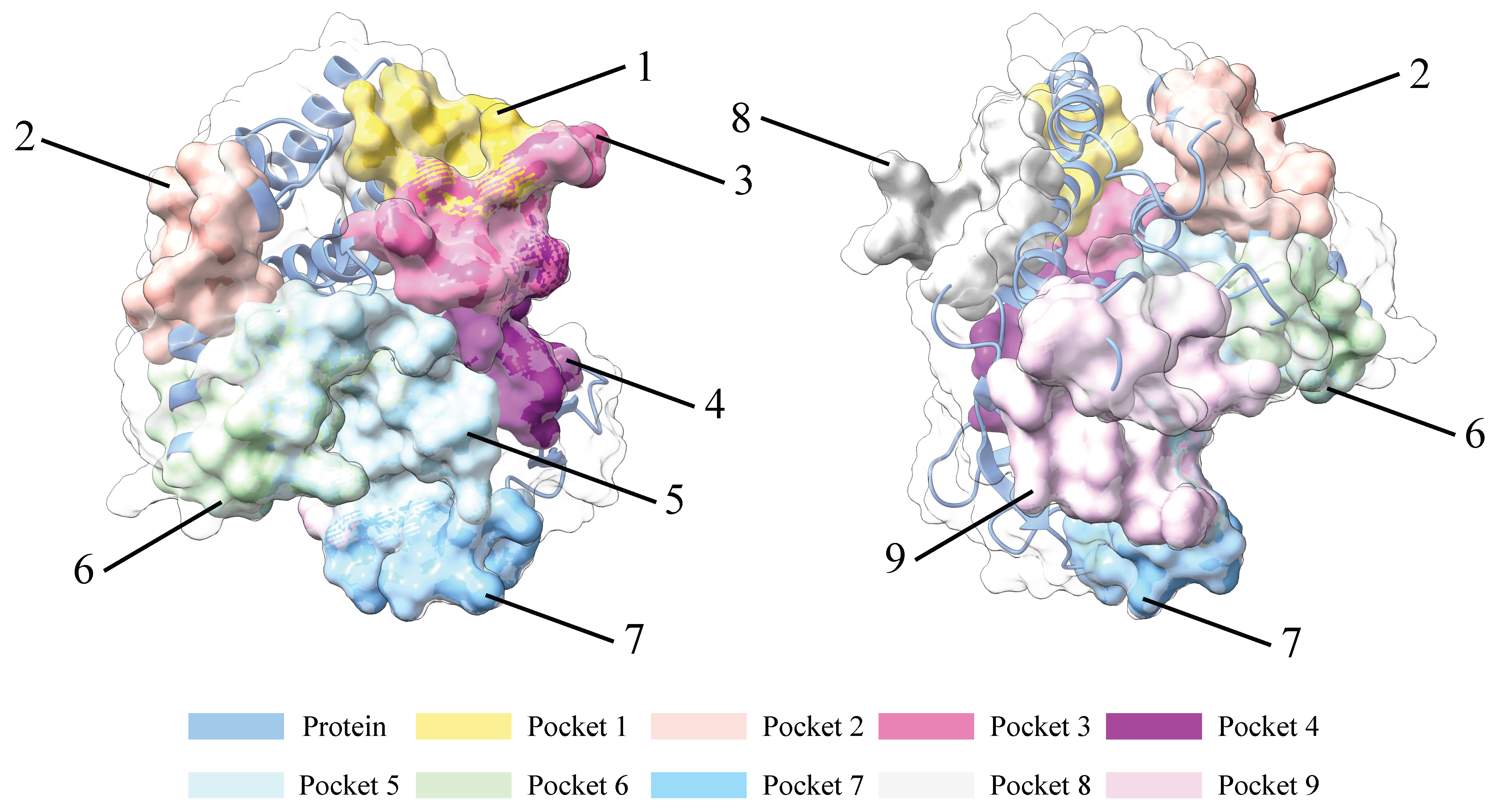
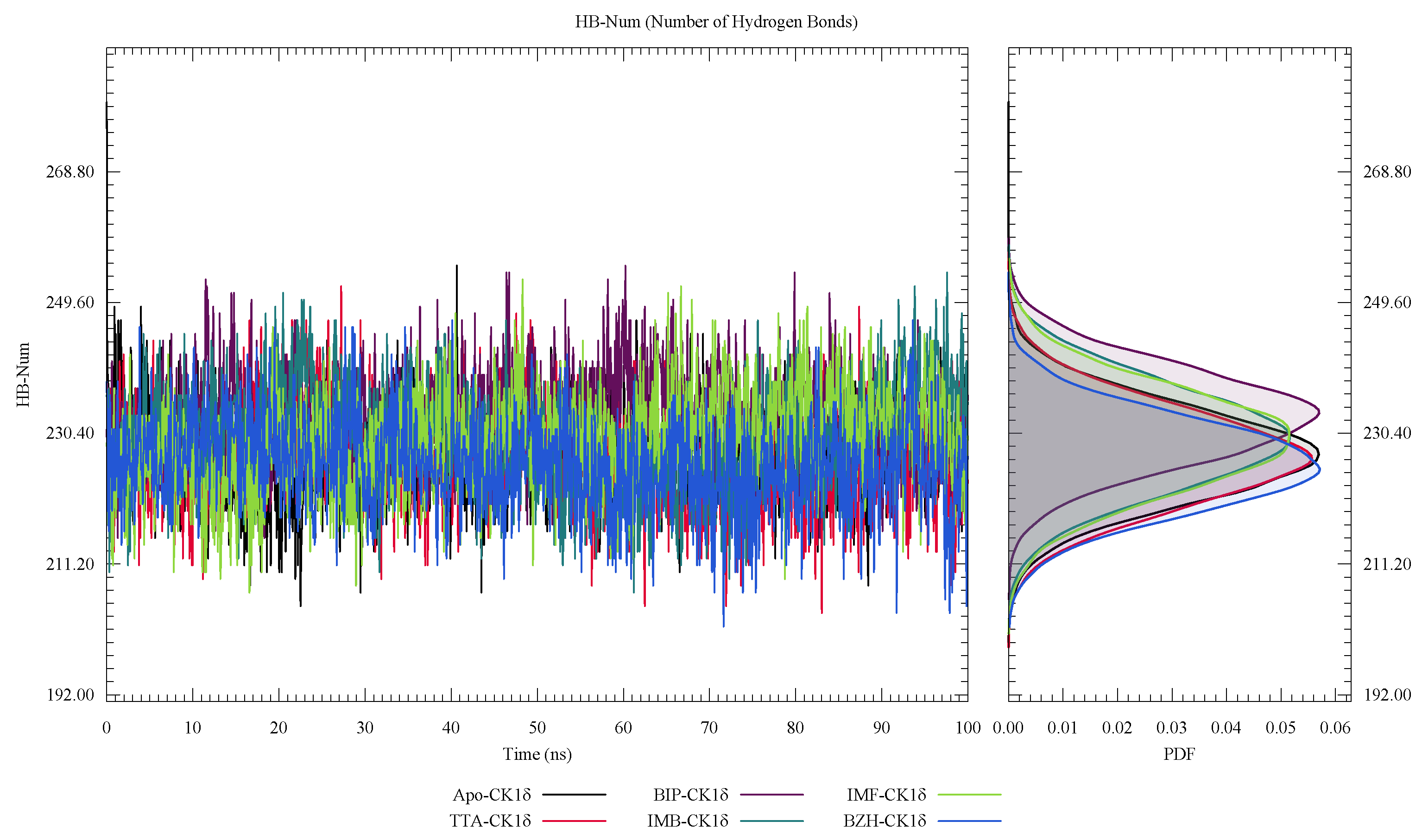
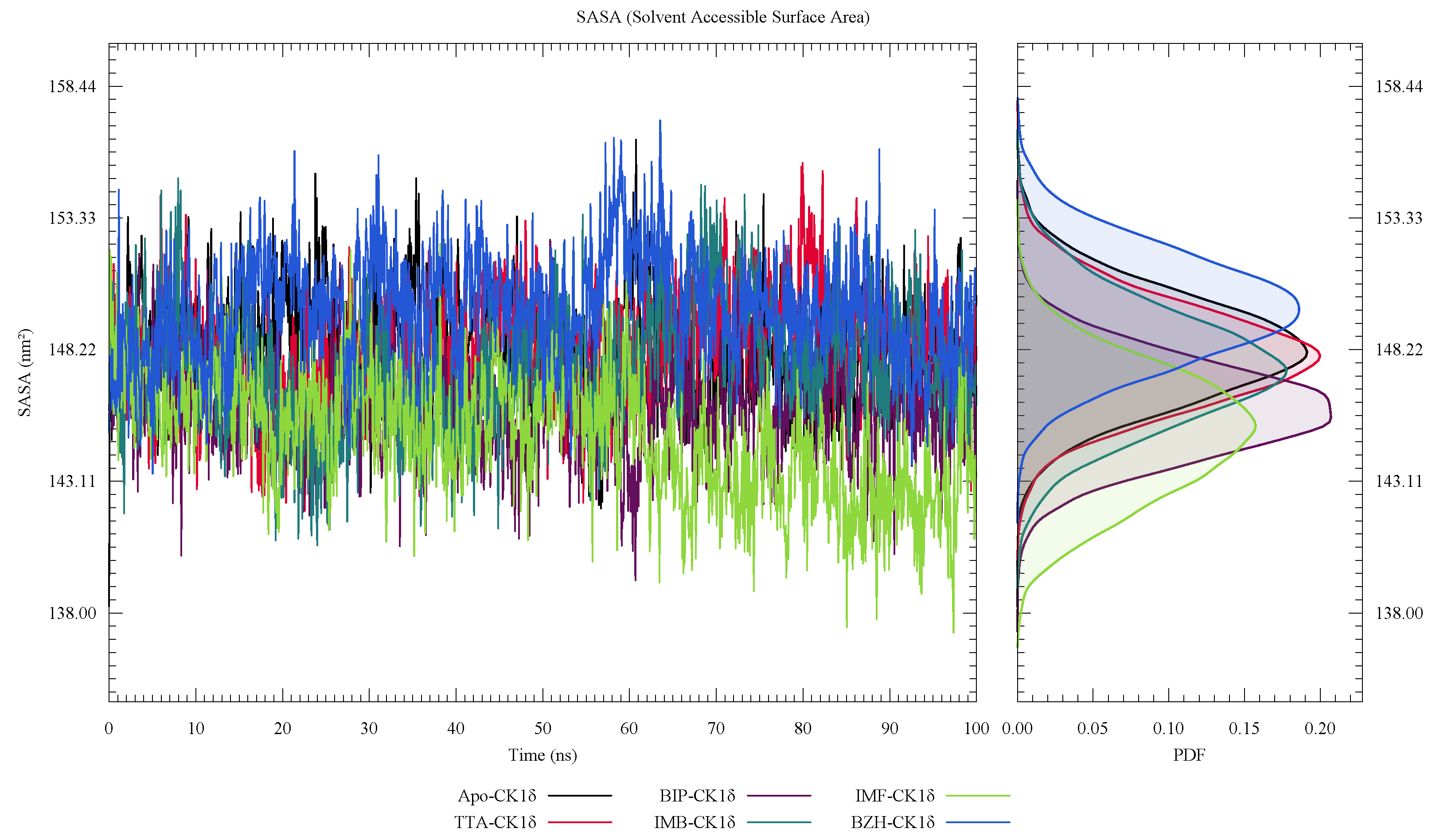
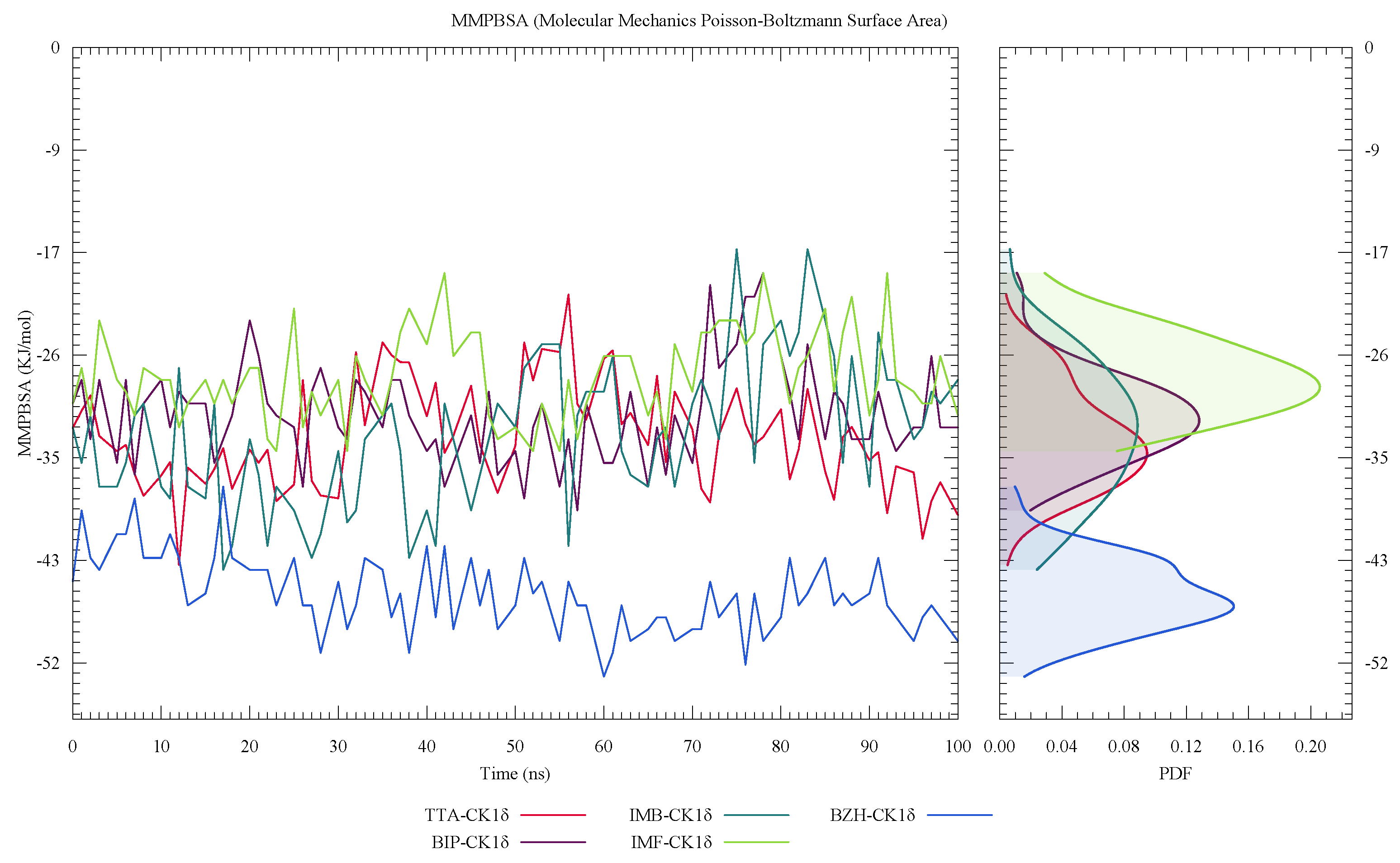
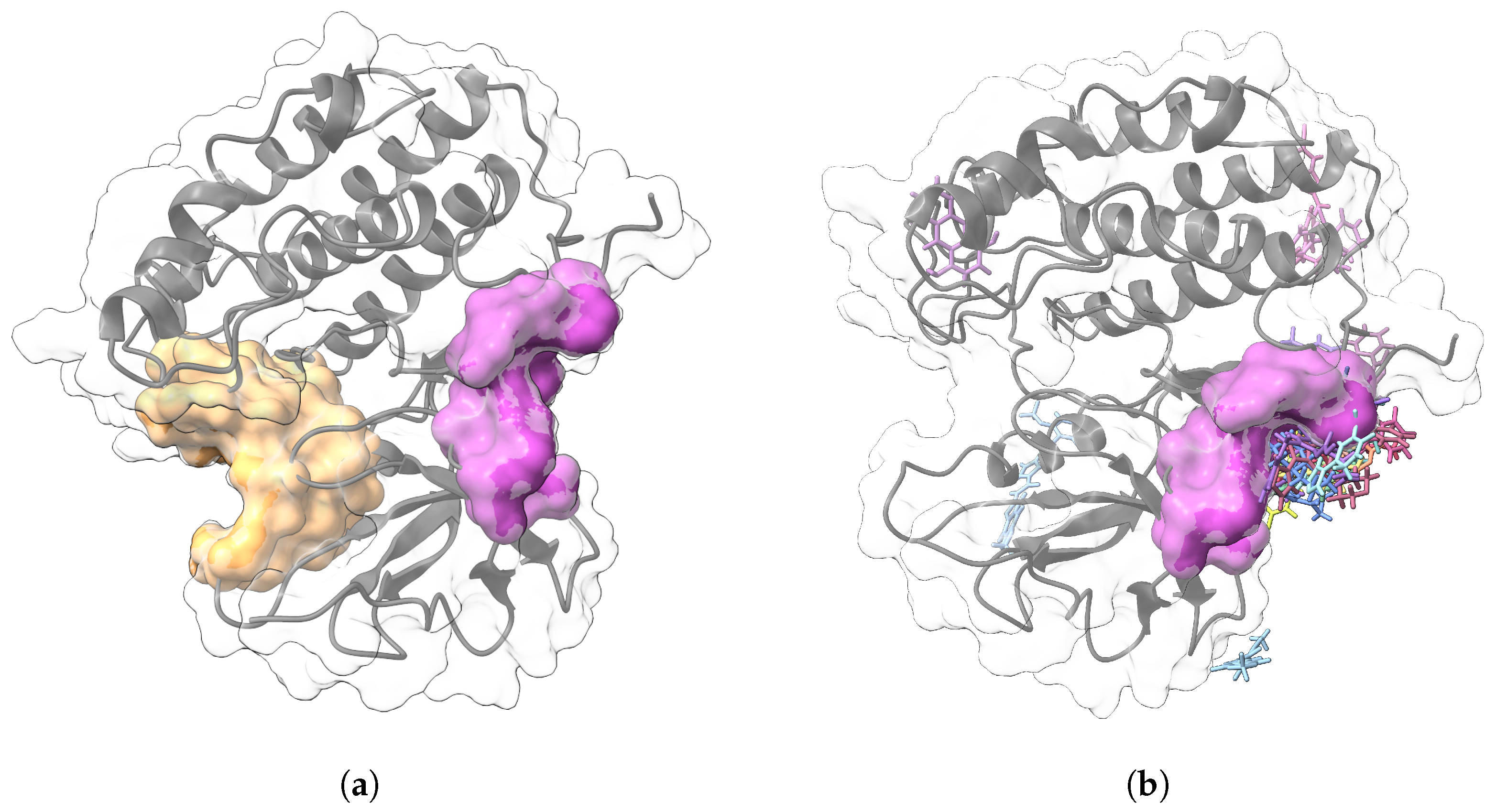
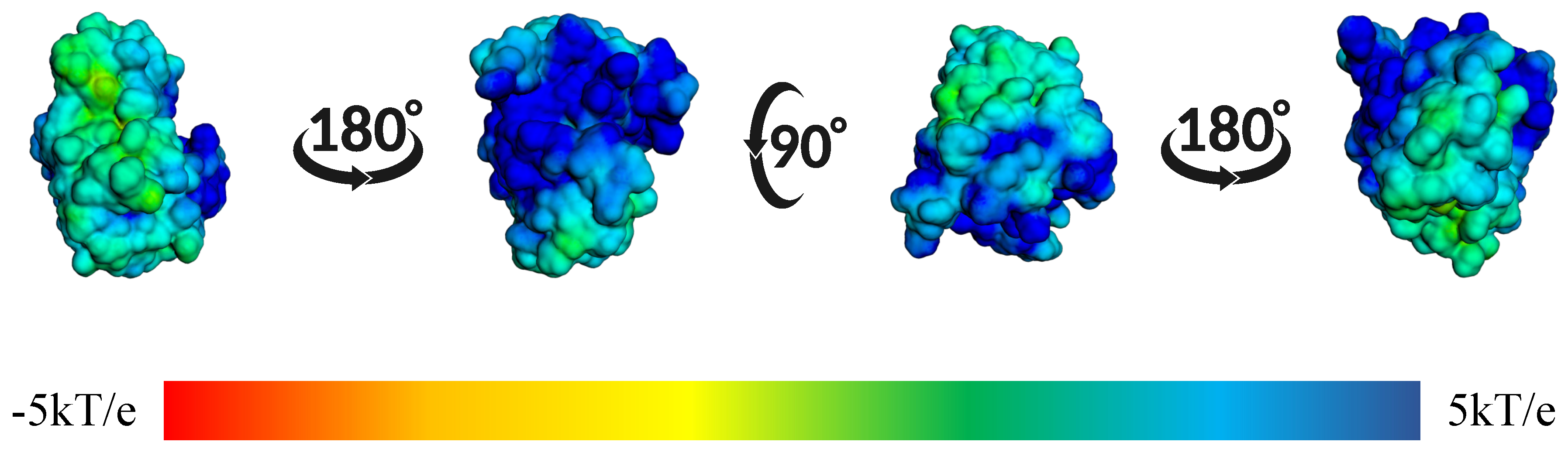
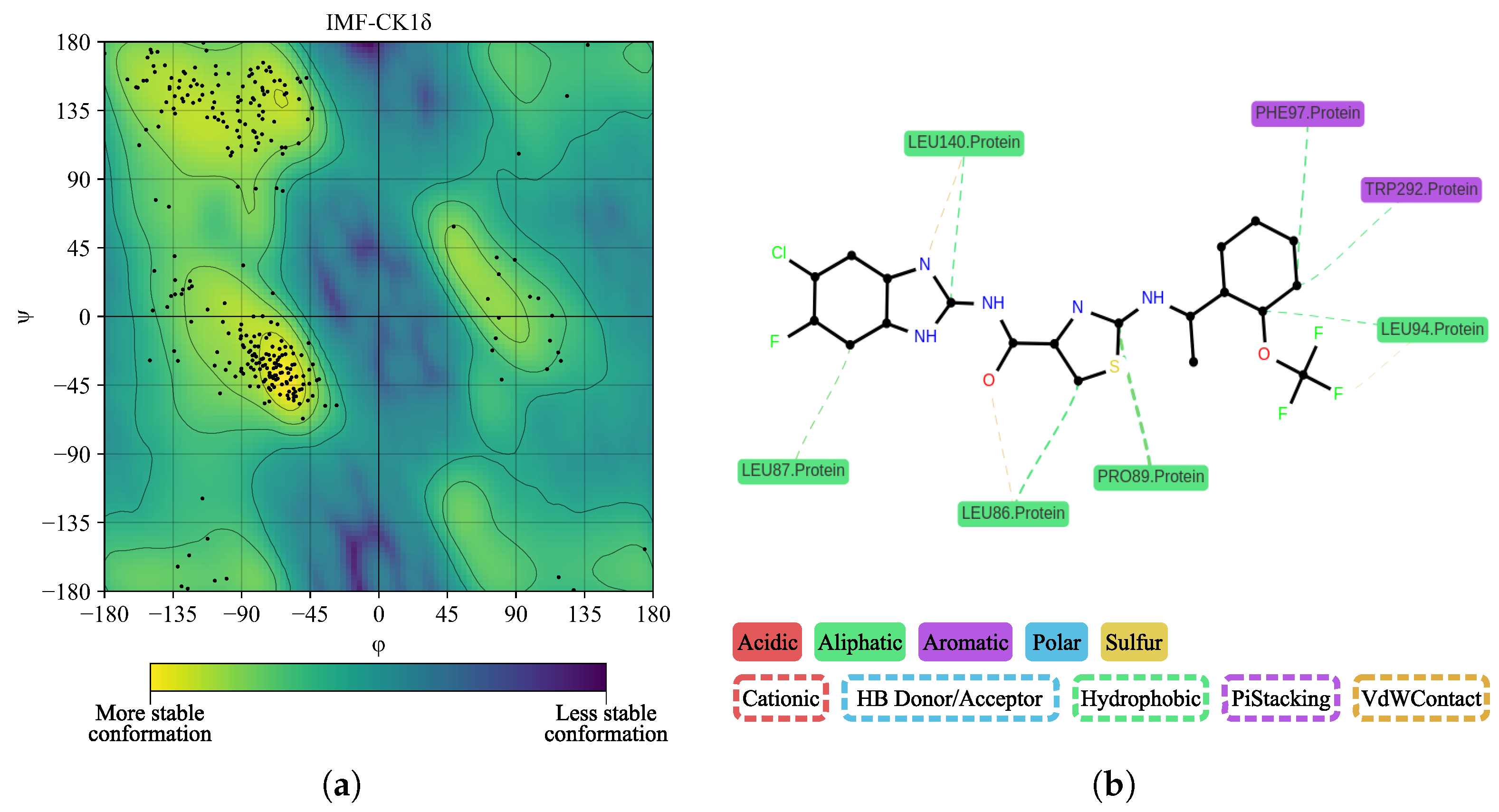
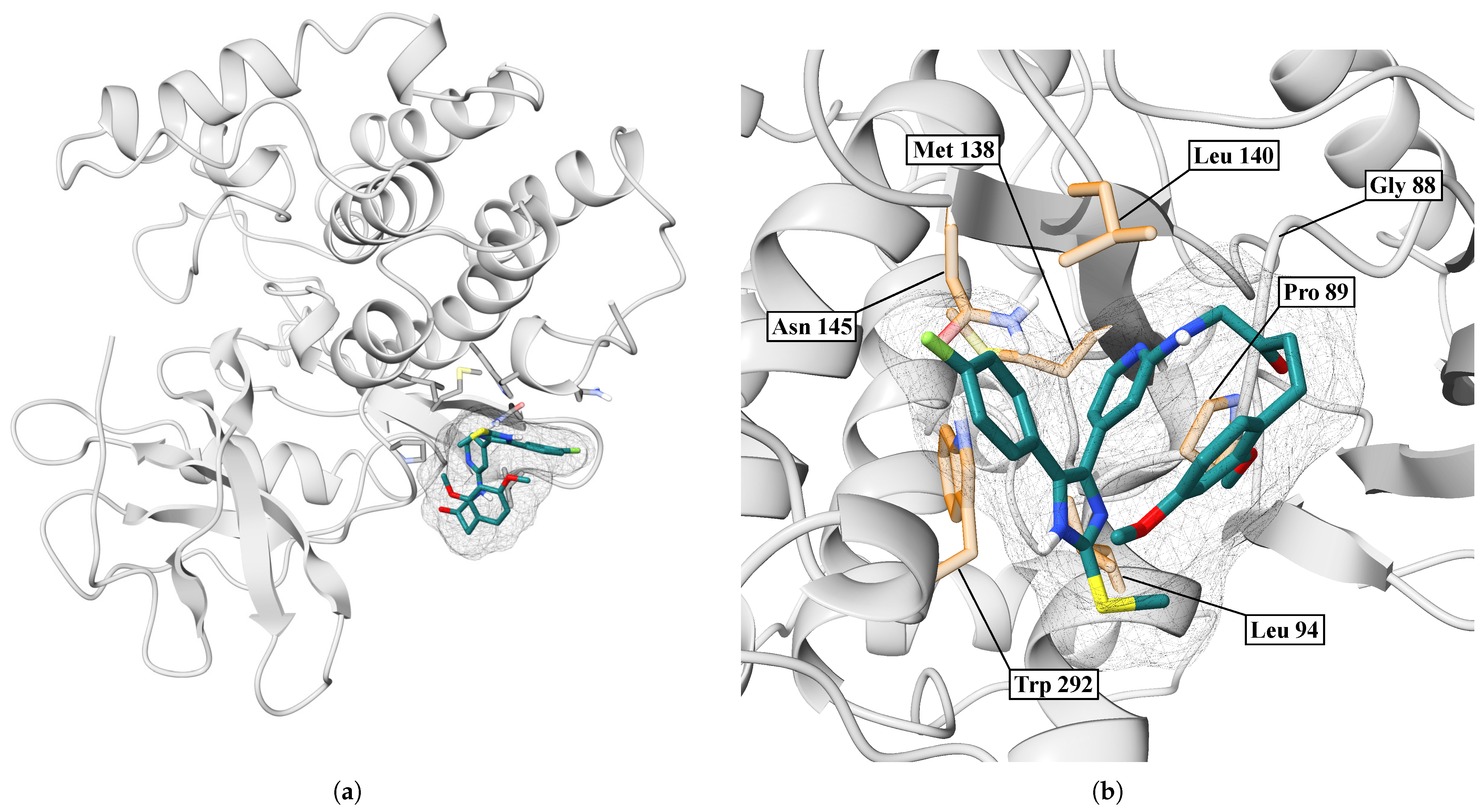
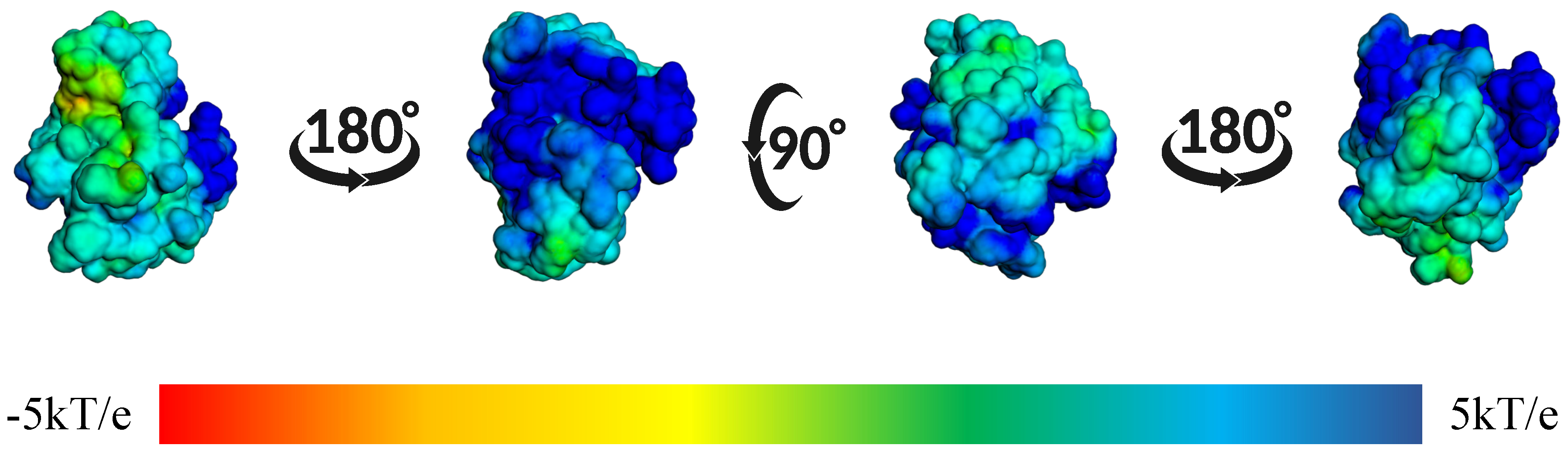
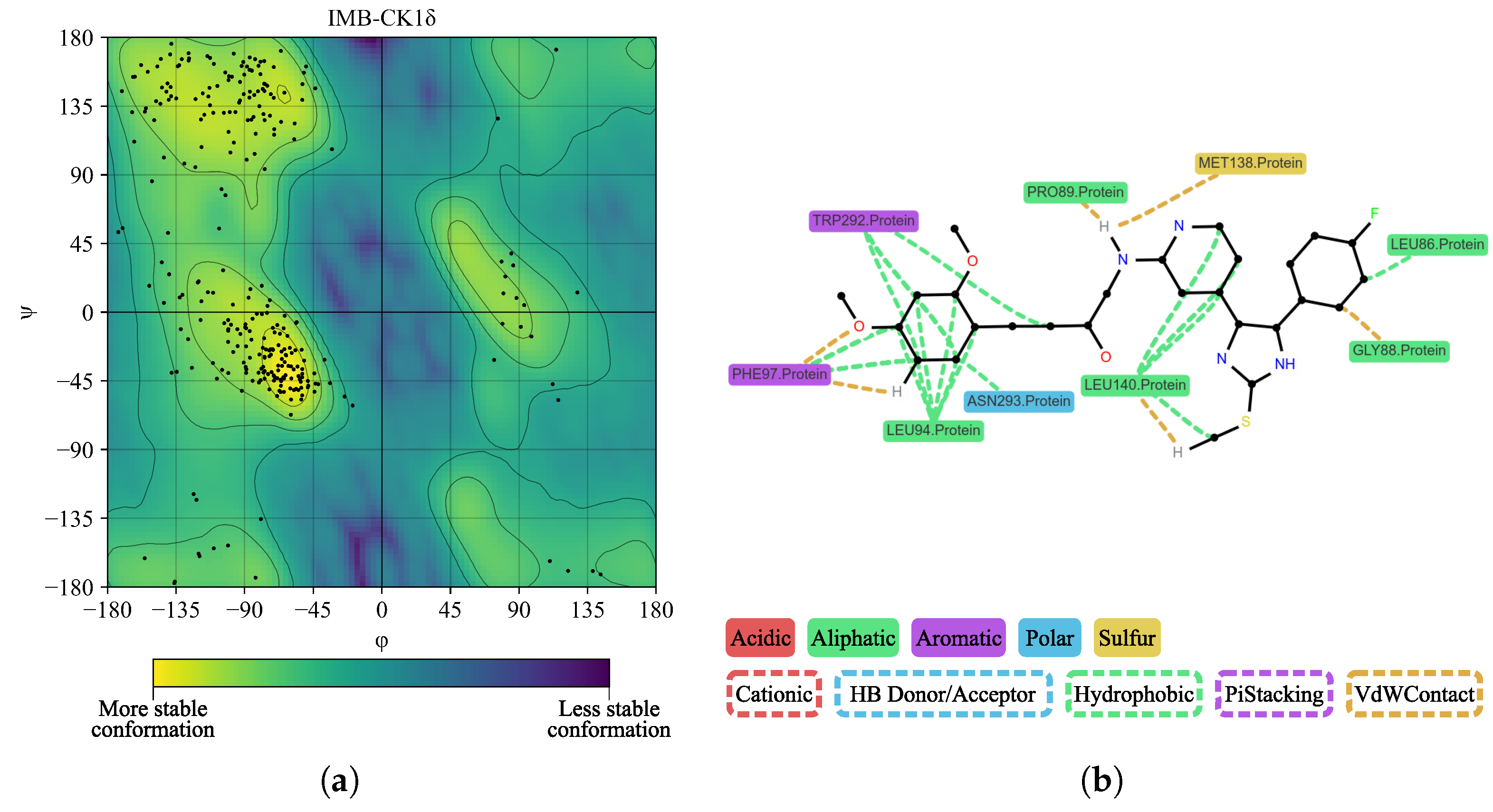
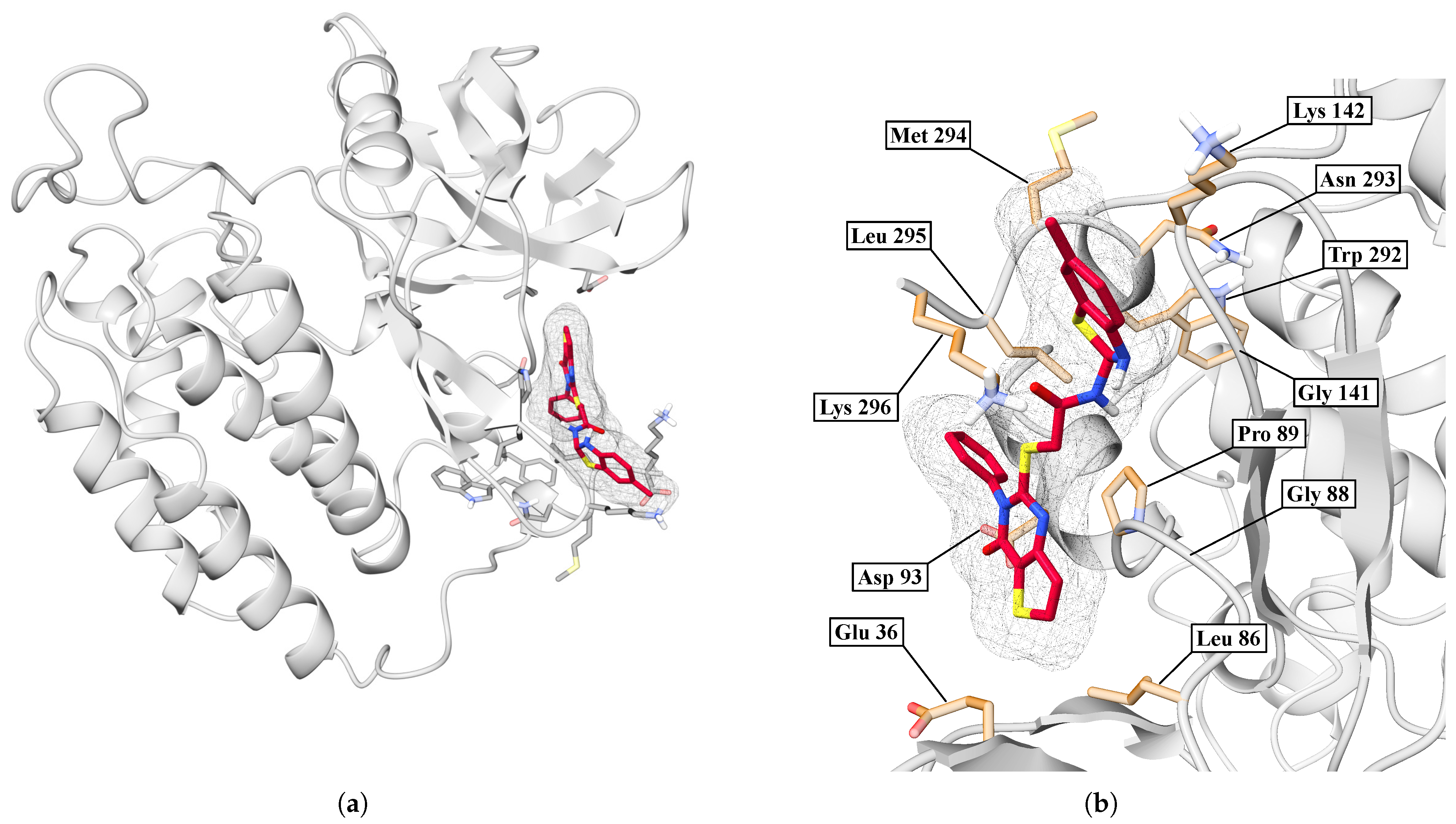
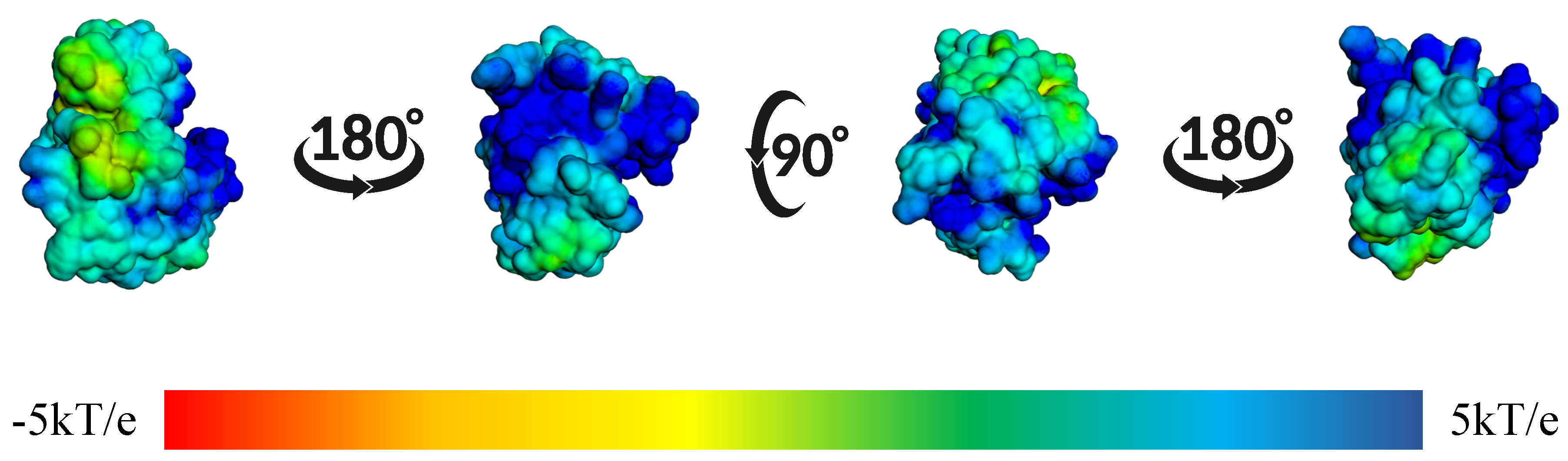
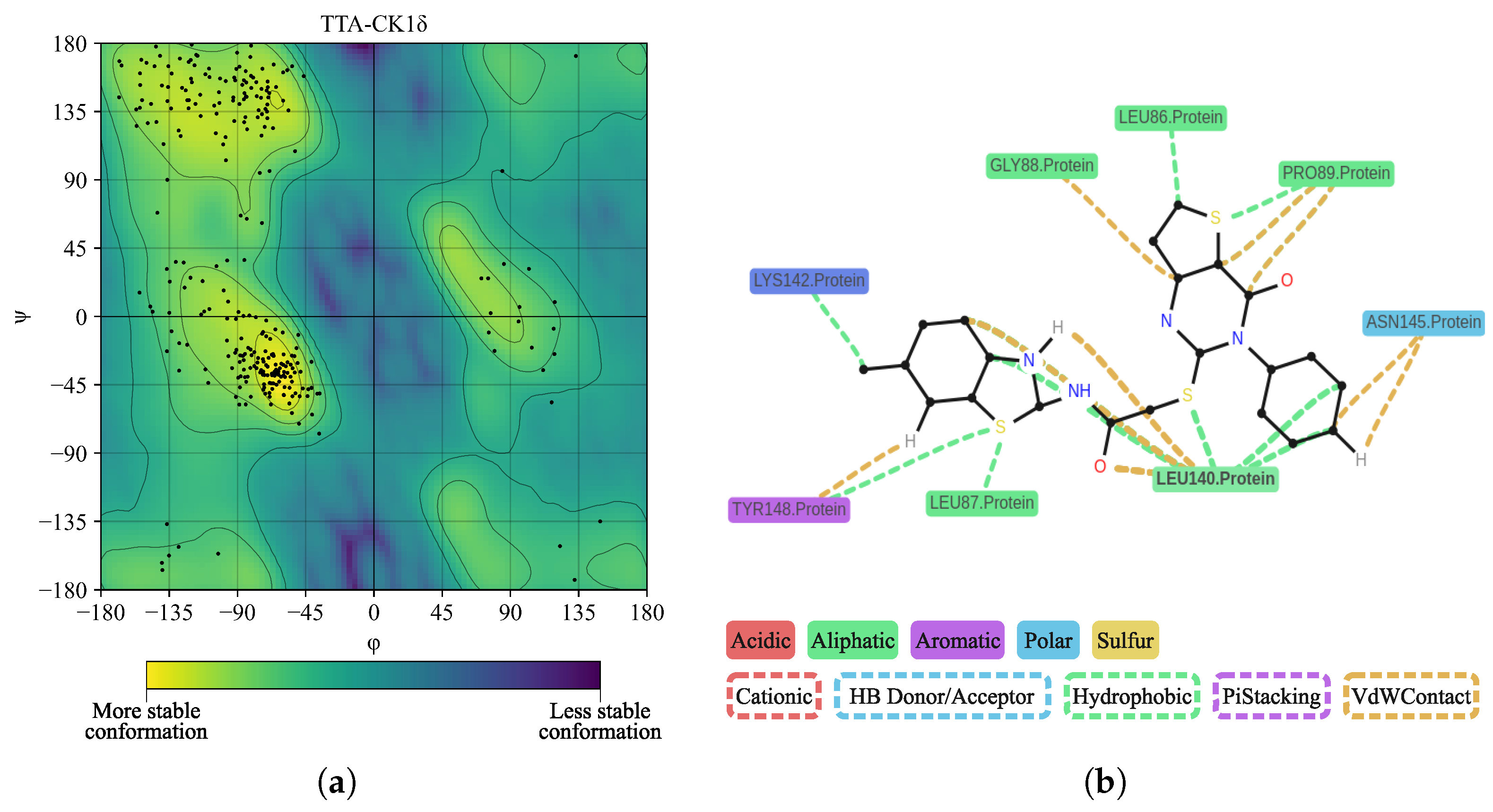
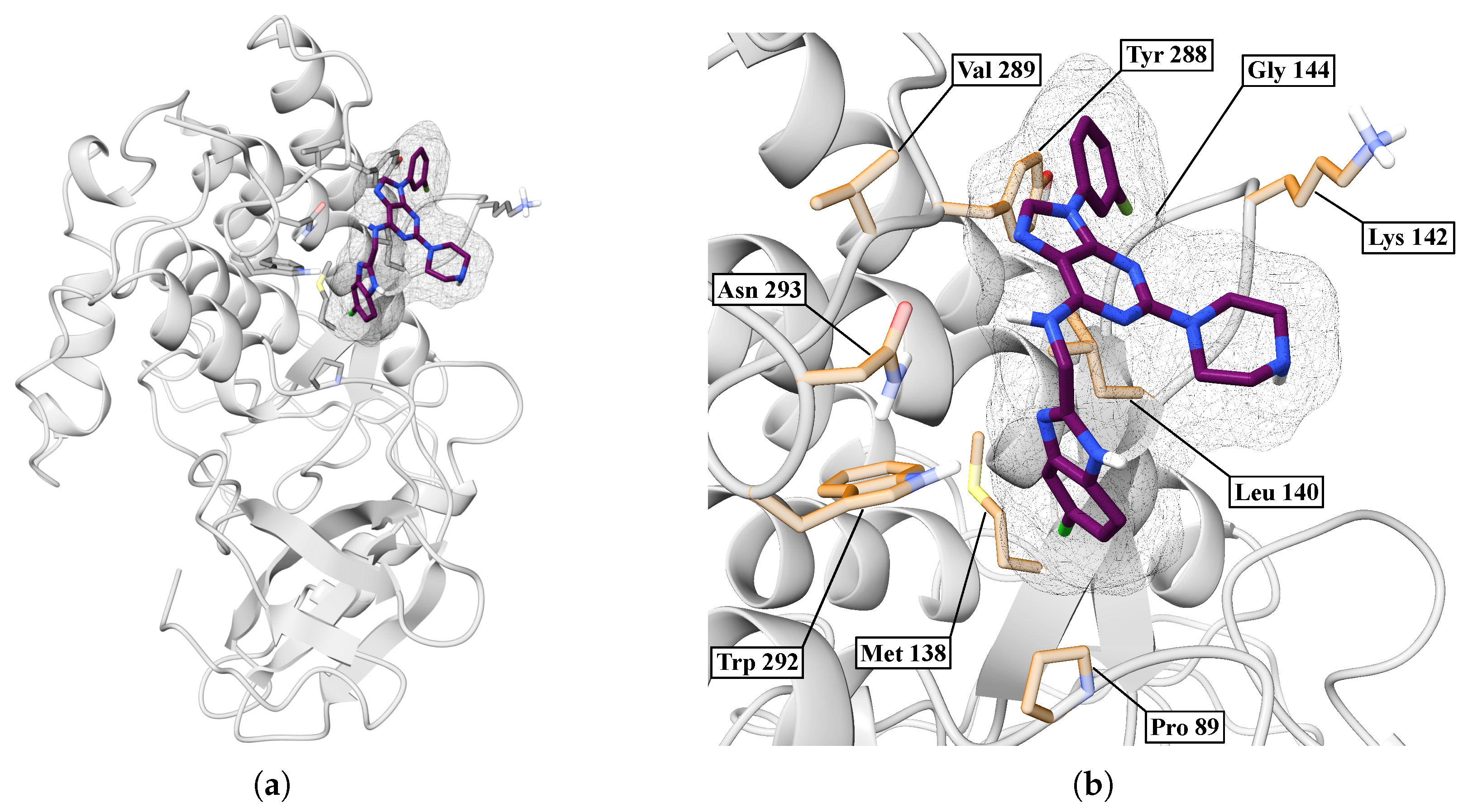
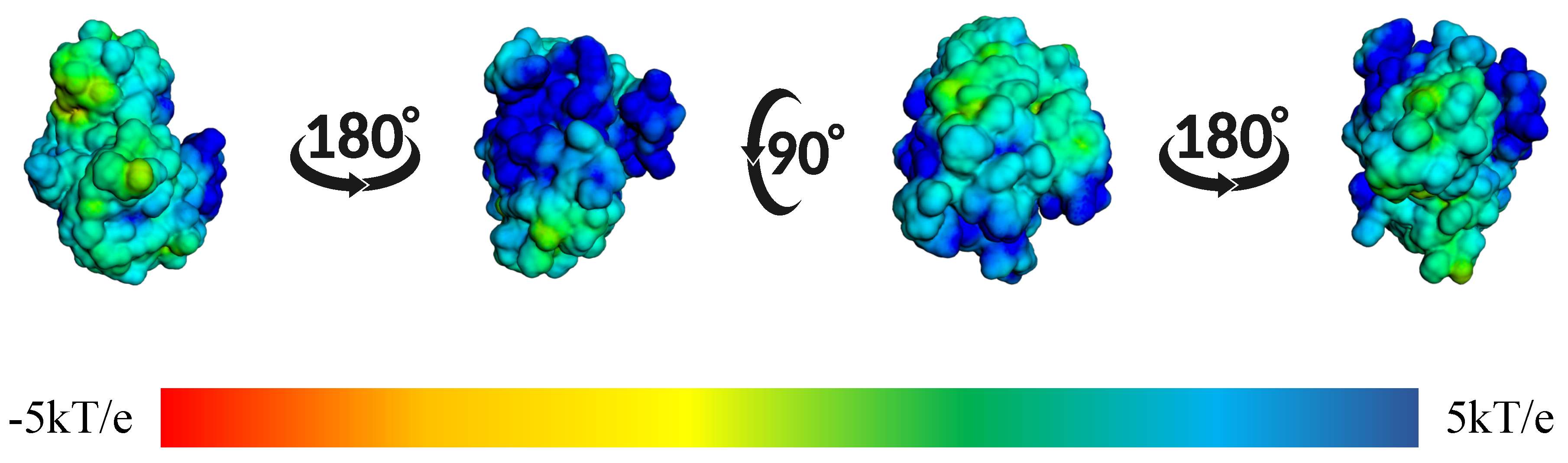
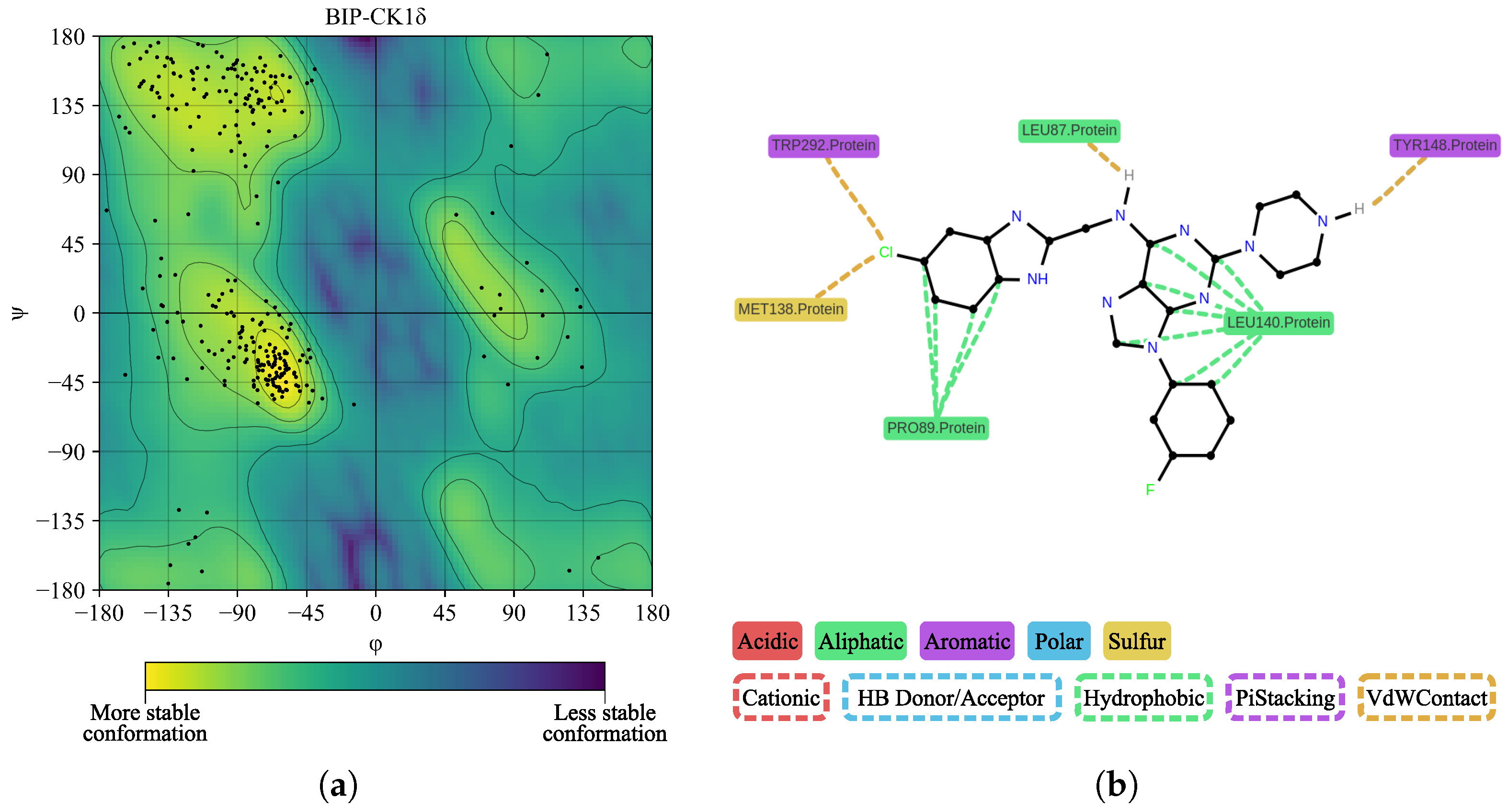
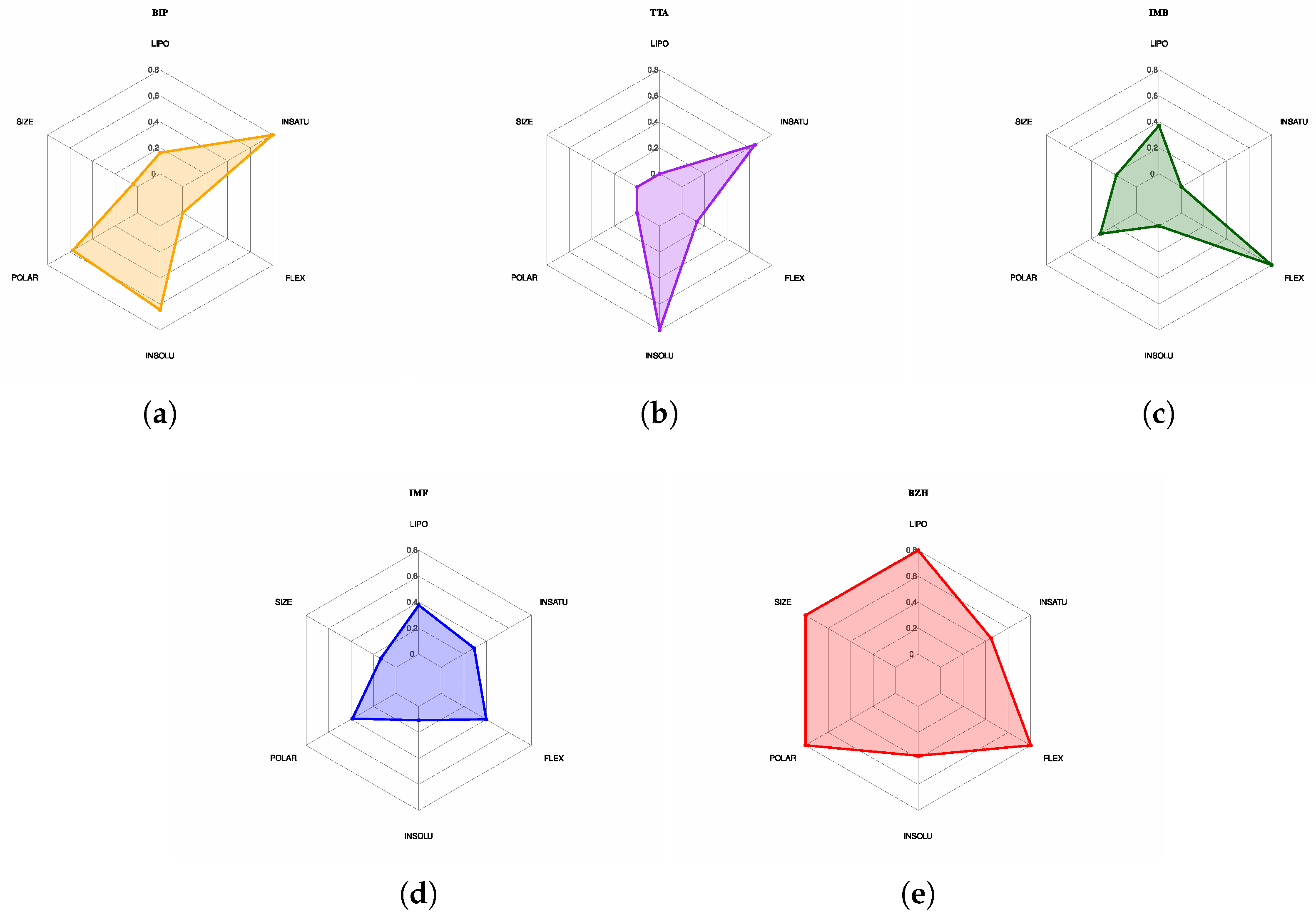
| Protein | DrugScore | Druggability | |
|---|---|---|---|
| CK1δ | 2 | 1058 | Strong |
| 4 | 22 | Medium | |
| 1 | 14 | Medium | |
| 5 | −375 | Weak | |
| 3 | −651 | Weak | |
| 7 | −782 | Weak | |
| 8 | −1200 | Weak | |
| 9 | −1183 | Weak | |
| 6 | −1274 | Weak |
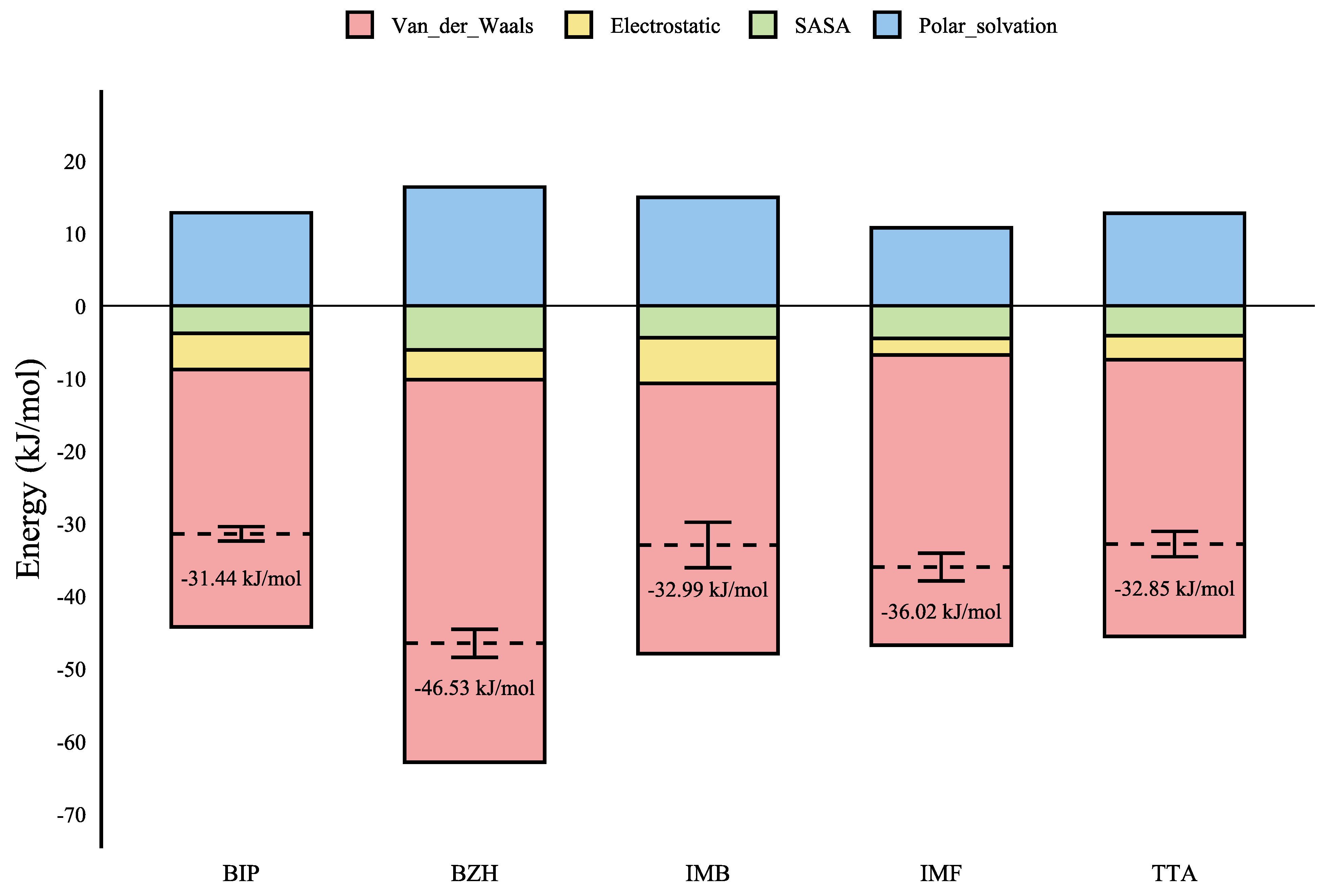
| Ligand | Dock | MMPBSA Components | |||||
|---|---|---|---|---|---|---|---|
| VdW | Elec | Pol | SASA | SAV | Total | ||
| BZH | −7.663 | ||||||
| IMF | −7.171 | ||||||
| IMB | −6.806 | ||||||
| TTA | −6.533 | ||||||
| BIP | −7.322 | ||||||
| BZC | −7.076 | ||||||
| IMT | −7.211 | ||||||
| IMD | −7.035 | ||||||
| BIM | −7.704 | ||||||
| PMP | −6.103 | ||||||
| PYP | −7.104 | ||||||
| LAX | −6.517 | ||||||
| LAM | −6.813 | ||||||
| BIT | −6.685 | ||||||
| BIC | −6.090 | ||||||
| PYR | −6.093 | ||||||
| PYI | −6.885 | ||||||
| AMI | −5.570 | ||||||
| BZF | −6.044 | ||||||
| BZT | −6.090 | ||||||
| BZM | −6.004 | ||||||
| DXB | −8.137 | ||||||
| HYA | −5.648 | ||||||
| AHQ | −5.276 | ||||||
| DXM | −6.435 | ||||||
| AMQ | −5.273 | ||||||
| Ligand | Interacting Residues | H. Bonds | Dist. (Å) |
|---|---|---|---|
| BZH | LEU86, LEU87, PRO89, LEU94, PHE97, MET138, LEU140, TRP292, ASN293 | — | 3.70 |
| IMF | LEU86, LEU87, GLY88, PRO89, LEU94, LEU140, TRP292, ASN293 | — | 3.64 |
| IMB | PRO89, LEU140, TRP292, MET138, GLY88, LEU94, ASN145 | — | 3.64 |
| TTA | LEU295, GLY88, LYS142, ASN293, GLU36, LEU86, PRO89, ASP93, GLY141, TRP292, MET294, LYS296 | — | 3.67 |
| BIP | TYR288, LEU140, GLY141, GLY144, PRO89, MET138, LYS142, TRP292, ASN293 | GLY144 | 3.65 |
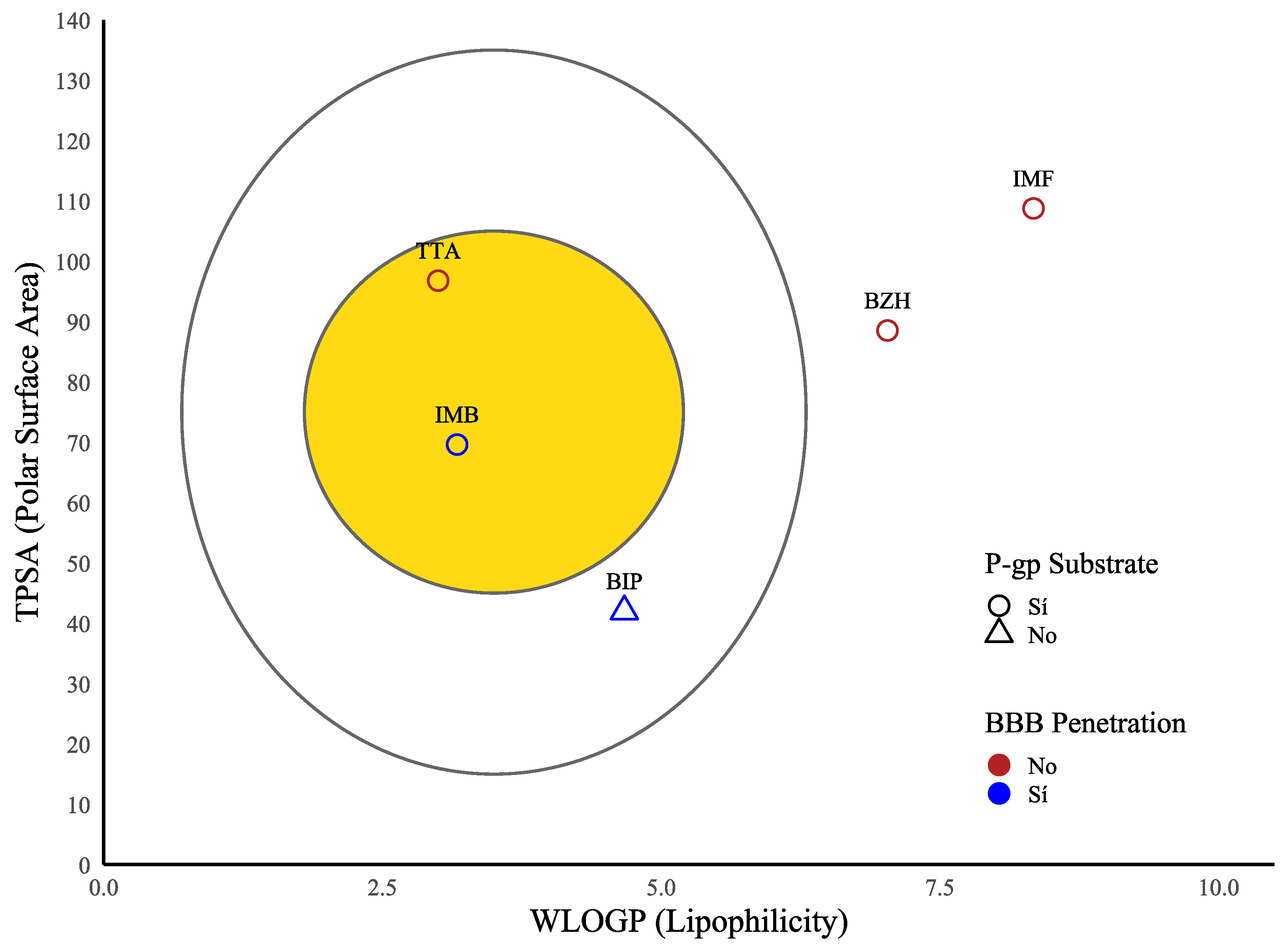
| Ligand | MW | HBD | HBA | LogP | TPSA | RB | Lipinski | Veber | HIA | BBB |
|---|---|---|---|---|---|---|---|---|---|---|
| BIP | 477.16 | 3 | 9 | 3.384 | 99.58 | 5 | Accepted | — | — | +++ |
| TTA | 468.07 | 2 | 6 | 2.113 | 76.02 | 6 | Accepted | — | — | — |
| IMB | 506.18 | 2 | 7 | 5.006 | 89.13 | 11 | Rejected | — | — | — |
| IMF | 497.03 | 3 | 7 | 5.054 | 91.93 | 8 | Accepted | — | — | ++ |
| BZH | 638.28 | 3 | 7 | 8.339 | 108.75 | 11 | Rejected | — | — | — |
| Compound | Hepatotoxicity | Carcinogenicity | Neurotoxicity | LD50 (Oral, Rat, mol/kg) | Toxicity Class |
|---|---|---|---|---|---|
| BIP | 0.768 | 0.040 | 0.271 | 0.503 | IV |
| TTA | 0.715 | 0.052 | 0.251 | 0.550 | IV |
| IMB | 0.849 | 0.032 | 0.212 | 0.497 | IV |
| IMF | 0.812 | 0.037 | 0.242 | 0.512 | IV |
| BZH | 0.831 | 0.039 | 0.262 | 0.567 | IV |
| Compound | Binding Energy (kcal/mol) | IC50 (μM) | Reference |
|---|---|---|---|
| BZH | ≈39.7000 | [40] | |
| IMF | [41] | ||
| IMB | [42] | ||
| TTA | [43] | ||
| BIP | [44] | ||
| BZC | ≈34.0000 | [40] | |
| IMT | [41,45] | ||
| IMD | [44,45,46] | ||
| BIM | [44,47] | ||
| PMP | – | [48] | |
| PYP | [49,50] | ||
| LAX | [51] | ||
| LAM | [51] | ||
| BIT | [52] | ||
| BIC | [53] | ||
| PYR | [39,54] | ||
| PYI | [39,44,55,56,57,58] | ||
| AMI | [59] | ||
| BZF | [60,61,62] | ||
| BZT | [22,60,61,63] | ||
| BZM | [64] | ||
| DXB | [65] | ||
| HYA | [66,67,68,69] | ||
| AHQ | [70] | ||
| DXM | [65] | ||
| AMQ | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turpo-Peqqueña, A.G.; Valencia-Arce, R.J.; Del-Carpio-Carrazco, F.L.; Quispe-Ppacco, D.J.; Carbajal-Llerena, P.F.; Loza-Chipa, H.R.; Vásquez-Macedo, A.S.; Gómez, B. Inhibition of Casein Kinase 1δ as a Novel Therapeutic Strategy for Amyotrophic Lateral Sclerosis: A Theoretical Study. Int. J. Mol. Sci. 2025, 26, 10188. https://doi.org/10.3390/ijms262010188
Turpo-Peqqueña AG, Valencia-Arce RJ, Del-Carpio-Carrazco FL, Quispe-Ppacco DJ, Carbajal-Llerena PF, Loza-Chipa HR, Vásquez-Macedo AS, Gómez B. Inhibition of Casein Kinase 1δ as a Novel Therapeutic Strategy for Amyotrophic Lateral Sclerosis: A Theoretical Study. International Journal of Molecular Sciences. 2025; 26(20):10188. https://doi.org/10.3390/ijms262010188
Chicago/Turabian StyleTurpo-Peqqueña, Albert Gabriel, Renato Javier Valencia-Arce, Fabio Leonardo Del-Carpio-Carrazco, David Jonatan Quispe-Ppacco, Pierina Fernanda Carbajal-Llerena, Harlly Romed Loza-Chipa, Antonella Sofia Vásquez-Macedo, and Badhin Gómez. 2025. "Inhibition of Casein Kinase 1δ as a Novel Therapeutic Strategy for Amyotrophic Lateral Sclerosis: A Theoretical Study" International Journal of Molecular Sciences 26, no. 20: 10188. https://doi.org/10.3390/ijms262010188
APA StyleTurpo-Peqqueña, A. G., Valencia-Arce, R. J., Del-Carpio-Carrazco, F. L., Quispe-Ppacco, D. J., Carbajal-Llerena, P. F., Loza-Chipa, H. R., Vásquez-Macedo, A. S., & Gómez, B. (2025). Inhibition of Casein Kinase 1δ as a Novel Therapeutic Strategy for Amyotrophic Lateral Sclerosis: A Theoretical Study. International Journal of Molecular Sciences, 26(20), 10188. https://doi.org/10.3390/ijms262010188








