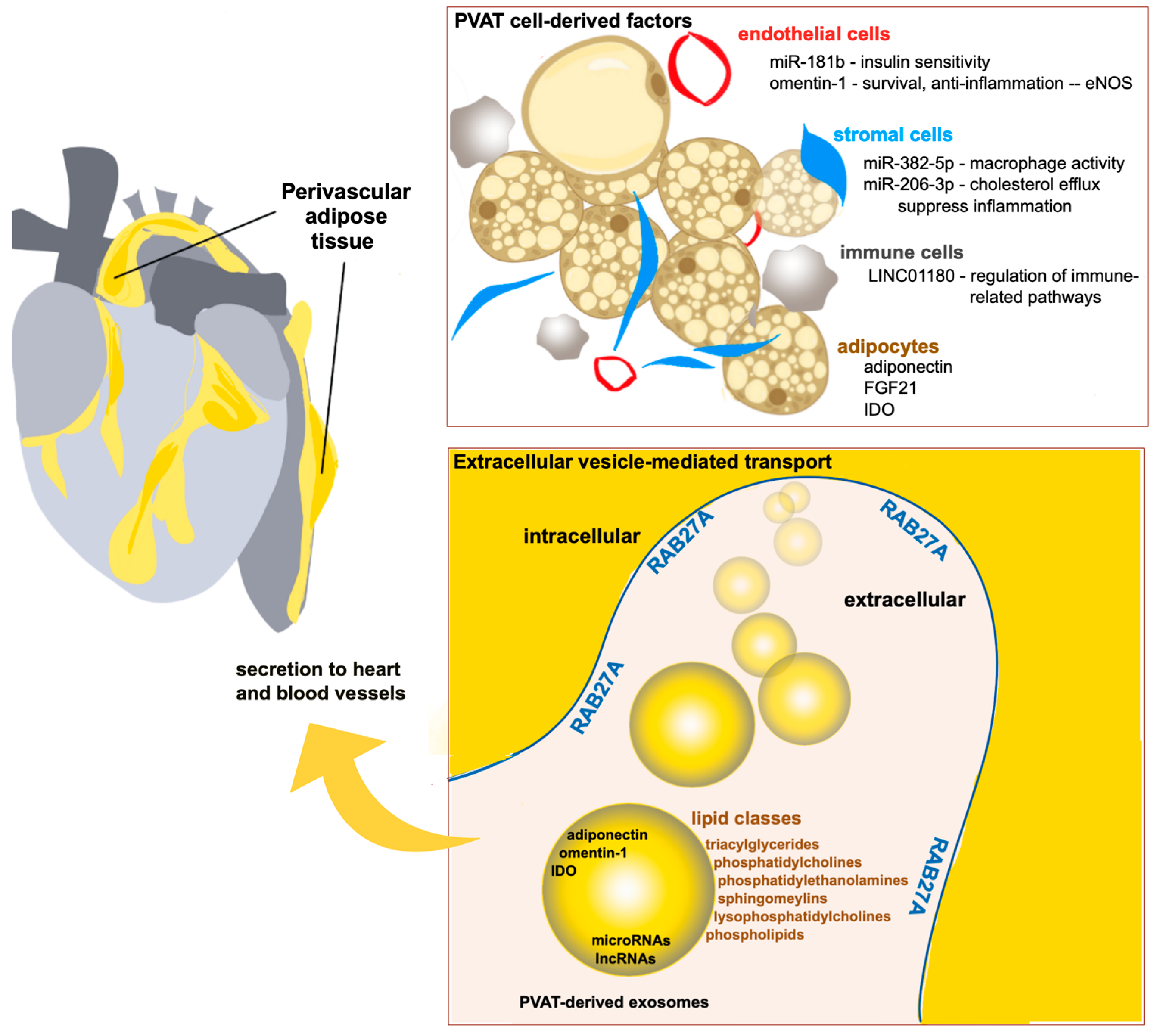
Promotion of Cardiovascular Homeostasis by the Perivascular Adipose Tissue Secretome
Abstract
1. Introduction
2. RAB27A Function in Exosome Regulation and Cardiovascular Health
3. Cardioprotective RNAs Secreted from PVAT
| RNA | Effects | Reference |
|---|---|---|
| miRNA-181b | Exerts anti-inflammatory effects; enhances production of endothelial NO synthase (eNOS); attenuates endothelial dysfunction | [33] |
| miR-382-5p | Reduces macrophage foam cell formation by mediating upregulation of cholesterol efflux transporters, ABCA1, and ABCg1 | [34] |
| miR-206-3p | Enhances cholesterol efflux via miR-206-3p-ABCA1-dependent signaling and upregulation of cholesterol efflux transporters, ABCA1, and ABCg1 | [35] |
| LINC01180 | Protective factor against the progression of atherosclerosis | [37] |
4. Cardioprotective Lipids Derived from PVAT
4.1. Sphingolipids
4.2. Palmitic Acid Methyl Ester
4.3. Phosopholipids
4.4. Plasmalogens
5. Protective PVAT-Derived Protein Secretome
5.1. Adiponectin
5.2. Endothelial Nitric Oxide Synthase
5.3. Omentin-1
5.4. Fibroblast Growth Factor-21
6. Discussion, Limitations, and Future Directions
7. Methods
7.1. Eligibility Criteria
7.2. Search Strategy
7.3. Selecting Studies and Data Extraction
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CVD | cardiovascular disease |
| eNOS | endothelial nitric oxide synthase |
| ESCRT | endosomal sorting complex required for transport |
| FABP4 | fatty acid-binding protein 4 |
| FGF21 | fibroblast growth factor 21 |
| ITLN1 | intelectin-1 |
| NVEV | neutrophil membrane-engineered extracellular vesicles |
| PAME | palmitic acid methyl ester |
| PPAR-γ | peroxisome proliferator-activated factor |
| PVAT | perivascular adipose tissue |
| TGF | transforming growth factor |
References
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2025, 151, e41–e660. [Google Scholar] [CrossRef]
- Antoniades, C.; Tousoulis, D.; Vavlukis, M.; Fleming, I.; Duncker, D.J.; Eringa, E.; Manfrini, O.; Antonopoulos, A.S.; Oikonomou, E.; Padró, T.; et al. Perivascular adipose tissue as a source of therapeutic targets and clinical biomarkers. Eur. Heart J. 2023, 44, 3827–3844. [Google Scholar] [CrossRef]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef]
- Kim, H.W.; Shi, H.; Winkler, M.A.; Lee, R.; Weintraub, N.L. Perivascular Adipose Tissue and Vascular Perturbation/Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2569–2576. [Google Scholar] [CrossRef]
- Zullo, J.; Matsumoto, K.; Xavier, S.; Ratliff, B.; Goligorsky, M.S. The cell secretome, a mediator of cell-to-cell communication. Prostaglandins Other Lipid Mediat. 2015, 120, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Arya, S.B.; Collie, S.P.; Parent, C.A. The ins-and-outs of exosome biogenesis, secretion, and internalization. Trends Cell Biol. 2024, 34, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Liese, S.; Wenzel, E.M.; Kjos, I.; Rojas Molina, R.; Schultz, S.W.; Brech, A.; Stenmark, H.; Raiborg, C.; Carlson, A. Protein crowding mediates membrane remodeling in upstream ESCRT-induced formation of intraluminal vesicles. Proc. Natl. Acad. Sci. USA 2020, 117, 28614–28624. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021, 31, 157–177. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, Y.; Song, C.; Cao, K.; Cai, K.; Chen, S.; Wu, Y.; Geng, D.; Sun, G.; Zhang, N.; et al. Advances in the study of exosomes in cardiovascular diseases. J. Adv. Res. 2024, 66, 133–153. [Google Scholar] [CrossRef]
- Guo, D.; Xu, Y.; Ding, J.; Dong, J.; Jia, N.; Li, Y.; Zhang, M. Roles and Clinical Applications of Exosomes in Cardiovascular Disease. BioMed Res. Int. 2020, 2020, 5424281. [Google Scholar] [CrossRef]
- Reiss, A.B.; Ahmed, S.; Johnson, M.; Saeedullah, U.; De Leon, J. Exosomes in Cardiovascular Disease: From Mechanism to Therapeutic Target. Metabolites 2023, 13, 479. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, D.; Han, X.; Han, S.; Zhang, W.; Zang, Z.; Gai, C.; Rong, R.; Gao, T. The role of perivascular adipose tissue-secreted adipocytokines in cardiovascular disease. Front. Immunol. 2023, 14, 1271051. [Google Scholar] [CrossRef]
- Le Lay, S.; Scherer, P.E. Exploring adipose tissue-derived extracellular vesicles in inter-organ crosstalk: Implications for metabolic regulation and adipose tissue function. Cell Rep. 2025, 44, 115732. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q.; Zhou, S.; Tan, P. Contents of exosomes derived from adipose tissue and their regulation on inflammation, tumors, and diabetes. Front. Endocrinol. 2024, 15, 1374715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhao, T.; He, Z.; Cai, R.; Pang, W. Composition, isolation, identification and function of adipose tissue-derived exosomes. Adipocyte 2021, 10, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Soucy, A.; Potts, C.; Kaija, A.; Harrington, A.; McGilvrey, M.; Sutphin, G.L.; Korstanje, R.; Tero, B.; Seeker, J.; Pinz, I.; et al. Effects of a Global Rab27a Null Mutation on Murine PVAT and Cardiovascular Function. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1601–1616. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zheng, X.; Cheng, C.; Guo, G.; Zhong, Y.; Liu, W.; Liu, K.; Chen, Y.; Liu, S.; Liu, S. Rab27a deletion impairs the therapeutic potential of endothelial progenitor cells for myocardial infarction. Mol. Cell. Biochem. 2021, 476, 797–807. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Guo, D.; Lui, G.Y.L.; Lai, S.L.; Wilmott, J.S.; Tikoo, S.; Jackett, L.A.; Quek, C.; Brown, D.L.; Sharp, D.M.; Kwan, R.Y.Q.; et al. RAB27A promotes melanoma cell invasion and metastasis via regulation of pro-invasive exosomes. Int. J. Cancer 2019, 144, 3070–3085. [Google Scholar] [CrossRef]
- Bahadoran, P.; Busca, R.; Chiaverini, C.; Westbroek, W.; Lambert, J.; Bille, K.; Valony, G.; Fukuda, M.; Naeyaert, J.M.; Ortonne, J.P.; et al. Characterization of the molecular defects in Rab27a, caused by RAB27A missense mutations found in patients with Griscelli syndrome. J. Biol. Chem. 2003, 278, 11386–11392. [Google Scholar] [CrossRef]
- Aslan, D.; Sari, S.; Derinöz, O.; Dalgiç, B. Griscelli syndrome: Description of a case with Rab27A mutation. Pediatr. Hematol. Oncol. 2006, 23, 255–261. [Google Scholar] [CrossRef]
- Bhattarai, D.; Banday, A.Z.; Joshi, S.; Adhikari, R.C.; Ali, I.; Rashid, M.; Kambay, A.H. Griscelli Syndrome Type 2 Secondary to a Novel RAB27A Variant Presenting With Dermatitis. J. Cutan. Pathol. 2025, 52, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Maimaris, J.; Roa-Bautista, A.; Sohail, M.; Booth, C.; Cugno, C.; Chenchara, L.; Omran, T.B.; Hacohen, Y.; Lim, M.; Gilmour, K.; et al. Griscelli Syndrome Type 2: Comprehensive Analysis of 149 New and Previously Described Patients with RAB27A Deficiency. J. Clin. Immunol. 2024, 45, 50. [Google Scholar] [CrossRef] [PubMed]
- Rausch, J.; Herold, S.; Liebhäuser, S.; Bülbül, Y.; Antunes Ferreira, E.; Wenz, T.; Legscha, K.J.; Bros, M.; Butsch, F.; Kriege, O.; et al. Case Report: Late-onset primary hemophagocytic lymphohistiocytosis leading to the diagnosis of Griscelli syndrome type 2 in a young woman with phenotypically inapparent partial albinism. Front. Immunol. 2025, 16, 1604460. [Google Scholar] [CrossRef]
- Dornbos, P.; Singh, P.; Jang, D.K.; Mahajan, A.; Biddinger, S.B.; Rotter, J.I.; McCarthy, M.I.; Flannick, J. Evaluating human genetic support for hypothesized metabolic disease genes. Cell Metab. 2022, 34, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.M.; Robich, M.; Scott, S.S.; Yang, X.; Ryzhova, L.; Turner, J.E.; Pinz, I.; Liaw, L. Rab27a Regulates Human Perivascular Adipose Progenitor Cell Differentiation. Cardiovasc. Drugs Ther. 2018, 32, 519–530. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhao, X.Y.; Jiang, R.C.; Chen, X.H.; Zhu, X.; Chen, K.F.; Chen, S.Y.; Zhang, X.L.; Qin, Y.; Liu, Y.H.; et al. Increased expression of Sonic hedgehog restores diabetic endothelial progenitor cells and improves cardiac repair after acute myocardial infarction in diabetic mice. Int. J. Mol. Med. 2019, 44, 1091–1105. [Google Scholar] [CrossRef]
- Hu, M.; Guo, G.; Huang, Q.; Cheng, C.; Xu, R.; Li, A.; Liu, N.; Liu, S. The harsh microenvironment in infarcted heart accelerates transplanted bone marrow mesenchymal stem cells injury: The role of injured cardiomyocytes-derived exosomes. Cell Death Dis. 2018, 9, 357. [Google Scholar] [CrossRef]
- Kasai, K.; Ohara-Imaizumi, M.; Takahashi, N.; Mizutani, S.; Zhao, S.; Kikuta, T.; Kasai, H.; Nagamatsu, S.; Gomi, H.; Izumi, T. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J. Clin. Investig. 2005, 115, 388–396. [Google Scholar] [CrossRef]
- Yamaoka, M.; Ishizaki, T.; Kimura, T. Interplay between Rab27a effectors in pancreatic β-cells. World J. Diabetes 2015, 6, 508–516. [Google Scholar] [CrossRef]
- Sun, X.; Lin, J.; Zhang, Y.; Kang, S.; Belkin, N.; Wara, A.K.; Icli, B.; Hamburg, N.M.; Li, D.; Feinberg, M.W. MicroRNA-181b Improves Glucose Homeostasis and Insulin Sensitivity by Regulating Endothelial Function in White Adipose Tissue. Circ. Res. 2016, 118, 810–821. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Lin, X.; Zhang, D.; Hu, C.; Liu, J.; Zhu, Y.; Gao, A.; Han, H.; Chai, M.; et al. Perivascular adipose-derived exosomes reduce macrophage foam cell formation through miR-382-5p and the BMP4-PPARγ-ABCA1/ABCG1 pathways. Vasc. Pharmacol. 2022, 143, 106968. [Google Scholar] [CrossRef]
- Wei, X.; Guan, X.; Ma, X.; He, X.; Wang, S.; Zhou, G.; Liu, H.; Fan, Y. Neutrophil membrane-engineered extrcellular vesicles from perivascular adipose tissue-derived stromal cells for atherosclerotic lesion resolution and enhanced vascular grant remodeling. Adv. Funct. Mater. 2025, 2503873. [Google Scholar] [CrossRef]
- Xie, X.; Wang, S.; Rao, J.; Xue, J.; Lin, K.; Lin, N.; Li, K.; Wu, S.; Liang, W.; Guo, Y. Comprehensive Analysis of Differentially Expressed lncRNAs in the Perivascular Adipose Tissue of Patients with Coronary Heart Disease. Rev. Cardiovasc. Med. 2022, 23, 341. [Google Scholar] [CrossRef] [PubMed]
- Ghadami, S.; Dellinger, K. The lipid composition of extracellular vesicles: Applications in diagnostics and therapeutic delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef]
- Durcin, M.; Fleury, A.; Taillebois, E.; Hilairet, G.; Krupova, Z.; Henry, C.; Truchet, S.; Trotzmuller, M.; Kofeler, H.; Mabilleau, G.; et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1305677. [Google Scholar] [CrossRef] [PubMed]
- Khanabdali, R.; Shojaee, M.; Johnson, J.; Law, S.Q.K.; Lim, M.B.L.; James, P.F.; Tester, A.; Kalionis, B. Profiling the extracellular vesicles of two human placenta-derived mesenchymal stromal cell populations. Exp. Cell Res. 2025, 444, 114387. [Google Scholar] [CrossRef]
- Gummadi, S.; Chitti, S.V.; Kang, T.; Shahi, S.; Mathivanan, S.; Fonseka, P. ExoCarta 2024: A Web-based Repository of Small Extracellular Vesicles Cargo. J. Mol. Biol. 2025, 437, 169218. [Google Scholar] [CrossRef]
- Anwar, M.Y.; Highland, H.M.; Palmer, A.B.; Duong, T.; Lin, Z.; Zhu, W.; Sprinkles, J.; Kim, D.; Young, K.L.; Chen, H.H.; et al. The Circulating Lipidome In Severe Obesity. medRxiv 2025. [Google Scholar] [CrossRef]
- Pizzinat, N.; Ong-Meang, V.; Bourgailh-Tortosa, F.; Blanzat, M.; Perquis, L.; Cussac, D.; Parini, A.; Poinsot, V. Extracellular vesicles of MSCs and cardiomyoblasts are vehicles for lipid mediators. Biochimie 2020, 178, 69–80. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tatsuno, I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020, 27, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Cartier, A.; Hla, T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science 2019, 366, eaar5551. [Google Scholar] [CrossRef] [PubMed]
- Cartier, A.; Leigh, T.; Liu, C.H.; Hla, T. Endothelial sphingosine 1-phosphate receptors promote vascular normalization and antitumor therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 3157–3166. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Vasile, V.C.; Donato, L.J.; Hurme, R.; Laaksonen, R. Ceramides and Ceramide Scores: Clinical Applications for Cardiometabolic Risk Stratification. Front. Endocrinol. 2020, 11, 570628. [Google Scholar] [CrossRef]
- Kopprasch, S.; Dheban, S.; Schuhmann, K.; Xu, A.; Schulte, K.M.; Simeonovic, C.J.; Schwarz, P.E.; Bornstein, S.R.; Shevchenko, A.; Graessler, J. Detection of Independent Associations of Plasma Lipidomic Parameters with Insulin Sensitivity Indices Using Data Mining Methodology. PLoS ONE 2016, 11, e0164173. [Google Scholar] [CrossRef]
- Bhat, O.M.; Mir, R.A.; Nehvi, I.B.; Wani, N.A.; Dar, A.H.; Zargar, M.A. Emerging role of sphingolipids and extracellular vesicles in development and therapeutics of cardiovascular diseases. Int. J. Cardiol. Heart Vasc. 2024, 53, 101469. [Google Scholar] [CrossRef]
- Jiang, X.C.; Liu, J. Sphingolipid Metabolism and Atherosclerosis. In Sphingolipids in Disease; Handbook of Experimental Pharmacology; Springer: Vienna, Austria, 2013; pp. 133–146. [Google Scholar] [CrossRef]
- Donoso-Quezada, J.; Ayala-Mar, S.; González-Valdez, J. The role of lipids in exosome biology and intercellular communication: Function, analytics and applications. Traffic 2021, 22, 204–220. [Google Scholar] [CrossRef]
- Bieberich, E. Sphingolipids and lipid rafts: Novel concepts and methods of analysis. Chem. Phys. Lipids 2018, 216, 114–131. [Google Scholar] [CrossRef]
- Verderio, C.; Gabrielli, M.; Giussani, P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. J. Lipid Res. 2018, 59, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Mirza, I.; Metwally, E.; Morsy, M.H.; Scichilone, G.; Asada, M.C.; Mostafa, A.; Bianco, F.M.; Ali, M.M.; Masrur, M.A.; et al. Lipidomic profiling of human adiposomes identifies specific lipid shifts linked to obesity and cardiometabolic risk. JCI Insight 2025, 10, e191872. [Google Scholar] [CrossRef] [PubMed]
- Quinville, B.M.; Deschenes, N.M.; Ryckman, A.E.; Walia, J.S. A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis. Int. J. Mol. Sci. 2021, 22, 5793. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Bo, S.; Ruscica, M.; Sahebkar, A. Ceramides and diabetes mellitus: An update on the potential molecular relationships. Diabet. Med. 2020, 37, 11–19. [Google Scholar] [CrossRef]
- Zhu, Q.; Scherer, P.E. Ceramides and Atherosclerotic Cardiovascular Disease: A Current Perspective. Circulation 2024, 149, 1624–1626. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Vessey, D.A.; Li, L.; Kelley, M.; Zhang, J.; Karliner, J.S. Sphingosine can pre- and post-condition heart and utilizes a different mechanism from sphingosine 1-phosphate. J. Biochem. Mol. Toxicol. 2008, 22, 113–118. [Google Scholar] [CrossRef]
- Vessey, D.A.; Li, L.; Honbo, N.; Karliner, J.S. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H1429–H1435. [Google Scholar] [CrossRef] [PubMed]
- Egom, E.E.; Mohamed, T.M.; Mamas, M.A.; Shi, Y.; Liu, W.; Chirico, D.; Stringer, S.E.; Ke, Y.; Shaheen, M.; Wang, T.; et al. Activation of Pak1/Akt/eNOS signaling following sphingosine-1-phosphate release as part of a mechanism protecting cardiomyocytes against ischemic cell injury. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H1487–H1495. [Google Scholar] [CrossRef]
- Egea-Jimenez, A.L.; Zimmermann, P. Lipids in Exosome Biology. Handb. Exp. Pharmacol. 2020, 259, 309–336. [Google Scholar] [CrossRef]
- Subra, C.; Grand, D.; Laulagnier, K.; Stella, A.; Lambeau, G.; Paillasse, M.; De Medina, P.; Monsarrat, B.; Perret, B.; Silvente-Poirot, S.; et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 2010, 51, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Molina, Y.L.; García-Seisdedos, D.; Babiy, B.; Lerma, M.; Martínez-Botas, J.; Casarejos, M.J.; Vallejo, M.T.; Gómez-Coronado, D.; Lasunción, M.A.; Pastor, Ó.; et al. Rottlerin Stimulates Exosome/Microvesicle Release Via the Increase of Ceramide Levels Mediated by Ampk in an In Vitro Model of Intracellular Lipid Accumulation. Biomedicines 2022, 10, 1316. [Google Scholar] [CrossRef]
- Elmallah, M.I.Y.; Ortega-Deballon, P.; Hermite, L.; Pais-De-Barros, J.P.; Gobbo, J.; Garrido, C. Lipidomic profiling of exosomes from colorectal cancer cells and patients reveals potential biomarkers. Mol. Oncol. 2022, 16, 2710–2718. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.F.; Aalberts, M.; Jansen, J.W.; van Niel, G.; Wauben, M.H.; Stout, T.A.; Helms, J.B.; Stoorvogel, W. Distinct lipid compositions of two types of human prostasomes. Proteomics 2013, 13, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Samouillan, V.; Martinez de Lejarza Samper, I.M.; Amaro, A.B.; Vilades, D.; Dandurand, J.; Casas, J.; Jorge, E.; de Gonzalo Calvo, D.; Gallardo, A.; Lerma, E.; et al. Biophysical and Lipidomic Biomarkers of Cardiac Remodeling Post-Myocardial Infarction in Humans. Biomolecules 2020, 10, 1471. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chang, H.H.; Chiang, C.L.; Liu, C.H.; Yeh, J.I.; Chen, M.F.; Chen, P.Y.; Kuo, J.S.; Lee, T.J. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation 2011, 124, 1160–1171. [Google Scholar] [CrossRef]
- Omachi, D.O.; Aryee, A.N.A.; Onuh, J.O. Functional Lipids and Cardiovascular Disease Reduction: A Concise Review. Nutrients 2024, 16, 2453. [Google Scholar] [CrossRef]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef]
- Su, H.; Rustam, Y.H.; Masters, C.L.; Makalic, E.; McLean, C.A.; Hill, A.F.; Barnham, K.J.; Reid, G.E.; Vella, L.J. Characterization of brain-derived extracellular vesicle lipids in Alzheimer’s disease. J. Extracell. Vesicles 2021, 10, e12089. [Google Scholar] [CrossRef]
- Simbari, F.; McCaskill, J.; Coakley, G.; Millar, M.; Maizels, R.M.; Fabriás, G.; Casas, J.; Buck, A.H. Plasmalogen enrichment in exosomes secreted by a nematode parasite versus those derived from its mouse host: Implications for exosome stability and biology. J. Extracell. Vesicles 2016, 5, 30741. [Google Scholar] [CrossRef]
- Lydic, T.A.; Townsend, S.; Adda, C.G.; Collins, C.; Mathivanan, S.; Reid, G.E. Rapid and comprehensive ‘shotgun’ lipidome profiling of colorectal cancer cell derived exosomes. Methods 2015, 87, 83–95. [Google Scholar] [CrossRef]
- Almsherqi, Z.A. Potential Role of Plasmalogens in the Modulation of Biomembrane Morphology. Front. Cell Dev. Biol. 2021, 9, 673917. [Google Scholar] [CrossRef] [PubMed]
- Messias, M.C.F.; Mecatti, G.C.; Priolli, D.G.; de Oliveira Carvalho, P. Plasmalogen lipids: Functional mechanism and their involvement in gastrointestinal cancer. Lipids Health Dis. 2018, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Compartmentalized regulation of lipid signaling in oxidative stress and inflammation: Plasmalogens, oxidized lipids and ferroptosis as new paradigms of bioactive lipid research. Prog. Lipid Res. 2023, 89, 101207. [Google Scholar] [CrossRef]
- Chatterjee, T.K.; Stoll, L.L.; Denning, G.M.; Harrelson, A.; Blomkalns, A.L.; Idelman, G.; Rothenberg, F.G.; Neltner, B.; Romig-Martin, S.A.; Dickson, E.W.; et al. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circ. Res. 2009, 104, 541–549. [Google Scholar] [CrossRef]
- Takaoka, M.; Nagata, D.; Kihara, S.; Shimomura, I.; Kimura, Y.; Tabata, Y.; Saito, Y.; Nagai, R.; Sata, M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ. Res. 2009, 105, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, C.; Ebrahimian, T.; Angulo, O.; Paradis, P.; Schiffrin, E.L. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 2009, 54, 1384–1392. [Google Scholar] [CrossRef]
- Karastergiou, K.; Evans, I.; Ogston, N.; Miheisi, N.; Nair, D.; Kaski, J.C.; Jahangiri, M.; Mohamed-Ali, V. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1340–1346. [Google Scholar] [CrossRef]
- Virdis, A.; Duranti, E.; Rossi, C.; Dell’Agnello, U.; Santini, E.; Anselmino, M.; Chiarugi, M.; Taddei, S.; Solini, A. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: Role of perivascular adipose tissue. Eur. Heart J. 2015, 36, 784–794. [Google Scholar] [CrossRef]
- Cheng, K.H.; Chu, C.S.; Lee, K.T.; Lin, T.H.; Hsieh, C.C.; Chiu, C.C.; Voon, W.C.; Sheu, S.H.; Lai, W.T. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int. J. Obes. 2008, 32, 268–274. [Google Scholar] [CrossRef]
- Weston, A.H.; Egner, I.; Dong, Y.; Porter, E.L.; Heagerty, A.M.; Edwards, G. Stimulated release of a hyperpolarizing factor (ADHF) from mesenteric artery perivascular adipose tissue: Involvement of myocyte BKCa channels and adiponectin. Br. J. Pharmacol. 2013, 169, 1500–1509. [Google Scholar] [CrossRef]
- Irie, D.; Kawahito, H.; Wakana, N.; Kato, T.; Kishida, S.; Kikai, M.; Ogata, T.; Ikeda, K.; Ueyama, T.; Matoba, S.; et al. Transplantation of periaortic adipose tissue from angiotensin receptor blocker-treated mice markedly ameliorates atherosclerosis development in apoE-/- mice. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 67–78. [Google Scholar] [CrossRef]
- Park, K.; Li, Q.; Lynes, M.D.; Yokomizo, H.; Maddaloni, E.; Shinjo, T.; St-Louis, R.; Li, Q.; Katagiri, S.; Fu, J.; et al. Endothelial Cells Induced Progenitors Into Brown Fat to Reduce Atherosclerosis. Circ. Res. 2022, 131, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.Y.; Qu, S.L.; Xiong, W.H.; Rom, O.; Chang, L.; Jiang, Z.S. Perivascular adipose tissue (PVAT) in atherosclerosis: A double-edged sword. Cardiovasc. Diabetol. 2018, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Horke, S.; Habermeier, A.; Closs, E.I.; Reifenberg, G.; Gericke, A.; Mikhed, Y.; Münzel, T.; Daiber, A.; Förstermann, U.; et al. Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 78–85. [Google Scholar] [CrossRef]
- Leandro, A.; Queiroz, M.; Azul, L.; Seiça, R.; Sena, C.M. Omentin: A novel therapeutic approach for the treatment of endothelial dysfunction in type 2 diabetes. Free. Radic. Biol. Med. 2021, 162, 233–242. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, F.; Han, F.; Lv, L.; Tang, C.E.; Xie, Z.; Luo, F. Omentin-1 is associated with atrial fibrillation in patients with cardiac valve disease. BMC Cardiovasc. Disord. 2020, 20, 214. [Google Scholar] [CrossRef]
- Du, Y.; Ji, Q.; Cai, L.; Huang, F.; Lai, Y.; Liu, Y.; Yu, J.; Han, B.; Zhu, E.; Zhang, J.; et al. Association between omentin-1 expression in human epicardial adipose tissue and coronary atherosclerosis. Cardiovasc. Diabetol. 2016, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Shibata, R.; Ouchi, N.; Tokuda, Y.; Funakubo, H.; Suzuki, M.; Kataoka, T.; Nagao, T.; Okumura, S.; Shinoda, N.; et al. Increased expression of the adipocytokine omentin in the epicardial adipose tissue of coronary artery disease patients. Atherosclerosis 2016, 251, 299–304. [Google Scholar] [CrossRef]
- Mestres-Arenas, A.; Villarroya, J.; Giralt, M.; Villarroya, F.; Peyrou, M. A Differential Pattern of Batokine Expression in Perivascular Adipose Tissue Depots from Mice. Front. Physiol. 2021, 12, 714530. [Google Scholar] [CrossRef]
- Chang, L.; Villacorta, L.; Li, R.; Hamblin, M.; Xu, W.; Dou, C.; Zhang, J.; Wu, J.; Zeng, R.; Chen, Y.E. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-γ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 2012, 126, 1067–1078. [Google Scholar] [CrossRef]
- Liu, F.; Fang, S.; Liu, X.; Li, J.; Wang, X.; Cui, J.; Chen, T.; Li, Z.; Yang, F.; Tian, J.; et al. Omentin-1 protects against high glucose-induced endothelial dysfunction via the AMPK/PPARδ signaling pathway. Biochem. Pharmacol. 2020, 174, 113830. [Google Scholar] [CrossRef]
- Watts, S.W.; Shaw, S.; Burnett, R.; Dorrance, A.M. Indoleamine 2,3-diooxygenase in periaortic fat: Mechanisms of inhibition of contraction. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H1236–H1247. [Google Scholar] [CrossRef]
- Jüttner, A.A.; Ataei Ataabadi, E.; Golshiri, K.; de Vries, R.; Garrelds, I.M.; Danser, A.H.J.; Visser, J.A.; Roks, A.J.M. Adiponectin secretion by perivascular adipose tissue supports impaired vasodilation in a mouse model of accelerated vascular smooth muscle cell and adipose tissue aging. Vasc. Pharmacol. 2024, 154, 107281. [Google Scholar] [CrossRef]
- Saxton, S.N.; Ryding, K.E.; Aldous, R.G.; Withers, S.B.; Ohanian, J.; Heagerty, A.M. Role of Sympathetic Nerves and Adipocyte Catecholamine Uptake in the Vasorelaxant Function of Perivascular Adipose Tissue. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 880–891. [Google Scholar] [CrossRef]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Agabiti-Rosei, C.; Saxton, S.N.; De Ciuceis, C.; Lorenza Muiesan, M.; Rizzoni, D.; Agabiti Rosei, E.; Heagerty, A.M. Influence of Perivascular Adipose Tissue on Microcirculation: A Link Between Hypertension and Obesity. Hypertension 2024, 81, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kihara, S.; Ouchi, N.; Nishida, M.; Arita, Y.; Kumada, M.; Ohashi, K.; Sakai, N.; Shimomura, I.; Kobayashi, H.; et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 2002, 106, 2767–2770. [Google Scholar] [CrossRef] [PubMed]
- Astapova, O.; Leff, T. Adiponectin and PPARγ: Cooperative and interdependent actions of two key regulators of metabolism. Vitam. Horm. 2012, 90, 143–162. [Google Scholar] [CrossRef]
- Ayers, S.D.; Nedrow, K.L.; Gillilan, R.E.; Noy, N. Continuous nucleocytoplasmic shuttling underlies transcriptional activation of PPARgamma by FABP4. Biochemistry 2007, 46, 6744–6752. [Google Scholar] [CrossRef]
- Margaritis, M.; Antonopoulos, A.S.; Digby, J.; Lee, R.; Reilly, S.; Coutinho, P.; Shirodaria, C.; Sayeed, R.; Petrou, M.; De Silva, R.; et al. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 2013, 127, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Greif, M.; Becker, A.; von Ziegler, F.; Lebherz, C.; Lehrke, M.; Broedl, U.C.; Tittus, J.; Parhofer, K.; Becker, C.; Reiser, M.; et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 781–786. [Google Scholar] [CrossRef]
- Schroeter, M.R.; Eschholz, N.; Herzberg, S.; Jerchel, I.; Leifheit-Nestler, M.; Czepluch, F.S.; Chalikias, G.; Konstantinides, S.; Schäfer, K. Leptin-dependent and leptin-independent paracrine effects of perivascular adipose tissue on neointima formation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 980–987. [Google Scholar] [CrossRef]
- Gil-Ortega, M.; Condezo-Hoyos, L.; García-Prieto, C.F.; Arribas, S.M.; González, M.C.; Aranguez, I.; Ruiz-Gayo, M.; Somoza, B.; Fernández-Alfonso, M.S. Imbalance between pro and anti-oxidant mechanisms in perivascular adipose tissue aggravates long-term high-fat diet-derived endothelial dysfunction. PLoS ONE 2014, 9, e95312. [Google Scholar] [CrossRef]
- Hiramatsu-Ito, M.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kanemura, N.; Kambara, T.; Enomoto, T.; Yuasa, D.; Matsuo, K.; Ito, M.; et al. Omentin attenuates atherosclerotic lesion formation in apolipoprotein E-deficient mice. Cardiovasc. Res. 2016, 110, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.F.; Li, S.M.; Ren, G.P.; Zheng, W.; Lu, Y.J.; Yu, Y.H.; Xu, W.J.; Li, T.H.; Zhou, L.H.; Liu, Y.; et al. Recombinant murine fibroblast growth factor 21 ameliorates obesity-related inflammation in monosodium glutamate-induced obesity rats. Endocrine 2015, 49, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zheng, Q.; Chen, J.; Tan, X.; Li, Q.; Ding, L.; Zhang, R.; Lin, X. FGF21 mitigates atherosclerosis via inhibition of NLRP3 inflammasome-mediated vascular endothelial cells pyroptosis. Exp. Cell Res. 2020, 393, 112108. [Google Scholar] [CrossRef]
- Jin, L.; Lin, Z.; Xu, A. Fibroblast Growth Factor 21 Protects against Atherosclerosis via Fine-Tuning the Multiorgan Crosstalk. Diabetes Metab. J. 2016, 40, 22–31. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Y.; Gu, J.; Wang, S.; Zhou, S.; Wang, Y.; Tan, Y.; Feng, W.; Fu, Y.; Mellen, N.; et al. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of Type 1 diabetic mice. Clin. Sci. 2016, 130, 625–641. [Google Scholar] [CrossRef]
- Wu, L.; Qian, L.; Zhang, L.; Zhang, J.; Zhou, J.; Li, Y.; Hou, X.; Fang, Q.; Li, H.; Jia, W. Fibroblast Growth Factor 21 is Related to Atherosclerosis Independent of Nonalcoholic Fatty Liver Disease and Predicts Atherosclerotic Cardiovascular Events. J. Am. Heart Assoc. 2020, 9, e015226. [Google Scholar] [CrossRef] [PubMed]
- Emont, M.P.; Jacobs, C.; Essene, A.L.; Pant, D.; Tenen, D.; Colleluori, G.; Di Vincenzo, A.; Jørgensen, A.M.; Dashti, H.; Stefek, A.; et al. A single-cell atlas of human and mouse white adipose tissue. Nature 2022, 603, 926–933. [Google Scholar] [CrossRef]
- Sun, W.; Dong, H.; Balaz, M.; Slyper, M.; Drokhlyansky, E.; Colleluori, G.; Giordano, A.; Kovanicova, Z.; Stefanicka, P.; Balazova, L.; et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 2020, 587, 98–102. [Google Scholar] [CrossRef]
- Angueira, A.R.; Sakers, A.P.; Holman, C.D.; Cheng, L.; Arbocco, M.N.; Shamsi, F.; Lynes, M.D.; Shrestha, R.; Okada, C.; Batmanov, K.; et al. Defining the lineage of thermogenic perivascular adipose tissue. Nat. Metab. 2021, 3, 469–484. [Google Scholar] [CrossRef]
- Potts, C.M.; Yang, X.; Lynes, M.D.; Malka, K.; Liaw, L. Exploration of Conserved Human Adipose Subpopulations Using Targeted Single-Nuclei RNA Sequencing Data Sets. J. Am. Heart Assoc. 2025, 14, e038465. [Google Scholar] [CrossRef]
- Vargas, D.; López, C.; Acero, E.; Benitez, E.; Wintaco, A.; Camacho, J.; Carreño, M.; Umaña, J.; Jimenez, D.; Díaz, S.; et al. Thermogenic capacity of human periaortic adipose tissue is transformed by body weight. PLoS ONE 2018, 13, e0194269. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.; Camacho, J.; Duque, J.; Carreño, M.; Acero, E.; Pérez, M.; Ramirez, S.; Umaña, J.; Obando, C.; Guerrero, A.; et al. Functional Characterization of Preadipocytes Derived from Human Periaortic Adipose Tissue. Int. J. Endocrinol. 2017, 2017, 2945012. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404, Correction in J. Extracell. Vesicles 2024, 13, e12451. [Google Scholar] [CrossRef]
- Lee, J.E.; Moon, P.G.; Lee, I.K.; Baek, M.C. Proteomic Analysis of Extracellular Vesicles Released by Adipocytes of Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Protein J. 2015, 34, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yin, F.; Guo, M.; Gan, G.; Lin, G.; Wen, C.; Wang, J.; Song, P.; Wang, J.; Qi, Z.Q.; et al. Quantitative proteomic analysis of exosomes from umbilical cord mesenchymal stem cells and rat bone marrow stem cells. Proteomics 2023, 23, e2200204. [Google Scholar] [CrossRef]
- Men, Y.; Yelick, J.; Jin, S.; Tian, Y.; Chiang, M.S.R.; Higashimori, H.; Brown, E.; Jarvis, R.; Yang, Y. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat. Commun. 2019, 10, 4136. [Google Scholar] [CrossRef]
- Fordjour, F.K.; Abuelreich, S.; Hong, X.; Chatterjee, E.; Lallai, V.; Ng, M.; Saftics, A.; Deng, F.; Carnel-Amar, N.; Wakimoto, H.; et al. Exomap1 mouse: A transgenic model for in vivo studies of exosome biology. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Neckles, V.N.; Morton, M.C.; Holmberg, J.C.; Sokolov, A.M.; Nottoli, T.; Liu, D.; Feliciano, D.M. A transgenic inducible GFP extracellular-vesicle reporter (TIGER) mouse illuminates neonatal cortical astrocytes as a source of immunomodulatory extracellular vesicles. Sci. Rep. 2019, 9, 3094. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, Y.; Han, C.; Fan, C.; Wang, L.; Chen, W.; Du, Y.; Han, D.; Arnone, B.; Xu, S.; et al. Visualization and Identification of Bioorthogonally Labeled Exosome Proteins Following Systemic Administration in Mice. Front. Cell Dev. Biol. 2021, 9, 657456. [Google Scholar] [CrossRef]
- Cersosimo, A.; Longo Elia, R.; Condello, F.; Colombo, F.; Pierucci, N.; Arabia, G.; Matteucci, A.; Metra, M.; Adamo, M.; Vizzardi, E.; et al. Cardiac rehabilitation in patients with atrial fibrillation. Minerva Cardiol. Angiol. 2025. [Google Scholar] [CrossRef]
- Abelhad, N.I.; Kachur, S.M.; Sanchez, A.; Lavie, C.J.; Milani, R.V. Impact of cardiac rehabilitation on psychological factors, cardiorespiratory fitness, and survival: A narrative review. Heart Mind 2023, 7, 13–17. [Google Scholar] [CrossRef]
- Kim, H.; Jung, J.; Park, S.; Joo, Y.; Lee, S.; Sim, J.; Choi, J.; Lee, H.; Hwang, G.; Lee, S. Exercise-Induced Fibroblast Growth Factor-21: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 7284. [Google Scholar] [CrossRef]
- Liu, C.; Yan, X.; Zong, Y.; He, Y.; Yang, G.; Xiao, Y.; Wang, S. The effects of exercise on FGF21 in adults: A systematic review and meta-analysis. PeerJ 2024, 12, e17615. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Geng, L.; Ying, L.; Shu, L.; Ye, K.; Yang, R.; Liu, Y.; Wang, Y.; Cai, Y.; Jiang, X.; et al. FGF21-Sirtuin 3 Axis Confers the Protective Effects of Exercise Against Diabetic Cardiomyopathy by Governing Mitochondrial Integrity. Circulation 2022, 146, 1537–1557. [Google Scholar] [CrossRef]
| Lipid Class | Example Species | Cardioprotective Action | References |
|---|---|---|---|
| Triacylglycerides (TAGs) containing polyunsaturated fatty acids | TAG(18:1/18:1/22:6), | Anti-inflammatory PUFA supply; improve endothelial NO signaling | [42] |
| Prostaglandins containing polyunsaturated fatty acids | Prostaglandin E2-EP4 | Protection from ischemic events | [43] |
| Phosphatidylcholines (PC) and phosphatidylethanolamines (PE) with n3-PUFA chains | PC(22:6-n3) PE(22:6-n3) | Anti-oxidant capacity and protection from ischemic events, linked with better vascular/metabolic outcomes | [44] |
| Plasmalogens (ether-linked Pls) | PlsPE(16:0/22:6), PlsPC(18:0/20:5) | Anti-oxidant; protect endothelial NO | [42] |
| Sphingosine-1-phosphate (S1P) | S1P(d18:1) | Enhances NO production, endothelial barrier, and survival signaling | [45,46] |
| Hexosylceramides (HexCer) | HexCer(d18:1/16:0), HexCer(d18:1/24:0) | Inverse association with CVD risk in some cohorts | [47] |
| Unsaturated lysophosphatidylcholines (Lyso-PCs) | Lyso-PC(18:2), Lyso-PC(20:4) | Some species linked with better vascular function | [48] |
| Sphingomyelins (SM, long-chain unsaturated) | SM(d18:1/24:1) | Some species inversely associated with atherosclerosis | [49,50] |
| Protein | Effects | Species | References |
|---|---|---|---|
| Adiponectin | An anti-inflammatory adipokine. | Human | [77] |
| Protects against vascular neointima lesion formation. | Mice | [78] | |
| Decreased adiponectin induced inflammatory pathophysiological conditions. | Mice | [79] | |
| Reversed molecular interactions associated with CAD, such as the adhesion of THP-1 cells to endothelial cells and reduced expression of intercellular adhesion molecules. | Human | [80] | |
| Reduced expression in obese PVAT; absence contributes to NO inhibition in obesity. | Human | [81] | |
| An anti-inflammatory adipokine; potentially elicits beneficial effects in the pathogenesis of CAD | Human | [82] | |
| Contributes to myocyte hyperpolarization; releases NO to induce vasorelaxation | Mice | [83] | |
| Reduced pro-inflammatory cytokines like TNF-α and IL-6; acts as an anti-inflammatory, and anti-atherogenic. | Mice | [84] | |
| Endothelial Nitric Oxide Synthase (eNOS) | Produces NO that is anti-atherogenic, by controlling vascular smooth muscle proliferation, inhibiting platelet aggregation, leucocyte adhesion, and vascular inflammation. | Mice and Humans | [85,86] |
| Produces NO; Uncoupling of eNOS increases ROS production, leading to oxidative stress and inflammation; Obese/metabolic syndrome mice had higher eNOS uncoupling. | Mice | [79] | |
| Uncoupling contributes to generation of superoxide and impairs tonic NO release; Obese tissue has decreased NO. | Human | [81] | |
| Uncoupling of eNOS diminishes superoxide production; Uncoupling is a function of arginase induction and l-arginine deficiency; Diet-induced obesity leads to l-arginine and NO deficiency, and eNOS uncoupling. | Mice | [87] | |
| Omentin-1 | Recovers anti-contractile action; Improves pro-inflammatory and pro-oxidant PVAT phenotype; Restores NO and inhibits oxidative stress. | Rat | [88] |
| Downregulated in atrial fibrillation; Inhibit TGF-beta1-induced cardiac fibroblast activation; Antifibrotic adipocytokine. | Human | [89] | |
| Decreased in patients with coronary artery disease; Decreased in fat next to coronary stenotic segments. | Human | [90] | |
| Increased expression in epicardial adipose in patients with coronary artery disease; plays cardioprotective role. | Human | [91] | |
| Increased expression in response to cold (brown fat). Mild cold-induced PVAT activation attenuates age-dependent and obesity-induced endothelial dysfunction. | Mice | [92,93] | |
| Caused a relaxation response in vessels. | Mice | [94] | |
| Indoleamine 2,3-dioxygenase metabolite (IDO) | Enzymes primarily in brown fat surrounding the thoracic aorta; Depresses aortic contractility. | Rats | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whittaker, O.R.; Lynes, M.D.; Pinz, I.; Liaw, L. Promotion of Cardiovascular Homeostasis by the Perivascular Adipose Tissue Secretome. Int. J. Mol. Sci. 2025, 26, 10173. https://doi.org/10.3390/ijms262010173
Whittaker OR, Lynes MD, Pinz I, Liaw L. Promotion of Cardiovascular Homeostasis by the Perivascular Adipose Tissue Secretome. International Journal of Molecular Sciences. 2025; 26(20):10173. https://doi.org/10.3390/ijms262010173
Chicago/Turabian StyleWhittaker, Olivia R., Matthew D. Lynes, Ilka Pinz, and Lucy Liaw. 2025. "Promotion of Cardiovascular Homeostasis by the Perivascular Adipose Tissue Secretome" International Journal of Molecular Sciences 26, no. 20: 10173. https://doi.org/10.3390/ijms262010173
APA StyleWhittaker, O. R., Lynes, M. D., Pinz, I., & Liaw, L. (2025). Promotion of Cardiovascular Homeostasis by the Perivascular Adipose Tissue Secretome. International Journal of Molecular Sciences, 26(20), 10173. https://doi.org/10.3390/ijms262010173






