The Prevalence of Second Neoplasms in Patients with Non-Aldosterone Producing Adrenocortical Lesions
Abstract
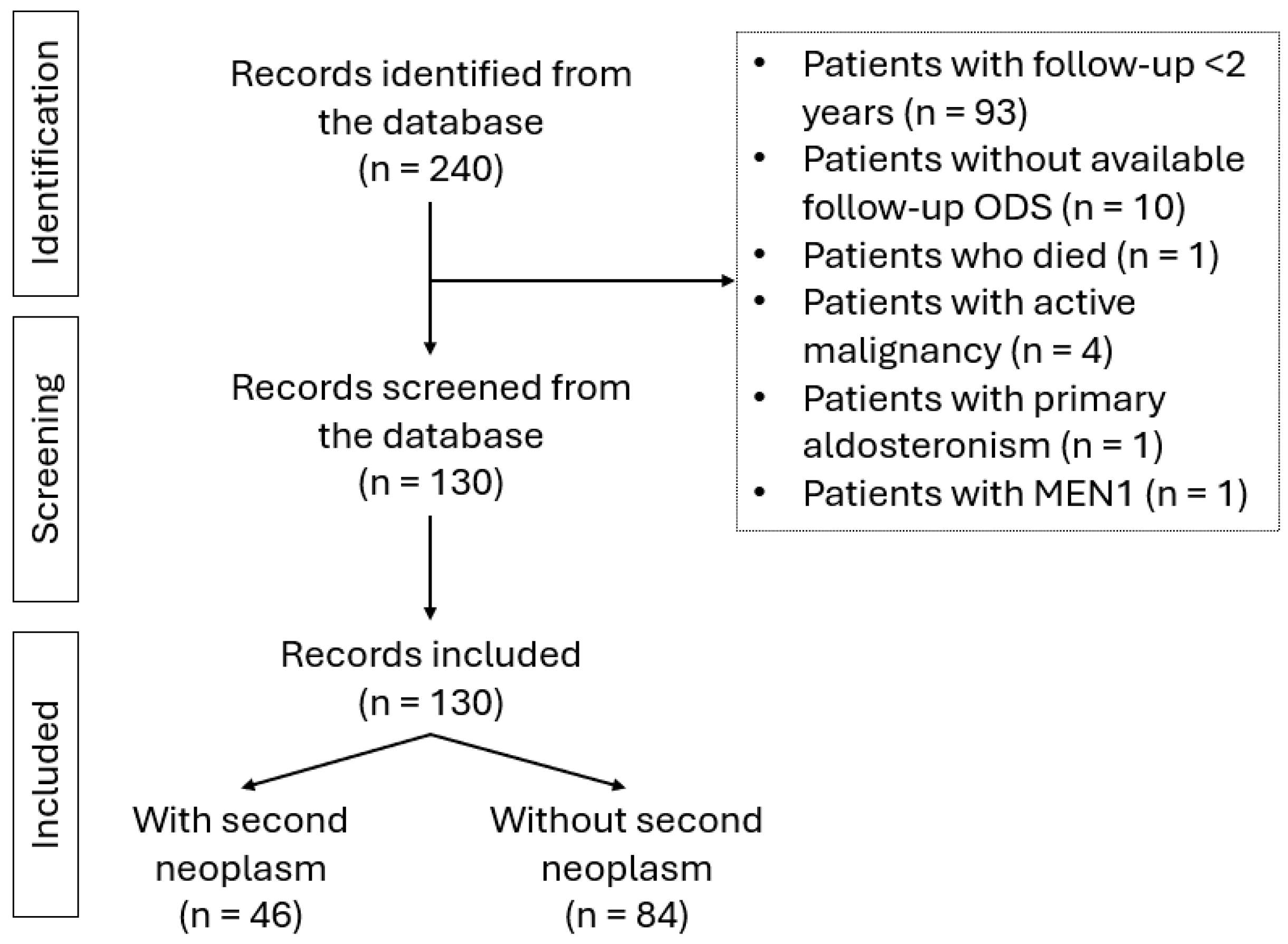
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bovio, S.; Cataldi, A.; Reimondo, G.; Sperone, P.; Novello, S.; Berruti, A.; Borasio, P.; Fava, C.; Dogliotti, L.; Scagliotti, G.V.; et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J. Endocrinol. Investig. 2006, 29, 298–302. [Google Scholar] [CrossRef]
- Reimondo, G.; Castellano, E.; Grosso, M.; Priotto, R.; Puglisi, S.; Pia, A.; Pellegrino, M.; Borretta, G.; Terzolo, M. Adrenal Incidentalomas are Tied to Increased Risk of Diabetes: Findings from a Prospective Study. J. Clin. Endocrinol. Metab. 2020, 105, e973–e981. [Google Scholar] [CrossRef]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef]
- Fassnacht, M.; Tsagarakis, S.; Terzolo, M.; Tabarin, A.; Sahdev, A.; Newell-Price, J.; Pelsma, I.; Marina, L.; Lorenz, K.; Bancos, I.; et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2023, 189, G1–G42. [Google Scholar] [CrossRef]
- Song, J.H.; Chaudhry, F.S.; Mayo-Smith, W.W. The Incidental Adrenal Mass on CT: Prevalence of Adrenal Disease in 1,049 Consecutive Adrenal Masses in Patients with No Known Malignancy. Am. J. Roentgenol. 2008, 190, 1163–1168. [Google Scholar] [CrossRef]
- Deutschbein, T.; Reimondo, G.; Di Dalmazi, G.; Bancos, I.; Patrova, J.; Vassiliadi, D.A.; Nekić, A.B.; Debono, M.; Lardo, P.; Ceccato, F.; et al. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: An international, retrospective, cohort study. Lancet Diabetes Endocrinol. 2022, 10, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Spyroglou, A.; Konstantakou, P.; Minnetti, M.; Altieri, B.; Nowak, E.; Papalexis, P.; Angelousi, A.; Vasiliadi, D.; Kimpel, O.; Macut, D.; et al. Metabolic phenotype in non-aldosterone producing adrenal adenomas with co-existent polycystic ovary syndrome: A joint Ens@t project. Endocrine 2025, 1–11. [Google Scholar] [CrossRef]
- Sconfienza, E.; Tetti, M.; Forestiero, V.; Veglio, F.; Mulatero, P.; Monticone, S. Prevalence of Functioning Adrenal Incidentalomas: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2023, 108, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Bancos, I. Mild autonomous cortisol secretion: Pathophysiology, comorbidities and management approaches. Nat. Rev. Endocrinol. 2024, 20, 460–473. [Google Scholar] [CrossRef]
- Papanastasiou, L.; Alexandraki, K.Ι.; Androulakis, I.I.; Fountoulakis, S.; Kounadi, T.; Markou, A.; Tsiavos, V.; Samara, C.; Papaioannou, T.G.; Piaditis, G.; et al. Concomitant alterations of metabolic parameters, cardiovascular risk factors and altered cortisol secretion in patients with adrenal incidentalomas during prolonged follow-up. Clin. Endocrinol. 2017, 86, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Jiang, Y. Second primary neoplasms after 19281 endocrine gland tumours. Eur. J. Cancer 2001, 37, 1886–1894. [Google Scholar] [CrossRef]
- Tsvetov, G.; Shimon, I.; Benbassat, C. Adrenal incidentaloma: Clinical characteristics and comparison between patients with and without extra-adrenal malignancy. J. Endocrinol. Investig. 2007, 30, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, A.D.; Román, Á.R.; Corrales, E.P.; Idrobo, C.; Ramírez, P.P.; Rojas-Marcos, P.M.; Lázaro, C.R.; Marginean, D.L.; Araujo-Castro, M. Adrenal Incidentalomas and Other Endocrine-Related Adenomas: How Much Does Cortisol Secretion Matter? Cancers 2023, 15, 4735. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Lee, W.-H.; Kar, S.; Burgess, S.; Allara, E. Assessing the role of cortisol in cancer: A wide-ranged Mendelian randomisation study. Br. J. Cancer 2021, 125, 1025–1029. [Google Scholar] [CrossRef]
- Bernabé, D.G.; Tamae, A.C.; Biasoli, É.R.; Oliveira, S.H. Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain, Behav. Immun. 2011, 25, 574–583. [Google Scholar] [CrossRef]
- Dobbie, J.W. Adrenocortical nodular hyperplasia: The ageing adrenal. J. Pathol. 1969, 99, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sakayama, K.; Mashima, N.; Kidani, T.; Miyazaki, T.; Yamamoto, H.; Masuno, H. Effect of cortisol on cell proliferation and the expression of lipoprotein lipase and vascular endothelial growth factor in a human osteosarcoma cell line. Cancer Chemother. Pharmacol. 2007, 61, 471–479. [Google Scholar] [CrossRef]
- Russell, R.P.; Masi, A.T.; Richter, E.D. Adrenal cortical adenomas and hypertension. A clinical pathologic analysis of 690 cases with matched controls and a review of the literature. Medicine 1972, 51, 211–225. [Google Scholar] [CrossRef]
- Prete, A.; Subramanian, A.; Bancos, I.; Chortis, V.; Tsagarakis, S.; Lang, K.; Macech, M.; Delivanis, D.A.; Pupovac, I.D.; Reimondo, G.; et al. Cardiometabolic Disease Burden and Steroid Excretion in Benign Adrenal Tumors. Ann. Intern. Med. 2022, 175, 325–334. [Google Scholar] [CrossRef]
- Mitre-Aguilar, I.B.; Moreno-Mitre, D.; Melendez-Zajgla, J.; Maldonado, V.; Jacobo-Herrera, N.J.; Ramirez-Gonzalez, V.; Mendoza-Almanza, G. The Role of Glucocorticoids in Breast Cancer Therapy. Curr. Oncol. 2022, 30, 298–314. [Google Scholar] [CrossRef]
- Khadka, S.; Druffner, S.R.; Duncan, B.C.; Busada, J.T. Glucocorticoid regulation of cancer development and progression. Front. Endocrinol. 2023, 14, 1161768. [Google Scholar] [CrossRef]
- Tan, L.; Ye, Y.; Xiao, K.; Xu, X.; Liang, H.; Zheng, F.; Qin, Z. A clinicopathological analysis of adrenal tumors in patients with history of extra-adrenal cancers. BMC Cancer 2019, 19, 1–6. [Google Scholar] [CrossRef]
- Samsel, R.; Nowak, K.; Papierska, L.; Karpeta, E.; Roszkowska-Purska, K.; Smiertka, W.; Ostrowski, T.; Chrapowicki, E.; Grabowski, A.; Leszczyńska, D.; et al. Risk of malignancy in adrenal tumors in patients with a history of cancer. Front. Oncol. 2023, 13, 1018475. [Google Scholar] [CrossRef] [PubMed]
- Rashed, W.M.; Saad, A.; Al-Husseini, M.; Galal, A.M.; Ismael, A.M.; Al-Tayep, A.M.; El Shafie, A.; Ali, M.A.; Alfaar, A.S. Incidence of adrenal gland tumor as a second primary malignancy: SEER-based study. Endocr. Connect. 2018, 7, 1040–1048. [Google Scholar] [CrossRef]
- Falhammar, H.; Stenman, A.; Juhlin, C.C.; Kistner, A. Adrenal tumors in patients with neuroendocrine neoplasms. Endocrine 2024, 85, 356–362. [Google Scholar] [CrossRef]
- Massironi, S.; Campana, D.; Pusceddu, S.; Albertelli, M.; Faggiano, A.; Panzuto, F.; Smiroldo, V.; Andreasi, V.; Rossi, R.; Maggio, I.; et al. Second primary neoplasms in patients with lung and gastroenteropancreatic neuroendocrine neoplasms: Data from a retrospective multi-centric study. Dig. Liver Dis. 2021, 53, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Woliński, K.; Komarnicki, P.; Maciejewski, A.; Musiałkiewicz, J.; Gut, P.; Ruchała, M. Prevalence of Second Primary Malignancies in Patients with Well-Differentiated Neuroendocrine Tumors. Endocr. Pract. 2025, 31, 426–432. [Google Scholar] [CrossRef] [PubMed]
| Malignant Neoplasms | N | Pre | Syn | Meta |
|---|---|---|---|---|
| Breast Carcinoma | 6 | 4 | 2 | |
| Colorectal Carcinoma | 6 | 5 | 1 | |
| Differentiated Thyroid Carcinoma | 4 | 3 | 1 | |
| Prostate Carcinoma | 3 | 3 | ||
| Endometrial Carcinoma | 3 | 1 | 2 | |
| Pancreatic Neuroendocrine Tumor | 3 | 2 | 1 | |
| Skin Carcinoma (Squamous or Basal Cell Carcinoma) | 3 | 1 | 2 | |
| Gastric Neuroendocrine Neoplasm Type 1 | 2 | 1 | 1 | |
| Renal Carcinoma | 2 | 1 | 1 | |
| Lung Carcinoma | 1 | 1 | ||
| Melanoma | 2 | 2 | ||
| Bladder Carcinoma | 1 | 1 | ||
| Pancreatic Carcinoma | 1 | 1 | ||
| Appendiceal Neuroendocrine Neoplasm | 1 | 1 | ||
| Medullary Thyroid Cancer | 1 | 1 | ||
| TOTAL | 39 | 25 | 7 | 7 |
| Benign Neoplasms | N | Pre | Syn | Meta |
| ECL-cell Gastric Hyperplasia | 3 | 2 | 1 | |
| Pituitary Adenoma | 4 | 1 | 3 | |
| Lipoma (Retroarticular Area/Cecum) | 2 | 1 | 1 | |
| Parathyroid Adenoma | 3 | 2 | 1 | |
| Parotid Neoplasm (Pleiomorphic Adenoma, Warthin’s Tumor) | 2 | 2 | ||
| Breast Neoplasm (Phyllodes Tumor) | 1 | 1 | ||
| Angiolipoma | 1 | 1 | ||
| Meningioma | 1 | 1 | ||
| Myeloma | 1 | 1 | ||
| IPMN | 1 | 1 | ||
| Colon Polyp | 1 | 1 | ||
| TOTAL | 20 | 10 | 4 | 6 |
| BASELINE | FOLLOW-UP | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients N = 130 | WITH 2nd Neoplasm N = 46 | NO 2nd Neoplasm N = 84 | p | WITH 2nd Malignant Neoplasm N = 35 | NO 2nd Malignant Neoplasm N = 95 | p | All Patients N = 130 | WITH 2nd Neoplasm N = 46 | NO 2nd Neoplasm N = 84 | p | WITH 2nd Malignant Neoplasm N = 35 | NO 2nd Malignant Neoplasm N = 95 | p | |
| Age (years) | 61 (18–89) | 63 (25–85) | 61 (18–89) | 0.285 | 61 (25–81) | 61 (18–89) | 0.711 | 67 (28–91) | 68 (30–89) | 65 (28–91) | 0.497 | 67 (30–89) | 66.5 (28–91) | 0.975 |
| Male (n,%) | 28 (21.5%) | 11 (23.9%) | 17 (20.2%) | 0.626 | 9 (25.7%) | 19 (20.0%) | 0.482 | Not applicable | ||||||
| BMI (kg/m2) | 28.70 (17.14–63.29) ** | 28.19 (17.14–45.61) ** | 28.74 (18.03–63.29) | 0.445 | 27.70 (17.14–45.61) | 28.75 (18.03–63.29) | 0.537 | 28.68 (17.40–55.08) | 27.88 (17.40–44.50) | 28.91 (17.63–55.08) | 0.234 | 27.78 (17.40–44.50) | 28.84 (17.63–55.08) | 0.299 |
| F-ODS (μg/dL) | 1.15 (0.30–4.33) ** | 1.00 (0.41–3.00) | 1.25 (0.30–4.33) | 0.02 | 1.00 (0.41–3.00) | 1.20 (0.30–4.33) | 0.02 | 1.10 (0.30–10.70) | 1.03 (0.49–4.9) | 1.20 (0.30–10.70) | 0.087 | 1.01 (0.49–4.9) | 1.20 (0.30–10.70) | 0.082 |
| ACTH (pg/mL) | 16.7 (3.8–200) | 19.4 (5–107) | 16.6 (3.8–200) | 0.302 | 21.6 (5–107) | 16.6 (3.8–200) | 0.297 | 18.4 (4.52–97.9) | 20.75 (5–97.9) | 16.75 (4.52–65.3) | 0.056 | 23.6 (5–97.9) | 17.1 (4.52–65.3) | 0.031 |
| DHEA-S to LLN | 2.18 (0.42–13.04) | 2.15 (0.51–10.17) | 2.20 (0.42–13.04) | 0.868 | 2.15 (0.51–8.00) | 2.18 (0.42–13.04) | 0.840 | 2.54 (0.34–13.04) | 2.6 (0.51–12.28) | 2.53 (0.34–13.04) | 0253 | 3.02 (0.51–12.28) | 2.52 (0.34–13.04) | 0.339 |
| MACS (n,%) | 33 (25.4%) | 8 (17.4%) | 25 (29.8%) | 0.121 | 5 (14.3%) | 28 (29.5%) | 0.078 | 38 (29.2%) | 7 (15.2%) | 31 (36.9%) | 0.195 | 11 (31.4%) | 27 (28.4%) | 0.420 |
| BMI/F-ODS (kg/m2/μg/dL) | 25.09 (6.27–120.17) | 28.94 (6.84–68.75) | 22.76 (6.27–120.17) | 0.069 | 28.44 (8.45–68.75) | 23.85 (6.27–120.17) | 0.094 | 25.02 (2.68–128.09) | 27.25 (6.13–69.53) | 23.53 (2.68–128.09) | 0.148 | 28.09 (7.60–69.53) | 23.55 (2.68–128.09) | 0.101 |
| ACTH/F-ODS (pg/mL/μg/dL) | 14.20 (0.92–222.22) | 17.62 (2.50–214.00) | 13.00 (0.92–222.22) | 0.256 | 23.65 (2.50–214.00) | 12.64 (0.92–222.22) | 0.046 | 13.35 (0.50- 163.25) | 23.04 (2.43–163.17) | 12.42 (0.50–163.25) | 0.036 | 23.38 (2.43–163.17) | 12.18 (0.50–163.25) | 0.014 |
| Tumor size (mm) | 8 (4.5–42) * | 8 (4.5–35) | 10 (5–42) * | 0.420 | 8 (4.5–35) | 8.5 (5–42) * | 0.769 | 11 (4.5–48) | 8.5 (4.5–35) | 13 (4.7–48) | 0.244 | 10 (4.5–35) | 11.5 (4.7–48) | 0.492 |
| Delta size (mm) | Not applicable | 0 (−8 to 25.5) | 0 (−6 to 8) | 0 (−8 to 25.5) | 0.129 | 0 (−6 to 8) | 0 (−8 to 25.5) | 0.03 | ||||||
| Bilateral (n,%) | 44/129 (34.1) | 16 (34.8) | 28/83 (33.7) | 0.528 | 13 (37.1) | 31/94 (33.0) | 0.524 | 51/129 (39.5) | 18 (39.1) | 33/83(39.8) | 0.548 | 14 (40.0) | 37/94 (39.4) | 0.508 |
| Smokers (n,%) | 52/125 (41.6) | 12/43 (27.9) | 40/82 (48.8) | 0.024 | 10/33 (30.3) | 42/92 (45.7) | 0.125 | Not assessed | ||||||
| HTN (n,%) | 64 (49.2) * | 24 (52.2) | 40 (47.6) * | 0.619 | 17 (48.6) | 47 (49.5) * | 0.927 | 71 (54.6) | 26 (56.5%) | 45 (53.6) | 0.735 | 18 (51.4) | 53 (55.8) | 0.731 |
| T2DM (n,%) | 20/129 (15.5) *,# | 9/45 (20.0) * | 11 (13.1) * | 0.302 | 7/34 (20.6) ** | 13 (13.7) * | 0.340 | 33/129(25.5) | 17/45 (37.0) | 16 (19) | 0.025 | 11/34 (31.4) | 22 (23.2) | 0.337 |
| Dyslipidemia (n,%) | 58 (44.6) * | 17 (37) | 41 (48.8) * | 0.174 | 11 (31.4) | 47 (49.4) * | 0.059 | 72 (55.4) | 21 (45.7) | 51 (60.7) | 0.099 | 15 (42.9) | 57 (60.0) | 0.081 |
| Obesity (n,%) | 57 (43.8) | 19 (41.3) | 38 (45.2) | 0.665 | 14 (40%) | 43 (45.3) | 0.591 | 52 (40) | 17 (37) | 35 (41.7) | 0.599 | 13 (37.1) | 39 (41.1) | 0.686 |
| CKD (n,%) | 5 (3.8) ** | 1 (2.2) | 4 (4.8) | 0.463 | 1 (2.9) | 4/95 (4.2) | 0.722 | 9 (6.9) | 4 (8.7) | 5 (6.0) | 0.556 | 4 (11.4) | 5 (5.3) | 0.219 |
| Osteoporosis (n,%) | 23 (17.7) * | 7 (15.2) | 16 (19.0) * | 0.584 | 4 (11.4) | 19 (20.0) * | 0.256 | 30 (23.1) | 8 (17.4) | 22 (26.2) | 0.255 | 5 (14.3) | 25 (26.3) | 0.149 |
| Fractures (n,%) | 8 (6.2) | 2 (4.3) | 6 (7.1) | 0.526 | 1 (2.9) | 7 (7.4) | 0.342 | 9 (6.9) | 2 (4.3) | 7 (8.3) | 0.518 | 1 (2.9) | 8 (8.4) | 0.442 |
| Dementia (n,%) | 3 (2.3) | 3 (6.5) | 0 (0) | 0.018 | 2 (5.7) | 1 (1.1) | 0.116 | 3 (2.3) | 3 (6.5) | 0 (0) | 0.018 | 2 (5.7) | 1 (1.1) | 0.116 |
| Psychiatric diagnosis (n,%) | 19 (14.6) ** | 7 (15.2) | 12 (14.3) | 0.886 | 5 (14.3) | 14 (14.7) | 0.949 | 23 (17.7) | 9 (19.6) | 14 (16.7) | 0.679 | 7 (20.0) | 16 (16.8) | 0.676 |
| CV thrombosis (n,%) | 8 (6.2) | 2 (4.3) | 6 (7.1) | 0.526 | 2 (5.7) | 6 (6.3) | 0.899 | 9 (6.9) | 3 (6.5) | 6 (7.1) | 0.894 | 3 (8.6) | 6 (6.3) | 0.653 |
| Myocardial infarction (n,%) | 5 (3.8) | 3 (6.5) | 2 (2.4) | 0.240 | 2 (5.7) | 3 (3.2) | 0.501 | 5 (3.8) | 3 (6.5) | 2 (2.4) | 0.240 | 2 (5.7) | 3 (3.2) | 0.501 |
| PTCA (n,%) | 3 (2.3) | 1 (2.2) | 2 (2.4) | 0.940 | 1 (2.9) | 2 (2.1) | 0.800 | 4 (3.1) | 1 (2.2) | 3 (3.6) | 0.659 | 1 (2.9) | 3 (3.2) | 0.930 |
| Heart failure | 1 (0.8) | 1 (2.2) | 0 (0) | 0.175 | 1 (2.9) | 0 (0) | 0.098 | 2 (1.5) | 2 (4.3) | 0 (0) | 0.054 | 2 (5.7) | 0 (0) | 0.019 |
| Atrial fibrillation (n,%) | 6 (4.6) | 3 (6.5) | 3 (3.6) | 0.443 | 2 (5.7) | 4 (4.2) | 0.717 | 6 (4.6) | 3 (6.5) | 3 (3.6) | 0.443 | 2 (5.7) | 4 (4.2) | 0.717 |
| Stroke (n,%) | 2 (1.5) | 0 (0) | 2 (2.4) | 0.292 | 0 (0) | 2 (2.1) | 0.387 | 2 (1.5) | 0 (0) | 2 (2.4) | 0.292 | 0 (0%) | 2 (2.1) | 0.387 |
| DVT (n,%) | 1 (0.8) | 1 (2.2) | 0 (0) | 0.175 | 1 (2.9) | 0 (0) | 0.098 | 3 (2.3) | 1 (2.2) | 2 (2.4) | 0.940 | 1 (2.9) | 2 (2.1) | 0.800 |
| Pulmonary embolism (n,%) | 0 (0) | 0 (0) | 0 (0) | 1.0 | 0 (0) | 0 (0) | 1.0 | 1 (0.8) | 0 (0) | 1 (1.2) | 0.458 | 0 (0) | 1(1.1) | 0.542 |
| BASELINE | FOLLOW-UP | |||||
|---|---|---|---|---|---|---|
| Non-MACS N = 97 | MACS N = 33 | p | Non-MACS N = 97 | MACS N = 33 | p | |
| ANY NEOPLASMS (n,%) | 38 (39.18) | 8 (24.24) | 0.089 | Not applicable | ||
| MALIGNANCY (n,%) | 30 (30.93) | 5 (15.15) | 0.058 | Not applicable | ||
| Age (years) | 59 (18–89) | 65 (42–85) | 0.005 | 63.5 (28–91) | 70 (48–88) | 0.004 |
| Male (n,%) | 18 (18.6) | 10 (30.3) | 0.122 | Not applicable | ||
| BMI (kg/m2) | 29.09 (17.14–51.67) | 28.16 (18.59–63.29) | 0.913 | 29.26 (17.40–55.08) | 28.58 (17.63–50.3) | 0.808 |
| F-ODS (μg/dL) | 1.01 (0.3–1.9) | 2.2 (1.8–4.33) | <0.001 | 1.01 (0.3–4.9) | 2.4 (0.62–10.7) | <0.001 |
| ACTH (pg/mL) | 20.7 (5.6–200) | 9.3 (3.8–36.4) | <0.001 | 20.2 (5–97.9) | 12.2 (4.52–29.8) | <0.001 |
| DHEAS to LLN | 2.96 (0.51–13.0) | 2.15 (0.51–10.2) | 0.013 | 2.62 (0.34–13.0) | 2.62 (0.51–12.3) | 0.098 |
| BMI/F-ODS | 30.02 (13.88–120.17) | 13.49 (6.27–28.77) | <0.001 | 28.26 (7.6–128.09) | 11.37 (2.68–46.08) | <0.001 |
| ACTH/F-ODS | 19.29 (4–222.22) | 4.38 (0.92–15.05) | <0.001 | 20 (3.05–163.25) | 5 (0.5–48.35) | <0.001 |
| Tumor size (mm) | 8 (4.5–40.9) * | 19 (7–42.0) | <0.001 | 8.05 (4.5–48.0) | 19 (7–44.0) | 0.002 |
| Delta size (mm) | Not applicable | 0 (−8 →25.5) | 0 (−4 →7) | 0.931 | ||
| Bilateral (n,%) | 26/96 (27.1) | 18 (54.5) | 0.012 | 33/96 (34.4) | 18 (54.5) | 0.033 |
| Smokers (n,%) | 34/93 (36.6) | 18/32 (56.3) | 0.041 | Not assessed | ||
| Hypertension (n,%) | 41 (42.3) * | 23 (69.7) | 0.009 | 47 (48.5) | 24 (72.7) | 0.036 |
| T2DM (n,%) | 10/96 (10.4) *,# | 10 (30.3) | 0.010 | 22/96 (22.9) | 11 (33.3) | 0.251 |
| Dyslipidemia (n,%) | 36 (37.5) * | 22 (66.7) * | 0.005 | 45 (46.4) | 27 (81.8) | <0.001 |
| Obesity (n,%) | 44 (45.4) | 13 (39.4) ** | 0.550 | 43 (44.3) | 9 (27.3) | 0.079 |
| CKD (n,%) | 2 (2.1) | 3 (9.1) | 0.103 | 4 (4.1) | 5 (15.2) | 0.046 |
| Osteoporosis (n,%) | 15 (15.5) | 8 (24.2) | 0.188 | 18 (18.6) | 12 (36.4) | 0.054 |
| Fractures (n,%) | 6 (6.2) | 2 (6.1) | 0.671 | 6 (6.2) | 3 (9.1) | 0.771 |
| Dementia (n,%) | 3 (3.1) | 0 (0) | 0.412 | 3 (3.1) | 0 (0) | 0.571 |
| Psychiatric disorders (n,%) | 10 (10.3) | 9 (27.3) | 0.022 | 12 (12.4) | 11 (33.3) | 0.015 |
| CV thrombosis (n,%) | 3 (3.1) | 5 (15.2) | 0.025 | 3 (3.1) | 6 (18.2) | 0.008 |
| Myocardial infarction (n,%) | 2 (2.1) | 3 (9.1) | 0.103 | 2 (2.1) | 3 (9.1) | 0.103 |
| PTCA (n,%) | 0 (0) | 3 (9.1) | 0.015 | 0 (0) | 4 (12.1) | 0.004 |
| Heart failure (n,%) | 0 (0) | 1 (3) | 0.254 | 0 (0) | 2 (6.1) | 0.063 |
| Atrial fibrillation (n,%) | 1 (1) | 5 (15.2) | 0.004 | 1 (1) | 4 (12.1) | 0.004 |
| Stroke (n,%) | 0 (0) | 2 (6.1) | 0.063 | 0 (0) | 2 (6.1) | 0.063 |
| DVT (n,%) | 1 (1) | 0 (0) | 1.000 | 3 (3.1) | 0 (0) | 0.571 |
| Pulmonary embolism (n,%) | 0 (0) | 0 (0) | N/A | 0 (0) | 1 (3) | 0.254 |
| Univariate Logistic Regression Analysis | Multivariate Logistic Regression Analysis | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | p | 95% CI | Odds Ratio | p | 95% CI | |
| Factors associated with the presence of any second neoplasms | ||||||
| F-ODS baseline | 0.562 | 0.046 | 0.320–0.989 | 0.614 | 0.112 | 0.336–1.121 |
| DELTA SIZE | 0.842 | 0.05 | 0.708–1.000 | 0.849 | 0.077 | 0.708–1.018 |
| Factors associated with the presence of second malignant neoplasms | ||||||
| F-ODS baseline | 0.468 | 0.027 | 0.239–0.916 | 0.528 | 0.098 | 0.248–1.125 |
| DELTA SIZE | 0.823 | 0.051 | 0.677–1001 | 0.826 | 0.070 | 0.672–1.016 |
| ACTH follow-up | 1.029 | 0.025 | 1.004–1.055 | 1.024 | 0.099 | 0.996–1.052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripolitsioti, P.; Spyroglou, A.; Violetis, O.; Konstantakou, P.; Chouliara, E.; Betsi, G.; Iliakopoulos, K.; Memi, E.; Bramis, K.; Kolomodi, D.; et al. The Prevalence of Second Neoplasms in Patients with Non-Aldosterone Producing Adrenocortical Lesions. Int. J. Mol. Sci. 2025, 26, 10167. https://doi.org/10.3390/ijms262010167
Tripolitsioti P, Spyroglou A, Violetis O, Konstantakou P, Chouliara E, Betsi G, Iliakopoulos K, Memi E, Bramis K, Kolomodi D, et al. The Prevalence of Second Neoplasms in Patients with Non-Aldosterone Producing Adrenocortical Lesions. International Journal of Molecular Sciences. 2025; 26(20):10167. https://doi.org/10.3390/ijms262010167
Chicago/Turabian StyleTripolitsioti, Paraskevi, Ariadni Spyroglou, Odysseas Violetis, Panagiota Konstantakou, Eleni Chouliara, Grigoria Betsi, Konstantinos Iliakopoulos, Eleni Memi, Konstantinos Bramis, Denise Kolomodi, and et al. 2025. "The Prevalence of Second Neoplasms in Patients with Non-Aldosterone Producing Adrenocortical Lesions" International Journal of Molecular Sciences 26, no. 20: 10167. https://doi.org/10.3390/ijms262010167
APA StyleTripolitsioti, P., Spyroglou, A., Violetis, O., Konstantakou, P., Chouliara, E., Betsi, G., Iliakopoulos, K., Memi, E., Bramis, K., Kolomodi, D., Xekouki, P., Konstadoulakis, M., Mastorakos, G., & Alexandraki, K. I. (2025). The Prevalence of Second Neoplasms in Patients with Non-Aldosterone Producing Adrenocortical Lesions. International Journal of Molecular Sciences, 26(20), 10167. https://doi.org/10.3390/ijms262010167









